Abstract
An increasing number of infants with end-stage kidney disease (ESKD) are surviving and receiving renal replacement therapy (RRT). Unique clinical issues specific to this age group of patients influence their short- and long-term outcomes.
This review summarizes current epidemiology, clinical characteristics, ethical dilemmas, management concerns, and outcomes of infants requiring chronic dialysis therapy.
Optimal care during infancy requires a multidisciplinary team working closely with the patient’s family. Nutritional management, infection prevention and attention to cardiovascular status are important treatment targets. Although mortality rates remain higher among infants on dialysis compared to older pediatric dialysis patients, outcomes have improved over time. Most importantly, infants who subsequently receive a kidney transplant are now experiencing graft survival rates that are comparable to older pediatric patients.
Keywords: peritoneal dialysis, hemodialysis, chronic peritoneal dialysis, chronic hemodialysis, pediatric ESKD, infants, neonates, growth, nutrition
Introduction
Dialysis was first used as a modality for renal replacement therapy (RRT) in children more than 60 years ago, initially for the management of acute renal failure. Whereas the use of dialysis for the chronic management of children with end-stage kidney disease (ESKD) proved challenging early on as a result of many technical obstacles, the development of permanent peritoneal catheters for adults, which were subsequently used for children as well, allowed for chronic peritoneal dialysis (CPD) to be established as a treatment modality for pediatric ESKD [1]. Similarly, in the early 1960s the availability of the Scribner arteriovenous shunt supported the development of chronic hemodialysis programs for children as young as 2 years of age [2]. Initially, there were many complications associated with both chronic therapies, including repeated infections, difficulty with maintaining dialysis access, and challenges with ultrafiltration monitoring. For these reasons and others, many pediatric nephrology centers were unable to support the smallest infants and children with ESKD with chronic dialysis. However, with advancements in dialysis technology and clinical expertise over the past several decades, and data confirming improved survival statistics and transplantation outcomes in the infant ESKD population, chronic RRT has become a well-accepted option for infants previously not considered to be candidates for chronic dialysis therapy [3]. Despite these achievements, the initiation and maintenance of chronic dialysis in infants with ESKD is still impacted by a variety of medical management challenges and ethical debates that are, in part, the result of an increasing number of infants with complex co-morbidities being considered as candidates for renal replacement therapy [3, 4]. This educational review will, in turn, summarize current literature on the epidemiology, diagnoses, ethical dilemmas, management, and outcomes of infants requiring chronic dialysis therapy.
Epidemiology
While the total number of children who are receiving chronic dialysis in some countries has decreased recently as a result of a growing number of children receiving preemptive kidney transplants, the proportion of patients who are infants who initiated chronic dialysis in the first year of life has increased. Data from the United States Renal Data System (USRDS) indicate that the number of children and adolescents initiating dialysis has steadily decreased from a rate as high as 14.8 per million population in 2004 to 11.3 per million population in 2015 [5]. However, there were a total of 1,723 infants ≤1 year of age who initiated chronic peritoneal dialysis (CPD) and who were recorded in the USRDS database from 1990–2014; 685 (39.8%) from 1990–2001 and 1,038 (60.2%) over the more recent 12-year period of 2002–2014 [5].
When assessing the age distribution of the entire pediatric dialysis population, the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) reported in 2011 that 13% of all children who initiated dialysis from 1992 to 2010 were <1 year of age [6]. Similar data are available from the European Society of Pediatric Nephrology and European Renal Association-European Dialysis and Transplant Association (ESPN/ERA-EDTA) registry which have reported that 11% of all children who initiated chronic dialysis from 1991 to 2013 were infants [7, 8].
In addition to an increase in the proportion of children initiating dialysis being infants, the USRDS has also demonstrated an increase in the percentage of ESKD patients less than 4 years of age and on dialysis with greater than 1 co-morbidity (3.9% with ≥1 comorbidity from 1990–1994 vs. 31.1% with ≥1 co-morbidity from 2005–2010), indicating that the youngest patients on dialysis have become more medically complex [9].
Diagnoses
The most common primary diagnoses in the infant dialysis population are congenital anomalies of the kidney and urinary tract (CAKUT) including renal hypoplasia/dysplasia and obstructive uropathy, comprising 40–60% of the cohort [6, 10, 11]. Infants with ESKD are also more likely to be diagnosed with disorders such as congenital nephrotic syndrome, autosomal recessive polycystic kidney disease and cortical necrosis related to perinatal asphyxia, when compared to patients greater than 5 years of age at the time of ESKD treatment initiation [9]. Recent literature has demonstrated that a substantial proportion of children with advanced chronic kidney disease (CKD) have an excess burden of rare genomic imbalances, especially in children diagnosed clinically with renal hypodysplasia, which may overlap with neurologic, skeletal, and cardiac disorders [12, 13]. In the future, the regular use of genomic sequencing may not only better define the underlying etiologies of CKD/ESKD in children, but it may also better define the risks for extrarenal comorbidities and in turn facilitate enhanced disease screening and management.
Management of the infant with ESKD
Ethical considerations
The management options for infants with ESKD consist of palliative care or initiation of renal replacement therapy (RRT). Advanced ultrasounds can facilitate the prenatal diagnosis of kidney disease in most neonates with ESKD, especially in the setting of oligohydramnios. Ideally, when a prenatal diagnosis of a severe kidney disorder is made, a multidisciplinary consultation with the child’s family should take place, with participants to include a pediatric nephrologist, neonatologist, and a maternal fetal medicine physician. The consultation provides an opportunity for the healthcare team to present the family with information about the center-specific intrauterine and postnatal morbidity and survival outcomes that accompany the specific kidney disorder so that well informed decisions can be made. Decisions regarding continuation of pregnancy for families in which there is evidence of severe prenatal kidney disease may in part be dependent upon local pediatric policy and experience. In a study of parental decision making in the setting of fetal renal oligohydramnios, 37% of parents decided to terminate the pregnancy, while 60% opted to continue the pregnancy after multidisciplinary counseling [14].
Following birth, pulmonary stabilization is often the primary clinical focus for the infant born with ESKD, and delay in decision making regarding the institution of chronic dialysis while the child is stabilized is feasible in many instances. Of course, the decision regarding whether or not to initiate RRT in the infant born with ESKD can be a most difficult decision for the infant’s parents, nephrologists, neonatologists, and other members of the multi-disciplinary healthcare team. The ethics surrounding the provision or withdrawal of dialysis have been well discussed within the nephrology literature, both pediatric and adult [4, 15–17]. While improvements in dialysis technology and clinical expertise have contributed to improved long-term outcomes for infants with ESKD, comorbidities such as neurocognitive delay, infectious complications, cardiorespiratory disorders, the need for supplemental tube feedings, the frequent need for hospitalizations and the “burden of care” for the family, all contribute to the dilemma that can arise in the shared decision making process between the family and the medical team regarding the decision to institute a lifetime of ESKD care.
The aforementioned comorbidities that are frequently present in infants with ESKD play a significant role in the decision process regarding long-term RRT. Registry data reveals that up to 30–70% of infants with ESKD have one or more non-renal co-morbidities which may further increase the mortality risk [8, 9, 18]. Interestingly, attitudes among pediatric nephrologists have, in turn, become more cautious over time regarding the recommendation for dialysis initiation during infancy. In a 1998 survey, 41% of nephrologists reported they would offer dialysis to all infants <1 month of age with ESKD and 53% of nephrologists would offer dialysis to all infants aged 1–12 months of age with ESKD [3]. In contrast, a very similar survey conducted in 2011 reported that only 30% of nephrologists would offer dialysis to all infants <1 month of age with ESKD and 50% of nephrologist would offer dialysis to all infants 1–12 months. An even smaller percentage of nurses believed that dialysis should be offered to all infants [16]. Not surprisingly, the most influential factor associated with the decision not to offer dialysis was the presence of a non-renal comorbidity. In the same surveys from 1998 and 2011, approximately 70–80% of pediatric nephrologists considered parental rights to refuse RRT acceptable, a decision supported by ethical principles [3, 16].
Overall, most experts believe that the decision to provide or withhold dialysis for the infant with ESKD is best made following in-depth discussions held between the parents and the multidisciplinary health care team, on occasion with additional information provided by parents of other infants/young children who have received chronic dialysis. In the professional responsibility model, neonatal outcomes including neonatal mortality, survival to dialysis, survival to transplantation, and long-term survival should be presented to families [19]. In most cases, this process will result in the development of a consensus opinion based on what action is deemed to be in the best interest of the child, taking into account both the short and long-term outcomes. On occasion, a hospital ethics committee may need to be consulted when the development of a consensus opinion proves difficult.
When should RRT be initiated?
The timing and indications for dialysis initiation in infants with ESKD is dependent upon multiple clinical criteria, which may include the presence of oligoanuria, severe electrolyte disturbances (i.e. hyperkalemia), critical fluid overload, and uremic symptomatology, some or all of which are non-responsive to medical management. Noteworthy is the lack of a general consensus regarding the serum creatinine or blood urea nitrogen levels at which dialysis initiation is absolutely indicated, especially in infants for whom an accurate means of estimating GFR is not available. Although European and American guidelines recommend starting dialysis in pediatric ESKD patients when the eGFR is between 10 and 15 ml/min/1.73m2, the eGFR of healthy infants doesn’t achieve the normal values of older children and adults until age 2 years [20–22]. More important factors to consider regarding the timing of dialysis initiation during infancy include the infant’s nutritional status, which may be compromised by severe oliguria/anuria and/or feeding intolerance, and the growth velocity. Malnutrition can result in poor growth, neurocognitive dysfunction and an increased risk of mortality [23, 24]. Given that infants on dialysis have been shown to demonstrate increases in weight and height as a result of enhanced nutritional management based on data collected by the International Pediatric Peritoneal Dialysis Network (IPPN) and the NAPRTCS, a suboptimal nutritional status (associated with fluid overload) and poor growth of an infant with ESKD are often the primary stimuli to initiate dialysis therapy.
Which modality should be initiated?
Renal transplantation is the preferred RRT for all patients with ESKD; however, transplantation in the youngest infants is technically limited by patient and vasculature size. In turn, transplantation is most often delayed until an infant reaches 8–10 kg, thus prompting the need for dialysis in virtually all infants with ESKD with plans for future transplantation [25]. Whereas either PD or hemodialysis (HD) may serve as the initial RRT for the infant with ESKD, the great majority of infants who receive chronic dialysis are initiated on chronic peritoneal dialysis (CPD) because of the relative technical simplicity of the procedure in contrast to HD. NAPRTCS recently reported that 857 (92%) of a cohort of 928 patients less than 1 year of age initiating dialysis were prescribed CPD as their index dialysis modality [6]. Similarly, the ERA-EDTA and European Society of Pediatric Nephrology (ESPN) registry have revealed that approximately 85–90% of ESKD patients <1 year of age received PD as their first chronic RRT modality [7, 8]. At the same time, it should be emphasized that it is not unusual for infants to require a change in dialysis modality prior to transplantation. Van Stralen et al., reported data from several international dialysis registries which characterized patient outcome during the first 2 years of life on dialysis. Nearly 30% of infants on dialysis in the combined registry analysis switched dialysis treatment modalities before transplant [10]. Vidal et al., reported a 5-year cumulative 25% incidence of dialysis modality change among infants <12 months of age at dialysis initiation [8]. In contrast, NAPRTCS found that only 15.8% of all infants <1 year of age and on chronic dialysis underwent a change in dialysis modality prior to transplantation [6]. Primary reasons reported for modality changes were peritonitis (63%) in infants on PD and patient/family choice (56%) in infants on HD [8].
Peritoneal dialysis
Chronic peritoneal dialysis (CPD) is the most common dialysis modality prescribed to infants with ESKD worldwide, and, as noted above, is the initial dialysis modality in 85–92 % of infants who initiate chronic dialysis [8, 10]. Advantages of CPD, which are especially pertinent for infants, include avoidance of vascular access, few dietary and fluid restrictions, infrequent hemodynamic instability, better preservation of residual kidney function compared to HD, and the feasibility for families living substantial distances from a pediatric dialysis center to conduct the procedure from home following training by the dialysis team. Disadvantages of CPD include the increased responsibility on the part of the families to conduct the care, which more specifically includes the requirement of a clean home environment with adequate temperature-controlled storage space for supplies, in addition to the physical and emotional demand to conduct nightly dialysis, administer medications multiple times per day, and to frequently provide repeated bolus and supplemental tube feeding, all of which can have a significant impact on the lifestyle of the parents and siblings. Absolute contraindications to PD include conditions that compromise the abdominal cavity and peritoneum such as gastroschisis, obliterated peritoneal cavity, omphalocele, and bladder exstrophy. Relative contraindications may include the lack of a suitable caregiver or home environment or a history of extensive abdominal procedures.
For successful initiation of CPD, the majority of infants are able to undergo placement of a double cuffed PD catheter with a downward or lateral positioned exit site that is placed at a distance from any ostomy site and the general diaper area to decrease the risk of peritonitis [26]. Whereas infants >2–3 kg are candidates for a double cuffed PD catheter, smaller infants <2–3 kg often use a single cuffed catheter because of their lack of substantial subcutaneous tissue [22]. Ideally, PD catheters should be placed by dedicated surgeons with expertise in the placement of CPD catheters, especially in infants [27]. Successful placement of the PD catheter depends upon an exit site supported by subcutaneous tissue to prevent leakage. The thin abdominal wall of low birth weight infants can lead to a high incidence of leakage and the need for catheter replacement [28, 29]. In the smallest infants (<1000 grams), single cuff vascular catheters, intravenous cannulas, and/or umbilical venous catheters placed in the abdomen have been used until infants are large enough for double cuffed CPD catheters [30] [31, 32]. The presence of omentum can create additional challenges for good catheter function and at least a partial omentectomy can be particularly beneficial [33]. Finally, the importance of dedicated surgeons committed to placement of unique, atypical catheters in the smallest of infants cannot be overemphasized.
A gastrostomy tube/button is often necessary to support nutrition and medication administration in infants on dialysis, and ideally it should be placed before or at the time of PD catheter placement as another means to reduce the risk of infection, specifically peritonitis [27, 34–36]. Recent data from the Standardized Care to Improve Outcomes in Pediatric End-Stage Renal Disease (SCOPE) collaborative demonstrated that gastrostomy tube placement after PD catheter insertion was associated with a 3-fold increased risk of peritonitis [34]. As a result of this risk, prophylactic antibiotic and antifungal therapy should be considered whenever a gastrostomy is placed after CPD has been initiated [27]. In addition, there is evidence that an open gastrostomy (Stamm procedure) in contrast to a percutaneous procedure (PEG) is preferred in this setting as well, to decrease the risk of peritonitis associated with gastrostomy placement [22, 36]. PD should be held for 2–3 days following the gastrostomy procedure if possible [36].
The need for an access revision early after PD catheter placement is most common during infancy and is often secondary to mechanical dysfunction [37]. Borzych-Duzalka et al., reported that nearly 40% of infants initiated on CPD at <1 year of age required PD catheter revision, most frequently >60 days after the initiation of CPD [37].
The PD prescription for the infant takes into account the fill volume, the number of dialysis exchanges that are required to meet the ultrafiltration and solute clearance needs of the patient, and the dextrose concentration of the dialysate. Most experts initiate PD in infants with a fill volume of 10–20 ml/kg and advance as tolerated. As many automated cycler machines require a minimum fill volume of >100 mL per exchange to account for tubing “dead space”, infants initiating chronic PD at a weight <10 kg often initially require PD via a manual, gravity-based closed system. The system typically includes a buretrol device to permit precise measurement of the dialysate fill volume and a urine meter drainage bag connected to the outflow tubing to precisely measure the peritoneal effluent. In this setting, dwell times are initially every 30–60 minutes and the dialysis is provided continuously, a schedule which most often requires an intensive care unit one-to-one level of nursing support. This approach is continued until the infant is able to tolerate fill volumes >100 mL. Newer automated cyclers are becoming available which allow for the initiation of PD with smaller fill volumes (i.e. Fresenius Medical Care PD-Paed Plus). The target fill volume for infants is generally 600–800 ml/m2 body surface area until 2 years of age [21, 27]. Greater volumes may increase the risk of hernias, leakage, and emesis/gastro-esophageal reflux.
CPD modalities that are available for infants on chronic home PD are automated PD (APD), which is typically conducted at night over a 10–12-hour period with or without a single daytime exchange, and continuous ambulatory peritoneal dialysis (CAPD), in which 4–5 manual exchanges are delivered intermittently over 24 hours. Automated PD is the preferred option (except when APD is not available because of technical and/or fiscal limitations) for infants, in part because of the ease in which the fill volume can be gradually increased to accompany the growth of the child. Data from the ESPN/ERAEDTA registry and the USRDS have revealed that APD is performed by 71% and 96% of infants initiating CPD, respectively [5, 8, 38]. The flexibility of exchange frequency with the cycler is also particularly advantageous when caring for the infant patient. Anuric infants usually require high ultrafiltration rates to allow for the provision of adequate nutrition [39] and these patients do best with more frequent exchanges and shorter dwell times. Alternatively, in infants with polyuric renal failure, the PD prescription may be characterized by longer dwell times with an emphasis on solute removal [40] [41].
The composition of PD solutions is important and the use of dialysate with the highest dextrose concentrations should be restricted because of the associated risk of complications, such as encapsulating peritoneal sclerosis and the potential long-term need of a functional peritoneum for the youngest patients [42]. Guidelines from the European Pediatric Dialysis Working (EPDW) Group recommend use of biocompatible multi-chambered PD fluids which are low in glucose degradation products (GDP) and reduce lactate exposure [43]. Interestingly, while both first generation single chamber PD solutions and biocompatible fluids with low GDP have been shown to impair peritoneal mesothelial morphology and function after only 12–24 months of PD exposure, Rees et al. have also shown that children <24 months of age treated with biocompatible PD solutions experienced more significant catch-up growth ((+0.52 +/−1.82 SDS/year) compared to children receiving PD with conventional single-chamber solutions (−0.06 +/−1.96 SDS/year) [24, 44–46]. Special consideration of the risks and benefits associated with the use of either single chamber PD solutions or biocompatible PD fluids may be of particular importance to infants, who on average spend >2 years on dialysis while experiencing the growth necessary for transplant. To date, while the biocompatible, low GDP solutions are available in Europe, they are not available in the U.S.
Finally, while current cyclers permit the review of dialysis performance with data collected over the monthly clinic interval, newer cyclers with the capacity for daily remote patient monitoring may enhance the safety, efficacy, and adherence of the treatment modality for infants receiving dialysis therapy at home [47].
Hemodialysis
Hemodialysis (HD) is feasible in infants, but is rarely the initial renal replacement modality of choice. The NAPRTCS registry has shown that 2.7% of children with ESKD and <1 years of age were initiated on chronic HD, while data from Europe (ESPN/ERAEDTA registry) revealed that up to 13.5% of infants received chronic HD as their initial form of RRT [6, 8]. An advantage of chronic HD compared to PD includes the decreased burden of care for families, as virtually all infants on chronic HD receive incenter treatments. Although HD offers a decreased treatment time compared to CPD for older children, infants are formula/volume dependent and regularly require 16–24 hours per week of chronic in-center HD to maintain ultrafiltration rates at less than 5–10% of body weight per treatment and still maintain good control of their fluid and hemodynamic status [48–50]. Hemodialysis prescriptions also usually involve blood flows of 20–100 ml/min or a median of 8 ml/kg/min to achieve urea clearances of 3–5 ml/min [48, 50]. Paglialonga et al. reported from the Italian Pediatric Dialysis registry that nearly 60–70% of infants on chronic dialysis received >4 sessions of HD per week [50]. Disadvantages of HD for infants are the need for central venous access and the associated risks for vascular injury and infection. Single center data have revealed that greater than 90% of infants receiving chronic HD are accessed via a right or left internal jugular central venous catheter (CVC) 5–8 French in diameter with a median 2.2 catheter changes/patient year and catheter survival times ranging from 1 to 13 months [48, 51–53]. Catheters are generally locked with heparin, citrate based solutions, and/or alteplase solutions, based on data from various single center studies with no specific reference to the experience in infants [50, 54].
Additional risks for the infant on HD include those related to significant blood loss and hemodynamic instability during the procedure, as well as repeated exposure to blood products for blood priming the HD circuit and the resultant impact on allosensitization and future transplantation. In a retrospective review by Al-Hermi et al. of ten infants with ESKD who received in-center HD, the authors described frequent challenges with HD line clotting, catheter-related infections, and poor dialysis adequacy [55]. As a result, there are few circumstances in which infants will preferentially initiate dialysis with chronic HD, such as in the patient with primary hyperoxaluria, or when PD is anatomically contraindicated or unable to be performed. Reports of successful use of intermittent hemodialysis with smaller volume circuits (i.e. Newcastle Infant Dialysis System, NIDUS), which reduce the need for blood priming and provide more precise ultrafiltration with adequate clearance, are promising [56]. There are also reports of successfully dialyzing infants via conventional HD devices with smaller dialyzers (0.2–0.6 m2) and priming volumes of only 18–35 ml with a much lower need for blood priming [48, 53].
Growth
Nearly 30% of postnatal growth occurs during the first 2 years of life in all children. This makes early growth particularly important for infants with ESKD, whose long-term clinical course may be complicated by a variety of factors which have a negative influence on growth and which may, in turn, impact final adult height [24]. Moreover, poor linear growth in pediatric ESKD patients has been associated with a decreased quality of life during childhood and as adults, in addition to excessive patient mortality [57, 58]. Unfortunately, linear growth is often severely impaired in infants with ESKD due to inadequate protein and calorie intake, water and electrolyte losses, metabolic acidosis, and poorly controlled mineral bone disease [59]. It remains unclear to what extent abnormalities of the GH-IGF1 axis contribute to poor linear growth during early infancy in patients with ESKD. In a review of 9 retrospective studies on growth in infants with stages 4 and 5 CKD, conservative measures consisting of adequate nutritional intake, correction of metabolic acidosis and electrolyte abnormalities, and management of fluid status, renal osteodystrophy and anemia resulted in greater median longitudinal growth patterns for the majority of infants [60]. However, the studies demonstrated inconsistencies in the ability of infants with CKD to actually experience catch up growth with the management noted above, emphasizing the importance of attention being directed early at the treatment of modifiable growth-related factors. Finally, reports on the use of recombinant human growth hormone (rhGH) in growth-retarded infants with CKD have consistently demonstrated significantly enhanced height velocity following the initiation of therapy [60, 61]. The provision of rhGH for growth-retarded infants on dialysis can be challenging because of fiscal/insurance-related obstacles, and the therapy is typically not instituted until the child is >1 year old and accompanied by evidence that the height and growth velocity are poor despite the correction of all modifiable risk factors [62, 63]. The most encouraging data regarding the growth of infants with ESKD and on dialysis was demonstrated in the 2011 NAPRTCS annual report which showed improvement in both height and weight SDS scores for patients less than 1 year of age, in contrast to the lack of catch up growth in the older patient cohorts [6, 26]. Similar data has been demonstrated by the IPPN [24].
Nutrition
Adequate nutrition is one of the most important contributors to growth during infancy [64]. Also noteworthy is the fact that the nutritional needs of infants with ESKD and on CPD can be greater compared to healthy infants as a result of the protein losses that occur via the peritoneal effluent, gastroesophageal reflux and frequent emesis, and periods of inadequate intake or increased requirements because of hospitalizations, infections, or surgeries. The KDOQI nutrition guidelines thus suggest that infants on CPD should receive 100% of the estimated energy requirements for normal age-dependent needs, with supplements to be provided to those with poor growth [39, 65]. The KDOQI guidelines also recommend that infants on dialysis should maintain a dietary protein intake of 100% of the daily recommended intake for ideal body weight, plus additional protein intake to address dialytic protein losses. Thus, most infants below the age of one year should receive at least 1.5–1.8 g/kg/day of dietary protein [66, 67]. Frequent monitoring of the nutritional status is imperative, and is best conducted in consultation with a pediatric dietitian. The importance of monitoring for evidence of malnutrition as well as obesity was recently emphasized in a European report in which 15.8% of dialysis patients <1 year of age were underweight, but 9% were obese/overweight [68]. The additional calories (10–20 kcal/kg/day) provided through the absorption of the osmotic agent glucose across the peritoneum in infants receiving CPD certainly contributes to the risk for excessive weight gain as well, and should be taken into consideration [22].
In order to meet these nutritional requirements, supplemental tube feedings (nasogastric or gastrostomy) are frequently required [23, 29, 33]. When the majority of nutritional needs are provided through a feeding tube, it is highly recommended to provide regular oral stimulation to the infant as well [69, 70].
Many infants on CPD are also at risk for excessive sodium and water losses as a result of their primary kidney disorder (e.g. CAKUT and cystic kidney disease), PD-related losses, and poor intake that often occurs in association with inter-current illnesses [71, 72]. Chronic sodium depletion can have an adverse effect on growth velocity, in addition to contributing to low blood pressure and the possible development of ischemic optic neuropathy [73]. Therefore, sodium supplementation is recommended in many infants on CPD, with close monitoring of blood pressure and serum sodium levels [74].
Mineral bone disorder
Management of CKD-Mineral Bone Disorder (CKD-MBD) is exceedingly important because of its impact on the growth of the infant receiving chronic dialysis. Calcium requirements are increased due to rapid growth, bone turnover, and poor enteral calcium absorption. The KDOQI nutrition guideline recommends that total oral and/or enteral calcium intake from nutritional sources and phosphate binders be 100–200% of the age-specific DRI for calcium [65]. At the same time, data from the IPPN demonstrates that higher serum calcium levels are independently associated with younger patient age and thus the potential for adynamic bone disease during infancy should be recognized [75]. The prevalence of hyperphosphatemia has been reported as only 6% in infants on CPD compared to nearly 80% in adolescents on CPD, likely the result of a diet dependent upon formula or breastmilk that tends to contain lower phosphorous concentrations than processed foods [70]. On the other hand, hypophosphatemia can occur as a result of the low phosphorus-containing diet during infancy and may require phosphate supplementation [76]. Activated vitamin D is important to support the insufficient 1α-hydroxylase activity associated with CKD, and supplementation with 25(OH)D is also often required in infants on dialysis, in particular those infants receiving breastmilk [77]. Replacement is indicated to achieve 25(OH)D >30 ng/mL, although no data specific to the needs of infants is available [78].
Finally, intact PTH levels are often elevated in children on dialysis, but to a lesser extent in infants than adolescents, likely owing to the better phosphorus management experienced by infants. Parathyroid hormone levels can be titrated with activated vitamin D supplementation to maintain PTH levels to less than twice the upper limit of normal [79]. Marked elevation of PTH has been associated with worsening of CKD-MBD and anemia in children on PD [75].
Cardiovascular disease
The USRDS has shown that cardiovascular disease is one of the most common causes of death among infants initiated on CPD [80]. Single center data indicates that 70–80% of infants on dialysis demonstrate systolic and/or diastolic blood pressure values >2 standard deviations above the mean despite the frequent use of chronic antihypertensive therapy [48]. ESPN/ERA-EDTA data demonstrates that younger age on dialysis is a risk factor for poorly controlled hypertension in children [81]. This trend is likely influenced by the formula fed infant who is hypervolemic with little or no urine output. Treatment strategies include dialytic volume removal, appropriate salt management and treatment with various combinations of antihypertensive drugs. There is no general consensus regarding target blood pressures in children with CKD [82]. The European Society of Hypertension and the American Academy of Pediatrics guidelines recommend BP targets ranging from <90th%ile to <50th%ile for age, sex, and height [83, 84]. Nevertheless, given the significant morbidity and mortality associated with cardiovascular disease in this population, careful monitoring and management of hypertension in infants on dialysis is essential.
Anemia
Anemia is a common manifestation of ESKD during infancy, being present in nearly 60% of infants from several international databases who initiated dialysis within the first month of life [10]. Iron and erythropoiesis-stimulating agents (ESA) are the mainstays of therapy for infants. Pharmacokinetic data on iron therapy in infants on dialysis is sparse, although both intravenous and oral iron have proven to be safe and effective means to maintain sufficient iron levels in non-dialysis requiring infants receiving ESA agents [85, 86]. ESA therapy is required in many infants with ESKD, and often at significantly higher weight-related dosages compared to older pediatric dialysis patients. Of interest, whereas studies have demonstrated that weekly ESA doses are inversely correlated with age when scaled to body weight, the same is not true when the ESA is prescribed per body surface area [6, 87]. Borzych-Duzalka et al. reported an average erythropoietin equivalent dose of 4300 IU/m2/week independent of age in pediatric PD patients [87].
Complications and outcomes
Pediatric patients with ESKD demonstrate significantly higher rates of hospitalization, morbidity, and mortality when compared to the general pediatric population. The higher hospitalization and mortality rates among infants on dialysis can partially be explained by the higher frequency of infection and comorbid conditions seen in the youngest patients on dialysis [10]. For instance, the 2011 NAPRTCS report revealed an annualized peritonitis rate of 0.85 in infants on CPD compared to a rate of 0.6 in older children [6]. A recent publication from the SCOPE collaborative also reported a dismal in-hospital annualized peritonitis rate of 1.73 episodes per patient year and an associated increased mortality rate for infants who experienced peritonitis during their initial hospitalization, reflective of the complex nature of this patient population [34]. Likewise, single center studies of infants on chronic HD report a catheter-related infection rate of 0.3–10 CVC infections per 1000 catheter days compared to a rate of 0.5–1.6 CVC infections per 1000 catheter days in older children [8].
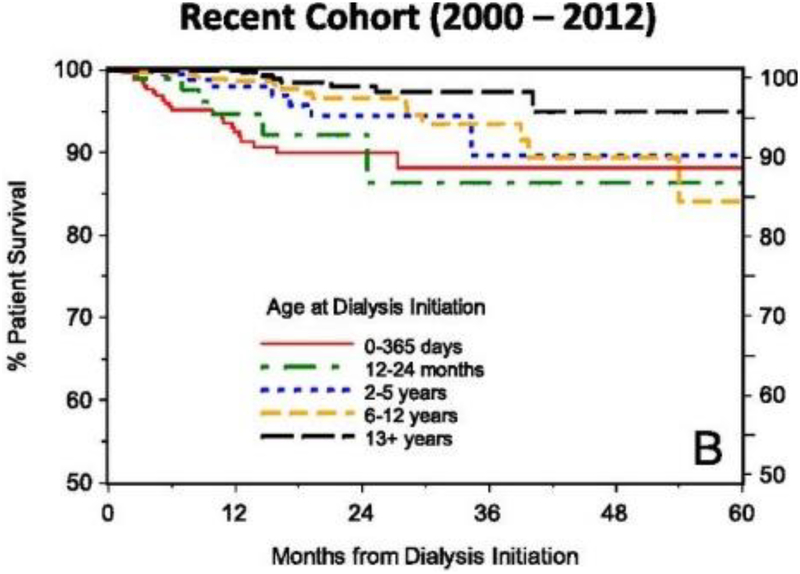
Whereas cardiovascular disease and infection serve as the most frequent primary causes of death for all children with ESKD and on dialysis irrespective of age, survival rates continue to be lowest in infants. However, there is recent evidence of improved outcomes in infants on dialysis, with the NAPRTCS, ESPN/ERA-EDTA, IPPN, ANZDATA, and Japanese RRT registries recently reporting 1 and 5 year survival ranges from 70–80% and 60–70%, respectively [8, 10, 11, 50, 88–90]. However, patient survival data remain poorer for infants compared to older children on dialysis (Figure 1). Predictors of an increased mortality risk for infants include younger age at dialysis initiation and having a primary diagnosis of polycystic kidney disease [11, 50]. Vidal et al. also reported a 5% lower risk of death for every month of later initiation on chronic dialysis for infants who were placed on dialysis during the first year of life [8]. Infection (23.8–35.6%) and cardiorespiratory failure (8.9–25.8%) are the predominant causes of mortality for infants on PD and HD alike [5, 8, 10, 80].
Figure 1:
Survival of children <18 years during initial course of chronic dialysis by age from 0 to 60 months. Image courtesy of NAPRTCS, reproduced with permission.
Finally, the primary goal for the vast majority of infants placed on chronic dialysis is kidney transplantation. The median time to transplant has ranged from 2–3 years from the time of dialysis initiation [5, 50, 80, 88]. Allograft rejection ratios are better in the youngest transplant patients compared to older children and adolescents receiving kidney transplants. NAPRTCS reported allograft rejection ratios of 0.21–0.35 in infants and young children compared to allograft rejection ratios of 0.5–0.65 in pre-adolescent/adolescent aged children, with the difference likely influenced by medication non-adherence in the latter patients [91].
Conclusion
The care of the infant on chronic dialysis remains a significant challenge for the patient’s family and the medical team. The need for dialysis support, the frequent presence of patient comorbidities, and a goal for excellent growth and development and subsequent transplantation, mandates a substantial effort from the multidisciplinary healthcare team and the patient’s caregivers if the best possible outcome is to be achieved. Continued efforts to decrease the frequency of infection and cardiovascular disease-related complications, and the ongoing sharing of clinical experiences and expertise from collaboratives, such as the International Pediatric Dialysis Network (IPDN), NAPRTCS, ESPN/ERA/EDTA, EPDWG and SCOPE, will hopefully result in continued improvements in the care provided to these children [92].
Table 1:
Summary comparison of complications and outcomes in infants receiving chronic hemodialysis and peritoneal dialysis
| Hemodialysis | Peritoneal Dialysis | |
|---|---|---|
| Percent Requiring Catheter Revisions | 40–70% | 30–40% |
| Infectious Complications | 0.3–10 CVC infections per 1000 catheter days | 0.85–1.73 annualized peritonitis rate |
| Percent Requiring Dialysis Modality Switch | 30–70% | 20–30% |
| Cumulative Survival On Dialysis | 2 year survival 80% | 2 year survival of 8090% |
| Frequency Receiving Transplant | 25–88% | 19–85% |
| Time to Transplant | Median 3 years | Median 2.7–3 years |
Key summary points.
The number of infants on chronic dialysis and the complexity of infants receiving chronic dialysis are increasing worldwide.
A multidisciplinary health care team should support an in-depth discussion with families of infants with ESKD to develop a consensus opinion on the decision to provide chronic dialysis.
Timing of chronic dialysis initiation during infancy is frequently influenced by growth status and nutritional needs.
Peritoneal dialysis is the most common chronic dialysis modality prescribed for infants with ESKD.
Patient survival data for infants receiving chronic dialysis are improving and approaching the results experienced by older children on dialysis
Allograft rejection ratios are better in the youngest transplant patients compared to older children and adolescents receiving kidney transplants
Acknowledgments
Funding source: The primary author is supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2TR001109. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations:
- ESKD
end-stage kidney disease
- CPD
chronic peritoneal dialysis
- HD
hemodialysis
- RRT
renal replacement therapy
- NAPRTCS
North American Pediatric Renal Trials and Collaborative Studies
- ANZDATA
Australia and New Zealand Dialysis and Transplant Registry
- KDOQI
Kidney Disease Outcomes Quality Initiative
- IPPN
International Pediatric Peritoneal Dialysis Network
- USRDS
United States Renal Data System
- EPDWG
European Pediatric Dialysis Working Group
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Conflicts of Interest: The authors have no conflicts of interest relevant to this article to disclose.
References
- 1.Hodson EM, Najarian JS, Kjellstrand CM, Simmons RL, Mauer SM (1978) Renal transplantation in children ages 1 to 5 years. Pediatrics 61:458–464 [DOI] [PubMed] [Google Scholar]
- 2.Morse T (1970) Synthetic arteriovenous shunts for hemodialysis in children. J Pediatr Surg 5:23–31 [DOI] [PubMed] [Google Scholar]
- 3.Geary DF (1998) Attitudes of pediatric nephrologists to management of end-stage renal disease in infants. J Pediatr 133:154–156 [DOI] [PubMed] [Google Scholar]
- 4.Lantos JD, Warady BA (2013) The evolving ethics of infant dialysis. Pediatr Nephrol 28:1943–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.2017 Annual Data Report: atlas of pediatric end-stage renal disease in the United States. United States Renal Data System (USRDS). 2017. [cited 2018 January 3,2018]; Available from: https://www.usrds.org/2017/view/v2_07.aspx.
- 6.North American Pediatric Renal Trials and Collaborative Studies: NAPRTCS 2011 Annual Dialysis Report. 2011. [cited 2017 December 17, 2017]; Available from: https://web.emmes.com/study/ped/annlrept/annualrept2011.pdf.
- 7.van der Heijden BJ, van Dijk PC, Verrier-Jones K, Jager KJ, Briggs JD (2004) Renal replacement therapy in children: data from 12 registries in Europe. Pediatr Nephrol 19:213–221 [DOI] [PubMed] [Google Scholar]
- 8.Vidal E, van Stralen KJ, Chesnaye NC, Bonthuis M, Holmberg C, Zurowska A, Trivelli A, Da Silva JEE, Herthelius M, Adams B, Bjerre A, Jankauskiene A, Miteva P, Emirova K, Bayazit AK, Mache CJ, Sanchez-Moreno A, Harambat J, Groothoff JW, Jager KJ, Schaefer F, Verrina E (2017) Infants Requiring Maintenance Dialysis: Outcomes of Hemodialysis and Peritoneal Dialysis. Am J Kidney Dis. 69:617–625 [DOI] [PubMed] [Google Scholar]
- 9.Mitsnefes MM, Laskin BL, Dahhou M, Zhang X, Foster BJ (2013) Mortality risk among children initially treated with dialysis for end-stage kidney disease, 1990–2010. JAMA 309:1921–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Stralen KJ, Borzych-Duzalka D, Hataya H, Kennedy SE, Jager KJ, Verrina E, Inward C, Ronnholm K, Vondrak K, Warady BA, Zurowska AM, Schaefer F, Cochat P (2014) Survival and clinical outcomes of children starting renal replacement therapy in the neonatal period. Kidney Int 86:168–174 [DOI] [PubMed] [Google Scholar]
- 11.Carey WA, Martz KL, Warady BA (2015) Outcome of Patients Initiating Chronic Peritoneal Dialysis During the First Year of Life. Pediatrics 136:e615–622 [DOI] [PubMed] [Google Scholar]
- 12.Verbitsky M, Sanna-Cherchi S, Fasel DA, Levy B, Kiryluk K, Wuttke M, Abraham AG, Kaskel F, Kottgen A, Warady BA, Furth SL, Wong CS, Gharavi AG (2015) Genomic imbalances in pediatric patients with chronic kidney disease. J Clin Invest 125:2171–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanna-Cherchi S, Kiryluk K, Burgess KE, Bodria M, Sampson MG, Hadley D, Nees SN, Verbitsky M, Perry BJ, Sterken R, Lozanovski VJ, Materna-Kiryluk A, Barlassina C, Kini A, Corbani V, Carrea A, Somenzi D, Murtas C, Ristoska-Bojkovska N, Izzi C, Bianco B, Zaniew M, Flogelova H, Weng PL, Kacak N, Giberti S, Gigante M, Arapovic A, Drnasin K, Caridi G, Curioni S, Allegri F, Ammenti A, Ferretti S, Goj V, Bernardo L, Jobanputra V, Chung WK, Lifton RP, Sanders S, State M, Clark LN, Saraga M, Padmanabhan S, Dominiczak AF, Foroud T, Gesualdo L, Gucev Z, Allegri L, Latos-Bielenska A, Cusi D, Scolari F, Tasic V, Hakonarson H, Ghiggeri GM, Gharavi AG (2012) Copy-number disorders are a common cause of congenital kidney malformations. Am J Hum Genet 91:987–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehler K, Gottschalk I, Burgmaier K, Volland R, Buscher AK, Feldkotter M, Keller T, Weber LT, Kribs A, Habbig S (2018) Prenatal parental decision-making and postnatal outcome in renal oligohydramnios. Pediatr Nephrol 33:651–659 [DOI] [PubMed] [Google Scholar]
- 15.Bunchman TE (1996) The ethics of infant dialysis. Perit Dial Int 16 Suppl 1:S505–508 [PubMed] [Google Scholar]
- 16.Teh JC, Frieling ML, Sienna JL, Geary DF (2011) Attitudes of caregivers to management of end-stage renal disease in infants. Perit Dial Int 31:459–465 [DOI] [PubMed] [Google Scholar]
- 17.Ledermann SE, Scanes ME, Fernando ON, Duffy PG, Madden SJ, Trompeter RS (2000) Long-term outcome of peritoneal dialysis in infants. J Pediatr 136:24–29 [DOI] [PubMed] [Google Scholar]
- 18.Neu AM, Sander A, Borzych-Duzalka D, Watson AR, Valles PG, Ha IS, Patel H, Askenazi D, Balasz-Chmielewska I, Lauronen J, Groothoff JW, Feber J, Schaefer F, Warady BA (2012) Comorbidities in chronic pediatric peritoneal dialysis patients: a report of the International Pediatric Peritoneal Dialysis Network. Perit Dial Int 32:410–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas AN, McCullough LB, Chervenak FA, Placencia FX (2017) Evidence-based, ethically justified counseling for fetal bilateral renal agenesis. J Perinat Med 45:585–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watson AR, Gartland C (2001) Guidelines by an Ad Hoc European Committee for Elective Chronic Peritoneal Dialysis in Pediatric Patients. Perit Dial Int 21:240–244 [PubMed] [Google Scholar]
- 21.National Kidney Foundation (2006) K/DOQI clinical practice recommendations for peritoneal dialysis adequacy. Am J Kidney Dis 48:S130–S158 [DOI] [PubMed] [Google Scholar]
- 22.Zurowska AM, Fischbach M, Watson AR, Edefonti A, Stefanidis CJ (2013) Clinical practice recommendations for the care of infants with stage 5 chronic kidney disease (CKD5). Pediatr Nephrol 28:1739–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson RJ, Warady BA (2013) Long-term neurocognitive outcomes of patients with end-stage renal disease during infancy. Pediatr Nephrol 28:1283–1291 [DOI] [PubMed] [Google Scholar]
- 24.Rees L, Azocar M, Borzych D, Watson AR, Buscher A, Edefonti A, Bilge I, Askenazi D, Leozappa G, Gonzales C, van Hoeck K, Secker D, Zurowska A, Ronnholm K, Bouts AH, Stewart H, Ariceta G, Ranchin B, Warady BA, Schaefer F (2011) Growth in very young children undergoing chronic peritoneal dialysis. J Am Soc Nephrol 22:2303–2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rees L (2009) Long-term outcome after renal transplantation in childhood. Pediatr Nephrol 24:475–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaritsky J, Warady BA (2011) Peritoneal dialysis in infants and young children. Semin Nephrol 31:213–224 [DOI] [PubMed] [Google Scholar]
- 27.Warady BA, Bakkaloglu S, Newland J, Cantwell M, Verrina E, Neu A, Chadha V, Yap HK, Schaefer F (2012) Consensus guidelines for the prevention and treatment of catheter-related infections and peritonitis in pediatric patients receiving peritoneal dialysis: 2012 update. Perit Dial Int 32 Suppl 2:S32–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coulthard MG, Vernon B (1995) Managing acute renal failure in very low birthweight infants. Arch Dis Child Fetal Neonatal Ed 73:F187–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vidal E, Edefonti A, Murer L, Gianoglio B, Maringhini S, Pecoraro C, Sorino P, Leozappa G, Lavoratti G, Ratsch IM, Chimenz R, Verrina E (2012) Peritoneal dialysis in infants: the experience of the Italian Registry of Paediatric Chronic Dialysis. Nephrol Dial Transplant 27:388–395 [DOI] [PubMed] [Google Scholar]
- 30.Harshman LA, Muff-Luett M, Neuberger ML, Dagle JM, Shilyansky J, Nester CM, Brophy PD, Jetton JG (2014) Peritoneal dialysis in an extremely low-birth-weight infant with acute kidney injury. Clin Kidney J 7:582–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macchini F, De Carli A, Testa S, Arnoldi R, Ghirardello S, Ardissino G, Mosca F, Torricelli M, Leva E (2012) Feasibility of peritoneal dialysis in extremely low birth weight infants. J Neonatal Surg 1:52. [PMC free article] [PubMed] [Google Scholar]
- 32.Stojanovic VD, Bukarica SS, Antic JB, Doronjski AD (2017) Peritoneal Dialysis in Very Low Birth Weight Neonates. Perit Dial Int 37:389–396 [DOI] [PubMed] [Google Scholar]
- 33.Clark KR, Forsythe JL, Rigg KM, Sharp J, Rangecroft L, Wagget J, Parrott NR, Lennard TW, Coulthard MG (1992) Surgical aspects of chronic peritoneal dialysis in the neonate and infant under 1 year of age. J Pediatr Surg 27:780–783 [DOI] [PubMed] [Google Scholar]
- 34.Zaritsky JJ, Hanevold C, Quigley R, Richardson T, Wong C, Ehrlich J, Lawlor J, Rodean J, Neu A, Warady BA (2018) Epidemiology of peritonitis following maintenance peritoneal dialysis catheter placement during infancy: a report of the SCOPE collaborative. Pediatr Nephrol 33:713–722 [DOI] [PubMed] [Google Scholar]
- 35.Ledermann SE, Spitz L, Moloney J, Rees L, Trompeter RS (2002) Gastrostomy feeding in infants and children on peritoneal dialysis. Pediatr Nephrol 17:246–250 [DOI] [PubMed] [Google Scholar]
- 36.von Schnakenburg C, Feneberg R, Plank C, Zimmering M, Arbeiter K, Bald M, Fehrenbach H, Griebel M, Licht C, Konrad M, Timmermann K, Kemper MJ (2006) Percutaneous endoscopic gastrostomy in children on peritoneal dialysis. Perit Dial Int 26:69–77 [PubMed] [Google Scholar]
- 37.Borzych-Duzalka D, Aki TF, Azocar M, White C, Harvey E, Mir S, Adragna M, Serdaroglu E, Sinha R, Samaille C, Vanegas JJ, Kari J, Barbosa L, Bagga A, Galanti M, Yavascan O, Leozappa G, Szczepanska M, Vondrak K, Tse KC, Schaefer F, Warady BA (2017) Peritoneal Dialysis Access Revision in Children: Causes, Interventions, and Outcomes. Clin J Am Soc Nephrol 12:105–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alexander SR, Salusky IB, Warady BA, Watkins SL (1997) Peritoneal dialysis workshop: pediatrics recommendations. Perit Dial Int 17 Suppl 3:S25–27 [PubMed] [Google Scholar]
- 39.Schmitt CP, Zaloszyc A, Schaefer B, Fischbach M (2011) Peritoneal dialysis tailored to pediatric needs. Int J Nephrol 2011:940267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fischbach M (1996) Peritoneal dialysis prescription for neonates. Perit Dial Int 16 Suppl 1:S512–514 [PubMed] [Google Scholar]
- 41.Fischbach M, Warady BA (2009) Peritoneal dialysis prescription in children: bedside principles for optimal practice. Pediatr Nephrol 24:1633–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Honda M, Warady BA (2010) Long-term peritoneal dialysis and encapsulating peritoneal sclerosis in children. Pediatr Nephrol 25:75–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmitt CP, Bakkaloglu SA, Klaus G, Schroder C, Fischbach M (2011) Solutions for peritoneal dialysis in children: recommendations by the European Pediatric Dialysis Working Group. Pediatr Nephrol 26:1137–1147 [DOI] [PubMed] [Google Scholar]
- 44.Williams JD, Craig KJ, Topley N, Von Ruhland C, Fallon M, Newman GR, Mackenzie RK, Williams GT (2002) Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol 13:470–479 [DOI] [PubMed] [Google Scholar]
- 45.Yoshino A, Honda M, Fukuda M, Araki Y, Hataya H, Sakazume S, Tanaka Y, Kawamura K, Murai T, Kamiyama Y (2001) Changes in peritoneal equilibration test values during long-term peritoneal dialysis in peritonitis-free children. Perit Dial Int 21:180–185 [PubMed] [Google Scholar]
- 46.Schaefer B, Bartosova M, Macher-Goeppinger S, Sallay P, Voros P, Ranchin B, Vondrak K, Ariceta G, Zaloszyc A, Bayazit AK, Querfeld U, Cerkauskiene R, Testa S, Taylan C, VandeWalle J, Yap Y, Krmar RT, Buscher R, Muhlig AK, Drozdz D, Caliskan S, Lasitschka F, Fathallah-Shaykh S, Verrina E, Klaus G, Arbeiter K, Bhayadia R, Melk A, Romero P, Warady BA, Schaefer F, Ujszaszi A, Schmitt CP (2018) Neutral pH and low-glucose degradation product dialysis fluids induce major early alterations of the peritoneal membrane in children on peritoneal dialysis. Kidney Int 94:419–429 [DOI] [PubMed] [Google Scholar]
- 47.Chua AN, Warady BA (2011) Adherence of pediatric patients to automated peritoneal dialysis. Pediatr Nephrol 26:789–793 [DOI] [PubMed] [Google Scholar]
- 48.Pollack S, Eisenstein I, Tarabeih M, Shasha-Lavski H, Magen D, Zelikovic I (2016) Long-term hemodialysis therapy in neonates and infants with end-stage renal disease: a 16-year experience and outcome. Pediatr Nephrol 31:305–313 [DOI] [PubMed] [Google Scholar]
- 49.Raina R, Vijayaraghavan P, Kapur G, Sethi SK, Krishnappa V, Kumar D, Bunchman TE, Bolen SD, Chand D (2018) Hemodialysis in neonates and infants: A systematic review. Semin Dial 31:289–299 [DOI] [PubMed] [Google Scholar]
- 50.Paglialonga F, Consolo S, Pecoraro C, Vidal E, Gianoglio B, Puteo F, Picca S, Saravo MT, Edefonti A, Verrina E (2016) Chronic haemodialysis in small children: a retrospective study of the Italian Pediatric Dialysis Registry. Pediatr Nephrol 31:833–841 [DOI] [PubMed] [Google Scholar]
- 51.Lopez PJ, Troncoso B, Grandy J, Reed F, Ovalle A, Celis S, Reyes D, Letelier N, Zubieta R (2014) Outcome of tunnelled central venous catheters used for haemodialysis in children weighing less than 15 kg. J Pediatr Surg 49:1300–1303 [DOI] [PubMed] [Google Scholar]
- 52.Quinlan C, Bates M, Sheils A, Dolan N, Riordan M, Awan A (2013) Chronic hemodialysis in children weighing less than 10 kg. Pediatr Nephrol 28:803–809 [DOI] [PubMed] [Google Scholar]
- 53.Shroff R, Wright E, Ledermann S, Hutchinson C, Rees L (2003) Chronic hemodialysis in infants and children under 2 years of age. Pediatr Nephrol 18:378–383 [DOI] [PubMed] [Google Scholar]
- 54.Novljan G, Rus RR, Premru V, Ponikvar R, Battelino N (2016) Chronic Hemodialysis in Small Children. Ther Apher Dial 20:302–307 [DOI] [PubMed] [Google Scholar]
- 55.Al-Hermi BE, Al-Saran K, Secker D, Geary DF (1999) Hemodialysis for end-stage renal disease in children weighing less than 10 kg. Pediatr Nephrol 13:401–403 [DOI] [PubMed] [Google Scholar]
- 56.Coulthard MG, Crosier J, Griffiths C, Smith J, Drinnan M, Whitaker M, Beckwith R, Matthews JN, Flecknell P, Lambert HJ (2014) Haemodialysing babies weighing <8 kg with the Newcastle infant dialysis and ultrafiltration system (Nidus): comparison with peritoneal and conventional haemodialysis. Pediatr Nephrol 29:1873–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Al-Uzri A, Matheson M, Gipson DS, Mendley SR, Hooper SR, Yadin O, Rozansky DJ, Moxey-Mims M, Furth SL, Warady BA, Gerson AC (2013) The impact of short stature on health-related quality of life in children with chronic kidney disease. J Pediatr 163:736–741.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosenkranz J, Reichwald-Klugger E, Oh J, Turzer M, Mehls O, Schaefer F (2005) Psychosocial rehabilitation and satisfaction with life in adults with childhood-onset of end-stage renal disease. Pediatr Nephrol 20:1288–1294 [DOI] [PubMed] [Google Scholar]
- 59.Kopple JD (2001) National kidney foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am J Kidney Dis 37(1 Suppl 2):S66–70 [DOI] [PubMed] [Google Scholar]
- 60.Haffner D, Fischer DC (2009) Growth hormone treatment of infants with chronic kidney disease: requirement, efficacy, and safety. Pediatr Nephrol 24:1097–1100 [DOI] [PubMed] [Google Scholar]
- 61.Mencarelli F, Kiepe D, Leozappa G, Stringini G, Cappa M, Emma F (2009) Growth hormone treatment started in the first year of life in infants with chronic renal failure. Pediatr Nephrol 24:1039–1046 [DOI] [PubMed] [Google Scholar]
- 62.Mahan JD, Warady BA, Frane J, Rosenfeld RG, Swinford RD, Lippe B, Davis DA (2010) First-year response to rhGH therapy in children with CKD: a National Cooperative Growth Study Report. Pediatr Nephrol 25:1125–1130 [DOI] [PubMed] [Google Scholar]
- 63.Mahan JD, Warady BA (2006) Assessment and treatment of short stature in pediatric patients with chronic kidney disease: a consensus statement. Pediatr Nephrol 21:917–930 [DOI] [PubMed] [Google Scholar]
- 64.Rees L, Shaw V (2007) Nutrition in children with CRF and on dialysis. Pediatr Nephrol 22:1689–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.(2009) KDOQI Clinical Practice Guideline for Nutrition in Children with CKD: 2008 update. Executive summary. Am J Kidney Dis 53(3 Suppl 2):S11–104 [DOI] [PubMed] [Google Scholar]
- 66.Ronnholm KA, Holmberg C (2006) Peritoneal dialysis in infants. Pediatr Nephrol 21:751–756 [DOI] [PubMed] [Google Scholar]
- 67.Warady BA, Neu AM, Schaefer F (2014) Optimal care of the infant, child, and adolescent on dialysis: 2014 update. Am J Kidney Dis 64:128–142 [DOI] [PubMed] [Google Scholar]
- 68.Bonthuis M, van Stralen KJ, Verrina E, Groothoff JW, Alonso Melgar A, Edefonti A, Fischbach M, Mendes P, Molchanova EA, Paripovic D, Peco-Antic A, Printza N, Rees L, Rubik J, Stefanidis CJ, Sinha MD, Zagozdzon I, Jager KJ, Schaefer F (2013) Underweight, overweight and obesity in paediatric dialysis and renal transplant patients. Nephrol Dial Transplant 28 Suppl 4:iv195–iv204 [DOI] [PubMed] [Google Scholar]
- 69.Greene Z, O’Donnell CP, Walshe M (2016) Oral stimulation for promoting oral feeding in preterm infants. Cochrane Database Syst Rev 9:Cd009720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McCain GC (2003) An evidence-based guideline for introducing oral feeding to healthy preterm infants. Neonatal Netw 22:45–50 [DOI] [PubMed] [Google Scholar]
- 71.Rippe B, Venturoli D (2008) Optimum electrolyte composition of a dialysis solution. Perit Dial Int 28 Suppl 3:S131–136 [PubMed] [Google Scholar]
- 72.Koletzko B, Baker S, Cleghorn G, Neto UF, Gopalan S, Hernell O, Hock QS, Jirapinyo P, Lonnerdal B, Pencharz P, Pzyrembel H, Ramirez-Mayans J, Shamir R, Turck D, Yamashiro Y, Zong-Yi D (2005) Global standard for the composition of infant formula: recommendations of an ESPGHAN coordinated international expert group. J Pediatr Gastroenterol Nutr 41:584–599 [DOI] [PubMed] [Google Scholar]
- 73.Vidal E, Schaefer F (2015) Hypotension in Infants on Chronic Peritoneal Dialysis: Mechanisms, Complications, and Management. Adv Perit Dial 31:54–58 [PubMed] [Google Scholar]
- 74.Schaefer F (2015) Peritoneal dialysis in infants: never lose sight of-and from-arterial hypotension! Perit Dial Int 35:123–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Borzych D, Rees L, Ha IS, Chua A, Valles PG, Lipka M, Zambrano P, Ahlenstiel T, Bakkaloglu SA, Spizzirri AP, Lopez L, Ozaltin F, Printza N, Hari P, Klaus G, Bak M, Vogel A, Ariceta G, Yap HK, Warady BA, Schaefer F (2010) The bone and mineral disorder of children undergoing chronic peritoneal dialysis. Kidney Int 78:1295–1304 [DOI] [PubMed] [Google Scholar]
- 76.Roodhooft AM, Van Hoeck KJ, Van Acker KJ (1990) Hypophosphatemia in infants on continuous ambulatory peritoneal dialysis. Clin Nephrol 34:131–135 [PubMed] [Google Scholar]
- 77.vieth Streym S, Hojskov CS, Moller UK, Heickendorff L, Vestergaard P, Mosekilde L, Rejnmark L (2016) Vitamin D content in human breast milk: a 9-mo follow-up study. Am J Clin Nutr 103:107–114 [DOI] [PubMed] [Google Scholar]
- 78.(2003) K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42(4 Suppl 3):S1–201 [PubMed] [Google Scholar]
- 79.Isakova T, Nickolas TL, Denburg M, Yarlagadda S, Weiner DE, Gutierrez OM, Bansal V, Rosas SE, Nigwekar S, Yee J, Kramer H (2017) KDOQI US Commentary on the 2017 KDIGO Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Am J Kidney Dis 70:737–751 [DOI] [PubMed] [Google Scholar]
- 80.Sanderson KR, Yu Y, Dai H, Willig LK, Warady BA (2018) Outcomes of infants receiving chronic peritoneal dialysis: an analysis of the USRDS registry. Pediatr Nephrol. doi: 10.1007/s00467-018-4056-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kramer AM, van Stralen KJ, Jager KJ, Schaefer F, Verrina E, Seeman T, Lewis MA, Boehm M, Simonetti GD, Novljan G, Groothoff JW (2011) Demographics of blood pressure and hypertension in children on renal replacement therapy in Europe. Kidney Int 80:1092–1098 [DOI] [PubMed] [Google Scholar]
- 82.Halbach S, Flynn J (2015) Treatment of hypertension in children with chronic kidney disease. Curr Hypertens Rep 17:503. [DOI] [PubMed] [Google Scholar]
- 83.Lurbe E, Agabiti-Rosei E, Cruickshank JK, Dominiczak A, Erdine S, Hirth A, Invitti C, Litwin M, Mancia G, Pall D, Rascher W, Redon J, Schaefer F, Seeman T, Sinha M, Stabouli S, Webb NJ, Wuhl E, Zanchetti A (2016) 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens 34:1887–1920 [DOI] [PubMed] [Google Scholar]
- 84.Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, de Ferranti SD, Dionne JM, Falkner B, Flinn SK, Gidding SS, Goodwin C, Leu MG, Powers ME, Rea C, Samuels J, Simasek M, Thaker VV, Urbina EM; Subcommittee on screening and management of high blood pressure in children (2017) Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics 140. doi: 10.1542/peds.2017-1904 [DOI] [PubMed] [Google Scholar]
- 85.El-Shimi MS, El-Farrash RA, Ismail EA, El-Safty IA, Nada AS, El-Gamel OA, Salem YM, Shoukry SM (2015) Renal functional and structural integrity in infants with iron deficiency anemia: relation to oxidative stress and response to iron therapy. Pediatr Nephrol 30:1835–1842 [DOI] [PubMed] [Google Scholar]
- 86.Meyer MP, Haworth C, Meyer JH, Commerford A (1996) A comparison of oral and intravenous iron supplementation in preterm infants receiving recombinant erythropoietin. J Pediatr 129:258–263 [DOI] [PubMed] [Google Scholar]
- 87.Borzych-Duzalka D, Bilginer Y, Ha IS, Bak M, Rees L, Cano F, Munarriz RL, Chua A, Pesle S, Emre S, Urzykowska A, Quiroz L, Ruscasso JD, White C, Pape L, Ramela V, Printza N, Vogel A, Kuzmanovska D, Simkova E, Muller-Wiefel DE, Sander A, Warady BA, Schaefer F (2013) Management of anemia in children receiving chronic peritoneal dialysis. J Am Soc Nephrol 24:665–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alexander RT, Foster BJ, Tonelli MA, Soo A, Nettel-Aguirre A, Hemmelgarn BR, Samuel SM (2012) Survival and transplantation outcomes of children less than 2 years of age with end-stage renal disease. Pediatr Nephrol 27:1975–1983 [DOI] [PubMed] [Google Scholar]
- 89.Chavers BM, Molony JT, Solid CA, Rheault MN, Collins AJ (2015) One-year mortality rates in US children with end-stage renal disease. Am J Nephrol 41:121–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hijazi R, Abitbol CL, Chandar J, Seeherunvong W, Freundlich M, Zilleruelo G (2009) Twenty-five years of infant dialysis: a single center experience. J Pediatr 155:111–117 [DOI] [PubMed] [Google Scholar]
- 91.Foster BJ, Dahhou M, Zhang X, Platt RW, Samuel SM, Hanley JA (2011) Association between age and graft failure rates in young kidney transplant recipients. Transplantation 92:1237–1243 [DOI] [PubMed] [Google Scholar]
- 92.Vidal E (2018) Peritoneal dialysis and infants: further insights into a complicated relationship. Pediatr Nephrol 33:547–551 [DOI] [PubMed] [Google Scholar]