Abstract
Sirtuins (SIRTs) are NAD+-dependent deacylases that play a key role in transcription, DNA repair, metabolism, and oxidative stress resistance. Increasing NAD+ availability regulates endogenous SIRT activity, leading to increased resistance to oxidative stress and decreased mitochondrial reactive oxygen production in multiple cell types and disease models. This protection, at least in part, depends on the activation of antioxidant mitochondrial proteins. We now show that increasing total NAD+ content in astrocytes leads to the activation of the transcription factor nuclear factor, erythroid-derived 2, like 2 (Nfe2l2 or Nrf2) and up-regulation of the antioxidant proteins heme oxygenase 1 (HO-1) and sulfiredoxin 1 (SRXN1). Nrf2 activation also occurs as a result of SIRT6 overexpression. Mutations in Cu-Zn superoxide dismutase 1 (SOD1) cause familial forms of amyotrophic lateral sclerosis (ALS). Astrocytes isolated from mutant human SOD1–overexpressing mice induce motor neuron death in coculture. Treatment with nicotinamide mononucleotide or nicotinamide riboside increases total NAD+ content in ALS astrocytes and abrogates their toxicity toward cocultured motor neurons. The observed neuroprotection depends on SIRT6 expression in astrocytes. Moreover, overexpression of SIRT6 in astrocytes by itself abrogates the neurotoxic phenotype of ALS astrocytes. Our results identify SIRT6 as a potential therapeutic target to prevent astrocyte-mediated motor neuron death in ALS.—Harlan, B. A., Pehar, M., Killoy, K. M., Vargas, M. R. Enhanced SIRT6 activity abrogates the neurotoxic phenotype of astrocytes expressing ALS-linked mutant SOD1.
Keywords: amyotrophic lateral sclerosis, NAD+, nicotinamide mononucleotide, nicotinamide riboside, Nrf2
Amyotrophic lateral sclerosis (ALS) is characterized by the progressive degeneration of motor neurons in the spinal cord, brain stem, and motor cortex. Motor neuron death leads to muscle weakness and paralysis, causing death within 1–5 yr from the time of the onset of symptoms. Approximately 5–10% of ALS cases have a familial history of the disease [familial ALS (FALS)] and are most frequently linked to dominant mutations. The rest of the cases do not have a familial history [sporadic ALS (SALS)] and may result from a yet-unidentified environmental exposure or genetic mutations (1). The first ALS-linked gene identified was superoxide dismutase 1 (SOD1) (2). Mutations in SOD1 account for up to 20% of FALS and 1–2% of apparent SALS cases. Mutations in several other genes, including TAR DNA binding protein, fused-in sarcoma, and a repeat expansion in chromosome 9 open-reading frame 72, have now been identified in many FALS pedigrees (1, 3, 4).
Astrocytes play a key role in determining motor neuron fate in ALS models, and primary astrocytes overexpressing mutant human SOD1 (hSOD1) or mutant hFUS induce motor neuron death in coculture (5–7). In line with these observations, astrocytes differentiated from human post mortem ALS spinal cord–derived progenitor cells and astrocytes obtained from the transdifferentiation of fibroblasts from FALS and SALS patients are also toxic for motor neurons in coculture (8, 9). Moreover, therapeutic strategies aimed at reducing astrocyte-mediated toxicity increase motor neuron survival and improve motor performance in ALS mouse models (10–12).
NAD+ is an essential redox molecule and a key cosubstrate for a range of regulatory proteins that govern fundamental biological processes (13, 14). NAD+ synthesis can occur de novo from l-tryptophan (via the kynurenine pathway) or from precursor molecules like nicotinic acid (via the Priess-Handler pathway), nicotinamide (NAM), or nicotinamide riboside (NR) (15–17). Because all the major NAD+-consuming enzymes generate NAM as a by-product, eukaryotic cells constantly resynthesize NAD+ from NAM. The enzyme NAM phosphoribosyltransferase (NAMPT) catalyzes the conversion of NAM and 5′-phosphoribosyl-1-pyrophosphate to nicotinamide mononucleotide (NMN). Subsequently, NMN adenylyl transferases (NMNATs) transfer adenine from ATP to NMN in order to generate NAD+ (18, 19). NR is also converted into NMN by NR kinases (16). All the biosynthetic pathways converge at the level of dinucleotide formation catalyzed by the NMNATs. However, NAMPT is the rate-limiting enzyme in the salvage pathway, and overexpression of NAMPT (but not NMNATs) increases cellular NAD+ levels (20–22).
Increasing NAD+ availability leads to increased resistance to oxidative stress and decreased mitochondrial reactive oxygen production in multiple cell types. This protection is mediated, at least in part, by increased activity of endogenous sirtuins (SIRTs) (23–29). SIRTs are NAD+-dependent deacylases that play a key role in transcription, DNA repair, metabolism, and oxidative stress resistance (30). Modulating NAD+ availability appears to regulate endogenous SIRT activity and has been shown to be a potential therapeutic approach for age-related diseases (31, 32).
Activation of the transcription factor nuclear factor, erythroid-derived 2, like 2 (Nrf2) has also been suggested to occur as a result of supplementation with NAD+ precursors (33). Nrf2 controls the expression of antioxidant and phase II detoxifying genes containing a cis-acting regulatory element named antioxidant response element (ARE) (34, 35). Nrf2 activation plays a central role regulating antioxidant defenses, and Nrf2 activation in astrocytes confers protection to neighboring neurons in culture and in vivo (10, 36–38).
Treatment with NMN or NR increases NAD+ availability in mutant hSOD1-expressing astrocytes, leading to increased resistance to oxidative stress and reversion of their toxicity toward cocultured motor neurons (26). Increased mitochondrial NAD+ pool and SIRT3 activation appear to play a role in the neuroprotective effect conferred by the treatment with NAD+ precursors (26). Here, we investigated the involvement of an additional protective pathway linking NMN treatment and increased SIRT6 activity to Nrf2 activation and up-regulation of antioxidant defenses in astrocytes. In addition, we present evidence for a central role of SIRT6 expression preventing the neurotoxic phenotype of mutant hSOD1-expressing astrocytes after increasing NAD+ availability.
MATERIALS AND METHODS
Reagents
All chemicals and reagents were obtained from MilliporeSigma (Burlington, MA, USA) unless otherwise specified. Culture media and serum were obtained from Thermo Fisher Scientific (Waltham, MA, USA). Primers and a mouse Nrf2 small interfering RNA (siRNA) (5′-AUAUACGCAGGAGAGGUAAGAAUAA-3′) were obtained from Integrated DNA Technologies (Coralville, IA, USA). Mouse SIRT6 siRNAs (L-061392-01) were obtained from Dharmacon (Lafayette, CO, USA). NR was obtained from BOC Sciences (Shirley, NY, USA).
Animals and primary cultures
B6.Cg-Tg(SOD1*G93A)1Gur/J mice (39) were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). Primary astrocyte cultures were prepared from the cortex or spinal cord of 1-d-old mice as previously described in Vargas et al. (5). Motor neuron cultures were prepared from embryonic d 12.5 (E12.5) mouse spinal cords as previously described in Vargas et al. (10). For coculture experiments, motor neurons were plated on mouse astrocyte monolayers at a density of 300 cells/cm2 and maintained in supplemented L15 medium (Thermo Fisher Scientific) (5). Motor neurons were identified by immunostaining with antineurofilament (MilliporeSigma), and survival was determined by counting all cells displaying intact neurites longer than the diameter of 4 cells. Counts were performed over an area of 0.90 cm2 in 24-well plates.
Cell treatment and transfections
Confluent astrocyte monolayers were treated with 5 mM NMN, 5 mM NR, or vehicle control 24 h before motor neuron plating or biochemical analysis. Adenovirus-expressing green fluorescent protein (GFP) and mouse SIRT6 under a cytomegalovirus promoter were obtained from Vector Biolabs (Malvern, PA, USA). Adenovirus-mediated transfections were performed at a multiplicity of infection of 50 and astrocytes were used 72 h post-transfection. siRNA transfection was performed using Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer’s instructions. Astrocytes were transfected with 25 nM of a target-specific siRNA or a negative control (NC)–siRNA 48 h before subsequent treatment with NMN, NR, or adenovirus-mediated transfections.
Western blot, chromatin immunoprecipitation, and real-time PCR
Western blots were performed as previously described in Vargas et al. and Pehar et al. (10, 40). Membranes were incubated overnight with one of the following antibodies: heme oxygenase 1 (HO-1; 70081, lot: 1; Cell Signaling Technology, Danvers, MA, USA), sulfiredoxin 1 (SRXN1; 14273-1-AP, lot: 39; Proteintech, Rosemont, IL, USA), NRF2 (12721, lot: 6; Cell Signaling Technology), SIRT6 (12486, lot: 1; Cell Signaling Technology), or actin (A5441, lot: 061M4808; MilliporeSigma). Because the Nrf2 protein’s half-life is very short, cells used to analyze Nrf2 levels by Western blot were incubated with the proteasome inhibitor MG-132 (10 μM; MilliporeSigma) for 10 h before protein harvesting and analysis. Image acquisition was performed in a chemiluminescent Western blot scanner (Li-Cor Biosciences, Lincoln, NE, USA) or exposed on Kodak BioMax Light film (Kodak, Rochester, NY, USA). Quantifications were performed using Image Studio Software (Li-Cor Biosciences) or ImageJ Software (National Institutes of Health, Bethesda, MD, USA). Chromatin immunoprecipitation (ChIP) was performed with the SimpleChIP enzymatic ChIP kit (Cell Signaling Technology) using the NRF2 antibody indicated above and analyzed by real-time PCR. Primers designed to amplify a sequence containing an Nrf2 binding site in the Hmox1 and Srxn1 promoters were as follows: Hmox ARE (5′-GGGCTAGCATGCGAAGTGAG-3′ and 5′-GGGCTAGCATGCGAAGTGAG-3′) and Srxn1 ARE (5′-CCTGCAAATTCACCCTGAGT-3′ and 5′-TGTCTCACTCTGACCTAGCTG-3′). Because of the limited yield of astrocytes obtained from mice spinal cord cultures, and because the response of cortical and spinal cord astrocytes to NMN treatment is similar (26), ChIP experiments were performed with cortical astrocytes and noted accordingly in the figure legends. RNA extraction, RNA retrotranscription, and real-time PCR were performed as previously described in Vargas et al. (10).
Statistical analysis
Each experiment was repeated in at least 3 independent primary culture preparations, and values from each independent experiment were combined for data reporting. Multiple-group comparison was performed by 1-way ANOVA with Tukey’s posttest. When comparing the effect of genotype and treatments, 2-way ANOVA was used, followed by Tukey’s posttest. Differences were declared statistically significant if P ≤ 0.05. All statistical computations were performed using Prism v.6.0 (GraphPad Software, La Jolla, CA, USA).
RESULTS
NMN induces ARE-driven gene expression in astrocytes
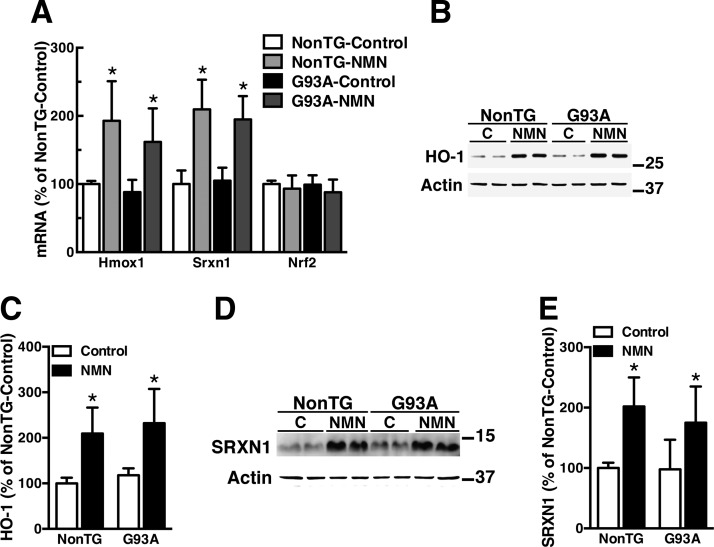
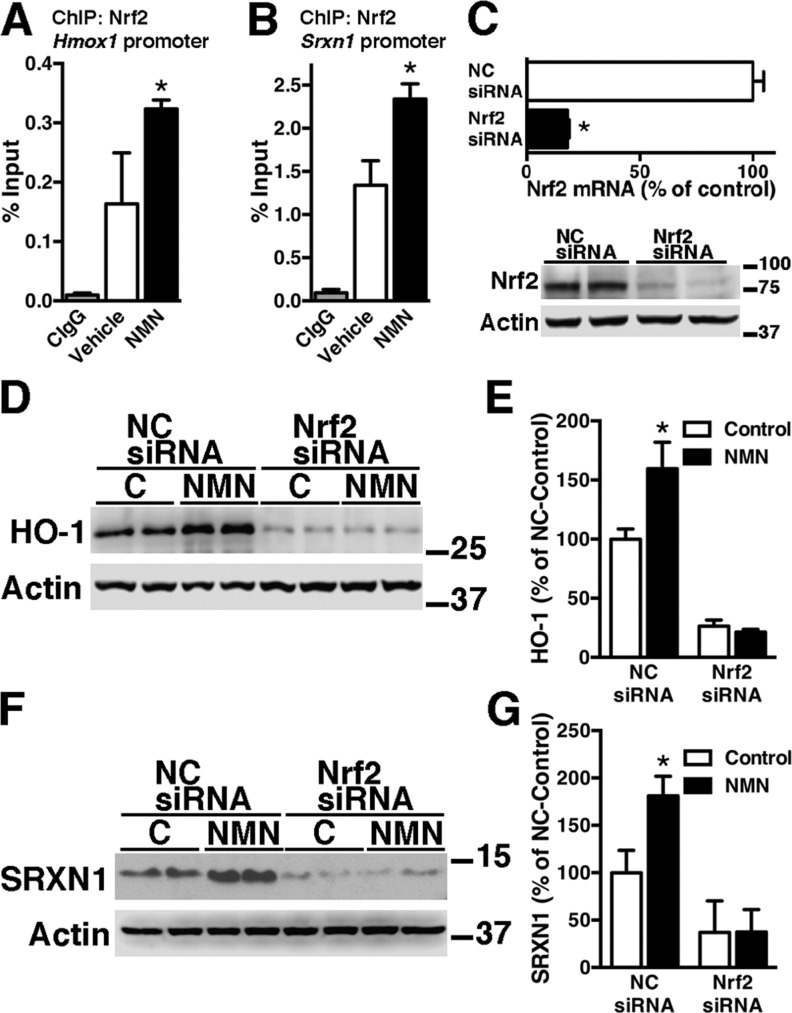
We previously showed that NMN supplementation significantly increases total NAD+ in primary astrocytes obtained from nontransgenic and hSOD1G93A-expressing mice (26). In addition, treatment of confluent spinal cord astrocyte cultures with NMN significantly increases the expression of HO-1 and SRXN1 (Fig. 1), 2 well-known targets of Nrf2. In turn, Nrf2 mRNA expression levels remain unchanged following NMN treatment. Induction levels were similar in both nontransgenic and hSOD1G93A-expressing astrocytes. ChIP–quantitative PCR (qPCR) was used to investigate a direct role of Nrf2 in the induction of these genes. Astrocytes were treated with NMN, and ChIP-qPCR analysis was performed with an antibody against Nrf2 and primers flanking ARE sequences in the promoters of the Hmox1 and Srxn1 genes. ChIP-qPCR analysis demonstrates that after NMN treatment, Nrf2 is recruited to both promoters (Fig. 2A, B). In line with its key role in the transcriptional regulation of ARE-driven genes, silencing Nrf2 expression decreases basal HO-1 and SRXN1 expression in astrocyte cultures (Fig. 2C–G). Importantly, silencing Nrf2 expression before NMN treatment completely prevents HO-1 and SRXN1 up-regulation (Fig. 2C–G).
Figure 1.
NMN induces HO-1 and SRXN1 expression in astrocytes. A) Primary confluent spinal cord astrocyte cultures obtained from nontransgenic (NonTG) or hSOD1G93A (G93A) mice were treated with vehicle (control) or 5 mM NMN. Twenty-four hours later, Hmox1, Srxn1, and Nrf2 mRNA levels were determined by real-time PCR and corrected by actin mRNA levels. B) Western blot analysis of HO-1 protein levels in NonTG and G93A astrocytes following 24 h of treatment with 5 mM NMN. C) Quantification of HO-1 expression after correction by actin levels. D) Western blot analysis of SRXN1 protein levels in NonTG and G93A astrocytes following 24 h of treatment with 5 mM NMN. E) Quantification of SRXN1 expression after correction by actin levels. For all graph panels, each data bar represents the mean ± sd of at least 3 independent experiments. *P < 0.05, significantly different from vehicle (control)-treated NonTG astrocytes.
Figure 2.
NMN induces Nrf2-dependent up-regulation of HO-1 and SRXN1 expression in astrocytes. A, B) Confluent cortical astrocytes were treated with 5 mM NMN, and, 24 h later, ChIP was performed with a preimmune IgG (CIgG) or an anti-Nrf2 antibody. Purified DNA was analyzed by real-time PCR with specific primers flanking an ARE sequence in the Hmox1 (A) or Srxn1 promoter (B). *P<0.05, significantly different from vehicle-treated astrocytes. C) Confluent astrocyte cultures were transfected with an NC-siRNA or Nrf2-siRNA. Forty-eight hours later, Nrf2 mRNA levels were determined by real-time PCR and corrected by actin mRNA levels. *P < 0.05, significantly different from NC-treated astrocytes. The lower panel shows decreased Nrf2-protein expression following Nrf2-siRNA treatment in astrocytes. D) Astrocytes were transfected with NC-siRNA or Nrf2-siRNA and, 48 h later, treated with 5 mM NMN. Twenty-four hours after NMN treatment, HO-1 protein levels were analyzed by Western blot. E) Quantification of HO-1 expression after correction by actin levels. F) Astrocytes were treated as in D, and SRXN1 protein levels were analyzed by Western blot. G) Quantification of SRXN1 expression after correction by actin levels. *P < 0.05, significantly different from its respective vehicle (control). For all graph panels, each data bar represents the mean ± sd of at least 3 independent experiments.
SIRT6 induces ARE-driven gene expression in astrocytes
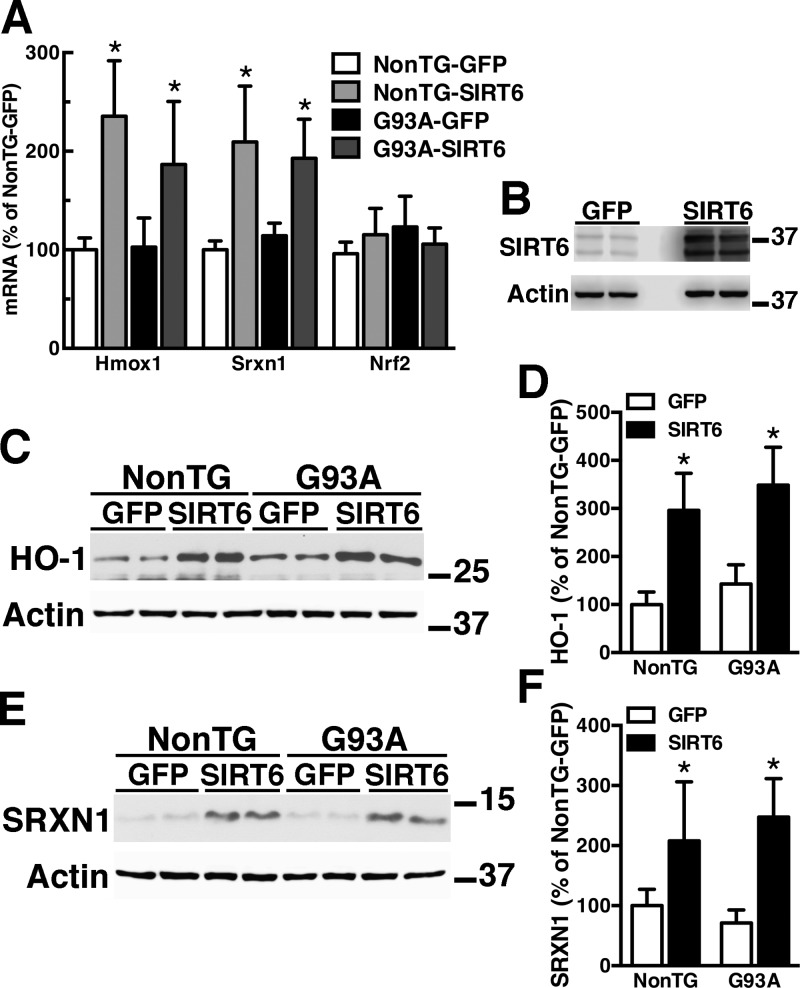
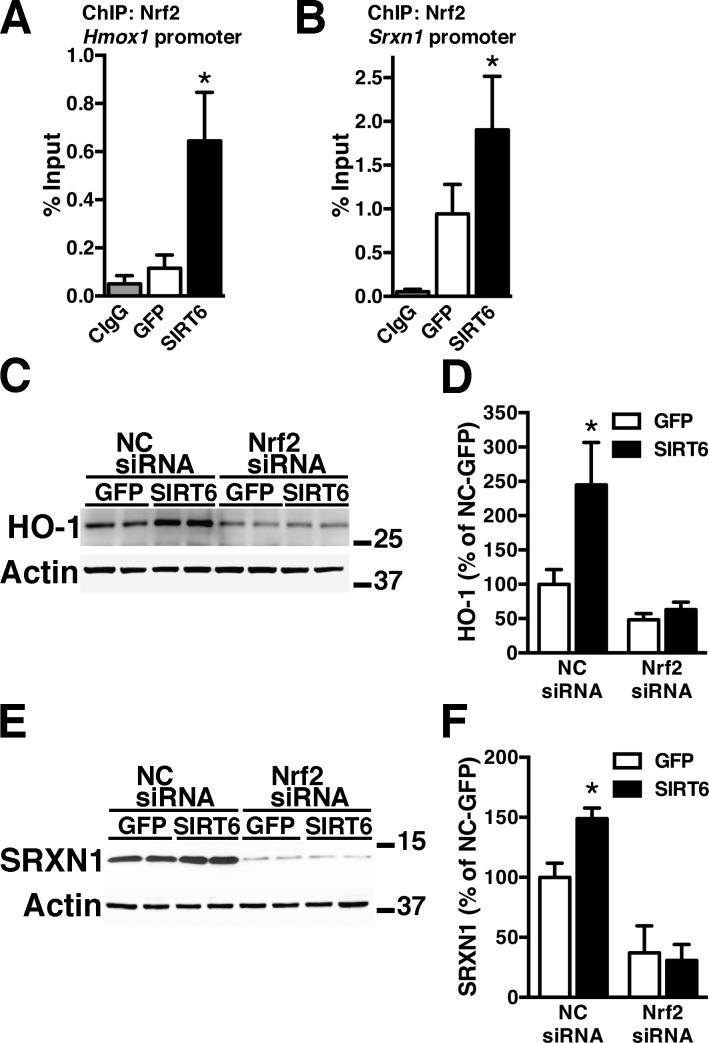
NMN treatment does not cause a change in Nrf2 mRNA expression (Fig. 1A), suggesting that Nrf2 activation occurs at a posttranscriptional level. In principle, an increase in NAD+ availability could lead to Nrf2 activation through an increase in SIRT activity. Of the 7 mammalian SIRTs, SIRT1 and SIRT6 have been directly implicated in Nrf2 activation (41, 42). However, our previous data demonstrating the lack of effect of SIRT1 overexpression on the neurotoxic phenotype of hSOD1G93A astrocytes (26) prompt us to investigate the role of SIRT6 in Nrf2-mediated antioxidant defenses. In addition, SIRT6 has been shown to form a complex with Nrf2 and to promote the recruitment of the basal transcription machinery necessary for the subsequent transactivation of ARE-driven genes (42). SIRT6 overexpression in confluent spinal cord astrocyte cultures significantly increases HO-1 and SRXN1 expression without changing Nrf2 mRNA expression levels (Fig. 3). ChIP-qPCR analysis confirmed that, following SIRT6 overexpression, Nrf2 is recruited to the promoter of both genes (Fig. 4A, B), whereas Nrf2 silencing before SIRT6 overexpression prevents HO-1 and SRXN1 up-regulation (Fig. 4C–F).
Figure 3.
SIRT6 induces HO-1 and SRXN1 expression in astrocytes. A) Primary confluent spinal cord astrocyte cultures obtained from nontransgenic (NonTG) or hSOD1G93A (G93A) mice were transfected with adenovirus-expressing GFP or SIRT6. Forty-eight hours later, Hmox1, Srxn1, and Nrf2 mRNA levels were determined by real-time PCR and corrected by actin mRNA levels. B) Representative Western blot showing SIRT6 overexpression level in astrocytes. Note overexpression of both isoforms of SIRT6. C) Western blot analysis of HO-1 protein levels in NonTG and G93A astrocytes 72 h after transfection with adenovirus-expressing GFP or SIRT6. D) Quantification of HO-1 levels after correction by actin levels. E) Western blot analysis of SRXN1 protein levels in NonTG and G93A astrocytes 72 h after transfection with adenovirus-expressing GFP or SIRT6. F) Quantification of SRXN1 levels after correction by actin levels. For all graph panels, each data bar represents the mean ± sd of at least 3 independent experiments. *P < 0.05, significantly different from NonTG-GFP astrocytes.
Figure 4.
SIRT6 induces Nrf2-dependent up-regulation of HO-1 and SRXN1 expression in astrocytes. Confluent cortical astrocyte cultures were transfected with adenovirus-expressing GFP or SIRT6 and, 48 h later, ChIP was performed with a preimmune IgG (CIgG) or an anti-Nrf2 antibody. Purified DNA was analyzed by real-time PCR with specific primers flanking an ARE sequence in the Hmox1 (A) or Srnx1 promoter (B). *P<0.05, significantly different from GFP astrocytes. C) Astrocytes were treated with an NC-siRNA or Nrf2-siRNA and, 48 h later, transfected with adenovirus-expressing GFP or SIRT6. Seventy-two hours after transfection, HO-1 protein levels were analyzed by Western blot. D) Quantification of HO-1 levels after correction by actin levels. E). Astrocytes were treated as in C and SRXN1 protein levels were analyzed by Western blot. F) Quantification of SRXN1 levels after correction by actin levels. *P < 0.05, significantly different from its respective GFP-control astrocytes. For all graph panels, each data bar represents the mean ± sd of at least 3 independent experiments.
SIRT6 overexpression abrogates the toxicity of primary astrocytes expressing ALS-linked mutant SOD1
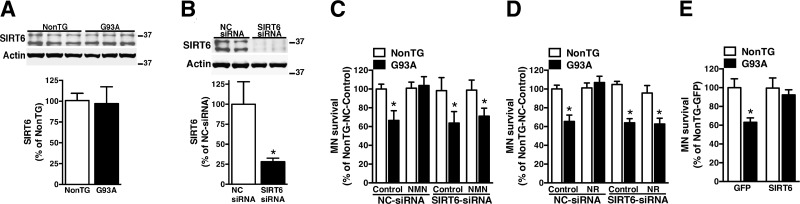
NAD+ metabolism (26) and SIRT6 expression levels are similar in nontransgenic and hSOD1G93A astrocytes (Fig. 5A). Astrocytes isolated from hSOD1G93A mice induce ∼40% decrease in the survival of cocultured motor neurons, but pretreatment with NMN or NR completely blocks the neurotoxic phenotype of hSOD1G93A astrocytes (26). To test if SIRT6 activation participates in the reversion of the neurotoxic phenotype, we silenced SIRT6 expression before NMN or NR treatments (Fig. 5B). In the presence of an NC-siRNA, pretreatment of hSOD1G93A astrocytes with NMN or NR prevents the death of cocultured motor neurons (Fig. 5C, D; NC-siRNA bars). However, silencing SIRT6 before NMN or NR treatments eliminates the protection conferred by both treatments (Fig. 5C, D; SIRT6-siRNA bars). Importantly, silencing of SIRT6 and treatment with the NAD+ precursors occurs exclusively in the astrocyte compartment, and motor neurons are not exposed to either treatment. Together, these results indicate that SIRT6 plays a central role in the protection conferred by enhancing NAD+ availability in hSOD1G93A astrocytes. Moreover, SIRT6 overexpression by itself abrogates the neurotoxic phenotype of hSOD1G93A astrocytes (Fig. 5E).
Figure 5.
SIRT6 is necessary for the protection conferred by NAD+ precursors in astrocyte motor neuron cocultures. A) SIRT6 protein levels in confluent nontransgenic (NonTG) or hSOD1G93A (G93A) spinal cord astrocyte cultures. Actin levels were used as loading control. Each lane represents an independent biological replicate. For quantification (lower panel), both SIRT6 isoforms were measured. B) Confluent astrocyte cultures were transfected with an NC-siRNA or SIRT6-siRNA and, 48 h later, SIRT6 protein levels were analyzed by Western blot. The lower panel shows the quantification of SIRT6 expression after correction by actin levels. C) Confluent NonTG or G93A spinal cord astrocyte cultures were transfected with NC-siRNA or SIRT6-siRNA and, 48 h later, treated with vehicle (control) or 5 mM NMN. Twenty-four hours after NMN treatment, astrocyte cultures were washed, and purified motor neurons from E12.5 mice were plated on top of the astrocyte monolayer. Motor neuron survival was assessed 72 h later. *P < 0.05, significantly different from NonTG-NC control astrocytes. D) The same experimental set up as in C, except that astrocytes were treated with 5 mM NR. *P < 0.05, significantly different from NonTG-NC control astrocytes. E) Confluent NonTG and G93A spinal cord astrocyte cultures were transfected with adenovirus-expressing GFP or SIRT6 and, 48 h later, purified motor neurons from E12.5 mice were plated on top of the astrocyte monolayer. *P < 0.05, significantly different from NonTG-GFP astrocytes. For C–E, each data bar represents the mean ± sd of at least 3 independent coculture experiments.
DISCUSSION
Astrocyte-mediated motor neuron toxicity is a feature observed in ALS animal models and is likely a pathogenic component of the human disease (5–9). Although the exact nature of the mechanism responsible for this toxicity remains under investigation, we have previously shown that several strategies aimed at modifying astrocyte antioxidant defenses are able to prevent astrocyte-mediated motor neuron death in cocultures (5, 10, 26, 43). We have previously shown that boosting NAD+ availability in astrocytes decreases isocitrate dehydrogenase 2 acetylation and increases NADPH content, an essential reducing equivalent for cellular and mitochondrial antioxidant defenses (26). Here, we report an additional protective pathway involving Nrf2-mediated transactivation of antioxidant genes in astrocytes following treatment with NAD+ precursors or SIRT6 overexpression.
Nrf2 plays a key role in the activation of antioxidant defenses in astrocytes through its ability to coordinate the transactivation of an array of genes that code for antioxidant and phase II detoxifying enzymes (34, 35). We have previously shown that Nrf2 activation in astrocytes abrogates the toxicity of hSOD1G93A astrocytes toward motor neurons and extends the survival in ALS animal models (5, 10). The activation of Nrf2 observed following NMN treatment is likely a component that contributes to both the enhancement of antioxidant defenses and the reversion of the neurotoxic phenotype of hSOD1G93A astrocytes. Silencing Nrf2 decreases HO-1 and SRXN1 basal expression in astrocytes (Figs. 2 and 4) but also clearly prevents the increase in expression observed following NMN treatment or SIRT6 overexpression. Additionally, increased recruitment of Nrf2 to regulatory sequences in the promoter of these 2 genes implies a direct participation of Nrf2 in the observed up-regulation. Interestingly, although NMN treatment and SIRT6 overexpression induce Nrf2-mediated Hmox1 and Srxn1 gene expression, the expression of NADPH dehydrogenase quinone 1, another prototypical target gene of Nrf2, did not change (unpublished results). This suggests that enhancing NAD+ availability in astrocytes may modulate the expression of only a specific subset of genes under the control of Nrf2, although the mechanism for that specificity remains to be established.
Neither NMN treatment nor SIRT6 overexpression causes a change in Nrf2 mRNA expression (Figs. 1 and 3), suggesting that activation occurs at a posttranscriptional level. Of the 7 mammalian SIRTs, SIRT1 and SIRT6 have been directly implicated in Nrf2 activation (41, 42). Both SIRT1 and SIRT6 localize to the nucleus. SIRT1 displays robust deacetylation activity (44), whereas SIRT6 displays weak deacetylase activity in vitro. Instead, SIRT6 can catalyze ADP-ribosylation and lysine defatty acylation (45–47). SIRT1-mediated deacetylation of Nrf2 has been linked to a decrease in Nrf2-dependent gene transcription, whereas SIRT6 appears to increase the recruitment of RNA polymerase II to ARE-containing promoters to form a complex with Nrf2 and promote gene expression (41, 42). However, it is important to note that more recent reports have linked SIRT1 expression to increased Nrf2-dependent transactivation (48, 49). In addition, the model proposed to explain SIRT6-mediated Nrf2 activation involves deacetylation of the lysine residue 56 in histone 3, which is generally linked to transcriptional repression (42). Thus, the mechanism linking SIRT activity to Nrf2-activation regulation is not yet completely understood. However, our data clearly show that SIRT6 mediates Nrf2-dependent transactivation of ARE-driven genes, and its overexpression is enough to revert the neurotoxic phenotype of hSOD1G93A astrocytes (Fig. 5).
We have previously attempted to test if the reversion of the neurotoxic phenotype conferred by increasing NAD+ availability in hSOD1G93A astrocytes was mediated by SIRT1 or SIRT3 activation. Decreasing SIRT1 or SIRT3 expression induces a neurotoxic phenotype in nontransgenic astrocytes, reducing the survival of cocultured motor neurons to the levels observed in cocultures with untreated hSOD1G93A astrocytes (26). Thus, SIRT1 and SIRT3 expression in astrocytes has a major role shaping astrocyte motor neuron interaction in basal conditions, but their role in NAD+-mediated protection cannot be directly tested. On the other hand, decreasing SIRT6 expression in untreated nontransgenic astrocytes does not have an effect on the survival of cocultured motor neurons (Fig. 5C, D). Hence, in basal (control) conditions, different SIRTs appear to have different roles in astrocyte motor neuron interaction. However, it is worth noting that hSOD1G93A astrocytes do not display decreased expression of SIRT1, SIRT3, SIRT6, or decreased NAD+ levels [Fig. 5A and (26)]. Thus, the toxicity of hSOD1G93A astrocytes is not linked to basal changes in these parameters, whereas the protection conferred by treatment with NAD+ precursors is linked to an increase in NAD+ availability above normal levels.
Regardless of whether SIRTs act as bona fide NAD+ sensors, the current paradigm indicates that other NAD+-consuming enzymes [like poly (ADP-ribose) polymerases and cyclic ADP-ribose synthases] limit NAD+ availability to endogenous SIRTs. In line with this concept, the beneficial effect that enhancing NAD+ availability confers in multiple pathologic situations has been linked to increased activity of endogenous SIRTs (14, 24, 50–52). This effect, in principle, will affect the activity of all SIRTs, leading us to try to identify which SIRT is critical for the neuroprotection conferred by NMN or NR treatment in hSOD1G93A astrocyte–motor neuron cocultures. Interestingly, silencing SIRT6 expression before increasing NAD+ availability with NMN or NR treatments inhibits the protective effect that these NAD+ precursors have in the reversion of the neurotoxic phenotype of hSOD1G93A astrocytes (Fig. 5C, D; SIRT6-siRNA bars). SIRT6 has multiple roles regulating genome stability, inflammation, aging, and metabolism (53, 54). Thus, changes in SIRT6 expression will likely affect multiple pathways, in addition to Nrf2 activation, that could be relevant in the context of astrocyte-mediated neurotoxicity. Our data show that SIRT6 expression is required for the neuroprotection exerted by NAD+ precursors, and SIRT6 overexpression is sufficient to revert the toxicity of hSOD1G93A astrocytes. These results highlight the potential therapeutic role of SIRT6 activation to prevent astrocyte-mediated motor neuron death in ALS.
Overall, our data demonstrate that enhancing NAD+ availability has the capacity to modulate multiple cytoprotective mechanisms that result in increased antioxidant defenses in astrocytes. Several of these pathways have been shown to be important in the regulation of astrocyte motor neuron interaction (e.g., Nrf2, SIRT3, and SIRT6 activation). Hence, further investigation is deserved regarding the therapeutic potential of enhancing NAD+ availability in ALS.
ACKNOWLEDGMENTS
This study was funded by U.S. National Institutes of Health (NIH), National Institute of Neurological Disorders and Stroke Grant R01NS089640. The authors declare no conflicts of interest.
Glossary
- ALS
amyotrophic lateral sclerosis
- ARE
antioxidant response element
- ChIP
chromatin immunoprecipitation
- E12.5
embryonic d 12.5
- FALS
familial ALS
- GFP
green fluorescent protein
- hSOD1
human SOD1
- HO-1
heme oxygenase 1
- NAM
nicotinamide
- NAMPT
NAM phosphoribosyltransferase
- NC
negative control
- NMN
nicotinamide mononucleotide
- NMNAT
NMN adenylyl transferase
- NR
nicotinamide riboside
- Nrf2
nuclear factor, erythroid-derived 2, like 2
- qPCR
quantitative PCR
- SOD1
superoxide dismutase 1
- SALS
sporadic ALS
- siRNA
small interfering RNA
- SIRT
sirtuin
- SRXN1
sulfiredoxin 1
AUTHOR CONTRIBUTIONS
B. A. Harlan, M. Pehar, K. M. Killoy, and M. R. Vargas performed experiments; B. A. Harlan, M. Pehar, and M. R. Vargas analyzed data; B. A. Harlan, M. Pehar, and M. R. Vargas wrote the manuscript; and all authors reviewed the content of the manuscript.
REFERENCES
- 1.Renton A. E., Chiò A., Traynor B. J. (2014) State of play in amyotrophic lateral sclerosis genetics. Nat. Neurosci. 17, 17–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosen D. R., Siddique T., Patterson D., Figlewicz D. A., Sapp P., Hentati A., Donaldson D., Goto J., O’Regan J. P., Deng H. X., et al. (1993) Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362, 59–62; erratum: 364, 362 [DOI] [PubMed] [Google Scholar]
- 3.Al-Chalabi A., Jones A., Troakes C., King A., Al-Sarraj S., van den Berg L. H. (2012) The genetics and neuropathology of amyotrophic lateral sclerosis. Acta Neuropathol. 124, 339–352 [DOI] [PubMed] [Google Scholar]
- 4.Ravits J., Appel S., Baloh R. H., Barohn R., Brooks B. R., Elman L., Floeter M. K., Henderson C., Lomen-Hoerth C., Macklis J. D., McCluskey L., Mitsumoto H., Przedborski S., Rothstein J., Trojanowski J. Q., van den Berg L. H., Ringel S. (2013) Deciphering amyotrophic lateral sclerosis: what phenotype, neuropathology and genetics are telling us about pathogenesis. Amyotroph. Lateral Scler. Frontotemporal Degener. 14 (Suppl 1), 5–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vargas M. R., Pehar M., Cassina P., Beckman J. S., Barbeito L. (2006) Increased glutathione biosynthesis by Nrf2 activation in astrocytes prevents p75NTR-dependent motor neuron apoptosis. J. Neurochem. 97, 687–696 [DOI] [PubMed] [Google Scholar]
- 6.Nagai M., Re D. B., Nagata T., Chalazonitis A., Jessell T. M., Wichterle H., Przedborski S. (2007) Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat. Neurosci. 10, 615–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kia A., McAvoy K., Krishnamurthy K., Trotti D., Pasinelli P. (2018) Astrocytes expressing ALS-linked mutant FUS induce motor neuron death through release of tumor necrosis factor-alpha. Glia 66, 1016–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haidet-Phillips A. M., Hester M. E., Miranda C. J., Meyer K., Braun L., Frakes A., Song S., Likhite S., Murtha M. J., Foust K. D., Rao M., Eagle A., Kammesheidt A., Christensen A., Mendell J. R., Burghes A. H., Kaspar B. K. (2011) Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat. Biotechnol. 29, 824–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer K., Ferraiuolo L., Miranda C. J., Likhite S., McElroy S., Renusch S., Ditsworth D., Lagier-Tourenne C., Smith R. A., Ravits J., Burghes A. H., Shaw P. J., Cleveland D. W., Kolb S. J., Kaspar B. K. (2014) Direct conversion of patient fibroblasts demonstrates non-cell autonomous toxicity of astrocytes to motor neurons in familial and sporadic ALS. Proc. Natl. Acad. Sci. USA 111, 829–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vargas M. R., Johnson D. A., Sirkis D. W., Messing A., Johnson J. A. (2008) Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J. Neurosci. 28, 13574–13581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song S., Miranda C. J., Braun L., Meyer K., Frakes A. E., Ferraiuolo L., Likhite S., Bevan A. K., Foust K. D., McConnell M. J., Walker C. M., Kaspar B. K. (2016) Major histocompatibility complex class I molecules protect motor neurons from astrocyte-induced toxicity in amyotrophic lateral sclerosis. Nat. Med. 22, 397–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pehar M., Harlan B. A., Killoy K. M., Vargas M. R. (2017) Role and therapeutic potential of astrocytes in amyotrophic lateral sclerosis. Curr. Pharm. Des. 23, 5010–5021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berger F., Ramírez-Hernández M. H., Ziegler M. (2004) The new life of a centenarian: signalling functions of NAD(P). Trends Biochem. Sci. 29, 111–118 [DOI] [PubMed] [Google Scholar]
- 14.Cantó C., Menzies K. J., Auwerx J. (2015) NAD(+) metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab. 22, 31–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belenky P., Bogan K. L., Brenner C. (2007) NAD+ metabolism in health and disease. Trends Biochem. Sci. 32, 12–19 [DOI] [PubMed] [Google Scholar]
- 16.Bieganowski P., Brenner C. (2004) Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell 117, 495–502 [DOI] [PubMed] [Google Scholar]
- 17.Ruddick J. P., Evans A. K., Nutt D. J., Lightman S. L., Rook G. A., Lowry C. A. (2006) Tryptophan metabolism in the central nervous system: medical implications. Expert Rev. Mol. Med. 8, 1–27 [DOI] [PubMed] [Google Scholar]
- 18.Garten A., Petzold S., Körner A., Imai S., Kiess W. (2009) Nampt: linking NAD biology, metabolism and cancer. Trends Endocrinol. Metab. 20, 130–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Stefano M., Conforti L. (2013) Diversification of NAD biological role: the importance of location. FEBS J. 280, 4711–4728 [DOI] [PubMed] [Google Scholar]
- 20.Revollo J. R., Grimm A. A., Imai S. (2004) The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J. Biol. Chem. 279, 50754–50763 [DOI] [PubMed] [Google Scholar]
- 21.Yang H., Yang T., Baur J. A., Perez E., Matsui T., Carmona J. J., Lamming D. W., Souza-Pinto N. C., Bohr V. A., Rosenzweig A., de Cabo R., Sauve A. A., Sinclair D. A. (2007) Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell 130, 1095–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nikiforov A., Dölle C., Niere M., Ziegler M. (2011) Pathways and subcellular compartmentation of NAD biosynthesis in human cells: from entry of extracellular precursors to mitochondrial NAD generation. J. Biol. Chem. 286, 21767–21778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu W., Dittenhafer-Reed K. E., Denu J. M. (2012) SIRT3 protein deacetylates isocitrate dehydrogenase 2 (IDH2) and regulates mitochondrial redox status. J. Biol. Chem. 287, 14078–14086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cantó C., Houtkooper R. H., Pirinen E., Youn D. Y., Oosterveer M. H., Cen Y., Fernandez-Marcos P. J., Yamamoto H., Andreux P. A., Cettour-Rose P., Gademann K., Rinsch C., Schoonjans K., Sauve A. A., Auwerx J. (2012) The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 15, 838–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown K. D., Maqsood S., Huang J. Y., Pan Y., Harkcom W., Li W., Sauve A., Verdin E., Jaffrey S. R. (2014) Activation of SIRT3 by the NAD+ precursor nicotinamide riboside protects from noise-induced hearing loss. Cell Metab. 20, 1059–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harlan B. A., Pehar M., Sharma D. R., Beeson G., Beeson C. C., Vargas M. R. (2016) Enhancing NAD+ salvage pathway reverts the toxicity of primary astrocytes expressing amyotrophic lateral sclerosis-linked mutant superoxide dismutase 1 (SOD1). J. Biol. Chem. 291, 10836–10846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Picciotto N. E., Gano L. B., Johnson L. C., Martens C. R., Sindler A. L., Mills K. F., Imai S., Seals D. R. (2016) Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell 15, 522–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schöndorf D. C., Ivanyuk D., Baden P., Sanchez-Martinez A., De Cicco S., Yu C., Giunta I., Schwarz L. K., Di Napoli G., Panagiotakopoulou V., Nestel S., Keatinge M., Pruszak J., Bandmann O., Heimrich B., Gasser T., Whitworth A. J., Deleidi M. (2018) The NAD+ precursor nicotinamide riboside rescues mitochondrial defects and neuronal loss in iPSC and fly models of Parkinson’s disease. Cell Rep. 23, 2976–2988 [DOI] [PubMed] [Google Scholar]
- 29.Hou Y., Lautrup S., Cordonnier S., Wang Y., Croteau D. L., Zavala E., Zhang Y., Moritoh K., O’Connell J. F., Baptiste B. A., Stevnsner T. V., Mattson M. P., Bohr V. A. (2018) NAD+ supplementation normalizes key Alzheimer’s features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency. Proc. Natl. Acad. Sci. USA 115, E1876–E1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imai S., Guarente L. (2014) NAD+ and sirtuins in aging and disease. Trends Cell Biol. 24, 464–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pehar M., Harlan B. A., Killoy K. M., Vargas M. R. (2018) Nicotinamide adenine dinucleotide metabolism and neurodegeneration. Antioxid. Redox Signal. 28, 1652–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katsyuba E., Auwerx J. (2017) Modulating NAD+ metabolism, from bench to bedside. EMBO J. 36, 2670–2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei C. C., Kong Y. Y., Li G. Q., Guan Y. F., Wang P., Miao C. Y. (2017) Nicotinamide mononucleotide attenuates brain injury after intracerebral hemorrhage by activating Nrf2/HO-1 signaling pathway. Sci. Rep. 7, 717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vargas M. R., Johnson J. A. (2009) The Nrf2-ARE cytoprotective pathway in astrocytes. Expert Rev. Mol. Med. 11, e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baxter P. S., Hardingham G. E. (2016) Adaptive regulation of the brain’s antioxidant defences by neurons and astrocytes. Free Radic. Biol. Med. 100, 147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kraft A. D., Resch J. M., Johnson D. A., Johnson J. A. (2007) Activation of the Nrf2-ARE pathway in muscle and spinal cord during ALS-like pathology in mice expressing mutant SOD1. Exp. Neurol. 207, 107–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen P. C., Vargas M. R., Pani A. K., Smeyne R. J., Johnson D. A., Kan Y. W., Johnson J. A. (2009) Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson’s disease: critical role for the astrocyte. Proc. Natl. Acad. Sci. USA 106, 2933–2938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vargas M. R., Burton N. C., Kutzke J., Gan L., Johnson D. A., Schäfer M., Werner S., Johnson J. A. (2013) Absence of Nrf2 or its selective overexpression in neurons and muscle does not affect survival in ALS-linked mutant hSOD1 mouse models. PLoS One 8, e56625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gurney M. E., Pu H., Chiu A. Y., Dal Canto M. C., Polchow C. Y., Alexander D. D., Caliendo J., Hentati A., Kwon Y. W., Deng H. X., et al. (1994) Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science 264, 1772–1775; erratum: 269, 149 [DOI] [PubMed] [Google Scholar]
- 40.Pehar M., Ball L. E., Sharma D. R., Harlan B. A., Comte-Walters S., Neely B. A., Vargas M. R. (2016) Changes in protein expression and lysine acetylation induced by decreased glutathione levels in astrocytes. Mol. Cell. Proteomics 15, 493–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawai Y., Garduño L., Theodore M., Yang J., Arinze I. J. (2011) Acetylation-deacetylation of the transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) regulates its transcriptional activity and nucleocytoplasmic localization. J. Biol. Chem. 286, 7629–7640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan H., Guan D., Liu X., Li J., Wang L., Wu J., Zhou J., Zhang W., Ren R., Zhang W., Li Y., Yang J., Hao Y., Yuan T., Yuan G., Wang H., Ju Z., Mao Z., Li J., Qu J., Tang F., Liu G. H. (2016) SIRT6 safeguards human mesenchymal stem cells from oxidative stress by coactivating NRF2. Cell Res. 26, 190–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pehar M., Beeson G., Beeson C. C., Johnson J. A., Vargas M. R. (2014) Mitochondria-targeted catalase reverts the neurotoxicity of hSOD1G93A astrocytes without extending the survival of ALS-linked mutant hSOD1 mice. PLoS One 9, e103438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feldman J. L., Dittenhafer-Reed K. E., Denu J. M. (2012) Sirtuin catalysis and regulation. J. Biol. Chem. 287, 42419–42427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liszt G., Ford E., Kurtev M., Guarente L. (2005) Mouse Sir2 homolog SIRT6 is a nuclear ADP-ribosyltransferase. J. Biol. Chem. 280, 21313–21320 [DOI] [PubMed] [Google Scholar]
- 46.Feldman J. L., Baeza J., Denu J. M. (2013) Activation of the protein deacetylase SIRT6 by long-chain fatty acids and widespread deacylation by mammalian sirtuins. J. Biol. Chem. 288, 31350–31356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang H., Khan S., Wang Y., Charron G., He B., Sebastian C., Du J., Kim R., Ge E., Mostoslavsky R., Hang H. C., Hao Q., Lin H. (2013) SIRT6 regulates TNF-α secretion through hydrolysis of long-chain fatty acyl lysine. Nature 496, 110–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang K., Huang J., Xie X., Wang S., Chen C., Shen X., Liu P., Huang H. (2013) Sirt1 resists advanced glycation end products-induced expressions of fibronectin and TGF-β1 by activating the Nrf2/ARE pathway in glomerular mesangial cells. Free Radic. Biol. Med. 65, 528–540 [DOI] [PubMed] [Google Scholar]
- 49.Matsui S., Sasaki T., Kohno D., Yaku K., Inutsuka A., Yokota-Hashimoto H., Kikuchi O., Suga T., Kobayashi M., Yamanaka A., Harada A., Nakagawa T., Onaka T., Kitamura T. (2018) Neuronal SIRT1 regulates macronutrient-based diet selection through FGF21 and oxytocin signalling in mice. Nat. Commun. 9, 4604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoshino J., Mills K. F., Yoon M. J., Imai S. (2011) Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 14, 528–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bai P., Cantó C., Oudart H., Brunyánszki A., Cen Y., Thomas C., Yamamoto H., Huber A., Kiss B., Houtkooper R. H., Schoonjans K., Schreiber V., Sauve A. A., Menissier-de Murcia J., Auwerx J. (2011) PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 13, 461–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mills K. F., Yoshida S., Stein L. R., Grozio A., Kubota S., Sasaki Y., Redpath P., Migaud M. E., Apte R. S., Uchida K., Yoshino J., Imai S. I. (2016) Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell Metab. 24, 795–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mostoslavsky R., Chua K. F., Lombard D. B., Pang W. W., Fischer M. R., Gellon L., Liu P., Mostoslavsky G., Franco S., Murphy M. M., Mills K. D., Patel P., Hsu J. T., Hong A. L., Ford E., Cheng H. L., Kennedy C., Nunez N., Bronson R., Frendewey D., Auerbach W., Valenzuela D., Karow M., Hottiger M. O., Hursting S., Barrett J. C., Guarente L., Mulligan R., Demple B., Yancopoulos G. D., Alt F. W. (2006) Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 124, 315–329 [DOI] [PubMed] [Google Scholar]
- 54.Tasselli L., Zheng W., Chua K. F. (2017) SIRT6: novel mechanisms and links to aging and disease. Trends Endocrinol. Metab. 28, 168–185 [DOI] [PMC free article] [PubMed] [Google Scholar]