Abstract
Conditions of extended bed rest and limb immobilization can initiate rapid and significant loss of skeletal muscle mass and function. Physical rehabilitation is standard practice following a period of disuse, yet mobility may be severely compromised, and recovery is commonly delayed or incomplete in special populations. Thus, a novel approach toward recovery of muscle mass is highly desired. Pericytes [neuron-glial antigen 2 (NG2)+CD31−CD45− (Lineage− [Lin−]) and CD146+Lin−] demonstrate capacity to facilitate muscle repair, yet the ability to enhance myofiber growth following disuse is unknown. In the current study, 3–4-mo-old mice were unilaterally immobilized for 14 d (IM) or immobilized for 14 d followed by 14 d of remobilization (RE). Flow cytometry and targeted gene expression analyses were completed to assess pericyte quantity and function following IM and RE. In addition, a transplantation study was conducted to assess the impact of pericytes on recovery. Results from targeted analyses suggest minimal impact of disuse on pericyte gene expression, yet NG2+Lin− pericyte quantity is reduced following IM (P < 0.05). Remarkably, pericyte transplantation recovered losses in myofiber cross-sectional area and the capillary-to-fiber ratio following RE, whereas deficits remained with vehicle alone (P = 0.01). These findings provide the first evidence that pericytes effectively rehabilitate skeletal muscle mass following disuse atrophy.—Munroe, M., Dvoretskiy, S., Lopez, A., Leong, J., Dyle, M. C., Kong, H., Adams, C. M., Boppart, M. D. Pericyte transplantation improves skeletal muscle recovery following hindlimb immobilization.
Keywords: disuse atrophy, rehabilitation, stem cells, muscle growth, capillary
Injury and illness require a period of immobilization (IM) that can be general to the whole body (bed rest) or localized to individual limbs (casting). IM is essential for healing of fractures or recovery from illness yet can result in rapid and significant loss of skeletal muscle mass and function (1–5). Physical rehabilitation is standard practice following a period of disuse (6), yet mobility may be severely compromised, and recovery is commonly delayed or incomplete in special populations. Specifically, older adults often fail to recover losses in muscle mass and are at high risk for disability (7–14). The biologic mechanisms responsible for lack of recovery are not clear but may include anabolic resistance, diminished satellite cell (SC) activation, increased fibrosis, endoplasmic reticulum stress, and other unidentified factors (7–14). Pharmacological approaches to prevent muscle atrophy or improve recovery are not available in the clinic. Thus, novel regenerative therapies are necessary to maximize positive outcomes associated with rehabilitation to prevent or treat long-term disability associated with IM.
Muscle-resident stem/stromal cells demonstrate significant potential to repair skeletal muscle following injury. The role for the SC Pax7+ in repair of skeletal muscle injury is well acknowledged (15, 16), but the extent to which these cells influence growth in response to loading remains controversial (17–21). SCs may not fuse or provide nuclear support for protein synthesis in adult muscle as previously proposed, but they may fine-tune the growth process via control of the extracellular matrix (ECM) environment (18, 21). SCs also appear to have a limited to nonexistent role in the regrowth process following disuse (22). Overall, these findings suggest that manipulation of SCs may not be the optimal approach to improve recovery postdisuse. Alternative stem and stromal cells, including mesenchymal stem/stromal cells and pericytes, exist in skeletal muscle that similarly demonstrate capacity for repair following injury, yet the ability of these cells to induce growth and recover mass following disuse remains unexplored (23–26).
Pericytes are vascular supportive cells residing in the abluminal space of microvessels (precapillary arterioles, capillaries, postcapillary venules) in a wide variety of tissues (27, 28). Pericyte cytoplasmic extensions form intimate connections with endothelial cells via tight junctions, gap junctions and adhesions, and subsequently provide important structural and paracrine factor support necessary for regulation of vascular permeability, vessel diameter and blood flow, and stabilization of newly formed capillaries (27). Recent studies suggest that pericytes possess mesodermal differentiation potential and contribute to skeletal muscle repair following injury or disease (23–25). However, they also secrete important paracrine factors necessary for regulation of baseline muscle mass (29). The extent to which capillarity or associated pericytes directly contribute to muscle mass gains in response to resistance exercise is not known (30), but we speculate that an important relationship exists between pericytes and the promotion of growth in response to load.
The purpose of this study was to evaluate the pericyte response to disuse and its contribution to skeletal muscle recovery following IM. We hypothesized that pericytes would be depleted during disuse and that exogenous pericyte transplantation would enhance recovery during the period of remobilization (RE).
MATERIALS AND METHODS
Animals
Three- to four-month-old male C57BL/6 mice obtained from Charles River Laboratories (Wilmington, MA, USA) were used for all experiments. Mice were housed in a pathogen-free animal room under controlled conditions (12-h light/dark cycle, 25°C) and maintained on a standard diet (Rodent Diet 7012; Harlan Teklad, Madison, WI, USA). Upon arrival, mice were acclimatized to their new environment for 7 d prior to initiation of the study. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Illinois at Urbana-Champaign (UIUC).
Hindlimb IM and RE procedures
Unilateral, lower-limb IM was performed on mice as previously described in refs. 31 and 32. While anesthetized with isoflurane, the right foot was securely pinned in full dorsiflexion using a Covidien Royal AutoSuture 35W skin stapler (eSutures, Mokena, IL, USA) exploiting normal murine dorsotibial flexion to induce significant atrophy of the tibialis anterior (TA) muscle. One tine of the metal clip was inserted into the plantar portion of the foot with the other tine being inserted into the distal portion of the gastrocnemius muscle. The left, contralateral limb was untreated and served as an internal control. Mice (n = 5–6 per experiment) were immobilized for 14 d (IM). A separate group of mice were immobilized for 14 d, then the metal clip was removed under anesthesia using a surgery staple remover, allowing for normal ambulation (RE) for an additional 14 d prior to euthanasia. Mice were euthanized using carbon dioxide asphyxiation, which was confirmed by cervical dislocation.
Skeletal muscle mononuclear cell isolation procedure
Each TA muscle was dissected and weighed then washed in cold 1× PBS with penicillin-streptomycin antibiotic (Thermo Fisher Scientific, Waltham, MA, USA). The cleaned muscle tissue was mechanically minced and enzymatically digested for ∼30 min at 37°C. The enzyme solution contained 250 U/ml collagenase Type 2 (Worthington Biochemical, Lakewood, NJ, USA), 2 U/ml neutral protease (Dispase) (Worthington Biochemical), 60 U/ml DNase (MilliporeSigma, Burlington, MA, USA), and 2.5 mM CaCl2 in 1× PBS. The tissue solution was filtered through a 70-μm then 40-μm filter, resulting in a mononuclear cell suspension. Cells were incubated for 10 min on ice with anti-mouse CD16/CD32 (Thermo Fisher Scientific) to block Fc-mediated nonspecific binding. Cells were incubated with a cocktail of antibodies designed for either flow cytometry analysis or fluorescence-activated cell sorting (FACS). Following antibody incubation, cells were centrifuged for 5 min at 300 g and supernatant discarded. The resulting cell pellet was resuspended in 2% fetal bovine serum (FBS) in 1× PBS and filtered through a 40 μm filter then placed on ice.
Flow cytometry and FACS
Skeletal muscle perivascular stem/stromal cell quantity was analyzed using multiplex flow cytometry. Isolated mononuclear cells were incubated for 45 min at 4°C with conjugated anti-mouse neuron-glial antigen 2 (NG2) (chondroitin sulfate proteoglycan-4)–Alexa Fluor 488 (1:50, AB5320A4; MilliporeSigma), CD146 (melanoma cell adhesion molecule)-phyocerythrin (clone ME-9F1) (1:50, 34704; BioLegend, San Diego, CA, USA), CD140A [platelet-derived growth factor receptor α (PDGFRα)]-BV421 (clone APA5) (1:50, 566293; BD Biosciences, San Jose, CA, USA), CD31 (PECAM-1)-allophycoctanin (APC) (clone MEC-13.3) (1:100,561814; BD Biosciences), and CD45-APC (clone 30-F11) (1:100, 561018; BD Biosciences) antibodies diluted in 2% FBS in 1× PBS. Stained cells were analyzed using a 4-laser BD LSRFortessa Cell Analyzer (403-nm violet, 488-nm blue, 561-nm yellow/green, 640-nm red lasers) (BD Biosciences) at the Flow Cytometry Center at the UIUC Roy J. Carver Biotechnology Center (CBC) (Urbana, IL, USA). Unstained and single-stained control samples were analyzed in order to account for potential spectral overlap between fluorophores and compensate accordingly. Fluorescence minus one (FMO) controls were utilized to establish proper gating parameters. Approximately 50,000 events were recorded for each sample and FMO. All flow cytometry data were analyzed using FCS Express Flow Cytometry Software v.5 (De Novo Software, Glendale, CA, USA).
NG2+ Lineage− (Lin−) (CD31−CD45−) and CD146+Lin− skeletal muscle pericytes were sorted using a BD FACS Aria II sorter by the Flow Cytometry Center at the UIUC CBC. NG2+Lin− pericytes were stained with conjugated anti-mouse NG2–Alexa Fluor 488 (1:50), CD31 (PECAM-1)-APC (clone MEC-13.3) (1:100), and CD45-APC (clone 30-F11) (1:100) antibodies. CD146+Lin− pericytes were stained with conjugated anti-mouse CD146-phyocerythrin (clone ME-9F1) (1:50), CD31-FITC (clone 390) (1:100, 11-0311-81; Thermo Fisher Scientific), and CD45-FITC (clone 30-F11) (1:100, 11-0451-81; Thermo Fisher Scientific) antibodies. All antibodies were diluted in 2% FBS in 1× PBS. Appropriate unstained and single-stained samples were run to establish proper gating. For gene expression analysis, NG2+Lin− and CD146+Lin− pericytes were sorted separately. Briefly, ∼20,000 cells were sorted directly into 500 μl of Buffer RLT (Qiagen, Hilden, Germany), with 5 μl β-mercaptoethanol being added immediately after collection followed by RNA extraction. For transplantation experiments, pericytes were collected separately in pericyte-specific growth medium (high glucose DMEM, 10% FBS, 1% penicillin-streptomycin) and seeded on tissue culture dishes incubated at 37°C and 5% CO2 for up to 10 d to allow for recovery and expansion, resulting in 70–80% confluency.
Gene expression analysis using real-time quantitative PCR
RNA was extracted from cell lysates using RNeasy Micro Kit (Qiagen) following the manufacturer’s instruction. Quantity of isolated RNA was assessed in duplicate on a Take-3 application plate using a Synergy H1 Hybrid Multi-Mode Microplate Reader (BioTek Instruments, Winooski, VT, USA). Starting RNA concentration of 25 ng was used to perform reverse transcription via the High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific) per the manufacturer’s instructions. Preamplification of cDNA was completed using TaqMan PreAmp Master Mix Kit (Thermo Fisher Scientific). Quantitative PCR was performed using the 7900HT Fast Real-Time PCR System with TaqMan Universal PCR Master Mix and inventoried TaqMan primers (Thermo Fisher Scientific) at the Functional Genomics Unit in the UIUC CBC. For each time point, genes were normalized to the geometric mean of β-actin, glyceraldehyde-3-phosphate dehydrogenase, and hypoxanthine-guanine phosphoribosyltransferase, except for CD146+Lin− pericytes post-IM, which was analyzed relative to β-actin only. Genes were expressed relative to the respective contralateral limb control. Gene expression data were calculated using the ΔΔCt method.
DNA plasmid extraction and pericyte transfections
For pericyte transplantation experiments, separately cultured NG2+Lin− and CD146+Lin− pericytes were transfected with either ZsGreen1-N1 or tdTomato-N1 plasmids purchased from Addgene (Watertown, MA, USA). ZsGreen1-N1 was a gift provided by Michael Davidson [Addgene investigator (Addgene plasmid 54702; http://n2t.net/addgene:54702; RRID:Addgene_54702)], and tdTomato-N1 was a gift provided by Michael Davidson, Nathan Shaner, and Roger Tsien [Addgene investigators (Addgene plasmid 54642; http://n2t.net/addgene:54642; RRID:Addgene_54642)]. Plasmids were obtained as bacteria in agar stab, which was streaked on Luria broth (LB) agar plates with Kanamycin antibiotics (50 μg/ml). Streaked LB agar plates were incubated overnight at 37°C. Single colonies were then selected and inoculated in liquid LB with Kanamycin (50 μg/ml) overnight at 37°C on a shaker (∼200 rpm). Plasmid was extracted using the PureYield Plasmid Miniprep System (A1223; Promega, Madison, WI, USA) following the manufacturer’s instructions. Quantity of isolated DNA was assessed in duplicate on a Take-3 application plate using a Synergy H1 Hybrid Multi-Mode Microplate Reader (BioTek Instruments).
After 9 d of culturing, FACS-isolated pericytes were detached using StemPro Accutase Cell Dissociation Reagent (Thermo Fisher Scientific) and replated onto separate 35 mm2 culture dishes (∼500,000 cells/population) and incubated at 37°C/5% CO2. After 24 h, pericytes were transfected with isolated plasmids (1 μg total) (NG2+Lin−: ZsGreen1-N1; CD146+Lin−: tdTomato-N1) using Effectene Transfection Reagent (Qiagen) following the manufacturer’s instructions. Twenty-four hours posttransfecction, pericytes were dissociated using Accutase and prepared for transplantation. Pericyte transfection was visualized and imaged with an inverted fluorescence and brightfield microscope using a Zeiss Axiocam digital camera and Axiovision software (Carl Zeiss, Oberkochen, Germany).
Conjugation of arginylglycylaspartic acid–oligopeptides to alginate
As previously described by Lee et al. (33), 1% weight alginate (MW: ∼50,000 g/mol) was dissolved in (N-morpholino)ethanesulfonic acid (MES) buffer at pH 6.5 (MilliporeSigma). Then, sulfonated N-hydroxysuccimide (Thermo Fisher Scientific), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (Thermo Fisher Scientific) and the oligopeptide sequence, GGGGRGDSP, termed arginylglycylaspartic acid (RGD) peptide (Mimotopes, Richmond, VA, USA), was sequentially added to the alginate solution and then stirred for 24 h. The molar ratio of uronic acid from alginate, sulfonated N-hydroxysuccimide, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide, and RGD peptide was 1:0.025:0.05:0.0016, corresponding to 2 RGD peptides for every 5 alginate chains. The resulting RGD-alginate solution was dialyzed against distilled water for 40 h, water was replaced 3 times a day. The RGD-alginate solution was then sterilized via 0.22 μm filtration, lyophilized, and stored at −20°C until use.
Pericyte transplantation using RGD-alginatehydrogel
To prepare the hydrogel, RGD-alginate was reconstituted in 1× PBS to obtain a 4% w/v solution prior to gelation. After transfection, both pericyte types were collected together and resuspended in the hydrogel solution, which was cross-linked with aqueous slurries of calcium sulfate (0.21 g CaSO4/ml distilled water) at a molar ratio of 1:0.25 (40 μl of CaSO4 per 1 ml of 4% w/v alginate solution). The solutions were mixed with 2, 1 ml syringes connected via a syringe connector, and by pushing the solutions from 1 syringe to the other 20 times (34). On d 14 of IM, immediately prior to RE, immobilized hindlimbs were unclipped and the immobilized TA was intramuscularly injected in the midbelly with either pericyte-loaded hydrogel (∼50,000 cells in 30 μl hydrogel, comprising ∼25,000 cells per population) or RGD-alginate hydrogel alone. The contralateral TA was untreated. Mice were remobilized for 14 d; TA muscles were then collected and preserved for immunofluorescence analysis.
Skeletal muscle immunofluorescence
Excised TA muscles were weighed and rapidly frozen in liquid nitrogen–cooled isopentane and stored at −80°C. For tissue sectioning, muscles were separated at the midbelly along the axial plane and embedded in optimum cutting temperature (OCT) (Tissue-Tek; Thermo Fisher Scientific). Transverse sections (10 μm nonserial sections, separated by at least 50–80 μm) were then cut using a CM3050S cryostat (Leica, Wetzlar, Germany), placed on microscope slides (Superfrost; Thermo Fisher Scientific), and stored at −80°C until staining.
Muscle sections were fixed in ice-cold acetone for 15 min and then washed with 1× PBS. Sections were blocked for 1 h at room temperature with AffiniPure Fab Fragment goat anti-mouse IgG (Heavy + Light chains) (55 μg/ml, 115-007-003; Jackson ImmunoResearch Laboratories, West Grove, PA, USA) in 5% bovine serum albumin with 0.05% Tween-20 in 1× PBS. To assess myofiber cross-sectional area (CSA), myonuclei content, and muscle capillarization, sections were incubated with rat anti-mouse CD31 (clone 390) (1:100, 14-0311-82; Thermo Fisher Scientific) and rabbit anti-mouse dystrophin (1:100, ab15277; Abcam, Cambridge, MA, USA). To assess myosin heavy chain (MHC) myofiber type, sections were incubated with either mouse IgM anti-Type 2b MHC (BF-F3) (1:50; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA, USA), mouse IgG1 anti-Type 2a MHC (Sc-71) (1:50; Hybridoma), and rabbit anti-mouse dystrophin (1:100, ab15277; Abcam) or mouse IgG2b anti-Type 1 MHC (BA-D5) (1:20; Hybridoma), mouse IgM anti-Type 2 times MHC (6H1) (1:20; Hybridoma), and rabbit anti-mouse dystrophin (1:100, ab15277; Abcam). To assess collagen accumulation and macrophage infiltration, sections were incubated with rat anti-mouse CD11b (1:200, 14-0112-81; Thermo Fisher Scientific) and rabbit anti-mouse collagen type I (1:100, ab34710; Abcam). To assess ZsGreen plasmid (NG2+Lin− pericyte) and tdTomato plasmid (CD146+Lin− pericyte) localization posttransplantation, sections were incubated with rat anti-mouse laminin α-2 (Clone 4H8-2) (1:100, sc-59854; Santa Cruz Biotechnology, Dallas, TX, USA) and either rabbit anti-mouse ZsGreen (1:75, 632474; Takara Bio, Kusatsu, USA) or rabbit anti-mouse DsRed (1:100, 632496; Takara Bio). All primary antibodies were applied for 1 h at room temperature in wash buffer (1% bovine serum albumin with 0.05% Tween-20 in 1× PBS). Species-specific secondary antibodies were diluted in wash buffer and applied as appropriate for 1 h at room temperature. Finally, sections were stained with DAPI (1:20,000; MilliporeSigma) to identify nuclei. After each staining procedure, slides were mounted with Vectashield mounting medium (Vector Laboratories, Burlingame, CA, USA) and stored at 4°C. Tissue sections were visualized using an inverted fluorescent microscope and images were acquired at ×20 magnification with a Zeiss Axiocam digital camera and Axiovision software (Carl Zeiss).
Immunofluorescence image analysis
To quantify myofiber CSA, dystrophin–Alexa Fluor 633 images were imported into Adobe Photoshop (CS5 Extended; Adobe, San Jose, CA, USA), and an average of 400 fibers were circled using the magnetic lasso tool, which decreases subjectivity and interassessment error (26). To determine MHC myofiber CSA, ∼100–150 myofibers per type were circled on average as described above. To assess myonuclei content, the number of total myonuclei per myofiber (∼200 fibers/sample) was recorded using ImageJ (National Institutes of Health, Bethesda, MD, USA). Muscle capillarization was determined using 5 random ×20 images per sample as previously described by Huntsman et al. (35). Measures of capillary-to-fiber (C/F) ratio (number of capillaries per total number of myofibers) and capillary density (number of capillaries per muscle fiber area) were determined for each image by quantifying (per image) the total number of transversely cut capillaries, the number of myofibers, and the total area (square millimeters) of the imaged muscle section.
TA collagen content was quantified with a threshold intensity program from ImageJ. Red, green, and black channels were separated, and the red channel was thresholded to remove background in order to determine the percent of collagen observed within each imaged muscle section. Six random ×20 images were analyzed per sample and normalized to muscle area. Skeletal muscle macrophage infiltration was analyzed by quantifying the number of CD11b+DAPI+ cells per field of view. Approximately 10 images per sample were obtained at ×20 magnification and number of CD11b-positive cells colocalizing with DAPI was recorded. Any punctate CD11b staining not overlapping with DAPI was excluded.
Statistical analysis
Data are presented as means ± sem. Paired sample t tests were conducted for all within subject analyses (stapled/treated vs. mobile/nontreated limbs). Independent sample t tests were used to compare the magnitude of the paired IM differences between groups (stapled-mobile for IM vs. RE or control-hydrogel vs. pericyte-hydrogel). All statistical analyses were completed using SPSS v.24 (IBM SPSS, Chicago, IL, USA). Differences were considered statistically significant at P ≤ 0.05.
RESULTS
Unilateral hindlimb IM reduces perivascular stem/stromal cell quantity
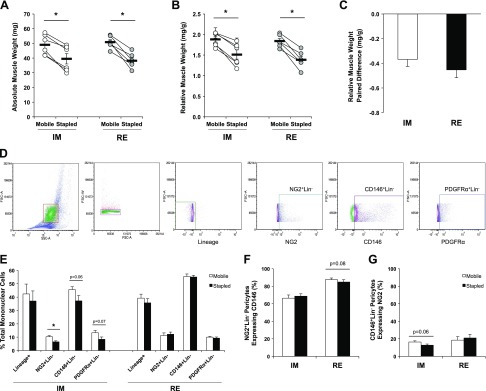
Following 14 d of unilateral IM, both absolute (milligrams) and relative (milligrams per gram body weight) TA muscle weight was significantly reduced in the immobilized limb (stapled) compared with the contralateral control limb (mobile) (P = 0.001) (Fig. 1A, B). As previously observed by Caron et al. (31), following 14 d of hindlimb RE, the stapled muscle weights remained significantly reduced compared with mobile (P = 0.001) (Fig. 1A, B). The magnitude of the paired difference in relative muscle weights between stapled and mobile limbs for both IM and RE was not significantly different (Fig. 1C), demonstrating lack of recovery following 2 wk of normal ambulation. In order to confirm previous findings regarding concurrent reductions to myofiber CSA with muscle weight loss, we quantified CSA following both IM and RE using dystrophin immunofluorescence on separate cohorts of mice. We observed significant reductions to TA myofiber CSA between hindlimbs (mobile vs. stapled) following IM and RE (IM: 1835 ± 134 μm2 vs. 1335 ± 143 μm2, P = 0.02, n = 4; RE: 1833 ± 194 μm2 vs. 1371 ± 63 μm2, P = 0.05, n = 4) that were concurrent with the TA muscle weight loss.
Figure 1.
Perivascular stem/stromal cell quantity is reduced following IM but restored upon RE. A, B) Absolute (A) and relative (B) TA muscle weights are significantly reduced in immobilized (Stapled) compared with contralateral control (Mobile) limbs after 14 d IM and 14 d RE. C) The paired difference between limbs (Stapled-Mobile) is not significantly different between IM and RE. D) Representative gating schematic for flow cytometry analysis. Gate 1 = mononuclear cell population as determined by forward scatter (FSC) and side scatter (SSC) size relationship; Gate 2 = selection of single cells from Gate 1 to exclude cell doublets or clumping; Gate 3 = exclusion (∼99%) of any CD31+/CD45+ expressing single cells (Lin− population) using FMO gating; gating of cells from the Lin− population expressing NG2+, CD146+, or PDGFRα+. E) Marker expression as a percentage of gated single cells extracted from Mobile and Stapled TAs after IM or RE excluding Lin+ population. F, G) Percentage of NG2+Lin− pericytes coexpressing CD146 (F) and the percentage of CD146+Lin− coexpressing NG2 (G) in both Mobile and Stapled TAs after IM or RE. A, B) Connected circles represent the immobilized and contralateral limbs from an individual sample. Data are means ± sem (n = 5–6). *P < 0.05, difference between Stapled and Mobile (with paired t test).
Figure 1D presents the gating parameters for quantification of perivascular stem/stromal cell content using multiplex flow cytometry. After excluding any doublets from gated mononuclear cells, all CD31 (endothelial)/CD45 (immune) expressing cells were likewise excluded (CD31−CD45−; Lin−). From the Lin− population, NG2+, CD146+, and PDGFRα+ cells were selected, and the relative percentages of pericytes (NG2+Lin− and CD146+Lin−) and fibroadipogenic progenitor cells (FAPs; PDGFRα+Lin−) are reported for mobile and stapled limbs. In the mobile limbs, the relative percentages of NG2+Lin− pericytes was 12.6 ± 1.2%, CD146+Lin− pericytes was 54.8 ± 1.9%, and PDGFRα+Lin− FAPs was 10.6 ± 1.4%. After 14 d of IM, no difference in the percentage of Lin+ (CD31+/CD45+) expressing cells was observed between stapled and mobile limbs (Fig. 1E). A significant reduction in the percentage of NG2+Lin− pericytes was detected with IM (P = 0.01), and there was a trend for a decrease in the number of CD146+Lin− pericytes and PDGFRα+Lin− FAPs (CD146+Lin−, P = 0.06; PDGFRα+Lin−, P = 0.07). Post-RE, no significant differences in quantity were observed between mobile and stapled muscles (Fig. 1E).
No difference was detected in the percentage of NG2+Lin− pericytes expressing CD146+ after 14 d of IM (Fig. 1F). Following IM, the percentage of CD146+Lin− pericytes expressing NG2 trended for a decrease (P = 0.06) but was recovered with RE (Fig. 1G). The significant overlap of CD146 expression within the NG2+Lin− pericyte population concurrent with the minimal overlap of NG2 expression with the CD146+Lin− pericyte population suggests that NG2+Lin− pericytes represent a subpopulation of the larger CD146+Lin− pericyte population.
IM minimally impacts pericyte gene expression but specifically increases activating transcription factor 4 and tumor protein p53 transcription
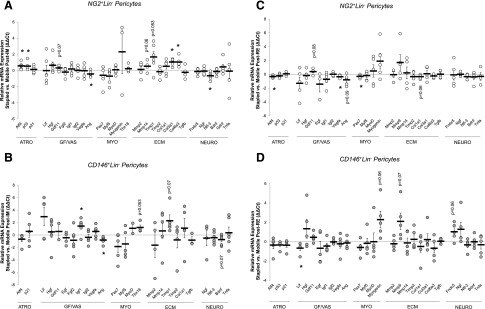
Gene expression profiles for both NG2+Lin− and CD146+Lin− pericytes were examined to determine the impact of hindlimb IM on pericyte function (Fig. 2). For both pericyte types, targeted genes associated with stress (STR), growth and vascularization factors (GF/VAS), myogenic factors (MYO), ECM proteins (ECM), and neurotrophic factors (NEURO) were examined. The pericyte-specific transcription factor, T-box transcription factor 18 (Tbx18) (36), was placed under the category of MYO. Minimal changes were observed in the NG2+Lin− pericyte gene expression profile, with significant decreases in the relative expression between limbs observed for angiogenin (Ang) (P = 0.03) and neurotrophin-3 (Ntf-3) (P = 0.02), whereas activating transcription factor 4 (Atf4) (P = 0.02), tumor protein p53 (p53) (P = 0.04), and various factors associated with ECM remodeling (collagen type 3 a1, collagen type 6 a1, and tissue inhibitor of metalloproteinase 1) were increased (P = 0.001, P = 0.006, P = 0.05, respectively). A trend for an increase was observed for matrix metalloproteinase 14 (Mmp14) (P = 0.06) (Fig. 2A). Minimal changes were also noted in the CD146+Lin− pericyte gene expression profile, with only an increase in insulin like growth factor 1 (Igf1) (P = 0.03) and a significant decrease in Ang (P = 0.02) detected (Fig. 2B). Trends for increases in Tbx18 and tissue inhibitor of metalloproteinase 1 (Timp1) and a decrease in brain-derived neurotrophic factor (Bdnf) were detected (P = 0.053, P = 0.07, P = 0.07, respectively).
Figure 2.
The pericyte gene expression profile is minimally impacted by IM and RE. Following both IM (A, B) and RE (C, D), the relative difference in mRNA expression of stress-related factors (STRs), growth and vascularization factors (GF/VASs), myogenic factors (MYOs), ECM remodeling factors (ECM), and neurotrophic factors (NEURO) between Stapled and Mobile TAs for both NG2+Lin− (A, C) and CD146+Lin− (B, D) pericytes. Each individual circle is the ΔΔCt value calculated for 1 mouse (ΔCt stapled − ΔCt mobile). Data are means ± sem (n = 3–6). *P < 0.05, difference between Stapled and Mobile (with paired t test). Atf4, activating transcription factor 4; p53, tumor protein 53; Lif, leukemia inhibitory factor; Hgf, hepatocyte growth factor, Gdf11, growth differentiation factor 11; Egf, epidermal growth factor; Fgf2, fibroblast growth factor 2; Igf1/2, insulin-like growth factor 1/2; Vegfa, vascular endothelial growth factor alpha; Ang, angiogenin; Pax7, paired box protein 7; Myf5, myogenic factor 5; MyoD, myogenic differentiation 1; Tbx18, T-box transcription factor 18; Mmp2/9/14, matrix metalloproteinase 2/9/14; Timp1/2, tissue inhibitor of metalloproteinase 1/2; Col1a1, collagen type 1 alpha-1; Col3a1, collagen type 3 alpha 1; Col6a3, collagen type 6 alpha 3; Tgfb, transforming growth factor beta; Fndc5, fibronectin type III domain containing protein-5; Ngf, nerve growth factor; Ntf-3, neurotrophin-3; Bdnf, brain-derived neurotrophic factor; Gdnf, glial cell-derived neurotrophic factor; Tnfa, tumor necrosis factor alpha.
RE minimally impacts pericyte gene expression but specifically decreases Atf4 transcription
Pericyte gene expression profiles were evaluated following 14 d of RE. Comparing the relative mRNA expression of stapled to mobile limbs revealed few differences. However, the NG2+Lin− pericytes demonstrated a significant reduction in the relative expression of Atf4 (P = 0.02), with no differences observed for p53 or p21. Vascular endothelial growth factor (Vegfa) (P = 0.04) and the myogenic transcription factor, paired box protein 7 (Pax7) (P = 0.05), were both reduced following RE, and nonsignificant reductions in the expression of Ang (P = 0.09) and collagen type 1 α1 (Col1a1) (P = 0.06) (Fig. 2C) were observed. A trend for increased growth differentiation factor 11 (Gdf11) expression was also noted (P = 0.08). Similarly, CD146+Lin− pericytes exhibited minimal differences in relative mRNA expression between limbs (Fig. 2D). A significant reduction in leukemia inhibitory factor (Lif) expression was detected (P = 0.05) with nonsignificant increases in expression observed for myogenin (Myogenin) (P = 0.06), matrix metalloproteinase 9 (Mmp9) (P = 0.07), and the irisin precursor Fndc5 (P = 0.06).
Pericyte transplantation effectively restores myofiber CSA following IM
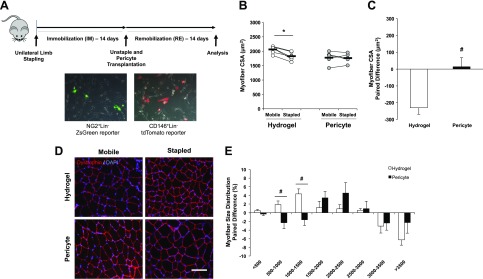
The pericyte transplantation experimental design is presented in Fig. 3A. One day prior to transplantation, donor NG2+Lin− pericytes were transiently transfected with a ZsGreen1-N1 plasmid, and CD146+Lin− pericytes were transiently transfected with a tdTomato-N1 plasmid. Figure 3A presents representative images of the pericytes 24 h after transfection. On the day of transplantation, pericytes were resuspended in a low MW RGD-alginate hydrogel solution for intramuscular transplantation into atrophied TA muscles followed by 14 d of RE. Cells can experience shear stress during the injection procedure and can encounter factors in the microenvironment that restrict migration and function. Thus, the low MW hydrogel solution was used to optimize cell viability during and postdelivery (33, 34).
Figure 3.
Pericyte transplantation restores myofiber CSA following IM. A) Experimental outline of IM and transfection procedures for NG2+Lin− and CD146+Lin− i.m. transplantation using a low MW RGD-alginate hydrogel. Representative images of transfected pericytes are shown at original magnification value of ×10. NG2+Lin− pericytes were transfected with a ZsGreen-N1 reporter plasmid and CD146+Lin− pericytes were transfected with a tdTomato-N1 reporter plasmid separately for 24 h. B) Global myofiber CSA was significantly reduced in the stapled TA following IM in the hydrogel group that was recovered with pericyte transplantation. C) The paired difference in myofiber CSA was significantly different between control and treated groups. D) Representative cross section images of stapled and mobile TA muscle from both hydrogel control and pericyte-treated groups. Dystrophin (red) and DAPI (blue) merged images at ×20 magnification (scale bar, 20 μm) are illustrated. E) The paired difference between limbs for each group comparing the percentage change (stapled-mobile) at each size range. B) Connected circles represent the immobilized and contralateral limbs from an individual sample. *P < 0.05, difference between Stapled and Mobile (with paired t test). Data are means ± sem (n = 5–6). #P < 0.05, paired difference between Hydrogel and Pericyte (with independent samples t test).
Both the control (Hydrogel) and treated (Pericyte) mice exhibited no significant differences in either absolute or relative muscle weight after RE with the relative TA muscle weight paired limb differences similar regardless of treatment (Hydrogel, 0.03 ± 0.06 mg vs. Pericyte, −0.02 ± 0.03 mg). This was likely due to additional weight gain associated with injection of the hydrogel material. However, global myofiber CSA was significantly reduced in the stapled limb of the Hydrogel group (P = 0.002), whereas no difference in CSA was detected between limbs in the Pericyte-treated group (Fig. 3B). The paired limb difference was significantly different between the control and treated groups (Hydrogel, −230.43 ± 39.23 μm2 vs. Pericyte, +14.52 ± 53.79 μm2; P = 0.01) (Fig. 3C). Representative images of mobile and stapled muscle sections stained with dystrophin (red) and DAPI (blue) in both Hydrogel and Pericyte groups are presented in Fig. 3D. The myofiber size distribution was evaluated comparing the paired limb difference between the Hydrogel and Pericyte groups. The Hydrogel control group had a relative increase in the percentage of fibers with smaller CSA (500–1000 and 1000–1500 μm2) (P = 0.02, P = 0.01, respectively), whereas the Pericyte group had a higher, albeit nonsignificant (P = 0.17), percentage of medium-sized fibers at the 2000–2500 μm2 range (Fig. 3E).
Pericyte transplantation preferentially restores Type IIa myofiber size following IM
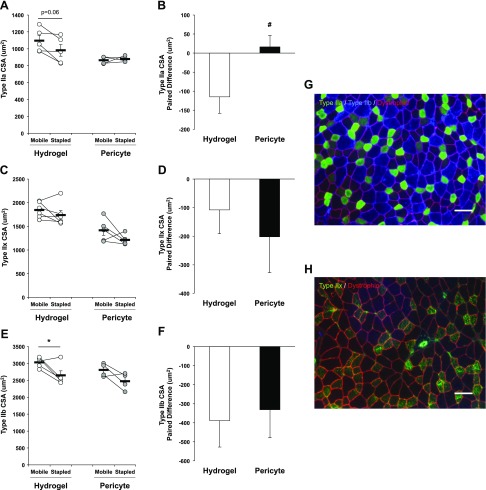
Fiber type specific CSA was preferentially altered by pericyte transplantation. Type IIa MHC fibers were smaller in the Hydrogel control group (nonsignificant, P = 0.06) but not different in the Pericyte-treated group following 14 d of IM and 14 d of RE (Fig. 4A). The paired limb difference was significantly different between control and treated groups (P = 0.05) (Fig. 4B). Type IIx MHC fiber size was variable as a result of IM, resulting in no detectable differences between mobile and stapled legs for either the control or treated groups (Hydrogel, −108.52 ± 83.13 μm2 vs. Pericyte −202.18 ± 125.44 μm2) (Fig. 4C, D). A significant reduction in the size of Type IIb MHC fibers was detected in the stapled limb of the Hydrogel control group (P = 0.05) (Fig. 4E), but no differences between treatment groups were observed (Fig. 4F). Representative images of immunofluorescent staining of Type IIa (green) and IIb (blue) with dystrophin (red) and Type IIx (green) with dystrophin (red) are presented in Fig. 4G, H, respectively. Type I MHC fibers were detected rarely in the predominantly fast twitch (Type II) TA muscle and thus were not quantified.
Figure 4.
Type IIa myofiber size is recovered with pericyte transplantation following IM. A) Type IIa myofiber CSA trends for a significant reduction in the stapled TA of the hydrogel control group but is maintained with pericyte transplantation. B) The paired limb difference between groups is significantly different for Type IIa myofibers. C) Type IIx fiber size is not significantly decreased with IM nor affected by transplantation. D) The magnitude of the paired limb difference is not different between groups. E) A significant reduction in Type IIb fiber size is observed between limbs of the hydrogel control group. F) No significant difference is present in the paired limb difference between groups. G) Representative cross section images of Type IIa (green), Type IIb (blue), and Dystrophin (red) myofibers. H) Representative cross section image of Type IIx (green) and dystrophin (red) myofibers. Images in G and H are presented at an original magnification value of ×20 (scale bars, 20 μm). *P < 0.05, difference between Stapled and Mobile (with paired t test). Data are means ± sem (n = 4–6). #P < 0.05, paired difference between Hydrogel and Pericyte (with independent samples t test).
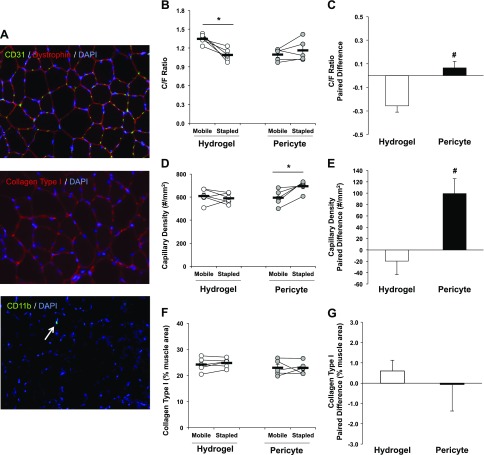
Pericyte transplantation significantly improves skeletal muscle capillarization following IM
Figure 5A (top) is a representative image of the CD31/dystrophin immunofluorescence staining used to quantify TA capillarization. Following IM and RE, the C/F ratio remained significantly reduced in the stapled TA of the Hydrogel control group (P = 0.01), which was recovered with pericyte transplantation (Fig. 5B). The paired limb difference was significantly decreased in the Hydrogel group, although it remained unchanged in the pericyte-treated group (Hydrogel, −0.26 ± 0.05 vs. Pericyte, 0.06 ± 0.06 number/fiber; P = 0.003) (Fig. 5C). Capillary density was not reduced in the stapled limb of the Hydrogel group, although it was significantly increased with pericyte transplantation (P = 0.02) (Fig. 5D). A significant increase in the capillary density between limbs was observed in the Pericyte group compared with controls (Hydrogel, −19.61 ± 23.96 vs. Pericyte, 99.24 ± 26.17 number/mm2; P = 0.01) (Fig. 5E). The percentage of collagen type I present in immobilized TA muscles was not significantly different from contralateral limbs (Fig. 5F, G). Figure 5A (middle) provides a representative image of collagen type I (red) and DAPI (blue) costaining. Total CD11b+ macrophages were minimally present in muscle and not affected by either IM or treatment. A representative image of a CD11b+DAPI+ macrophage within a TA muscle section is presented in Fig. 5A (bottom).
Figure 5.
Pericyte transplantation improves skeletal muscle capillarization following IM. A) Representative cross section images of capillaries (CD31), collagen type 1, and immune cells (CD11b+). Merged images at original magnification value of ×20. B) C/F ratio is significantly reduced in the stapled muscle of the hydrogel control group. Pericyte transplantation leads to a recovery of the C/F ratio. C) The paired limb difference between Hydrogel and Pericyte groups are significantly different. D) Capillary density is significantly increased in the immobilized TA with pericyte transplantation. E) The paired limb difference demonstrates a significant increase in capillary density in the pericyte group. F, G) Collagen type I content is not altered by RE or pericyte transplantation. B, D, F) Connected circles represent the immobilized and contralateral limbs from an individual sample. *P < 0.05, difference between Stapled and Mobile (with paired t test). Data are means ± sem (n = 5–6). #P < 0.05, paired difference between Hydrogel and Pericyte (with independent samples t test).
Myonuclear accretion is not altered following IM and RE
The myonuclei-to-fiber ratio, which can be used as an indicator of myonuclear accretion, was not different between limbs or as a result of treatment. Likewise, the percentage of myofibers containing centrally located nuclei was not significantly altered following IM and RE in either the control or treated groups (data not shown).
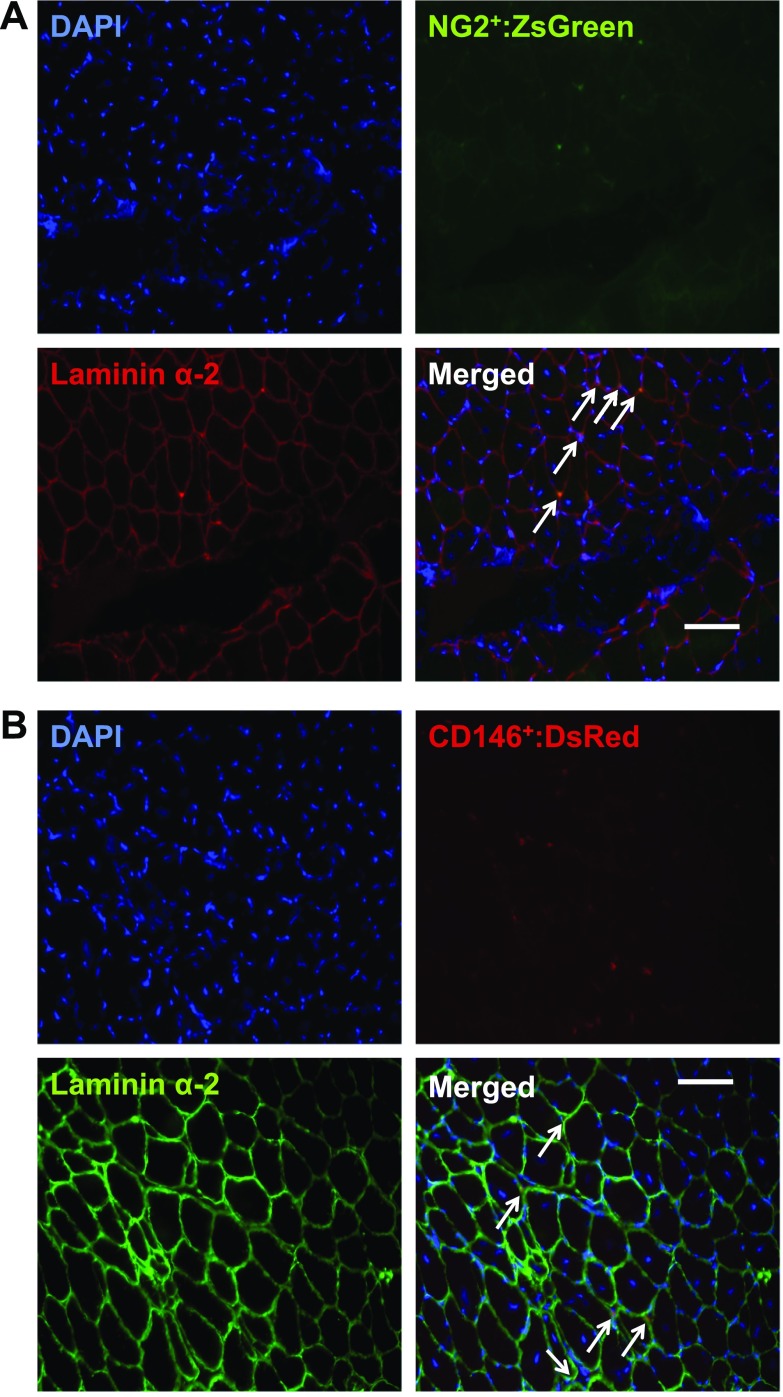
Transplanted pericytes localize to different areas of the interstitium in skeletal muscle
Both pericyte types were transfected with reporter plasmids prior to injection in an attempt to evaluate localization post-IM and RE. Unfortunately, reporter fluorescence was not detected 14 d following transplantation. Thus, we applied anti-ZsGreen and DsRed antibodies to tissue sections to indirectly retrieve this information (Fig. 6). Whereas NG2+ pericytes appeared to reside in close contact with the basal lamina, as identified by overlap of laminin α2 with the ZsGreen plasmid (Fig. 6A), CD146+ pericytes appear to localize in the interstitum between myofibers (Fig. 6B). ZsGreen and DsRed were not detected within the myofiber.
Figure 6.
NG2+ and CD146+ pericyte localization in muscle is divergent following transplantation. A) Cross section image of DAPI (blue), ZsGreen (green), Laminin α-2 (red), and merged image illustrating localization of transplanted NG2+Lin− pericytes following RE. B) Cross section image of DAPI (blue), DsRed (red), Laminin α-2 (green), and merged image illustrating localization of transplanted CD146+Lin− pericytes following RE. A, B) Images at ×20 magnification value; scale bars, 20 μm.
DISCUSSION
The purpose of this study was to assess the pericyte response to disuse and the efficacy of pericyte transplantation on skeletal muscle recovery following hindlimb IM. Targeted analyses suggest minimal impact of disuse on pericyte gene expression, yet NG2+Lin− pericyte quantity was significantly decreased. Intramuscular transplantation of NG2+Lin− and CD146+Lin− pericytes fully restored TA myofiber size and capillarization. Overall, these findings suggest that skeletal muscle–derived pericytes represent a viable therapeutic option for improving skeletal muscle recovery after limb IM.
Pericytes were first identified structurally as a prominent mural cell surrounding microvessels using standard microscopy. The recent verification of chondroitin sulfate proteoglycan (Cspg4 or neural-glial antigen 2, NG2) and melanoma cell adhesion molecule (CD146) expression on the pericyte using single-cell transcriptomic analysis is a step in the right direction toward investigation of cell function (37). With proper exclusion of other cell types that express NG2 or CD146, including immune cells (CD45) and endothelial cells (CD31), a mostly pure population of pericytes can be isolated and phenotyped. In the overwhelming majority of studies that utilize NG2 or CD146 as a pericyte marker for isolation, no information is provided on the relative expression or overlap of cell surface marker expression, as well as the relative quantity of each. In the current work, we report that the majority of NG2+Lin− pericytes express CD146, yet only a small percentage of CD146+Lin− pericytes express NG2, suggesting that NG2+Lin− pericytes represent a subfraction of the larger CD146+Lin− pericyte pool at baseline. In the context of disuse, both pericyte fractions are reduced with hindlimb immobilization (significant at P < 0.05 for NG2+Lin− pericytes and borderline significant at P = 0.06 for CD146+Lin− pericytes). No change in overlay of marker expression was observed with disuse, suggesting that both fractions maintain their identity. Surprisingly, pericyte quantity was restored with 2 wk of RE (normal ambulation) despite a persistent deficit in muscle weight. This discrepancy prompted us to evaluate pericyte function following both IM and RE based on gene expression profile.
Pericytes possess myogenic potential and the ability to directly repair or regenerate muscle with injury or neuromuscular disease (mdx) in mice (23–25). Pericytes also synthesize and secrete a wide variety of growth and immunomodulatory factors that are essential for SC quiescence and maintenance of baseline muscle mass (29). Capacity to influence angiogenesis, ECM remodeling and nerve structure has also been noted (23, 38–41; unpublished results). Therefore, a targeted screen was completed as a first step toward revealing pericyte response to disuse and capacity to influence regrowth. The gene expression profiles suggest that neither IM nor RE robustly impacted pericyte function. A few changes were observed, however, that were of interest. First, Ang gene expression was significantly decreased in both NG2+Lin− and CD146+Lin− pericytes with IM and barely recovered following RE. We have previously reported increased ANG protein secretion by perivascular stem/stromal cells following mechanical strain in vitro, as well as enhanced gene expression in a transgenic mouse model endowed with the capacity for muscle growth following mechanical load (42, 43). Kostallari et al. (29) similarly reported enrichment of ANG protein in NG2+ pericyte conditioned medium. As an RNase, ANG facilitates an increase in cap-independent translation during times of stress and may influence rRNA synthesis (44). ANG is also a potent stimulator of angiogenesis, specifically up-regulating the production of proteases that degrade laminin and fibronectin, allowing endothelial cells to migrate and form new vessels (45, 46). This information suggests that lack of ANG production by pericytes during IM may contribute to suppression of protein synthesis and atrophy, as well as reductions to capillary number (C/F ratio) or capillary density (capillary per square millimeter tissue) following disuse (47–50). In addition to the change in Ang gene expression, the increases in Atf4 and p53 in NG2+Lin− pericytes post-IM were also striking. ATF4 can suppress protein synthesis and initiate atrophy when overexpressed in mouse skeletal muscle (51, 52). As a stress-inducible basic leucine zipper transcription factor, it is possible that an increase in Atf4 is not cell specific, but its induction in a variety of cell types initiates a molecular program that conserves energy for the purpose of survival. Follow-up analyses are justified for Ang, Atf4, and p53, as well as unbiased screening of pericyte gene expression post-IM and RE. An earlier time point post-RE would also be informative regarding pericyte contribution to regrowth during recovery.
The decline in relative pericyte quantity following IM suggested an important role for pericytes in the regulation of muscle mass and provided justification for cell replacement. Given the potential for perivascular stem/stromal cells to sense mechanical strain and promote skeletal muscle adaptation in response to an exercise stimulus (26, 35, 42, 53), we hypothesized that transplantation of pericytes immediately prior to RE would optimize cell function and subsequent recovery of muscle mass. In addition, based on our observation that cell survival is consistently low in muscle posttransplantation, pericytes (NG2+Lin− and CD146+Lin−) were encapsulated in an injectable hydrogel formed from cross-linking of alginate conjugated with integrin-binding RGD peptides. The average MW of alginate was tuned to be 50,000 g/mol to optimize cell viability during and after delivery (33, 34). Alginate is a naturally occurring polymer obtained from brown seaweed that is frequently used for biomedical applications because of its unique biocompatibility, low toxicity, and low cost (54). Alginate hydrogels are structurally similar to ECMs of living tissues and facilitate cell viability and migration posttransplantation. As predicted, pericyte transplantation fully restored myofiber CSA by the end of the 2-wk mobilization period when comparing the paired difference between legs, whereas deficits were sustained in the hydrogel-only control group. Baseline CSA (mobile leg) was different between treated groups due to different cohorts of mice used to complete the study, but there were clear differences between legs by treatment by the end of the study. We initially collected muscle from mice that did not undergo surgery and served as a “complete control” but did not observe significant differences from the mobile leg using the unilateral design, and collection was discontinued. As further evidence for a preferential effect, we found that pericyte transplantation specifically recovered the CSA of Type IIa fibers, the more oxidative fast twitch fiber type that is susceptible to atrophy with IM (1), whereas no differences were noted in Type IIx or Type IIb fiber CSA. Therefore, we are confident that pericyte transplantation significantly improved recovery of muscle mass following disuse. The extent to which the hydrogel was necessary for positive outcomes observed was not determined, but it will be the focus of a future study that will provide several detailed measures of cell viability and function, endogenous cell responses, and rate of hydrogel degradation.
The paired differences in C/F ratio and capillary density also suggest recovery from capillary refraction (C/F ratio) and enhancement of angiogenesis (capillary density) following disuse. Hayes et al. (41) recently reported the ability for muscle-derived CD146+CD45−CD34− pericytes to augment blood flow recovery and collateral artery enlargement, but not angiogenesis, in wild-type mice following ischemia. Neovascularization and recovery postischemia have been reported in mice using NG2+nestin+ pericytes and pericytes derived from human pluripotent stem cells (38, 40). We have similarly demonstrated the potential for perivascular stem/stromal cells to augment exercise-induced arteriogenesis in young mice (35) and improve perfusion in aged skeletal muscle (42). In the study by Hayes et al. (41), a small percentage of GFP+CD146+ pericytes engrafted into collateral arteries and S100+ Schwann cells but not skeletal muscle fibers. We attempted to evaluate engraftment of our cells by transfecting pericytes with ZsGreen and tdTomato reporter plasmids prior to injection. Unfortunately, transfection was either low or the cytomegalovirus promoter was methylated and silenced because very few cells were detected with fluorescence microscopy. Using antibodies against the reporters, we found that pericytes did not directly engraft with existing fibers or form new fibers but resided in the interstitial space between fibers. Interestingly, NG2+ pericytes were intimately associated with the basal lamina, whereas CD146+ pericytes localized outside the basal lamina. It is not clear if the difference in localization was due to engraftment with vessels surrounding the fibers (NG2+ pericytes) or simply due to differences in preference for cues provided by the niche. Regardless, pericytes did not demonstrate any capacity for myogenesis. These results are consistent with a recent study that performed lineage tracing of pericytes in multiple tissues using a newly identified pericyte transcriptional factor, TBX18 (36). Specifically, Tbx18-CreERT2 lineage traced cells did not demonstrate myogenic capacity in muscle in response to barium chloride–induced injury and maintained cellular identity across the lifespan. It will be important to use this new molecular tool to verify pericyte fate in the context of disuse atrophy and mechanical loading.
Importantly, pericyte transplantation did not result in an inflammatory response or collagen accumulation, which can occur with nonautologous transplantation. CD11b+ immune cells were rarely present in muscle, mitigating the need for quantitation. Collagen content similarly did not increase as a result of either atrophy or pericyte transplantation. Based on studies that suggest an improvement in ECM turnover and decreased fibrosis with pericyte transplantation in the context of muscle atrophy following rotator cuff tear (55) and cardiac ischemia (56), we suspect that pericytes stimulate collagen degradation and that remodeling is a primary mechanism by which pericytes improve recovery following disuse.
CONCLUSIONS
The findings from this study provide the first evidence that pericytes contribute to skeletal muscle recovery following IM. This study provides justification for further development of a pericyte therapy as well as parallel studies that identify the mechanistic basis by which pericytes increase capillarization and myofiber size following disuse atrophy.
ACKNOWLEDGMENTS
The authors thank Dr. Barbara Pilas and the Flow Cytometry Center at the Roy J. Carver Biotechnology Center (University of Illinois–Urbana-Champaign) for advice and assistance. Research reported in this publication was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the U.S. National Institutes of Health (NIH) under award number R01AR072735 (to M.D.B.), National Heart, Lung, and Blood Institute (NHLBI) under award number R21HL131469 (to H.K. and M.D.B.), and a University of Illinois–Urbana-Champaign (UIUC) Research Board Grant (to M.D.B.). M.M. was supported by a UIUC Dissertation Completion Award. S.D. was supported by National Institute of Biomedical Imaging and Bioengineering (NIBIB) of the NIH under award number T32EB019944 and an American College of Sports Medicine National Aeronautics and Space Administration (NASA) Space Physiology Research Grant (18-00664). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors declare no conflicts of interest.
Glossary
- APC
allophycoctanin
- Ang
angiogenin
- Atf4
activating transcription factor 4
- CBC
Roy J. Carver Biotechnology Center
- CD
cluster of differentiation
- CD146
melanoma cell adhesion molecule
- C/F
capillary-to-fiber
- CSA
cross-sectional area
- ECM
extracellular matrix
- FACS
fluorescence-activated cell sorting
- FAP
fibroadipogenic progenitor cell
- FBS
fetal bovine serum
- FMO
fluorescence minuse one
- Fndc5
fibronectin type III domain-containing protein 5
- IM
immobilization
- LB
Luria broth
- Lin
Lineage
- MHC
myosin heavy chain
- NG2
neuron-glial antigen 2
- p53
tumor protein p53
- Pax7
paired box protein 7
- PDGFRα
platelet-derived growth factor receptor α
- PECAM-1
platelet endothelial cell adhesion molecule
- RE
remobilization
- RGD
arginylglycylaspartic acid
- SC
satellite cell
- TA
tibialis anterior
- Tbx18
T-box transcription factor 18
- UIUC
University of Illinois at Urbana-Champaign
AUTHOR CONTRIBUTIONS
M. Munroe, M. C. Dyle, H. Kong, C. M. Adams, and M. D. Boppart conceived and designed the research; M. Munroe, S. Dvoretskiy, and A. Lopez performed experiments; J. Leong and H. Kong designed and supplied the alginate hydrogel; M. Munroe, S. Dvoretskiy, A. Lopez, and M. D. Boppart analyzed data and interpreted the results of the experiments; M. Munroe and M. D. Boppart prepared the figures and drafted the manuscript; and all authors revised and approved the final version of the manuscript.
REFERENCES
- 1.Bodine S. C. (2013) Disuse-induced muscle wasting. Int. J. Biochem. Cell Biol. 45, 2200–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brooks N. E., Myburgh K. H. (2014) Skeletal muscle wasting with disuse atrophy is multi-dimensional: the response and interaction of myonuclei, satellite cells and signaling pathways. Front. Physiol. 5, 99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dirks M. L., Wall B. T., Nilwik R., Weerts D. H., Verdijk L. B., van Loon L. J. (2014) Skeletal muscle disuse atrophy is not attenuated by dietary protein supplementation in healthy older men. J. Nutr. 144, 1196–1203 [DOI] [PubMed] [Google Scholar]
- 4.Hvid L. G., Suetta C., Aagaard P., Kjaer M., Frandsen U., Ørtenblad N. (2013) Four days of muscle disuse impairs single fiber contractile function in young and old healthy men. Exp. Gerontol. 48, 154–161 [DOI] [PubMed] [Google Scholar]
- 5.Wall B. T., Snijders T., Senden J. M., Ottenbros C. L., Gijsen A. P., Verdijk L. B., van Loon L. J. (2013) Disuse impairs the muscle protein synthetic response to protein ingestion in healthy men. J. Clin. Endocrinol. Metab. 98, 4872–4881 [DOI] [PubMed] [Google Scholar]
- 6.Valenzuela P. L., Morales J. S., Pareja-Galeano H., Izquierdo M., Emanuele E., de la Villa P., Lucia A. (2018) Physical strategies to prevent disuse-induced functional decline in the elderly. Ageing Res. Rev. 47, 80–88 [DOI] [PubMed] [Google Scholar]
- 7.Baehr L. M., West D. W. D., Marcotte G., Marshall A. G., De Sousa L. G., Baar K., Bodine S. C. (2016) Age-related deficits in skeletal muscle recovery following disuse are associated with neuromuscular junction instability and ER stress, not impaired protein synthesis. Aging (Albany N.Y.) 8, 127–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drummond M. J., Dickinson J. M., Fry C. S., Walker D. K., Gundermann D. M., Reidy P. T., Timmerman K. L., Markofski M. M., Paddon-Jones D., Rasmussen B. B., Volpi E. (2012) Bed rest impairs skeletal muscle amino acid transporter expression, mTORC1 signaling, and protein synthesis in response to essential amino acids in older adults. Am. J. Physiol. Endocrinol. Metab. 302, E1113–E1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hvid L., Aagaard P., Justesen L., Bayer M. L., Andersen J. L., Ørtenblad N., Kjaer M., Suetta C. (2010) Effects of aging on muscle mechanical function and muscle fiber morphology during short-term immobilization and subsequent retraining. J. Appl. Physiol. 109, 1628–1634 [DOI] [PubMed] [Google Scholar]
- 10.Hwee D. T., Bodine S. C. (2009) Age-related deficit in load-induced skeletal muscle growth. J. Gerontol. A. Biol. Sci. Med. Sci. 64A, 618–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kortebein P., Ferrando A., Lombeida J., Wolfe R., Evans W. J. (2007) Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA 297, 1772–1774 [DOI] [PubMed] [Google Scholar]
- 12.Suetta C., Frandsen U., Mackey A. L., Jensen L., Hvid L. G., Bayer M. L., Petersson S. J., Schrøder H. D., Andersen J. L., Aagaard P., Schjerling P., Kjaer M. (2013) Ageing is associated with diminished muscle re-growth and myogenic precursor cell expansion early after immobility-induced atrophy in human skeletal muscle. J. Physiol. 591, 3789–3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanner R. E., Brunker L. B., Agergaard J., Barrows K. M., Briggs R. A., Kwon O. S., Young L. M., Hopkins P. N., Volpi E., Marcus R. L., LaStayo P. C., Drummond M. J. (2015) Age-related differences in lean mass, protein synthesis and skeletal muscle markers of proteolysis after bed rest and exercise rehabilitation. J. Physiol. 593, 4259–4273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White J. R., Confides A. L., Moore-Reed S., Hoch J. M., Dupont-Versteegden E. E. (2015) Regrowth after skeletal muscle atrophy is impaired in aged rats, despite similar responses in signaling pathways. Exp. Gerontol. 64, 17–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lepper C., Partridge T. A., Fan C. M. (2011) An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 138, 3639–3646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sambasivan R., Yao R., Kissenpfennig A., Van Wittenberghe L., Paldi A., Gayraud-Morel B., Guenou H., Malissen B., Tajbakhsh S., Galy A. (2011) Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development 138, 3647–3656 [DOI] [PubMed] [Google Scholar]
- 17.Egner I. M., Bruusgaard J. C., Gundersen K. (2016) Satellite cell depletion prevents fiber hypertrophy in skeletal muscle. Development 143, 2898–2906 [DOI] [PubMed] [Google Scholar]
- 18.Fry C. S., Kirby T. J., Kosmac K., McCarthy J. J., Peterson C. A. (2017) Myogenic progenitor cells control extracellular matrix production by fibroblasts during skeletal muscle hypertrophy. Cell Stem Cell 20, 56–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goh Q., Millay D. P. (2017) Requirement of myomaker-mediated stem cell fusion for skeletal muscle hypertrophy. eLife 6, 1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCarthy J. J., Mula J., Miyazaki M., Erfani R., Garrison K., Farooqui A. B., Srikuea R., Lawson B. A., Grimes B., Keller C., Van Zant G., Campbell K. S., Esser K. A., Dupont-Versteegden E. E., Peterson C. A. (2011) Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development 138, 3657–3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murach K. A., White S. H., Wen Y., Ho A., Dupont-Versteegden E. E., McCarthy J. J., Peterson C. A. (2017) Differential requirement for satellite cells during overload-induced muscle hypertrophy in growing versus mature mice. Skelet. Muscle 7, 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson J. R., Mula J., Kirby T. J., Fry C. S., Lee J. D., Ubele M. F., Campbell K. S., McCarthy J. J., Peterson C. A., Dupont-Versteegden E. E. (2012) Satellite cell depletion does not inhibit adult skeletal muscle regrowth following unloading-induced atrophy. Am. J. Physiol. Cell Physiol. 303, C854–C861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birbrair A., Zhang T., Wang Z. M., Messi M. L., Enikolopov G. N., Mintz A., Delbono O. (2013) Role of pericytes in skeletal muscle regeneration and fat accumulation. Stem Cells Dev. 22, 2298–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crisan M., Yap S., Casteilla L., Chen C. W., Corselli M., Park T. S., Andriolo G., Sun B., Zheng B., Zhang L., Norotte C., Teng P. N., Traas J., Schugar R., Deasy B. M., Badylak S., Buhring H. J., Giacobino J. P., Lazzari L., Huard J., Péault B. (2008) A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3, 301–313 [DOI] [PubMed] [Google Scholar]
- 25.Sacchetti B., Funari A., Remoli C., Giannicola G., Kogler G., Liedtke S., Cossu G., Serafini M., Sampaolesi M., Tagliafico E., Tenedini E., Saggio I., Robey P. G., Riminucci M., Bianco P. (2016) No identical “mesenchymal stem cells” at different times and sites: human committed progenitors of distinct origin and differentiation potential are incorporated as adventitial cells in microvessels. Stem Cell Reports 6, 897–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zou K., Huntsman H. D., Carmen Valero M., Adams J., Skelton J., De Lisio M., Jensen T., Boppart M. D. (2015) Mesenchymal stem cells augment the adaptive response to eccentric exercise. Med. Sci. Sports Exerc. 47, 315–325 [DOI] [PubMed] [Google Scholar]
- 27.Armulik A., Genové G., Betsholtz C. (2011) Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 21, 193–215 [DOI] [PubMed] [Google Scholar]
- 28.Geevarghese A., Herman I. M. (2014) Pericyte-endothelial crosstalk: implications and opportunities for advanced cellular therapies. Transl. Res. 163, 296–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kostallari E., Baba-Amer Y., Alonso-Martin S., Ngoh P., Relaix F., Lafuste P., Gherardi R. K. (2015) Pericytes in the myovascular niche promote post-natal myofiber growth and satellite cell quiescence. Development 142, 1242–1253 [DOI] [PubMed] [Google Scholar]
- 30.Snijders T., Nederveen J. P., Joanisse S., Leenders M., Verdijk L. B., van Loon L. J., Parise G. (2017) Muscle fibre capillarization is a critical factor in muscle fibre hypertrophy during resistance exercise training in older men. J. Cachexia Sarcopenia Muscle 8, 267–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caron A. Z., Drouin G., Desrosiers J., Trensz F., Grenier G. (2009) A novel hindlimb immobilization procedure for studying skeletal muscle atrophy and recovery in mouse. J. Appl. Physiol. 106, 2049–2059 [DOI] [PubMed] [Google Scholar]
- 32.Ebert S. M., Dyle M. C., Kunkel S. D., Bullard S. A., Bongers K. S., Fox D. K., Dierdorff J. M., Foster E. D., Adams C. M. (2012) Stress-induced skeletal muscle Gadd45a expression reprograms myonuclei and causes muscle atrophy. J. Biol. Chem. 287, 27290–27301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee M. K., Rich M. H., Lee J., Kong H. (2015) A bio-inspired, microchanneled hydrogel with controlled spacing of cell adhesion ligands regulates 3D spatial organization of cells and tissue. Biomaterials 58, 26–34 [DOI] [PubMed] [Google Scholar]
- 34.Kong H. J., Smith M. K., Mooney D. J. (2003) Designing alginate hydrogels to maintain viability of immobilized cells. Biomaterials 24, 4023–4029 [DOI] [PubMed] [Google Scholar]
- 35.Huntsman H. D., Zachwieja N., Zou K., Ripchik P., Valero M. C., De Lisio M., Boppart M. D. (2013) Mesenchymal stem cells contribute to vascular growth in skeletal muscle in response to eccentric exercise. Am. J. Physiol. Heart Circ. Physiol. 304, H72–H81 [DOI] [PubMed] [Google Scholar]
- 36.Guimarães-Camboa N., Cattaneo P., Sun Y., Moore-Morris T., Gu Y., Dalton N. D., Rockenstein E., Masliah E., Peterson K. L., Stallcup W. B., Chen J., Evans S. M. (2017) Pericytes of multiple organs do not behave as mesenchymal stem cells in vivo. Cell Stem Cell 20, 345–359.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vanlandewijck M., He L., Mäe M. A., Andrae J., Ando K., Del Gaudio F., Nahar K., Lebouvier T., Laviña B., Gouveia L., Sun Y., Raschperger E., Räsänen M., Zarb Y., Mochizuki N., Keller A., Lendahl U., Betsholtz C. (2018) A molecular atlas of cell types and zonation in the brain vasculature. Nature 554, 475–480; erratum: 560, E3 [DOI] [PubMed] [Google Scholar]
- 38.Birbrair A., Zhang T., Wang Z. M., Messi M. L., Olson J. D., Mintz A., Delbono O. (2014) Type-2 pericytes participate in normal and tumoral angiogenesis. Am. J. Physiol. Cell Physiol. 307, C25–C38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Birbrair A., Zhang T., Wang Z. M., Messi M. L., Mintz A., Delbono O. (2014) Pericytes: multitasking cells in the regeneration of injured, diseased, and aged skeletal muscle. Front. Aging Neurosci. 6, 245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dar A., Domev H., Ben-Yosef O., Tzukerman M., Zeevi-Levin N., Novak A., Germanguz I., Amit M., Itskovitz-Eldor J. (2012) Multipotent vasculogenic pericytes from human pluripotent stem cells promote recovery of murine ischemic limb. Circulation 125, 87–99 [DOI] [PubMed] [Google Scholar]
- 41.Hayes K. L., Messina L. M., Schwartz L. M., Yan J., Burnside A. S., Witkowski S. (2018) Type 2 diabetes impairs the ability of skeletal muscle pericytes to augment postischemic neovascularization in db/db mice. Am. J. Physiol. Cell Physiol. 314, C534–C544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huntsman H. D., Rendeiro C., Merritt J. R., Pincu Y., Cobert A., De Lisio M., Kolyvas E., Dvoretskiy S., Dobrucki I. T., Kemkemer R., Jensen T., Dobrucki L. W., Rhodes J. S., Boppart M. D. (2018) The impact of mechanically stimulated muscle-derived stromal cells on aged skeletal muscle. Exp. Gerontol. 103, 35–46 [DOI] [PubMed] [Google Scholar]
- 43.Mahmassani Z. S., Son K., Pincu Y., Munroe M., Drnevich J., Chen J., Boppart M. D. (2017) α7β1 integrin regulation of gene transcription in skeletal muscle following an acute bout of eccentric exercise. Am. J. Physiol. Cell Physiol. 312, C638–C650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lyons S. M., Fay M. M., Akiyama Y., Anderson P. J., Ivanov P. (2017) RNA biology of angiogenin: current state and perspectives. RNA Biol. 14, 171–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu G., Riordan J. F., Vallee B. L. (1994) Angiogenin promotes invasiveness of cultured endothelial cells by stimulation of cell-associated proteolytic activities. Proc. Natl. Acad. Sci. USA 91, 12096–12100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miyake M., Goodison S., Lawton A., Gomes-Giacoia E., Rosser C. J. (2015) Angiogenin promotes tumoral growth and angiogenesis by regulating matrix metallopeptidase-2 expression via the ERK1/2 pathway. Oncogene 34, 890–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaneguchi A., Ozawa J., Kawamata S., Kurose T., Yamaoka K. (2014) Intermittent whole-body vibration attenuates a reduction in the number of the capillaries in unloaded rat skeletal muscle. BMC Musculoskelet. Disord. 15, 315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kano Y., Shimegi S., Takahashi H., Masuda K., Katsuta S. (2000) Changes in capillary luminal diameter in rat soleus muscle after hind-limb suspension. Acta Physiol. Scand. 169, 271–276 [DOI] [PubMed] [Google Scholar]
- 49.Roudier E., Gineste C., Wazna A., Dehghan K., Desplanches D., Birot O. (2010) Angio-adaptation in unloaded skeletal muscle: new insights into an early and muscle type-specific dynamic process. J. Physiol. 588, 4579–4591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tyml K., Mathieu-Costello O. (2001) Structural and functional changes in the microvasculature of disused skeletal muscle. Front. Biosci. 6, D45–D52 [DOI] [PubMed] [Google Scholar]
- 51.Adams C. M., Ebert S. M., Dyle M. C. (2017) Role of ATF4 in skeletal muscle atrophy. Curr. Opin. Clin. Nutr. Metab. Care 20, 164–168 [DOI] [PubMed] [Google Scholar]
- 52.Fox D. K., Ebert S. M., Bongers K. S., Dyle M. C., Bullard S. A., Dierdorff J. M., Kunkel S. D., Adams C. M. (2014) p53 and ATF4 mediate distinct and additive pathways to skeletal muscle atrophy during limb immobilization. Am. J. Physiol. Endocrinol. Metab. 307, E245–E261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Valero M. C., Huntsman H. D., Liu J., Zou K., Boppart M. D. (2012) Eccentric exercise facilitates mesenchymal stem cell appearance in skeletal muscle. PLoS One 7, e29760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee K. Y., Mooney D. J. (2012) Alginate: properties and biomedical applications. Prog. Polym. Sci. 37, 106–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eliasberg C. D., Dar A., Jensen A. R., Murray I. R., Hardy W. R., Kowalski T. J., Garagozlo C. A., Natsuhara K. M., Khan A. Z., McBride O. J., Cha P. I., Kelley B. V., Evseenko D., Feeley B. T., McAllister D. R., Péault B., Petrigliano F. A. (2017) Perivascular stem cells diminish muscle atrophy following massive rotator cuff tears in a small animal model. J. Bone Joint Surg. Am. 99, 331–341 [DOI] [PubMed] [Google Scholar]
- 56.Chen C. W., Okada M., Proto J. D., Gao X., Sekiya N., Beckman S. A., Corselli M., Crisan M., Saparov A., Tobita K., Péault B., Huard J. (2013) Human pericytes for ischemic heart repair. Stem Cells 31, 305–316 [DOI] [PMC free article] [PubMed] [Google Scholar]