Abstract
Fetal cardiomyocytes shift from glycolysis to oxidative phosphorylation around the time of birth. Myeloid ecotropic viral integration site 1 (MEIS1) is a transcription factor that promotes glycolysis in hematopoietic stem cells. We reasoned that MEIS1 could have a similar role in the developing heart. We hypothesized that suppression of MEIS1 expression in fetal sheep cardiomyocytes leads to a metabolic switch as found at birth. Expression of MEIS1 was assayed in left ventricular cardiac tissue and primary cultures of cardiomyocytes from fetal (100- and 135-d gestation, term = 145 d), neonatal, and adult sheep. Cultured cells were treated with short interfering RNA (siRNA) to suppress MEIS1. Oxygen consumption rate was assessed with the Seahorse metabolic flux analyzer, and mitochondrial activity was assessed by staining cells with MitoTracker Orange. Cardiomyocyte respiratory capacity increased with advancing age concurrently with decreased expression of MEIS1. MEIS1 suppression with siRNA increased maximal oxygen consumption in fetal cells but not in postnatal cells. Mitochondrial activity was increased and expression of glycolytic genes decreased when MEIS1 expression was suppressed. Thus, we conclude that MEIS1 is a key regulator of cardiomyocyte metabolism and that the normal down-regulation of MEIS1 with age underlies a gradual switch to oxidative metabolism.—Lindgren, I. M., Drake, R. R., Chattergoon, N. N., Thornburg, K. L. Down-regulation of MEIS1 promotes the maturation of oxidative phosphorylation in perinatal cardiomyocytes.
Keywords: fetal heart, development, metabolic shift, glycolysis, mitochondrial respiration
The cardiovascular system undergoes a series of rapid changes at birth. In large mammals, including humans and sheep, the mean arterial blood pressure increases from ∼40 mmHg in the fetus to 70–80 mmHg after delivery (1), prompting the newborn left ventricle to generate increased contractile force to pump against an increased systemic load. Concomitantly, the partial pressure of arterial oxygen increases from ∼20 mmHg in the fetus to ∼80 mmHg in the newborn with the first breaths of air (2–4). This provides the oxygen required for ATP generation by the working myocardium as workload increases. As the fetus becomes more mature toward term, the myocardium prepares for its increased metabolic requirements by shifting away from glycolysis to a greatly augmented capacity for mitochondrial oxidative phosphorylation, which generates ATP much more efficiently than glycolysis. The switch to oxidative phosphorylation in the developing myocardium is also linked to a switch of the metabolic substrate from mainly glucose and lactate to free fatty acids as the primary fuel (5). The transition from the use of glucose for cytosolic glycolysis to the use of fatty acids for mitochondrial β-oxidation requires that the machinery for mitochondrial respiration be in place before term. Thus, the regulatory machinery that supports the metabolic switch does not appear instantaneously at the moment of birth but accompanies the gradual maturation of cardiomyocytes as term approaches.
The metabolic switch at birth is not merely a process of academic interest; it also applies to clinical problems. For example, the preterm birth rate in the U.S. was 9.8% in 2016 (6), and babies born prematurely suffer an increased risk for cardiovascular complications throughout their lives (7). These cases contribute to the increasing burden of cardiovascular disease. Heart disease causes personal suffering and is projected to cost over $1 trillion annually in the U.S. by 2035 (8, 9). The timing of feeding and the composition of nutrient supplements for premature babies is important to ensure optimal postnatal growth and long-term health (10–12). The postnatal growth rate of premature babies is predictive of the individual’s cardiovascular performance in adulthood, with perinatal growth restriction and rapid postnatal catch-up growth being synergistically detrimental (12–14). Prematurely born babies have immature hearts that may not be metabolically prepared for the lipid load they will receive from breast milk, formula, or intravenous feeding. Although lipids are the heart’s main energy source after birth, fatty acid concentrations are generally low in fetal plasma (15). Thus, the cellular machinery of the premature heart may not be ready for a lipid-rich diet. Little is known about how the switch in substrates affects the growth and development of the premature heart. However, it is likely that failure to provide nutrients optimized for cardiac metabolism could hamper both cardiac growth and function, which could harm other organs if cardiac output is inadequate. Therefore, there is an urgent need to understand the physiologic mechanisms that regulate cardiac metabolism during the perinatal period.
The transcription factor myeloid ecotropic viral integration site 1 (MEIS1) is a powerful regulator of metabolism in hematopoietic stem cells (HSCs). Suppression of MEIS1 expression in HSCs leads to a switch from glycolysis to oxidative phosphorylation (16–18), somewhat similar to the metabolic switch taking place in the heart around birth. We reasoned that MEIS1 might play the same role in the developing myocardium of large mammals as it does in HSCs, a concept that has not been previously tested. If so, suppression of MEIS1 expression would promote the perinatal increase in oxidative phosphorylation. Thus, we sought to test the hypothesis that suppression of MEIS1 expression in cardiomyocytes leads to a metabolic switch that promotes oxidative phosphorylation. Here, we report on our investigation of the role of MEIS1 in metabolic maturation in ovine cardiomyocytes that resemble cardiomyocyte ontogeny in humans more closely than rodent cells (19, 20).
MATERIALS AND METHODS
Animals
All animal work was performed in accordance with the Institutional Animal Care and Use Committee at Oregon Health and Science University (Portland, OR, USA). All animal groups contained both males and females. Hearts were obtained from fetal sheep (Ovis aries, mixed breed) at gestational d 100 or 135 (term = 145 d). For fetal samples, the pregnant ewe was euthanized by an intravenous bolus injection of sodium pentobarbital (SomnaSol, ∼80 mg/kg; Henry Schein Animal Health, Dublin, OH, USA). The fetus was given a 10,000 U injection of heparin (Baxter, Deerfield, IL, USA) into the umbilical vein, followed by 10 ml saturated potassium chloride (KCl) to stop the heart in diastole. Fetal mass was recorded, and the heart was excised, trimmed in a standardized manner, weighed, and further processed for cardiomyocyte isolation. Hearts from 24 h to 5 d neonates were obtained by euthanizing the lamb in the same fashion as described above for the ewes after giving a 10,000 U bolus injection of heparin. Neonatal hearts were dissected and treated the same as fetal hearts. Adult, neonatal, and fetal whole-tissue samples (n = 7–8) were obtained from the euthanized animals and flash frozen in liquid nitrogen.
Cardiomyocyte isolation and culture
Five to six hearts per age group were cannulated through the ascending aorta and retrograde-perfused with gassed solutions (100% O2, 39°C) in the following order: 1) calcium-free Tyrode’s buffer (140 mM NaCl, 5 mM KCl, 1 mM, 153 MgCl20.6H2O, 10 mM glucose, and 10 mM HEPES; pH adjusted to 7.35 with NaOH) to clear the heart of blood (5–10 min), 2) Tyrode’s buffer containing 160 U/ml type II collagenase and 0.78 U/ml type XIV protease to enzymatically disaggregate the tissue (2–5 min), and finally 3) a high-potassium and calcium-free Kraftbrühe (KB) solution (74 mM glutamic acid, 30 mM KCl, 30 mM KH2PO4, 20 mM taurine, 3 mM MgSO4, 0.5 mM EGTA, 10 mM HEPES, and 10 mM glucose; pH adjusted to 7.37 using KOH) to rinse out the enzymes and ensure relaxed cardiomyocytes (10–15 min). The 2 ventricles were separated from the septum, and all 3 parts were treated separately. Gentle agitation of each tissue piece in KB-filled Falcon tubes released the cardiomyocytes into the solution and the remaining tissue was discarded.
Following the isolation, the cell slurry was left to rest for 30 min in KB at room temperature. The slurry was centrifuged (5 min, 2000 rpm, room temperature) and resuspended in low-glucose DMEM (5.5 mM glucose) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, MA, USA), 1 ml/L antibiotic-antimycotic solution (MilliporeSigma, Burlington, MA, USA), and 10 mg/L insulin-transferrin-sodium selenite supplement (MilliporeSigma). Cardiomyocytes were identified as large rod-shaped cells and made up >95% of the cell population (21). To further enhance cell purity, the cells were preplated twice for 2 h to remove the majority of noncardiomyocytes, such as endothelial cells and fibroblasts (39°C, 5% CO2), before the cardiomyocytes were used in experiments or frozen down for later processing.
All cultures were performed with cardiomyocytes from the left ventricular (LV) free wall only. A modified ultra-low-glucose DMEM supplemented with lactate (0.9 mM glucose, 2 mM l-lactate, 1 mM sodium pyruvate, 2 mM glutamine, and 22 mM NaHCO3) was used for fetal cell culture to mimic the composition of fetal plasma. Neonatal cultures were maintained in regular low-glucose DMEM as previously described. Fetal cultures were incubated in hypoxic conditions at 3% O2 to mimic a fetal arterial oxygen of 20 mmHg, whereas neonatal cultures were incubated in ambient oxygen. All cultures were maintained at 39°C and 5% CO2.
For later RNA isolation for quantitative PCR (qPCR), cells were plated in a 6-well plate (BioLite; Thermo Fisher Scientific) at a density of 3 × 105 cardiomyocytes per well. For Seahorse experiments, cells were plated in 96-well Seahorse culture plates (Agilent Technologies, Santa Clara, CA, USA) at 2 × 104 cells per well in 8 replicates per animal.
Short interfering RNA transfection
We designed and verified a customized short interfering RNA (siRNA) oligo targeting sheep MEIS1 (sense sequence CCCUUACCCUUCUGAAGAATT; Thermo Fisher Scientific). The transfection agent Lipofectamine 2000 (1:100; Thermo Fisher Scientific) and the MEIS1 siRNA (120 nM) were separately mixed with Opti-Minimum Essential Medium (MEM) (Thermo Fisher Scientific), incubated at room temperature for 5 min, mixed together, and then incubated at room temperature for another 10 min before applying to cells (500 μl/well in 6-well plates and 50 μl/well for 96-well Seahorse culture plates). Cells were washed with Opti-MEM before transfection, because antibiotic residue from the growth medium can decrease the transfection efficiency (per the manufacturer’s instructions). Transfection was carried out for 2 h, after which the transfection medium was removed, regular serum medium was added, and cells were incubated for 48 h. The following 3 groups were included in each experiment: 1) control cells treated with only Opti-MEM during the transfection (control group), 2) transfection control cells treated only with 1:100 Lipofectamine in Opti-MEM, and 3) MEIS1 siRNA–transfected cells. Lipofectamine alone had no effect on metabolic activity or gene expression compared with controls, so Lipofectamine-treated group data are therefore not presented.
Seahorse metabolic assay
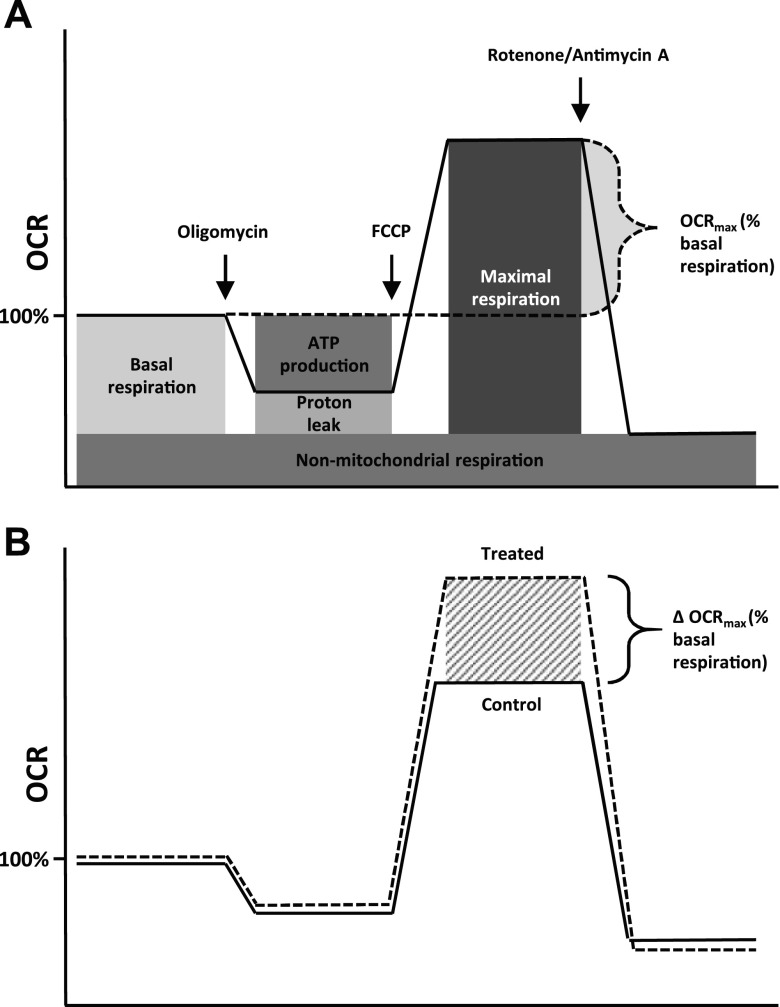
Basal and maximal oxygen consumption rates (OCRmax) in cultured cardiomyocytes were measured with the XFe96 Seahorse Extracellular Flux Analyzer (Agilent Technologies). One hour before the assay, the cells were washed twice with and then left to equilibrate for 1 h in nonbuffered base medium (Agilent Technologies) in a CO2-free incubator at 37 and 39°C for neonatal and fetal cells, respectively. The base medium was supplemented with 2 mM glutamine, 200 μM sodium pyruvate, 2% FBS, 1 or 5 mM glucose (fetal vs. neonatal cells, respectively), and 2 or 1 mM l-lactate (fetal vs. neonatal cells, respectively). The FBS was added to supplement the cells with low levels of fatty acid to promote the cells’ ability to display their true metabolic maximum. Drugs were made up in the same media used for the assay. At the same temperature as the previous incubation culture, 3-min measurements were taken 3 times at the baseline and after serial injections of oligomycin (1 μM ATP synthase inhibitor), carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (1 μM ATP uncoupling agent), and rotenone–antimycin A (1 μM complex I-cytochrome c reductase inhibitors) with 3 min mixing after each injection. This combination of sequential drug injections is referred to as the “mitochondrial stress test,” and a visual guide on how to interpret the resulting curves can be found in Fig. 1.
Figure 1.
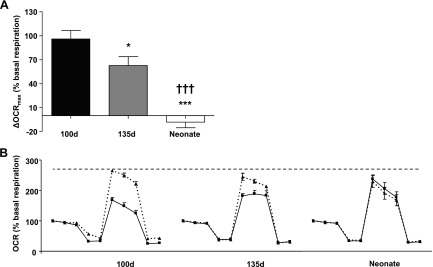
Seahorse mitochondrial stress test. Schematic overview of Seahorse metabolic flux assay using the mitochondrial stress test. The test assesses mitochondrial respiration in cultured cells by measuring the OCR. A) Basal respiration, ATP production, proton leak, and maximal respiration can be measured by adding successive doses of oligomycin (ATP synthase inhibitor), carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP; ionophore; ATP production inhibitor), and antimycin A plus rotenone (electron transport chain inhibitors). B) When basal respiration level is set to 100%, the difference between maximal respiration level and basal respiration level is spare respiratory capacity and referred to as OCRmax (%basal respiration). When cells are treated with agents that increase OCRmax, the magnitude of the increase in OCR between treatment and control is expressed as the ΔOCRmax.
By normalizing the Seahorse data to the basal respiration level and subtracting the maximal respiration level, the spare respiratory capacity was obtained [OCRmax (%basal respiration)] (Fig. 1A). When siRNA-transfected cells showed an increase in OCRmax, the magnitude of the increase in oxygen consumption rate (OCR) between treatment and control was expressed as the difference in OCRmax [ΔOCRmax (%basal respiration)] (Fig. 1B).
RNA isolation and gene expression
RNA was isolated from cultured cells by adding Trizol (Thermo Fisher Scientific) directly into the well after removing the medium. For RNA isolation from LV tissue, 30–50 μg of tissue was homogenized in Trizol with a steel bead in a TissueLyser LT (Qiagen, Germantown, MD, USA). All samples were further processed through RNeasy Mini Columns (Qiagen) to ensure pure RNA. Samples prepared from whole LV tissue was passed through additional RNeasy MinElute Columns (Qiagen) to exclude small RNAs, because the adult and neonatal tissue was shown to contain much higher amounts of small RNAs than fetal samples, which eventually interfered with cDNA synthesis (unpublished results).
cDNA was synthesized from 1 μg of total RNA template with the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific) and all samples were diluted 1:20 before being used for PCR. Real-time qPCR was performed using ABI Power SYBR Green (Thermo Fisher Scientific) in the Stratagene Mx3005P PCR instrument (Thermo Fisher Scientific). We used the ΔCt method (22) to analyze the qPCR results and used ribosomal protein L37a as housekeeper, because it was shown not to change significantly with animal age or cell-culture treatments. Primer sequences can be found in Table 1.
TABLE 1.
Primer sequences used for real-time qPCR
| Primer sequence, 5′–3′ |
||
|---|---|---|
| Gene ID | Forward | Reverse |
| MEIS1 | ATATCATGAGGGCGTGGCTG | ATAGGTCCTGGTGCCCTGAT |
| HIF-1α | AGCCGCTTAGAAGTAGTGCC | GGGACTGTTAGGCTCAGGTG |
| ALDO | CCTCTCCTGTGCCAGGAACT | TGGTAGAGCGTCTCGTGGAA |
| ENO | TAAAACTATTGCGCCTGCCC | CTTGTTGCCAGCATGAGAGC |
| LDHB | TTTCAGGGCACAATGGCAAC | CCTGCAGTTACCACCACGAT |
| CD36 | CTGGTGGAAATGGTCTTGCT | ATGTGCTGCTGCTTATGGGT |
Western blot
Cardiac tissue was lysed in RIPA buffer (MilliporeSigma) supplemented with a Complete Mini Protease 227 Inhibitor tablet (Roche, Basel, Switzerland) and phosphatase inhibitor cocktails I and II (MilliporeSigma). Frozen tissue pieces of 20–30 μg were coarsely crushed in a mortar chilled with liquid nitrogen and were put in prechilled RIPA in 2-ml tubes containing a stainless steel bead. Tissue samples were then lysed by 4-min shaking at 50 Hz in a TissueLyser LT. Total protein content of each sample was quantified by a bicinchoninic acid assay (Thermo Fisher Scientific) and diluted to the same concentration across all groups (0.5 μg/μl). A total of 5 μg per sample was loaded on 10% EZ-Run Gels (Thermo Fisher Scientific) and separated by SDS-PAGE. The protein was transferred to nitrocellulose membranes (Optitran BA-S 83; MilliporeSigma) and blocked in 5% milk in 1× Tris-buffered saline plus 0.01% Tween 20 (TBS-T) for 1 h at room temperature. After washing, membranes were incubated for 1 h at room temperature with a rabbit monoclonal anti-MEIS1 antibody (EPR5781; Abcam, Cambridge, MA, USA) at a 1:4000 dilution in TBS-T buffer plus 4% bovine serum albumin and 0.1% sodium azide, followed by wash and incubation with horseradish peroxidase–linked secondary antibody (goat anti-rabbit; Cell Signaling Technology, Danvers, MA, USA) at a 1:10,000 dilution in TBS-T plus 5% milk. Membranes were visualized using SuperSignal chemiluminescence substrate (Thermo Fisher Scientific) and developed on film in a darkroom for 20 s. Blots were scanned and analyzed using the gel analysis tool in ImageJ (1.49 g; National Institutes of Health, Bethesda, MD, USA). Individual band intensities were expressed as area under the curve and as a percentage of total intensity of all bands. Protein bands were normalized against total protein stained with amido black (MilliporeSigma) as the loading control.
In MEIS1 knockdown experiments, protein for Western blots was isolated from cultured cells 48 h after transfection. Medium was aspirated and cardiomyocytes were rinsed in ice-cold 1× PBS, lysed in supplemented RIPA buffer, and collected into prechilled tubes. Five micrograms per sample was separated by SDS-PAGE and probed for MEIS1 as previously described, stripped with Restore Western Blot Stripping Buffer (Thermo Fisher Scientific), and probed again with α-tubulin (α-Tub; 2144; Cell Signaling Technology). Blots were developed and analyzed, and MEIS1 was normalized to α-Tub as the loading control in corresponding samples.
Mitochondrial staining, immunofluorescence, and ATP assay
Mitochondrial morphology and activity were assessed by staining with fluorescent MitoTracker Orange (MitoO) CMTMRos (Thermo Fisher Scientific). MitoO is dependent on mitochondrial membrane potential and is thus not only a general mitochondrial stain but an indicator of mitochondrial activity. Cardiomyocytes from 135-d fetuses were cultured in 8-well cell culture–treated imaging chambers (Ibidi, Martinsried, Germany) at a density of 5 × 103 cells per well (control or transfected with siRNA against MEIS1, see previous section for transfection details). Following culture and transfection, growth medium was replaced with 500 nM MitoO in serum-free low-glucose DMEM for 30 min at 39°C. MitoO medium was aspirated and replaced with 4% paraformaldehyde in serum-free DMEM, incubated for 30 min at 39°C, and washed 3 times for 5 min each with HBSS at room temperature.
Following fixation, cells were blocked in 1% BSA in HBSS containing 0.5% saponin and 200 mM glycine for 1 h at room temperature. After washing, cells were incubated with primary antibodies against α-Tub [2125S anti-rabbit (1:200 dilution); Cell Signaling Technology] and cardiac troponin T [MS-295-P1 (1:50 dilution); Thermo Fisher Scientific]. Cells were finally incubated with 10 mg/ml Hoescht 33258 (Thermo Fisher Scientific) in HBSS for 20 min at room temperature to stain nuclei. Hoescht was aspirated and replaced with fresh HBSS prior to imaging. Z stacks were acquired using a Zeiss LSM 880 confocal microscope with Airyscan (Carl Zeiss, Oberkochen, Germany). ImageJ was used for image processing, and images were obtained by maximum intensity projection of the confocal z stacks. Background reduction (rolling ball radius of 110 pixels and noise reduction by despeckling) and brightness and contrast adjustments were performed equally across all images.
ATP levels were measured in cultured control and MEIS1 siRNA–transfected cardiomyocytes from 135-d hearts using the CellTiter-Glo assay (Promega, Madison, WI, USA). Cells were cultured in 96-well plates with 4 replicates per animal (n = 6) under the same culture and transfection conditions as described above. At the time of the assay, cells were treated according to the manufacturer’s protocol. Biological samples, together with a dilution series from a commercial ATP standard (Promega), were transferred onto a black, clear-bottomed 96-well plate. Luminescence from standards and samples were read on the same plate in a plate reader after a 10-min incubation period.
Statistical analysis
All data are presented as means ± sem if not stated otherwise. A 1-way ANOVA followed by a Newman-Keuls post hoc test, when justified, was used for all analyses except for qPCR data from siRNA-transfected cells and ATP data, where a Student’s t test was used to compare the control with transfected cells within the same age. Statistical significance was set at P < 0.05.
RESULTS
MEIS1 and hypoxia-inducible factor 1α expression decreases with age
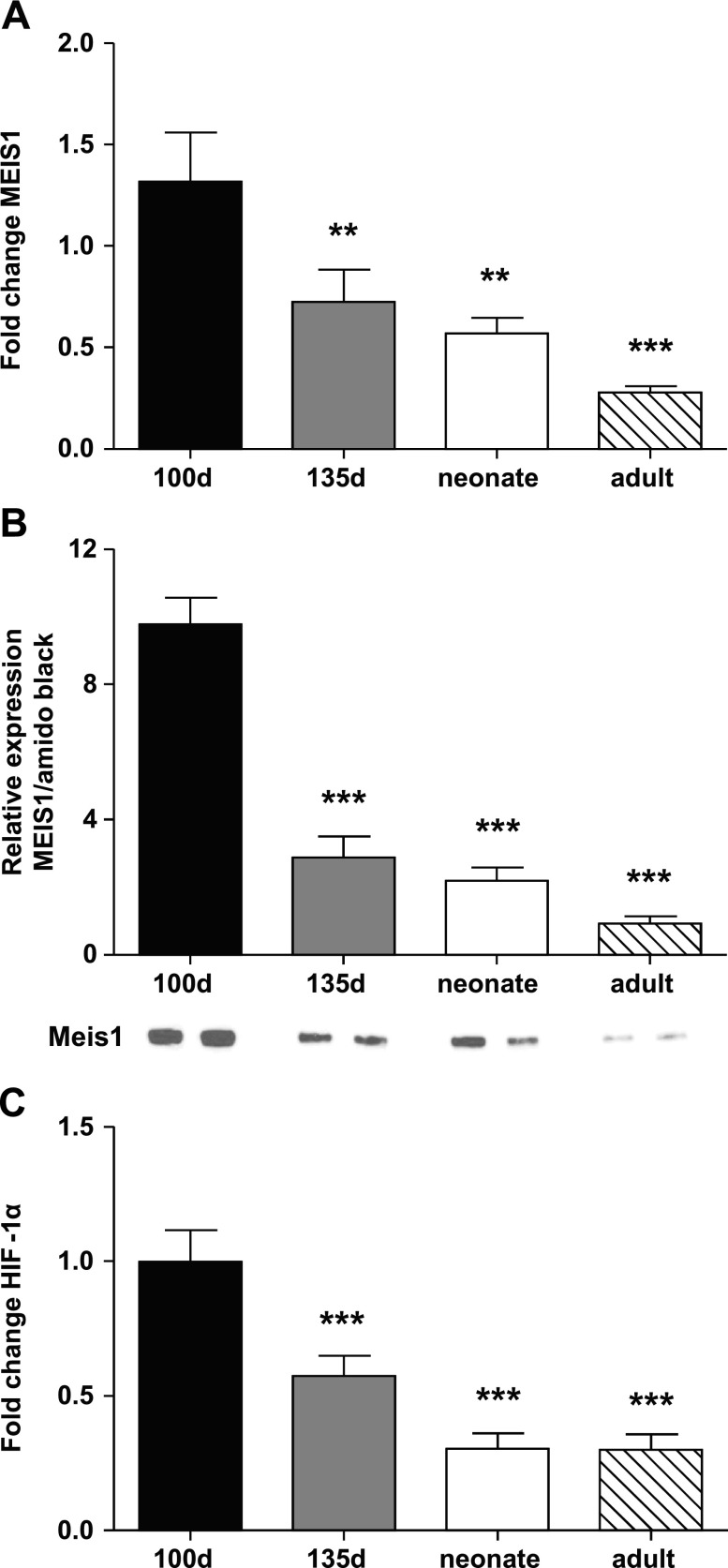
The level of expression of MEIS1 and hypoxia-inducible factor 1α (HIF-1α) in whole LV tissue from sheep was highest in the youngest fetuses (100 d) (Fig. 2A, C). Expression declined with age and was lowest in the adult heart. The same trend was seen for MEIS1 protein expression, where the expression was highest in the 100-d fetus compared with the other ages [9.78 ± 0.77 vs. 0.92 ± 0.21 (100-d fetus vs. adult, respectively); Fig. 2B].
Figure 2.
Ontogeny of MEIS1 and HIF-1α expression. A) Real-time qPCR gene expression data for MEIS1. B) MEIS1 protein expression data from Western blot. Representative blots from 2 animals can be found under each respective bar. C) Real-time qPCR gene expression data for HIF-1α. Data are from whole LV tissue from 100-d fetal (black), 135-d fetal (gray), 24-h to 5-d neonatal (white), and adult (diagonal stripes) sheep. Data presented as means ± se; n = 7–8. **P < 0.01, ***P < 0.001 compared with 100 d.
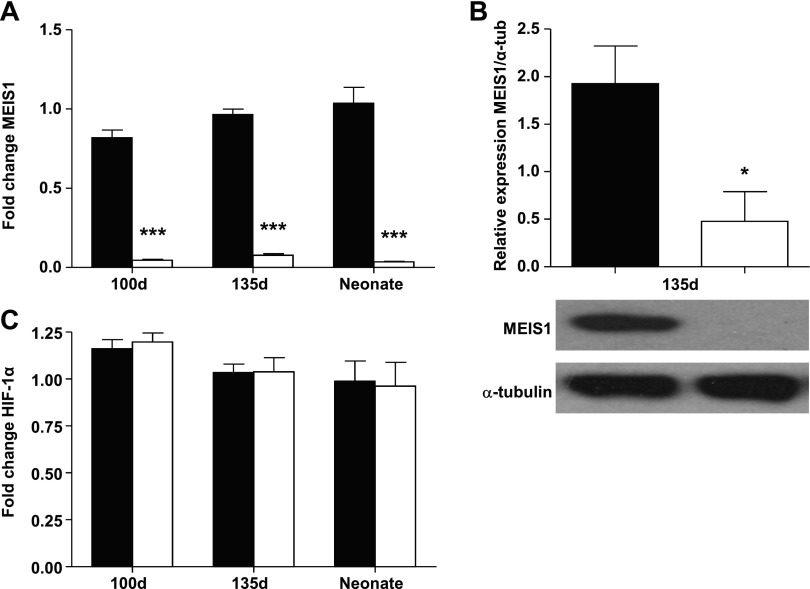
Primary cultures of fetal and neonatal cardiomyocytes transfected with siRNA against MEIS1 displayed an ∼90% decrease in MEIS1 expression, as verified by both qPCR and Western blot (Fig. 3A, B). Previous studies of MEIS1 in stem cells have suggested that MEIS1 exerts its metabolic effects through transcriptional regulation of HIF-1α (16, 23). We therefore determined the expression of HIF-1α in cultures where MEIS1 expression had been suppressed by siRNA. However, no effect was found on cardiomyocyte HIF-1α expression when MEIS1 was suppressed (Fig. 3C).
Figure 3.
Suppressing MEIS1 expression with siRNA. Real-time qPCR gene expression (A) and protein (B) of MEIS1 and HIF-1α (C) in cultured cardiomyocytes from 100-d fetal, 135-d fetal, and 24-h neonatal sheep (protein-only, 135-d). Black bars represent control cells, and white bars represent cells transfected with siRNA against MEIS1. Data presented as means ± se; n = 5. *P < 0.05, ***P < 0.001 vs. same-age control.
Suppressed MEIS1 expression leads to increased oxygen consumption in cardiomyocytes
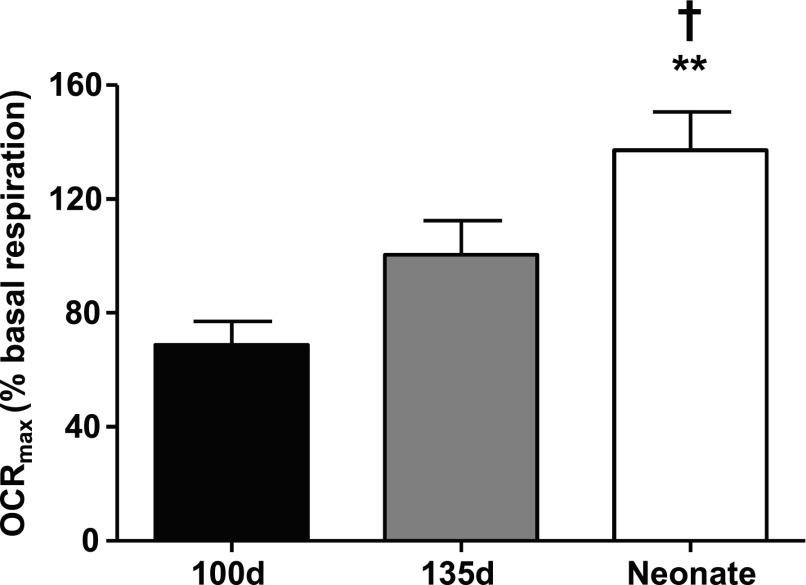
The OCRmax of cultured, untreated cardiomyocytes after normalization to basal respiration level [OCRmax (% basal respiration)] increased incrementally with age and was significantly higher in neonates compared with fetuses (Fig. 4). In cell cultures where MEIS1 expression was suppressed by siRNA, OCRmax (%basal respiration) increased in fetal, but not neonatal, cardiomyocytes (Fig. 5A). The effect of the transfection was the largest in the 100-d fetal cells (95.95 ± 10.58%) and significantly lower in both the late fetus (62.66 ± 11.16) and the neonate (−8.09 ± 7.04). This is further illustrated in Fig. 5B, where the full mitochondrial stress test curves are compared between the different ages. When analyzing baseline oxygen consumption before baseline normalization, MEIS1 knockdown caused an ∼7% increase in 100 d (84.97 ± 2.61 vs. 91.38 ± 1.87 pmol O2/min control vs. MEIS1 knockdown, respectively; P < 0.05), ∼10% in 135 d (86.32 ± 6.32 vs. 96.18 ± 6.03 pmol O2/min control vs. MEIS1 knockdown, respectively; P = 0.09), and no change in neonatal cardiomyocytes. Fetal cardiomyocytes reached the same level of OCRmax as untreated neonatal cardiomyocytes when treated with MEIS1 siRNA (Fig. 5B). This demonstrates a suppressive action of MEIS1 on mitochondrial respiration in the younger cells and the normal loss of that suppression with age.
Figure 4.
OCRmax. Seahorse oxygen consumption data from 100-d fetal (black), 135-d fetal (gray), and 24-h neonatal (white) sheep cardiomyocytes. By setting the basal respiration level to 100% and subtracting the maximal respiration level, the spare respiratory capacity was obtained [OCRmax (%basal respiration)]. Data are shown as means ± sem; n = 5–6. **P < 0.01 vs. 100 d, †P < 0.05 vs. 135 d.
Figure 5.
Difference in OCRmax between control sheep cardiomyocytes and cardiomyocytes with suppressed MEIS1 expression. A) Seahorse oxygen consumption data in control cells and cells transfected with siRNA against MEIS1 from 100-d fetal (black), 135-d fetal (gray), and 24-h neonatal (white) sheep cardiomyocytes. By setting the basal respiration level to 100% and subtracting the maximal respiration level, the spare respiratory capacity was obtained [OCRmax (%basal respiration)]. The ΔOCRmax (%basal respiration) between control and siRNA-transfected cells is shown. B) The individual mitochondrial stress test curves for control (solid line) vs. siRNA-transfected cells (dashed line) for each age studied. Data presented as means ± se; n = 5. *P < 0.05 vs. 100 d, ***P < 0.001 vs. 100 d, †††P < 0.001 vs. 135 d.
Mitochondrial activity increases with MEIS1 suppression
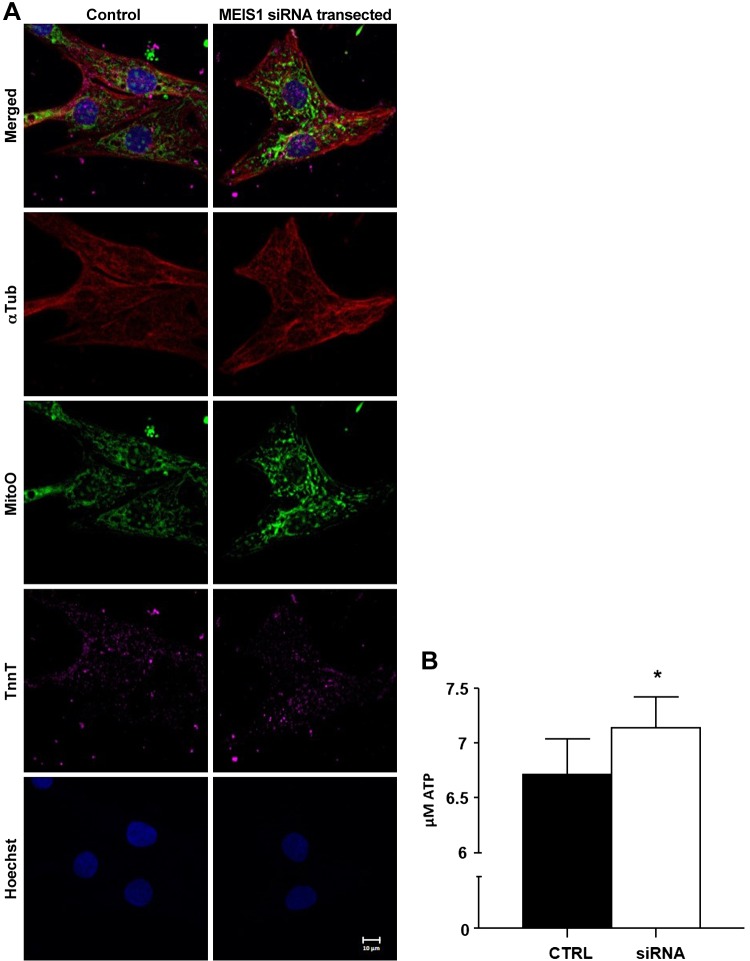
MitoO was used to stain mitochondria in control and MEIS1-suppressed cardiomyocytes. This stain was chosen because the intensity of the fluorescence is proportional to mitochondrial activity (i.e., brighter staining indicates higher mitochondrial activity). Mitochondria in 135-d cardiomyocytes were visually brighter when MEIS1 was suppressed compared with control cells (Fig. 6A). Additionally, baseline ATP levels increased ∼6% (6.72 ± 0.32 vs. 7.14 ± 0.29 μmol ATP, control vs. MEIS1 knockdown, respectively; P < 0.05) in 135-d cardiomyocytes (Fig. 6B).
Figure 6.
Mitochondrial activity. A) Cultured 135-d fetal sheep cardiomyocytes (control and MEIS1 siRNA–transfected) were exposed to MitoO before they were fixed and stained with antibodies against α-Tub and cardiac troponin T (TnnT) and nuclei were stained with Hoechst 33258. MitoO accumulation is dependent upon membrane potential and is therefore an indicator of mitochondrial activity. All images were obtained by a maximum intensity projection of confocal z stacks. Scale bar, 10 μm. B) The same culture conditions were used on 96-well plates for luminescent ATP assessment. Data presented as means ± se; n = 5. *P < 0.05.
Metabolic gene expression changes with MEIS1 suppression to favor mitochondrial respiration
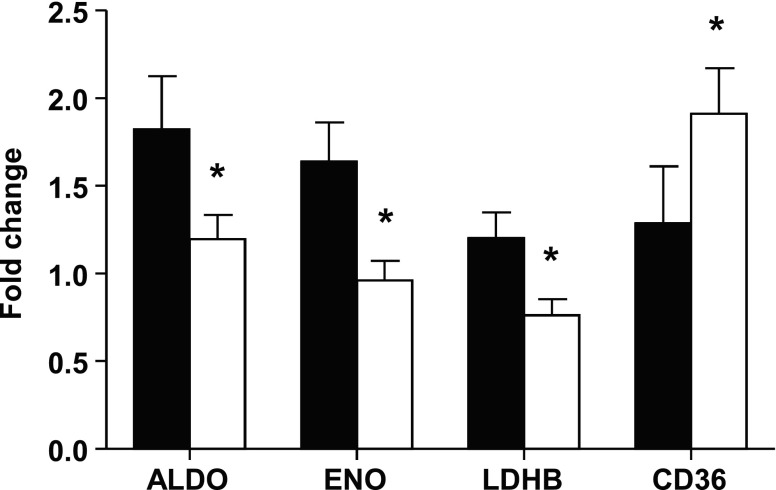
Expression of a few selected key metabolic genes was studied in control cardiomyocytes following MEIS1 suppression and their unsuppressed controls. The glycolytic genes aldolase (ALDO) and enolase (ENO) and the postglycolytic gene lactate dehydrogenase B (LDHB) that catalyzes the interconversion of pyruvate and lactate were all down-regulated in cells that had a lower MEIS1 expression (Fig. 7). The expression of the fatty acid translocase (CD36), however, was up-regulated in MEIS1 knockdown cells (Fig. 7).
Figure 7.
Metabolic gene expression after MEIS1 siRNA transfection. Real-time qPCR gene expression data of selected metabolic genes ALDO, ENO, LDHB, and CD36 in cultured cardiomyocytes from 100-d fetal sheep. Black bars represent control cells, and white bars represent cells transfected with siRNA against MEIS1. Data presented as means ± se; n = 5. *P < 0.05 vs. control.
DISCUSSION
We tested the hypothesis that MEIS1 is a key regulator of the metabolic transition at birth. We showed that cardiac MEIS1 expression is high during fetal life and that it gradually decreases as term approaches and further into adulthood, when it is hardly detectable (Fig. 2A, B). Suppressing MEIS1 with siRNA caused a significant increase in mitochondrial respiration in fetal cells, but not in postnatal cells where expression is already low. Finally, we showed that suppression of MEIS1 leads to a down-regulation of important glycolytic genes and increased mitochondrial activity. We conclude that MEIS1 is a powerful suppressant of oxidative phosphorylation in the heart that is developmentally down-regulated during fetal development. We speculate that MEIS1 plays an important role in the transition of the fetal myocardial phenotype to its more mature postnatal form.
Normal fetal development is a series of timed, regulated processes and events. Developmental gene expression patterns regulate the complex hormonal environment as it changes throughout fetal life. Hormones like angiotensin II, cortisol, and thyroid hormone have powerful effects on heart growth and maturation. Angiotensin II, for example, decreases with increasing age, whereas hormones like cortisol and thyroid hormones increase drastically at the end of gestation (19). These changes in the hormonal milieu direct cardiomyocyte maturation, including proliferation and differentiation. We therefore studied hearts and cardiomyocytes from the following 2 well-studied stages in terms of cardiovascular maturity: 100 d, when cardiomyocytes are immature and proliferative, and again at 135 d, when the heart is much more mature with cardiomyocytes that are less proliferative and almost ready for birth.
Cell proliferation and metabolic strategy are closely linked. Glycolytic metabolism is evidently a required condition in cells that are proliferating (24, 25). This is consistent with the associations of MEIS1 expression in the developing, proliferative heart. As proliferating cells differentiate, there is a concurrent shift toward oxidative phosphorylation (5, 26). Conversely, if oxidative metabolism is induced in proliferating cells, it promotes differentiation (5, 27). The cardiomyocytes in the 100-d sheep heart are virtually all proliferating (28). At gestational d 135 (term at 145 d), ∼70% of sheep cardiomyocytes are fully differentiated, as are nearly 100% of cardiomyocytes in the neonate (28, 29). Because differentiation is linked to an increased oxidative metabolic activity, one would expect to see an increase in oxygen consumption, of cardiomyocytes with increasing developmental age. Indeed, oxygen consumption did increase postnatally (Fig. 4), which is consistent with the increase in differentiation and maturation of the working myocytes. Furthermore, Fisher et al. (30) showed that the myocardial oxygen consumption of the resting near-term fetal sheep heart in vivo is about 70% of the neonatal heart, which corresponds to our results when comparing 135-d fetal with neonatal cardiomyocytes (Fig. 4).
In contrast with our findings, Mahmoud et al. (31) found that MEIS1 increases with postnatal age in the mouse heart and suppresses cardiomyocyte proliferation in the perinatal period. Murine cardiac development differs significantly compared with that of large mammals; the newborn mouse heart is immature, and the cardiomyocytes possess the ability to proliferate for more than a week after birth (20). In contrast, cardiomyocytes from large precocial mammals, such as the human and the sheep, mature and proliferate very little after birth; the heart grows mainly through cell hypertrophy during postnatal life (28, 32). Because the normal suppression of cellular proliferation happens mostly before birth in the sheep but after birth in the mouse, the opposing expression patterns of MEIS1 in the sheep vs. the mouse make it unlikely that MEIS1 has the same suppressing effect on proliferation in the sheep, and probably the human, as observed in the mouse heart. It is also possible that the timing of MEIS1 expression is different in the 2 species, resulting in different functions around birth. This study did not address the role of MEIS1 as a regulator of cardiomyocyte growth, and thus it cannot be compared directly with studies in the mouse cardiomyocytes.
The phosphate and oxygen ratio in isolated ovine fetal and newborn mitochondria has been shown to be equal under the same oxygen conditions. Thus, the isolated mitochondria from fetal and neonatal hearts are equally efficient at producing ATP (33). In other words, the mitochondrial activity may be lower in the immature cardiomyocyte, although its potential capacity is equivalent to the adult. We found that siRNA suppression of MEIS1 had less effect on OCRmax as the cells became more mature. These findings suggest that the high levels of MEIS1 found in the youngest hearts are suppressing oxygen consumption in cardiomyocytes. Lowering the levels of MEIS1 allowed oxygen consumption to increase to the levels found in mature cardiomyocytes where MEIS1 is normally low (Fig. 5B). These experiments suggest that, under these experimental conditions, there is an upper limit of oxygen consumption that the cardiomyocytes can reach and that even immature fetal mitochondria can be impelled to reach neonatal levels of oxygen consumption by removing the influence of MEIS1.
The low oxygen tension in the fetus, together with the high rate of glycolysis, has been suggested to be a prerequisite for appropriate prenatal development (34, 35). The oxidative activity that does exist in the fetal heart mainly utilizes lactate produced by the placenta (36, 37). In HSCs, the metabolic effects of MEIS1 are transmitted via its positive regulation of HIF-1α expression (23), a well-described master regulator of glycolysis that is sensitive to oxygen. The dominance of glycolysis in the fetal heart appears to be actively maintained by such a transcriptional regulation of metabolic control factors. However, HIF-1α expression was not affected in cells treated with siRNA against MEIS1 (Fig. 3B). We therefore concluded that any effect on metabolism from MEIS1 is not because of its downstream regulation of HIF-1α. However, it is possible that MEIS1 is regulated under the influence of HIF-1α. This possibility, which we have not yet pursued, is supported by the RNA expression pattern of HIF-1α which follows the same pattern as MEIS1 over the different stages of development (Fig. 2A, C).
Cellular proliferation in several types of stem cells is accompanied by an increased glycolytic rate, lactate production, and decreased oxygen consumption, even in the presence of adequate oxygen (aerobic glycolysis, also known as the Warburg effect) (5, 25, 38). Both lactate production and glucose uptake are higher in fetal skeletal muscle than adult muscle under aerobic conditions, suggesting that glycolytic activity is higher during development, even under supraphysiologic oxygen levels (39). Because HIF-1α would be degraded in such an oxygen environment, this speaks for active genetic regulatory mechanisms that maintain proliferating immature cardiomyocytes in a glycolytic state, independent of HIF-1α. Based on our results, we propose that MEIS1 is a highly important transcription factor that regulates a host of genes that maintains the fetal cardiomyocyte in a glycolytic state, all while suppressing β-oxidation. We further suggest that our experiments demonstrate for the first time that the natural reduction in MEIS1 protein in cardiomyocytes with maturation allows the gradual switch to oxidative metabolism, starting before birth. We have shown in previous studies that tri-iodo-l-thyronine is the primary driver of the metabolic maturation of the cardiomyocyte (40). The increase in tri-iodo-l-thyronine concentration in fetal plasma that occurs as the concentration of MEIS1 decreases in the cardiomyocyte fits temporally with the smooth transition of glycolysis to β-oxidation as cardiomyocytes prepare for the metabolic switch to free fatty acids as the primary fuel for ATP synthesis at birth.
In order for a rapid transition from glycolysis to mitochondrial oxidative metabolism to occur within the first minutes of postnatal life, the main metabolic machinery must already be in place, ready to go, by the time of delivery. The machinery includes structured and mature mitochondria with functional electron transport chain complexes. It is possible that the high expression of MEIS1 in the fetal sheep heart not only affects glycolysis by up-regulating glycolytic gene expression (Fig. 7), but actively suppresses oxidative phosphorylation through repressing genes required for mitochondrial respiration and fatty acid β-oxidation. This speculation is based on the evidence that mitochondrial activity (Fig. 6) and expression of CD36 (Fig. 7) is up-regulated in cardiomyocytes following MEIS1 suppression. If MEIS1 is not only a driver of glycolysis but also a suppressor of mitochondrial metabolism, the decreased MEIS1 expression with developmental age would gradually allow the up-regulation of oxidative phosphorylation–regulatory genes and increase oxidative phosphorylation. Such negative regulation of oxidative activity has been previously described; Breckenridge et al. (41) showed that the Heart and Neural Crest Derivatives Expressed 1 (HAND1) transcription factor HAND1 regulates the oxygen consumption of cardiomyocytes by actively repressing lipid-metabolizing genes, leading to decreased mitochondrial activity and subsequent ATP generation. HIF-1α has been ascribed a similar function where, on top of its positive regulation of glycolysis, it actively suppresses mitochondrial function and oxygen consumption by stimulating pyruvate dehydrogenase kinase 1 (42). Furthermore, inactivation of AMPK by PI3K has been shown to promote glycolysis at the expense of oxidative phosphorylation caused by suppression of the tricarboxylic acid cycle (43). MEIS1 has in turn been suggested to be positively regulated by PI3K in HSCs (44) and could potentially be part of this regulatory pathway.
In summary, we found our hypothesis to be supported by the experimental evidence generated in this study. We suggest that MEIS1 is both a driver of glycolysis and a master suppressor of oxidative phosphorylation in the immature myocardium. We encourage further investigation of this gene in immature human cardiomyocytes, because the gene may be important for cardiomyocyte maturation in babies born prematurely and facing high levels of lipids caused by to iatrogenic therapies.
ACKNOWLEDGMENTS
The authors thank Sonnet Jonker and Samantha Louey for helpful comments on the manuscript and Haeri Choi for technical support (all from Oregon Health and Science University). This research was supported by the M. Lowell Edwards Endowment, and the U.S. National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development (Grants 408 P01HD034430 and R21 HD090529 to K.L.T.). I.M.L. was supported by training grants from the Sixten Gemzéus Foundation, the Carl Tryggers Foundation, and the Sweden-America Foundation. The authors declare no conflicts of interest.
Glossary
- α-Tub
α-tubulin
- ALDO
aldolase
- CD36
fatty acid translocase
- ENO
enolase
- FBS
fetal bovine serum
- HIF-1α
hypoxia-inducible factor 1α
- HSC
hematopoietic stem cell
- KB
Kraftbrühe
- LDHB
lactate dehydrogenase B
- LV
left ventricular
- MEIS1
myeloid ecotropic viral integration site 1
- MEM
minimum essential medium
- MitoO
MitoTracker Orange
- OCR
oxygen consumption rate
- OCRmax
maximal oxygen consumption rate
- qPCR
quantitative PCR
- siRNA
short interfering RNA
- TBS-T
Tris-buffered saline plus 0.01% Tween 20
AUTHOR CONTRIBUTIONS
I. M. Lindgren designed the study; I. M. Lindgren, R. R. Drake, and N. N. Chattergoon performed the experiments; I. M. Lindgren analyzed the data; K. L. Thornburg supervised the study; and I. M. Lindgren, R. R. Drake, N. N. Chattergoon, and K. L. Thornburg wrote the manuscript.
REFERENCES
- 1.Dawes G. S., Johnston B. M., Walker D. W. (1980) Relationship of arterial pressure and heart rate in fetal, new-born and adult sheep. J. Physiol. 309, 405–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudolph A. M. (1970) The changes in the circulation after birth. Their importance in congenital heart disease. Circulation 41, 343–359 [DOI] [PubMed] [Google Scholar]
- 3.Klopfenstein H. S., Rudolph A. M. (1978) Postnatal changes in the circulation and responses to volume loading in sheep. Circ. Res. 42, 839–845 [DOI] [PubMed] [Google Scholar]
- 4.Reller M. D., Morton M. J., Reid D. L., Thornburg K. L. (1987) Fetal lamb ventricles respond differently to filling and arterial pressures and to in utero ventilation. Pediatr. Res. 22, 621–626 [DOI] [PubMed] [Google Scholar]
- 5.Lopaschuk G. D., Jaswal J. S. (2010) Energy metabolic phenotype of the cardiomyocyte during development, differentiation, and postnatal maturation. J. Cardiovasc. Pharmacol. 56, 130–140 [DOI] [PubMed] [Google Scholar]
- 6.March of Dimes (2016) 2016 premature birth report card. Accessed August 1, 2018, at: https://www.marchofdimes.org/materials/premature-birth-report-card-united-states.pdf
- 7.Rogers L. K., Velten M. (2011) Maternal inflammation, growth retardation, and preterm birth: insights into adult cardiovascular disease. Life Sci. 89, 417–421 [DOI] [PubMed] [Google Scholar]
- 8.Dunbar S. B., Khavjou O. A., Bakas T., Hunt G., Kirch R. A., Leib A. R., Morrison R. S., Poehler D. C., Roger V. L., Whitsel L. P. (2018) Projected costs of informal caregiving for cardiovascular disease: 2015 to 2035: a policy statement from the American Heart Association. Circulation 137:e558–e577 [DOI] [PubMed] [Google Scholar]
- 9.American Heart Association (2016) Projections of cardiovascular disease prevalence and costs: 2015–2035. Accessed August 1, 2018, at: http://www.heart.org/idc/groups/heart-public/%40wcm/%40adv/documents/downloadable/ucm_491513.pdf
- 10.Gross S. J. (1983) Growth and biochemical response of preterm infants fed human milk or modified infant formula. N. Engl. J. Med. 308, 237–241 [DOI] [PubMed] [Google Scholar]
- 11.Thureen P. J., Hay W. W., Jr (2001) Early aggressive nutrition in preterm infants. Semin. Neonatol. 6, 403–415 [DOI] [PubMed] [Google Scholar]
- 12.Hay W. W., Jr (2008) Strategies for feeding the preterm infant. Neonatology 94, 245–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singhal A., Cole T. J., Fewtrell M., Deanfield J., Lucas A. (2004) Is slower early growth beneficial for long-term cardiovascular health? Circulation 109, 1108–1113 [DOI] [PubMed] [Google Scholar]
- 14.Barker D. J. P., Osmond C., Forsén T. J., Kajantie E., Eriksson J. G. (2005) Trajectories of growth among children who have coronary events as adults. N. Engl. J. Med. 353, 1802–1809 [DOI] [PubMed] [Google Scholar]
- 15.Comline R. S., Silver M. (1972) The composition of foetal and maternal blood during parturition in the ewe. J. Physiol. 222, 233–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kocabas F., Zheng J., Thet S., Copeland N. G., Jenkins N. A., DeBerardinis R. J., Zhang C., Sadek H. A. (2012) Meis1 regulates the metabolic phenotype and oxidant defense of hematopoietic stem cells. Blood 120, 4963–4972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Unnisa Z., Clark J. P., Roychoudhury J., Thomas E., Tessarollo L., Copeland N. G., Jenkins N. A., Grimes H. L., Kumar A. R. (2012) Meis1 preserves hematopoietic stem cells in mice by limiting oxidative stress. Blood 120, 4973–4981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang C. C., Sadek H. A. (2014) Hypoxia and metabolic properties of hematopoietic stem cells. Antioxid. Redox Signal. 20, 1891–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jonker S. S., Louey S. (2016) Endocrine and other physiologic modulators of perinatal cardiomyocyte endowment. J. Endocrinol. 228, R1–R18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soonpaa M. H., Kim K. K., Pajak L., Franklin M., Field L. J. (1996) Cardiomyocyte DNA synthesis and binucleation during murine development. Am. J. Physiol. 271, H2183–H2189 [DOI] [PubMed] [Google Scholar]
- 21.Sundgren N. C., Giraud G. D., Stork P. J., Maylie J. G., Thornburg K. L. (2003) Angiotensin II stimulates hyperplasia but not hypertrophy in immature ovine cardiomyocytes. J. Physiol. 548, 881–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmittgen T. D., Livak K. J. (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3, 1101–1108 [DOI] [PubMed] [Google Scholar]
- 23.Simsek T., Kocabas F., Zheng J., Deberardinis R. J., Mahmoud A. I., Olson E. N., Schneider J. W., Zhang C. C., Sadek H. A. (2010) The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell 7, 380–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perales-Clemente E., Folmes C. D., Terzic A. (2014) Metabolic regulation of redox status in stem cells. Antioxid. Redox Signal. 21, 1648–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Folmes C. D., Dzeja P. P., Nelson T. J., Terzic A. (2012) Metabolic plasticity in stem cell homeostasis and differentiation. Cell Stem Cell 11, 596–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung S., Dzeja P. P., Faustino R. S., Perez-Terzic C., Behfar A., Terzic A. (2007) Mitochondrial oxidative metabolism is required for the cardiac differentiation of stem cells. Nat. Clin. Pract. Cardiovasc. Med. 4 (Suppl 1), S60–S67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mills R. J., Titmarsh D. M., Koenig X., Parker B. L., Ryall J. G., Quaife-Ryan G. A., Voges H. K., Hodson M. P., Ferguson C., Drowley L., Plowright A. T., Needham E. J., Wang Q. D., Gregorevic P., Xin M., Thomas W. G., Parton R. G., Nielsen L. K., Launikonis B. S., James D. E., Elliott D. A., Porrello E. R., Hudson J. E. (2017) Functional screening in human cardiac organoids reveals a metabolic mechanism for cardiomyocyte cell cycle arrest. Proc. Natl. Acad. Sci. USA 114, E8372–E8381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jonker S. S., Zhang L., Louey S., Giraud G. D., Thornburg K. L., Faber J. J. (2007) Myocyte enlargement, differentiation, and proliferation kinetics in the fetal sheep heart. J. Appl. Physiol. 102, 1130–1142 [DOI] [PubMed] [Google Scholar]
- 29.Jonker S. S., Louey S., Giraud G. D., Thornburg K. L., Faber J. J. (2015) Timing of cardiomyocyte growth, maturation, and attrition in perinatal sheep. FASEB J. 29, 4346–4357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fisher D. J., Heymann M. A., Rudolph A. M. (1981) Myocardial consumption of oxygen and carbohydrates in newborn sheep. Pediatr. Res. 15, 843–846 [DOI] [PubMed] [Google Scholar]
- 31.Mahmoud A. I., Kocabas F., Muralidhar S. A., Kimura W., Koura A. S., Thet S., Porrello E. R., Sadek H. A. (2013) Meis1 regulates postnatal cardiomyocyte cell cycle arrest. Nature 497, 249–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burrell J. H., Boyn A. M., Kumarasamy V., Hsieh A., Head S. I., Lumbers E. R. (2003) Growth and maturation of cardiac myocytes in fetal sheep in the second half of gestation. Anat. Rec. A. Discov. Mol. Cell. Evol. Biol. 274, 952–961 [DOI] [PubMed] [Google Scholar]
- 33.Wells R. J., Friedman W. F., Sobel B. E. (1972) Increased oxidative metabolism in the fetal and newborn lamb heart. Am. J. Physiol. 222, 1488–1493 [DOI] [PubMed] [Google Scholar]
- 34.Breckenridge R. A. (2014) Molecular control of cardiac fetal/neonatal remodeling. J. Cardiovasc. Dev. Dis. 1, 29–36 [Google Scholar]
- 35.Patterson A. J., Zhang L. (2010) Hypoxia and fetal heart development. Curr. Mol. Med. 10, 653–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sparks J. W., Hay W. W., Jr., Bonds D., Meschia G., Battaglia F. C. (1982) Simultaneous measurements of lactate turnover rate and umbilical lactate uptake in the fetal lamb. J. Clin. Invest. 70, 179–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fisher D. J., Heymann M. A., Rudolph A. M. (1980) Myocardial oxygen and carbohydrate consumption in fetal lambs in utero and in adult sheep. Am. J. Physiol. 238, H399–H405 [DOI] [PubMed] [Google Scholar]
- 38.Neary M. T., Ng K. E., Ludtmann M. H., Hall A. R., Piotrowska I., Ong S. B., Hausenloy D. J., Mohun T. J., Abramov A. Y., Breckenridge R. A. (2014) Hypoxia signaling controls postnatal changes in cardiac mitochondrial morphology and function. J. Mol. Cell. Cardiol. 74, 340–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beatty C. H., Basinger G. M., Bocek R. M. (1968) Oxygen consumption and glycolysis in fetal, neonatal, and infant muscle of the rhesus monkey. Pediatrics 42, 5–16 [PubMed] [Google Scholar]
- 40.Chattergoon N. N., Giraud G. D., Louey S., Stork P., Fowden A. L., Thornburg K. L. (2012) Thyroid hormone drives fetal cardiomyocyte maturation. FASEB J. 26, 397–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Breckenridge R. A., Piotrowska I., Ng K. E., Ragan T. J., West J. A., Kotecha S., Towers N., Bennett M., Kienesberger P. C., Smolenski R. T., Siddall H. K., Offer J. L., Mocanu M. M., Yelon D. M., Dyck J. R., Griffin J. L., Abramov A. Y., Gould A. P., Mohun T. J. (2013) Hypoxic regulation of hand1 controls the fetal-neonatal switch in cardiac metabolism. PLoS Biol. 11, e1001666; erratum 11(12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Papandreou I., Cairns R. A., Fontana L., Lim A. L., Denko N. C. (2006) HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 3, 187–197 [DOI] [PubMed] [Google Scholar]
- 43.Antico Arciuch V. G., Russo M. A., Kang K. S., Di Cristofano A. (2013) Inhibition of AMPK and Krebs cycle gene expression drives metabolic remodeling of Pten-deficient preneoplastic thyroid cells. Cancer Res. 73, 5459–5472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kirito K., Fox N., Kaushansky K. (2004) Thrombopoietin induces HOXA9 nuclear transport in immature hematopoietic cells: potential mechanism by which the hormone favorably affects hematopoietic stem cells. Mol. Cell. Biol. 24, 6751–6762 [DOI] [PMC free article] [PubMed] [Google Scholar]