Abstract
Systemic lupus erythematosus is an autoimmune disease characterized by overproduction of type 1 IFN that causes multiple organ dysfunctions. Plasmacytoid dendritic cells (pDCs) that secrete large amounts of IFN have recently been implicated in the initiation of the disease in preclinical mouse models. Sphingosine-1-phosphate, a bioactive sphingolipid metabolite, is produced by 2 highly conserved isoenzymes, sphingosine kinase (SphK) 1 and SphK2, and regulates diverse processes important for immune responses and autoimmunity. However, not much is known about the role of SphK2 in autoimmune disorders. In this work, we examined the role of SphK2 in pDC development and activation and in the pristane-induced lupus model in mice that mimics the hallmarks of the human disease. Increases in pDC-specific markers were observed in peripheral blood of SphK2 knockout mice. In agreement, the absence of SphK2 increased the differentiation of FMS-like tyrosine kinase 3 ligand dendritic cells as well as expression of endosomal TLRs, TLR7 and TLR9, that modulate production of IFN. Surprisingly, however, SphK2 deficiency did not affect the initiation or progression of pristane-induced lupus. Moreover, although absence of SphK2 increased pDC frequency in pristane-induced lupus, there were no major changes in their activation status. Additionally, SphK2 expression was unaltered in lupus patients. Taken together, our results suggest that SphK2 may play a role in dendritic cell development. Yet, because its deletion had no effect on the clinical lupus parameters in this preclinical model, inhibitors of SphK2 might not be useful for treatment of this devastating disease.—Mohammed, S., Vineetha, N. S., James, S., Aparna, J. S., Lankadasari, M. B., Allegood, J. C., Li, Q.-Z., Spiegel, S., Harikumar, K. B. Examination of the role of sphingosine kinase 2 in a murine model of systemic lupus erythematosus.
Keywords: S1P, pDCs, pristane, IFN, autoimmunity
Innate immune responses involve the prompt recognition of pathogenic microorganisms that cross the mechanical barriers of infection and enter the body. Plasmacytoid dendritic cells (pDCs) are an important class of sentinel cells that monitor the cellular milieu for any foreign microbes. pDCs are endowed with endosomal TLRs, TLR7 and 9, that recognize the nucleic acids of invading viruses (1). In response to infections, pDCs secrete and are the highest known producers of type I IFNs, thereby having a dominant role in deciding the outcome of an immune response (2, 3). Immune responses are largely beneficial, but if left unchecked, they can lead to a state of autoimmunity. Overproduction of IFNs is involved in the development of autoimmune disorders, such as systemic lupus erythematosus (SLE) (4, 5). pDCs are associated with the early stages of SLE, when they are involved in the initiation of the disease in preclinical models (6, 7). There is still no cure for SLE, and it has been suggested that targeting pDCs represents a potential new therapeutic strategy to deter the disease from its onset (6, 7).
A delicate balance is maintained between an optimal immune response and autoimmunity. This highlights the necessity of robust regulatory mechanisms that maintain the equilibrium. Sphingosine-1-phosphate (S1P) is a pleiotropic bioactive sphingolipid metabolite that regulates diverse physiologic and pathologic processes important for immune responses and autoimmunity (8). S1P, mainly acting through a family of 5 specific cell-surface S1P receptors, has been shown to regulate lymphocyte trafficking (9), endocytosis (10), dendritic cell (DC) migration (11), and cell growth and survival (12), and it has also been linked to the pathophysiology of arthritis (13) and inflammation (14). S1P is formed by phosphorylation of sphingosine by 2 evolutionarily conserved isoenzymes, sphingosine kinase (SphK) 1 and SphK2 (12, 15). Although they catalyze the same reaction and may have some overlapping functions, the tissue expression and cellular localization of the 2 SphKs vary (16). The subcellular localization of SphKs has been shown to be an important determinant of their functions (17). SphK2 is also localized in the nucleus, and S1P generated there is an endogenous inhibitor of histone deacetylase (HDAC) 1 and 2 and regulates gene expression (18). Because HDAC 1 and 2 can regulate the stability of the retinoid-related orphan receptor γ (the master transcriptional regulator of Th17 polarization), it has been suggested that SphK2 inhibitors might be beneficial for attenuating autoimmune and inflammatory disorders (19). Intriguingly, previous studies demonstrated that a SphK2 inhibitor ABC294640 (Yeliva; RedHill Biopharma, Tel Aviv, Israel) has significant anti-inflammatory and antiarthritic effects in preclinical models (20, 21). However, although treatment with ABC294640 decreased glomerular pathology, it did not improve vascular or interstitial pathology associated with lupus nephritis (22). Further interest in the role of SphK2 in lupus stemmed from the promising effects of the S1P receptor 1 (S1PR1) modulator FTY720-fingolimod, a prodrug that is phosphorylated in vivo by SphK2, in improving lupus-associated symptoms in mice (23–25). However, the roles of SphK2 in innate and autoimmune responses are not well understood. Therefore, in this study, we examined the involvement of SphK2 in the initiation and progression of SLE in a murine model and particularly examined its role in regulation of development and functions of pDCs.
MATERIALS AND METHODS
Mice
SphK2+/+ (005304) and SphK2−/− (019140) mice were procured from The Jackson Laboratory (Bar Harbor, ME, USA). Mice were bred and housed in individually ventilated cages with access to standard rodent chow and water ad libitum in a 12-h light/dark cycle. All animal experiments were performed after receiving prior approval from the Institutional Animal Ethics Committee of the Rajiv Gandhi Centre for Biotechnology and followed the rules and regulations prescribed by the Indian Committee for the Purpose of Control and Supervision of Experiments on Animals.
For in vivo experiments, SphK2+/+ and SphK2−/−mice (n = 5/group, age- and gender-matched) were tail-vein injected with the TLR7 ligand resiquimod (R848; ApexBio, Houston, TX, USA) at a dose of 2 µg per mouse. After a period of 2 h, mice were euthanized by CO2 asphyxiation. Blood was collected by cardiac puncture, serum separated, and used for ELISA analyses.
Generation of FMS-like tyrosine kinase 3 ligand pDCs
Femurs were removed from 6- to 8-wk-old SphK2+/+ (n = 5) and SphK2−/− mice (n = 5; no gender preference given, but age- and sex-matched mice from both groups were used for the study), and the bone marrow was flushed out with PBS containing 1% serum. The cells were recollected by centrifugation and washed twice with PBS with 1% serum. Red blood cells (RBCs) were lysed with RBC lysis solution (R7757; MilliporeSigma, Burlington, MA, USA). Cells were counted and plated in Roswell Park Memorial Institute (RPMI) 1640 medium (23400-013; Thermo Fisher Scientific, Waltham, MA, USA) containing 10% serum (10082-147, 1× penicillin-streptomycin, 15140122; Thermo Fisher Scientific), 2 mM l-glutamine (25030081; Thermo Fisher Scientific), 10 mM HEPES (H3375; MilliporeSigma), 5 µM 2-mercaptoethanol (M3148; MilliporeSigma) and 100 ng/ml FMS-like tyrosine kinase 3 ligand (Flt3L) (250-31L; Peprotech, Rocky Hill, NJ, USA). The cells were cultured for 8 d with a medium change at d 5, when half of the spent medium was replaced with fresh complete medium. After 8 d, the cells were collected, washed, and resuspended in 100 µl PBS with 1% serum. A total of 1 × 106 cells were stained with FITC anti-mouse CD11c (117305; BioLegend, San Diego, CA, USA) and allophycocyanin (APC) anti-mouse CD317 [pDC antigen 1 (PDCA-1); 127015; BioLegend] antibodies (0.5 µg each) and incubated at 4°C for 60 min in the dark. CD11c is a pan-DC marker, and PDCA-1 specifically stains pDCs. pDCs were isolated by sorting out the double-positive cells with the BD FacsAria II flow cytometer (BD Biosciences, San Jose, CA, USA). The population was selected using forward scatter (FSC) vs. side scatter (SSC) plots; unstained controls were used for setting the gates. Double-positive cells stained for APC and FITC were collected, washed, counted, and plated in 12-well plates in RPMI 1640 medium containing 2% serum and 1× antibiotic. Flt3L-DCs, after sorting (1 × 105 cells/well), were treated with TLR9 ligand, murine cytomegalovirus (MCMV) (multiplicity of infection = 1; VR-1399, Smith MSGV strain from the American Type Culture Collection, Manassas, VA, USA) in RPMI 1640 medium containing 2% serum for 18 h. The cell culture supernatants were used for ELISA analyses.
Collection of peritoneal lavage
After euthanasia, mice were injected in the peritoneal cavity with 3 ml ice-cold sterile PBS. The peritoneum was gently massaged, and the fluid was then collected into centrifuge tubes. Cell pellets were washed with PBS containing 1% serum; cells were counted and stained for pDCs. Peritoneal lavage from 2 to 3 mice was pooled and used for fluorescence-activated cell sorting (FACS) analyses because the number of peritoneal cells is too low to be analyzed from single mice. Activation of pDCs was assessed by determining expression of the activation markers CD80 and CD86 by staining with PE–anti-mouse CD80 (104708; BioLegend) and PE-Cy7 anti-mouse CD86 (105014; BioLegend). Appropriate isotypic and unstained controls were used to rule out nonspecific staining. The activation status of different groups was compared, and data were shown as quadrant plots.
Isolation of splenocytes
Mice were euthanized by CO2 asphyxiation, and spleens were collected into sterile PBS. The organ was washed thoroughly with PBS and macerated in PBS containing 1% serum. This was followed by centrifugation at 2000 rpm for 3 min. The pellets were washed with PBS containing 1% serum and treated with RBC lysis solution to disrupt the erythrocytes. Cells were washed and resuspended in PBS containing 1% serum and counted for FACS analysis of the splenic pDCs by staining for CD11C and PDCA-1.
Annexin and propidium iodide staining
To examine the cell death profile of peritoneal cells, cells were resuspended in 1X Binding buffer using the annexin V–FITC Apoptosis Detection Kit (B32117a; Biotool, Houston, TX, USA) after counting. Five microliters of annexin V–FITC were added, and cells were incubated in the dark for 15 min at room temperature. This was followed by the addition of 5 µl propidium iodide (PI) staining solution and further incubation for 5 min in the dark. The cells were then immediately analyzed by FACS with the BD FacsAria II flow cytometer. Populations for analysis were selected from FSC and SSC scatter plots and quadrant gating adjusted based on an unstained sample. The different forms of cell death were assigned based on the ability of annexin V–FITC to bind to phosphatidylserine in the outer leaflet of the cell membrane, which is a measure of apoptosis. The ability of PI to penetrate the cell indicates cell death. The absence of an FITC signal together with the presence of a red signal (PI) is a characteristic of necrosis. All populations were determined with BD FacsDiva software.
Measurement of type 1 IFN
The culture medium obtained after treatment of cells with MCMV for 18 h was centrifuged to remove cellular debris. Sandwich ELISA was used to assess the amount of IFN-α (BMS6027; Thermo Fisher Scientific) and IFN-β (439407; BioLegend). Similarly, serum obtained from mice after 2-h treatment with R848 was used for type I IFN ELISA as recommended by the manufacturer. The readings were compared to an established standard graph and results obtained.
Real-time quantitative PCR
RNA was isolated from Flt3L-DCs and mouse blood by using an RNeasy Micro Kit (74004; Qiagen, Germantown, MD, USA) or an RNeasy Protect Animal Blood Kit (73224; Qiagen), following the manufacturer’s instructions. For isolating RNA from whole blood, blood was collected from mice by cardiac puncture into RNAprotect Animal Blood Tubes (76554; Qiagen) and processed after 2 h at room temperature. An additional DNase step was incorporated to remove genomic DNA contamination. Equal concentrations of RNA were converted into cDNA using a High-Capacity cDNA Reverse Transcription Kit (4368814; Thermo Fisher Scientific). Gene expression was measured using Power SYBR Green PCR Master Mix (4367659; Thermo Fisher Scientific) with the respective primers and analyzed by the 2(−∆∆Ct) method after normalizing with the housekeeping gene and internal control. The results are expressed as fold change in expression. The primer sequences used are as follows:
Glyceraldehyde-3-phosphate dehydrogenase (Gapdh) forward (F) 5′-TGCCCCCATGTTTGTGATG-3′, reverse (R) 5′-GTGGTCATGAGCCCTTCC-3′;
SpiB (F) 5′-CCACACTTAAGCTGTTTGTACCC-3′, (R) 5′TGAACAGTTTGGGAGTGGCT-3′; sialic acid–binding Ig-like lectin H (Siglec H) (F) 5′-TGTGCATGTGACAGACCTCA-3′, (R) 5′-GCTGACATCCAGGAAAAGATGG-3′; E2-2 (F) 5′-AAACCGAGCCAGGTGCATAA-3′, (R) 5′-CACAAACCTTCAATGGCCACA-3′; Tlr7 (F) 5′-CTCAGTGGGTGTTTTCGATGTG-3′, (R) 5′-CACATGGGCCTCTGGGATATTT-3′; Tlr9 (F) 5′-ACTTACTGTTGGAGGTGCAGAC-3′, (R) 5′-AAAGGCCAAAGCAGTCCCAA-3′; U1 small nuclear ribonucleoprotein A (U1a) (F) 5′-CGGGGAAGATAGTTGTGTGTCT-3′, (R) 5′-AGGGACTTCTTGAGCTCATCCT-3′; stem cell antigen 1 (Sca-1) (F) 5′-ACTGTGTGCAGAAAGAGCTCAG-3′, (R) 5′-TGCCTCCTGAGTAACACAGACT-3′;
Oas-3 (F) 5′-TGATAAGGATTGCCAA GGGAGG-3′, (R) 5′-TCACCAAAGCTCTGGAAGCA -3′; Mx1 (F) 5′-GCAGACGGAATATTGGGAGA-3′, (R) 5′-GAGCCTCATCCAGCCTTAAA-3′; Ifit1 (F) 5′-CAACCATGGGAGAGAATGCTGA-3′, (R) 5′-TGATGTCAAGGAACTGGACCTG-3′; human β-actin (F) 5′-CCAGCTCACCATGGATGATG-3′, (R) 5′-ATGCCGGAGCCGTTGTC; human SphK2 (F) 5′-CTGTCTGCTCCGAGGACTGC-3′, (R) 5′-CAAAGGGATTGACCAATAGAAGC-3′.
Pristane-induced murine model of lupus
To induce lupus, mice (SphK2+/+ and SphK2−/−) received a single intraperitoneal injection of 0.5 ml pristane (sc-281684; Santa Cruz Biotechnology, Dallas, TX, USA) as described in Satoh et al. (26). Female mice (n = 7/group, 6–8 wk) were selected for the study because females are more susceptible to the disease. Untreated SphK2+/+ and SphK2−/− mice were used as controls. The mice were continuously monitored for signs of lethargy and change in body weight. Blood was collected routinely through tail-vein bleeding. mRNA was isolated from peripheral blood and assessed for the expression of different genes by quantitative PCR. At the end of 2 mo, the mice were euthanized by CO2 asphyxiation and the organs and blood were collected for analyses.
Analysis of renal deposition of IgG
To assess for the deposition of IgG in kidney sections, cryosections were prepared from snap-frozen kidneys. The sections were fixed with ice-cold acetone for 10 min followed by PBS washes. Blocking was done with 3% bovine serum albumin for 20 min at room temperature. After washing with PBS, diluted FITC goat anti-mouse IgG antibody (1:50; 405305; BioLegend) prepared in 3% bovine serum albumin was added to the sections and incubated overnight at 4°C in the dark. The next day, the sections were washed 3 times with PBS. After mounting with glycerol, the sections were analyzed with an A1R laser-scanning confocal microscope (Nikon, Tokyo, Japan) using ×60 oil-immersion objective and NIS-Elements Advanced Research analysis imaging software (Nikon). A minimum of 6 sections were prepared for each experimental group. The fluorescence intensities were quantified for 8 random fields for each group using ImageJ software (National Institutes of Health, Bethesda, MD, USA) and the data were presented graphically.
Measurement of proteinuria
Kidneys are severely affected by lupus, and the presence of protein in the urine (proteinuria) indicates abnormal renal function. Urine samples were collected from mice housed in metabolic cages overnight. The collected urine samples were then spotted directly on Uristix strips (2855B; Siemens, Mumbai, India). The color developed was compared with the color code provided by the manufacturer, which corresponds to the amount of protein present in the urine. The following scoring was used to assess severity of proteinuria: 0–trace, 1–30, 2–100, 3–300, and 4–2000 mg/ml. Three mice from each group were checked for proteinuria.
Autoantigen microarray for assessing autoantibody formation
Lupus is characterized by the presence of autoantibodies directed against self-antigens. Autoantigen array analyses of mouse serum were carried out by the University of Texas Southwestern Medical Center Genomics and Microarray Core Facility (https://microarray.swmed.edu/products/product/autoantigen-microarray-panel-i/) with the Autoantigen Microarray Panel I. The serum samples (n = 2 for the untreated SphK2+/+ and SphK2−/− mice; n = 4 for the pristane-injected groups) were incubated with the array, and the IgG autoantibodies in the samples were measured fluorometrically with a laser wavelength of 532 nm. The results were obtained as tagged image file format images and quantified by Genepix Pro v.6.0 software (Molecular Devices, Sunnyvale, CA, USA) (27). The average net fluorescence intensity (average signal) that corresponds to the signal-to-noise ratio, which is normalized to a PBS-negative control, is used for comparing the samples and has been represented graphically.
Human lupus samples and isolation of peripheral blood mononuclear cells
Peripheral blood was collected from 10 normal healthy volunteers and 22 lupus patients into lithium-heparin–coated tubes (367880; Becton Dickinson, Franklin Lakes, NJ, USA). The samples were kept at 4°C for a maximum of 2 h or were processed immediately. The study was conducted after receiving prior approval of the Institutional Review Board and the Human Ethical Committee (protocol numbers IHEC/01/2017/01 and 04/18/2018/MCT). Peripheral blood mononuclear cells (PBMCs) were isolated from the blood samples by gradient centrifugation by overlaying the blood samples onto Histopaque (10771; MilliporeSigma). The tubes were centrifuged at 400 g for 30 min at room temperature. Buffy coats were collected and RNA isolated using the RNeasy Micro Kit (Qiagen). cDNA was prepared and quantitative PCR measurements of mRNA levels measured as previously described.
Measurement of sphingolipids
Sphingolipids in mouse serum after the pristane-induced lupus were quantified by liquid chromatography–electrospray ionization–tandem mass spectrometry (LC-ESI-MS/MS) at the Virginia Commonwealth University Lipidomics Facility using Qtrap (ABI Sciex, Framingham, MA, USA) as described in Newton et al. (28).
Statistical analyses
Statistical analyses were performed using an unpaired, 2-tailed Student’s t test for comparison of 2 groups or ANOVA with post hoc Tukey’s test analyses for multiple groups (Prism 7; GraphPad Software, La Jolla, CA, USA). A value of P < 0.05 was considered significant.
RESULTS
SphK2 in pDC development and function
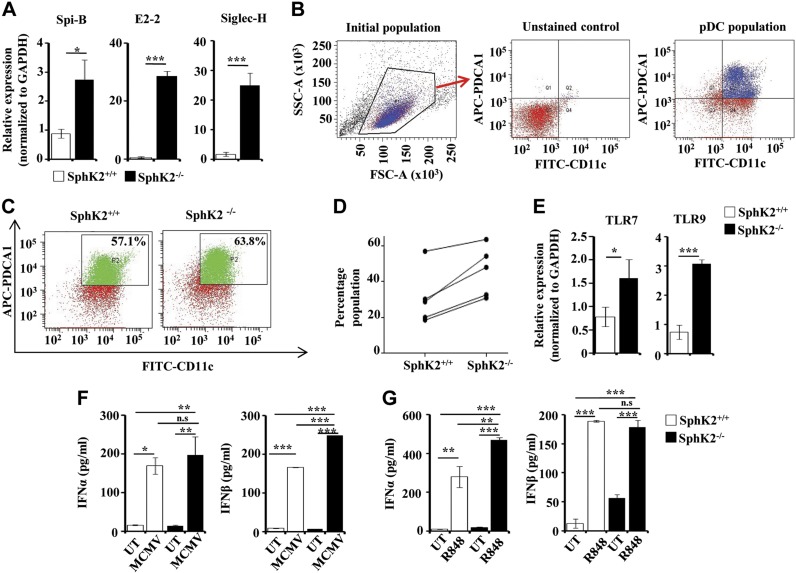
To examine the involvement of SphK2 in the development of pDCs, we first analyzed the expression of the pDC-specific markers E2-2, SpiB, and Siglec H (29–32) in peripheral blood of SphK2+/+ and SphK2−/− mice. These markers were significantly up-regulated in the SphK2-null mice compared with the wild-type mice (Fig. 1A), suggesting that SphK2 may be an endogenous negative regulator of development of pDCs. To examine this further, we determined the percentage of pDCs in Flt3L-DCs, which were generated from hematopoietic cells by differentiation into pDC lineage by incubation with the growth factor Flt3L. Although small, an increased pDC population (double-positive for CD11C and PDCA-1) was consistently observed in the Flt3L-DCs from the SphK2 knockout animals assessed by FACS (Fig. 1B–D), corresponding to a 1.8 ± 0.3–fold increase in numbers of Flt3L-pDCs compared with those obtained from wild-type mice. pDCs are known to be important players in the defense against viruses and express the endosomal TLRs, TLR7 and TLR9 (33, 34). Increased expression of TLR7 and TLR9 was observed in Flt3L-DCs isolated from SphK2−/− mice (Fig. 1E). Type I IFNs (IFN-α and β) are known to be produced by pDCs in response to a viral attack (35, 36). A deficiency of SphK2 did not drastically affect the ability of the pDCs to secrete IFN-α because Flt3L-DCs produced IFN-α levels comparable with the wild-type cells when infected with MCMV, a natural TLR9 ligand. However, SphK2 deficiency significantly increased secretion of IFN-β (Fig. 1F). Moreover, injection of the TLR7 ligand R848 caused a significantly greater increase in secretion of IFN-α in SphK2−/− mice compared with wild-type mice (Fig. 1G). Nevertheless, the IFN-β response to R848 was not affected by the deletion of SphK2 (Fig. 1G). Thus, although the absence of SphK2 increased the differentiation of pDCs and expression of immunoreceptors, type I IFNs were differentially affected.
Figure 1.
Effect of SphK2 on pDC development and function. A) pDC-specific markers SpiB, E2-2, and Siglec H were examined in the peripheral blood of SphK2+/+ and SphK2−/− mice by quantitative PCR. The fold change was calculated after normalizing with GAPDH. Data are expressed as means ± sd of 3 independent experiments (n = 7 mice/group). B–D) FACS analysis of pDCs. Flt3L-DCs from SphK2+/+ and SphK2−/− mice were analyzed by FACS by staining for both CD11c and PDCA-1. B) The gating strategy of FACS is shown. The cells were first gated using FSC and SSC plots. Unstained samples were used to determine the gating for both FITC and APC. See Materials and Methods section for more details. C) The dot plots are representative of 5 different experiments showing Flt3L-pDCs generated from SphK2+/+ and SphK2−/− mice. D) Percent populations of Flt3L-pDCs positive for both CD11c and PDCA-1, sorted out by FACS (D). Data from 5 independent experiments are shown. E) Expression of TLR7 and TLR9 in Flt3L-DCs generated from SphK2+/+ and SphK2−/− mice was determined by quantitative PCR and expressed as fold change after normalizing with GAPDH. Similar results were obtained in 3 independent experiments (n = 5 mice/group). F) Flt3L-DCs (1 × 105 cells) isolated by cell sorting from SphK2+/+ to SphK2−/− mice were treated without or with MCMV (multiplicity of infection = 1). After 18 h, cell supernatants were collected and analyzed by ELISA for IFN-α and β. Similar results were obtained in 3 independent experiments (n = 5 mice/group). G) SphK2+/+ and SphK2−/− mice were tail-vein injected with 2 µg of R848 per mouse, and blood was collected after 2 h. Serum was separated and analyzed by ELISA for type I IFN secretion. Statistical significance was assessed by a Student's unpaired t test (A, E) or by ANOVA (F, G) followed by a Tukey’s post hoc test; n.s., not significant. *P < 0.05, **P < 0.005, ***P < 0.001.
SphK2 deficiency does not decrease lupus disease markers
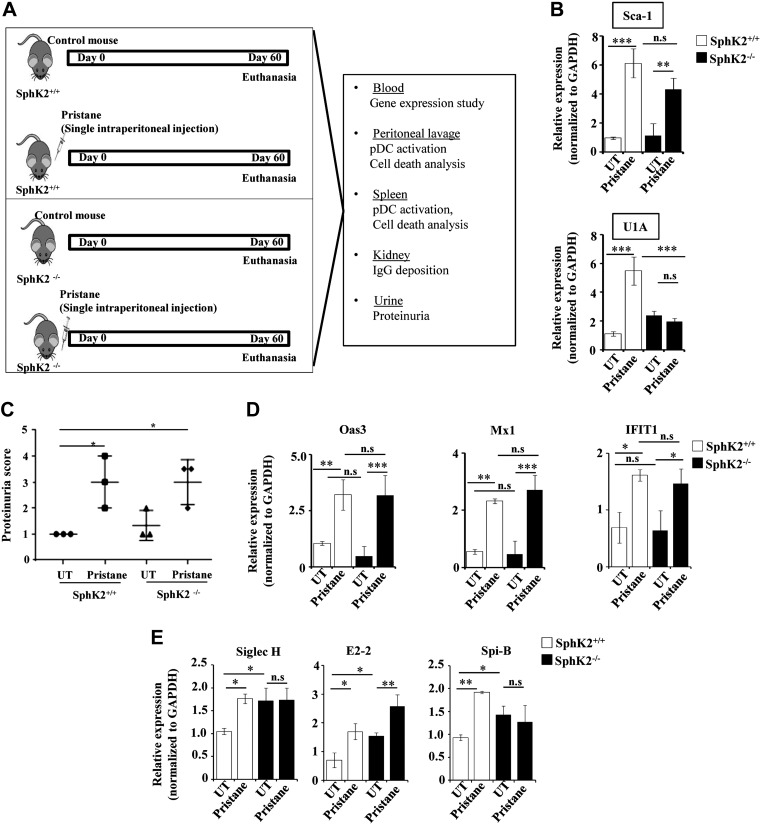
Next, we extended our finding to pristane-induced lupus, an excellent in vivo lupus model that recapitulates the symptoms of SLE (37). The schematic depicting the model and the endpoint analyses is shown in Fig. 2A. Because pDCs are considered important for the initiation of the disease in animal models (6, 7), mice were euthanized 2 mo after pristane treatment, when the disease just starts to be manifested. Sca-1 and U1A are considered to be disease markers expressed during the onset of the disease (38). However, expression of Sca-1 in peripheral blood was similar in both SphK2+/+ and SphK2−/− mice that had been treated with pristane (Fig. 2B). In contrast, U1A was reduced in the pristane-treated SphK2−/− mice compared with the wild-type mice (Fig. 2B).
Figure 2.
Lupus disease markers are similar in pristane-treated SphK2+/+ and SphK2−/− mice. A) Schematic representation of the pristane-induced lupus model, depicting the experimental conditions and the endpoint analyses. B) Expression of disease markers Sca-1 and U1A was determined in peripheral blood of mice by quantitative PCR and expressed as fold change after normalizing with GAPDH (n = 7/group). C) Renal function was evaluated by measurement of urinary protein levels using Uristix colorimetric strips. Proteinuria scores were based on color development that signifies the amount of protein in urine (n = 3/group). D) Expression of IFN-stimulated genes 2'-5′-oligoadenylate synthase 3 (Oas3), myxovirus resistance 1 (Mx1), and IFN-induced protein with tetratricopeptide repeats (IFIT1) in peripheral blood from SphK2+/+ to SphK2−/− mice (n = 7/group) was determined by quantitative PCR. Data were expressed as relative expressions normalized to GAPDH. E) Expression of the pDC markers SpiB, E2-2, and Siglec H in peripheral blood was determined by quantitative PCR and expressed as relative levels after normalizing to GAPDH. Data are expressed as means ± sd from 3 independent experiments. Statistical significance was determined by ANOVA with a post hoc Tukey’s test; n.s., not significant. *P < 0.05, **P < 0.005, ***P < 0.001.
Kidney functions are well known to be strongly affected by SLE and can be assessed by measurements of proteinuria. We observed increased protein content in the urine samples from both wild-type and SphK2−/− pristane-injected mice (Fig. 2C). Because a heightened IFN signature is associated with the severity of lupus, we also analyzed the expression of IFN-stimulated genes (2'-5′-oligoadenylate synthase 3, myxovirus resistance 1, and IFN-induced protein with tetratricopeptide repeats) in the peripheral blood of these mice (Fig. 2D). The IFN signature was comparable between pristane-treated wild-type and SphK2 knockout mice, indicating that increased expression of these disease markers is independent of SphK2 expression. Furthermore, we evaluated the expression pattern of pDC functional markers in blood, Siglec H, E2-2, and SpiB, which were increased after pristane administration and not decreased in knockout of SphK2 (Fig. 2E).
Absence of SphK2 increases pDC frequency in lupus but does not affect their activation status
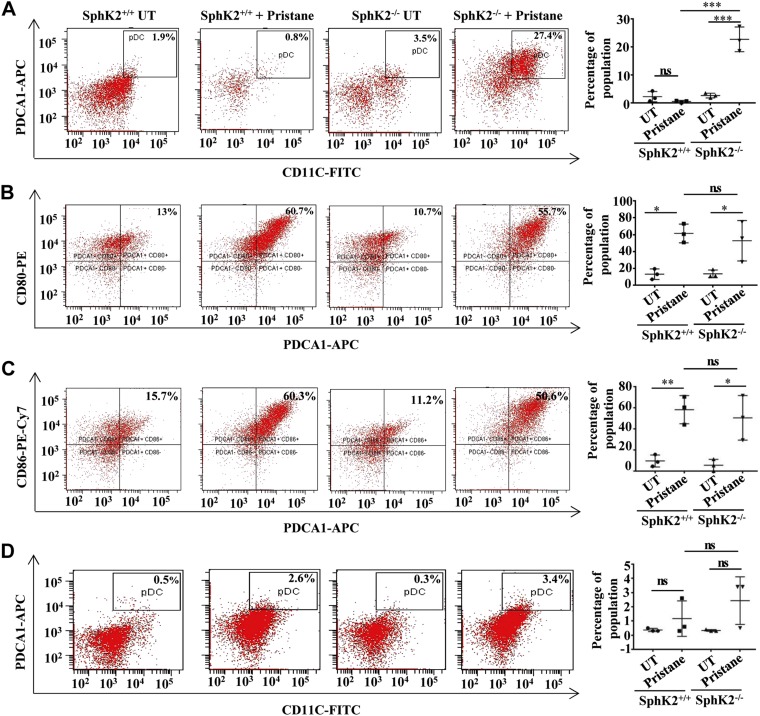
The influence of SphK2 expression on frequency and activation of pDCs in lupus mice was next examined. FACS analysis revealed that, although pristane did not have a significant effect on frequency of pDCs in the peritoneal lavage of wild-type mice, the percentage of pDC population markedly increased in the SphK2 knockout animals (Fig. 3A). It is well known that pDCs are activated in response to TLR stimulation, either by invading microorganisms or by immune complexes composed of self-antigens, and then express the typical pDC activation markers CD80 and CD86 (39). Evaluation of the activation profile of pDCs revealed a significant increase in the expression of both CD80 and CD86 in mice injected with pristane (Fig. 3B, C). However, activation of pDCs in SphK2−/− mice injected with pristane was similar to that of the wild-type animals (Fig. 3B, C). The increase in pDC frequency in the SphK2−/− pristane group was also observed in splenic pDCs, albeit not to the same extent and not reaching statistical significance (Fig. 3D).
Figure 3.
SphK2 deficiency increases the pDC population in a diseased state but not their activation status. A) The percentage of pDC population identified as CD11c+ and PDCA-1+ in the peritoneal lavage of the experimental mice was assessed by FACS. B, C) The activation status of peritoneal pDCs indicated by the expression of CD80 (B) and CD86 (C) was assessed by FACS analysis. Representative dot plots of 1 of 3 independent experiments are shown in the left panels. Data shown are averages of 3 independent experiments (A–C). D) Percentage of pDC population double-positive for CD11c and PDCA-1 in splenocytes isolated from SphK2+/+ to SphK2−/− mice. Representative dot plots of 1 of 3 independent experiments are shown in the left panels, and averaged data from 3 independent experiments are shown in the right panel. Statistical significance was determined by ANOVA with a post hoc Tukey’s test; n.s., not significant. *P < 0.05, **P < 0.005, ***P < 0.001.
Pristane enhances apoptosis of peritoneal-lavage cells from SphK2 knockout mice
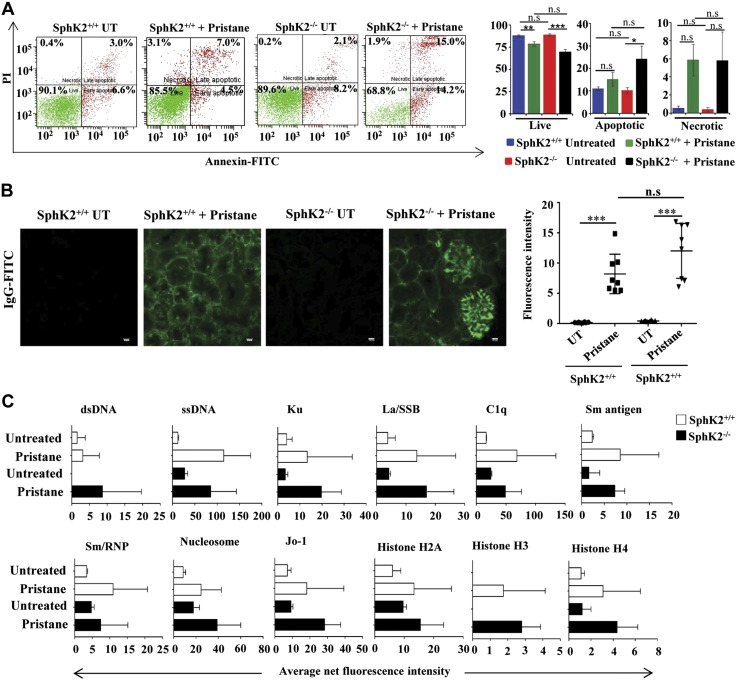
The accumulation of uncleared cellular debris is a causative factor of SLE (40). We next examined the effect of pristane on peritoneal-lavage cell death by annexin V–PI staining followed by FACS. Pristane administration caused a significant reduction of live cell population in the peritoneal lavage of both SphK2+/+ and SphK2−/− mice compared with respective untreated controls (Fig. 4A). Annexin staining that represents early and late apoptotic cells were significantly increased in peritoneal-lavage cells from pristane-treated SphK2−/− mice compared with those from wild-type mice (Fig. 4A). However, no significant differences were observed in necrotic populations. Hence, a deficiency of SphK2 results in a greater apoptotic cell population in response to an immune assault, such as pristane treatment, in peritoneal lavage, which is the site of injection of pristane.
Figure 4.
Absence of SphK2 enhances pristane-induced apoptosis of peritoneal cells. A) Peritoneal-lavage cells from SphK2+/+ to SphK2−/− mice untreated or injected with pristane were stained with PI and annexin V–FITC and analyzed by FACS. Representative FACS analyses are shown in the left panels. The right panel shows average populations of live cells (PI and annexin negative), necrotic cells (PI positive and annexin negative), and early and late apoptotic cells (annexin positive). Data are expressed as means ± sem (n = 7 mice each in SphK2+/+ groups, and n = 6 mice each in SphK2−/− groups). Statistical significance was determined by ANOVA with a post hoc Tukey’s test. B) Glomerulonephritis in renal sections were determined by deposition of IgG-FITC immunofluorescence after staining with goat anti-mouse IgG antibody. Scale bars, 10 µm. Data from 8 fields were quantified by ImageJ software. Statistical significance was determined by ANOVA with a post hoc Tukey’s test. C) IgG autoantibodies in serum from SphK2+/+ to SphK2−/− mice untreated or injected with pristane were measured fluorometrically with an autoantigen microarray as described in Materials and Methods. Data are presented as average net fluorescence intensities; n.s., not significant. *P < 0.05, **P < 0.005, ***P < 0.001.
SphK2 knockout does not protect against the disease outcome in pristane-induced lupus
It was then important to determine whether SphK2 deficiency affects the disease outcome in the mouse lupus model. We next investigated the effect of SphK2 deficiency in pristane-treated mice on renal IgG deposition, a hallmark of SLE. IgG in kidney sections was clearly increased by pristane treatment. However, no significant differences were observed between pristane-injected wild-type and SphK2 knockout mice (Fig. 4B). Autoantibodies directed against self-antigens are also an important characteristic of lupus (7, 38) and are considered to be the gold standard in clinical analyses. An autoantigen array (27) used to examine the levels of pathogenic IgG autoantibodies showed similar pristane-induced increases in autoantibody production in both the wild-type and knockout mice (Fig. 4C).
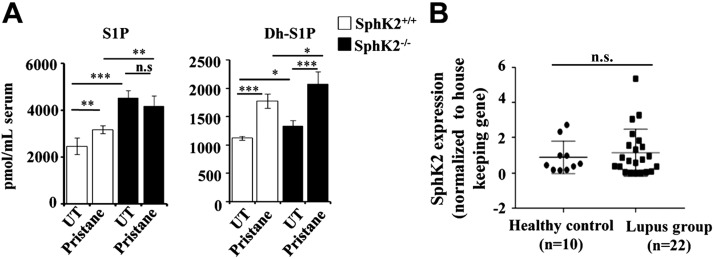
Effects of pristane-induced lupus on levels of S1P and dihydro-S1P and SphK2 expression in lupus patient samples
Finally, we measured the levels of S1P and dihydro-S1P by LC-ESI-MS/MS in serum from untreated and pristane-treated wild-type and SphK2 knockout mice. In agreement with a previous study (41), levels of S1P and dihydro-S1P were increased in serum from SphK2 knockout mice (Fig. 5A). Although pristane significantly increased serum S1P levels in wild-type mice compared with untreated mice, the pristane-induced increase was blunted in SphK2−/− mice, perhaps because of high basal levels (Fig. 5A). Nevertheless, dihydro-S1P was further increased by SphK2 deletion in pristane-injected animals. It was then important to also measure the expression of SphK2 in PBMCs from human lupus patients by quantitative PCR. Notably, there were no significant differences in SphK2 expression in lupus patients compared with healthy volunteers (Fig. 5B and Table 1).
Figure 5.
Levels of the sphingolipid metabolites S1P and dihydro-S1P in pristane-induced lupus in mice. A) S1P (left panel) and dihydro-S1P (right panel) levels in the serum from untreated and pristane-treated SphK2+/+ and SphK2−/− mice were measured by LC-ESI-MS/MS. Data are expressed as picomoles per milliliter and are means ± sd (n = 4 mice/group). B) Expression of SphK2 in clinical lupus patients. Expression of SphK2 in PBMCs isolated from healthy volunteers (n = 10) and lupus patients (n = 22). mRNA levels of SphK2 were determined by quantitative PCR and normalized to β-actin. Statistical significance was determined by ANOVA with a post hoc Tukey’s test; n.s., not significant. *P < 0.05, **P < 0.005, ***P < 0.001.
TABLE 1.
Clinical characteristics of SLE patients
| Clinical characteristics |
|||||
|---|---|---|---|---|---|
| Sample no. | Gender | Age | SLEDAI score | dsDNA titer | C3 complement titer |
| 1 | Female | 24 | 21 | 92 | 32 |
| 2 | Female | 28 | 16 | 824 | 67 |
| 3 | Female | 27 | 25 | 800 | 30 |
| 4 | Female | 17 | 25 | 610 | 25 |
| 5 | Female | 24 | 10 | 391 | 44 |
| 6 | Female | 22 | 28 | 560 | 23 |
| 7 | Female | 38 | 26 | 252 | 31 |
| 8 | Female | 38 | 12 | 45 | 66 |
| 9 | Female | 18 | 11 | 84.8 | 24 |
| 10 | Female | 47 | 21 | 292 | 62 |
| 11 | Female | 13 | 27 | 300 | 47 |
| 12 | Female | 43 | 20 | 302 | 39 |
| 13 | Female | 19 | 16 | 300 | 30 |
| 14 | Female | 37 | 18 | 145 | 55 |
| 15 | Female | 20 | 15 | 244 | 30 |
| 16 | Female | 25 | 12 | 399 | 43 |
| 17 | Female | 28 | 11 | 760 | 42 |
| 18 | Female | 24 | 18 | 269 | 46 |
| 19 | Female | 46 | 19 | 38 | 30 |
| 20 | Female | 23 | 19 | 300 | 30 |
| 21 | Female | 18 | 19 | 310 | 68 |
| 22 | Female | 13 | 23 | 800 | 29 |
dsDNA, double-stranded DNA; SLEDAI, SLE Disease Activity Index.
DISCUSSION
In this study, we investigated the role of SphK2 in pDC development, functional responses, and the pathologic conditions that are mediated by type I IFNs. Our data indicate for the first time that SphK2 expression is an important determinant of pDC development. We found that deletion of SphK2 increased expression of pDC-specific markers, likely because of increased differentiation of pDCs from hematopoietic progenitors controlled by the growth factor Flt3-L, suggesting that SphK2 has a negative regulatory role. Furthermore, increased expression of TLR7 and TLR9, which are important for pathogen recognition and activation of innate immunity, was correlated with increased pDC development in SphK2−/− animals. The functional aspect of the accompanying increased secretion of IFNs also supported the influence of SphK2 on pDC development and function. S1P generated in the nucleus by SphK2 has been suggested to be an endogenous inhibitor of HDACs (18), and it is tempting to speculate that increased HDAC activity caused by deletion of SphK2 might be involved, as DC development requires HDAC activity (42).
The pristane model of lupus is known to be dependent on the IFN system, and this murine model phenocopies the clinically manifested symptoms in humans (37). Pristane treatment–increased pDCs in the peritoneum of SphK2 knockout mice further support the notion that SphK2 is a negative regulator of pDC differentiation. However, the onset of lupus symptoms in pristane-treated mice was unaffected by the deletion of SphK2, as shown by the expression of the disease marker Sca-1, compared with the wild-type pristane-treated mice; however, another disease marker, U1A, was decreased by the deletion of SphK2, suggesting a different regulatory mechanism for this particular marker. Moreover, the absence of SphK2 had no apparent effects on the IFN signature that is typical of lupus. Accumulation of cellular debris because of defective clearance is known to be a causative SLE mechanism (43). Moreover, greater accumulation of uncleared cellular refuse would trigger DC maturation (44), leading to a heightened immune response and autoimmunity. Release of autoantigens sets off the production of autoantibodies that are characteristic of lupus. An assessment of the pathogenic IgG in the serum showed similar levels of autoantibodies in the pristane-treated wild-type and SphK2−/− mice. Glomerulonephritis is marked by the glomerular deposition of immune complexes containing IgG. However, an assessment of the renal function by measuring proteinuria revealed that deletion of SphK2 did not protect against renal damage induced by pristane treatment. Thus, our data indicate that the loss of SphK2 does not protect against the initiation of lupus symptoms.
Several studies have explored the importance of S1P receptors in the progression of lupus, utilizing preclinical animal models. For example, treatment with a modulator of S1PR1, KRP-203, or FTY720 reduced renal injury and end-stage glomerular inflammation in the spontaneous lupus mouse models MRL-lpr (45) and BXSB (25). Likewise, female MRL-lpr mice showed reduced immune complex deposition and autoantibody titers when treated with FTY720 (24). Similarly, deficiency of microRNA-155, which targets S1PR1, reduced autoimmune inflammation in Faslpr/lpr mice (46). Moreover, ozanimod, a selective S1PR1 and S1PR5 modulator, also attenuated chronic inflammation and alleviated kidney pathology in the NZBWF1 preclinical animal model (47). In agreement with the previous study showing that S1P and dihydro-S1P were elevated in serum from lupus nephritis mice, we also observed elevated S1P and dihydro-S1P in pristane-injected mice (22). However, the increase in these bioactive sphingolipid metabolites did not correlate with lupus severity because SphK2 knockout mice have higher levels of S1P and dihydro-S1P. It is still not completely clear why levels of S1P are elevated in SphK2 knockout mice, but it has been suggested that this might be due to up-regulation of SphK1, which could mask the effects of SphK2 deletion (48). It is interesting to also note that it is still a matter of debate whether S1P is elevated in lupus patients. Although it was reported that S1P was increased in juvenile-onset SLE patients and correlated with increased renal involvement (49), in another study, it was suggested that its precursor C16-ceramide, rather than S1P, was increased in female lupus patients and was associated with severity of the disease (50). Taken together, our results show that SphK2 may play a role in DC development. However, because SphK2 deletion had no effect on the clinical parameters in pristane-induced lupus and SphK2 was not overexpressed in lupus patients, our work suggests that inhibitors of SphK2 might not be beneficial for treatment of SLE.
ACKNOWLEDGMENTS
The authors are thankful to the personnel of the Flow Cytometry Core Facility and Bioimaging Facility for excellent technical assistance, and the Animal Research Facility for mouse colony maintenance. S.M. and M.B.L. acknowledge the senior research fellowship from the Indian Council of Medical Research and the University Grant Commission, respectively. S.M. acknowledges the Doctoral Advisory Committee for valuable suggestions. The work was supported by the U.S. National Institutes of Health, National Institute of General Medical Sciences Grant R01GM043880 (to S.S.), a fast-track grant from the Indian Government’s Department of Science and Technology (SR/FT/LS-159/2012), a grand-in-aid scheme of the Council for Scientific and Industrial Research [37 (1720)/18/EMR-II], and, in part, by a faculty start-up grant and Department of Biotechnology (DBT) Ramalingaswami Re-Entry Fellowship (BT/RLF/Re-entry/38/2011) to K.B.H. The authors declare no conflicts of interest.
Glossary
- APC
allophycocyanin
- DC
dendritic cell
- FACS
fluorescence-activated cell sorting
- Flt3L
FMS-like tyrosine kinase 3 ligand
- FSC
forward scatter
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HDAC
histone deacetylase
- LC-ESI-MS/MS
liquid chromatography–electrospray ionization–tandem mass spectrometry
- MCMV
murine cytomegalovirus
- PBMC
peripheral blood mononuclear cell
- pDC
plasmacytoid dendritic cell
- PDCA-1
pDC antigen 1
- PI
propidium iodide
- R848
resiquimod
- RBC
red blood cell
- RPMI
Roswell Park Memorial Institute
- S1P
sphingosine-1-phosphate
- S1PR1
S1P receptor 1
- Sca-1
stem cell antigen 1
- Siglec H
sialic acid–binding Ig-like lectin H
- SLE
systemic lupus erythematosus
- SphK
sphingosine kinase
- SSC
side scatter
- U1a
U1 small nuclear ribonucleoprotein A
AUTHOR CONTRIBUTIONS
S. Mohammed designed and performed the experiments, analyzed data, and prepared the manuscript; N. S. Vineetha recruited the lupus patients for the study; S. James, J. S. Aparna, and M. B. Lankadasari provided technical assistance to the study; J. C. Allegood performed liquid chromatography–mass spectrometry (LCMS) analysis of sphingolipids; Q.-Z. Li performed autoantigen microarray analysis; S. Spiegel performed LCMS analysis of sphingolipids, analyzed the data, provided intellectual contributions, and edited the manuscript; K. B. Harikumar conceived the idea, designed the experiments, analyzed the data, provided overall project coordination, and prepared the manuscript; and all authors read and approved the final manuscript.
REFERENCES
- 1.Swiecki M., Colonna M. (2015) The multifaceted biology of plasmacytoid dendritic cells. Nat. Rev. Immunol. 15, 471–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegal F. P., Kadowaki N., Shodell M., Fitzgerald-Bocarsly P. A., Shah K., Ho S., Antonenko S., Liu Y. J. (1999) The nature of the principal type 1 interferon-producing cells in human blood. Science 284, 1835–1837 [DOI] [PubMed] [Google Scholar]
- 3.Fitzgerald-Bocarsly P., Feng D. (2007) The role of type I interferon production by dendritic cells in host defense. Biochimie 89, 843–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rönnblom L., Alm G. V. (2003) Systemic lupus erythematosus and the type I interferon system. Arthritis Res. Ther. 5, 68–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rönnblom L., Eloranta M. L., Alm G. V. (2006) The type I interferon system in systemic lupus erythematosus. Arthritis Rheum. 54, 408–420 [DOI] [PubMed] [Google Scholar]
- 6.Sisirak V., Ganguly D., Lewis K. L., Couillault C., Tanaka L., Bolland S., D’Agati V., Elkon K. B., Reizis B. (2014) Genetic evidence for the role of plasmacytoid dendritic cells in systemic lupus erythematosus. J. Exp. Med. 211, 1969–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rowland S. L., Riggs J. M., Gilfillan S., Bugatti M., Vermi W., Kolbeck R., Unanue E. R., Sanjuan M. A., Colonna M. (2014) Early, transient depletion of plasmacytoid dendritic cells ameliorates autoimmunity in a lupus model. J. Exp. Med. 211, 1977–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maceyka M., Spiegel S. (2014) Sphingolipid metabolites in inflammatory disease. Nature 510, 58–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matloubian M., Lo C. G., Cinamon G., Lesneski M. J., Xu Y., Brinkmann V., Allende M. L., Proia R. L., Cyster J. G. (2004) Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427, 355–360 [DOI] [PubMed] [Google Scholar]
- 10.Lima S., Milstien S., Spiegel S. (2017) Sphingosine and sphingosine kinase 1 involvement in endocytic membrane trafficking. J. Biol. Chem. 292, 3074–3088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Czeloth N., Bernhardt G., Hofmann F., Genth H., Förster R. (2005) Sphingosine-1-phosphate mediates migration of mature dendritic cells. J. Immunol. 175, 2960–2967 [DOI] [PubMed] [Google Scholar]
- 12.Maceyka M., Payne S. G., Milstien S., Spiegel S. (2002) Sphingosine kinase, sphingosine-1-phosphate, and apoptosis. Biochim. Biophys. Acta 1585, 193–201 [DOI] [PubMed] [Google Scholar]
- 13.Lai W. Q., Irwan A. W., Goh H. H., Howe H. S., Yu D. T., Valle-Oñate R., McInnes I. B., Melendez A. J., Leung B. P. (2008) Anti-inflammatory effects of sphingosine kinase modulation in inflammatory arthritis. J. Immunol. 181, 8010–8017 [DOI] [PubMed] [Google Scholar]
- 14.Tsai H. C., Han M. H. (2016) Sphingosine-1-phosphate (S1P) and S1P signaling pathway: therapeutic targets in autoimmunity and inflammation. Drugs 76, 1067–1079 [DOI] [PubMed] [Google Scholar]
- 15.Kohama T., Olivera A., Edsall L., Nagiec M. M., Dickson R., Spiegel S. (1998) Molecular cloning and functional characterization of murine sphingosine kinase. J. Biol. Chem. 273, 23722–23728 [DOI] [PubMed] [Google Scholar]
- 16.Mohammed S., Harikumar K. B. (2017) Sphingosine 1-phosphate: a novel target for lung disorders. Front. Immunol. 8, 296; erratum: 9, 1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maceyka M., Sankala H., Hait N. C., Le Stunff H., Liu H., Toman R., Collier C., Zhang M., Satin L. S., Merrill A. H., Jr., Milstien S., Spiegel S. (2005) SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J. Biol. Chem. 280, 37118–37129 [DOI] [PubMed] [Google Scholar]
- 18.Hait N. C., Allegood J., Maceyka M., Strub G. M., Harikumar K. B., Singh S. K., Luo C., Marmorstein R., Kordula T., Milstien S., Spiegel S. (2009) Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science 325, 1254–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pyne N. J., Adams D. R., Pyne S. (2017) Sphingosine kinase 2 in autoimmune/inflammatory disease and the development of sphingosine kinase 2 inhibitors. Trends Pharmacol. Sci. 38, 581–591 [DOI] [PubMed] [Google Scholar]
- 20.French K. J., Zhuang Y., Maines L. W., Gao P., Wang W., Beljanski V., Upson J. J., Green C. L., Keller S. N., Smith C. D. (2010) Pharmacology and antitumor activity of ABC294640, a selective inhibitor of sphingosine kinase-2. J. Pharmacol. Exp. Ther. 333, 129–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fitzpatrick L. R., Green C., Frauenhoffer E. E., French K. J., Zhuang Y., Maines L. W., Upson J. J., Paul E., Donahue H., Mosher T. J., Smith C. D. (2011) Attenuation of arthritis in rodents by a novel orally-available inhibitor of sphingosine kinase. Inflammopharmacology 19, 75–87 [DOI] [PubMed] [Google Scholar]
- 22.Snider A. J., Ruiz P., Obeid L. M., Oates J. C. (2013) Inhibition of sphingosine kinase-2 in a murine model of lupus nephritis. PLoS One 8, e53521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alperovich G., Rama I., Lloberas N., Franquesa M., Poveda R., Gomà M., Herrero-Fresneda I., Cruzado J. M., Bolaños N., Carrera M., Grinyó J. M., Torras J. (2007) New immunosuppresor strategies in the treatment of murine lupus nephritis. Lupus 16, 18–24 [DOI] [PubMed] [Google Scholar]
- 24.Okazaki H., Hirata D., Kamimura T., Sato H., Iwamoto M., Yoshio T., Masuyama J., Fujimura A., Kobayashi E., Kano S., Minota S. (2002) Effects of FTY720 in MRL-lpr/lpr mice: therapeutic potential in systemic lupus erythematosus. J. Rheumatol. 29, 707–716 [PubMed] [Google Scholar]
- 25.Ando S., Amano H., Amano E., Minowa K., Watanabe T., Nakano S., Nakiri Y., Morimoto S., Tokano Y., Lin Q., Hou R., Ohtsuji M., Tsurui H., Hirose S., Takasaki Y. (2010) FTY720 exerts a survival advantage through the prevention of end-stage glomerular inflammation in lupus-prone BXSB mice. Biochem. Biophys. Res. Commun. 394, 804–810 [DOI] [PubMed] [Google Scholar]
- 26.Satoh M., Reeves W. H. (1994) Induction of lupus-associated autoantibodies in BALB/c mice by intraperitoneal injection of pristane. J. Exp. Med. 180, 2341–2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q. Z., Xie C., Wu T., Mackay M., Aranow C., Putterman C., Mohan C. (2005) Identification of autoantibody clusters that best predict lupus disease activity using glomerular proteome arrays. J. Clin. Invest. 115, 3428–3439; erratum: 116, 548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newton J., Hait N. C., Maceyka M., Colaco A., Maczis M., Wassif C. A., Cougnoux A., Porter F. D., Milstien S., Platt N., Platt F. M., Spiegel S. (2017) FTY720/fingolimod increases NPC1 and NPC2 expression and reduces cholesterol and sphingolipid accumulation in Niemann-Pick type C mutant fibroblasts. FASEB J. 31, 1719–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cisse B., Caton M. L., Lehner M., Maeda T., Scheu S., Locksley R., Holmberg D., Zweier C., den Hollander N. S., Kant S. G., Holter W., Rauch A., Zhuang Y., Reizis B. (2008) Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell 135, 37–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schotte R., Nagasawa M., Weijer K., Spits H., Blom B. (2004) The ETS transcription factor Spi-B is required for human plasmacytoid dendritic cell development. J. Exp. Med. 200, 1503–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J., Raper A., Sugita N., Hingorani R., Salio M., Palmowski M. J., Cerundolo V., Crocker P. R. (2006) Characterization of Siglec-H as a novel endocytic receptor expressed on murine plasmacytoid dendritic cell precursors. Blood 107, 3600–3608 [DOI] [PubMed] [Google Scholar]
- 32.Blasius A. L., Cella M., Maldonado J., Takai T., Colonna M. (2006) Siglec-H is an IPC-specific receptor that modulates type I IFN secretion through DAP12. Blood 107, 2474–2476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saitoh S. I., Abe F., Kanno A., Tanimura N., Mori Saitoh Y., Fukui R., Shibata T., Sato K., Ichinohe T., Hayashi M., Kubota K., Kozuka-Hata H., Oyama M., Kikko Y., Katada T., Kontani K., Miyake K. (2017) TLR7 mediated viral recognition results in focal type I interferon secretion by dendritic cells. Nat. Commun. 8, 1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Combes A., Camosseto V., N’Guessan P., Argüello R. J., Mussard J., Caux C., Bendriss-Vermare N., Pierre P., Gatti E. (2017) BAD-LAMP controls TLR9 trafficking and signalling in human plasmacytoid dendritic cells. Nat. Commun. 8, 913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Izaguirre A., Barnes B. J., Amrute S., Yeow W. S., Megjugorac N., Dai J., Feng D., Chung E., Pitha P. M., Fitzgerald-Bocarsly P. (2003) Comparative analysis of IRF and IFN-alpha expression in human plasmacytoid and monocyte-derived dendritic cells. J. Leukoc. Biol. 74, 1125–1138 [DOI] [PubMed] [Google Scholar]
- 36.Colonna M., Trinchieri G., Liu Y. J. (2004) Plasmacytoid dendritic cells in immunity. Nat. Immunol. 5, 1219–1226 [DOI] [PubMed] [Google Scholar]
- 37.Reeves W. H., Lee P. Y., Weinstein J. S., Satoh M., Lu L. (2009) Induction of autoimmunity by pristane and other naturally occurring hydrocarbons. Trends Immunol. 30, 455–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu Y., Zhuang H., Han S., Liu C., Wang H., Mathews C. E., Massini J., Yang L., Reeves W. H. (2015) Mechanisms of tumor necrosis factor α antagonist-induced lupus in a murine model. Arthritis Rheumatol. 67, 225–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dilioglou S., Cruse J. M., Lewis R. E. (2003) Function of CD80 and CD86 on monocyte- and stem cell-derived dendritic cells. Exp. Mol. Pathol. 75, 217–227 [DOI] [PubMed] [Google Scholar]
- 40.Muñoz L. E., Lauber K., Schiller M., Manfredi A. A., Herrmann M. (2010) The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat. Rev. Rheumatol. 6, 280–289 [DOI] [PubMed] [Google Scholar]
- 41.Nagahashi M., Yamada A., Aoyagi T., Allegood J., Wakai T., Spiegel S., Takabe K. (2016) Sphingosine-1-phosphate in the lymphatic fluid determined by novel methods. Heliyon 2, e00219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chauvistré H., Küstermann C., Rehage N., Klisch T., Mitzka S., Felker P., Rose-John S., Zenke M., Seré K. M. (2014) Dendritic cell development requires histone deacetylase activity. Eur. J. Immunol. 44, 2478–2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaipl U. S., Munoz L. E., Grossmayer G., Lauber K., Franz S., Sarter K., Voll R. E., Winkler T., Kuhn A., Kalden J., Kern P., Herrmann M. (2007) Clearance deficiency and systemic lupus erythematosus (SLE). J. Autoimmun. 28, 114–121 [DOI] [PubMed] [Google Scholar]
- 44.Rovere P., Vallinoto C., Bondanza A., Crosti M. C., Rescigno M., Ricciardi-Castagnoli P., Rugarli C., Manfredi A. A. (1998) Bystander apoptosis triggers dendritic cell maturation and antigen-presenting function. J. Immunol. 161, 4467–4471 [PubMed] [Google Scholar]
- 45.Wenderfer S. E., Stepkowski S. M., Braun M. C. (2008) Increased survival and reduced renal injury in MRL/lpr mice treated with a novel sphingosine-1-phosphate receptor agonist. Kidney Int. 74, 1319–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xin Q., Li J., Dang J., Bian X., Shan S., Yuan J., Qian Y., Liu Z., Liu G., Yuan Q., Liu N., Ma X., Gao F., Gong Y., Liu Q. (2015) miR-155 deficiency ameliorates autoimmune inflammation of systemic lupus erythematosus by targeting S1pr1 in Faslpr/lpr mice. J. Immunol. 194, 5437–5445 [DOI] [PubMed] [Google Scholar]
- 47.Taylor Meadows K. R., Steinberg M. W., Clemons B., Stokes M. E., Opiteck G. J., Peach R., Scott F. L. (2018) Ozanimod (RPC1063), a selective S1PR1 and S1PR5 modulator, reduces chronic inflammation and alleviates kidney pathology in murine systemic lupus erythematosus. PLoS One 13, e0193236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liang J., Nagahashi M., Kim E. Y., Harikumar K. B., Yamada A., Huang W. C., Hait N. C., Allegood J. C., Price M. M., Avni D., Takabe K., Kordula T., Milstien S., Spiegel S. (2013) Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell 23, 107–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watson L., Tullus K., Marks S. D., Holt R. C., Pilkington C., Beresford M. W. (2012) Increased serum concentration of sphingosine-1-phosphate in juvenile-onset systemic lupus erythematosus. J. Clin. Immunol. 32, 1019–1025 [DOI] [PubMed] [Google Scholar]
- 50.Checa A., Idborg H., Zandian A., Sar D. G., Surowiec I., Trygg J., Svenungsson E., Jakobsson P. J., Nilsson P., Gunnarsson I., Wheelock C. E. (2017) Dysregulations in circulating sphingolipids associate with disease activity indices in female patients with systemic lupus erythematosus: a cross-sectional study. Lupus 26, 1023–1033 [DOI] [PubMed] [Google Scholar]