Abstract
Herpes simplex virus 1 (HSV-1) is a contagious neurotropic herpesvirus responsible for oral lesions and herpesviral encephalitis. The HSV-1 envelope contains N-glycosylated proteins involved in infection and that are candidate drug targets. NGI-1 is a small-molecule inhibitor of oligosaccharyltransferase (OST) complexes STT3A-OST and STT3B-OST, which catalyze cotranslational and post-translational N-glycosylation, respectively. Because host OSTs attach HSV-1 glycans, NGI-1 might have anti–HSV-1 activity. We evaluated HSV-1 function using NGI-1 and human embryonic kidney 293 knockout lines for OST isoform-specific catalytic and accessory subunits. N-glycosylation of 2 representative envelope proteins (gC and gD) was primarily dependent upon STT3A-OST, but to a large extent replaceable by STT3B-OST. Knockouts impairing STT3A- or STT3B-OST activity, by themselves, did not appreciably affect HSV-1 function (plaque-forming units, normalized to viral particles measured by unglycosylated capsid protein VP5 content). However, with cells lacking STT3B-OST activity (missing the catalytic subunit STT3B or the oxidoreductase subunits magnesium transporter 1/tumor suppressor candidate 3) and thus solely dependent upon STT3A-OST for N-glycosylation, NGI-1 treatment resulted in HSV-1 having cell type–dependent dysfunction (affecting infectivity with Vero cells much more than with the 293 lines). Ablation of post-translational N-glycosylation can therefore make HSV-1 infectivity, and possibly masking of immunogenic peptide epitopes by glycans, highly sensitive to pharmacological inhibition of cotranslational N-glycosylation.—Lu, H., Cherepanova, N. A., Gilmore, R., Contessa, J. N., Lehrman, M. A. Targeting STT3A-oligosaccharyltransferase with NGI-1 causes herpes simplex virus 1 dysfunction.
Keywords: glycobiology, glycosylation, glycoprotein, herpesvirus, lipid-linked oligosaccharide
The human pathogen herpes simplex virus 1 (HSV-1) causes cold sores, lesions of the oral mucosal epithelium and the skin, and herpesviral encephalitis. Infections involve oral or dermal exposure, followed by neurotropic latency from which HSV-1 can later emerge. At several levels, there has been strong interest in the glycobiology of HSV-1, which has as many as 11 N-glycosylated envelope proteins (1). The abundant HSV-1 envelope glycoproteins gC and gD, which are readily detected by metabolic labeling with [35S]-methionine and autoradiography, enabled early insights into the basic mechanisms of cotranslational N-glycosylation and glycan processing (2, 3). Impairment of the host cell N-glycosylation machinery, upon which HSV-1 depends, has also long been known to suppress its replication and cytopathology (4, 5). However, depending upon the context, HSV-1 N-glycans are not always necessary for infectivity. Propagation of HSV-1 in N-acetylglucosaminyl transferase I-deficient baby hamster kidney (BHK) cells, which altered its envelope protein N-glycans, did not reduce subsequent infectivity on normal BHK cells (6). However, removal of N-glycans by endoglycosidase F treatment from a truncated form of gC reduced its binding to BHK cells (7), and treatment of HSV-1 with the same enzyme reduced infectivity with RC-37 cells (8). Extensive analyses of gD with COS-1 cells (9), Vero cells (10, 11), and a mouse eye model (12) did not identify essential roles of its N-glycans in a number of aspects of HSV-1 replication and pathogenesis, but reduced plaque size with Vero cells and alteration of gD conformation were reported. In contrast with gC and gD, an N-glycan on envelope glycoprotein gK is important for HSV-1 pathogenesis because gK must be properly N-glycosylated for cytopathic effects associated with plaque formation. Mutation of gK, to prevent glycosylation of Asn58, leads to obscured plaques and greater syncytia formation on Vero cell monolayers (13). In summary, there is a substantial body of evidence supporting the importance of N-glycans of envelope proteins in HSV-1 function, but potential roles may be host cell and context dependent. In a recent review, a more comprehensive overview of the roles of N-glycans in infectivity of a number of enveloped viruses is provided (14).
Host cell N-glycosylation is therefore an appealing anti–HSV-1 therapeutic target. Approaches for depleting the N-glycosylation precursor, the lipid-linked oligosaccharide (LLO) glucose3mannose9N-acetylglucosamine2-P-P-dolichol (G3M9Gn2), unfortunately bring significant complications. Tunicamycin (TN), for example, is an inhibitor of uridine diphosphate N-acetylglucosamine:dolichol phosphate GlcNAc-1-P transferase (15), the committed step for synthesis of G3M9Gn2-LLO (16). However, TN has potentially toxic effects. By inhibiting LLO synthesis, TN can cause endoplasmic reticulum stress with a robust unfolded protein response (17), and high TN concentrations inhibit protein palmitoylation (18). 2-Deoxyglucose (2DG) impairs N-glycosylation by causing the accumulation of aberrant or truncated LLOs (19) and can hinder HSV-1 infectivity (4). Moreover, 2DG has been considered for cancer chemotherapy because of its dual effects on N-glycosylation and glycolysis, which may be effective for tumors containing both normoxic and hypoxic regions (20), but such broad effects would likely be problematic for antiviral therapy in otherwise healthy individuals.
Recently a new class of small-molecule inhibitors that partially inhibit N-glycosylation by targeting oligosaccharyltransferase (OST) complexes has been identified. Unlike TN and 2DG, these inhibitors leave LLO biosynthesis unaffected, and their concentrations appear to be well controlled, allowing host cell viability to be maintained (21, 22). The lead compound NGI-1 (394.5 Da) inhibits both mammalian OST isoforms, which are defined by their signature catalytic subunits: STT3A-OST, which conducts cotranslational N-glycosylation and forms a complex with the translocon, and STT3B-OST, which catalyzes post-translational glycosylation and is unassociated with the translocon (23–25). A second-generation analog, C19 (415.5 Da), is highly selective for STT3B-OST (21). Potentially, when used in parallel, NGI-1 and C19 could differentiate roles of STT3A-OST and STT3B-OST. NGI-1–type inhibitors are generally nontoxic to cultured mammalian cells at concentrations that appreciably inhibit OST function but do alter glycoprotein-driven cellular processes, such as receptor tyrosine kinase-dependent tumor cell growth (22). In this study, vide infra, human embryonic kidney (HEK) 293 lines were treated with ≤5 μM drug. Previously, HEK293 cells showed no evidence of toxicity or viability after 72 h with 30 μM NGI-1 (22) or after 48 h with 10 μM C19 (21). Perhaps not surprisingly as a partial inhibitor of N-glycosylation, NGI-1 is capable of inducing a mild unfolded protein response (with activation of binding immunoglobulin protein expression and X-box binding protein 1 splicing but not activating transcription factor 6 cleavage) (21). There is no evidence of acceptor substrate bias by NGI-1, which inhibited N-glycosylation of several STT3A- and STT3B-specific exogenous reporter substrates, as well as endogenous N-glycoproteins (epidermal growth factor receptor, fibroblast growth factor receptor, and cyclooxygenase 2) (21, 22). NGI-1 interacts physically with STT3B-OST (22) and thus most likely interacts with STT3A-OST, but its detailed mechanism of OST inhibition remains unclear. Because neither inhibitor bears obvious structural similarities to saccharides, dolichol phosphate, or peptides suggestive of competitive inhibition (22), it is possible that NGI-1 and C19 act noncompetitively.
Replication of several pathogenic mosquito-borne flaviviruses (dengue, West Nile, and yellow fever) is suppressed by loss of STT3A, as well as by loss of STT3B in the case of dengue virus (26–28). Infection by these viruses is also inhibited by NGI-1 in the concentration range that impairs N-glycosylation of their envelope glycoproteins. Interestingly, although OST complexes are the targets of NGI-1, NGI-1’s ability to inhibit dengue virus replication is not directly linked to its inhibition of actual OST enzymatic activity (29). Moreover, OST complexes with catalytic subunit mutations still support dengue virus replication (26, 27). This indicates an unanticipated complexity of the effects of OST manipulation on viral replication. It is also unclear to what extent mutation of OST subunits, or their inhibition with NGI-1, either reduces the amount of otherwise infectious viral particles or promotes production of less infectious (dysfunctional) virus. These possibilities have been difficult to distinguish because, in most cases, luminescent reporters were used to measure viral infectivity in place of direct comparisons of viral particle function with particle quantity. Because 2 enveloped viruses of the alphavirus family (Chikungunya virus and Venezuelan equine encephalitis virus) were not affected by NGI-1 (29), it is also unclear whether NGI-1 has an antiviral range beyond flaviviruses.
Here we examine the consequence of inhibiting host N-glycosylation on propagation of HSV-1 by treating HEK293 cells harboring knockouts (KOs) of OST catalytic and accessory subunits with OST inhibitors. We report that N-glycosylation of gC and gD was primarily dependent upon STT3A-OST but largely replaceable by STT3B-OST. Impairment of the OST activities themselves had little effect on HSV-1 function. However, in the absence of STT3B-OST activity (such that N-glycosylation was fully dependent upon STT3A-OST), NGI-1 treatment caused significant HSV-1 dysfunction while viral particle quantity was only mildly affected. Loss of post-translational N-glycosylation in host cells thus made HSV-1 function highly sensitive to pharmacological inhibition of cotranslational N-glycosylation. The range of NGI-1 as a potential antiviral agent therefore includes HSV-1 and extends beyond flaviviruses.
MATERIALS AND METHODS
HEK293 cell culture
Human HEK293 kidney lines [wild type (WT), STT3A-KO, STT3B-KO, and magnesium transporter 1 (MagT1)/tumor suppressor candidate 3 (TUSC3)–double KO (DKO) (23); DC2-KO (30)] were grown as indicated in DMEM (low glucose, 11885092; Thermo Fisher Scientific, Waltham, MA, USA, or high glucose, 11995065; Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (FBS; Atlanta Biologicals, Flowery Branch, GA, USA). Cells were cultured in a humidified 5% CO2 atmosphere and then used for fluorophore-assisted carbohydrate electrophoresis (FACE) analysis, immunoblots, OST assays, or HSV-1 infections.
FACE
LLOs, N-glycans, and free oligosaccharides isolated from methanolic sonicates of intact or permeabilized cells, as well as glucose from culture media, were analyzed by FACE (31, 32). LLO glycans were released from dolichol with mild acid, and N-glycans were released from glycopeptides with endo H (P0703L; New England Biolabs, Ipswich, MA, USA). Glycans were labeled with 7-amino-1,3-naphthalenedisulfonic acid (81529; AnaSpec, Fremont, CA, USA), and samples were normalized to total cellular protein for FACE analysis. Gel images were acquired with a UVP Chemidoc-It II Scanner (Analytik Jena, Jena, Germany), and saccharides quantified with UVP VisionWorks Software (Analytik Jena).
HSV-1 infection and plaque assay
HSV-1 propagation and plaque assays were performed by standard procedures as described (33). HSV-1 strain 17 and Vero cells were obtained from Beth Levine (University of Texas Southwestern Medical Center). HSV-1 stocks from Vero cells were titered by serial dilution with methylcellulose overlay plaque assays. Infections of HEK lines (cultured in DMEM with high glucose, heat-inactivated 10% FBS, and 50 μg/ml gentamicin) were initiated with monolayers at ∼70% confluence, and with virus at the indicated multiplicity. Controls revealed that negligible virus was passively released into the medium from gently handled, infected HEK monolayers. Thus, at the indicated times, either of 2 harvest methods were used. Method A: the conditioned (spent) medium was carefully removed, and the entire infected monolayer was harvested into fresh medium with a mechanical scraper, subjected to 1–3 cycles of freezing (−80°C) and thawing (37°C), and then briefly disrupted with a bath sonicator. Method B: the conditioned medium was repeatedly pipetted over infected monolayers and recovered as fraction I (a population of loosely associated and thus more mobile virus), followed by harvest of the remaining monolayer by mechanical scraping in fresh medium, 1–3 cycles of freezing (−80°C) and thawing (37°C), and brief disruption with a bath sonicator to yield fraction II (a population of tightly associated virus). For both methods A and B, to concentrate virus, lysates were centrifuged for 15 min at 1000 g to remove debris, then at 15 min at 20,000 g to collect viral particles. Viral yields were typically in the range of 103–108 plaque-forming unit (pfu)/ml in experimental lysates compared with 109–1010 in high-titer stocks.
Immunoblot analysis of HSV-1 samples
Whole viral lysates or concentrated particles were suspended in 1× SDS-PAGE Laemmli buffer, then sonicated and heated at 100°C for 5 min prior to gel loading. Proteins were separated in polyacrylamide gels (7% resolving gel, 3.5% stacking gel) and transferred onto PVDF membrane for immunoblot analysis. Primary antibodies for HSV-1 proteins were anticapsid protein VP5 (ab6508; Abcam, Cambridge, United Kingdom), anti-envelope protein gC (ab6509; Abcam), and anti-envelope protein gD (sc-21719; Santa Cruz Biotechnology, Dallas, TX, USA). Anti-actin (4970s; Cell Signaling Technology, Danvers, MA, USA) was used as a cellular protein marker. Horseradish peroxidase–conjugated secondary antibodies were used and detected with SuperSignal West Pico chemiluminescent substrate (34077; Thermo Fisher Scientific) in a UVP Chemidoc-It II Scanner.
VP5 signals (7) were employed to quantify viral particles with a 2-step process. Viral sample volumes for initial immunoblots were used that were estimated to fall within the linear detection range of the UVP Chemidoc-It II Scanner. After quantification of the VP5 signals with UVP VisionWorks software, secondary immunoblots were performed with sample volumes calculated to give uniform amounts of VP5 across all lanes of the gel. This permitted verification that VP5 measurements from the initial immunoblots were accurate or, if needed, recalibration of the VP5 concentrations.
gC and gD blots were used to resolve their glycoforms, and where indicated samples were de–N-glycosylated with N-glycosidase F (PNGase F) (GKE-5003; Prozyme, Hayward, CA, USA). To calculate glycosylation indices when glycoforms were sufficiently well resolved, in each lane, individual glycoforms were quantified, and these values were normalized arithmetically to add up to 1 arbitrary unit. Normalized glycoform values were then multiplied by the number of glycans on each glycoform, and the products were summed to calculate a glycosylation index. Thus, fully glycosylated gC and gD had indices of 8.0 and 3.0, respectively.
Permeabilization of plasma membranes with pore-forming toxin
HEK293 lines were re-fed within 24 h prior to anthrolysin O (ALO) treatment to achieve 70–90% confluence, and permeabilized with ALO (34, 35) as described for streptolysin O (36). Dishes on ice were rinsed twice with ice-cold PBS, 20 nM ALO (in ice-cold PBS) was added for 4 min with periodic swirling on ice, and dishes were rinsed again with ice-cold PBS. Dishes were then incubated 4 min at 37°C with prewarmed transport buffer (78 mM KCl, 4 mM MgCl2, and 50 mM K–4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, pH 7.2), then placed directly on ice for 10 min. The cytosol-free cell bodies were used for OST reactions.
OST reactions
Assays for OST transferase and LLO hydrolysis activities were performed exactly as previously described (35). ALO-permeabilized cells were incubated 30–60 min in a reaction buffer consisting of transport buffer plus 0 or 400 μM uridine diphosphate–glucose (UDP-Glc), 200 μM uridine diphosphate N-acetylglucosamine, 50 μM guanosine diphosphate mannose, 0.2 mM AMP, 10 μg/ml castanospermine, and 10 μg/ml deoxymannojirimycin, in the absence or presence of 50 μM control (acetyl-Gln-Tyr-Thr-CONH2) or acceptor peptide (acetyl-Asn-Tyr-Thr-CONH2) as indicated. After discarding the reaction buffer, cell bodies were scraped into methanol with sonication, followed by isolation of glycopeptides, free oligosaccharides, and LLOs for FACE analysis.
Statistics
Prism 7 (GraphPad Software, La Jolla, CA, USA) was used to calculate means ± sd and perform statistical analyses. Where 2 groups were compared, a 2-tailed Student’s t test was used. For multiple groups, ordinary 1-way ANOVA (no matching or pairing) was performed using Tukey’s multiple comparisons test. For both the ANOVA and the Student’s t test, P < 0.05 was considered significant.
RESULTS
HSV-1 as an experimental model for OST function
To understand the roles of OST isoforms in HSV-1 replication, we used NGI-1 and C19 to treat HEK293 cells with previously reported KOs of OST subunits: for STT3A-OST, its catalytic subunit STT3A and its accessory subunit DC2; for STT3B-OST, its catalytic subunit STT3B and its accessory oxidoreductase subunits MagT1 and TUSC3 (DKO) (23, 30). Glycoforms of envelope proteins gC and gD were resolved by gel electrophoresis and detected by immunoblotting with commercial antibodies. The nonglycosylated capsid protein VP5, also detected by immunoblotting, was used to quantify viral particles. Particle infectivity was measured in pfu after adjusting multiplicity of infection (MOI), depending upon the need to favor multi- (virus < cells) or single-cycle (virus > cells) infections (such as MOI = 0.1 or MOI = 20, respectively). In pfu assays, controls were arbitrarily scored as 100% and used for side-by-side comparisons of vehicle (control) vs. OST inhibitor treatment, or WT (control) vs. OST subunit KO.
OST inhibitors implicate the STT3A-OST isoform in HSV-1 envelope N-glycosylation
In healthy intact cells, NGI-1 inhibits both STT3A-OST and STT3B-OST (22, 29), whereas C19 is selective for STT3B-OST (21). Because HSV-1–infected cells may undergo extensive cytopathic effects to determine whether these inhibitors were also generally efficacious with disrupted cells, we employed a system for assaying OST isoforms using cells with permeabilized plasma membranes (35). Consistent with prior results from intact cells, in permeabilized cells, NGI-1 inhibited the transferase activities of both STT3A-OST and STT3B-OST as well as the LLO hydrolysis activity of STT3B-OST, and C19 inhibited the transferase and hydrolase activities of STT3B-OST (Fig. 1). This supported the use (below) of NGI-1 and C19 in cells disrupted by HSV-1 infection. We noted that unlike intact cells, C19 inhibited STT3A-OST in permeabilized cells. This apparent off-target effect may be related to the assay using high concentrations of small peptide acceptors presented in solution rather than cotranslationally, a situation not naturally encountered by STT3A-OST. As expected (35), STT3A-OST had no significant LLO hydrolysis activity, and neither NGI-1 nor C19 affected LLO synthesis.
Figure 1.
NGI-1 and C19 inhibit STT3A-OST and STT3B-OST enzymatic activities in plasma membrane–permeabilized cell assays. The plasma membranes of STT3A-KO and STT3B-KO HEK lines were permeabilized with ALO, followed by OST assays for transferase or LLO hydrolysis activity with DMSO (vehicle; no inhibitor), 5 μM C19, or 5 μM NGI-1. A) Representative FACE gels showing endo-H liberated glycans (retaining a single N-acetylglucosamine) from peptide transferase assays [upper gels, acceptor peptide (AP)], or glycans from LLO hydrolysis assays [retaining 2 N-acetylglucosamine residues, center gels, control peptide (CP)]. LLOs remaining after 30 min reactions are shown (lower gels). Positions of glycan standards are indicated. Left: STT3A-KO cells. Right: STT3B-KO cells. As expected (35), LLO hydrolysis in STT3B-KO cells is negligible and is suppressed by AP in STT3A-KO cells. B) Bar graphs quantifying transferase and hydrolysis results from multiple assays with significance values shown (ns, not significant).
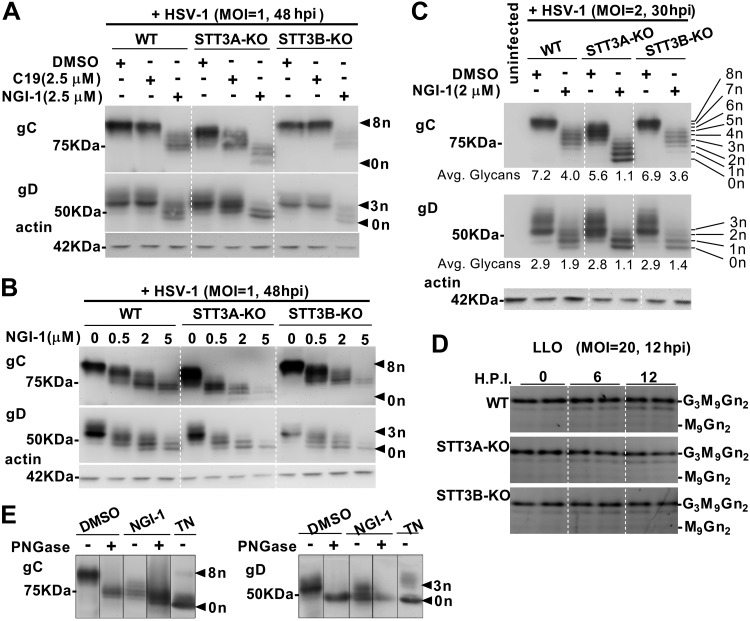
To test NGI-1 and C19 as inhibitors of HSV-1 envelope N-glycosylation, cells were infected at MOI of 1–2, and cultures were harvested 30–48 h postinfection (hpi). Widespread cytopathic effect was evident at harvest. Independent controls verified that the inoculating virus itself was undetectable in the subsequent assays. Shown in Fig. 2A, 2.5 μM C19 had a mild inhibitory effect toward glycosylation of gC and gD when only STT3B-OST was present (STT3A-KO cells) but no detectable effect when STT3A-OST was present (WT HEK and STT3B-KO cells). In contrast, 2.5 μM NGI-1 inhibited both gC and gD glycosylation whether they were dependent upon STT3A-OST (STT3B-KO cells) or STT3B-OST (STT3A-KO cells). Because C19 is selective for STT3B-OST, these results indicated that STT3A-OST was primarily responsible for gC and gD glycosylation, and were consistent with failure of STT3B-OST alone (STT3A-KO cells) to fully glycosylate gC.
Figure 2.
STT3A-OST is primarily responsible for glycosylating HSV-1 envelope proteins gC and gD. HEK293 lines were infected with HSV-1 with the indicated MOI and hpi conditions, and harvested by method A (Materials and Methods). A–C) gC and gD and host actin were analyzed with immunoblots after infection in the indicated concentrations of C19 or NGI-1. Nine gC glycoforms (0–8 N-glycans) and 4 gD glycoforms (0–3 N-glycans) were discerned. Glycosylation indices were calculated (C). D) LLO (M9Gn2-LLO through G3M9Gn2-LLO) from HEK lines infected for different times are shown. E) PNGase digestion of gC and gD from virus propagated in the absence or presence of 2 μM NGI-1 or 0.05 μg/ml TN added at the beginning of infection (MOI = 10; harvest at 16 hpi). Samples are from the same gels, with irrelevant lanes removed electronically as indicated by lines. Positions of 50 and 75 kDa markers are shown. gC is normally O-glycosylated, and we noted that PNGase-treated mature gC migrates slower than gC generated in the presence of TN, suggesting that gC that is not N-glycosylated in endoplasmic reticulum fails to acquire O-glycans. Some gD glycoforms migrate more slowly than expected for 3 N-glycans, which we attribute to processing in the Golgi apparatus because they appear to merge with unglycosylated gD after PNGase treatment. Avg., average.
Inhibition of gC and gD N-glycosylation was dependent upon NGI-1 concentration (Fig. 2B). Laddered glycoforms corresponding to 8 N-glycosylation sites on gC and 3 sites on gD could be discerned by comparing samples from the various infected and NGI-1–treated lines (Fig. 2C). Hypoglycosylation of gC and gD was most severe in NGI-1–treated STT3A-KO cells. We considered that HEK lines might become depleted of LLO in response to HSV-1 infection, contributing to hypoglycosylation. Due to extensive cytopathic effects and cell death at 30–48 hpi, reliable LLO analysis was not plausible under these conditions. Instead, we evaluated shorter periods (6 and 12 hpi) during 1 round of envelope glycoprotein synthesis, using a large viral excess (MOI = 20) to achieve infection of essentially every cell. Under this condition, we saw no evidence that HSV-1 infection of HEK cells caused LLO depletion or accumulation of LLO assembly intermediates (Fig. 2D). The total absence of glycans on some gC and gD glycoforms was corroborated with PNGase F and TN treatments (Fig. 2E). Electrophoresis of PNGase F–digested gC (but not gC from TN-treated cells) was slightly hindered, presumably due to remaining O-glycans (37). This suggested that O-glycan attachment and/or extension on gC was hindered when TN prevented acquisition of N-glycans in the endoplasmic reticulum.
In summary: 1) the dual STT3A/B-OST inhibitor NGI-1 impaired gC and gD N-glycosylation in WT, STT3A-KO, and STT3B-KO cells; 2) the STT3B-OST selective inhibitor C19 altered gC and gD N-glycosylation only in the absence of STT3A-OST; 3) KO of STT3A resulted in more hypoglycosylation of gC and gD than KO of STT3B, in the absence and presence of NGI-1; and 4) KO of STT3B alone caused no loss of gC or gD N-glycosylation. Taken together, STT3A-OST (acting cotranslationally) is of primary importance for N-glycosylation of gC and gD, whereas STT3B-OST (acting post-translationally) can partially substitute for an absence of STT3A-OST.
NGI-1 impairs HSV-1 infection
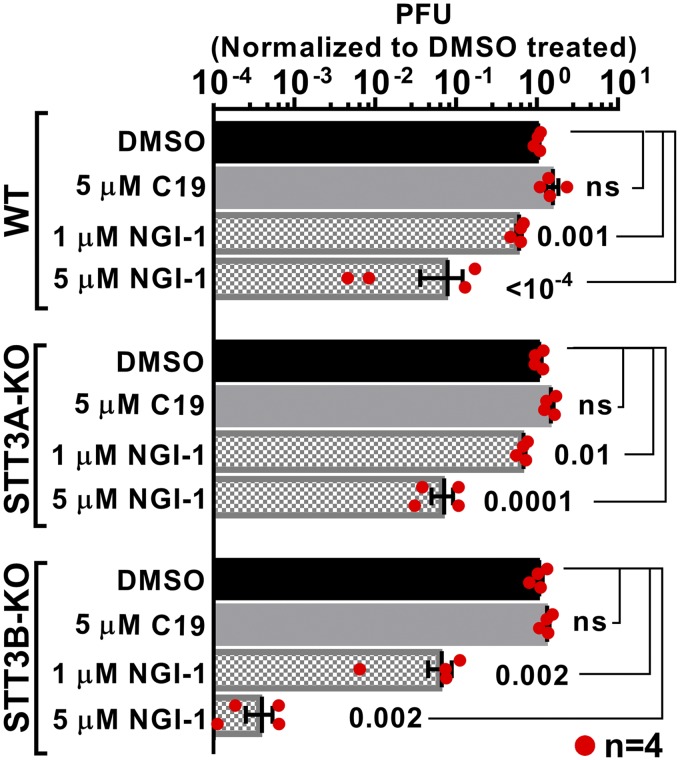
WT HEK, STT3A-KO, and STT3B-KO cells were next infected with HSV-1 in the absence or presence of NGI-1 or C19, for pfu assays on Vero cell monolayers. C19 (5 μM) had no effect on HSV-1 infectivity (Fig. 3). In contrast, over the general range (1–5 μM) that suppressed N-glycosylation, NGI-1 inhibited HSV-1 function in all 3 lines. Interestingly, NGI-1 suppressed infectivity most potently when STT3B-OST was ablated. Sensitivity of infectivity to NGI-1 did not have an obvious correlation with effects of NGI-1 on envelope protein glycosylation. Glycoform quantitation (glycosylation indices) in both Fig. 2C (MOI = 1–2) and Fig. 4 (MOI = 0.1) showed that 2 μM NGI-1 treatment of STT3A-KO cells resulted in more total loss of gC and gD N-glycosylation than with similarly treated STT3B-KO cells. However, the net suppression of gC and gD N-glycosylation by 2 μM NGI-1 treatment compared to DMSO controls was at least as strong for STT3A-KO cells as for STT3B-KO cells.
Figure 3.
NGI-1 treatment of STT3B-KO cells potently suppresses HSV-1 plaques. HSV-1 pfu were measured 48 hpi (MOI = 0.1) after treatment of WT, STT3A-KO, or STT3B-KO cells with 5 μM C19 or the indicated concentrations of NGI-1 and then harvested by method A. Pfu values for DMSO treatments were arbitrarily set at unity. Each bar represents n = 4 replicates, with statistical analysis (including use of a 2-tailed Student’s t test; ns, not significant) described in Materials and Methods.
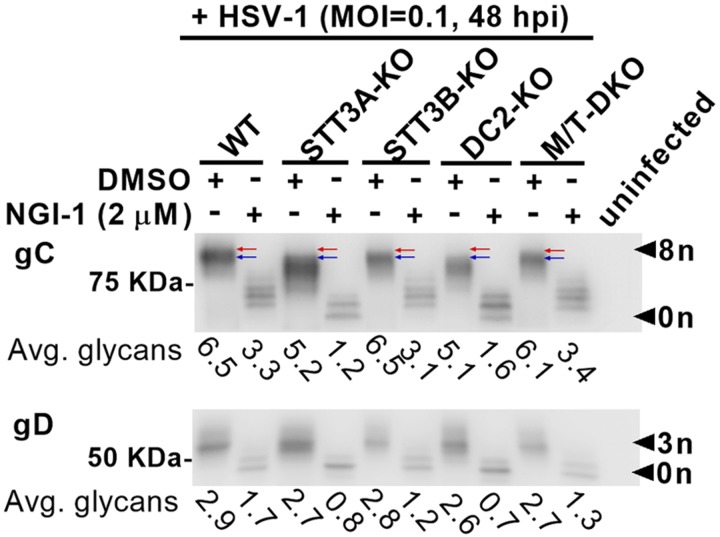
Figure 4.
KOs of OST accessory subunits corroborate effects of catalytic subunit KOs on HSV-1 glycosylation. WT HEK, HEK lines lacking STT3A-OST function (STT3A-KO and DC2-KO), and lines lacking STT3B-OST function (STT3B-KO and MagT1/TUSC3-DKO) were infected with HSV-1 (MOI = 0.1) in the absence or presence of 2 μM NGI-1. Extracts (method B, fraction II) for immunoblots of gC, gD, and VP5 were prepared 48 hpi. Glycosylation indices were calculated for all lanes. In the absence of NGI-1, red arrows distinguish the most highly glycosylated forms of gC visible in WT and STT3B-OST–impaired cells from the corresponding slightly hypoglycosylated forms of gC in STT3A-OST–impaired cells (blue arrows). In the presence of NGI-1, the most severe hypoglycosylation of both gC and gD occurred in STT3A-OST–deficient cells. Avg., average.
These results reaffirmed that NGI-1 can inhibit both STT3A-OST and STT3B-OST in HSV-1–infected HEK293 cells and that STT3A-OST is of primary importance for gC and gD N-glycosylation. Moreover, sensitivity of HSV-1 infectivity to pharmacological inhibition of STT3A-OST was greatest in the absence of STT3B-OST. We hypothesize that NGI-1 inhibits addition of N-glycans on HSV-1 that are particularly important for infectivity and/or affects a host cell process involved in viral replication. In either case, 2 predictions would follow: Other manipulations of co- and post-translational N-glycosylation should emulate the results obtained by knocking out OST catalytic subunits, and NGI-1 treatment should link STT3A-OST activity to a specific aspect of HSV-1 infection.
Manipulation of OST accessory subunits supports a primary role for STT3A-OST in HSV-1 N-glycosylation
Work with flaviviruses showed that although both STT3A-OST and STT3B-OST were necessary for dengue virus infection, these enzyme complexes acted as structural scaffolds for replicating viral components rather than as catalytic OSTs per se (26, 27, 29). This counterintuitive result was echoed by showing that NGI-1 impaired dengue virus replication by interaction with OST independent of its enzymatic activity (29). Therefore, we sought alternatives to genetic manipulation of STT3A and STT3B to further query the roles of OST complexes in HSV-1 infection.
Beside their namesake catalytic subunits, the STT3A-OST and STT3B-OST complexes each have isoform-specific accessory subunits. STT3A-OST contains 2 subunits (DC2 and KCP2) involved in physical association with the translocon, and necessary for efficient cotranslational N-glycosylation (30). STT3B-OST contains only 1 additional isoform-specific subunit, alternatively MagT1 or TUSC3, that prevents thiols in fully translated acceptors from forming disulfides that interfere with access to sequons at the catalytic site (38). This includes acceptors with Asn-Xaa-Ser/Thr sequons near disulfides, or rare Asn-Xaa-Cys sequons. However, the oxidoreductase subunits are also required for STT3B-OST acceptors having extreme C-terminal sequons unaffected by disulfide bonds (39). This indicates that MagT1/TUSC3 might assist STT3B-OST by helping place some acceptors at the transferase catalytic site, independent of its oxidoreductase properties.
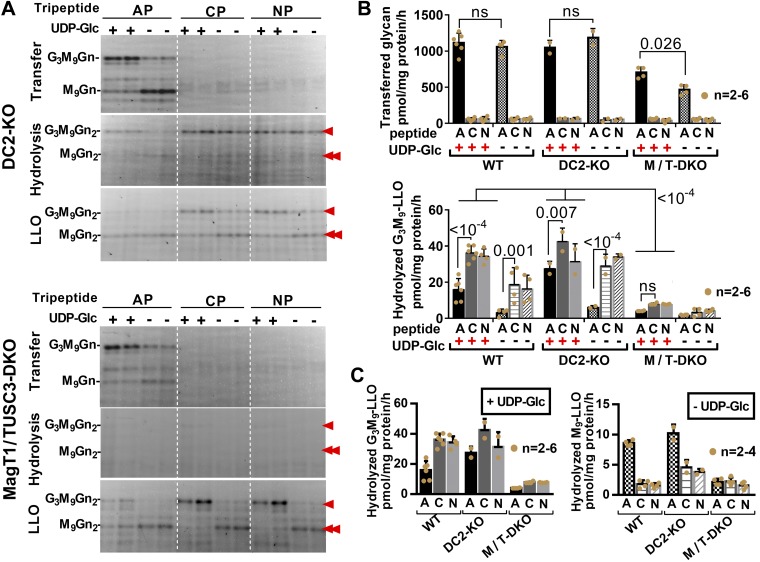
Although DC2-deficient STT3A-OST cannot access polypeptides cotranslationally, its catalytic site is active toward small synthetic acceptors in detergent-based assays (30). Similarly, MagT1/TUSC3-deficient STT3B-OST is ineffective for post-translational glycosylation, but solubilized and isolated STT3B-OST from which oxidoreductase subunits have dissociated retains transferase activity toward small synthetic acceptors (40). Thus, in live cells, accessory subunit-deficient OSTs emulate their catalytic subunit-deficient counterparts, although they retain active catalytic subunits. Furthemorer, as presented in Fig. 5, the membrane-bound OST transferase activities of DC2 and MagT1/TUSC3-deficient permeabilized cells resembled their solubilized counterparts, forming G3M9Gn2 products with synthetic acceptor peptides. DC2-KO cells had transferase activity levels similar to STT3A-KO cells but approximately double the activity of MagT1/TUSC3-DKO cells which, in turn, were similar to the previously reported activity in STT3B-KO cells (35). LLO hydrolysis in MagT1/TUSC3-DKO cells was greatly reduced and also reminiscent of STT3B-KO cells. By a number of criteria, absences of either accessory subunits or catalytic subunits of OST isoforms consequently resulted in similar consequences for glycosylation.
Figure 5.
OST complexes lacking accessory subunits phenocopy catalytic subunit KOs in enzyme assays. OST transferase and LLO hydrolysis activities (see Fig. 1) were assayed in WT, DC2-KO, and MagT1/TUSC3-DKO lines. AP, CP, and NP indicate acceptor, control, and no peptide, respectively. Assays were performed in the absence or presence of UDP-Glc to favor synthesis of M9Gn2-LLO (double red arrowhead) or G3M9Gn2-LLO (single red arrowhead), respectively. Even without UDP-Glc, some G3M9Gn2-LLO is evident (35). A) Representative FACE gels. Like STT3B-KO cells, LLO hydrolysis was nearly negligible in MagT1/TUSC3-DKO cells. Like STT3A-KO cells, DC2-KO cells expressing intact STT3B-OST had less hydrolysis of M9Gn2-LLO in the absence of acceptor peptide (35). B) Quantification of transferase and G3M9Gn2-LLO hydrolysis assays. These results were part of a larger data set, from which the WT data (35) are reproduced in this figure to aid the reader, and which included FACE images of primary WT data, as well as FACE and graphed data obtained with STT3A-OST and STT3B-OST cells. C) “+UDP-Glc” and “−UDP-Glc” data are replotted for comparisons of G3M9Gn2-LLO with M9Gn2-LLO hydrolysis, emphasizing that M9Gn2-LLO hydrolysis in DC2-KO cells [like STT3A-KO cells (35)] is counterintuitively increased by acceptor peptide; ns, not significant.
As shown in Fig. 4, KO of DC2 caused mild hypoglycosylation of gC, comparable to that in STT3A-KO cells. In contrast, gC glycosylation appeared normal with KOs of STT3B and MagT1/TUSC3. Treatment with 2 μM NGI-1 resulted in similarly robust hypoglycosylation of both gC and gD in STT3A-KO and DC2-KO cells. By comparison, hypoglycosylation of gC and gD caused by NGI-1 in both STT3B-KO and MagT1/TUSC3-DKO cells was milder. Results with DC2-KO therefore supported the above conclusion that HSV-1 envelope protein glycosylation was most sensitive to NGI-1 when STT3A-OST function was genetically impaired. With the criteria reported here and previously (30) taken together, we conclude that in infected cells, DC2-deficient STT3A-OST complexes behave functionally as if the STT3A subunit is absent, and MagT1/TUSC3-deficient STT3B-OST complexes behave as if the STT3B subunit is absent.
HSV-1 infectivity, not particle number, is primarily susceptible to NGI-1 in cells lacking STT3B-OST function
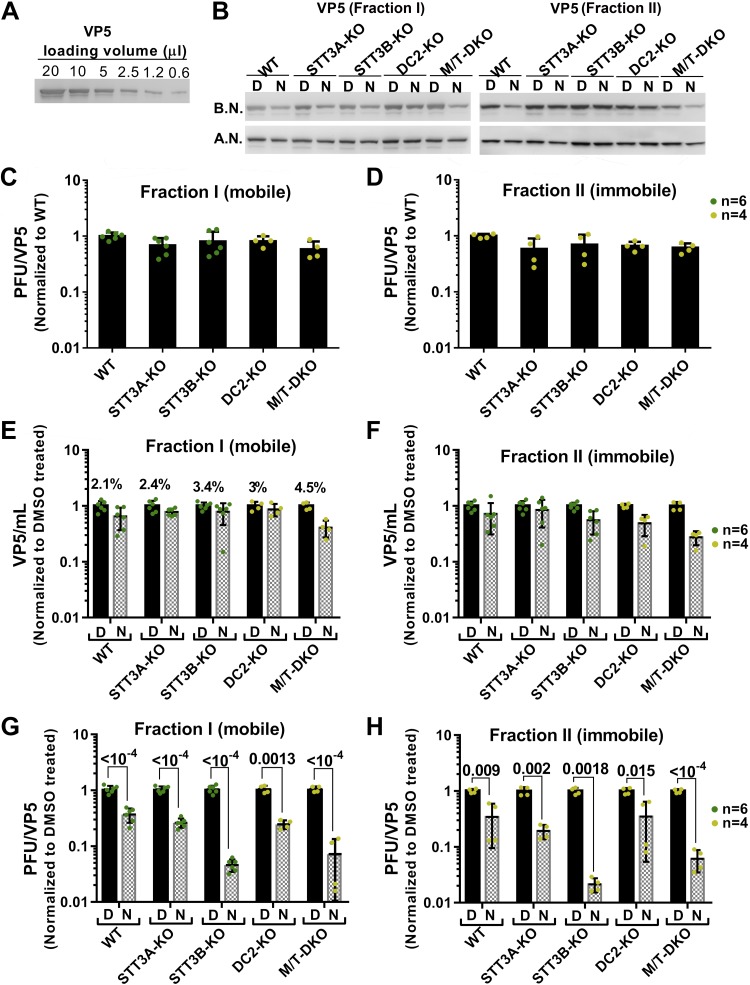
HEK293 KOs of OST catalytic and accessory subunits were next used to identify the basis for suppression of HSV-1 plaque formation by NGI-1 shown in Fig. 3, which did not distinguish between effects on viral infectivity or viral particle number. As presented in Fig. 6, the nonglycosylated capsid protein VP5 was immunoblotted as a measure of viral particle quantity, with a 2-step process to ensure reliability of VP5 quantification (Fig. 6A, B). Unlike the experiment displayed in Fig. 3 (using method A, Materials and Methods), in Fig. 6, HSV-1 was separated into 2 fractions (method B): loosely associated virus (fraction I, mobile) recovered by gentle pipetting of the conditioned culture medium over the cell monolayer (Fig. 6B, C, E, G), and tightly associated virus (fraction II, immobile) recovered by subsequently scraping and sonicating the remaining monolayer (Fig. 6B, D, F, H). Our goal was to distinguish HSV-1 particles that had exited cells (fraction I) and were possibly more infectious from HSV-1 that remained intracellular or trapped within cell debris, including any premature particles (fraction II).
Figure 6.
NGI-1 primarily suppresses HSV-1 infectivity, not particle yield, in STT3B-OST–impaired cells. The conditions presented in Fig. 4 were also used here (MOI = 0.1, harvest at 48 hpi, 0–2 μM NGI-1). A) Immunoblot with different volumes of viral lysate mixed 5:1 with concentrated loading buffer, showing how VP5 detection varied appropriately with viral quantity. B) Examples of immunoblots of VP5 used with the following panels before normalization (B.N.) and after normalization (A. N.) by a 2-step process (Materials and Methods). Left: fraction I [relatively mobile particles loosely associated with HEK293 monolayers (C, E, G)]. Right: fraction II [relatively immobile particles avidly associated with HEK293 monolayers (D, F, H)]. C, D) Infectivity (pfu divided by VP5) of virus harvested from vehicle-treated WT cells was arbitrarily set to unity and compared with infectivity of virus harvested from vehicle-treated KO lines. E, F) Particle yields (VP5 divided by lysate volume) of virus from vehicle-treated lines were arbitrarily set to unity, and compared with particle yields after NGI-1 treatment. G, H). Infectivities (pfu divided by VP5) of virus harvested from vehicle-treated cell lines were arbitrarily set to unity, and compared with infectivities after NGI-1 treatment. As indicated by percentage values above control bars (DMSO, “D”) (E), based upon VP5 content fraction I was a minor portion of total viral particles (G, H). For both fractions, NGI-1 (bars labeled “N”) had its most potent effects with cells lacking STT3B-OST activity and mainly caused HSV-1 dysfunction (G, H) rather than particle loss (E, F). Data (C–H) represent n = 4–6 as indicated, with statistical analysis (including use of a 2-tailed Student’s t test) described in Materials and Methods.
HSV-1 particle recoveries from various HEK293 lines exhibited considerable day-to-day variability. This required that infectivity data be appropriately normalized before results from multiple independent experiments could be integrated. For example, for all cell lines, effects of NGI-1 on particle (VP5) yield were normalized to results with vehicle (DMSO) treatments done at the same time (Fig.6 E, F). For infectivity, pfu values were normalized to VP5 and used in either of 2 ways. In some cases, the normalized pfu data from various vehicle-treated KO lines were compared with vehicle-treated WT controls done at the same time (Fig. 6C, D). Alternatively, normalized pfu data for individual cell lines after NGI-1 treatment were compared with normalized vehicle data obtained at the same time (Fig. 6G, H).
Compared with WT HEK293 cells, KOs of OST catalytic or accessory subunits had only modest effects on infectivity of virus in multicycle infections (Fig. 6C, D). Additionally, 2 μM NGI-1 had mild effects compared with vehicle on particle yields in the various HEK293 WT and KO lines (Fig. 6E, F). These findings held true for both the mobile and immobile viral fractions [the latter being more abundant and with similar relative ratios among cell types (Fig. 6E)]. Taken together HSV-1 infection and morphogenesis in HEK293 host cells was fairly tolerant to partial impairment of N-glycosylation by either genetic or pharmacologic means. In contrast, NGI-1 treatment combined with OST subunit KO caused substantially more suppression of infectivity than the individual maneuvers alone (Fig. 6G, H), depending upon the OST isoform that was impaired. A particularly synergistic inhibition of HSV-1 infectivity was observed with NGI-1 treatment of cells lacking STT3B-OST function by KO of either STT3B or MagT1/TUSC3. The loss of infectivity resulting from NGI-1 treatment of cells lacking STT3A-OST function (STT3A or DC2 KOs), however, was similar to that of WT cells.
The results presented in Fig. 6 confirmed and extended the findings of Fig. 3. NGI-1 was most potent as an inhibitor of HSV-1 infectivity with cells in which post-translational glycosylation was already ablated by loss of an essential catalytic or accessory STT3B-OST subunit. In this scenario, skipped opportunities for cotranslational glycosylation that were due to NGI-1 action could not be compensated post-translationally. The primary effect of combining NGI-1 treatment with genetic hindrance of STT3B-OST function in HEK293 cells was to prevent attachment of viral and/or host N-glycans necessary for subsequent HSV-1 infectivity with Vero cells. However, because particle numbers produced by the HEK293 KO lines were not strongly affected, it appears that impaired infectivity was cell-type specific (see Discussion).
Culture variability affects HSV-1 glycosylation
Although normalized pfu data were highly reproducible over multiple independent experiments (Figs. 3 and 6), raw (unnormalized) pfu data had large variations among cultures seeded on different days. For insight into factors that might vary on an experiment-to-experiment basis to influence HSV-1 replication, we reexamined a previously unexplained loss of glycosylation of HSV-1 envelope proteins occurring late in infection (2, 41). Specifically, gB and gC became largely unglycosylated 8–10 hpi in Vero cells and 20–24 hpi in human epithelial type 2 cells (41). Similarly, gD appeared unglycosylated 18 hpi in human epithelial type 2 cells (2). A hallmark of these experiments was an acute transition from fully glycosylated to nonglycosylated viral glycoform populations, with little accumulation of detectable intermediate glycoforms.
Here we designate this phenomenon “late-stage hypoglycosylation” (LSH). By screening culture conditions (Fig. 7), we observed: 1) LSH occurred with gC and gD in WT, STT3A-KO, and STT3B-KO cells without appreciable accumulation of intermediate glycoforms (Fig. 7A); 2) LSH was suppressed by glucose supplementation (Fig. 7A) or with large volumes of medium to delay glucose depletion, and correlated with <5 mM glucose (Fig. 7B); 3) G3M9Gn2-LLO was partially depleted when LSH occurred (Fig. 7C), although we did not detect accumulation of unassembled LLO intermediates; 4) LSH was exacerbated with aged HEK293 cells after continuous culture for ∼4 mo, which appeared to have less contact inhibition and more rapid growth than fresh cultures; 5) at MOI ≥10, LSH was evident at 24 hpi but not at 12 hpi; and 6) unlike the unfolded protein response (42, 43), the glucose depletion and LLO loss associated with LSH were not accelerated by viral infection (Fig. 7D).
Figure 7.
Late-stage HSV-1 hypoglycosylation is emulated by glucose depletion with LLO loss. LSH was emulated by plating 5 × 105 cells in 12-well plates with 1 ml DMEM plus 10% FBS. Twenty four hours later, cells were infected with HSV-1 at MOI = 1 or MOI = 10, and harvested 24 hpi. A) WT, STT3A-KO, and STT3B-KO cells were cultured with either 5 or 25 mM glucose. gC, gD (with glycoforms indicated), and VP5 were detected by immunoblotting. One set of samples was incubated with 2 μg/ml TN. B) Glucose concentrations in media either prior to infection or 24 hpi, for DMEM formulated with 5 or 25 mM glucose. C) G3M9Gn2-LLO at 24 hpi, normalized to the value for 5 mM glucose medium. D) Glucose consumption by WT HEK cells grown in medium with 5 mM glucose and infected with HSV-1 at MOI = 1 or 10 for 12 or 24 h. Calibration of the assay showed that the limit of sensitivity was 98–99% consumption (i.e., 1–2% glucose remaining); ns, not significant.
Taken together, we hypothesize that the LSH of HSV-1 envelope proteins previously reported (2, 41) might have been due to LLO loss resulting from glucose depletion under conventional culture conditions. To exclude a potential influence of LSH from the HSV-1 experiments displayed in Figs. 2–4 and 6, we therefore routinely verified that gC in vehicle-treated WT cells was fully N-glycosylated, and, where indicated, we used media with high (25 mM) glucose. The absence of intermediate glycoforms in LSH strongly contrasted with the glycoform laddering of gC and gD obtained with NGI-1 (Figs. 2 and 4). The basis for this difference is unclear, but one possibility is that during glucose depletion, glucose concentrations supporting intermediate glycoforms might exist for only a brief time, with the majority of envelope proteins produced either before (i.e., fully glycosylated) or after (i.e., unglycosylated) this interval.
DISCUSSION
In this study, we tested whether blocking N-glycosylation by targeting host OSTs hinders HSV-1 infection. NGI-1, an inhibitor of both STT3A-OST and STT3B-OST in cellular glycosylation assays (21, 22) and biochemical transferase assays (35), inhibited N-glycosylation of HSV-1 envelope proteins gC and gD. By utilizing NGI-1 with KOs of OST catalytic subunits, we found that STT3A-OST had a primary role for glycosylating HSV-1 envelope proteins but that STT3B-OST could partly substitute. These results were corroborated with C19 (21), a second-generation OST inhibitor selective for STT3B-OST. Importantly, NGI-1 suppressed HSV-1 infection in culture mainly by reducing infectivity of viral particles, not particle number, and did so more strongly with cells lacking post-translational glycosylation machinery (STT3B-KO and MagT1/TUSC3-DKO) than with cells possessing it (WT, STT3A-KO, and DC2-KO).
To better understand how NGI-1 affected HSV-1, we took advantage of the dysfunctional phenotypes of OST complexes missing isoform-specific accessory subunits [(23, 30, 40) and Fig. 5]. HSV-1 abnormalities in cells missing DC2, a subunit of STT3A-OST, resembled abnormalities in cells missing STT3A. Similarly, HSV-1 abnormalities in cells missing MagT1/TUSC3, alternative subunits of STT3B-OST, exhibited abnormalities resembling those in cells lacking STT3B. OST catalytic subunits, therefore, support HSV-1 replication (and are the targets of NGI-1) by providing an important catalytic function, not a noncatalytic structural role. This contrasted with the finding that noncatalytic structural features of OST catalytic subunits were critical for dengue virus replication (26, 27). Glycosylation indices for gC (Figs. 2 and 4) revealed similar net losses of N-glycosylation due to NGI-1 treatment of WT, STT3A-OST–deficient, and STT3B-OST–deficient cells. Although this demonstrates that NGI-1 is similarly efficacious in both HSV-1–infected and uninfected cells for STT3A-OST and STT3B-OST, it remains to be determined why NGI-1 had its greatest effects on HSV-1 infectivity in STT3B-OST–deficient cells. The results are further complicated by the possibility that NGI-1 affects host factors, as well as an HSV-1 envelope proteins, involved in infection. Overall, chemical inhibition of cotranslational glycosylation in cells lacking post-translational backup for skipped sequons seems particularly detrimental to HSV-1.
HSV-1 dysfunction resulting from NGI-1 treatment was primarily manifested as a loss of pfus on Vero cell monolayers rather than a loss of viral particles recovered from HEK293 lines even in experiments (Figs. 4 and 6) designed for 2–3 cycles of infection. No appreciable difference was detected for effects of NGI-1 on mobile or immobile viral fractions, further indicating that hypoglycosylation did not result in altered viral spread or release in HEK293 lines. We suggest that although NGI-1 does not strongly reduce HSV-1 particles produced by STT3B-OST deficient HEK lines, such particles might inefficiently adhere to or fuse with Vero cells for infection. Consequently, the N-oligosaccharide units of HSV-1 envelope proteins might participate in cell specificity for infection. Alternatively, it is feasible that hypoglycosylated particles adhere or fuse with Vero cells, but that a downstream step necessary for viral replication or distribution fails. For example, HSV-1 induced cell fusion and syncytia formation increase with gK hypoglycosylation because of point mutation (13). NGI-1 treatment of HEK293 lines might similarly yield hypoglycosylated HSV-1 particles that infect Vero cells and replicate, but instead of causing cell death, the resultant nascent particles might lead to syncytia that do not result in typical plaques. Such an alternative, however, seems unlikely. Plaque assays were performed in the absence of NGI-1, so it would be necessary to invoke a “memory effect,” allowing hypoglycosylation of infecting HSV-1 particles to somehow interfere with later replication or dispersal. Indeed, inspection of Vero monolayers infected with large ranges of particles from the various DMSO- and NGI-1–treated HEK293 lines revealed only crystal violet-negative plaques with typical morphology, included some adjacent small syncytia. Large independent syncytia were not detected.
Our results indicate that the potential antiviral range of NGI-1 type “pan-OST” agents extend beyond flaviviruses and have unanticipated implications for the treatment of, and host response to, HSV-1 infection. In addition to any effect of hypoglycosylation on HSV-1 infectivity or replication, which may be cell-type specific (infectivity with Vero cells, but not HEK293 lines, was mainly altered) NGI-1 type agents could also increase HSV-1 particle immunogenicity by elimination of oligosaccharides that block immune recognition of the underlying polypeptide [the so-called viral glycan shield (44, 45)]. NGI-1 is expected to cause accumulation of mixtures of hypoglycosylated HSV-1 envelope proteins, but as discussed above (Fig. 7), depending upon local conditions, a portion may also be completely unglycosylated. Clinically, HSV-1 infections can be treated but not cured in part because viral pathogenesis involves neurotropic latency. We envision that OST inhibitors like NGI-1 administered topically at infected lesions could not only help resolve the infection but also yield unshielded viral particles to aid immune recognition and attenuate subsequent infections, with minimal risk of systemic toxicity.
Finally, we revisited the unexplained hypoglycosylation of gC and gD late in HSV-1 infection, first reported 35–40 yr ago (2, 41), in which envelope protein pools appeared to abruptly shift from fully glycosylated to fully unglycosylated. We identified a similar glycosylation shift that correlated with depletion of both medium glucose and cellular LLOs, which occurred over time under conventional culture conditions, and which was not accelerated by viral infection itself. The idea that diminished LLO levels lead to unglycosylated envelope proteins may provide insight into the evolution of human genetic congenital disorders of glycosylation (CDGs) (46). The CDGs encompass over 100 hereditary recessive disorders, many of which block steps in LLO synthesis. Homozygous and compound heterozygous CDG patients have varied clinical abnormalities that include neurologic, developmental, and metabolic problems. Heterozygous individuals (carriers) appear free of disease, and, in culture, their cells lack obvious glycosylation abnormalities. However, defective CDG alleles provide no known selective advantage for heterozygotes. We speculate that by capping the maximum ability to produce LLOs in virally infected cells, defective CDG alleles in heterozygous carriers might promote presentation of unglycosylated viral envelope proteins for enhanced immune recognition.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health (NIH) National Institute of General Medical Sciences Grants R01-GM038545 (to M.A.L.), R01-GM043768 (to R.G.), R01-GM127383 (to J.N.C.), and NIH National Cancer Institute Grant R01-CA172391 (to J.N.C.). Experiments with HSV-1 were performed with the oversight of the University of Texas Southwestern Medical Center Office of Safety and Business Continuity. J.N.C. has ownership interest (including stock, patents, etc.) in NGI-1. The remaining authors declare no other conflicts of interest.
Glossary
- 2DG
2-deoxyglucose
- ALO
anthrolysin O
- BHK
baby hamster kidney
- CDG
congenital disorder of glycosylation
- DKO
double KO
- FACE
fluorophore-assisted carbohydrate electrophoresis
- FBS
fetal bovine serum
- G3M9Gn2
glucose3mannose9N-acetylglucosamine2-P-P-dolichol
- HEK
human embryonic kidney
- hpi
hour postinfection
- HSV-1
herpes simplex virus 1
- KO
knockout
- LLO
lipid-linked oligosaccharide
- LSH
late-stage hypoglycosylation
- MagT1
magnesium transporter 1
- MOI
multiplicity of infection
- OST
oligosaccharyltransferase
- pfu
plaque-forming unit
- PNGase F
N-glycosidase F
- TN
tunicamycin
- TUSC3
tumor suppressor candidate 3
- UDP-Glc
uridine diphosphate–glucose
- WT
wild type
AUTHOR CONTRIBUTIONS
H. Lu performed the research; H. Lu, N. A. Cherepanova, R. Gilmore, J. N. Contessa, and M. A. Lehrman analyzed the data; and H. Lu and M. A. Lehrman designed the research and wrote the manuscript.
REFERENCES
- 1.Bagdonaite I., Nordén R., Joshi H. J., Dabelsteen S., Nyström K., Vakhrushev S. Y., Olofsson S., Wandall H. H. (2015) A strategy for O-glycoproteomics of enveloped viruses--the O-glycoproteome of herpes simplex virus type 1. PLoS Pathog. 11, e1004784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spear P. G. (1976) Membrane proteins specified by herpes simplex viruses. I. Identification of four glycoprotein precursors and their products in type 1-infected cells. J. Virol. 17, 991–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen G. H., Long D., Eisenberg R. J. (1980) Synthesis and processing of glycoproteins gD and gC of herpes simplex virus type 1. J. Virol. 36, 429–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Courtney R. J., Steiner S. M., Benyesh-Melnick M. (1973) Effects of 2-deoxy-D-glucose on herpes simplex virus replication. Virology 52, 447–455 [DOI] [PubMed] [Google Scholar]
- 5.Knowles R. W., Person S. (1976) Effects of 2-deoxyglucose, glucosamine, and mannose on cell fusion and the glycoproteins of herpes simplex virus. J. Virol. 18, 644–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campadelli-Fiume G., Poletti L., Dall’Olio F., Serafini-Cessi F. (1982) Infectivity and glycoprotein processing of herpes simplex virus type 1 grown in a ricin-resistant cell line deficient in N-acetylglucosaminyl transferase I. J. Virol. 43, 1061–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tal-Singer R., Peng C., Ponce De Leon M., Abrams W. R., Banfield B. W., Tufaro F., Cohen G. H., Eisenberg R. J. (1995) Interaction of herpes simplex virus glycoprotein gC with mammalian cell surface molecules. J. Virol. 69, 4471–4483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kühn J. E., Eing B. R., Brossmer R., Munk K., Braun R. W. (1988) Removal of N-linked carbohydrates decreases the infectivity of herpes simplex virus type 1. J. Gen. Virol. 69, 2847–2858 [DOI] [PubMed] [Google Scholar]
- 9.Sodora D. L., Cohen G. H., Eisenberg R. J. (1989) Influence of asparagine-linked oligosaccharides on antigenicity, processing, and cell surface expression of herpes simplex virus type 1 glycoprotein D. J. Virol. 63, 5184–5193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sodora D. L., Eisenberg R. J., Cohen G. H. (1991) Characterization of a recombinant herpes simplex virus which expresses a glycoprotein D lacking asparagine-linked oligosaccharides. J. Virol. 65, 4432–4441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sodora D. L., Cohen G. H., Muggeridge M. I., Eisenberg R. J. (1991) Absence of asparagine-linked oligosaccharides from glycoprotein D of herpes simplex virus type 1 results in a structurally altered but biologically active protein. J. Virol. 65, 4424–4431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tal-Singer R., Eisenberg R. J., Valyi-Nagy T., Fraser N. W., Cohen G. H. (1994) N-linked oligosaccharides on herpes simplex virus glycoprotein gD are not essential for establishment of viral latency or reactivation in the mouse eye model. Virology 202, 1050–1053 [DOI] [PubMed] [Google Scholar]
- 13.Rider P. J. F., Naderi M., Bergeron S., Chouljenko V. N., Brylinski M., Kousoulas K. G. (2017) Cysteines and N-glycosylation sites conserved among all alphaherpesviruses regulate membrane fusion in herpes simplex virus 1 infection. J. Virol. 91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagdonaite I., Wandall H. H. (2018) Global aspects of viral glycosylation. Glycobiology 28, 443–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu X., Zeng Y., Lehrman M. A. (1992) Evidence that the hamster tunicamycin resistance gene encodes UDP-GlcNAc:dolichol phosphate N-acetylglucosamine-1-phosphate transferase. J. Biol. Chem. 267, 8895–8902 [PubMed] [Google Scholar]
- 16.Lehrman M. A. (1991) Biosynthesis of N-acetylglucosamine-P-P-dolichol, the committed step of asparagine-linked oligosaccharide assembly. Glycobiology 1, 553–562 [DOI] [PubMed] [Google Scholar]
- 17.Marciniak S. J., Ron D. (2006) Endoplasmic reticulum stress signaling in disease. Physiol. Rev. 86, 1133–1149 [DOI] [PubMed] [Google Scholar]
- 18.Patterson S. I., Skene J. H. P. (1994) Novel inhibitory action of tunicamycin homologues suggests a role for dynamic protein fatty acylation in growth cone-mediated neurite extension. J. Cell Biol. 124, 521–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurtoglu M., Gao N., Shang J., Maher J. C., Lehrman M. A., Wangpaichitr M., Savaraj N., Lane A. N., Lampidis T. J. (2007) Under normoxia, 2-deoxy-D-glucose elicits cell death in select tumor types not by inhibition of glycolysis but by interfering with N-linked glycosylation. Mol. Cancer Ther. 6, 3049–3058 [DOI] [PubMed] [Google Scholar]
- 20.Merchan J. R., Kovács K., Railsback J. W., Kurtoglu M., Jing Y., Piña Y., Gao N., Murray T. G., Lehrman M. A., Lampidis T. J. (2010) Antiangiogenic activity of 2-deoxy-D-glucose. PLoS One 5, e13699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rinis N., Golden J. E., Marceau C. D., Carette J. E., Van Zandt M. C., Gilmore R., Contessa J. N. (2018) Editing N-glycan site occupancy with small-molecule oligosaccharyltransferase inhibitors. Cell Chem. Biol. 25, 1231–1241.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez-Sambrooks C., Shrimal S., Khodier C., Flaherty D. P., Rinis N., Charest J. C., Gao N., Zhao P., Wells L., Lewis T. A., Lehrman M. A., Gilmore R., Golden J. E., Contessa J. N. (2016) Oligosaccharyltransferase inhibition induces senescence in RTK-driven tumor cells. Nat. Chem. Biol. 12, 1023–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cherepanova N. A., Gilmore R. (2016) Mammalian cells lacking either the cotranslational or posttranslocational oligosaccharyltransferase complex display substrate-dependent defects in asparagine linked glycosylation. Sci. Rep. 6, 20946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruiz-Canada C., Kelleher D. J., Gilmore R. (2009) Cotranslational and posttranslational N-glycosylation of polypeptides by distinct mammalian OST isoforms. Cell 136, 272–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braunger K., Pfeffer S., Shrimal S., Gilmore R., Berninghausen O., Mandon E. C., Becker T., Förster F., Beckmann R. (2018) Structural basis for coupling protein transport and N-glycosylation at the mammalian endoplasmic reticulum. Science 360, 215–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin D. L., Cherepanova N. A., Bozzacco L., MacDonald M. R., Gilmore R., Tai A. W. (2017) Dengue virus hijacks a noncanonical oxidoreductase function of a cellular oligosaccharyltransferase complex. MBio 8, e00939-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marceau C. D., Puschnik A. S., Majzoub K., Ooi Y. S., Brewer S. M., Fuchs G., Swaminathan K., Mata M. A., Elias J. E., Sarnow P., Carette J. E. (2016) Genetic dissection of Flaviviridae host factors through genome-scale CRISPR screens. Nature 535, 159–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang R., Miner J. J., Gorman M. J., Rausch K., Ramage H., White J. P., Zuiani A., Zhang P., Fernandez E., Zhang Q., Dowd K. A., Pierson T. C., Cherry S., Diamond M. S. (2016) A CRISPR screen defines a signal peptide processing pathway required by flaviviruses. Nature 535, 164–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puschnik A. S., Marceau C. D., Ooi Y. S., Majzoub K., Rinis N., Contessa J. N., Carette J. E. (2017) A small-molecule oligosaccharyltransferase inhibitor with pan-flaviviral activity. Cell Reports 21, 3032–3039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shrimal S., Cherepanova N. A., Gilmore R. (2017) DC2 and KCP2 mediate the interaction between the oligosaccharyltransferase and the ER translocon. J. Cell Biol. 216, 3625–3638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao N., Holmes J., Lehrman M. A. (2013) Letter to the glycoforum: improved protocols for preparing lipid-linked and related saccharides for fluorophore-assisted carbohydrate electrophoresis (FACE). Glycobiology 23, 1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao N., Lehrman M. A. (2006) Non-radioactive analysis of lipid-linked oligosaccharide compositions by fluorophore-assisted carbohydrate electrophoresis. Methods Enzymol. 415, 3–20 [DOI] [PubMed] [Google Scholar]
- 33.Gao N., Shang J., Huynh D., Manthati V. L., Arias C., Harding H. P., Kaufman R. J., Mohr I., Ron D., Falck J. R., Lehrman M. A. (2011) Mannose-6-phosphate regulates destruction of lipid-linked oligosaccharides. Mol. Biol. Cell 22, 2994–3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gay A., Rye D., Radhakrishnan A. (2015) Switch-like responses of two cholesterol sensors do not require protein oligomerization in membranes. Biophys. J. 108, 1459–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu H., Fermaintt C. S., Cherepanova N. A., Gilmore R., Yan N., Lehrman M. A. (2018) Mammalian STT3A/B oligosaccharyltransferases segregate N-glycosylation at the translocon from lipid-linked oligosaccharide hydrolysis. Proc. Natl. Acad. Sci. USA 115, 9557–9562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anand M., Rush J. S., Ray S., Doucey M. A., Weik J., Ware F. E., Hofsteenge J., Waechter C. J., Lehrman M. A. (2001) Requirement of the Lec35 gene for all known classes of monosaccharide-P-dolichol-dependent glycosyltransferase reactions in mammals. Mol. Biol. Cell 12, 487–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson D. C., Spear P. G. (1983) O-linked oligosaccharides are acquired by herpes simplex virus glycoproteins in the Golgi apparatus. Cell 32, 987–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cherepanova N. A., Shrimal S., Gilmore R. (2014) Oxidoreductase activity is necessary for N-glycosylation of cysteine-proximal acceptor sites in glycoproteins. J. Cell Biol. 206, 525–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shrimal S., Trueman S. F., Gilmore R. (2013) Extreme C-terminal sites are posttranslocationally glycosylated by the STT3B isoform of the OST. J. Cell Biol. 201, 81–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelleher D. J., Karaoglu D., Mandon E. C., Gilmore R. (2003) Oligosaccharyltransferase isoforms that contain different catalytic STT3 subunits have distinct enzymatic properties. Mol. Cell 12, 101–111 [DOI] [PubMed] [Google Scholar]
- 41.Compton T., Courtney R. J. (1984) Evidence for post-translational glycosylation of a nonglycosylated precursor protein of herpes simplex virus type 1. J. Virol. 52, 630–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pouysségur J., Shiu R. P., Pastan I. (1977) Induction of two transformation-sensitive membrane polypeptides in normal fibroblasts by a block in glycoprotein synthesis or glucose deprivation. Cell 11, 941–947 [DOI] [PubMed] [Google Scholar]
- 43.Shiu R. P., Pouyssegur J., Pastan I. (1977) Glucose depletion accounts for the induction of two transformation-sensitive membrane proteinsin Rous sarcoma virus-transformed chick embryo fibroblasts. Proc. Natl. Acad. Sci. USA 74, 3840–3844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lavie M., Hanoulle X., Dubuisson J. (2018) Glycan shielding and modulation of hepatitis C virus neutralizing antibodies. Front. Immunol. 9, 910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doores K. J. (2015) The HIV glycan shield as a target for broadly neutralizing antibodies. FEBS J. 282, 4679–4691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ng B. G., Freeze H. H. (2018) Perspectives on glycosylation and its congenital disorders. Trends Genet. 34, 466–476 [DOI] [PMC free article] [PubMed] [Google Scholar]