Abstract
T cells expressing invariant γδ antigen receptors (γδ T cells) bridge innate and adaptive immunity and facilitate barrier responses to pathogens. Macrophage migration inhibitory factor (MIF) is an upstream mediator of host defense that up-regulates the expression of pattern recognition receptors and sustains inflammatory responses by inhibiting activation-induced apoptosis in monocytes and macrophages. Surprisingly, Mif−/− γδ T cells, when compared with wild type, were observed to produce >10-fold higher levels of the proinflammatory cytokine IL-17 after stimulation with gram-positive exotoxins. High–IL-17 expression was associated with the characteristic features of IL-17–producing γδ T (γδ17) cells, including expression of IL-23R, IL-1R1, and the transcription factors RORγt and Sox13. In the gram-positive model of shock mediated by toxic shock syndrome toxin (TSST-1), Mif−/− mice succumbed to death more quickly with increased pulmonary neutrophil accumulation and higher production of cytokines, including IL-1β and IL-23. Mif−/− γδ T cells also produced high levels of IL-17 in response to Mycobacterium lipomannan, and depletion of γδ T cells improved survival from acutely lethal Mycobacterium infection or TSST-1 administration. These data indicate that MIF deficiency is associated with a compensatory amplification of γδ17 cell responses, with implications for innate immunity and IL-17–mediated pathology in situations such as gram-positive toxic shock or Mycobacterium infection.—Kim, H. K., Garcia, A. B., Siu, E., Tilstam, P., Das, R., Roberts, S., Leng, L., Bucala, R. Macrophage migration inhibitory factor regulates innate γδ T-cell responses via IL-17 expression.
Keywords: MIF, TSST-1, M. bovis
The cytokine IL-17 plays an important role in immunity to infection and is involved in the inflammatory pathology of different autoimmune conditions (1–3). IL-17 regulates neutrophil homeostasis and increases neutrophil recruitment into inflammatory sites (4, 5), and although most studies have focused on IL-17–producing CD4 T cells, which express the αβ T-cell receptor (TCR) and are synonymous with T helper 17 (Th17) cells, T cells that express invariant γδ TCR (γδ T cells) also produce IL-17. IL-17–producing γδ T (γδ17) cells exhibit many of the differentiation hallmarks of Th17 cells, such as expression of the IL-23 and CCR6 receptors and the transcription factor RORγt (6, 7).
γδ T cells account for no more than 5% of lymphocytes in the circulation or in secondary lymphoid tissues and have an important role in the innate immune response against Mycobacterium (8, 9), Escherichia coli (10), Listeria (11, 12), and Staphylococci (13). The γδ TCR does not engage major histocompatibility complex–antigen complexes in the same manner as the αβ TCR and its ligands are less well characterized; stimulatory responses may follow from pattern recognition of bacterial phosphoantigens and the endogenous products of cell damage (14). These γδ T cells also contribute to the resolution of infection by facilitating bacterial clearance at barrier sites through neutrophil, macrophage, and NK-cell recruitment (15).
Macrophage migration inhibitory factor (MIF) is a pleiotropic innate cytokine that is released from preformed intracellular pools upon stimulation by microbial products or by cellular stress signals (16, 17). MIF up-regulates pattern recognition receptor expression by macrophages (18), counter-regulates the immunosuppressive effects of glucocorticoids (19), and sustains proinflammatory responses by inhibiting activation-induced p53-dependent apoptosis (18). MIF is centrally involved in the pathogenesis of acute respiratory distress syndrome (20) and septic shock (21) and contributes to chronic inflammatory conditions such as asthma (22) and systemic lupus erythematosus (23). Notably, MIF deficiency is protective in mouse models of infection in which inflammation contributes to pathogen dissemination or tissue damage (24, 25) but is deleterious to those infections in which inflammatory mechanisms are essential for pathogen clearance (26–30). In models of Mycobacterium infection, for instance, Mif−/− mice succumb more quickly from failure of macrophage function (31).
Inflammatory or infectious challenge of Mif-deficient mice uncovers features of immunodeficiency in hosts that are otherwise phenotypically normal; in selected cases, similar deficiencies have been reported to occur in low–genotypic MIF–expressing human subjects (31, 32). In the course of gene expression–profiling studies in mice, the genetic absence of Mif was unexpectedly observed to be associated with markedly higher production of IL-17 by γδ T cells. Mif−/− γδ T cells showed a more inflammatory Th17-like phenotype with up-regulated expression of IL-17A, IL-23R, IL-1R1, and the transcription factor RORγt. In vivo, Mif−/−mice demonstrated altered innate responses, including increased numbers of neutrophils and augmented γδ17-cell responses induced by gram-positive exotoxins or Mycobacterium infection.
MATERIALS AND METHODS
Experimental mice
Wild-type (WT) C57BL/6 and BALB/c mice were purchased from the National Cancer Institute (Bethesda, MD, USA). Mif−/− mice in the C57BL/6 or BALB/c backgrounds (32, 33) were bred by homozygous mating in a pathogen-free facility and were utilized with age and gender matching to the WT. γδ−/− Mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) (12). All animals were used for experiments at 8–12 wk of age. All procedures performed in these experiments complied with federal and Yale University guidelines. The γδ TCR depletion was performed by using anti-γδ TCR mAb as previously described by Usami et al. (34).
Murine model of exotoxin shock
Toxic shock syndrome toxin 1 (TSST-1) and streptococcal pyrogenic exotoxin A (SPEA) were obtained from Toxin Technology (Sarasota, FL, USA). The toxins were resuspended in pyrogen-free water at a concentration of 1 mg/ml, divided into aliquots, and stored at −80°C. WT or Mif−/− mice in the BALB/c background were weighed and randomly distributed into groups of 4–5 animals of equal mean body weight. For survival experiments, mice were injected intraperitoneally with TSST-1 (1 mg/kg body weight) and D-galactosamine (0.9 g/kg body weight; MilliporeSigma, Burlington, MA, USA) diluted into 0.25 ml saline (35). Mice were observed for at least 7 d. For all other experiments, mice were injected intraperitoneally with TSST-1 (1 mg/kg body weight) and euthanized at the indicated time points. For in vitro infection experiments, splenocytes from WT or Mif−/− mice in the BALB/c background were incubated for 24 or 72 h with TSST-1 or SPEA at concentrations ranging from 0.01 to 100 ng/ml. Cytokine production was determined by flow cytometry and ELISA using antibodies from Thermo Fisher Scientific (Waltham, MA, USA).
M. bovis infection and histopathology
M. bovis infection was performed with the bacillus Calmette-Guérin strain Connaught, grown to log phase in Middlebrook 7H9 broth, 0.2% glycerol, and 0.05% Tween 80 (31). The viable bacterial numbers were determined using a Middlebrook 7H10 agar plate supplemented with oleic acid–albumin-dextrose-catalase enrichment (BD Biosciences, San Jose, CA, USA). Briefly, WT or Mif−/− mice in the C57BL/6 background were infected intranasally with ∼1 × 105 CFU M. bovis in 30 μl PBS. M. bovis–infected mice were housed in biologic safety level 2 plus pathogen-free conditions. For in vitro infection experiments, splenocytes were plated in 6-well tissue culture plates and were infected with M. bovis, multiplicity of infection 50:1 for 24 or 72 h. For histopathology, segments of lung tissue were fixed in 10% buffered formalin (MilliporeSigma) and paraffin embedded. Histologic sections were stained with hematoxylin-eosin or Ziehl-Neelsen acid-fast stain for evaluation of pathology and mycobacterial load, respectively, as previously described (31).
Cell preparation
Cells in the bronchoalveolar lavage fluid (BAL) were recovered at the indicated time points. Briefly, the BAL was collected with PBS (0.8 ml × 2). Cells in the BAL were collected and resuspended in complete Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% fetal bovine serum. Single-cell suspensions from lung tissue were prepared by incubating minced tissue with collagenase IV (1 mg/ml; Worthington Biochemical, Lakewood, NJ, USA) and DNase I (25–50 U/ml; MilliporeSigma) for 1 h at 37°C. The digested lung was further disrupted by passage through a 70-μm strainer (BD Biosciences). Spleens and lymph nodes were harvested at the indicated days postinfection, homogenized, and passed through a 70-μm strainer to obtain single-cell suspensions. Red blood cells were lysed with ammonium-chloride-potassium lysis buffer. Prepared cells were used for in vitro culture or flow cytometric analysis. Bone marrow–derived macrophages (BMDMs) were prepared by differentiating cells flushed from the femur and tibias of BALB/c mice of the appropriate genotype (WT and Mif−/−) in the presence of supernatant from L929 cells for 1 wk. 1 × 106 BMDMs were plated in 12-well tissue culture plates and allowed to adhere overnight. Purified lipomannan (LM), NR-14850 from Mycobacterium tuberculosis strain H37Rv Biological and Emerging Infections (BEI) Resources, National Institute of Allergy and Infections Diseases, Bethesda, MD, USA], at a concentration of 1 μg/ml was added to the culture for 18 h. Culture supernatant was harvested for ELISA.
Magnetic cell separation
The γδ TCR T cells were purified by a 2-step procedure with magnetic-activated cell sorting beads (Miltenyi Biotec, Bergisch Gladbach, Germany). In brief, the B cells and macrophages were depleted using biotin-conjugated anti-B220 and anti-CD11b mAbs and anti-biotin microbeads used for depletion, then TCR γδ T cells were indirectly magnetically labeled with anti-biotin microbeads and isolated by positive selection from pre-enriched T-cell fraction. In some experiments, CD4 T cells were purified by magnetic selection (Miltenyi Biotec).
Microarray expression analysis
Spleen and lymph node cells from WT and Mif−/− mice were prepared and stimulated with LM at 1 mg/ml. At 18 h after stimulation, total RNA was isolated using the RNeasy Kit (Qiagen, Hilden, Germany). Afterward, cDNA and then cRNA were prepared, and cRNA was hybridized to the MouseWG-6 Bead-Chip (Illumina, San Diego, CA, USA) according to the manufacturer’s instructions. The hybridized chips were scanned using the Illumina BeadArray reader, and the images were analyzed with Beadstudio software. Data were downloaded into Partek Genomic Suite (Partek, St. Louis, MO, USA) for analysis. Principal component analysis was used to determine the relationships between the samples in the 4 groups: WT-untreated control, WT treated with LM, Mif−/−-untreated control, and Mif−/− treated with LM. A list of genes was extracted from the full transcriptional profile analysis by a combination of statistical testing of absolute and relative changes in expression across the different experimental conditions and controlling for false discovery in multiple testing, with genes with a false discovery rate of <0.05 and a fold change of >1.4 being considered differentially expressed. Statistical analysis between experimental groups was performed using a Student’s t test. Gene expression data are available upon publication in the International MIF Consortium database (http://imc.isd-muc.de/index.php/data-protocols-databases).
ELISA and real-time RT-PCR
Cytokine levels in BAL or culture supernatant from lungs and spleens were measured by ELISA (Thermo Fisher Scientific). Single-cell suspensions (2–4 × 106 cells/500 μl) from lungs or spleens were incubated in 24-well plates (Corning, Corning, NY. USA) with 1 μg/ml ionomycin (MilliporeSigma) and 50 ng/ml phorbol myristate acetate (MilliporeSigma) for 4 h. Culture supernatants were collected and stored at −20°C until analysis. IL-1β, IL-17A, IL-23 (Thermo Fisher Scientific), and macrophage inflammatory protein 2 (MIP-2) (R&D Systems, Minneapolis, MN, USA) were assayed by ELISA kits. For real-time RT-PCR, cDNA was reverse-transcribed from total RNA with random hexamers and Superscript II RT (Thermo Fisher Scientific). The synthesized first-strand cDNA was amplified using real-time PCR thermal cycler iCycler iQ (Bio-Rad, Hercules, CA, USA). The amplified PCR products were quantified by detecting SYBR Green incorporation. The primers were purchased from Qiagen (QuantiTect Primer Assays). The data from real-time RT-PCR were analyzed using Real-Time PCR Optical System Software (Bio-Rad). The cycle number at which the various transcripts became detectable, referred to as threshold cycle (Ct), was compared with that of glyceraldehyde 3-phosphate dehydrogenase and referred to as ΔCt.
Flow cytometry
Cells were pretreated with anti–FcγR II/III antibody (BD Biosciences) and then were surface stained with CD3-phycoerythrin–cyanine (Cy) 7, CD4–Pacific Blue, CD8–allophycocyanin–Cy7, γδ TCR–adenomatous polyposis coli, CD11b–peridinin-chlorophyll protein–Cy5.5, and Gr-1–FITC (Thermo Fisher Scientific). To analyze the cytokine expression, the cells were incubated with 1 μg/ml ionomycin and 50 ng/ml phorbol myristate acetate for 4 h in the presence of GolgiPlug (BD Biosciences). The surface-stained cells were subjected to intracellular cytokine staining with phycoerythrin-conjugated anti–IL-17A mAbs postpermeabilization by using Cytofix/Cytoperm kits (BD Biosciences). Stained cells were analyzed on LSR II or FACSCalibur flow cytometers (BD Bioscience). Data were analyzed with FlowJo software (TreeStar, Ashland, OR, USA).
Statistical analysis
Data were analyzed for normality of distribution, and significance was assessed by unpaired 2-tailed Student’s t test. Survival analysis was performed with Gehan-Breslow-Wilcoxon testing. Values of P < 0.05 were considered significant, and data shown represent replicate experimental results.
RESULTS
γδ T cells from Mif−/− mice express high levels of IL-17
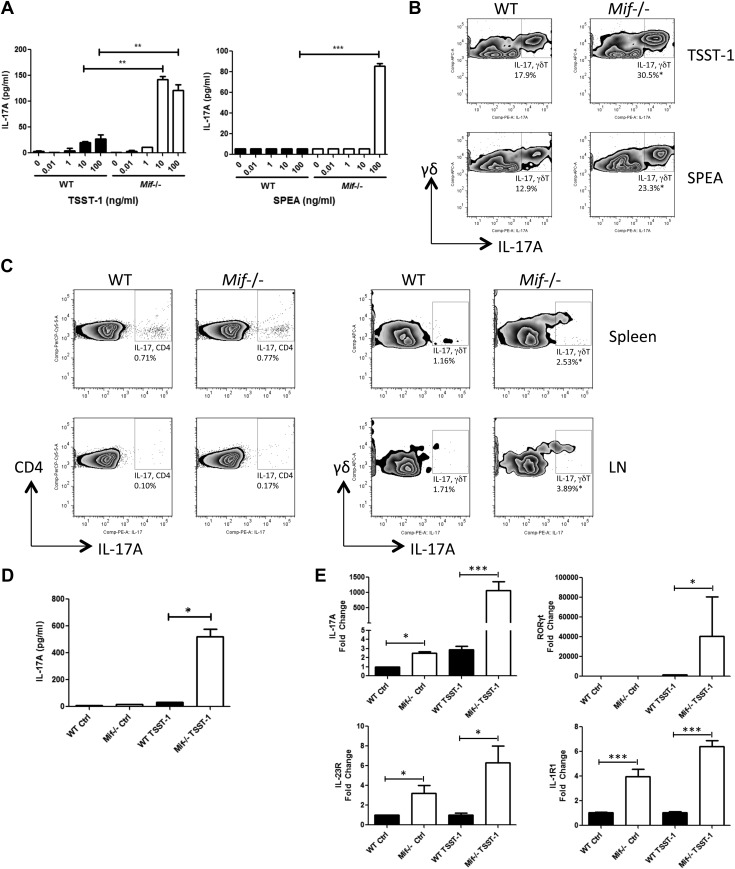
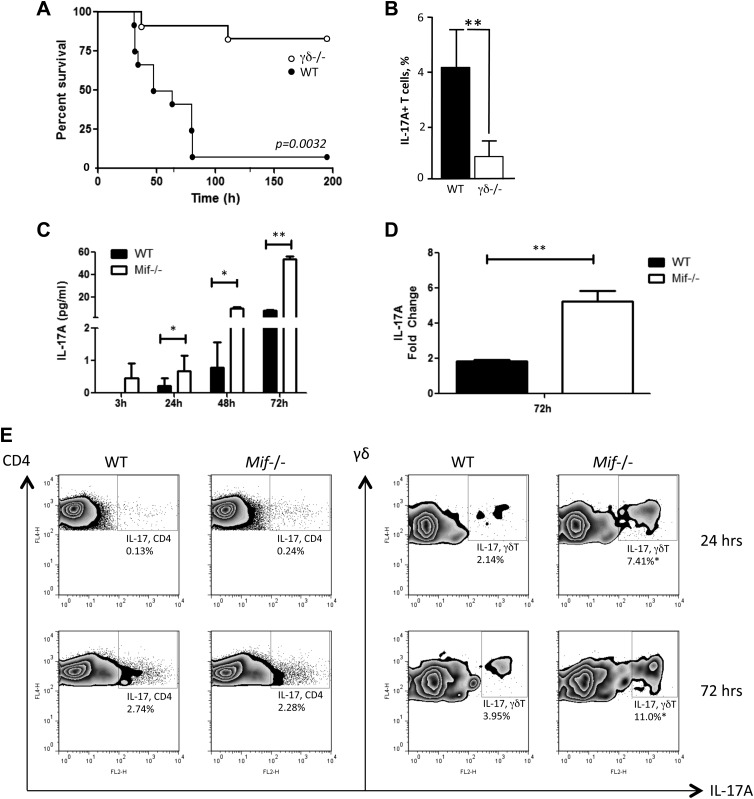
Gram-positive bacterial exotoxins such as TSST-1 or SPEA induce high-level secretion of proinflammatory cytokines including MIF from macrophages or T lymphocytes (35–38). We stimulated splenocytes from WT and Mif−/− mice with TSST-1 or SPEA and harvested the conditioned medium for measurement of IL-17. As shown in Fig. 1A, splenocytes from Mif−/− mice produced >10-fold higher quantities of IL-17 at 10–100 ng/ml of these exotoxins when compared with splenocytes from WT mice. Examination of the cellular phenotype by flow cytometry revealed a TSST-1– or SPEA-stimulated induction of IL-17 by both αβ T cells and γδ T cells, with higher IL-17 production by cells obtained from Mif−/− than from WT mice (Fig. 1B, measured as the IL-17A isoform). To examine IL-17 production in vivo, WT or Mif−/− mice were injected with TSST-1, the spleens and lymph nodes were harvested 72 h later, and single-cell suspensions were prepared for analysis. Consistent with the in vitro data, both αβ and γδ T cells from spleens and lymph nodes produced IL-17, and the level of IL-17 production by the CD3+CD4+ T-cell population was similar in WT and Mif−/− mice. By contrast, γδ T cells from Mif−/− mice produced IL-17 at >2 times frequency than those from WT mice (Fig. 1C).
Figure 1.
Mif-dependent production of IL-17A in response to gram-positive Staphylococcus TSST-1 and SPEA. Spleen cells from WT or Mif−/− BALB/c mice were incubated with TSST-1 or SPEA at various concentrations. A, B) After 72 h, IL-17A levels in the supernatants were measured by ELISA (A), and IL-17A production in γδ T cells was determined by flow cytometry (B). C) WT or Mif−/− BALB/c mice were injected i.p. with 1 mg/kg of TSST-1, and the spleen and draining lymph node cells were collected 72 h later and analyzed by flow cytometry for CD4, γδ TCR, and IL-17A without further stimulation. D, E) γδ T cells were isolated from spleens or lymph nodes (LNs) by magnetic cell sorting 72 h after toxin injection in vivo; supernatant IL-17A levels were measured by ELISA (D), and RNA was extracted for qPCR analysis for IL-17A, RORγt, IL-23R, and IL-1R1 (E). Comp, compensation; ctrl, control; PE, phycoerythrin. Representative flow cytometry plots are shown from 3 independent experiments. Error bars denote sd. *P < 0.05, **P < 0.01, ***P < 0.001 (unpaired Student’s t test).
To examine IL-17 production by isolated γδ T cells, spleens from mice injected 72 h previously with TSST-1 were harvested and the γδ T cells purified by immunomagnetic selection. Significantly more IL-17 production was observed in supernatants of γδ T cells isolated from Mif−/− mice than WT mice (Fig. 1D). We further examined in isolated γδ T cells the expression of genes known to be associated with Th17 cell differentiation. The characteristic mRNA transcripts for Th17 cells (e.g., IL-17A, the transcription factor RORγt, and the cytokine receptors IL-23R and IL-1R1) were more highly up-regulated by TSST-1 in γδ T cells from Mif−/− mice than in γδ T cells from WT mice (Fig. 1E). These results indicate a strong regulatory influence of MIF in suppressing a Th17-like or γδ17 cell expression profile (39).
Mif−/− mice show increased numbers of neutrophils, greater cytokine production, and reduced survival following TSST-1 injection
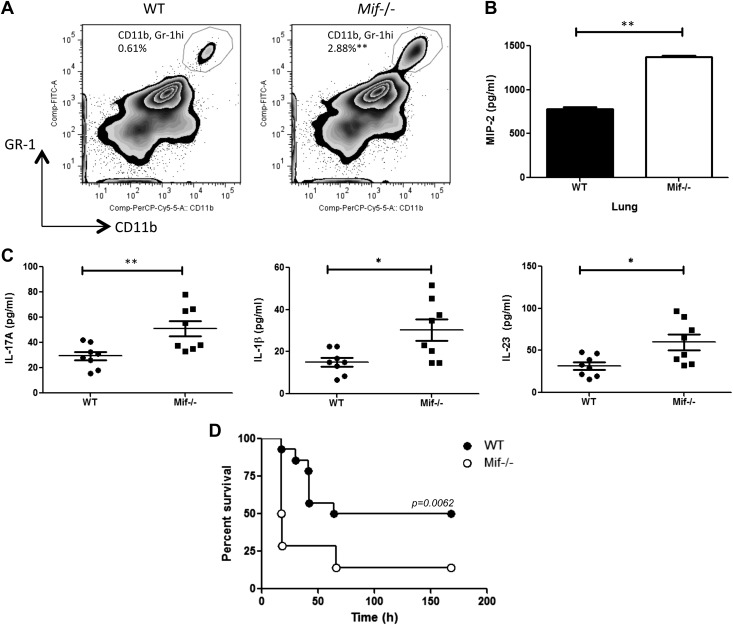
IL-17 regulates innate responses in part by promoting the recruitment and activation of neutrophils, and excessive neutrophilic inflammation underlies the lethal response to gram-positive exotoxins (4). To investigate the phenotypic impact of an Mif-deficient γδ17 cell response in exotoxin-mediated inflammation, we examined neutrophil mobilization in the lungs of mice that received systemic TSST-1, which leads to acute lung injury (40). An increased number of neutrophils was observed in the BAL of Mif−/− mice when compared with their WT counterparts at 72 h after TSST-1 injection (Fig. 2A). A higher concentration of the neutrophil chemoattractant MIP-2 [chemokine (C-X-C motif) ligand 2 (CXCL2)] (41) was evident in the lungs of Mif−/− mice (Fig. 2B), and there was increased production of IL-17A and the IL-17+ T-cell stimulatory cytokines IL-1β and IL-23 in the bronchoalveolar lavage of Mif−/− lungs than in WT lungs (Fig. 2C). That Mif−/− mice are more sensitive to IL-17+ γδ T cell–mediated inflammation was supported by analyzing the survival of TSST-1–injected mice. As shown in Fig. 2D, overall survival was 50% in WT mice but only 14% in Mif−/− mice, with 74% of the Mif−/− mice succumbing to lethality within 24 h of toxin administration.
Figure 2.
Mif−/− mice show increased pulmonary neutrophil accumulation, greater cytokine production, and lower survival after TSST-1 injection. WT or Mif−/− BALB/c mice were injected intraperitoneally with 1 mg/kg of TSST-1. A) BAL and lung cells were prepared at 72 h postinjection, and neutrophil recruitment was examined by flow cytometry. B, C) Levels of the neutrophil chemoattractant MIP-2 in the lungs (B), and cytokine levels in BAL determined by ELISA (C). Data are representative of 2 replicates. *P < 0.05, **P < 0.01, by unpaired Student’s t test. D) WT BALB/c or Mif−/− mice were injected intraperitoneally with 1 mg/kg of TSST-1 and 0.9 mg/kg of d-galactosamine and monitored for survival. PerCP, peridinin-chlorophyll protein. Data are representative of 2 experiments with n = 10 mice per group. P = 0.0062 (Gehan-Breslow-Wilcoxon Test).
Increased expansion of γδ17 cells in response to IL-1β or IL-23 in Mif−/− vs. WT mice
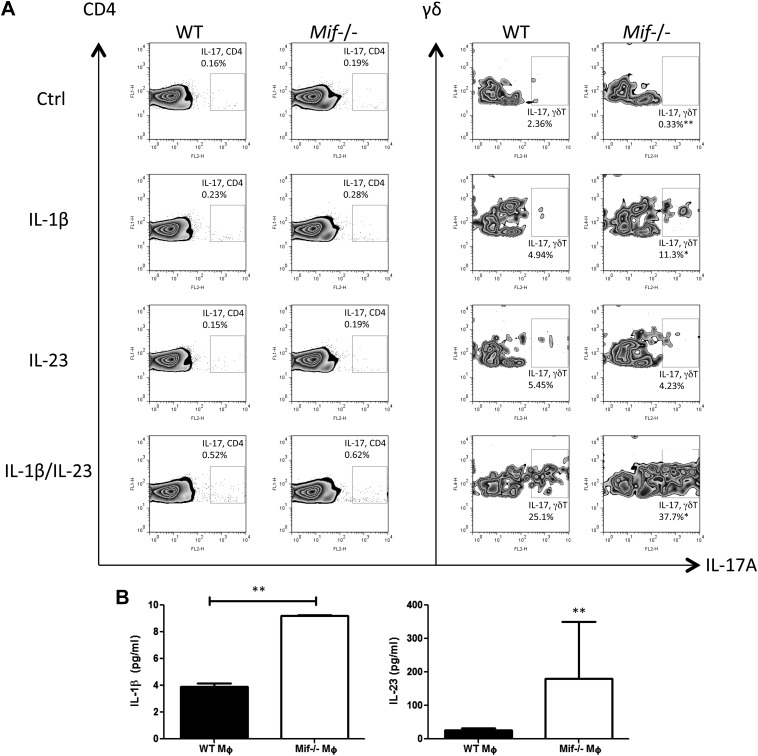
The inducible expression of receptors for IL-1β and IL-23 (Fig. 1E) together with prior evidence for a role for these receptors in TCR-independent IL-17 production by γδ17 cells (6) prompted us to examine the impact of IL-1β and IL-23 stimulation in MIF-dependent IL-17 expression. We found that IL-1β induced a greater level of IL-17 production by Mif−/− than by WT γδ T cells, with the most prominent effect observed after stimulation by both IL-1β and IL-23 (Fig. 3A). These results are consistent with a potential role for the up-regulated expression of IL-1R1 and IL-23R in the Mif-dependent expansion of γδ17 cells.
Figure 3.
IL-1β and IL-23 promote IL-17A production from γδ T cells in the absence of TCR engagement. A) Spleen cells from WT or Mif−/− BALB/c mice were stimulated with 20 ng/ml of IL-1β, 20 ng/ml of IL-23, or both for 72 h and stained for intracellular IL-17A and for surface CD3, CD4, and γδ TCRs. Numbers refer to percentage of CD3+CD4+IL-17A+ cells or CD3+γδTCR+IL-17A+ cells. B) LM induces IL-1β and IL-23 production by macrophages. BMDMs from WT or Mif−/− BALB/c mice were stimulated with 1 µg/ml of LM for 18 h. IL-1β and IL-23 concentrations in the supernatants were quantified by ELISA. Comp, compensation; ctrl, control; FL2-H, flourescence 2 channel height; Gr, granulocyte. Data shown are representative of 3 replicates with n = 4–5 per group. *P < 0.05, **P < 0.01 (unpaired Student’s t test).
IL-1β and IL-23 typically are produced by the innate response to pathogen-associated molecular patterns. Because γδ17 cells are activated during Mycobacterium infection (8), we tested whether the mycobacterial glycolipid LM stimulated IL-1β or IL-23 expression in Mif−/− macrophages. BMDMs from WT or Mif−/− BALB/c mice were stimulated with 1 µg/ml of LM in culture. As shown in Fig. 3B, macrophages from Mif−/− mice produced higher amounts of IL-1β and IL-23 into conditioned medium when compared with macrophages from WT mice, suggesting that innate stimuli serve to support the expansion the γδ17 cell population observed in Mif−/− mice.
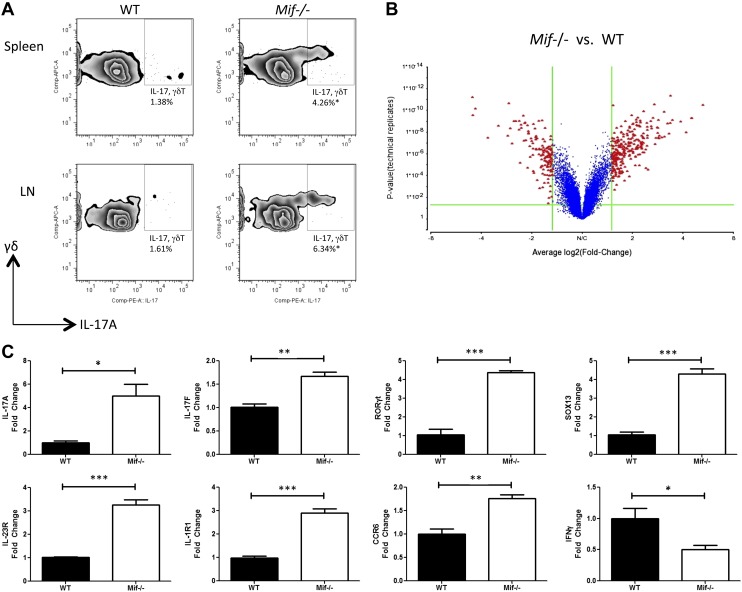
γδ T cells from Mif−/− mice show a γδ17 cell gene expression profile and increased IL-17 production in response to Mycobacterium stimulation
To explore more closely the cellular processes regulated by MIF that are associated with the development of γδ17 cells, we performed a comparative transcriptional microarray analysis of mRNA isolated from γδ T cells of WT and Mif−/− C57BL/6 mice. As shown in Fig. 4A, increased numbers of γδ17 cells were detected in spleen and lymph node cells from Mif−/− mice compared with WT mice after stimulation in vitro with LM. The γδ T cells then were isolated by magnetic cell sorting after stimulation with LM for 18 h, and mRNA was extracted and analyzed by microarray. Using an unbiased statistical and fold-change filtering procedure, an illustrative volcano plot was generated (Fig. 4B), and the highest-scoring genes in the IL-17 pathway are shown in Table 1. Of note, an up-regulation of Sox13, Rorc, Cd44, Ccr6, and Il-17f, which are known to be associated with Th17 and γδ17 cells, was evident, and a reduced level of Ifng was observed (7, 42). These changes were each independently confirmed by quantitative PCR (qPCR) analysis of replicate RNA samples (Fig. 4C). As expected, an increase in the expression levels of IL-17A, IL-23R, and IL-1R1 also was observed. This mRNA expression analysis is consistent with the interpretation that MIF is a central regulator of IL-17 production by γδ T cells cells in vivo.
Figure 4.
Higher production of IL-17A in γδ T cells of Mif−/− mice in response to the Mycobacterium glycolipid, LM. Spleen and lymph node (LN) cells of WT or Mif−/− C57BL/6 mice were stimulated in vitro with 1 µg/ml LM. A) After 72 h of culture, IL-17A expression was determined by flow cytometry. For microarray and real-time PCR analysis, the γδ T cells were separated by magnetic cell sorting after 18 h of culture. B) The microarray results are shown as a volcano plot. C) The levels of IL-17A, IL-17F, RORγt, SOX13, IL-23R, IL-1R1, CCR6, and IFN-γ mRNA were examined by qPCR. APC, allophycocyanin; comp, compensation; PE, phycoerythrin. Error bars denote sd. All data shown are representative of 3 replicates. *P < 0.05, **P < 0.01, ***P < 0.001 (unpaired Student’s t test).
TABLE 1.
Highest-scoring genes in the IL-17 pathway observed by transcriptional profiling of LM-treated Mif−/− vs. LM-treated WT γδ T cells
| Gene | LM-treated Mif−/− vs. LM-treated WT γδ T cells (fold change) | Description |
|---|---|---|
| Sox13 | 2.20 | SRY-box containing gene 13 (Mus musculus, mRNA) |
| Rorc | 2.60 | retineic-acid-receptor-related orphan receptor γ (M. musculus, mRNA) |
| Ccr6 | 1.61 | chemokine (C-C motif) receptor 6 (M. musculus, mRNA) |
| Il-17f | 1.48 | IL-17F (M. musculus, mRNA) |
| Ifng | −1.47 | Ifng (M. musculus, mRNA) |
Transcriptional fold change was calculated and normalized for the respective untreated controls. Genes were examined that were up- or down-regulated >1.4-fold in Mif−/− compared with WT cells, and the highest scoring IL-17 pathway–related genes are listed. Source file available at http://imc.isd-muc.de/index.php/data-protocols-databases.
To better understand the significance of the relationship between Mif and γδ17 cells, we next studied an established C57BL/6 mouse model of M. bovis (bacillus Calmette-Guérin) infection, which induces an increase in the frequency and activation of γδ T cells and in which IL-17 plays a pathogenic role (8, 9, 43). The primacy of γδ T cells in the host response to lethal intraperitoneal M. bovis infection was confirmed by infecting WT and γδ TCR−/− mice (γδ−/−), which lack functional γδ T cells (12), and observing significant protection from acute lethality (Fig. 5A). Protection also was associated with a significant reduction in IL-17A+–producing T cells in the γδ Τ cell–deficient mice when assessed 48 h after M. bovis infection (Fig. 5B).
Figure 5.
Production of IL-17A in response to M. bovis infection. A) Survival of WT and γδ TCR−/− (γδ−/−) C57BL/6 mice infected intraperitoneally with M. bovis (n = 9 mice/group, Gehan-Breslow-Wilcoxon test. P = 0.0032). B) Flow cytometry analysis of peritoneal lavage fluid content of total IL-17A+–expressing T cells as percent of total recorded cells (n = 4 samples per group). C–E) Spleen cells of WT or Mif−/− C57BL/6 mice were infected with M. bovis in vitro at a multiplicity of infection of 50. At indicated time points, the IL-17A level in the supernatants was measured by ELISA (C), and the cellular IL-17 mRNA level was examined by real-time PCR (D). Production of IL-17A by CD4 αβ T cells and γδ T cells was determined by flow cytometry (E). FL4-H, flourescence 4 channel height Error bars denote sd. *P < 0.05, **P < 0.01 (unpaired Student’s t test). All data shown are representative of replicate experiments.
γδ T cells are recruited and activated within the lungs and secondary lymphoid organs following disseminated M. bovis infection (44). Splenocytes from WT or Mif−/− mice were infected with M. bovis in vitro, and IL-17 production into culture supernatants was measured by ELISA (Fig. 5C). IL-17 release by Mif−/− splenocytes was significantly higher than in WTs at all time points that were examined; IL-17 was already detectable at 3 h in Mif−/− splenocytes, whereas WT cells produced detectable IL-17 at 24 h postinfection. Splenocytes from Mif−/− mice also expressed a more than 2-fold increased level of IL-17A mRNA when compared with WT cells (Fig. 5D). Because previous reports have indicated γδ T cells as major producers of IL-17 during Mycobacterium infection in vivo (8, 9, 43), we confirmed the cellular source of IL-17 production by flow cytometry. Whereas both CD4 T cells and γδ T cells produced IL-17, γδ T cells from Mif−/− mice showed a ∼3-fold higher frequency of IL-17 production than those from WT mice (Fig. 5E).
Mif−/− mice show enhanced IL-17 production, greater neutrophil accumulation, and increased levels of MIF-2 and keratinocyte chemoattractant chemokine expression after pulmonary infection by M. bovis
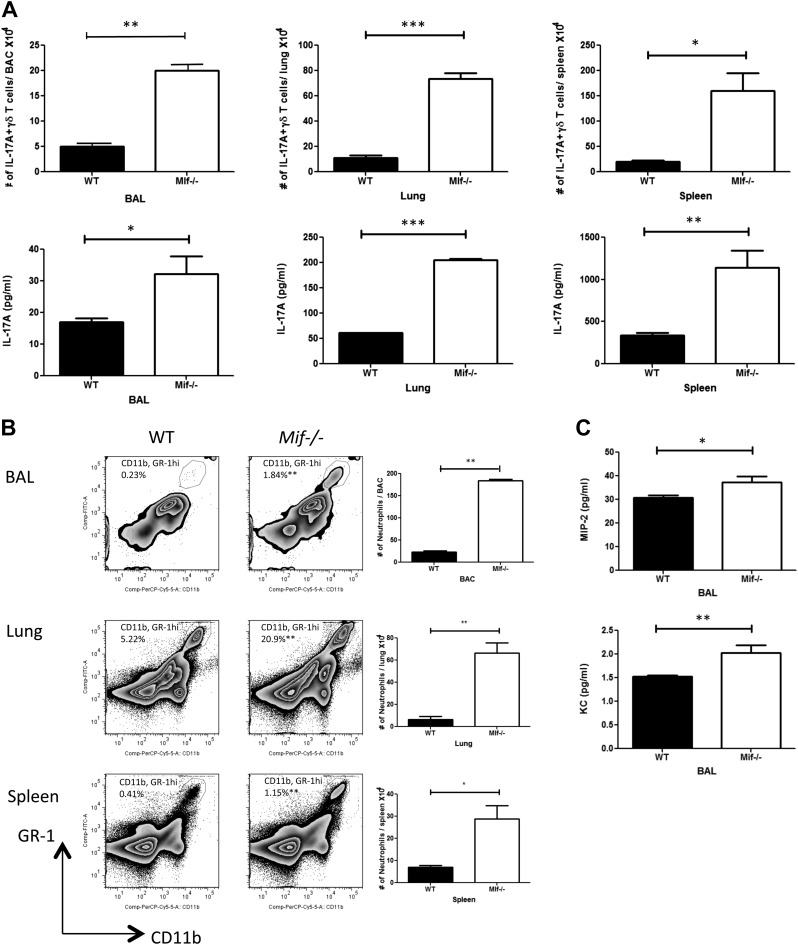
To further examine the role of MIF in IL-17 production by γδ T cells in vivo, WT and Mif−/− mice were infected with 1 × 105 CFU of M. bovis by the intranasal route. This model leads to a more chronic model of Mycobacterium pulmonary infection with mortality only after 200 d of infection (31). WT and Mif−/− mice produced IL-17 in response to M. bovis infection, and considerably increased numbers of γδ17 cells were observed in the BAL, lungs, and spleens from Mif−/− mice at 2 wk postinfection in comparison with WT mice (Fig. 6A). Significantly higher IL-17 production by BAL, lung, and spleen cells from Mif−/− mice than by corresponding cells from WT mice also was observed (Fig. 6A).
Figure 6.
Mif−/− mice demonstrate higher production of IL-17A, increased neutrophil accumulation, and higher levels of MIP-2 and KC upon pulmonary infection by M. bovis. WT or Mif−/− C57BL/6 mice were infected intranasally with 1 × 105 CFU M. bovis. At 2 wk postinfection, cellular production of IL-17A was determined by flow cytometry, and supernatant IL-17A levels were determined by ELISA (A). Neutrophil recruitment was determined by flow cytometry (B), and the levels of neutrophil attracting chemokines MIP-2 and KC were determined by ELISA (C). Comp, compensation; PerCP, peridinin-chlorophyll protein. Error bars denote sd. All data shown are representative of 3 replicates. *P < 0.05, **P < 0.01, ***P < 0.001 (unpaired Student’s t test).
We next investigated neutrophil appearance in M. bovis–infected WT and Mif−/− mice. An enhancement of neutrophil accumulation in the BAL, lungs, and spleens was observed in Mif−/− mice compared with WT mice (Fig. 6B), and among the major chemokines responsible for recruiting neutrophils, increased concentrations of MIP-2 and keratinocyte chemoattractant (KC) were evident in the BAL of Mif−/− mice when compared with WT mice (Fig. 6C). Taken together, these data suggest that MIF deficiency results in higher production of IL-17 by γδ T cells after M. bovis infection together with an expected increase in neutrophil numbers and in the expression of the neutrophil chemokines MIP-2 and KC.
γδ T cell–depleted mice show increased survival and reduced Mif-dependent IL-17 production after TSST-1 administration
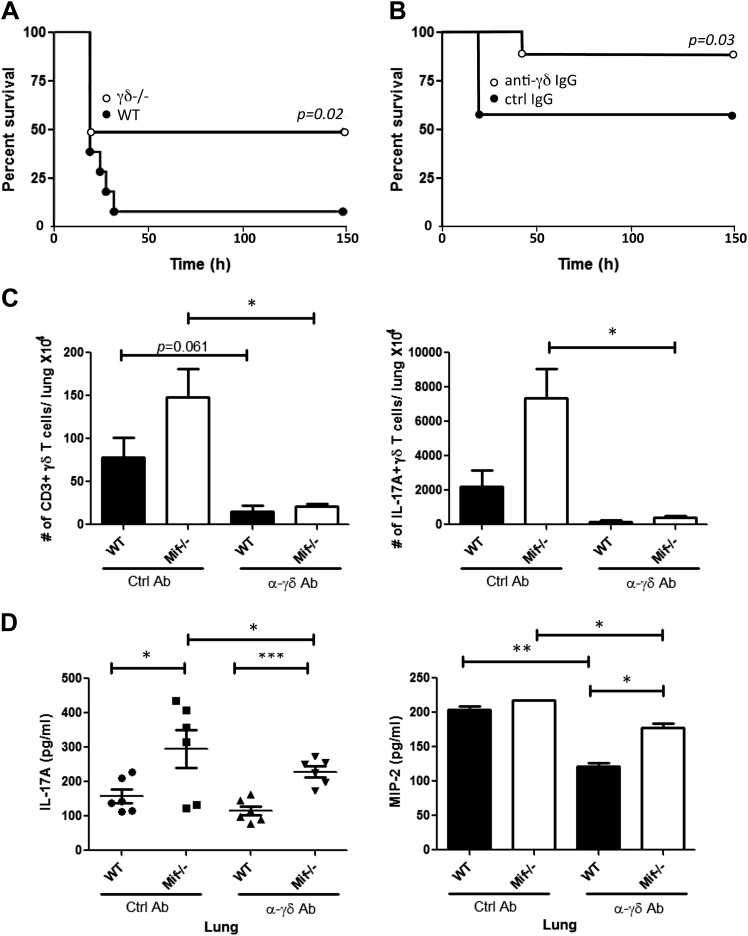
Finally, given the initial correlation observed between γδ T-cell IL-17 production and TSST-1 lethality (Fig. 2D), we examined the impact of γδ T-cell depletion in the BALB/c mouse model of TSST-1 shock. Genetic γδ T-cell deficiency was associated with enhanced survival (Fig. 7A), similar to the observation in M. bovis infection (Fig. 5A). WT mice that were pretreated with an anti–γδ TCR depletion protocol (34) also showed similar, albeit not as complete (20% vs. 40%), protection against TSST-1 lethality (Fig 7B). Anti–γδ TCR pretreatment of WT or γδ17 cell–hyperresponsive Mif−/− mice was associated with a measureable reduction in both total and γδ17 cells in lungs (Fig. 7C), as well as in BAL content of IL-17A and MIP-2 (Fig. 7D).
Figure 7.
γδ T-cell depletion increases survival and reduces Mif-dependent IL-17 production after TSST-1 administration. A) WT or γδ−/− BALB/c mice were injected intraperitoneally with TSST-1 and d-galactosamine as described in Materials and Methods and survival monitored (n = 18 mice). B) For antibody-mediated γδ T-cell depletion, mice were injected intraperitoneally with anti–γδ TCR mAb (UC7-13D5) or control isotypic Ab (200 μg) on d −3 (n = 20 mice; Gehan-Breslow-Wilcoxon test). Lung cells were prepared at 72 h postinjection of TSST-1/d-galactosamine. C, D) Number of CD3+ γδ TCR+ cells and IL-17A–producing γδ T cells was enumerated by flow cytometry (C), and concentrations of IL-17A and MIP-2 were determined in BAL by ELISA (D). Ctrl, control. Data are representative from 3 experiments. *P < 0.05, **P < 0.01, ***P < 0.001 (unpaired Student’s t test).
DISCUSSION
MIF is an upstream immmunoregulatory cytokine that is released from preformed cytoplasmic pools as part of the innate response (17). In the human genome, MIF is encoded in a functionally polymorphic locus that is linked to differences in the susceptibility or the clinical outcome of different infections (25, 29–31, 47). Investigation of genetic MIF deficiency in mice led to the unexpected observation that Mif−/− γδ T cells, when compared with WT cells, produce more than 10-fold higher levels of IL-17, which mediates inflammatory T-cell responses and downstream neutrophil activation. γδ T cells express a restricted repertoire of TCRs, which function mainly as pattern recognition receptors for a subset of microbial macromolecules that include gram-positive exotoxins and Mycobacterium components (7). The observation of high–IL-17 expression by γδ T cells, which bridge innate and adaptive responses, suggests a possible compensatory mechanism in mice with germline Mif deficiency. An enhancement in the development of a Th17-like subset of γδ T cells, which are synonymous with γδ17 cells (39), was further supported by RNA expression analysis, which showed an up-regulation in the levels of IL-1R1, IL-23R, and RORγt mRNAs relative to WT mice. Notably, the orphan nuclear receptor RORγt is essential for the induction of transcription of genes encoding IL-17, and mice lacking RORγt are deficient in barrier γδ T cells (42). Th17 T cells also are characterized by CCR6 expression, and γδ17 T cells have been reported to express both CCR6 and the activation marker CD44 (7). The transcription factor Sox13 (of the sry-related high-mobility group box family) has further been suggested to have a general role in γδ T-cell differentiation (45), and, consistent with these reports, our expression array data showed that the levels of CCR6, CD44, and Sox13 were each up-regulated in γδ T cells from Mif−/− mice. Taken together, these data support the interpretation of an enhancement of γδ 17 development or responsiveness in the setting of MIF deficiency. Notably, Mif−/− macrophages also were observed to exhibit increased production of IL-1β and IL-23, which promote IL-17 production by γδ T cells in the absence of TCR antigen engagement.
Our data do not address whether Mif directly influences the functional commitment of γδ17 cells, which occurs in the thymus. TCR engagement may drive γδ thymocyte differentiation into IFN-γ–expressing γδ T cells by suppressing a default IL-17 program (39). IL-17 is produced in the periphery in response to IL-1β and IL-23 and without TCR stimulation. MIF’s role in suppressing γδ T-cell IL-17 production appears relevant for innate responses to γδ T-cell stimuli, which include the exotoxin products of gram-positive bacteria and the conserved macromolecules of Mycobacterium. Prior reports in experimental model systems and from human genetic studies also are consistent with this conclusion. Calandra et al. first reported a critical role for MIF in the host response to SPEA and TSST-1 (38), and recent data have shown impaired innate responses to M. bovis and M. tuberculosis infection in Mif−/− mice or in low–genotypic MIF–expressing human subjects (31). We found genetic MIF deficiency to exacerbate lethality and γδ T-cell depletion to enhance survival in lethal TSST-1–induced toxic shock. γδ T cells from Mif−/− mice were activated in greater numbers to produce IL-17 and downstream inflammatory responses, including neutrophil accumulation. It should be noted that genetic Mif deficiency did not recapitulate the survival phenotype reported by Calandra et al. (38), in which anti-MIF elicited protection. This observation may reflect differences in the impact of MIF immunoneutralization in a WT host vs. the response of a host that has developed in the complete absence of MIF, in which compensatory pathways may become expressed. With respect to specific mechanisms, Mif−/− mice may maintain a larger population of γδ17 cells because of the lower levels of IL-12 in Mif−/− mice (26), and there is evidence that during development, IL-12 may reduce the population of γδ17 cells by promoting their differentiation into IFN-γ–producing cells (46). Human subjects exhibit an MIF genotype–dependent response to gram-positive infections (29, 47), and further studies to examine the influence of MIF alleles in patients with exotoxin-mediated shock should be considered.
Studies of Mycobacterium infection in mice support the notion that IL-17A production is primarily from γδ or other unconventional T cells rather than αβ T cells (8). Mice infected with M. bovis also accumulate γδ T cells in lungs before antigen-specific αβ T cells, which appear at ∼3 wk (48). In agreement with the primacy of an innate γδ T-cell–mediated inflammatory response to acute Mycobacterium infection, we observed protection in γδ−/− mice challenged with a lethal dose of M. bovis that was associated with a reduction in IL-17A+–expressing T cells. In a chronic model of pulmonary infection induced by the intranasal administration of M. bovis, an enhanced γδ T-cell–mediated IL-17 response also was observed in Mif−/− hosts, with increased neutrophil accumulation and expression of the neutrophilic chemokines MIP-2 and KC. This γδ T cell–dependent phenotype was in agreement with observations in the TSST-1 model of inflammatory shock, in which Mif−/− hosts showed increased γδ IL-17A+ T-cell accumulation and IL-17A, IL-1β, and IL-23 expression in lungs. Peripheral γδ T cells preferentially localize to barrier sites such as the lung. TSST-1 induces IL-1β and IL-23 production, leading to the expansion of these cells and increased IL-17 production. IL-17 in turn up-regulates the expression by lung epithelium of the potent neutrophil attractant MIP-2 (49), which we detected in BAL. γδ T-cell depletion thus reduced mortality and in Mif−/− hosts decreased γδ IL-17A+ T-cell accumulation and IL-17A and MIP-2 expression.
γδ T cells have a specialized role in innate defense and epithelial barrier function by rapidly recognizing and responding to conserved microbial products without the requirement for antigen-presenting cells and major histocompatibility complex–dependent antigen processing. Beyond a role in antimicrobial defense, IL-17 is an important mediator of autoimmunity and chronic arthritis (50), and it can be produced by conventional Th17 cells, NK T cells, or γδ T cells (3, 8, 51). Data from experimental autoimmune encephalomyelitis and mouse models of psoriasis implicate γδ17 cells in immunopathology (6, 52); however, the greater impact of Mif deficiency in suppressing these diseases (53, 54) and verification in multiple sclerosis by pharmacologic MIF antagonism (55) suggests the likely dominance of MIF action over γδ17 cell action. A mouse model of juvenile idiopathic arthritis has recently highlighted the role of γδ17 cells in immunopathology (56), and it has been proposed that in human juvenile idiopathic arthritis, in which anti–IL-1 therapy is highly effective, IL-1β favors the induction of γδ17 cells (57, 58). These observations, together with human genetic data implicating variant MIF alleles in juvenile idiopathic arthritis (59, 60) and other autoimmune arthritides (61, 62), may suggest an upstream role for MIF in regulating γδ17 cell responses by augmenting IL-1β expression. In these contexts, such a pathway could be pharmacologically targeted by a precision medicine approach in patients who are high–genotypic MIF expressers.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health (NIH) Grants AR049610 (National Institute of Arthritis and Musculoskeletal and Skin Diseases) and HL130669 (National Heart, Lung, and Blood Institute) and Arthritis Foundation Grant 548970. The authors declare no conflicts of interest.
Glossary
- γδ17
IL-17–producing γδ T
- BAL
bronchoalveolar lavage fluid
- BMDM
bone marrow–derived macrophage
- Cy
cyanine
- KC
keratinocyte chemoattractant
- LM
lipomannan
- MIF
macrophage migration inhibitory factor
- MIP-2
macrophage inflammatory protein 2
- qPCR
quantitative PCR
- SPEA
streptococcal pyrogenic exotoxin A
- TCR
T-cell receptor
- Th17
T helper 17
- TSST-1
toxic shock syndrome toxin 1
- WT
wild type
AUTHOR CONTRIBUTIONS
H. K. Kim and R. Bucala designed research; H. K. Kim, A. B. Garcia, E. Siu, P. Tilstam, R. Das, S. Roberts, and L. Leng performed experiments; and H. K. Kim and R. Bucala wrote the manuscript.
REFERENCES
- 1.Koenders M. I., Lubberts E., Oppers-Walgreen B., van den Bersselaar L., Helsen M. M., Kolls J. K., Joosten L. A., van den Berg W. B. (2005) Induction of cartilage damage by overexpression of T cell interleukin-17A in experimental arthritis in mice deficient in interleukin-1. Arthritis Rheum. 52, 975–983 [DOI] [PubMed] [Google Scholar]
- 2.Langrish C. L., Chen Y., Blumenschein W. M., Mattson J., Basham B., Sedgwick J. D., McClanahan T., Kastelein R. A., Cua D. J. (2005) IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201, 233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao Z., Painter S. L., Fanslow W. C., Ulrich D., Macduff B. M., Spriggs M. K., Armitage R. J. (1995) Human IL-17: a novel cytokine derived from T cells. J. Immunol. 155, 5483–5486 [PubMed] [Google Scholar]
- 4.Ye P., Rodriguez F. H., Kanaly S., Stocking K. L., Schurr J., Schwarzenberger P., Oliver P., Huang W., Zhang P., Zhang J., Shellito J. E., Bagby G. J., Nelson S., Charrier K., Peschon J. J., Kolls J. K. (2001) Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 194, 519–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stark M. A., Huo Y., Burcin T. L., Morris M. A., Olson T. S., Ley K. (2005) Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity 22, 285–294 [DOI] [PubMed] [Google Scholar]
- 6.Sutton C. E., Lalor S. J., Sweeney C. M., Brereton C. F., Lavelle E. C., Mills K. H. (2009) Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 31, 331–341 [DOI] [PubMed] [Google Scholar]
- 7.Martin B., Hirota K., Cua D. J., Stockinger B., Veldhoen M. (2009) Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity 31, 321–330 [DOI] [PubMed] [Google Scholar]
- 8.Lockhart E., Green A. M., Flynn J. L. (2006) IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J. Immunol. 177, 4662–4669 [DOI] [PubMed] [Google Scholar]
- 9.Umemura M., Yahagi A., Hamada S., Begum M. D., Watanabe H., Kawakami K., Suda T., Sudo K., Nakae S., Iwakura Y., Matsuzaki G. (2007) IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J. Immunol. 178, 3786–3796 [DOI] [PubMed] [Google Scholar]
- 10.Shibata K., Yamada H., Hara H., Kishihara K., Yoshikai Y. (2007) Resident Vdelta1+ gammadelta T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J. Immunol. 178, 4466–4472 [DOI] [PubMed] [Google Scholar]
- 11.Hiromatsu K., Yoshikai Y., Matsuzaki G., Ohga S., Muramori K., Matsumoto K., Bluestone J. A., Nomoto K. (1992) A protective role of gamma/delta T cells in primary infection with Listeria monocytogenes in mice. J. Exp. Med. 175, 49–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mombaerts P., Arnoldi J., Russ F., Tonegawa S., Kaufmann S. H. (1993) Different roles of alpha beta and gamma delta T cells in immunity against an intracellular bacterial pathogen. Nature 365, 53–56 [DOI] [PubMed] [Google Scholar]
- 13.Cho J. S., Pietras E. M., Garcia N. C., Ramos R. I., Farzam D. M., Monroe H. R., Magorien J. E., Blauvelt A., Kolls J. K., Cheung A. L., Cheng G., Modlin R. L., Miller L. S. (2010) IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J. Clin. Invest. 120, 1762–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eberl M., Altincicek B., Kollas A. K., Sanderbrand S., Bahr U., Reichenberg A., Beck E., Foster D., Wiesner J., Hintz M., Jomaa H. (2002) Accumulation of a potent gammadelta T-cell stimulator after deletion of the lytB gene in Escherichia coli. Immunology 106, 200–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zachariadis O., Cassidy J. P., Brady J., Mahon B. P. (2006) Gammadelta T cells regulate the early inflammatory response to bordetella pertussis infection in the murine respiratory tract. Infect. Immun. 74, 1837–1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calandra T., Roger T. (2003) Macrophage migration inhibitory factor: a regulator of innate immunity. Nat. Rev. Immunol. 3, 791–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merk M., Baugh J., Zierow S., Leng L., Pal U., Lee S. J., Ebert A. D., Mizue Y., Trent J. O., Mitchell R., Nickel W., Kavathas P. B., Bernhagen J., Bucala R. (2009) The Golgi-associated protein p115 mediates the secretion of macrophage migration inhibitory factor. J. Immunol. 182, 6896–6906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell R. A., Liao H., Chesney J., Fingerle-Rowson G., Baugh J., David J., Bucala R. (2002) Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: regulatory role in the innate immune response. Proc. Natl. Acad. Sci. USA 99, 345–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calandra T., Bernhagen J., Metz C. N., Spiegel L. A., Bacher M., Donnelly T., Cerami A., Bucala R. (1995) MIF as a glucocorticoid-induced modulator of cytokine production. Nature 377, 68–71 [DOI] [PubMed] [Google Scholar]
- 20.Donnelly S. C., Haslett C., Reid P. T., Grant I. S., Wallace W. A., Metz C. N., Bruce L. J., Bucala R. (1997) Regulatory role for macrophage migration inhibitory factor in acute respiratory distress syndrome. Nat. Med. 3, 320–323 [DOI] [PubMed] [Google Scholar]
- 21.Calandra T., Echtenacher B., Roy D. L., Pugin J., Metz C. N., Hültner L., Heumann D., Männel D., Bucala R., Glauser M. P. (2000) Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat. Med. 6, 164–170 [DOI] [PubMed] [Google Scholar]
- 22.Mizue Y., Ghani S., Leng L., McDonald C., Kong P., Baugh J., Lane S. J., Craft J., Nishihira J., Donnelly S. C., Zhu Z., Bucala R. (2005) Role for macrophage migration inhibitory factor in asthma. Proc. Natl. Acad. Sci. USA 102, 14410–14415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sreih A. G., Ezzeddine R., Leng L., LaChance A., Yu G., Mizue Y., Subrahmanyan L., Pons-Estel B., Abelson A. K., Svenungsson E., Gunnarsson I., Cavett J., Glenn S., Zhang L., Montgomery R., Perl A., Salmon J., Alacon-Riquelme M., Harley J., Bucala R. (2011) Dual effect of the macrophage migration inhibitory factor gene on the development and severity of human systemic lupus erythematosus. Arthritis Rheum. 63, 3942–3951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bozza M., Satoskar A. R., Lin G., Lu B., Humbles A. A., Gerard C., David J. R. (1999) Targeted disruption of migration inhibitory factor gene reveals its critical role in sepsis. J. Exp. Med. 189, 341–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Renner P., Roger T., Bochud P. Y., Sprong T., Sweep F. C. G. J., Bochud M., Faust S. N., Haralambous E., Betts H., Chanson A. L., Reymond M. K., Mermel E., Erard V., van Deuren M., Read R. C., Levin M., Calandra T. (2012) A functional microsatellite of the macrophage migration inhibitory factor gene associated with meningococcal disease. FASEB J. 26, 907–916 [DOI] [PubMed] [Google Scholar]
- 26.Flores M., Saavedra R., Bautista R., Viedma R., Tenorio E. P., Leng L., Sánchez Y., Juárez I., Satoskar A. A., Shenoy A. S., Terrazas L. I., Bucala R., Barbi J., Satoskar A. R., Rodriguez-Sosa M. (2008) Macrophage migration inhibitory factor (MIF) is critical for the host resistance against Toxoplasma gondii. FASEB J. 22, 3661–3671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koebernick H., Grode L., David J. R., Rohde W., Rolph M. S., Mittrücker H. W., Kaufmann S. H. (2002) Macrophage migration inhibitory factor (MIF) plays a pivotal role in immunity against Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 99, 13681–13686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Satoskar A. R., Bozza M., Rodriguez Sosa M., Lin G., David J. R. (2001) Migration-inhibitory factor gene-deficient mice are susceptible to cutaneous Leishmania major infection. Infect. Immun. 69, 906–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savva A., Brouwer M. C., Roger T., Valls Serón M., Le Roy D., Ferwerda B., van der Ende A., Bochud P. Y., van de Beek D., Calandra T. (2016) Functional polymorphisms of macrophage migration inhibitory factor as predictors of morbidity and mortality of pneumococcal meningitis. Proc. Natl. Acad. Sci. USA 113, 3597–3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yende S., Angus D. C., Kong L., Kellum J. A., Weissfeld L., Ferrell R., Finegold D., Carter M., Leng L., Peng Z. Y., Bucala R. (2009) The influence of macrophage migration inhibitory factor gene polymorphisms on outcome from community-acquired pneumonia. FASEB J. 23, 2403–2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Das R., Koo M. S., Kim B. H., Jacob S. T., Subbian S., Yao J., Leng L., Levy R., Murchison C., Burman W. J., Moore C. C., Scheld W. M., David J. R., Kaplan G., MacMicking J. D., Bucala R. (2013) Macrophage migration inhibitory factor (MIF) is a critical mediator of the innate immune response to Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 110, E2997–E3006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kevill K. A., Bhandari V., Kettunen M., Leng L., Fan J., Mizue Y., Dzuira J. D., Reyes-Mugica M., McDonald C. L., Baugh J. A., O’Connor C. L., Aghai Z. H., Donnelly S. C., Bazzy-Asaad A., Bucala R. J. (2008) A role for macrophage migration inhibitory factor in the neonatal respiratory distress syndrome. J. Immunol. 180, 601–608 [DOI] [PubMed] [Google Scholar]
- 33.Fingerle-Rowson G., Petrenko O., Metz C. N., Forsthuber T. G., Mitchell R., Huss R., Moll U., Müller W., Bucala R. (2003) The p53-dependent effects of macrophage migration inhibitory factor revealed by gene targeting. Proc. Natl. Acad. Sci. USA 100, 9354–9359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Usami J., Hiromatsu K., Matsumoto Y., Maeda K., Inagaki H., Suzuki T., Yoshikai Y. (1995) A protective role of gamma delta T cells in primary infection with Listeria monocytogenes in autoimmune non-obese diabetic mice. Immunology 86, 199–205 [PMC free article] [PubMed] [Google Scholar]
- 35.Parsonnet J., Hickman R. K., Eardley D. D., Pier G. B. (1985) Induction of human interleukin-1 by toxic-shock-syndrome toxin-1. J. Infect. Dis. 151, 514–522 [DOI] [PubMed] [Google Scholar]
- 36.Ikejima T., Okusawa S., van der Meer J. W., Dinarello C. A. (1988) Induction by toxic-shock-syndrome toxin-1 of a circulating tumor necrosis factor-like substance in rabbits and of immunoreactive tumor necrosis factor and interleukin-1 from human mononuclear cells. J. Infect. Dis. 158, 1017–1025 [DOI] [PubMed] [Google Scholar]
- 37.Uchiyama T., Kamagata Y., Wakai M., Yoshioka M., Fujikawa H., Igarashi H. (1986) Study of the biological activities of toxic shock syndrome toxin-1. I. Proliferative response and interleukin 2 production by T cells stimulated with the toxin. Microbiol. Immunol. 30, 469–483 [DOI] [PubMed] [Google Scholar]
- 38.Calandra T., Spiegel L. A., Metz C. N., Bucala R. (1998) Macrophage migration inhibitory factor is a critical mediator of the activation of immune cells by exotoxins of Gram-positive bacteria. Proc. Natl. Acad. Sci. USA 95, 11383–11388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akitsu A., Iwakura Y. (2018) Interleukin-17-producing γδ T (γδ17) cells in inflammatory diseases. Immunology 155, 418–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Floret D. (2001) Clinical aspects of streptococcal and staphylococcal toxinic diseases [in French]. Arch. Pediatr. 8(Suppl 4), 762s–768s [DOI] [PubMed] [Google Scholar]
- 41.Lee J., Cacalano G., Camerato T., Toy K., Moore M. W., Wood W. I. (1995) Chemokine binding and activities mediated by the mouse IL-8 receptor. J. Immunol. 155, 2158–2164 [PubMed] [Google Scholar]
- 42.Ivanov I. I., McKenzie B. S., Zhou L., Tadokoro C. E., Lepelley A., Lafaille J. J., Cua D. J., Littman D. R. (2006) The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 [DOI] [PubMed] [Google Scholar]
- 43.Okamoto Yoshida Y., Umemura M., Yahagi A., O’Brien R. L., Ikuta K., Kishihara K., Hara H., Nakae S., Iwakura Y., Matsuzaki G. (2010) Essential role of IL-17A in the formation of a mycobacterial infection-induced granuloma in the lung. J. Immunol. 184, 4414–4422 [DOI] [PubMed] [Google Scholar]
- 44.McGill J. L., Sacco R. E., Baldwin C. L., Telfer J. C., Palmer M. V., Waters W. R. (2014) Specific recognition of mycobacterial protein and peptide antigens by γδ T cell subsets following infection with virulent Mycobacterium bovis. J. Immunol. 192, 2756–2769 [DOI] [PubMed] [Google Scholar]
- 45.Melichar H. J., Narayan K., Der S. D., Hiraoka Y., Gardiol N., Jeannet G., Held W., Chambers C. A., Kang J. (2007) Regulation of gammadelta versus alphabeta T lymphocyte differentiation by the transcription factor SOX13. Science 315, 230–233 [DOI] [PubMed] [Google Scholar]
- 46.Ness-Schwickerath K. J., Morita C. T. (2011) Regulation and function of IL-17A- and IL-22-producing γδ T cells. Cell. Mol. Life Sci. 68, 2371–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doernberg S., Schaaf B., Dalhoff K., Leng L., Beitin A., Quagliarello V., Bucala R. (2011) Association of macrophage migration inhibitory factor (MIF) polymorphisms with risk of meningitis from Streptococcus pneumoniae. Cytokine 53, 292–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dieli F., Ivanyi J., Marsh P., Williams A., Naylor I., Sireci G., Caccamo N., Di Sano C., Salerno A. (2003) Characterization of lung gamma delta T cells following intranasal infection with Mycobacterium bovis bacillus Calmette-Guérin. J. Immunol. 170, 463–469 [DOI] [PubMed] [Google Scholar]
- 49.Fogli L. K., Sundrud M. S., Goel S., Bajwa S., Jensen K., Derudder E., Sun A., Coffre M., Uyttenhove C., Van Snick J., Schmidt-Supprian M., Rao A., Grunig G., Durbin J., Casola S., Rajewsky K., Koralov S. B. (2013) T cell-derived IL-17 mediates epithelial changes in the airway and drives pulmonary neutrophilia. J. Immunol. 191, 3100–3111; erratum: 5318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hirota K., Hashimoto M., Yoshitomi H., Tanaka S., Nomura T., Yamaguchi T., Iwakura Y., Sakaguchi N., Sakaguchi S. (2007) T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J. Exp. Med. 204, 41–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Michel M. L., Keller A. C., Paget C., Fujio M., Trottein F., Savage P. B., Wong C. H., Schneider E., Dy M., Leite-de-Moraes M. C. (2007) Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J. Exp. Med. 204, 995–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pantelyushin S., Haak S., Ingold B., Kulig P., Heppner F. L., Navarini A. A., Becher B. (2012) Rorγt+ innate lymphocytes and γδ T cells initiate psoriasiform plaque formation in mice. J. Clin. Invest. 122, 2252–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bezdek S., Leng L., Busch H., Mousavi S., Rades D., Dahlke M., Zillikens D., Bucala R., Sadik C. D. (2018) Macrophage migration inhibitory factor (MIF) drives murine psoriasiform dermatitis. Front. Immunol. 9, 2262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Benedek G., Meza-Romero R., Jordan K., Zhang Y., Nguyen H., Kent G., Li J., Siu E., Frazer J., Piecychna M., Du X., Sreih A., Leng L., Wiedrick J., Caillier S. J., Offner H., Oksenberg J. R., Yadav V., Bourdette D., Bucala R., Vandenbark A. A. (2017) MIF and D-DT are potential disease severity modifiers in male MS subjects. Proc. Natl. Acad. Sci. USA 114, E8421–E8429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fox R. J., Coffey C. S., Conwit R., Cudkowicz M. E., Gleason T., Goodman A., Klawiter E. C., Matsuda K., McGovern M., Naismith R. T., Ashokkumar A., Barnes J., Ecklund D., Klingner E., Koepp M., Long J. D., Natarajan S., Thornell B., Yankey J., Bermel R. A., Debbins J. P., Huang X., Jagodnik P., Lowe M. J., Nakamura K., Narayanan S., Sakaie K. E., Thoomukuntla B., Zhou X., Krieger S., Alvarez E., Apperson M., Bashir K., Cohen B. A., Coyle P. K., Delgado S., Dewitt L. D., Flores A., Giesser B. S., Goldman M. D., Jubelt B., Lava N., Lynch S. G., Moses H., Ontaneda D., Perumal J. S., Racke M., Repovic P., Riley C. S., Severson C., Shinnar S., Suski V., Weinstock-Guttman B., Yadav V., Zabeti A.; NN102/SPRINT-MS Trial Investigators (2018) Phase 2 trial of ibudilast in progressive multiple sclerosis. N. Engl. J. Med. 379, 846–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Avau A., Mitera T., Put S., Put K., Brisse E., Filtjens J., Uyttenhove C., Van Snick J., Liston A., Leclercq G., Billiau A. D., Wouters C. H., Matthys P. (2014) Systemic juvenile idiopathic arthritis-like syndrome in mice following stimulation of the immune system with Freund’s complete adjuvant: regulation by interferon-γ. Arthritis Rheum. 66, 1340–1351 [DOI] [PubMed] [Google Scholar]
- 57.Nigrovic P. A. (2014) Review: is there a window of opportunity for treatment of systemic juvenile idiopathic arthritis? Arthritis Rheum. 66, 1405–1413 [DOI] [PubMed] [Google Scholar]
- 58.Kessel C., Lippitz K., Weinhage T., Hinze C., Wittkowski H., Holzinger D., Fall N., Grom A. A., Gruen N., Foell D. (2017) Proinflammatory cytokine environments can drive interleukin-17 overexpression by γ/δ T cells in systemic juvenile idiopathic arthritis. Arthritis Rheum. 69, 1480–1494 [DOI] [PubMed] [Google Scholar]
- 59.Donn R., Alourfi Z., De Benedetti F., Meazza C., Zeggini E., Lunt M., Stevens A., Shelley E., Lamb R., Ollier W. E., Thomson W., Ray D.; British Paediatric Rheumatology Study Group (2002) Mutation screening of the macrophage migration inhibitory factor gene: positive association of a functional polymorphism of macrophage migration inhibitory factor with juvenile idiopathic arthritis. Arthritis Rheum. 46, 2402–2409 [DOI] [PubMed] [Google Scholar]
- 60.De Benedetti F., Meazza C., Vivarelli M., Rossi F., Pistorio A., Lamb R., Lunt M., Thomson W., Ravelli A., Donn R., Martini A.; British Paediatric Rheumatology Study Group (2003) Functional and prognostic relevance of the -173 polymorphism of the macrophage migration inhibitory factor gene in systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 48, 1398–1407 [DOI] [PubMed] [Google Scholar]
- 61.Radstake T. R. D. J., Sweep F. C. G. J., Welsing P., Franke B., Vermeulen S. H. H. M., Geurts-Moespot A., Calandra T., Donn R., van Riel P. L. C. M. (2005) Correlation of rheumatoid arthritis severity with the genetic functional variants and circulating levels of macrophage migration inhibitory factor. Arthritis Rheum. 52, 3020–3029 [DOI] [PubMed] [Google Scholar]
- 62.Baugh J. A., Chitnis S., Donnelly S. C., Monteiro J., Lin X., Plant B. J., Wolfe F., Gregersen P. K., Bucala R. (2002) A functional promoter polymorphism in the macrophage migration inhibitory factor (MIF) gene associated with disease severity in rheumatoid arthritis. Genes Immun. 3, 170–176 [DOI] [PubMed] [Google Scholar]