Abstract
Purpose
Wound represents a major health challenge as they consume a large amount of healthcare resources to improve patient's quality of life. Many scientific studies have been conducted in search of ideal biomaterials with wound-healing activity for clinical use and collagen has been proven to be a suitable candidate biomaterial. This study intended to investigate the wound healing activity of collagen peptides derived from jellyfish following oral administration.
Methods
In this study, collagen was extracted from the jellyfish--Rhopilema esculentum using 1% pepsin. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and fourier transform infrared (FTIR) were used to identify and determine the molecular weight of the jellyfish collagen. Collagenase II, papain and alkaline proteinase were used to breakdown jellyfish collagen into collagen peptides. Wound scratch assay (in vitro) was done to determine migration potential of human umbilical vein endothelial cells (HUVEC) covering the artificial wound created on the cell monolayer following treatment with collagen peptides. In vivo studies were conducted to determine the effects of collagen peptides on wound healing by examining wound contraction, re-epithelialization, tissue regeneration and collagen deposition on the wounded skin of mice. Confidence level (p < 0.05) was considered significant using GraphPad Prism software.
Results
The yield of collagen was 4.31%. The SDS-PAGE and FTIR showed that extracted collagen from jellyfish was type I. Enzymatic hydrolysis of this collagen using collagenase II produced collagen peptides (CP1) and hydrolysis with alkaline proteinase/papain resulted into collagen peptides (CP2). Tricine SDS-PAGE revealed that collagen peptides consisted of protein fragments with molecular weight <25 kDa. Wound scratch assay showed that there were significant effects on the scratch closure on cells treated with collagen peptides at a concentration of 6.25 μg/mL for 48 h as compared to the vehicle treated cells. Overall treatment with collagen peptide on mice with full thickness excised wounds had a positive result in wound contraction as compared with the control. Histological assessment of peptides treated mice models showed remarkable sign of re-epithelialization, tissue regeneration and increased collagen deposition. Immunohistochemistry of the skin sections showed a significant increase in β-fibroblast growth factor (β-FGF) and the transforming growth factor-β1 (TGF-β1) expression on collagen peptides treated group.
Conclusion
Collagen peptides derived from the jellyfish–Rhopilema esculentum can accelerate the wound healing process thus could be a therapeutic potential product that may be beneficial in wound clinics in the future.
Keywords: Collagen, Collagen peptides, Wound healing, Jellyfish
Introduction
Prolonged life expectancy due to advancement in human development is accompanied by increased risk of disease, aging and incidence of wounds/injuries requiring medical attention. Wounds may be acute or chronic depending on underlying condition. Chronic wounds, such as venous and arterial ulcers, pressure ulcers and diabetic ulcers, are often associated with advanced age, patient immobility, compromised blood circulation and systemic illnesses.1 According to the World Wound Care Markets 2008, chronic wounds (or skin ulcers) account for approximately 37 million skin wounds globally and 6 million skin wounds in the United States.2 Generally, chronic wounds are the challenge to health care system as they consume a great amount of healthcare resources and wound care professionals who often adopt multidisciplinary approaches in order to achieve effective results.3 Conventional medicines including pain killers and antibiotics are the common measures for wound treatment. Some biomaterials such as collagen are known to be effective in protecting wounds from infection and maintain moisture in the wound area. Together with antibiotics they accelerate wound healing aiding shorter healing time, reduced hospital stay and costs, and overall patient health improvement.4
Collagen has long been used in wound care and management as a wound dressing material in various forms such as powder, amorphous gels/pastes, gel-impregnated dressing and pad.4 Collagen in nature is a large protein molecule which upon enzymatic hydrolysis produces very small bioactive peptides with interesting biological functions.5 Depending on their specific amino acid composition, bioactive peptides derived from collagen have been proven to show immunomodulatory,6, 7 ACE inhibitory properties,8, 9, 10 antibacterial,11, 12 antioxidative13 etcetera. Unlike collagen, collagen peptides can easily be digested by gastric enzymes, absorbed and transported through human peptide transporter 1(PEPT-1) to systemic circulation where they can find their target and elicit their biological activities.14 In the skin, collagen peptides act as false collagen degradation peptides which send a false signal in the fibroblast cells to synthesize new collagen fibres. Moreover, collagen peptides possess chemotactic properties, they can promote cell migration and proliferation which is an important process in wound healing.15
Collagen is abundant in nature, it constitutes about 30% of animal protein. Over the years, bovine and porcine have been used as common source of collagen. However, the outbreak of bovine spongiform encephalopathy (BSE), transmissible spongiform encephalopathy (TSE) and foot and mouth disease (FMD) in last decades have limited their use. Marine organisms such as fish, fish wastes, starfish, sponges and jellyfish have recently been explored as the alternative source.16 As an alternative source, the jellyfish--Rhopilema esculentum has recently gained attention to researchers. This jellyfish is common in China and Japan but recently has become popular in other countries due to its nutritional and pharmacological values.6, 17 It is rich in collagenous protein and minerals with negligible fat contents and low calories.18 Zhao et al.19 and Zhuang et al.20 reported that proteins derived from jellyfish--Rhopilema esculentum have antioxidant activity and could be utilized in food and pharmaceutical industries. Zhuang et al.10 reported that peptides isolated from the jellyfish--Rhopilema esculentum have antihypertensive activity and could be applied in functional foods as antihypertensive compounds. Cheng et al.21 recently reported that collagen sponges derived from Rhopilema esculentum have potential hemostatic effects suggesting that it might be a suitable candidate for wound dressing applications. Other biomaterials derived from marine collagen including collagen gels films, and membranes have also been reported to have beneficial effects on wound healing. However, there is limited information regarding collagen peptides derived from jellyfish or the effects of these peptides on wound healing. This study aimed at extracting collagen from the jellyfish--Rhopilema esculentum and break it down using enzymes to make small molecular weight collagen peptides and investigate their wound healing potential. Here in we report collagen peptides prepared from the jellyfish collagen and their wound healing activities.
Methods
Raw materials
Fresh filaments of jellyfish--Rhopilema esculentum were bought from Auchan supermarket in Nanjing city and transported in ice to the laboratory and stored at −80°C.
Chemical reagents
All reagents used were of analytical grade. A high molecular weight marker was purchased from Tanon company and low molecular weight marker was purchased from Sigma–Aldrich chemical company.
Cells and animals
Human embryonic vein endothelial cell (HUVEC) was obtained from the laboratory at The Engineering Research Center of Peptide Drug Discovery and Development, China Pharmaceutical University. Adult male mice with a body weight of approximately 26 g each were used in this study. The mice were maintained under standard acclimatization conditions of 12-h dark/light circle at approximately 25°C and were provided with standard rodent food. All the experimental protocols to use animals were approved by Shanghai Sippr-Bk Laboratory animal Co., Ltd.
Extraction of collagen (jellyfish collagen)
The filaments of the jellyfish (203g) were washed with tap water at least thrice and then washed with ultra-purified water (three times). The clean jellyfish filaments were then homogenized in 0.5 mol/L acetic acid (50% v/w) for 10 min. Then 1% pepsin (1:30000 Dalian Meilun biological technology Co., Ltd.) was added, and the mixture was constantly stirred for 72 h. The mixture was then centrifuged at 8000 rpm for 15 min. The supernatant was salted-out by adding NaCl to obtain a final concentration of 2 mol/L in the presence of 0.05 mol/L tris (at pH 7). The resulting precipitate was collected by centrifugation at 10000 rpm for 20 min and then dissolved in 0.5 mol/L acetic acid. The final solution was dialyzed against 0.1 mol/L acetic acid for 2 h followed by ultra-purified water for 2 days. The dialysis solution was changed at least thrice a day and the samples were freeze-dried and stored at 4°C for further experimental use. The whole procedure was carried out at 4°C.
The percentage yield of the extracted collagen was obtained by using the following formula:22
Preparation of collagen peptides (CP1)
The extracted collagen (1 g) was suspended in 200 mL of ultra-purified water and put in a water bath at 37°C. Collagenase II enzyme (5% w/w) was then added and the mixture was stirred for 5 h. The enzymatic reaction was terminated by heating the mixture at 95°C for 10 min. The mixture was then cooled at room temperature and centrifuged at 3000 rpm for 30 min. The resulting supernatant was freeze-dried as collagen peptides CP1 and stored at 4°C for further experimental use.
Preparation of collagen peptides (CP2)
The extracted collagen (1 g) was suspended in 200 mL of ultra-purified water of which the pH was adjusted to 8.5 by adding 1 mol/L NaOH. The mixture was put in a water bath at 55°C. Added 2.5% alkaline protease, and stirred the mixture for 2 h. The temperature was then adjusted to 50°C and 3% papain was added to the mixture with stirring for 2 h. The enzymatic reaction was terminated by heating the mixture at 95°C for 10 min. The mixture was then cooled at room temperature and centrifuged at 5000 rpm for 15 min. The resulting supernatant (sample) was dialyzed against ultra-purified water for 2 h. The dialysis solution was changed after 1 h and the sample was freeze-dried as collagen peptides CP2 and stored at 4°C for further experimental use.
The molecular weight of collagen
The molecular weight and type of collagen were determined by using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS PAGE) and fourier transform infrared (FTIR) methods. SDS-PAGE was carried out according to the method of Barzideh et al.22 with some modifications. The gel consisting of 4% stacking gel and 8% resolving gel was used. The extracted collagen samples were dissolved in 0.02 mol/L sodium phosphate buffer (pH 7.2) containing SDS (1% w/v) and 3.5 mol/L urea. The samples were then mixed with loading buffer in equal volume with or without dithiothreitol (DTT) and heated at 95°C for 5 min. A high molecular weight pre-stained marker was used to estimate the molecular weight of collagen. The protein bands were visualized by Tanon 5200 multi-gel analysis (Tanon Shanghai. China). The FTIR spectrum of jellyfish collagen was recorded from 4000 to 650 cm−1 Bruker tensors 27 spectrophotometers.
The molecular weight of collagen peptides
The molecular weight of collagen peptides was determined by using tricine SDS PAGE, and the protocol used was adapted from Nature protocols (2006). The gel consisted of 6%resolving gel, 3% stacking gel and 3% preparative gel. Collagen peptide samples (CP1 & CP2) were dissolved in distilled water and mixed with sample loading buffer at the sample buffer ratio of 1:1 (v/v) with DTT and heated at 95°C for 5 min. Each sample (2 mg protein) was loaded into a well and run at 90 V for 2 h. Collagen peptide weight was then estimated by using low molecular weight pre-stained maker. After electrophoresis, the gel was washed with tap water and incubated in a fixing solution for 20 min then washed again and stained with coomassie blue. The protein bands were visualized by Tanon 5200 multi-gel analysis (Tanon Shanghai. China).
Wound scratch assay
The collagen peptides CP1 & CP2 were tested for wound healing activity by using wound scratch assay. HUVEC cells were used to determine the potential of collagen peptides on cell migration. These cells were cultured immediately after taking them out from the liquid nitrogen and then they were maintained in Dulbecco's modified eagles medium (DMEM) with 10% fetal bovine serum (FBS) supplemented with penicillin (120 U/mL) and streptomycin (75 mg/mL) at 37°C with 5% CO2. When the cells reached about 80% confluency the cells were treated with trypsin for passing. After 3 passages, HUVEC cells were seeded in a 96-well plate and cultured as monolayer to confluence overnight. Each well contained approximately 1 × 105 cells. The monolayer was then scratched with a white 100 μl pipette tip to create an approximate 0.7 mm-wide wound area and washed twice with PBS to remove floating cells. After the line scratch, 100 μl DMEM was added into every well. To observe the effect of collagen peptides on HUVEC cells migration, cells were incubated with collagen peptides (0–50 μg/mL) for various time periods (from 0 h to 48 h). Images of the wounded cell monolayer were taken using a microscope (Shanghai measurement dimension photoelectric technology Co., Ltd.) at 0, 18, 36 and 48 h after scratched wounding. Cell migration activity was expressed as the percentage of the gap relative to the total area of the cell-free region immediately after the scratch, named the repair rate of scarification.23
Animal study for wound healing
The mice were subjected to anesthesia with 1% pentobarbital sodium (0.25 mL/26 g body weight). The anesthetic was administered intraperitoneally. The mice were then shaved on their back and an excision wound of approximately 8.5 mm diameter was made by removing a full-thickness piece of skin using a scissor from a pre-determined shaved area. The wounds were left undressed to the open environment without any local or systemic anti-microbial agents administration. The mice were put in the cages in a cool environment at room temperature about 25°C until when they were fully recovered from anesthesia which was the next day (post wound day).
Animal grouping and treatment. Wounded mice were randomly divided into 7 groups of 10 mice each, that is the control (vehicle) group, CP1 and CP2. The collagen peptides (0.3, 0.6 and 0.9 g/kg body weight) or normal saline as a vehicle were administered orally (intragastric) every morning for 6 days. The wound contraction size was observed and measured on day 4, 6 and 8. Photos were taken using a digital camera on day 8. At day 6 three mice with larger wound area were sacrificed from the vehicle-treated group and the groups treated with collagen peptides at a dose of 0.9 g/kg body weight and their healing skins were collected and put in the 4% poly formaldehyde solution for histological and immunohistochemistry analysis.
Histological analysis. The skin samples were removed from the formalin solution and processed for paraffin embedding. The skin sections (4 μm thick) were then mounted on glass slides and stained with Masson's trichrome or hematoxylin and eosin. The morphological structures of the healing skin including re-epithelialization, signs of tissue regeneration, collagen bundles etc. were observed and described under the light microscope (NIKON Eclipse ci, imaging System: NIKON Digital Sight DS-FI2, made in JAPAN). The skin histological results interpretations were provided by Wuhan service biotechnology CO., Ltd.
Immunohistochemistry. The skin tissues embedded in paraffin were sectioned (4 μm thick) and were treated with sodium citrate buffer (10 mmol/L) for antigen retrieval. The skin sections were then treated with 0.3% hydrogen peroxide in methanol for at least 15 min to block the activity of endogenous peroxidases. The skin tissues were then treated with anti-basic fibroblast growth factor (β-FGF) and polyclonal rabbit anti-transforming growth factor beta 1 (TGF-β1) for overnight at 48°C for detection of β-FGF and TGF-β1 respectively. Slides were then mounted and the expression of β-FGF and TGF-β1 was assessed under the light microscope. The Image-pro plus 6.0 (Media Cybernetics, Inc., Rockville, MD, USA) software was used to select the same brown yellow as the uniform standard for judging all photo positives, each photo was analyzed to obtain a positive cumulative light density. One-way Anova was used to compare test groups to vehicle groups.
Statistical analysis
A computer software Graph pad prism version 5 was used to conduct statistical analysis. Two-way ANOVA was used to compare test groups to control groups. Data were presented as means ± SEM and the statistical significance level was set at p < 0.05.
Results
Collagen extraction yield
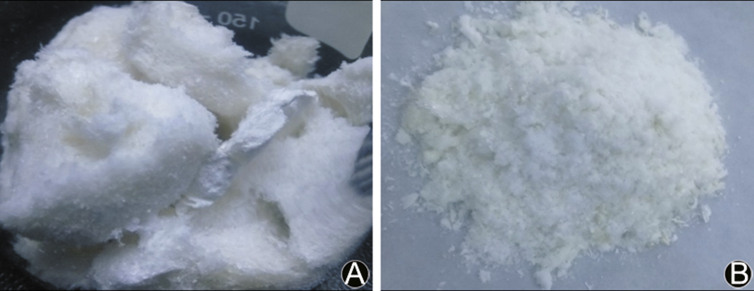
The yield of collagen extracted from the jellyfish Rhopilema esculentum was 4.31% (on a wet weight basis). This yield was higher than that reported for the same species using 1% pepsin.16, 19, 20, 24 The appearance of lyophilized collagen was off-white spongy/mesh-like material as shown in Fig. 1A. The digestion of jellyfish collagen using enzymes resulted in off-white agglomerated powder of collagen peptides as shown in Fig. 1B. On a dry weight basis, the percentage yield for CP1 and CP2 was 65% and 54% respectively.
Fig. 1.
Products obtained from the jellyfish (Rhopilema esculentum). (A) Collagen sponges, (B) Collagen peptides.
The molecular weight of collagen and collagen peptides
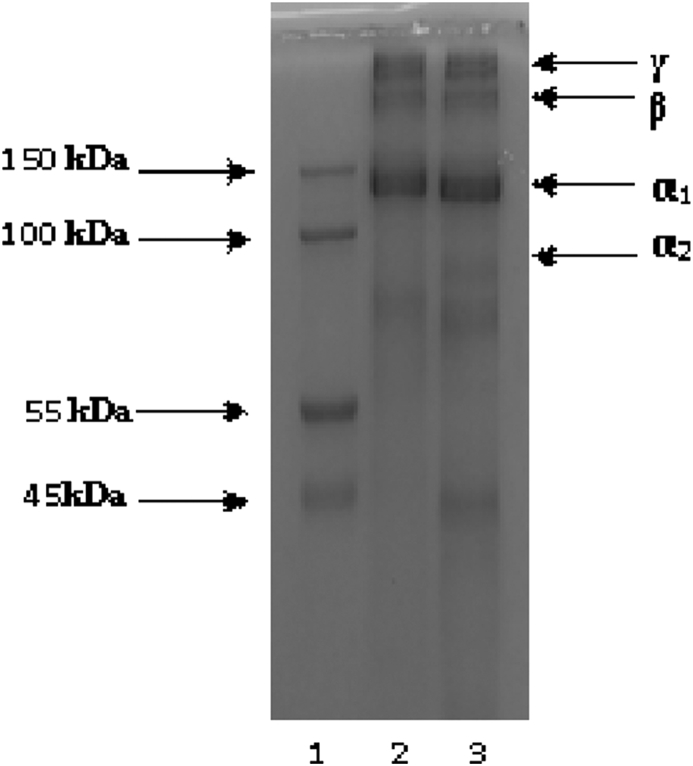
SDS-PAGE was used to determine the molecular weight of the extracted collagen, type of collagen and the purity of the product. The gel showed three distinct protein bands at the molecular weight above 100 kDa. From the literature, the molecular weight of a single collagen polypeptide chain is about 100 kDa.26 Thus, our electrophoretic pattern was similar to those findings in other studies.27, 28, 29 SDS-PAGE showed that the extracted collagen consisted of two α chains between 100 kDa and 150 kDa, β (dimer) chain and γ (trimer) chain located on the high molecular mass region (above 150 kDa) as shown in Fig. 2. The α chains were the major component of jellyfish collagen. Moreover, the α1 and α2 chains had different electrophoretic mobility, indicating that their molecular weights were different. From the SDS-PAGE it can be suggested that the jellyfish collagen was type I collagen as it consisted of two α1 chains and one α2 chain as the major component. Moreover, several studies have reported that marine invertebrate animals including sea urchin (Paracentrotus lividus),30 starfish (Acanthaster planci)31 and squid (Uroteuthis duvauceli)32 contain type I collagen. In addition, the present results were found to be similar to the previous studies.21 Amino acid analysis done by Cheng et al.21 shows that collagen obtained from the jellyfish Rhopilema esculentum contain cysteine, this confirms our SDS-PAGE results under reducing conditions of DTT treated collagen samples. Under the non-reducing condition, the pattern showed distinctive three polypeptide chains with less or no other protein band below 100 kDa showing the purity of the sample.
Fig. 2.
The electrophoretic patterns of collagen extracted from the jellyfish (Rhopilema esculentum). Lane 1: Molecular weight marker; Lane 2: Collagen sample without DTT; Lane 3: Collagen sample with DTT.
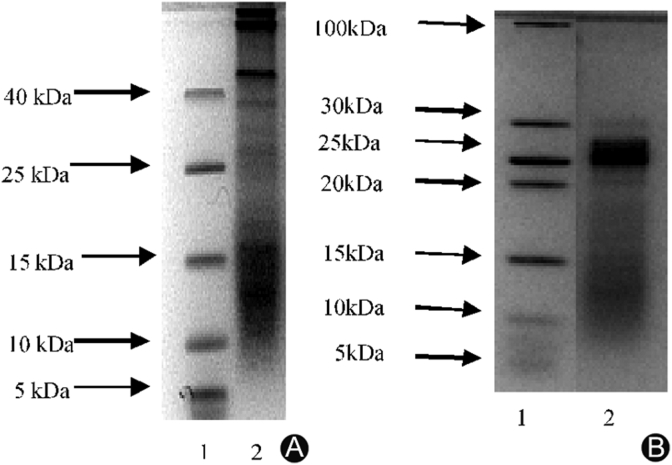
The tricine SDS-PAGE of the hydrolyzed collagen revealed the presence of collagen peptide chains with low molecular weight. CP1 showed intensive collagen peptide bands at 10–15 kDa (Fig. 3A) while CP2 showed the presence of collagen peptides with molecular weight <25 kDa (Fig. 3B). Different enzyme treatment on collagen can result into different products exhibiting similar or different bioactivities. Studies have reported different bioactive proteins/peptides derived from the jellyfish (Rhopilema esculentum). Li et al.25 and Zhuang et al.33 reported very low molecular weight peptides (<1 kDa) derived from the same species. These alcalase hydrolysis products were revealed to have strong antioxidant and ACE inhibitory activities. Zhuang et al.20 reported polypeptides with molecular weight 2 kDa from the hydrolysis of jellyfish gelatin with trypsin and properase E which showed strong antioxidant activity in vitro suggesting their utilization in food industry. The two products obtained in this study are different from the reported ones due to different enzyme treatments and conditions.
Fig. 3.
Electrophoretic patterns of collagen peptides. (A) Lane 1: low Molecular weight marker; lane 2: collagen peptides (CP1). (B) Lane 1: low molecular weight marker; lane 2: collagen peptides CP2.
FTIR spectroscopy
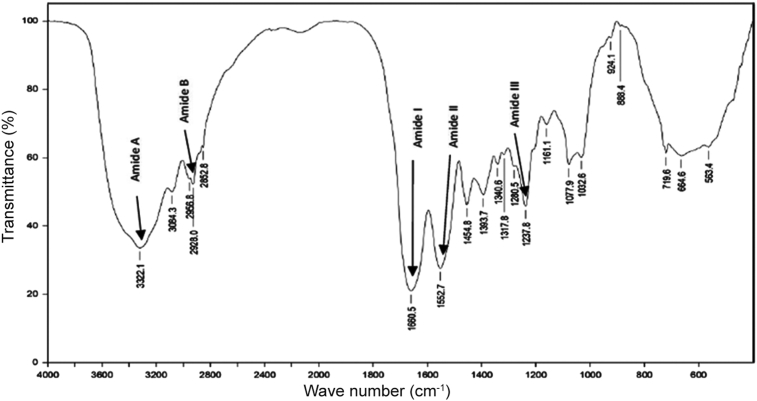
As shown in Fig. 4, the FTIR spectra of the collagen extracted from the jellyfish Rhopilema esculentum exhibited a characteristic peak of amide A, B and amide I, II, III. The band position of amide A was found at 3322.1 cm−1. This position is determined by the stretching vibrations of N—H which normally occurs at 3400-3440 cm−1 when the N—H group is free from hydrogen bonding. In the present results, the band position appears to have moved to the lower frequencies suggesting that the N—H groups were involved in the hydrogen bond formation which helps to hold the triple helical structures together within the collagen molecule.34 The band position of amide B was observed at ∼2926.8 cm−1 as a result of asymmetrical stretching of CH2.35 The wave numbers of the amide I, II and III bands are directly related to the structural configuration of collagen and they can be observed from (1200-1700 cm−1).36 The amide I band was found at a wave length of ∼1660.5 cm−1. This band occurs normally at wave number ranging from 1600 to 1700 cm−1.23 The Amide II band was observed at ∼1552.7 cm−1 as a result of N—H bending and C—H stretching.37 Amide I and II peaks depicted a high molecular order of the extracted collagen. It could be suggested that pepsin altered a non-helical part of telopeptide regions, resulting in an increased molecular order of collagen structure. Amide III peak was found at 1237.8 cm−1 which correspond to the stretching vibrations of C—H. Absorption bands around 1390-1455 cm−1 were also found. These bands extensively corresponded to pyrrolidine ring vibration of hydroxyproline and proline. This result indicated that the triple helical structure of collagen was well preserved. Moreover, the FTIR spectrum of Rhopilema esculentum collagen was found to be similar to FTIR spectra reported for the same species by Cheng et al.21 Altogether, the FTIR spectra indicate a well-maintained secondary structure in the collagen extracted from Rhopilema esculentum.
Fig. 4.
Fourier transform infrared spectrum of collagen from the jellyfish Rhopilema esculentum.
In vitro wound healing activity
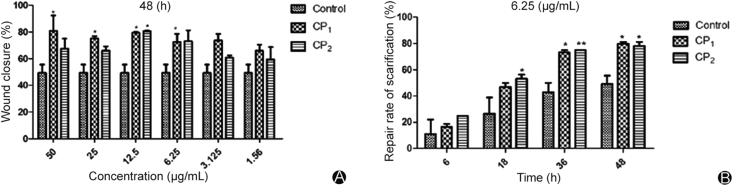
Effects of collagen peptides on the migration of HUVEC cells are illustrated in Fig. 5, Fig. 6. The wound-healing scratch assay in which the artificial wound was created by disrupting the monolayers of HUVEC cells, indicated that collagen peptides enhanced cell migration. Cell migration across the wound area was significantly enhanced in cells treated with collagen peptides compared to those treated with vehicle. The repair rate of scarification of cells treated with 6.25 μg/mL collagen peptides was reached to 75.49% for CP1 (p < 0.05) and 73.65% for CP2 (p < 0.05) after 48 h compared to the vehicle-treated cells which was 49.2% (Fig. 5B). For all concentrations 1.56–50 μg/mL there was no significant effect on the wound closure by collagen peptides treatment for 6 h whereas significant effects of collagen peptides on wound closure were observed 18 h, 36 h and 48 h after treatment compared with the control. The effect of collagen peptide on the wound closure was dose-dependent that is, the percentage of wound closure for the cell treated with collagen peptides increased as the concentration increased from of 1.56μg/mL-6.25 μg/mL but this dose-dependent manner is not seen in the concentration above 6.25 μg/mL where the wound closure effects remained constant (Fig. 5A) this means that 6.25 μg/mL was the minimum peptides concentration with significant wound closure effects. These results demonstrated that the collagen peptides from the jellyfish Rhopilema esculentum can induce cell migration. This might be due to the chemotactic effects of collagen peptides which enhanced migration of cells to the artificial wound area. Moreover, the abundant amino acid residues in the collagen peptides serve as additional nutrients for cellular growth and proliferation.38
Fig. 5.
The in vitro wound healing activity of collagen peptides derived from the jellyfish Rhopilema esculentum. (A) The percentage wound closure on the cells treated with collagen peptides and non-treated cells at different concentrations for 48 h; (B) The rate at which the artificial wound gap closes with time on the cells treated with collagen peptides (6.25 μg/mL) and non-treated cells.
Fig. 6.
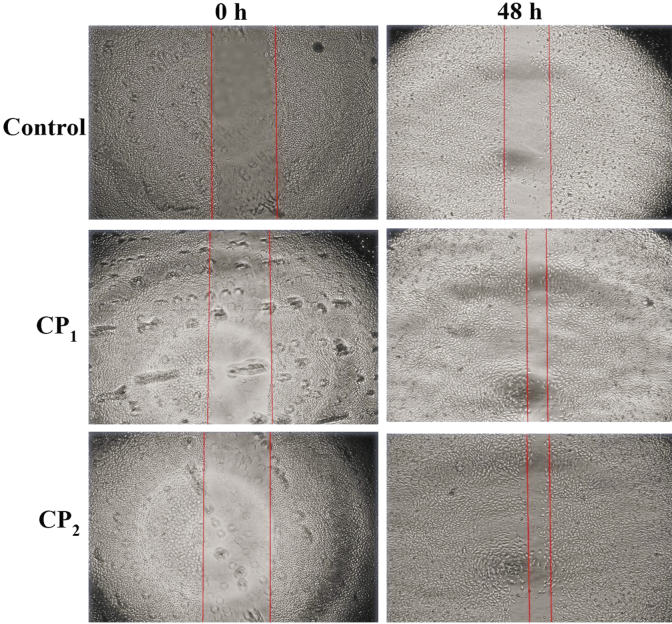
Photomicrograph showing the migration of cells treated with collagen peptides (6.25 μg/mL) against cells treated with vehicle (control) at 0 h and 48th hour. The red line denoted the margin of the wound gap.
In vivo wound healing activity
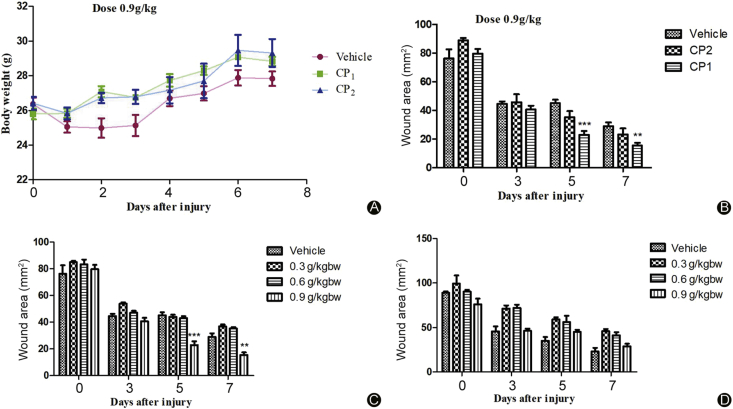
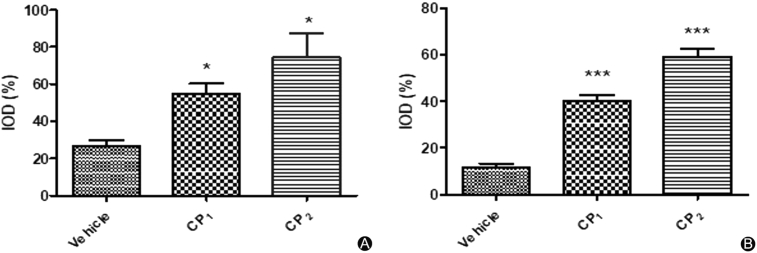
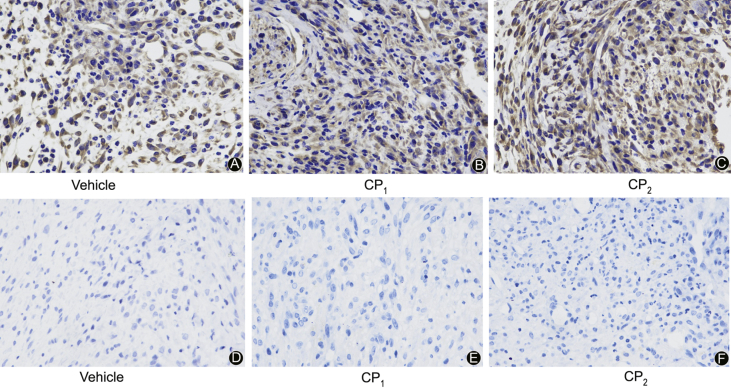
All mice appeared healthy throughout the study except for the day they were wounded most of the mice had minor weight loss due to pain caused by the wound. From the second day, when the treatment started all the mice regained their weight and the wound area started to decrease. From the graph in Fig. 7A, there was no significant difference observed in the mice body weight indicating that our sample did not have negative effects on mice appetite and health in general. The significant effect of wound healing on collagen peptides treated mice was observed after 4 days of treatment with collagen peptides at a dose of 0.9 g/kg while the mice treated with collagen peptides at a dose of 0.6 g/kg and 0.3 g/kg had similar results to the vehicle treated group (Fig. 7B). The mice groups treated with the highest dose had decreased wound area compared to the vehicle-treated group. Between the two samples, CP1 and CP2, CP1 appeared to have more effects on wound healing than CP2. This was observed at day 6 where by 0.9 g/kg-CP1 treated mice showed increased wound contraction compared to vehicle-treated groups (p < 0.001). From our observations, the wound healing time was 5–7 days, at this time, most mice had smaller wound area which was almost closed (Fig. 8). Histological analysis of the wounded skin showed increased signs of re-epithelialization, new tissue formation (regeneration) and collagen deposition in collagen peptides treated groups with more signs observed in CP1 treated groups compared to the vehicle-treated group (Fig. 9). As shown in (Fig. 10, Fig. 11), immunohistochemistry showed increased levels of β-FGF and TGF-β1 in collagen peptides treated mice than the vehicle group. Although the β-FGF expression was weak as shown in Table 1, the overall results showed that there was significant increase in TGF-β1 and β-FGF expression levels in the collagen treated mice as compared to the vehicle group with higher levels in CP2 than CP1 treated group implying that the former has more ability to enhance the production of these chemotactic factors.
Fig. 7.
The in vivo wound healing activity of collagen peptides derived from the jellyfish Rhopilema esculentum. (A) The effect of collagen peptides on the mice body weight; (B) The wound area of the mice treated with high dose of collagen peptides (0.9 g/kg) for 6 days; (C) The wound area of mice treated with CP1 for 6 days; (D) The wound area of mice treated with CP2 for 6 days (n = 6).
Fig. 8.
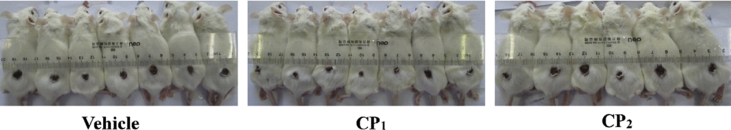
Contracted wound area on mice following intragastric administration of collagen peptides (0.9 g/kg body weight) or vehicle at day 8.
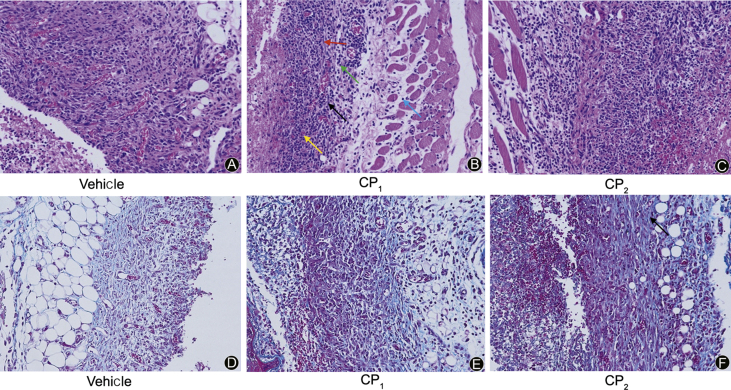
Fig. 9.
Microscopic images representing stained skin sections of mice treated with 0.9 g/kg body weight collagen peptides or vehicle at day 6. (A-C) Hematoxylin and eosin (H & E) stained skin sections showing fibroblasts (black arrows), fiber cells (red arrow), new blood vessels (yellow arrow), inflammatory cells (green arrow) and interstitial connective tissue of the muscle cells (blue arrow). (D-F) Masson stained skin sections showing blue stained collagen (black arrow) (magnification 200×.).
Fig. 10.
Semi quantitative immunohistochemical results of healing skin of mice treated with 0.9 g/kg collagen peptides or vehicle at day 6. (A) The mean percentage levels of TGF-β1 expression p ≤ 0.05; (B) The mean percentage levels of β-FGF expression p < 0.0001. IOD= Immunohistochemical integrated optical density.
Fig. 11.
Photomicrograph representation of immunohistochemistry skin sections of mice treated with 0.9 g/kg body weight collagen peptides or vehicle at day 6. (A-C) Expression of TGF-β1 on the healing skin of mice; (D-F) Expression of β-FGF on the healing skin of mice (Magnification 400 × ).
Table 1.
Immunohistochemical integrated optical density (IOD).
| TGF-β1 | β-FGF | |||||
|---|---|---|---|---|---|---|
| Vehicle treated mice | 19600.4 | 13173.4 | 15589.6 | 1.00819 | 1.06183 | 1.51521 |
| CP1 treated mice | 28284.2 | 39569.3 | 29091.9 | 3.51001 | 4.52302 | 4.01283 |
| CP2 treated mice | 57740.3 | 45211.6 | 31701.2 | 6.58691 | 5.47103 | 5.65609 |
Discussion
Wound healing is a complex process involving four distinct phases working interdependently, namely hemostasis, inflammation, proliferation and remodelling. The hemostasis phase commences immediately after injury and is characterized by platelet aggregation which lead to the release of chemotactic factors including platelet-derived growth factors (PDGF), TGF β1 & TGF β2 which further recruit inflammatory cells (neutrophils, leukocytes and macrophages) to the wound to protect it from infection. Together with epidermal and dermal cells, inflammatory cells secrete other mediators that regulate and stimulate proliferation and migration of smooth muscle cells, fibroblasts and keratinocytes within the wound. In response to hypoxia, angiogenic factors including vascular endothelial growth factor (VEGF), PDGF and β-FGF induce the formation of new blood vessels. Fibroblasts and keratinocytes synthesize new collagen fibers which form a provisional extracellular matrix (ECM) which is further remodeled and strengthened with the aid of matrix metalloproteinases (MMP-9) activity balanced with the activity of tissue inhibitors of metalloproteinases (TIMPs).39, 40 Collagen is known to play an important role in each of these phases mainly due to its chemotactic properties. In proliferation phase, it promotes the production of keratinocytes and fibroblasts and their migration to the wound site. In the present study, the collagen peptides derived from the jellyfish revealed their potential in accelerating the wound healing by promoting chemotactic factors and fibroblast production. Furthermore, the collagen peptides showed their ability in promoting collagen production in the mice skin hence enhancing the remodeling phase. The results obtained from this study are similar to the study done by Wang et al.41 who investigated the wound healing effects of collagen peptides from chum salmon (Oncorhynchus keta) in cesarean sectioned rats. Similar results were also demonstrated by Zhou et al.38 who reported the wound healing effect of collagen peptides from the skin of nile tilapia (Oreochromis niloticus) in rabbits. All these studies have revealed the wound healing potential of marine collagen peptides although the molecular mechanisms for their activity are still unclear.
Collagen is an interesting protein which can be broken down into small different proteins with various biological activity. The jellyfish Rhopilema esculentum is one of the marine organisms that can serve as safe collagen source. In addition to reported nutrition benefits and other pharmacological values, collagen peptides derived from the jellyfish Rhopilema esculentum have potential to accelerate wound healing thus could be a promising product for wound treatment in the future.
Funding
This work was supported by the Project Program of State Key Laboratory of Natural Medicines (no.SKLNMBZ201403), Natural Science Foundation of Jiangsu Province BK20160757, the Priority Academic Program Development of Jiangsu Higher Education Institutions and the Fundamental Research Funds for the Central Universities 26320NPY07.
Ethical statement
All the experimental protocols to use animals were approved by Shanghai Sippr-Bk Laboratory animal Co., Ltd.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Shilo S., Roth S., Amzel T. Cutaneous wound healing after treatment with plant-derived human recombinant collagen flowable gel. Tissue Eng. 2013;19:1519–1526. doi: 10.1089/ten.tea.2012.0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wild T., Rahbarnia A., Kellner M. Basics in nutrition and wound healing. Nutrition. 2010;26:862–866. doi: 10.1016/j.nut.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Frykberg R.G., Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care. 2015;4:560–582. doi: 10.1089/wound.2015.0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hochstein A.O., Bhatia A. Collagen : its role in wound healing. Podiatry Manag. 2014;103–106:109–110. [Google Scholar]
- 5.Gomez-Guillen M.C., Gimenez B., Lopez-Caballero M.E. Functional and bioactive properties of collagen and gelatin from alternative sources: a review. Food Hydrocolloids. 2011;25:1813–1827. [Google Scholar]
- 6.Morishige H., Sugahara T., Nishimoto S. Immunostimulatory effects of collagen from jellyfish in vivo. Cytotechnology. 2011;63:481–492. doi: 10.1007/s10616-011-9371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan J., Zhuang Y., Li B. Effects of collagen and collagen hydrolysate from jellyfish umbrella on histological and immunity changes of mice photoaging. Nutrients. 2013;5:223–233. doi: 10.3390/nu5010223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alemán A., Gómez-Guillén M.C., Montero P. Identification of ace-inhibitory peptides from squid skin collagen after in vitro gastrointestinal digestion. Food Res Int. 2013;54:790–795. [Google Scholar]
- 9.Barzideh Z., Latiff A.A., Gan C.Y. ACE inhibitory and antioxidant activities of collagen hydrolysates from the ribbon jellyfish (Chrysaora sp.) Food Technol Biotechnol. 2014;52:495–504. doi: 10.17113/ftb.52.04.14.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhuang Y., Sun L., Zhang Y. Antihypertensive effect of long-term oral administration of jellyfish (Rhopilema esculentum) collagen peptides on renovascular hypertension. Mar Drugs. 2012;10:417–426. doi: 10.3390/md10020417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ennaas N., Hammami R., Gomaa A. Collagencin, an antibacterial peptide from fish collagen: activity, structure and interaction dynamics with membrane. Biochem Biophys Res Commun. 2016;473:642–647. doi: 10.1016/j.bbrc.2016.03.121. [DOI] [PubMed] [Google Scholar]
- 12.Ennaas N., Hammami R., Beaulieu L. Purification and characterization of four antibacterial peptides from protamex hydrolysate of Atlantic mackerel (Scomber scombrus) by-products. Biochem Biophys Res Commun. 2015;462:195–200. doi: 10.1016/j.bbrc.2015.04.091. [DOI] [PubMed] [Google Scholar]
- 13.Chi C.F., Cao Z.H., Wang B. Antioxidant and functional properties of collagen hydrolysates from Spanish mackerel skin as influenced by average molecular weight. Molecules. 2014;19:11211–11230. doi: 10.3390/molecules190811211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asserin J., Lati E., Shioya T. The effect of oral collagen peptide supplementation on skin moisture and the dermal collagen network: eevidence from an ex vivo model and randomized, placebo-controlled clinical trials. J Cosmet Dermatol. 2015;14:291–301. doi: 10.1111/jocd.12174. [DOI] [PubMed] [Google Scholar]
- 15.Banerjee P., Suguna L., Shanthi C. Wound healing activity of a collagen-derived cryptic peptide. Amino Acids. 2015;47:317–328. doi: 10.1007/s00726-014-1860-6. [DOI] [PubMed] [Google Scholar]
- 16.Addad S., Exposito J.Y., Faye C. Isolation, characterization and biological evaluation of jellyfish collagen for use in biomedical applications. Mar Drugs. 2011;9:967–983. doi: 10.3390/md9060967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu H.H., Li R.F., Liu S. Amino acid composition and nutritional quality of gonad from jellyfish Rhopilema esculentum. Biomed Prev Nutr. 2014;4:399–402. [Google Scholar]
- 18.Khong N.M., Yusoff F.M., Jamilah B. Nutritional composition and total collagen content of three commercially important edible jellyfish. Food Chem. 2016;196:953–960. doi: 10.1016/j.foodchem.2015.09.094. [DOI] [PubMed] [Google Scholar]
- 19.Zhuang Y.L., Zhao X., Li B.F. Optimization of antioxidant activity by response surface methodology in hydrolysates of jellyfish (Rhopilema esculentum) umbrella collagen. J Zhejiang Univ - Sci B. 2009;10:572–579. doi: 10.1631/jzus.B0920081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhuang Y.L., Sun L.P., Zhao X. Investigation of gelatin polypeptides of jellyfish (Rhopilema esculentum) for their antioxidant activity in vitro. Food Technol Biotechnol. 2010;48:222–228. [Google Scholar]
- 21.Cheng X., Shao Z., Li C. Isolation, characterization and evaluation of collagen from jellyfish Rhopilema esculentum kishinouye for use in hemostatic applications. PLoS One. 2017;12 doi: 10.1371/journal.pone.0169731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alves A.L., Marques A.P.L., Martins E. Cosmetic Potential of marine fish skin collagen. Cosmetics. 2017;4:39. [Google Scholar]
- 23.Barzideh Z., Latiff A.A., Gan C.Y. Isolation and characterisation of collagen from the ribbon jellyfish (Chrysaora sp.) Int J Food Sci Technol. 2013;49:1490–1499. [Google Scholar]
- 24.Tang J., Liu H., Gao C. A small peptide with potential ability to promote wound healing. PLoS One. 2014;9 doi: 10.1371/journal.pone.0092082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J., Li Q., Li J. Peptides derived from Rhopilema esculentum hydrolysate exhibit angiotensin converting enzyme (ACE) inhibitory and antioxidant abilities. Molecules. 2014;19:13587–13602. doi: 10.3390/molecules190913587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bailey J.L., Critser P.J., Whittington C. Collagen oligomers modulate physical and biological properties of three-dimensional self-assembled matrices. Biopolymers. 2011;95:77–93. doi: 10.1002/bip.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang C.Y., Kuo J.M., Wu S.J. Isolation and characterization of fish scale collagen from tilapia (Oreochromis sp.) by a novel extrusion-hydro-extraction process. Food Chem. 2016;190:997–1006. doi: 10.1016/j.foodchem.2015.06.066. [DOI] [PubMed] [Google Scholar]
- 28.Sun L., Hou H., Li B. Characterization of acid- and pepsin-soluble collagen extracted from the skin of Nile tilapia (Oreochromis niloticus) Int J Biol Macromol. 2017;99:8–14. doi: 10.1016/j.ijbiomac.2017.02.057. [DOI] [PubMed] [Google Scholar]
- 29.Zhang F., Wang A., Li Z. Preparation and characterisation of collagen from freshwater fish scales. Food Nutr Sci. 2011;2:818–823. [Google Scholar]
- 30.Benedetto C.D., Barbaglio A., Martinello T. Production, characterization and biocompatibility of marine collagen matrices from an alternative and sustainable source: the sea urchin Paracentrotus lividus. Mar Drugs. 2014;12:4912–4933. doi: 10.3390/md12094912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan C.C., Karim A.A., Latiff A. Extraction and characterization of pepsin-solubilized collagen from the body wall of crown-of-thorns Starfish (Acanthaster planci) Int Food Res J. 2013;20:3013–3020. [Google Scholar]
- 32.Delphi L., Sepehri H., Motevaseli E.2. Collagen extracted from Persian gulf squid exhibits anti-cytotoxic properties on apple pectic treated cells: assessment in an in vitro bioassay model. Iran J Public Health. 2016;45:1054–1063. [PMC free article] [PubMed] [Google Scholar]
- 33.Zhuang Y.L., Sun L.P., Li B.F. Production of the angiotensin-I-converting enzyme (ACE)-inhibitory peptide from hydrolysates of jellyfish (Rhopilema esculentum) collagen. Food Bioprocess Technol. 2012;5:1622–1629. [Google Scholar]
- 34.Nagai T. Characterization of acid-soluble collagen from skins of surf smelt (hypomesus pretiosus japonicus brevoort) Food Nutr Sci. 2010;01:59–66. [Google Scholar]
- 35.Zhang J., Duan R., Huang L. Characterisation of acid-soluble and pepsin-solubilised collagen from jellyfish (Cyanea nozakii Kishinouye) Food Chem. 2014;150:22–26. doi: 10.1016/j.foodchem.2013.10.116. [DOI] [PubMed] [Google Scholar]
- 36.Pal G.K., Suresh P.V. Comparative assessment of physico-chemical characteristics and fibril formation capacity of thermostable carp scales collagen. Mater Sci Eng C Mater Biol Appl. 2017;70:32–40. doi: 10.1016/j.msec.2016.08.047. [DOI] [PubMed] [Google Scholar]
- 37.Nagai T., Suzuki N., Nagashima T. Collagen from common minke whale (Balaenoptera acutorostrata)unesu. Food Chem. 2008;111:296–301. doi: 10.1016/j.foodchem.2008.03.087. [DOI] [PubMed] [Google Scholar]
- 38.Hu Z., Yang P., Zhou C. Marine collagen peptides from the skin of Nile Tilapia (Oreochromis niloticus): characterization and wound healing evaluation. Mar Drugs. 2017;15 doi: 10.3390/md15040102. pii: E102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Demidova-Rice T.N., Hamblin M.R., Herman I.M. Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 2: role of growth factors in normal and pathological wound healing: therapeutic potential and methods of delivery. Adv Skin Wound Care. 2012;25:349–370. doi: 10.1097/01.ASW.0000418541.31366.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dickinson L.E., Gerecht S. Engineered biopolymeric scaffolds for chronic wound healing. Front Physiol. 2016;7:314. doi: 10.3389/fphys.2016.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J., Xu M., Liang R. Oral administration of marine collagen peptides prepared from chum salmon (oncorhynchus keta) improves wound healing Following Cesarean Section in Rats. Food Nutr Res. 2015;59:26411. doi: 10.3402/fnr.v59.26411. [DOI] [PMC free article] [PubMed] [Google Scholar]