Abstract
Cancer-derived exosomes are constitutively produced and secreted into the blood and biofluids of their host patients providing a liquid biopsy for early detection and diagnosis. Given their ubiquitous nature, cancer exosomes influence biological mechanisms that are beneficial to the tumor cells where they are produced and the microenvironment in which these tumors exist. Accumulating evidence suggests that exosomes transport proteins, lipids, DNA, mRNA, miRNA and long non coding RNA (lncRNA) for the purpose of cell-cell and cell-extracellular communication. These exosomes consistently reflect the status as well as identity of their cell of origin and as such may conceivably be affecting the ability of a functional immune system to recognize and eliminate cancer cells. Recognizing and mapping the pathways in which immune suppression is garnered through these tumor derived exosome (TEX) may lead to treatment strategies in which specific cell membrane proteins or receptors may be targeted, allowing for immune surveillance to once again help with the treatment of cancer. This Review focuses on how cancer exosomes interact with immune cells in the blood.
Keywords: Exosome, Non-Hodgkin’s lymphoma, B cell
Introduction
Exosomes are small 30–150 nm sized, extracellular vesicles (EVs) important in the intercellular communication between cells [1–4]. They belong to the nanovesicle family and originate from the intraluminal vesicles of the late endosome, named multivesicular bodies. The fusion of these multivesicular bodies with the cell membrane results in the release of exosomes into the extracellular space. Communication can occur both by transfer of nucleic acids and proteins, or by binding cell-surface receptors and inducing cell signaling pathways [5, 6]. Both normal and tumor cells release exosomes, although TEX have been the subject of a wide range of studies. Exosomes have been shown to be involved in many aspects of the tumor microenvironment (TME) including immune suppression [7, 8], antigen presentation [9–13], a means of acquiring chemotherapeutic resistance [14–18], as biomarker reservoirs [19–25], inducers of angiogenesis [26–28], and vehicles of niche preparation for metastasis [29–33] (Fig. 1). However, modes and mechanisms of uptake are not completely understood. Cells appear to internalize EVs through several endocytic pathways, including clathrin- and caveolin-dependent endocytosis, phagocytosis, and lipid raft–mediated internalization. It is likely that cells utilize multiple routes to take up exosomes, depending on the proteins, glycoproteins, and lipids found on the surface of the vesicles and the target cell itself [34]. Numerous studies show proficient uptake of exosomes by endothelial cells [35–37], epithelial cells [38], fibroblasts [39], myeloid precursors in bone marrow [32, 37], mesenchymal stem cells [37], and other tumor cells [40] (Fig. 2).
Fig. 1. Tumor derived exosomes (TEX) function in favor of metastasis, support angiogenesis, confer chemoresistance and promote immune-suppression and cellular proliferation.
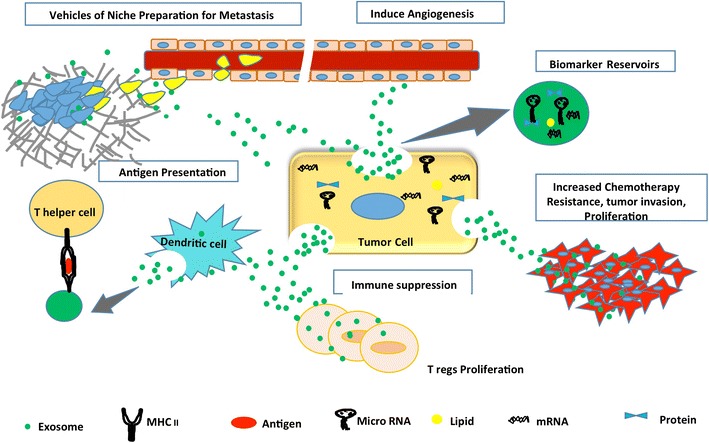
Exosomes from tumor cells were found to release functional biomolecules into the tumor microenvironment thereby affecting the biology of cancer
Fig. 2. Cancer cells release exosomes which are taken up by other cancer cells, endothelial cells, epithelial cells, fibroblasts, bone marrow myeloid precursor cells, and mesenchymal stem cells.
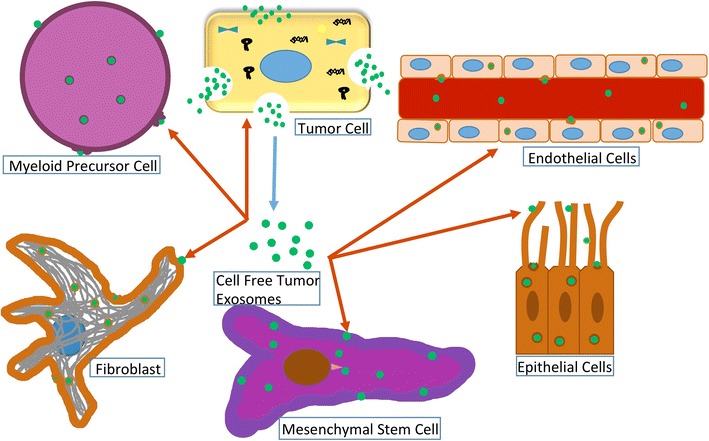
Exosomes from tumor cells were found to release functional biomolecules (protein, RNA, miRNA) into many cell types
Cancer and Exosomes
Cancer cell uptake of exosomes has been well documented and studies even show that exosomes can preferentially associate with cancer cells [41, 42]. Endocytic pathways are utilized by ovarian cancer cells to internalize exosomes from the SKOV3 ovarian cancer cell line [43], by glioblastoma cells [44], and by bladder cancer cells as demonstrated by dose and time dependent uptake of PKH26 labeled bladder exosomes [40]. Treatment with heparin can partially block the active and specific mechanism of uptake, implicating receptor-mediated endocytosis involving heparan sulfate proteoglycans (HSPGs) [40]. HSPGs were also shown to be critical in the internalization of glioblastoma exosomes by glioblastoma cells [45]. Other tumor types have demonstrated exosome uptake, such as colorectal cancer exosomes into lung cancer cells [46] and breast cancer exosomes into breast cancer cell lines [47, 48], although mechanisms underlying the uptake were not addressed.
Diffuse large B cell lymphoma (DLBCL), an aggressive form of lymphoma representing over 40% of adult lymphoma patients, has not until recently been investigated. In an attempt to close the gap in knowledge concerning lymphoma TME immunosuppression, normal human peripheral blood leukocytes were treated with PKH67-labeled lymphoma exosomes and monitored for uptake by measuring fluorescence at different time points using flow cytometry and fluorescent microscopy. Results show that of the four populations examined, B cells and monocytes demonstrated uptake of PKH67 labeled lymphoma exosomes, while T cells and NK cells displayed significantly less uptake [49].
Immune Influence
Macrophages
As exosomes have exhibited multiple forms of influence within the immune system, immune cells have also been investigated regarding their ability to interact with exosomes. Macrophages exhibit specialized capacity for internalizing exosomes, although they typically reside within tissue, not in circulation. Their blood counterparts, monocytes, also appear to have a relatively high level of exosome internalization. Consequently, numerous investigations have focused on exosomal interactions with these myeloid-derived cell types. Macrophages have been shown to internalize exosomes from both normal [50] and malignant sources [51], with differing effects. Macrophages exposed to breast cancer exosomes, but not normal exosomes, activated NF-κB pathways and released pro-inflammatory cytokines like IL-6 and TNFα, possibly due to TLR2 interacting with palmitoylated protein ligands on the exosomes [51]. There are reports of multiple ways macrophages use to interact with exosomes, such as CD169 (SIGLEC) to bind exosomes expressing sialic acids, as seen with B cell-derived exosomes expressing α2, 3-linked sialic acids [52]. Macrophages also utilize a dynamin-dependent phagocytic pathway to internalize vesicles [6, 53]. Leukemia exosomes were found to be efficiently internalized via phagocytosis by macrophages, while non-phagocytic cells such as T cells show few internalized vesicles, with most interaction being with surface-attached vesicles [6].
Monocytes
Monocytes also utilize phagocytic mechanisms to internalize exosomes, perhaps relying on tetraspanin targeting, as was shown by Rana et al. [36] Vesicle internalization by monocytes can induce changes such as production of cytokines like TNFα, which has important downstream ramifications on T cells [54]. Like macrophages and monocytes, other cells of the myeloid lineage such as neutrophils and dendritic cells (DCs) have the ability for exosome uptake. As one of the major infiltrators of the TME, neutrophil interactions with exosomes have been of interest. Investigations in leukemia demonstrate communication between tumor cells and neutrophils, transferring genomic DNAs (gDNAs) of the BCR/ABL hybrid gene from K562 cells to normal neutrophils [55] and even promoting leukemia tumorigenesis in rats [56].
Dendritic Cells
Early investigations in the exosome field recognized follicular DCs as interactors with exosomes [57], and even though no specific receptors had been demonstrated yet, it seemed that alpha 4-integrin on B cell-derived exosomes was important [58]. Integrin complexes with CD9 and CD81 tetraspanins, externalized phosphatidylserine (PS), and CD11a (LFA-1)/ICAM-1 interactions all participate in the binding and uptake processes of DCs [57, 59]. Uptake can be through endocytic mechanisms [60], including phagocytosis [61] and DCs may be even more efficient than macrophages at picking up exosome-sized particles [62, 63]. DCs are affected by their interactions with vesicles. Uptake of mast cell exosomes can induce bone-marrow precursors to acquire antigen presenting capacity to T cells [64], and CD11b + and CD11c + cells in mice began releasing IL-6 and TNFα and upregulated CD86 and MHC class II after exposure to exosomes [63]. Uptake of tumor exosomes by bone marrow precursor cells can inhibit maturation and promote induction of myeloid-derived suppressor cells [65, 66].
B Cells
In addition to APCs like macrophages and DCs, B cells are also capable of internalizing exosomes. B lymphocytes interact with exosomes containing MHC class II and ICAM-1 from mature DCs and obtain the ability to prime naïve T cells and trigger antigen-specific effector responses [67, 68]. B cells may need specific surface proteoglycans (HSPGs) such as syndecans and glypicans to aid exosome uptake. It was demonstrated that chronic lymphocytic leukemia (CLL) exosomes can be internalized via active uptake by various benign cell populations found in the TME such as endothelial cells, myeloid cells, bone marrow mesenchymal stem cells, and even some leukocytes. However, CLL B cells themselves did not show uptake of labeled exosomes – possibly due to a lack of surface HSPGs [37] or syndecan-1 [69]. Additionally, lymphoid cell differentiation from the pre-B-cells in the bone marrow to the plasma cells that produce and release antibody, and the multiple stages of development in between are accompanied by different syndecan-1 protein levels [69]. Syndecan-1 is present on the Pre-B cells and also on the plasma cells but it is absent on the circulating B cells. What this indicates is that syndecan-1 is needed when cells require tissue environments and interactions [70]. These studies support Syndecan-1’s importance to exosome uptake and why CLL B cells, lacking syndecan-1, are unable to, by themselves, uptake exosomes. In a separate study, malignant B cell exosomes showed a natural specificity for B lymphocytes while in another B cells are selectively targeted by exosomes carrying the EBV envelope glycoprotein gp350 [71]. Furthermore, the interaction between an EBV-transformed B cell line (LCL1)-derived exosomes and peripheral blood B cells could be blocked efficiently by anti gp350 antibodies and by anti-CD21 [71]. Mantle cell lymphoma (MCL) exosomes were efficiently internalized by both healthy and diseased B-lymphocytes utilizing a cholesterol dependent pathway independent of clathrin and caveolin [72]. Very little uptake was recorded in bone marrow stroma cell lines, T-cell leukemia cells, or NK cells.
Exosomes have been relatively well-studied in EBV-positive transformed human B cell lines, as these cells constitutively produce large numbers of MVBs and MHC class II molecules [73–75]. The WSU-DLCL2 B cell lymphoma cell line used in our own study [49] as a source of exosomes, is EBV-free. This may be of interest because the virus has been known to hijack and alter exosomes in infected cells. The internalization and subsequent effects of these exosomes may involve viral factors, such as latent membrane protein (LMP). One group examined epithelial uptake of exosomes from EBV-infected B lymphocytes and found uptake was through a dynamin and caveolae-dependent process. In addition, type III latency-derived exosomes were able to induce proliferation and upregulation of ICAM-1 in recipient cells [76]. LMP-1 was also harbored on exosomes from a Burkitt’s lymphoma cell line, and could mimic CD40 signaling to induce stimulatory changes in the B cells that efficiently bound them [77].
T Cells and NK Cells
LMP-1 can also produce an immunosuppressive effect by inhibiting T cell proliferation and NK cytotoxicity [78] and has been shown co-localized with MHC-II on exosomes. Though these studies appear supportive it is still unclear whether T cells can truly obtain antigen/MHC signals from exosomes or EV’s. Dendritic cells have been shown to require dendritic cell derived exosome or DEX’s in order to activate T cells [79, 80]. Specifically, these studies show that in addition to carrying antigen, exosomes promote DC exchange of functional peptide-MHC complexes [80]. T cells have been shown in defined conditions to be activated by antigen presenting cell (APC)-secreted exosomes. Under physiologic conditions however, T cell activation required simply contact with a viable APC. However, further supporting the co-stimulatory scenario is the fact that T cells, in order to be activated, must make contact with B7, ICAM-1 CD28 and LFA-1 [81]. What this might mean to exosome stimulation of T cells is that the signals are weak at best, or that specific requirements such as high MHC density and the presence of ICAM1 and B7 are critical.
There have been few studies investigating uptake of exosomes by peripheral blood cell populations. However, rat pancreatic adenocarcinoma exosomes could be taken up by all leukocyte subpopulations examined, with CD11b + cells demonstrating higher internalization than T or B cells [82]. At this time there is only one other publication addressing peripheral blood uptake of lymphoma exosomes - a study by Hazan-Halevy et al., looking at MCL exosomes and their preferential uptake by B-lymphocytes [72]. In this study, it was shown that exosomes isolated from a MCL cell line, when administered to B lymphocytes, NK cells, and various T lymphocytes, preferentially internalized into B lymphocytes.
While effector cells such as T cells and NK cells are less equipped to internalize vesicles, there is still evidence for a variety of interactions with exosomes. T lymphocytes are affected by exosomes from APCs harboring antigen in MHC class I and II molecules, and constitute an important aspect of immune system communication [73, 83]. The mechanisms of T cell internalization or binding of exosomes from APCs have been posited to involve the T-cell receptor (TCR), CD28, and LFA-1 (CD11a) [84]. Activated T cells use LFA-1 (leukocyte function-associated antigen-1) for binding of DC exosomes containing MHC class II [85]. This LFA-1/ICAM interaction is critical for priming of naïve T cells by exosomes from mature DCs [67]. CD4+ T cells can internalize DC exosomes and stimulate antigen-specific CD8+ CTL while overcoming Treg suppression, with a resultant shift in immune responses [11]. In contrast to T cell priming effects of exosomes, tumor exosomes (TEX) can suppress T cells. Surface ligands such as TRAIL, PD-L1 and FasL result in exosome-mediated cell death [86, 87].
Evidently, despite low exosome internalization, T cells are still subject to exosome-mediated effects. Likewise, even with little uptake, NK cells are influenced through exosomal interactions. NK cell cytotoxicity is frequently seen to be inhibited after exposure to exosomes derived from solid tumors and even EBV-immortalized B cells. It has been surmised that MICB and TGF-β1 expressed on exosomes are responsible [88–90]. One mechanism is through the downregulation of NK activating receptor NKG2D, as exemplified by plasma exosomes from AML patients [91]. In contrast to tumor derived exosomes, dendritic cell derived exosomes can promote NK activation and proliferation through copresentation of NKG2DL with IL-15Ra [92]. Some studies have found evidence of NK cell uptake of exosomes in a time dependent fashion, perhaps utilizing PS located on vesicle membranes as demonstrated in ovarian cancer model [93].
Conclusion
Exosomes are important mediators and regulators of cellular communication. Although they are involved in active immunosuppression, they can also facilitate tumor progression and are also a good source of tumor antigens. However, until a more full understanding of the interplay between the tumor microenvironment and the exosome occurs, effective strategies to mobilize the immune system as an effective anticancer modality will be limited. Recognizing the luminal and surface contents of the exosome is not enough to design exosome-associated therapy but understanding the communication patterns and types of communication (luminal protein delivered or surface to cell protein/protein interaction signaling) will be key. Moreover, identifying which exosome populations are communicating and which are providing additional ligands or receptors in order to facilitate communication will prove necessary to potentiate the immune response.
Funding
Research reported in this publication was supported by NIH awards P20MD006988.
References
- 1.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 2.Ratajczak J, Miekus K, Kucia M, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20(5):847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 3.Iero M, Valenti R, Huber V, et al. Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ. 2008;15(1):80–88. doi: 10.1038/sj.cdd.4402237. [DOI] [PubMed] [Google Scholar]
- 4.Bobrie A, Krumeich S, Reyal F, et al. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res. 2012;72(19):4920–4930. doi: 10.1158/0008-5472.CAN-12-0925. [DOI] [PubMed] [Google Scholar]
- 5.Kalluri R. The biology and function of exosomes in cancer. J Clin Invest. 2016;126(4):1208–1215. doi: 10.1172/JCI81135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng D, Zhao W-L, Ye Y-Y, et al. Cellular internalization of exosomes occurs through phagocytosis. Traffic. 2010;11(5):675–687. doi: 10.1111/j.1600-0854.2010.01041.x. [DOI] [PubMed] [Google Scholar]
- 7.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9(8):581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 8.Zhang HG, Grizzle WE. Exosomes and cancer: a newly described pathway of immune suppression. Clin Cancer Res. 2011;17(5):959–964. doi: 10.1158/1078-0432.CCR-10-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolfers J, Lozier A, Raposo G, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7(3):297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 10.Mallegol J, van Niel G, Heyman M. Phenotypic and functional characterization of intestinal epithelial exosomes. Blood Cell Mol Dis. 2005;35(1):11–16. doi: 10.1016/j.bcmd.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Hao S, Bai O, Li F, Yuan J, Laferte S, Xiang J. Mature dendritic cells pulsed with exosomes stimulate efficient cytotoxic T-lymphocyte responses and antitumour immunity. Immunology. 2007;120(1):90–102. doi: 10.1111/j.1365-2567.2006.02483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeelenberg IS, Ostrowski M, Krumeich S, et al. Targeting tumor antigens to secreted membrane vesicles in vivo induces efficient antitumor immune responses. Cancer Res. 2008;68(4):1228–1235. doi: 10.1158/0008-5472.CAN-07-3163. [DOI] [PubMed] [Google Scholar]
- 13.Zeelenberg IS, van Maren WWC, Boissonnas A, et al. Antigen localization controls T cell-mediated tumor immunity. J Immunol. 2011;187(3):1281–1288. doi: 10.4049/jimmunol.1003905. [DOI] [PubMed] [Google Scholar]
- 14.Safaei R, Larson BJ, Cheng TC, et al. Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Mol Cancer Ther. 2005;4(10):1595–1604. doi: 10.1158/1535-7163.MCT-05-0102. [DOI] [PubMed] [Google Scholar]
- 15.Shedden K, Xie XT, Chandaroy P, Chang YT, Rosania GR. Expulsion of small molecules in vesicles shed by Cancer cells: association with gene expression and chemosensitivity profiles. Cancer Res. 2003;63(15):4331–4337. [PubMed] [Google Scholar]
- 16.Khan S, Aspe JR, Asumen MG, et al. Extracellular, cell-permeable survivin inhibits apoptosis while promoting proliferative and metastatic potential. Br J Cancer. 2009;100(7):1073–1086. doi: 10.1038/sj.bjc.6604978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Hendrix A, Hernot S, et al. Bone marrow stromal cell–derived exosomes as communicators in drug resistance in multiple myeloma cells. Blood. 2014;124(4):555–566. doi: 10.1182/blood-2014-03-562439. [DOI] [PubMed] [Google Scholar]
- 18.D-d Y, Wu Y, Zhang X-h, et al. Exosomes from adriamycin-resistant breast cancer cells transmit drug resistance partly by delivering miR-222. Tumor Biol. 2016;37(3):3227–3235. doi: 10.1007/s13277-015-4161-0. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell P, Welton J, Staffurth J, et al. Can urinary exosomes act as treatment response markers in prostate cancer? J Transl Med. 2009;7(1):4. doi: 10.1186/1479-5876-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110(1):13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 21.Skog J, Wurdinger T, van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welton JL, Khanna S, Giles PJ, et al. Proteomics analysis of bladder cancer exosomes. Mol Cell Proteomics. 2010;9(6):1324–1338. doi: 10.1074/mcp.M000063-MCP201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keller S, Ridinger J, Rupp A-K, Janssen J, Altevogt P. Body fluid derived exosomes as a novel template for clinical diagnostics. J Transl Med. 2011;9(1):86. doi: 10.1186/1479-5876-9-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong C-S, Miller L, Whiteside TL, Boyiadzis M (2014) Plasma exosomes as markers of therapeutic response in patients with acute myeloid leukemia. Front Immunol 5:160 [DOI] [PMC free article] [PubMed]
- 25.Turay D, Khan S, Osterman CJD, et al. Proteomic profiling of serum-derived exosomes from ethnically diverse prostate Cancer patients. Cancer Investig. 2016;34(1):1–11. doi: 10.3109/07357907.2015.1081921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deregibus MC, Cantaluppi V, Calogero R, et al. Endothelial progenitor cell–derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110(7):2440–2448. doi: 10.1182/blood-2007-03-078709. [DOI] [PubMed] [Google Scholar]
- 27.Kawamoto T, Ohga N, Akiyama K, et al. Tumor-derived microvesicles induce proangiogenic phenotype in endothelial cells via endocytosis. PLoS One. 2012;7(3):e34045. doi: 10.1371/journal.pone.0034045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kosaka N, Iguchi H, Hagiwara K, Yoshioka Y, Takeshita F, Ochiya T. Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic MicroRNAs regulate cancer cell metastasis. J Biol Chem. 2013;288(15):10849–10859. doi: 10.1074/jbc.M112.446831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graves LE, Ariztia EV, Navari JR, Matzel HJ, Stack MS, Fishman DA. Proinvasive properties of ovarian cancer ascites-derived membrane vesicles. Cancer Res. 2004;64(19):7045–7049. doi: 10.1158/0008-5472.CAN-04-1800. [DOI] [PubMed] [Google Scholar]
- 30.Hood JL, San RS, Wickline SA. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011;71(11):3792–3801. doi: 10.1158/0008-5472.CAN-10-4455. [DOI] [PubMed] [Google Scholar]
- 31.Luga V, Zhang L, Viloria-Petit Alicia M, et al. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151(7):1542–1556. doi: 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 32.Peinado H, Aleckovic M, Lavotshkin S, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18(6):883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suetsugu A, Honma K, Saji S, Moriwaki H, Ochiya T, Hoffman RM. Imaging exosome transfer from breast cancer cells to stroma at metastatic sites in orthotopic nude-mouse models. Adv Drug Deliv Rev. 2013;65(3):383–390. doi: 10.1016/j.addr.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Mulcahy LA, Pink RC, Carter DRF (2014) Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles 3. 10.3402/jev.v3403.24641 [DOI] [PMC free article] [PubMed]
- 35.Nazarenko I, Rana S, Baumann A, et al. Cell surface Tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res. 2010;70(4):1668–1678. doi: 10.1158/0008-5472.CAN-09-2470. [DOI] [PubMed] [Google Scholar]
- 36.Rana S, Yue S, Stadel D, Zöller M. Toward tailored exosomes: the exosomal tetraspanin web contributes to target cell selection. Int J Biochem Cell Biol. 2012;44(9):1574–1584. doi: 10.1016/j.biocel.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 37.Paggetti J, Haderk F, Seiffert M, et al. Exosomes released by chronic lymphocytic leukemia cells induce the transition of stromal cells into cancer-associated fibroblasts. Blood. 2015;126(9):1106–1117. doi: 10.1182/blood-2014-12-618025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Obregon C, Rothen-Rutishauser B, Gerber P, Gehr P, Nicod LP. Active uptake of dendritic cell-derived exovesicles by epithelial cells induces the release of inflammatory mediators through a TNF-α-mediated pathway. Am J Pathol. 2009;175(2):696–705. doi: 10.2353/ajpath.2009.080716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clayton A, Turkes A, Dewitt S, Steadman R, Mason MD, Hallett MB. Adhesion and signaling by B cell-derived exosomes: the role of integrins. FASEB J. 2004;18:977–979. doi: 10.1096/fj.03-1094fje. [DOI] [PubMed] [Google Scholar]
- 40.Franzen CA, Simms PE, Van Huis AF, Foreman KE, Kuo PC, Gupta GN. Characterization of uptake and internalization of exosomes by bladder cancer cells. Biomed Res Int. 2014;2014:619829. doi: 10.1155/2014/619829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parolini I, Federici C, Raggi C, et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284(49):34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smyth TJ, Redzic JS, Graner MW, Anchordoquy TJ. Examination of the specificity of tumor cell derived exosomes with tumor cells in vitro. Biochim Biophys Acta Biomembr. 2014;1838(11):2954–2965. doi: 10.1016/j.bbamem.2014.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Escrevente C, Keller S, Altevogt P, Costa J. Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer. 2011;11(1):1–10. doi: 10.1186/1471-2407-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Svensson KJ, Christianson HC, Wittrup A, et al. Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid raft-mediated endocytosis negatively regulated by Caveolin-1. J Biol Chem. 2013;288(24):17713–17724. doi: 10.1074/jbc.M112.445403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Christianson HC, Svensson KJ, van Kuppevelt TH, Li J-P, Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci. 2013;110(43):17380–17385. doi: 10.1073/pnas.1304266110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chiba M, Kimura M, Asari S. Exosomes secreted from human colorectal cancer cell lines contain mRNAs, microRNAs and natural antisense RNAs, that can transfer into the human hepatoma HepG2 and lung cancer A549 cell lines. Oncol Rep. 2012;28(5):1551–1558. doi: 10.3892/or.2012.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Brien K, Rani S, Corcoran C, et al. Exosomes from triple-negative breast cancer cells can transfer phenotypic traits representing their cells of origin to secondary cells. Eur J Cancer. 2013;49(8):1845–1859. doi: 10.1016/j.ejca.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 48.Riches A, Campbell E, Borger E, Powis S. Regulation of exosome release from mammary epithelial and breast cancer cells – a new regulatory pathway. Eur J Cancer. 2014;50(5):1025–1034. doi: 10.1016/j.ejca.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 49.Bennit HF, Gonda A, Oppegard L, Chi D, Khan S, Wall NR (2017) Uptake of lymphoma-derived exosomes by peripheral blood leukocytes. Blood and Lymphatic Cancer: Targets and Therapy 7:9–23 [DOI] [PMC free article] [PubMed]
- 50.Lässer C, Seyed Alikhani V, Ekström K, et al. Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. J Transl Med. 2011;9(1):1–8. doi: 10.1186/1479-5876-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chow A, Zhou W, Liu L, et al. Macrophage immunomodulation by breast cancer-derived exosomes requires Toll-like receptor 2-mediated activation of NF-κB. Sci Rep. 2014;4:5750. doi: 10.1038/srep05750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saunderson SC, Dunn AC, Crocker PR, McLellan AD. CD169 mediates the capture of exosomes in spleen and lymph node. Blood. 2014;123(2):208–216. doi: 10.1182/blood-2013-03-489732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barrès C, Blanc L, Bette-Bobillo P, et al. Galectin-5 is bound onto the surface of rat reticulocyte exosomes and modulates vesicle uptake by macrophages. Blood. 2010;115(3):696–705. doi: 10.1182/blood-2009-07-231449. [DOI] [PubMed] [Google Scholar]
- 54.Danesh A, Inglis HC, Jackman RP, et al. Exosomes from red blood cell units bind to monocytes and induce proinflammatory cytokines, boosting T-cell responses in vitro. Blood. 2014;123(5):687–696. doi: 10.1182/blood-2013-10-530469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cai J, Han Y, Ren H, et al. Extracellular vesicle-mediated transfer of donor genomic DNA to recipient cells is a novel mechanism for genetic influence between cells. J Mol Cell Biol. 2013;5(4):227–238. doi: 10.1093/jmcb/mjt011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cai J, Wu G, Tan X, et al. Transferred BCR/ABL DNA from K562 extracellular vesicles causes chronic myeloid leukemia in immunodeficient mice. PLoS One. 2014;9(8):e105200. doi: 10.1371/journal.pone.0105200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morelli AE, Larregina AT, Shufesky WJ, et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104(10):3257–3266. doi: 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- 58.Wubbolts R, Leckie RS, Veenhuizen PT, et al. Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. J Biol Chem. 2003;278(13):10963–10972. doi: 10.1074/jbc.M207550200. [DOI] [PubMed] [Google Scholar]
- 59.Segura E, Guerin C, Hogg N, Amigorena S, Thery C. CD8+ dendritic cells use LFA-1 to capture MHC-peptide complexes from exosomes in vivo. J Immunol. 2007;179(3):1489–1496. doi: 10.4049/jimmunol.179.3.1489. [DOI] [PubMed] [Google Scholar]
- 60.Xie J, Zhu H, Guo L, et al. Lectin-like oxidized low-density lipoprotein receptor-1 delivers heat shock protein 60-fused antigen into the MHC class I presentation pathway. J Immunol. 2010;185(4):2306–2313. doi: 10.4049/jimmunol.0903214. [DOI] [PubMed] [Google Scholar]
- 61.Bastos-Amador P, Perez-Cabezas B, Izquierdo-Useros N, et al. Capture of cell-derived microvesicles (exosomes and apoptotic bodies) by human plasmacytoid dendritic cells. J Leukoc Biol. 2012;91(5):751–758. doi: 10.1189/jlb.0111054. [DOI] [PubMed] [Google Scholar]
- 62.Czernek L, Chworos A, Duechler M. The uptake of extracellular vesicles is affected by the differentiation status of myeloid cells. Scand J Immunol. 2015;82(6):506–514. doi: 10.1111/sji.12371. [DOI] [PubMed] [Google Scholar]
- 63.Sheng H, Hassanali S, Nugent C, et al. Insulinoma-released exosomes or microparticles are immunostimulatory and can activate autoreactive T cells spontaneously developed in nonobese diabetic mice. J Immunol. 2011;187(4):1591–1600. doi: 10.4049/jimmunol.1100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Skokos D, Botros HG, Demeure C, et al. Mast cell-derived exosomes induce phenotypic and functional maturation of dendritic cells and elicit specific immune responses in vivo. J Immunol. 2003;170(6):3037–3045. doi: 10.4049/jimmunol.170.6.3037. [DOI] [PubMed] [Google Scholar]
- 65.Yu S, Liu C, Su K, et al. Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J Immunol. 2007;178(11):6867–6875. doi: 10.4049/jimmunol.178.11.6867. [DOI] [PubMed] [Google Scholar]
- 66.Xiang X, Poliakov A, Liu C, et al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer. 2009;124(11):2621–2633. doi: 10.1002/ijc.24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Segura E, Amigorena S, Thery C. Mature dendritic cells secrete exosomes with strong ability to induce antigen-specific effector immune responses. Blood Cells Mol Dis. 2005;35(2):89–93. doi: 10.1016/j.bcmd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 68.Segura E, Nicco C, Lombard B, et al. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood. 2005;106(1):216–223. doi: 10.1182/blood-2005-01-0220. [DOI] [PubMed] [Google Scholar]
- 69.Kopper L, Sebestyen A, Gallai M, Kovalszky I. Syndecan-1 - a new piece in B-cell puzzle. Pathol Oncol Res. 1997;3(3):183–191. doi: 10.1007/BF02899919. [DOI] [PubMed] [Google Scholar]
- 70.Sanderson RD, Lalor P, Bernfield M. B lymphocytes express and lose syndecan at specific stages of differentiation. Cell Regul. 1989;1(1):27–35. doi: 10.1091/mbc.1.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vallhov H, Gutzeit C, Johansson SM, et al. Exosomes containing glycoprotein 350 released by EBV-transformed B cells selectively target B cells through CD21 and block EBV infection in vitro. J Immunol. 2011;186(1):73–82. doi: 10.4049/jimmunol.1001145. [DOI] [PubMed] [Google Scholar]
- 72.Hazan-Halevy I, Rosenblum D, Weinstein S, Bairey O, Raanani P, Peer D. Cell-specific uptake of mantle cell lymphoma-derived exosomes by malignant and non-malignant B-lymphocytes. Cancer Lett. 2015;364(1):59–69. doi: 10.1016/j.canlet.2015.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Raposo G, Nijman HW, Stoorvogel W, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183(3):1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem. 1998;273(32):20121–20127. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- 75.Dolcetti R. Cross-talk between Epstein-Barr virus and microenvironment in the pathogenesis of lymphomas. Semin Cancer Biol. 2015;34:58–69. doi: 10.1016/j.semcancer.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 76.Nanbo A, Kawanishi E, Yoshida R, Yoshiyama H. Exosomes derived from Epstein-Barr virus-infected cells are internalized via caveola-dependent endocytosis and promote phenotypic modulation in target cells. J Virol. 2013;87(18):10334–10347. doi: 10.1128/JVI.01310-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gutzeit C, Nagy N, Gentile M, et al. Exosomes derived from Burkitt's lymphoma cell lines induce proliferation, differentiation, and class-switch recombination in B cells. J Immunol. 2014;192(12):5852–5862. doi: 10.4049/jimmunol.1302068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dukers DF, Meij P, Vervoort MB, et al. Direct immunosuppressive effects of EBV-encoded latent membrane protein 1. J Immunol. 2000;165(2):663–670. doi: 10.4049/jimmunol.165.2.663. [DOI] [PubMed] [Google Scholar]
- 79.Vincent-Schneider H, Stumptner-Cuvelette P, Lankar D, et al. Exosomes bearing HLA-DR1 molecules need dendritic cells to efficiently stimulate specific T cells. Int Immunol. 2002;14(7):713–722. doi: 10.1093/intimm/dxf048. [DOI] [PubMed] [Google Scholar]
- 80.Thery C, Duban L, Segura E, Veron P, Lantz O, Amigorena S. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3(12):1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 81.Sprent J. Direct stimulation of naive T cells by antigen-presenting cell vesicles. Blood Cells Mol Dis. 2005;35(1):17–20. doi: 10.1016/j.bcmd.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 82.Zech D, Rana S, Buchler MW, Zoller M. Tumor-exosomes and leukocyte activation: an ambivalent crosstalk. Cell Commun Signal. 2012;10(1):37. doi: 10.1186/1478-811X-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Arnold PY, Mannie MD. Vesicles bearing MHC class II molecules mediate transfer of antigen from antigen-presenting cells to CD4+ T cells. Eur J Immunol. 1999;29(4):1363–1373. doi: 10.1002/(SICI)1521-4141(199904)29:04<1363::AID-IMMU1363>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 84.Hwang I, Ki D. Receptor-mediated T cell absorption of antigen presenting cell-derived molecules. Front Biosci (Landmark Ed) 2011;16:411–421. doi: 10.2741/3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nolte-'t Hoen EN, Buschow SI, Anderton SM, Stoorvogel W, Wauben MH. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood. 2009;113(9):1977–1981. doi: 10.1182/blood-2008-08-174094. [DOI] [PubMed] [Google Scholar]
- 86.Stenqvist AC, Nagaeva O, Baranov V, Mincheva-Nilsson L. Exosomes secreted by human placenta carry functional Fas ligand and TRAIL molecules and convey apoptosis in activated immune cells, suggesting exosome-mediated immune privilege of the fetus. J Immunol. 2013;191(11):5515–5523. doi: 10.4049/jimmunol.1301885. [DOI] [PubMed] [Google Scholar]
- 87.Wang R, Xu A, Zhang X, Wu J et al (2017) Novel exosome-targeted T-cell-based vaccine counteracts T-cell anergy and converts CTL exhaustion in chronic infection via CD40L signaling through the mTORC1 pathway. Cell Mol Immunol 14(6):529–545 [DOI] [PMC free article] [PubMed]
- 88.Clayton A, Mitchell JP, Court J, Linnane S, Mason MD, Tabi Z. Human tumor-derived exosomes down-modulate NKG2D expression. J Immunol. 2008;180(11):7249–7258. doi: 10.4049/jimmunol.180.11.7249. [DOI] [PubMed] [Google Scholar]
- 89.Clayton A, Tabi Z. Exosomes and the MICA-NKG2D system in cancer. Blood Cells Mol Dis. 2005;34(3):206–213. doi: 10.1016/j.bcmd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 90.Ashiru O, Boutet P, Fernandez-Messina L, et al. Natural killer cell cytotoxicity is suppressed by exposure to the human NKG2D ligand MICA*008 that is shed by tumor cells in exosomes. Cancer Res. 2010;70(2):481–489. doi: 10.1158/0008-5472.CAN-09-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hong CS, Muller L, Whiteside TL, Boyiadzis M. Plasma exosomes as markers of therapeutic response in patients with acute myeloid leukemia. Front Immunol. 2014;5:160. doi: 10.3389/fimmu.2014.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Viaud S, Terme M, Flament C, et al. Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15Ralpha. PLoS One. 2009;4(3):e4942. doi: 10.1371/journal.pone.0004942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Keller S, Konig AK, Marme F, et al. Systemic presence and tumor-growth promoting effect of ovarian carcinoma released exosomes. Cancer Lett. 2009;278(1):73–81. doi: 10.1016/j.canlet.2008.12.028. [DOI] [PubMed] [Google Scholar]


