Abstract
Objectives
To determine whether over the counter cough medicines are effective for acute cough in adults.
Design
Systematic review of randomised controlled trials.
Data sources
Search of the Cochrane Acute Respiratory Infections Group specialised register, Cochrane Controlled Trials Register, Medline, Embase, and the UK Department of Health National Research Register in all languages.
Included studies
All randomised controlled trials that compared oral over the counter cough preparations with placebo in adults with acute cough due to upper respiratory tract infection in ambulatory settings and that had cough symptoms as an outcome.
Results
15 trials involving 2166 participants met all the inclusion criteria. Antihistamines seemed to be no better than placebo. There was conflicting evidence on the effectiveness of antitussives, expectorants, antihistamine-decongestant combinations, and other drug combinations compared with placebo.
Conclusion
Over the counter cough medicines for acute cough cannot be recommended because there is no good evidence for their effectiveness. Even when trials had significant results, the effect sizes were small and of doubtful clinical relevance. Because of the small number of trials in each category, the results have to be interpreted cautiously.
What is already know on this topic
The NHS encourages self treatment of acute self limiting illnesses
Over the counter cough medicines are commonly used as first line treatment for acute cough
What this study adds
There is little evidence for or against the effectiveness of over the counter cough medicines
Although cough medicines are generally well tolerated, they may be an unnecessary expense
Recommendation of over the counter cough medicines to patients is not justified by current evidence
Introduction
General practitioners and other health professionals are encouraged to recommend over the counter cough medicines as a first line treatment for acute cough,1 but evidence regarding their effectiveness is inconclusive. The NHS direct healthcare guide also recommends simple cough medicines for dry cough.2
Acute cough is a common symptom. In 1991-2, there were over 4000 consultations per 10 000 patient years in general practice for acute respiratory infections.3 Cough medicines are widely available to the public without medical prescription in most countries, and retail sales rose by 3.0% to £94m between 1998 and 1999 in the United Kingdom.4 However, many studies of cough preparations have involved patients from different populations and included participants with chronic cough due to underlying disease or were carried out on healthy volunteers in whom cough had been induced artificially through chemical irritants.5–8 Previous systematic reviews have either focused on children or were limited to trials retrieved from Medline.9–11 We conducted this systematic review to determine whether over the counter cough medicines are effective for acute cough due to upper respiratory tract infections in adults. This review is based on a Cochrane systematic review of over the counter treatments in adults and children.12
Methods
Searching
We searched the Cochrane Acute Respiratory Infections Group specialised register (database of studies of acute respiratory infections based on regular database searches, personal contributions from Cochrane review group members, and hand searching of journals), the Cochrane Controlled Trials Register (issue 2, 2000, which includes randomised controlled trials published in Medline and Embase up to 1998), Medline (January 1998 to December 1999), Embase (January 1998 to December 1999), the UK Department of Health National Research Register (December 2000), personal collections of references, and reference lists of all retrieved articles for original randomised controlled trials (box). We wrote to study authors, the Proprietary Association of Great Britain, and pharmaceutical companies for information on unpublished studies. We considered studies in all languages regardless of publication status.
Search strategy
cough
cough*:ME
(#1 or #2)
antitussive-agents*:ME
expectorants*:ME
cholinergic-antagonists*:ME
drug-combinations*:ME
prescriptions-non-drug*:ME
#4 or #5 or #6 or #7 or #8 or #9
#3 and #10
cough
(common next cold)
colds
#12 or #13 or #14
antitussiv*
expectorant*
antihistamin*
anticholinergic*
suppressant*
mucolytic*
(drug next combinations)
over-the-counter
non-prescription*
#16 or #17 or #18 or #19 or #20 or #21 or #22 or #23
#15 and #24
#11 or #25
*for searching the Cochrane Controlled Trials Register. Slightly amended versions were used for searching Medline and Embase
Study selection and validity assessment
We selected studies for review if (a) the participants were adults (aged 16 years or older) with acute cough (less than three weeks' duration) due to upper respiratory tract infection (presumed to be viral in origin with no auscultatory chest signs or signs on chest radiography) in an ambulatory setting; (b) the interventions were over the counter cough preparations; (c) a reported outcome was cough (frequency or duration assessed with any assessment tool); and (d) studies were randomised controlled trials with a contemporaneous control group receiving placebo or no intervention. We excluded studies if participants had chronic cough (more than three weeks' duration or due to a chronic underlying disease such as asthma, tuberculosis, or bronchial malignancy); cough was artificially induced in healthy volunteers; or they used non-conventional (herbal or homoeopathic) or non-oral preparations.
Both authors assessed relevant citations independently and applied the selection criteria with the help of an in/out/pending sheet, which was filled out in duplicate. We resolved differences in opinion at any stage of the review by discussion. A study had to meet all our inclusion criteria to be included. We also extracted data and assessed the quality of studies independently. If necessary, we contacted study authors for additional information and data. For studies written in languages other than English or German we obtained translations of abstracts or papers. We did not mask studies with regard to trial authors or journals. We listed data on potential sources of bias such as randomisation, blinding, and follow up in a table (table 1) instead of applying a quality score. Drugs were divided into six categories according to their mode of action (table 2).
Table 1.
Quality assessment of included trials and potential sources of bias
| Study | Randomisation process used | Blinding to treatment allocation
|
No (%) of dropouts/losses to follow up
|
Power calculation reported | Hypothesis stated before data collection | Comments | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Provider | Outcome assessor | Total | Intervention group | Control group | Reasons given | ||||||||
| Antitussives | ||||||||||||||
| Eccles 1992 | NR | Yes | Yes | NR | 10/91 (11) | NR | NR | No | No | No | Two separate phases of study (laboratory and home) | |||
| Adams 1993 | NR | Yes | Yes | NR | 11/108 (10) | NR | NR | Yes | No | No | Newly developed peripherally acting antitussive, trial supported by pharmaceutical company | |||
| Parvez 1996 | Minimisation using computer program | Yes | Yes | NR | NR | NR | NR | NR | No | No | Many multiple comparisons with no corrections and high probability of type I error. Dropouts unlikely because of short length of follow up | |||
| Freestone 1997 | NR | Yes | Yes | NR | NR | NR | NR | NR | No | No | Potential sources of bias poorly reported | |||
| Lee 2000 | NR | Yes | Yes | NR | 1/44 (2) | NR | NR | Yes | No | Yes | ||||
| Expectorants | ||||||||||||||
| Robinson 1977 | NR | Yes | Yes | Yes | 27/239 (11) | 14/121 (12) | 13/118 (11) | No | No | Yes | ||||
| Kuhn 1982 | NR | Yes | Yes | NR | None | None | None | NR | No | Yes | Aspirin and paracetamol were allowed after inclusion in the study. Vehicle contained 95% alcohol | |||
| Mucolytics | ||||||||||||||
| Nesswetha 1967 | No | Yes | Yes | NR | 7/99 (7) | NR | NR | No | No | Yes | Potential sources of bias poorly reported | |||
| Antihistamine-decongestant combinations | ||||||||||||||
| Curley 1988 | Computer generated | Yes | Yes | NR | 13/86 (15) | 6/44 (14) | 7/42 (17) | Yes | No | No | Patients “randomised in a double-blind fashion”; dropouts due to inconvenience of study and none due to side effects | |||
| Berkowitz 1989 | Computer generated | Yes | Yes | Yes | 22/283 (8) | 9/142 (6) | 13/141 (9) | Yes | No | No | Many multiple comparisons made | |||
| Other combinations | ||||||||||||||
| Kurth 1978 | NR | Yes | NR | NR | 6/113 (5) | NR | NR | NR | No | Yes | High likelihood of bias | |||
| Thackray 1978 | “Random number code” | Yes | Yes | NR | 0 | NR | NR | NR | No | Yes | Investigator was medical director of the company supplying the drug for study. Crossover after 1 day, no washout period | |||
| Tukiainen 1986 | NR | Yes | Yes | NR | 0 | NR | NR | NR | No | Yes | Losses to follow up not reported | |||
| Antihistamines | ||||||||||||||
| Gaffey 1988 | NR | NR | NR | NR | 16/250 (6) | 7/126 (6) | 9/124 (7) | NR | No | No | Subjects were “compensated” for participation, blinding presumed but not clearly stated, subjects received “sequential admission numbers and were randomly assigned” active treatment or placebo. Non-compliers were considered dropouts. Other drugs taken: aspirin/non-steroidal anti-inflammatory in 7 patients, paracetamol 7 patients | |||
| Berkowitz 1991 | NR | NR | NR | NR | 4/100 (4) | NR | NR | Yes | Yes | Yes | Patients “randomly assigned,” blinding presumed but not clearly stated | |||
NR=not reported or unclear.
Table 2.
Method of action and examples of different types of over the counter cough medicines
| Group | Mechanism of action | Examples of proposed active ingredients | Examples of relevant preparations |
|---|---|---|---|
| Antitussives | Centrally acting opioid derivates or peripherally acting agents5 | Codeine, moguisteine | Famel original cough syrup |
| Dextromethorphan | Benylin dry coughs, Robitussin dry cough | ||
| Expectorants | Increased bronchial mucus production, making secretions easier to remove through cough or ciliary transport13 | Guaifenesin, ipecacuana | Adult Meltus expectorant, Beechams VENO's expectorant, Benylin chesty coughs (non-drowsy), Benylin children's chesty coughs, Hill's balsam chesty cough liquid, Vicks vaposyrup for chesty coughs |
| Mucolytics | Decrease the viscosity of bronchial secretions, making them easier to clear through coughing14 | Bromhexine hydrochloride | Bisolvon linctus |
| Antihistamine-decongestant combinations | Combine histamine H1 receptor antagonists and α adrenoceptor agonists, which cause vasoconstriction of mucosal blood vessels15 | Pseudoephedrine plus guaifenesin | Sudafed expectorant, Robitussin chesty cough with congestion |
| Other drug combinations | Fixed drug combinations using different ingredients | Dextromethorphan, ephedrine, doxylamine, paracetamol | Vicks Medinite |
| EM-Vier (containing thyme extract, eucalyptus oil, and menthol) | Minetten | ||
| Antihistamines | Histamine H1 receptor antagonists | Loratadine | Clarityn allergy syrup |
Results
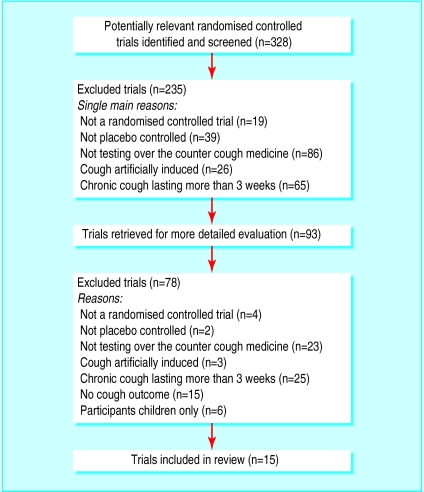
After evaluating 328 citations and abstracts from all sources, we included 15 trials involving 2166 participants (figure).16–30
Table 3 shows the main characteristics of the included randomised controlled trials. The number of studies for each type of drug was small, ranging from one to five. Outcomes included frequency and severity of cough and were measured in many different ways—for example, self report, physician assessment, cough sound pressure levels, and tape recordings. Ten studies reported data on adverse effects.
Table 3.
Characteristics of randomised controlled trials of over the counter cough preparations versus placebo for acute cough
| Study | Participants, setting, country | Definition of illness | Intervention
|
Method of measuring main cough outcomes | Results
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Dose (mg) | Frequency | Treatment duration | Efficacy | Adverse effects | |||||||
| Antitussives | ||||||||||||
| Eccles 1992 | 81 adults, mean age 23 years (18 to 71), 52% men; hospital research clinic, UK | Cough associated with URTI | Codeine | 30 | Four times daily | 4 days | Cough severity score (5 point scale) from diaries expressed as area under curve for 8 measures over 5 days | Mean cough scores 18.8 (placebo) v 17.2 (codeine), P=0.23 | No data provided | |||
| Adams 1993 | 108 adults, mean age 48 years, 70% women, 60% smokers; UK primary care (6 centres) | Acute dry or slightly productive cough | Moguisteine | 20 | Three times daily | 3.5 days | Patient reported cough scale from 0 to 9 | Mean score difference of about 0.5 between groups on days 2 and 3 in patients with severe cough, P<0.05, but no difference at final follow up | Mainly nausea, vomiting and abdominal pain; 22% (active) and 8% (placebo) | |||
| Parvez 1996 | 451 adults in 3 different studies, mean age 30 years, 65% men, mainly non-smokers; corporate healthcare centre, India (combined report of 3 studies) | URTI | Dextromethorphan | 30 | Single dose | — | Cough acoustic signals captured via microphone, visual analogue scales over 180 min | Differences in mean changes between cough counts varied from 19% to 36% (P<0.05) in 3 studies (up to a net difference of 8-10 coughing bouts every 30 min) | No data provided | |||
| Freestone 1997 | 82 university students and staff, mean age 24 years (18 to 46), 62% men; common cold centre at university department, UK | Cough associated with URTI | Codeine | 50 | Single dose | — | 5 point subjective rating scale, cough sound pressure levels, cough frequency | Mean score reductions from 2.0 to 1.0 in both treatment groups (P=0.8); no significant differences for cough sound pressure levels and cough frequency | No data provided | |||
| Lee 2000 | 44 adults aged 18 to 60 years (mean age 23 years), 70% women; university staff and students and general city population, UK | URTI | Dextromethorphan | 30 | Single dose | 3 hours | Cough frequency recordings, cough sound pressure levels, questionnaire on cough severity (scale 0-3) | Decline in cough frequency of 31 (active) v 21.5 (placebo), P=0.38; mean decline in cough score 1 (active) v 0.5 (placebo), P=0.08 | None reported from participants | |||
| Expectorants | ||||||||||||
| Robinson 1977 | 239 adults, mean age 38 years, smokers and non-smokers evenly distributed; office or clinic outpatients, US | URTI | Guaifenesin | 20 | Four times daily | 3 days | Patient questionnaires, cough scores from 0 to 3 | 79/105 (75%) found medicine helpful compared with 33/106 (31%) in the placebo group, P<0.01 | Nausea, hives (2 in active group); headache and drowsiness (2 in placebo group) | |||
| Kuhn 1982 | 65 adults (mostly university students), age range 18 to 30 years; university research centre, US | Acute respiratory illness with cough for <48 h | Guaifenesin | 480 | Every 6 hours | 30 hours | Tape recordings of cough frequency, questionnaire on 6 symptoms | Cough frequency: 33/33 (100%) improved in active group v 30/32 (94%) in placebo group, P=0.5. Cough severity: 33/33 (100%) improved in active treatment group v 29/32 (91%) in placebo group, P=0.2 | No data provided | |||
| Mucolytics | ||||||||||||
| Nesswetha 1967 | 99 factory workers in chemical industry, age range 15 to 44 years; Germany | URTI | Bisolvon linctus (bromhexine hydrochloride) | 4 | Three times daily | Average of 4 days | Not clearly described; used 4 point scale | Frequent cough (every 2-5 min) in 4/46 (9%) in active group v 7/46 (15%) in placebo group (P<0.02) | No data provided | |||
| Antihistamine-decongestant combinations | ||||||||||||
| Curley 1988 | 73 adults, mean age 31 years, 60% women, 19% active smokers; presumably outpatients, US | Common cold for <72 h | Dexbrompheniramine Pseudoephedrine | 6 120 | Twice daily | 1 week | Patient diary, cough score from 0 to 4 | Mean severity cough score 1.4 (active) v 2.0 (placebo) on days 3-5 (P<0.05) | Severity of dizziness and dry mouth significantly increased in active group (P<0.01), but no figures reported | |||
| Berkowitz 1989 | 283 adults, mean age 30 years, mainly white, 52% women; 3 centres in US | Common cold | Loratadine Pseudoephedrine | 5 120 | Twice daily | 5 days | Patient diaries, cough score from 0 to 3 | No significant difference in cough score reduction (0.8 in active group v 0.6 in placebo group, P>0.05) | Dry mouth, headache, and insomnia more common in active group (42/142, 30%) than placebo group (29/141, 21%) | |||
| Other combinations | ||||||||||||
| Kurth 1978 | 113 adults, 57% men, age range <30 to >70 years; primary care, Germany | Cough due to URTI | EM-Vier (Minetten): | Six times daily | 14 days | Unclear | 26/58 (45%) in active treatment group improved within first 3 days v 15/55 (27%) in placebo group (P=0.05) | No adverse effects in both groups | ||||
| Extract of thyme | 5 | |||||||||||
| Succus Liquiritiae depurat Inspiss | 2 | |||||||||||
| Menthol | 3.5 | |||||||||||
| Ephedrine | 2 | |||||||||||
| Eucalyptus oil | 2 | |||||||||||
| Menthae piperitae oil | 0.7 | |||||||||||
| Thackray 1978 | 70 adults, mean age 34 years (range 18 to 60), 61% women; 21 general practices, UK | Common cold | Vicks Medinite syrup: | Single dose at bedtime | 2 days | Questionnaire, 6 point rating scale | Crossover design: 34/59 (58%) subjects rated active treatment as good or better compared with 19/59 (32%) for placebo treatment (P<0.01) | Giddiness or drowsiness reported in 7 (active) and 4 (placebo) participants | ||||
| Dextromethorphan | 15 | |||||||||||
| Ephedrine | 8 | |||||||||||
| Doxylamine | 7.5 | |||||||||||
| Paracetamol | 600 | |||||||||||
| Tukiainen 1986 | 108 outpatients, mean age about 38 years, 55% women, 48% smokers, Finland | Cough associated with URTI | Dextromethorphan (D) | 30 | Three times daily | 4 days | Patient diary and symptom score from 0 to 3 | No significant differences between mean treatment scores for daytime cough on day 4 1.26 (D+S), 1.28 (D), and 1.15 (placebo); no exact P value given. Dextromethorphan/salbutamol more effective in suppressing cough at night than dextromethorphan alone (0.45 v 0.92, P<0.01) | Dextromethorphan/salbutamol led to more tremor than placebo (P<0.05), but no figures were given. No serious adverse effects reported | |||
| Dextromethorphan + salbutamol (D+S) | 30 + 2 | |||||||||||
| Antihistamines | ||||||||||||
| Gaffey 1988 | 250 adults, mean age 23 years, 65% women; internal medicine clinic, US | Common cold | Terfenadine | 60 | Twice daily | 3.5 days | Patient diary and symptom score from 0 to 3 | Syptom scores for cough “virtually the same in the terfenadine and placebo recipient,” but no exact scores reported | Low incidence of adverse effects; most common were sedation or excess fatigue (12% of active group and 10% of placebo group) | |||
| Berkowitz 1991 | 100 adults, mean age 32, 56% women, non-smokers; single centre (setting not stated), US | Common cold | Terfenadine | 120 | Twice daily | 4 to 5 days | Patient diary and symptom score from 0 to 3 | No significant difference in cough scores between active treatment (0.81, SE 0.13) and placebo (0.65, SE 0.12), Pp=0.35 | Low incidence of headache (6% in active group and 4% in placebo group) | |||
URTI=upper respiratory tract infection.
SE=standard error.
The methodological quality of included studies in terms of randomisation, blinding, and reports of losses to follow up was variable and generally not high (table 1). Four of the 15 studies reported the randomisation process, which was adequate in three trials. Only two studies reported blinding of outcome assessors. It was unclear for three trials whether participants or treatment providers were blinded. Loss to follow up was well documented in 12 studies, with differential loss to follow up in both treatment arms reported in four studies. One trial reported a power calculation, and only one study fulfilled all the quality criteria. Many trials were too small to detect clinically important differences.
Quantitative data synthesis
We could not pool the results because there was clear clinical heterogeneity between trials in terms of participants, interventions, and outcome measurements. Furthermore, the number of trials in each category was small and the amount of quantitative data available limited.
Antitussives
Five trials tested antitussives versus placebo (table 3). Two studies tested codeine and found it no more effective than placebo. One of two studies of dextromethorphan favoured active treatment over placebo (differences in mean changes of cough counts 19% to 36% in three substudies, P<0.05), whereas the other found no significant effect. Moguisteine (one trial) led to mean differences in cough scores of about 0.5 in groups with severe cough on days 2 and 3 (P<0.05), but there were no differences between groups at final follow up. Only two trials reported adverse effects.17,20 Nausea, vomiting, and abdominal pain were more common in participants treated with moguisteine than placebo (22% v 8%),17 and in one trial participants did not report any adverse effects from dextromethorphan.20
Expectorants
Participants in one study found guaifenesin more helpful than placebo (75% v 31%, P<0.01).21 However, a second trial found no significant differences between the groups (table 3).22 Guaifenesin led to a low incidence of nausea and urticaria in the active treatment group in one trial21; the other did not report on adverse effects.22
Mucolytics
In the only study of mucolytics, frequent cough was less prevalent in the Bisolvon linctus group than the placebo group (8.6% v 15.2%, P<0.02).23 This study did not report on adverse effects.
Antihistamine-decongestant combinations
One of the two trials of antihistamine-decongestant combinations showed a lower mean severity cough score in the active treatment group on days 3-5 (1.4 in active group v 2.0 in placebo group, P<0.05).24 The other trial found no significant differences between the two treatments (table 3).25 Antihistamine-decongestant combinations seemed to have a slightly higher incidence of adverse effects than placebo. These included dry mouth, dizziness, headache, and insomnia.
Other drug combinations
We included three studies of medicines containing fixed drug combinations (table 3).26–28 These studies were very heterogeneous and used different drug preparations, limiting their comparability. In a study of EM-Vier, more participants in the treatment group improved within the first three days than in the placebo group (26/58 v 15/55, P=0.05).26 In a crossover trial of Vicks Medinite syrup, 58% of participants rated active treatment good or better in relieving cough symptoms compared with 32% for placebo.27 Dextromethorphan plus salbutamol was better than placebo or dextromethorphan alone in relieving cough at night but there were no significant differences for cough symptoms during the day.28 Adverse effects for all preparations were rare and usually mild.
Antihistamines
Based on two trials, terfenadine was no more effective than placebo in relieving cough symptoms (table 3).29,30 The incidence of adverse effects, which included excess fatigue and headache, was low with no significant differences between the groups.
Discussion
We found only a small number of randomised controlled trials investigating each category of cough medicine, so evidence on effectiveness is limited. In nine out of 15 trials, active treatment was no better than placebo. The positive results in the other six studies were of questionable clinical relevance. Most over the counter cough preparations were generally well tolerated and did not lead to serious adverse effects.
Study limitations and potential sources of bias
The included studies varied with respect to settings, populations, interventions (drugs, doses, and frequency), and outcome measures, which makes comparison difficult. Our results should therefore be interpreted with caution. Potential sources of bias such as randomisation procedure, blinding of outcome assessment, and losses to follow up were inadequately reported in several studies, suggesting poor methodological quality. The effect sizes of active treatment over placebo were often reported as differences between cough scores, which are difficult to interpret in a clinically meaningful way. Several studies were supported by the pharmaceutical industry, and others did not report their sources of funding or conflicts of interest.
We tried to obtain information on unpublished studies from study authors and pharmaceutical companies but obtained a limited response. If studies with negative results were less likely to be submitted for publication, this could have led to publication bias.
Implications
It remains unclear whether over the counter cough preparations are helpful in acute cough. We therefore cannot yet recommend these medicines as first line treatment for cough associated with upper respiratory tract infection. The NHS encourages self treatment for acute self limiting illnesses, and the use of over the counter cough preparations as a home remedy.2 Although these medicines are generally well tolerated, their purchase could lead to unnecessary expense for the healthcare consumer. The advice to use over the counter cough medicines should therefore be restricted until more evidence becomes available on their effectiveness. Future studies should use outcome measures that can be easily assessed in a primary care setting and that produce clinically meaningful results, such as patient satisfaction, disturbance at night, side effects, or time to return to normal daily activities.
Figure.
Evaluation of trials for inclusion in review
Acknowledgments
We thank Steve McDonald and Ron D'Souza for their support in designing the search strategy and performing additional searches. We also thank Debbie Sharp and Massimo Pignatelli for help with the French and Italian translations and Bruce Arroll, Keith Dear, Warren McIsaac, and Amy Zelmer for their earlier comments on the review.
Footnotes
Funding: Division of Primary Health Care, University of Bristol and the South and West Research and Development Directorate. KS is funded through an MRC training fellowship in health services research. TF is funded through the NHS primary care career scientist fund.
Competing interests: None declared.
References
- 1.Moss C. OTC Directory 2001/2002. London: Proprietory Association of Great Britain; 2001. Putting self-care into your consultation. [Google Scholar]
- 2.Banks I. The NHS Direct healthcare guide. London: Stationery Office; 2001. [Google Scholar]
- 3.Royal College of General Practitioners; Office of Population Censuses and Surveys; Department of Health. Morbidity statistics from general practice. Fourth national study 1991-1992. London: HMSO; 1992. [Google Scholar]
- 4.Proprietary Association of Great Britain. Annual review and report. London: PAGB; 2000. [Google Scholar]
- 5.Irwin RS, Curley FJ, Bennett FM. Appropriate use of antitussives and protussives. A practical review. Drugs. 1993;46:80–91. doi: 10.2165/00003495-199346010-00006. [DOI] [PubMed] [Google Scholar]
- 6.Gastpar H, Cricuolo D, Dieterich HA. Efficacy and tolerability of glaucine as an antitussive agent. Curr Med Res Opin. 1984;9:21–27. doi: 10.1185/03007998409109554. [DOI] [PubMed] [Google Scholar]
- 7.Chernish SM, Lewis G, Kraft B, Howell J. Clinical evaluation of a new antitussive preparation. Ann Allergy. 1963;21:677–682. [PubMed] [Google Scholar]
- 8.Cass LJ, Frederik WS. Evaluation of a new antitussive agent. N Engl J Med. 1953;249:132–136. doi: 10.1056/NEJM195307232490402. [DOI] [PubMed] [Google Scholar]
- 9.Cough medications in children. Drug Ther Bull. 1999;37:19–21. doi: 10.1136/dtb.1999.37319. [DOI] [PubMed] [Google Scholar]
- 10.Banderali G, Riva E, Fiocchi A, Cordaro CI, Giovannini M. Efficacy and tolerability of levodropropizine and dropropizine in children with non-productive cough. J Int Med Res. 1995;23:175–183. doi: 10.1177/030006059502300304. [DOI] [PubMed] [Google Scholar]
- 11.Smith MBH, Feldman W. Over-the-counter cold medications. A critical review of clinical trials between 1950 and 1991. JAMA. 1993;269:2258–2263. doi: 10.1001/jama.269.17.2258. [DOI] [PubMed] [Google Scholar]
- 12.Schroeder K, Fahey T. Over-the-counter medications for acute cough in children and adults in ambulatory settings. Cochrane Database Syst Rev 2001;(3):CD001831. [DOI] [PubMed]
- 13.Ziment I. What to expect from expectorants. JAMA. 1976;236:193–194. [PubMed] [Google Scholar]
- 14.Reynolds JEF. Martindale's: the extra pharmacopeia. London: Pharmaceutical Press; 1993. Cough suppressants, expectorants and mucolytics; pp. 741–753. [Google Scholar]
- 15.Morice A, Abdul-Manap R. Drug treatments for coughs and colds. Prescriber. 1998;9:74–79. [Google Scholar]
- 16.Eccles R, Morris S, Jawad MS. Lack of effect of codeine in the treatment of cough associated with acute upper respiratory tract infection. J Clin Pharm Ther. 1992;17:175–180. doi: 10.1111/j.1365-2710.1992.tb01289.x. [DOI] [PubMed] [Google Scholar]
- 17.Adams R, Hosie J, James I, Khong T, Kohn H, Smith I, et al. Antitussive activity and tolerability of moguisteine in patients with acute cough: a randomized, double-blind, placebo-controlled study. Adv Ther. 1993;10:263–271. [Google Scholar]
- 18.Parvez L, Vaidya M, Sakhardande A, Subburaj S, Rajagopalan TG. Evaluation of antitussive agents in man. Pulm Pharmacol. 1996;9:299–308. doi: 10.1006/pulp.1996.0039. [DOI] [PubMed] [Google Scholar]
- 19.Freestone C, Eccles R. Assessment of the antitussive efficacy of codeine in cough associated with common cold. J Pharm Pharmacol. 1997;49:1045–1049. doi: 10.1111/j.2042-7158.1997.tb06039.x. [DOI] [PubMed] [Google Scholar]
- 20.Lee PC, Jawad MS, Eccles R. Antitussive efficacy of dextromethorphan in cough associated with acute upper respiratory tract infection. J Pharmacy Pharmacol. 2000;52:1137–1142. doi: 10.1211/0022357001774903. [DOI] [PubMed] [Google Scholar]
- 21.Robinson RE, Cummings WB, Deffenbaugh ER. Effectiveness of guaifenesin as an expectorant: a cooperative double-blind study. Curr Ther Res Clin Exp. 1977;22:284–296. [Google Scholar]
- 22.Kuhn JJ, Hendley JO, Adams KF, Clark JW, Gwaltney JM., Jr Antitussive effect of guaifenesin in young adults with natural colds. Chest. 1982;82:713–718. doi: 10.1378/chest.82.6.713. [DOI] [PubMed] [Google Scholar]
- 23.Nesswetha W. Kriterien der Arzneimittelpruefung in der werksaerztlichen Praxis, dargestellt am Beispiel eines Hustenloesers. Arzneimittelforschung. 1967;17:1324–1326. [PubMed] [Google Scholar]
- 24.Curley FJ, Irwin RS, Pratter MR, Stivers DH, Doern GV, Vernaglia PA, et al. Cough and the common cold. Am Rev Respir Dis. 1988;138:305–311. doi: 10.1164/ajrccm/138.2.305. [DOI] [PubMed] [Google Scholar]
- 25.Berkowitz RB, Connell JT, Dietz AJ, Greenstein SM, Tinkelman DG. The effectiveness of the nonsedating antihistamine loratadine plus pseudoephedrine in the symptomatic management of the common cold. Ann Allergy. 1989;63:336–339. [PubMed] [Google Scholar]
- 26.Kurth W. Gesicherte therapeutische Wirksamkeit des traditionellen Antitussivums Minetten im Doppelblindversuch. Med Welt. 1978;29:1906–1909. [PubMed] [Google Scholar]
- 27.Thackray P. A double-blind, crossover controlled evaluation of a syrup for the night-time relief of the symptoms of the common cold, containing paracetamol, dextromethorphan hydrobromide, doxylamine succinate and ephedrine sulphate. J Int Med Res. 1978;6:161–165. doi: 10.1177/030006057800600218. [DOI] [PubMed] [Google Scholar]
- 28.Tukiainen H, Karttunen P, Silvasti M, Flygare U, Korhonen R, Korhonen T. The treatment of acute transient cough: a placebo-controlled comparison of dextromethorphan and dextromethorphan-beta 2-sympathomimetic combination. Eur J Resp Dis. 1986;69:95–99. [PubMed] [Google Scholar]
- 29.Gaffey MJ, Kaiser DL, Hayden FG. Ineffectiveness of oral terfenadine in natural colds: evidence against histamine as a mediator of common cold symptoms. Pediatr Infect Dis J. 1988;7:223–228. doi: 10.1097/00006454-198803000-00032. [DOI] [PubMed] [Google Scholar]
- 30.Berkowitz RB, Tinkelman DG. Evaluation of oral terfenadine for treatment of the common cold. Ann Allergy. 1991;67:593–597. [PubMed] [Google Scholar]