Abstract
Though the existence of cancer stem cells remained enigmatic initially, over the time their participation in tumorigenesis and tumor progression has become highly evident. Today, they are also appreciated as the causal element for tumor heterogeneity and drug-resistance. Cancer stem cells activate a set of molecular pathways some of which are triggered by the unique mechanical properties of the tumor tissue stroma. A relatively new field called mechanobiology has emerged, which aims to critically evaluate the mechanical properties associated with biological events like tissue morphogenesis, cell-cell or cell-matrix interactions, cellular migration and also the development and progression of cancer. Development of more realistic model systems and biophysical instrumentation for observation and manipulation of cell-dynamics in real-time has invoked a hope for some novel therapeutic modalities against cancer in the future. This review discusses the fundamental concepts of cancer stem cells from an intriguing viewpoint of mechanobiology and some important breakthroughs to date.
Keywords: Stem cells, Niche, Stromal remodeling, Stiffness, Mechanotransduction
Introduction
The interaction of stem cells with their microenvironment, composed of both cellular and acellular components forms the notion of ‘stem cell niche’ that enables the stem cells to maintain their self-renewing ability, multipotency and undifferentiated state [1]. The same idea of niche is applicable to the cancer stem cells, a rare subpopulation of cancer cells thought to be accountable for tumor initiation, heterogeneity, drug resistance and reemergence following remission [2].
Cancer microenvironment often characterized by tissue hypoxia, reactive oxygen species, extracellular matrix remodeling and plethora of factors secreted by mesenchymal stem cells, immune cells and tumor cells actively modulates a multitude of signaling pathways like JAK/STAT, Hedgehog, Wnt, Notch, NF-κβ etc. to help CSCs in maintaining their stemness [3]. Besides the chemical players, physical properties like stiffness and tension of the microenvironment are also increasingly drawing attention to their roles in the development and progression of cancer [4, 5]. An increased risk of developing cancer in a variety of tissues is found to be associated with increased stiffness featured by high stromal collagen content and the presence of fibrotic lesions [6, 7]. The high stiffness of tumors facilitates the activation of mechanosensitive biochemical pathways enhancing the cell-cycle, EMT, cell motility and renders the tumor metastatic [8]. Now scientists are curious to understand how the mechanical properties of cancer microenvironment perturb the expression of CSC markers and associated traits.
Mainstream biologists find it difficult to assess these physicomechanical properties by conventional tools used in biology. In this context, the demand of a new discipline to fill the void of our knowledge is satiated by the advent of mechanobiology, an interdisciplinary field of science at the interface of biology, physics, and engineering. It employs both physical devices and biological techniques to look into how the changes in the mechanical properties of cells and tissues contribute to development, cell differentiation, cell physiology and diseases [9].
In order to unveil the biomechanical aspect of understanding the uniqueness of cancer stem cells and their niche, the basis of metastasis, neovasculogenesis and other important hallmarks of cancer, more reliable, as well as easily tractable model systems, are being designed [10–12]. This article critically reviews the participation of CSCs and the associated niche in cancer from a mechanobiological perspective.
Cancer Stem Cells
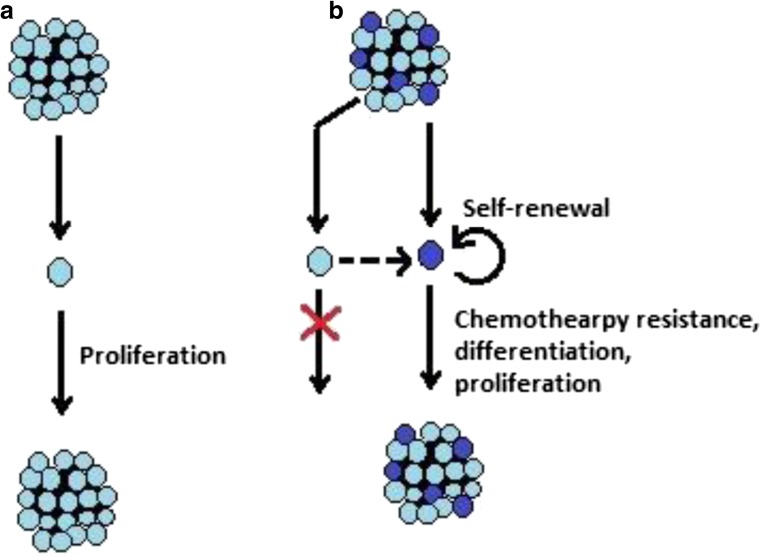
Since the discovery of cancer stem cells (CSCs) in the year 1997 by Bonnet and Dick in leukemia, they have been shown to exist in several other types of solid tumors including colon, breast, brain, and skin [13–17]. There are mainly two models best known to explain tumor initiation and progression, namely: the stochastic model and the hierarchical model. Whereas the stochastic model counts each tumor cell to have equal tumorigenicity, the hierarchical model suggests only CSCs have all the potential to proliferate and differentiate [18]. Figure 1 depicts the schema of these two models. The current review aims to examine the CSC hypothesis from a mechanobiological viewpoint not going into a comparison of the advantages and pitfalls of different models.
Fig. 1.
Schematic representation of the two familiar models of cancer initiation and progression. a. Stochastic model: According to this model, each cancer cell of a tumor is equally potent to continuously divide and form tumor. b. Hierarchical model: According to this model, only few cells called cancer stem cells of a tumor are capable of dividing, differentiating and reinitiating tumor. Other cells are not generally able to perpetuate a tumor, though they possess the ability to dedifferentiate into cancer stem cells
CSCs present a very small percentage of the overall cancer cell population [19, 20]. No single isolation protocol is exclusive and sufficient for delineating CSCs from other cancer cells. Tumor cells are considered as CSCs if they are simultaneously: (a) positive for specific surface markers (like CD133/ prominin1) (b) part of side population (c) able to form floating spheres in serum-free medium and (d) able to form new tumors when implanted in mice [21]. CSCs exhibit a less adhesive strength towards the basement membrane and hence can be mechanistically enriched from a majority of other cancer cells before being definitively validated by surface marker analysis [22]. Biomechanical profiling of CSCs of different cancers (ovarian, breast, hepatic) using atomic force microscopy or rheological technique like micropipette aspiration has demonstrated that these cells are significantly softer and highly deformable than non-malignant, intermediates and even aggressive late-stage cancer cells [23–25]. The soft phenotype has also been shown to be sensitive to the anti-cancer agents [23]. These findings may lead to reaching the goal of efficiently identifying and targeting CSCs from a host of other tumor cells exploiting their mechanical uniqueness.
Like adult stem cells, CSCs display ‘stemness’, a remarkable ability to self-renew, differentiate and to balance between quiescence, proliferation, and regeneration [26]. CSCs exhibit a substantially high capacity of self-renewal, a high degree of plasticity, continuous proliferation, ability to differentiate conforming to intra-tumor heterogeneity and a pronounced resistance to stressful factors and drugs [3, 26–30]. Because of their ability to initiate tumor formation, disseminate to other locations and to re-populate and grow into a new tumor mass, CSCs are interchangeably termed as tumor-initiating cells (TICs) or tumor-repopulating cells (TRCs) [31]. CSCs share many common pathways with adult stem cells like JAK/STAT, Hedgehog, Wnt, Notch, PTEN/AKT/P13K, NF-κB, MAPK/ERK, and TGFβ [26, 32]. Some of these pathways like Wnt and TGFβ cross-talk with mechanosensitive transcription factor YAP/TAZ [33, 34]. Growing evidence shows that mechanical properties of extracellular matrix (ECM) regulate the self-renewal and differentiation of adult stem cells and hence are important in tissue engineering [35, 36]. So, it is quite natural to be inquisitive about ‘if and how’ the tumor microenvironment modulates ECM composition and corresponding mechanical properties conferring to a hospitable niche for CSCs.
Tumor Microenvironment: A Mechanical Panorama
In 1889, Stephen Paget proposed that a favorable interaction between the metastatic cells (seed) and the tumor microenvironment (soil) lies in the root of an organ-preference pattern of breast cancer metastasis [37]. Before examining the applicability of this ‘seed-and-soil’ hypothesis with reference to CSCs and their niche, in this section, we will peep into the biomechanical aspects of the tumor microenvironment, the ‘home’-ground of CSCs.
It has long been practiced by medical doctors to diagnose a tumor on the basis of the differences in tissue rigidity sensed by palpation [38]. When the microenvironment is in a healthy state, it can help protect tumorigenesis while an unhealthy microenvironment becomes an accomplice [39]. Multiple studies, in recent days, have also confirmed that tumor tissue stiffness is much higher than that of its normal counterpart and is strongly correlated with disease progression and clinical outcome [40, 41]. This observation falls in with the fact that the TME experiences an increased deposition and dynamic remodeling of ECM proteins, the universal packing material of living tissues [42]. So, it is intriguing to look into the physicomechanical details of TME in conjunction with the key cellular components that actively sculpt the tumor-associated ECM.
Builders of the Mechanical Milieu
Tumor microenvironment (TME) is asymmetric aggression of a bunch of cellular and acellular components. Other than the cancer cells themselves, the cellular components include vascular cells (endothelial cells and pericytes), immune cells (mast cells, neutrophils, monocytes, macrophages, myeloid-derived suppressor cells) and most importantly the cancer-associated fibroblasts (CAFs). The acellular compartment consists of the extracellular matrix proteins (collagen, fibronectin, laminin etc.) and conditions like hypoxia [39, 43].
Fibroblasts are the cells specialized for secreting ECM proteins that provide the scaffold for the tissue morphogenesis and homeostasis [44]. During adult epidermal wound healing, otherwise quiescent fibroblast cells undergo differentiation and enormous expansion to smooth-muscle myosin (α-SMA)-positive myofibroblasts, expressing stress-fibers [45]. Cancers exceptionally allow the continuous recruitment and conversion of fibroblasts into active myofibroblasts, alternatively cited as CAFs to the tumor sites justifying the notion that “Cancers are wounds that do not heal”. Such kind of differentiation is often referred to as mesenchymal-to-mesenchymal transition (MMT). The CAFs not only originate from the fibroblast precursors but also from the other stromal cells by transdifferentiation, rendering them the most abundant cells in a tumor tissue stroma [46]. Also, there are reports showing that CAF activity is aggravated by the paracrine actions of cancer cells and other cancer-associated reactive stromal cells, especially mast cells, M2 macrophages and endothelial cells [43, 47, 48]. Other than their participation in ECM composition and remodeling, soluble factors form CAFs and other stromal cells are found to extensively cross-talk with the mechanically induced signaling pathways [49].
Hypervascularization and hypoxia, two more signature components of solid tumors, directly and indirectly, result in ECM realignment, elevation of interstitial fluid pressure and shear stress. These mechanical properties reciprocally act on their causative agents to cause a vicious cycle [50]. Concisely, the cellular components along with the acellular factors provide TME with a unique mechanical identity by setting a mutually interactive network in action.
Mechanical Properties of Tumor Microenvironment
Earlier reviews based on the published reports have distinguished four major mechanical perturbations of TME as follows: (a) ECM stiffening (b) elevated interstitial fluid pressure (IFP) (c) increased interstitial fluid flow and (d) compressive/solid stress imparted by confined growth [51].
The ‘core matrisome’ of mammalian ECM is characterized by having about 300 different proteins, of which collagen, proteoglycans, laminins, fibronectins, and elastin are worth mentioning [52]. ECM composition in cancerous tissue is quantitatively altered by both CAFs and the resident cancer cells leading to qualitative changes in terms of rigidity, density, porosity, solubility, and topography [53, 54]. The disruption of the equilibrium between ECM synthesis and secretion, and alterations in the amount and activity of matrix-remodeling enzymes namely MMPs and LOX are responsible for the desmoplastic appearance of solid tumors [42]. Collagen I and fibronectin are the most abundant ECM constituents in TME. Other ECM proteins namely tenascin, decorin, fibromodulin, SPARC, lumican, osteopontin, periostin, versican, and hyaluronan have shown to be implicated in biochemical and biomechanical alterations of TME. The metastatic transformation has been found to be closely associated with the remodeling of basement membrane (BM) proteins (collagen IV, laminin, entactin) and linearization of collagen fibers [54–56]. Recent evidence also accuses the tensile stress generated by cellular actomyosin contractility in response to high ECM stiffness for reciprocally regulating the ECM stiffness [57–59].
TME is the reservoir of pro-angiogenic agents including ECM components and fibronectin) and paracrine factors like vascular endothelial growth factor (VEGF) [60]. Excessive development of aberrant and leaky vasculature along with the deposition of a large amount of ECM proteins and retention and immobilization of liquid by negatively charged hyaluronan collectively give rise to an elevated IFP [50, 61]. Increased IFP and consequential increment of interstitial fluid flow are linked to heightened chemotherapeutic resistance, induction of epithelial-to-mesenchymal transition (EMT), myofibroblast, collagen alignment and tissue hypoxia [50, 62]. Hypoxia, in turn, promotes CSC self-renewal, excess angiogenesis and secretion along with collagen and collagen-remodeling enzymes by cancer cells [63, 64].
The solid stress in growing tumor develops as a result of: (a) an increased density of cancer cells, stromal cells and ECM components within a defined periphery of the host tissue (defined as residual stress), and (b) the reciprocal compression by the adjacent host tissue (defined as reciprocal stress). Such compressive stresses can regulate tumor morphology, growth, and metastasis [57].
So, from the above discussion, it is obvious that TME creates a preparative and supportive biomechanical atmosphere for the disease to perpetuate. Now, in accordance with the scope of our present review, we would like to dig out the studies regarding the effects of biomechanical anomalies of TME on the CSCs and the signaling pathways involved.
Tumor Microenvironment ‘Niche’s Cancer Stem Cells: Mechanomolecules in Action
Cells can sense the internal and external mechanical fluctuations in a similar manner, yet more quickly they can sense the chemical changes around or inside them, and decide to take a due course of action which encompasses maintenance of cell size and shape, cell migration, cell competition, cell division and what not! Cellular mechanotransduction is the fancy name to describe the transmission of mechanical signals in the form of chemical cascades by the cells [65]. With our previous understanding of the biomechanical characteristics of CSCs and TME, we hereby discuss how TME creates an advantageous ‘niche’ for CSCs to turn on different mechanosensory pathways that make them special. Extrinsic forces operated by ECM constituents can affect native conformation and related interactions of a great variety of molecules (mechanosensors) which, in response, trigger biologically important reactions leading to covalent modification of enzymes, protein-protein interaction, cytoskeletal rearrangement, changes in gene expressions, and beyond. Mechanosensors commonly consist of ion channels, cytoskeletal proteins, junctional proteins, receptors etc. [65, 66]. Figure 2 simplistically summarizes the common mechanosignaling pathways and their implications in CSCs.
Fig. 2.
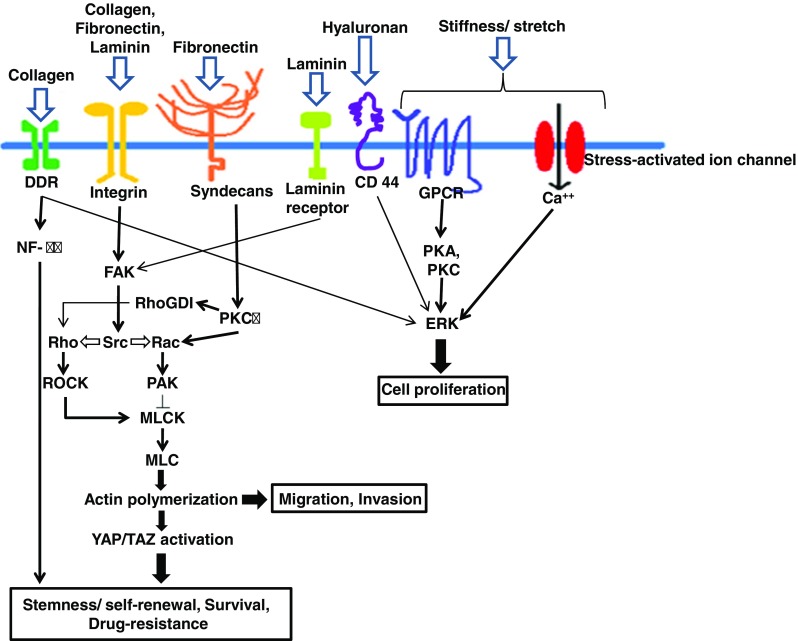
Mechanosensory molecular pathways in cancer stem cell. Mechanical stress induced by stiffened ECM, composed of collagen, laminin, fibronectin, hyaluronan etc. can be sensed by mechanosensors (integrins, laminin, CD44 receptors, syndecans, DDRs, GPCRs etc.) and transduced to induce pathways specific for CSC survival, self renewal, drug resistance and progression. DDR: discoidin domain receptor; GPCR: G protein coupled rector; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; FAK: focal adhesion kinase; RhoGDI: Rho GDP-dissociation inhibitor; MLCK: Myosin light-chain kinase; MLC: Myosin light-chain; PKA, PKC: Protein kinase A, C; ERK: extracellular signal-regulated kinase
Transmembrane glycoprotein CD44, a known receptor of hyaluronic acid (HA) is considered as an important CSC marker. On binding with growth factors of TME, CD44 and its isoforms form complexes with ezrin resulting in cytoskeletal remodeling and signaling to the nucleus. Tumor necrotic factor (TNF-α) mediated up-regulation of HA leads to the generation of CD44 variants by alternative splicing. The HA-CD44 interaction has been implicated in (a) the sustenance of stemness (Nanog expression), (b) tumor metastasis to liver, bone marrow and lungs and (c) drug resistance [67, 68]. CD133 or prominin is another transmembrane pentaspan glycoprotein and a known biomarker of CSCs. Type I collagen, the causal factor for increased ECM stiffness of tumor stroma, has been found to conditionally stimulate the CD133 expression in glioblastoma cells [69, 70]. CD133 expression is linked with CSC stemness, plasticity, and drug resistance [71]. Other such integral membrane proteins like syndecan-1 (CD138), discoidin domain receptor 1 (DDR1) act as receptors for ECM components like fibronectin and collagen respectively to induce mechanotransduction pathways in CSCs [72, 73].
Laminin (Lam)-α2, a non-collagenous ECM protein acts as a niche for glioblastoma stem cells by supporting their growth and self-renewal [74]. Breast CSCs produce Lam511 matrix which interacts with α6Bβ1 integrin to activate Hippo transducer TAZ which, in turn, induces the transcription of Lam511. This signaling also promotes CSC self-renewal and tumor initiation [75].
Versican, a large chondroitin sulfate proteoglycan is responsible for the emergence of various cancer hallmarks by its interaction with multiple membrane proteins including HA, integrins, CD44, microfibrillar fibulins and epidermal growth factor receptor (EGFR). CSC marker CD44 binds with versican to promote tumor progression and migration via expressing HA-mediated motility receptor (RHAMM) and MMP9 through the activation of JNK and NF-κB pathways [76].
Fibronectin, an essential component of ECM interacts with membrane integrins. Investigation on glioma stem-like cells revealed that fibronectin (Fn) favored cell survival via Erk activation; differentiation, proliferation and motility via the activation of Focal adhesion kinase/Paxillin/AKT signaling; and increased chemoresistance via upregulating P-glycoprotein expression [77].
Formation of macromolecular focal adhesion (FA) complexes is marked by engagement and clustering of integrins and associated proteins classified into (a) ‘integrin signaling layer’ consisting of focal adhesion kinase (FAK) and paxillin (b) ‘force-transduction layer’ made of talin and vinculin and (c) zyxin, VASP, α-actinin constituting the ‘actin regulatory layer’. The level of tyrosine phosphorylation of signaling molecules activates either Rac to protrusion and migration or Rho leading to adhesion growth and stabilization [78]. Binding of type I collagen of stiff ECM with Integrin β1 of CSC membrane is followed by the induction of FAK and subsequent autophosphorylation that recruits Src family kinases. These Src kinases activate the catalytic domain of FAK essential for the formation of the whole FA complex. FAK promotes CSC survival and metastasis in a kinase-dependent manner [79]. ILK, a serine-threonine kinase by nature has also been implicated in the assembly of FA and interaction of FA with actin cytoskeleton [80]. ECM stiffening and tissue hypoxia cooperatively generate the breast CSC pool via the activation of ILK and CD44 [31]. Activated ILK/PI3K/Akt pathway leads to up-regulation of self-renewal capacity in CSCs [81].
Caveolins (Cav) are integral membrane proteins densely populated over the lipid rafts and are involved in receptor-independent endocytosis [82]. Cav1 has been reported to mediate chemoresistance via the activation of Wnt-independent β-catenin/ABCG2 signaling pathway in breast CSCs [83]. Recently, Cav1 has been suggested to regulate a unique mechanotransduction response to substrate stiffness through an actin-dependent control of Yes-associated protein (YAP) [84]. This particular pathway needs further investigation to uncover its contribution to CSC hallmarks.
In several types of cancers, YAP/TAZ helps to sustain CSC features via its increased activity specifically within tissue regions exhibiting higher collagen cross-linking [85]. TAZ induces the self-renewal of non-CSCs and expansion of the pool of CSCs [86]. YAP expression marks CSCs and maintains CSC phenotype through Sox2-Hippo signaling pathway [87]. YAP/TAZ is also important for CSCs to display other hallmarks like EMT and chemoresistance. Shear stress-induced migration and invasion of cancer cells also require YAP onstage [88]. Fluid shear stress has also been shown to induce CSC-like phenotype in epithelial cell adhesion molecule (EpCAM) expressing MCF-7 breast cancer cells without induction of EMT, though the pathways involved are poorly understood [89].
Nuclear architecture and chromatin remodeling are closely related to gene expression and cell differentiation. A specialized multimolecular assembly called linker of nucleoskeleton and cytoskeleton (LINC) containing nesprin, SUN proteins and lamins, constitutes a functional connection of membrane adhesion molecules with nucleoplasm via actin and intermediate filament network [90]. LINC proteins have a close association with the differentiation status of embryonic stem cells, and their loss-of-function is proven to have roles in cancer metastasis [91, 92]. So, their roles in shaping the plasticity and metastatic ability of CSCs in response to external biophysical cues should be thoroughly probed.
The discovery by Tan et al. has pointed at epigenetic changes for melanoma CSCs’ self-renewal capacity and tumorigenic potential that soft matrices bring in through a mechanism involving reduced H3K9 methylation and increased Sox2 expression [93]. Conversely, glioblastoma CSCs show little change in proliferation, migration and spreading as a function of ECM stiffness [94]. Hence, the exact routes operational within the CSCs in response to extrinsic biomechanical cues of tumor ECM to modulate their intrinsic properties and showcase specified hallmarks require in-depth analysis across different cancer types by employing both in vivo and in vitro state-of-the-art approaches.
Tools for Novel Therapeutic Discovery
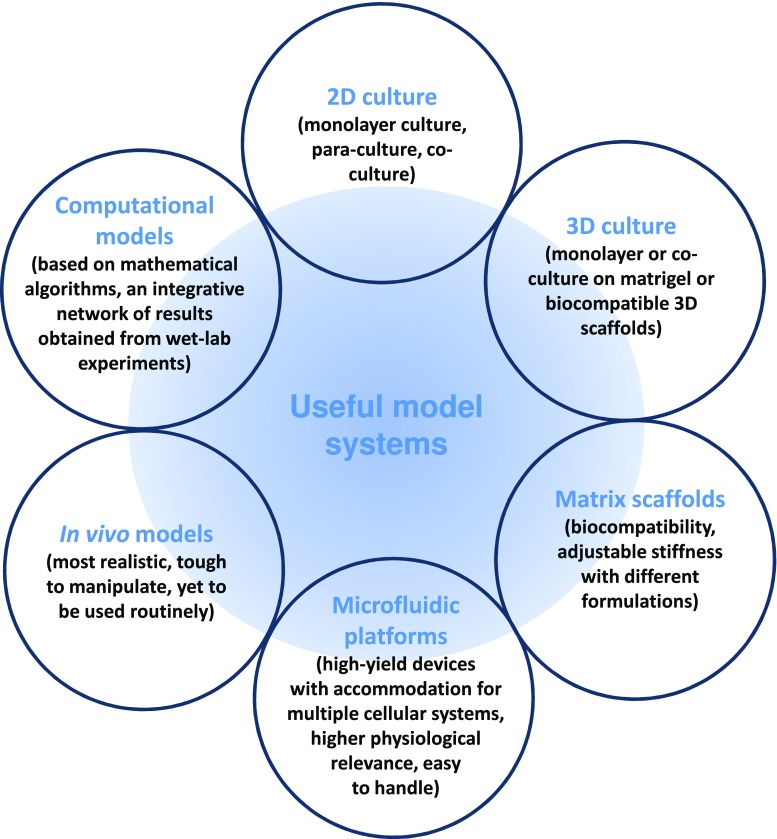
The tumor microenvironment provides cancer cells with a diverse set of extracellular cues to influence tumor cell behavior and function. Recent developments in the arena of tumor biomechanics and CSC biology, in particular, have chalked out an alternative explanation and potential therapeutic targets of tumor progression and metastasis. Hence, a detailed understanding of the mechanisms of mechanical cues in this context demands some realistic model systems, suitable for experimental set-ups. The model systems currently being used in this field of research are summarized in Fig. 3.
Fig. 3.
Model systems and platforms commonly used in mechanobiological experiments
Stromal remodeling, tensional redistribution between tumor cells and the surrounding stroma and angiogenesis-driven fluid flux and shear stress in tumor-stroma: all these changes result in a significantly stiffer ECM than that of a normal tissue. Despite the classical 2D and 3D cell culture platforms, in order to genuinely mimic the mechanical nature of normal and tumor stroma, researchers have been dealing with a spectrum of biocompatible as well as widely tunable biomaterials, broadly grouped into naturally derived, synthetic and hybrid [95].
High-throughput microfluidics-based platforms have come up with three remarkable advantages: (a) the ability of co-culturing cells in a spatially controlled manner (b) generation of and control over (signaling) gradients (c) the integration of perfusion/flow. Recently, a couple of experiments on microfluidic platforms have shown that migration of the tumor cells through microtracks laid down by the migrating CAFs and macrophages, is very much dependent on the overall topography of ECM, including its porosity, stiffness etc. [96]. As mentioned earlier in this review, CSCs are softer and deformable than non-CSCs [23]. So, it is quite possible to distinguish CSCs on the basis of their unique cellular mechanics. The use of high-throughput microfluidic devices instead of high-precision but low-throughput laboratory techniques like micropipette aspiration, atomic force microscopy and optical tweezers have created a new hope of dealing regular diagnostic affairs with a mechanistic approach [97, 98]. Such screening applications must be coupled with transcriptomic analyses in order to characterize the molecular mechanisms that regulate mechanical features of the CSCs in response to surrounding tissue stiffness.
Experiments require mimicking the kind of solid stress that tumor cells experience in order to replicate the situation in vitro. For this purpose 3D model systems with adjustable mechanical properties by varying the ECM elements are commonly employed. There are few specialized instruments to artificially generate mechanical force, like the commerciallyavailable FlexCell FX-5000 compression system [99]. Scientists have invested in biomaterials and parameters like differential drug-sensitivity to enrich and perpetually culture CSCs in vitro [100].
Particle-tracking microrheology (PTMR) uses ballistic fluorescent polystyrene tracer beads and statistically analyzes their brownian motion to provide a quantitative measurement of fluctuating intracellular stresses. Employing this novel technique and a prostate cancer cell line, a group of researchers from the University of Texas has shown and quantified how ECM stiffness regulates effective intracellular stiffness of cancer cells in a 3D matrix environment [101]. The possibility of using such techniques to pinpoint CSCs and related ECM based on their unique biomechanical identities needs fervent scientific enquiry.
Recently, a research group from University of California, Santa Barbara (UCSB) has created a biocompatible magnetic microdroplet of ferrofluid oil to investigate mechanical forces in cellular microenvironments and their spatiotemporal variations in vivo. Using this technique, they have found that tissue stiffness in live developing zebrafish embryos varies along the tail bud of the animals [102]. They aim to use this platform to study the mechanisms of tumor formation in multicellular spheroids and hope to understand how abnormal biomechanics can cause or promote cancer and other diseases. These kinds of technologies can be exploited to investigate mechanical responses of CSCs in vivo.
Recent in vivo experiments, though few, have shown how ECM stiffness influences CAF activation, tumor cell invasion etc. via the mechanobiologically important molecules like LOXL2, FAK, YAP, ROCK, Cav1and actomyosin [103]. By chemotherapeutically targeting important mechanosignaling pathways or by instructing the stromal cells to cause changes in ECM composition and thereby stiffness, one can manage to get rid of the CSCs and chances of relapse [104–107]. Nowadays, scientists are also attempting to develop integrative systems biology-models in order to analyze complex mechanobiological interactions across all levels of biological organization i.e. from atomistic to systemic scales.
Conclusion
The current state-of-the-art technologies hold great promises for better understanding and prospective application of mechanobiological modulations during tumorigenesis and tumor progression both in fundamental and translational research arenas. Since the discovery of cancer stem cells, they attracted much scientific attention because of their utmost biochemical and biomechanical uniqueness amongst the whole bunch of other cells in a malignant tumor. These cells are not solitary entities. Though representing a small subset of tumor cells, cancer stem cells are supposed to constantly interact with other cells and tumor ECM. Biophysical properties like rigidity, porosity, density etc. of tumor microenvironment are actively architected by its cellular and acellular constituents. The review has critically discussed how the tumor microenvironment provides a hospitable niche for the cancer stem cells by inducing several cross-connected mechanotransduction pathways to support exhibiting their distinctive phenotype. Simply targeting the intrinsic pathways (Wnt, Notch, Hedgehog) implicated in the self-renewal and survival of CSCs can lead to their differentiation and proliferation, and may also impact normal stem cell functions. As normal tissue stroma is mechanically very different from the CSC niche, researchers are endeavoring to come up with more targeted intervention against CSCs by perturbing the pathways activated by extrinsic mechanical cues. Innovations in terms of tools, platforms and model systems for the study of mechanobiology of CSCs and their niche are also gathering pace. Present trends are encouraging and it is well expected that there will be many breakthroughs in the coming years.
Acknowledgements
This study was supported by Intramural Research Grant (A-523) from AIIMS, New Delhi.
Abbreviations
- CSC
Cancer stem cell
- EMT
Epithelial-mesenchymal transition
Compliance with Ethical Standards
The manuscript does not contain clinical studies or patient data.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang J, Li L. Stem cell niche: microenvironment and beyond. J Biol Chem. 2008;283(15):9499–9503. doi: 10.1074/jbc.R700043200. [DOI] [PubMed] [Google Scholar]
- 2.Clarke MF, Dick JE, Dirks PB, et al. Cancer stem cells—perspectives on current status and future directions: AACR workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 3.Plaks V, Kong N, Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16(3):225–238. doi: 10.1016/j.stem.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levental KR, Yu H, Kass L, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;3:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paszek MJ, Zahir N, Johnson KR, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8(3):241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Boyd NF, Rommens JM, Vogt K, et al. Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol. 2005;6(10):798–808. doi: 10.1016/S1470-2045(05)70390-9. [DOI] [PubMed] [Google Scholar]
- 7.Daniels CE, Jett JR. Does interstitial lung disease predispose to lung cancer? Curr Opin Pulm Med. 2005;11(5):431–437. doi: 10.1097/01.mcp.0000170521.71497.ba. [DOI] [PubMed] [Google Scholar]
- 8.Broders-Bondon F, Ho-Bouldoires THN, Fernandez-Sanchez ME, Farge E. Mechanotransduction in tumor progression: the dark side of the force. J Cell Biol. 2018;217(5):1571–1587. doi: 10.1083/jcb.201701039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs CR, Hayden H, Kwon RY (2012) Cell Mechanics in the laboratory. In: Scholl S (ed) Introduction to cell mechanics and mechanobiology. 1st edn. Garland Science, New York and London, pp 151–179
- 10.McLane JS, Ligon LA. Stiffened extracellular matrix and signaling from stromal fibroblasts via osteoprotegerin regulate tumor cell Invasionin a 3-D tumor in situ model. Cancer Microenviron. 2016;9:127–139. doi: 10.1007/s12307-016-0188-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Z, Li E, Guo Z, et al. Design and construction of a multi-organ microfluidic chip mimicking the in vivo microenvironment of lung cancer metastasis. ACS Appl Mater Interfaces. 2016;8(39):25840–25847. doi: 10.1021/acsami.6b08746. [DOI] [PubMed] [Google Scholar]
- 12.Duinen VV, Trietsch SJ, Joore J, Vulto P, Hankemeier T. Microfluidic 3D cell culture: from tools to tissue models. Curr Opin Biotechnol. 2015;35:118–126. doi: 10.1016/j.copbio.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Bonnet D, Dick J. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 14.Schepers AG, Snippert HJ, Stange DE, et al. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science. 2012;337:730–735. doi: 10.1126/science.1224676. [DOI] [PubMed] [Google Scholar]
- 15.Al-Hajj M, Wicha M, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh S, Hawkins C, Clarke I, Squire J. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 17.Luo Y, Dallaglio K, Chen Y, et al. Aldh1a isozymes are markers of human melanoma stem cells and potential therapeutic targets. Stem Cells. 2012;30:2100–2113. doi: 10.1002/stem.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shackleton M, Quintana E, Fearon ER, Morrison SJ. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell. 2009;138:822–829. doi: 10.1016/j.cell.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Kong D, Ahmad A, et al. Pancreatic cancer stem cells: emerging target for designing novel therapy. Cancer Lett. 2012;338(1):94–100. doi: 10.1016/j.canlet.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huntly BJ, Gilliland DG. Leukaemia stem cells and the evolution of cancer-stem-cell research. Nat Rev Cancer. 2005;5:311–321. doi: 10.1038/nrc1592. [DOI] [PubMed] [Google Scholar]
- 21.Tirino V, Desiderio V, Paino F, et al. Methods for cancer stem cell detection and isolation. Methods Mol Biol. 2012;879:513–529. doi: 10.1007/978-1-61779-815-3_32. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Wu M, Han X, et al. High-throughput, label-free isolation of cancer stem cells on the basis of cell adhesion capacity. Angew Chem Int Ed Engl. 2015;54(37):10838–10842. doi: 10.1002/anie.201505294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Babahosseini H, Ketene AN, Schmelz EM, et al. Biomechanical profile of cancer stem-like/tumor initiating cells derived from a progressive ovarian cancer model. Nanomedicine. 2014;10(5):1013–1019. doi: 10.1016/j.nano.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohammadalipour A, Burdick MM, Tees DFJ. Deformability of breast cancer cells in correlation with surface markers and cell rolling. FASEB J. 2018;32(4):1806–1817. doi: 10.1096/fj.201700762R. [DOI] [PubMed] [Google Scholar]
- 25.Sun J, Luo Q, Liu L, et al. Biomechanical profile of cancer stem like cells derived from MHCC97H cell lines. J Biomech. 2016;49(1):45–52. doi: 10.1016/j.jbiomech.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Aponte PM, Caicedo A. Stemness in cancer: stem cells, cancer stem cells, and their microenvironment. Stem Cells Int. 2017;2017:5619472. doi: 10.1155/2017/5619472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cabrera MC, Hollingsworth RE, Hurt EM. Cancer stem cell plasticity and tumor hierarchy. World J Stem Cells. 2015;7(1):27–36. doi: 10.4252/wjsc.v7.i1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaffer CL, Brueckmann I, Scheel C, et al. Normal and neoplastic nonstem cells can spontaneously convert to a stemlike state. Proc Natl Acad Sci U S A. 2011;108(19):7950–7955. doi: 10.1073/pnas.1102454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borovski T, Melo FSE, Vermeulen L, Medema JP. Cancer stem cell niche: the place to be. Cancer Res. 2011;71(3):634–639. doi: 10.1158/0008-5472.CAN-10-3220. [DOI] [PubMed] [Google Scholar]
- 30.Alison MR, Lim S, Nicholoson L. Cancer stem cells: problems for therapy? J Pathol. 2010;223:147–161. doi: 10.1002/path.2793. [DOI] [PubMed] [Google Scholar]
- 31.Pang M-F, Siedlik MJ, Han S, et al. Tissue stiffness and hypoxia modulate the integrin-linked kinase ILK to control breast cancer stem-like cells. Cancer Res. 2016;76:5277–5287. doi: 10.1158/0008-5472.CAN-16-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsui WH. Cancer stem cell signaling pathways. Medicine. 2016;95(1):S8–S19. doi: 10.1097/MD.0000000000004765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varelas X, Miller BW, Sopko R, et al. The Hippo pathway regulates Wnt/beta-catenin signaling. Dev Cell. 2010;18(4):579–591. doi: 10.1016/j.devcel.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 34.Varelas X, Sakuma R, Samavarchi-Tehrani P, et al. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat Cell Biol. 2008;10(7):837–848. doi: 10.1038/ncb1748. [DOI] [PubMed] [Google Scholar]
- 35.McMurray RJ, Dalby MJ, Tsimbouri PM. Using biomaterials to study stem cell mechanotransduction, growth and differentiation. J Tissue Eng Regen Med. 2015;9(5):528–539. doi: 10.1002/term.1957. [DOI] [PubMed] [Google Scholar]
- 36.Faulk DM, Johnson SA, Zhang L, Badylak SF. Role of the extracellular matrix in whole organ engineering. J Cell Physiol. 2014;229(8):984–989. doi: 10.1002/jcp.24532. [DOI] [PubMed] [Google Scholar]
- 37.Paget S (1989) The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev 8(2):98–101. 10.1016/S0140-6736(00)49915-0 [PubMed]
- 38.Huang S, Ingber DE. Cell tension, matrix mechanics, and cancer development. Cancer Cell. 2005;8:175–176. doi: 10.1016/j.ccr.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 39.Wang MN, Zhao JZ, Zhang LS, et al. Role of tumor microenvironment in tumorigenesis. J Cancer. 2017;8:761–773. doi: 10.7150/jca.17648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Correas JM, Tissier AM, Khairoune A et al. (2015) Prostate cancer: diagnostic performance of real-time shear-wave elastography. Radiology 275(1):280–289. 10.1148/radiol.14140567 [DOI] [PubMed]
- 41.Boyd NF, Li Q, Melnichouk O, et al. Evidence that breast tissue stiffness is associated with risk of breast cancer. PLoS One. 2014;9(7):e100937. doi: 10.1371/journal.pone.0100937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and diseases. Nat Rev Mol Cell Biol. 2014;15(12):786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dasari S, Fang Y, Mitra AK. Cancer associated fibroblasts: naughty neighbors that drive ovarian Cancer progression. Cancers. 2018;10(11):406. doi: 10.3390/cancers10110406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sriram G, Bigliardi PL, Bigliardi-Qi M. Fibroblast heterogeneity and its implications for engineering organotypic skin models in vitro. Eur J Cell Biol. 2015;94:483–512. doi: 10.1016/j.ejcb.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Li B, Wang JH. Fibroblasts and myofibroblasts in wound healing: force generation and measurement. J Tissue Viability. 2011;20:108–120. doi: 10.1016/j.jtv.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cirri P, Chiarugi P. Cancer associated fibroblasts: the dark side of the coin. Am J Cancer Res. 2011;1(4):482–497. [PMC free article] [PubMed] [Google Scholar]
- 47.Chiarugi P. Cancer-associated fibroblasts and macrophages: friendly conspirators for malignancy. OncoImmunology. 2013;2(9):e25563. doi: 10.4161/onci.25563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ronca R, Van Ginderachter J, Turtoi A. Paracrine interactions of cancer-associated fibroblasts, macrophages and endothelial cells. Curr Opin Oncol. 2018;30(1):45–53. doi: 10.1097/CCO.0000000000000420. [DOI] [PubMed] [Google Scholar]
- 49.De VK, Rao L, De BE, et al. Cancer associated fibroblasts and tumor growth: focus on multiple myeloma. Cancers (Basel) 2014;6(3):1363–1381. doi: 10.3390/cancers6031363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yeldag G, Rice A, Hernández ADR. Chemoresistance and the self-maintaining tumor microenvironment. Cancers (Basel) 2018;10(12):471. doi: 10.3390/cancers10120471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zanotelli MR, Reinhart-King CA. Mechanical forces in tumor angiogenesis. Adv Exp Med Biol. 2018;1092:91–112. doi: 10.1007/978-3-319-95294-9_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hynes RO, Naba A. Overview of the Matrisome--an inventory of extracellular matrix constituents and functions. Cold Spring Harb Perspect Biol. 2011;4(1):a004903–a004903. doi: 10.1101/cshperspect.a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yue B. Biology of the extracellular matrix: an overview. J Glaucoma. 2014;23(8):S20–S23. doi: 10.1097/IJG.0000000000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walker C, Mojares E, del Río Hernández A. Role of extracellular matrix in development and cancer progression. Int J Mol Sci. 2018;19(10):3028. doi: 10.3390/ijms19103028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cox TR, Erler JT. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech. 2011;4(2):165–178. doi: 10.1242/dmm.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kalli M, Stylianopoulos T. Defining the role of solid stress and matrix stiffness in cancer cell proliferation and metastasis. Front Oncol. 2018;8:55. doi: 10.3389/fonc.2018.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leung DY, Glagov S, Mathews MB. Cyclic stretching stimulates synthesis of matrix components by arterial smooth muscle cells in vitro. Science. 1976;191:475–477. doi: 10.1126/science.128820. [DOI] [PubMed] [Google Scholar]
- 59.Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol. 2014;15(12):802–812. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17:1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 61.Ferretti S, Allegrini PR, Becquet MM, McSheehy PMJ. Tumor interstitial fluid pressure as an early-response marker for anticancer therapeutics. Neoplasia. 2009;11:874–881. doi: 10.1593/neo.09554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ng CP, Hinz B, Swartz MA. Interstitial fluid flow induces myofibroblast differentiation and collagen alignment in vitro. J Cell Sci. 2005;118:4731–4739. doi: 10.1242/jcs.02605. [DOI] [PubMed] [Google Scholar]
- 63.Bao B, Azmi AS, Ali S, et al. The biological kinship of hypoxia with CSC and EMT and their relationship with deregulated expression of miRNAs and tumor aggressiveness. Biochim Biophys Acta. 2012;1826:272–296. doi: 10.1016/j.bbcan.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the extracellular matrix: drivers of tumor metastasis. Nat Rev Cancer. 2014;14:430–439. doi: 10.1038/nrc3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luis Alonso J, Goldmann WH. Cellular mechanotransduction. AIMS Biophysics. 2016;3(1):50–62. [Google Scholar]
- 66.Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nat Rev Mol Cell Biol. 2009;10:63–73. doi: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang L, Zuo X, Xie K, Wei D. The role of CD44 and cancer stem cells. Cancer Stem Cells. 2017;1692:31–42. doi: 10.1007/978-1-4939-7401-6_3. [DOI] [PubMed] [Google Scholar]
- 68.Kamble SC, Bapat SA. Stem cell and cancer stem cell games on the ECM field. J Cancer Stem Cell Res. 2013;1:e1002. [Google Scholar]
- 69.Glumac PM, LeBeau AM. The role of CD133 in cancer: a concise review. Clin Transl Med. 2018;7:18. doi: 10.1186/s40169-018-0198-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Motegi H, Kamoshima Y, Terasaka S, et al. Type 1 collagen as a potential niche component for CD133-positive glioblastoma cells. Neuropathology. 2014;34:378–385. doi: 10.1111/neup.12117. [DOI] [PubMed] [Google Scholar]
- 71.Song WS, Yang YP, Huang CS, et al. Sox2, a stemness gene, regulates tumor-initiating and drug-resistant properties in CD133-positive glioblastoma stem cells. J Chin Med Assoc. 2016;79(10):538–545. doi: 10.1016/j.jcma.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 72.Toy KA, Valiathan RR, Nunez F, Kidwell KM, et al. Tyrosine kinase discoidin domain receptors DDR1 and DDR2 are coordinately deregulated in triple-negative breast cancer. Breast Cancer Res Treat. 2015;150:9–18. doi: 10.1007/s10549-015-3285-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shimada K, Anai S, Fujii T, et al. Syndecan-1 (CD138) contributes to prostate cancer progression by stabilizing tumour-initiating cells. J Pathol. 2013;231(4):495–504. doi: 10.1002/path.4271. [DOI] [PubMed] [Google Scholar]
- 74.Lathia JD, Li M, Hall PE, et al. Laminin alpha 2 enables glioblastoma stem cell growth. Ann Neurol. 2012;72:766–778. doi: 10.1002/ana.23674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chang C, Goel HL, Gao H, et al. A laminin 511 matrix is regulated by TAZ and functions as the ligand for the α6Bβ1 integrin to sustain breast cancer stem cells. Genes Dev. 2015;29:1–6. doi: 10.1101/gad.253682.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Keire PA, Kang I, Wight TN. Versican: role in cancer tumorigenesis. In: Brekken R, Stupack D, editors. Extracellular matrix in tumor biology. Biology of Extracellular Matrix. Cham: Springer; 2017. pp. 51–74. [Google Scholar]
- 77.Yu Q, Xue Y, Liu J, Xi Z, Li Z, Liu Y. Fibronectin promotes the malignancy of glioma stem-like cells via modulation of cell adhesion, differentiation, proliferation and chemoresistance. Front Mol Neurosci. 2018;11:130. doi: 10.3389/fnmol.2018.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stutchbury B, Atherton P, Tsang R, et al. Distinct focal adhesion protein modules control different aspects of mechanotransduction. J Cell Sci. 2017;130:1612–1624. doi: 10.1242/jcs.195362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Begum A, Ewachiw T, Jung C, et al. The extracellular matrix and focal adhesion kinase signaling regulate cancer stem cell function in pancreatic ductal adenocarcinoma. PLoS One. 2017;12:e0180181. doi: 10.1371/journal.pone.0180181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu C, Dedhar S. Integrin-linked kinase (ILK) and its interactors: a new paradigm for the coupling of extracellular matrix to actin cytoskeleton and signaling complexes. J Cell Biol. 2001;155:505–510. doi: 10.1083/jcb.200108077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Prasetyanti PR, Medema JP. Intra-tumor heterogeneity from a cancer stem cell perspective. Mol Cancer. 2017;16(1):41. doi: 10.1186/s12943-017-0600-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Parton RG, Richards AA. Lipid rafts and caveolae as portals for endocytosis: new insights and common mechanisms. Traffic. 2003;4:724–738. doi: 10.1034/j.1600-0854.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- 83.Wang Z, Wang N, Li W, et al. Caveolin-1 mediates chemoresistance in breast cancer stem cells via beta-catenin/ABCG2 signaling pathway. Carcinogenesis. 2014;35:2346–2356. doi: 10.1093/carcin/bgu155. [DOI] [PubMed] [Google Scholar]
- 84.Moreno-Vicente R, Pavón DM, Martín-Padura I, et al. Caveolin-1 modulates mechanotransduction responses to substrate stiffness through actin-dependent control of YAP. Cell Rep. 2018;25(6):1622–1635.e6. doi: 10.1016/j.celrep.2018.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Acerbi I, Cassereau L, Dean I, et al. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr Biol (Camb) 2015;7:1120–1134. doi: 10.1039/c5ib00040h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cordenonsi M, Zanconato F, Azzolin L, et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147:759–772. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 87.Basu-Roy U, Bayin NS, Rattanakorn K, et al. Sox2 antagonizes the Hippo pathway to maintain stemness in cancer cells. Nat Commun. 2015;6:6411. doi: 10.1038/ncomms7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Park JH, Shin JE, Park HW. The role of Hippo pathway in cancer stem cell biology. Mol Cell. 2018;41:83–92. doi: 10.14348/molcells.2018.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Triantafillu U, et al. Fluid shear stress induces cancer stem cell-like phenotype in MCF7 breast cancer cell line without inducing epithelial to mesenchymal transition. Int J Oncol. 2017;50:993–1001. doi: 10.3892/ijo.2017.3865. [DOI] [PubMed] [Google Scholar]
- 90.Hieda M. Implications for diverse functions of the LINC complexes based on the structure. Cells. 2017;6(1):3. doi: 10.3390/cells6010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Constantinescu D, Gray HL, Sammak PJ, et al. Lamin A/C expression is a marker of mouse and human embryonic stem cell differentiation. Stem Cells. 2006;24:177–185. doi: 10.1634/stemcells.2004-0159. [DOI] [PubMed] [Google Scholar]
- 92.Matsumoto A, Hieda M, Yokoyama Y, et al. Global loss of a nuclear lamina component, Lamin A/C, and LINC complex components SUN1, SUN2, and nesprin-2 in breast cancer. Cancer Med. 2015;4:1547–1557. doi: 10.1002/cam4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tan Y, Tajik A, Chen J, et al. Matrix softness regulates plasticity of tumour-repopulating cells via H3K9 demethylation and Sox2 expression. Nat Commun. 2014;5:717–728. doi: 10.1038/ncomms5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen J, Kumar S. Biophysical regulation of cancer stem/initiating cells: implications for disease mechanisms and translation. Curr Opin Biomed Eng. 2017;1:87–95. doi: 10.1016/j.cobme.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xu X, Farach-Carson MC, Jia X. Three-dimensional in vitro tumor models for cancer research and drug evaluation. Biotechnol Adv. 2014;32(7):1256–1268. doi: 10.1016/j.biotechadv.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Irimia D, Toner M. Spontaneous migration of cancer cells under conditions of mechanical confinement. Integr Biol. 2009;1(8–9):506–512. doi: 10.1039/b908595e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gossett DR, Tse HTK, Lee SA, et al. Hydrodynamic stretching of single cells for large population mechanical phenotyping. Proc Natl Acad Sci U S A. 2012;109:7630–7635. doi: 10.1073/pnas.1200107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Darling EM, Di Carlo D. High-throughput assessment of cellular mechanical properties. Annu Rev Biomed Eng. 2015;17:35–62. doi: 10.1146/annurev-bioeng-071114-040545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yongming Xu, Shujuan Xu, Zhi Gao et al. (2018) Degree of endplate chondrocyte degeneration in different tension regions during mechanical stimulation. Mol Med Rep 17(3):4415–4421. 10.3892/mmr.2018.8435 [DOI] [PMC free article] [PubMed]
- 100.Ordikhani F, Kim Y, Zustiak SP. The role of biomaterials on cancer stem cell enrichment and behavior. JOM. 2015;67(11):2543–2549. [Google Scholar]
- 101.Baker EL, Bonnecaze RT, Zaman MH. Extracellular matrix stiffness and architecture govern intracellular rheology in cancer. Biophys J. 2009;97(4):1013–1021. doi: 10.1016/j.bpj.2009.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Serwane F, Mongera A, Rowghanian P, et al. In vivo quantification of spatially varying mechanical properties in developing tissues. Nat Methods. 2009;97(4):1013–1021. doi: 10.1038/nmeth.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Barker HE, Bird D, Lang G, Erler JT. Tumor-secreted LOXL2 activates fibroblasts through FAK signaling. Mol Cancer Res. 2013;11(11):1425–1436. doi: 10.1158/1541-7786.MCR-13-0033-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Venkatesh V, Nataraj R, Thangaraj GS, et al. Targeting notch signalling pathway of cancer stem cells. Stem Cell Investig. 2018;5:5. doi: 10.21037/sci.2018.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lang T, Ding X, Kong L, et al. NFATC2 is a novel therapeutic target for colorectal cancer stem cells. Onco Targets Ther. 2018;11:6911–6924. doi: 10.2147/OTT.S169129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang S, Zhang H, Ghia EM, et al. Inhibition of chemotherapy resistant breast cancer stem cells by a ROR1 specific antibody. Proc Natl Acad Sci USA. 2019;116(4):1370–1377. doi: 10.1073/pnas.1816262116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cazet AS, Hui MN, Elsworth BL, et al. Targeting stromal remodeling and cancer stem cell plasticity overcomes chemoresistance in triple negative breast cancer. Nat Commun. 2018;9:2897. doi: 10.1038/s41467-018-05220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]