Abstract
Post-transplant lymphoproliferative disorders (PTLDs) cover a broad spectrum of lymphoproliferative lesions arising after solid organ or allogeneic hematopoietic stem cell transplantation. The composition and function of the tumor microenvironment (TME), consisting of all non-malignant constituents of a tumor, is greatly impacted in PTLD through a complex interplay between 4 factors: 1) the graft organ causes immune stimulation through chronic antigen presentation; 2) the therapy to prevent organ rejection interferes with the immune system; 3) the oncogenic Epstein-Barr virus (EBV), present in 80% of PTLDs, has a causative role in the oncogenic transformation of lymphocytes and influences immune responses; 4) interaction with the donor-derived immune cells accompanying the graft. These factors make PTLDs an interesting model to look at cancer-microenvironment interactions and current findings can be of interest for other malignancies including solid tumors. Here we will review the current knowledge of the TME composition in PTLD with a focus on the different factors involved in PTLD development.
Keywords: Post-transplant lymphoproliferative disorders; Epstein-Barr virus; Microenvironment, immunosuppression
Introduction
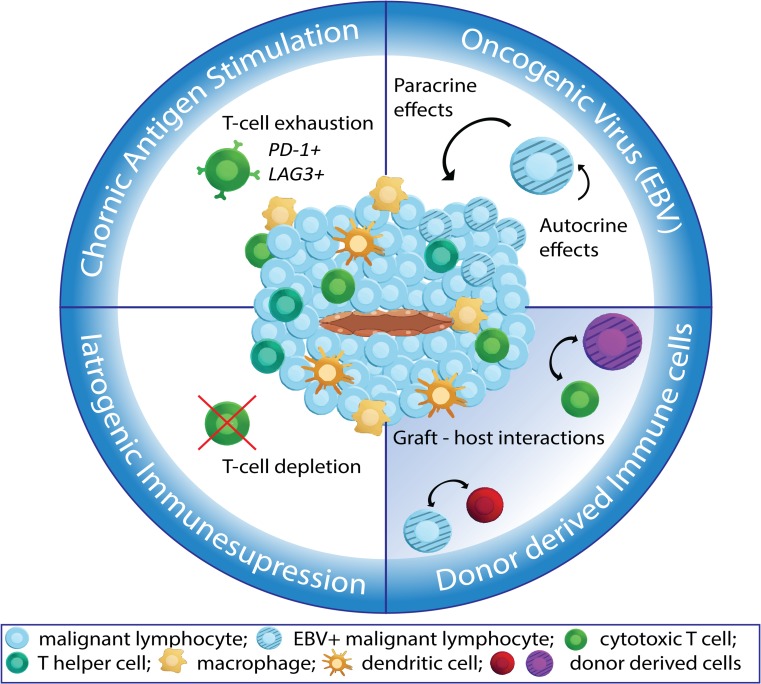
Post-transplant lymphoproliferative disorders (PTLDs) cover a broad spectrum of lymphoproliferative lesions arising after solid organ or allogeneic hematopoietic stem cell transplantation [1]. The life-long incidence of this disease varies between 2 and 20% [2] and the 3-year overall survival is about 50%–60% since the introduction of rituximab/anti-CD20 monoclonal antibody treatment [3]. Considering the high life-long risk in the post-transplant (PT) population and poor overall survival, there is an urgent need for better therapies. Interference with the immune system by immunotherapy has been suggested in recent years [3], because of the interplay of 4 major factors (Fig. 1) in PTLD pathogenesis, each with a direct impact on the immune system: 1) the graft organ causes chronic immune stimulation through chronic antigen presentation; 2) the therapy to prevent organ rejection causes chronic immunosuppression; 3) the oncogenic Epstein-Barr virus (EBV), present in 80% of PTLD [2], has a causative role in the oncogenic transformation of lymphocytes and influences immune responses; 4) donor-derived immune cells accompanying the graft interact with the host immune cells [4]. PTLD in most cases develops from lymphocytes from host origin, but in some cases from co-transplanted immune cells. The role for the TME in donor- versus host-derived PTLD remains enigmatic but might have prognostic significance [4].
Fig. 1.
Major microenvironment influencing factors. A schematic overview of the 4 major factors influencing the tumor microenvironment in post-transplant lymphoproliferative disorders. The Epstein-Barr virus has multiple para- and autocrine effects; chronic antigen stimulation leads to T-cell exhaustion; iatrogenic immunosuppression causes T-cell depletion and possible interactions between host derived and (co-)transplanted donor immune cells need to be further investigated
The tumor microenvironment (TME) consists of all non-malignant cellular constituents of a tumor including mesenchymal cells, immune cells and also non-cell components such as extracellular matrix. In lymphoma the focus has been mainly on immune cells and more specifically the adaptive immunity and T cells, but innate immune cells such as natural killer (NK) cells might play a role as well [5–7]. This is particularly true for PTLD since different immune cells are all influenced by the mentioned factors that drive or influence pathogenesis.
The importance of immunity and consequently the TME in PTLD is illustrated by the following facts: 1) reduction of immunosuppression (RIS) is considered the first step in PTLD treatment whenever possible and a subset of PTLDs shows partial to even complete regression after RIS-only, irrespective of the EBV status [8]; 2) EBV is asymptomatically present in around 95% of the adult world population [9] including the population of PT-patients and yet only a subset of PT-patients develop EBV-driven lymphoproliferations. The TME is considered to play a crucial role in the (failed) control of virus-induced replication in patients developing PTLD; 3) in contrast to lymphomas in immunocompetent patients, PTLD often develops in various extranodal sites such as the gastrointestinal tract (20–30% of cases) or the central nervous system (5–20% of cases) [3], which are characterized by a different composition of TME compared to lymph nodes.
Despite the abovementioned evidence, studies investigating the TME in PTLD remain scarce. Prognostic biomarkers described for other lymphomas (e.g. classical Hodgkin’s lymphoma [10]), such as the amount of tumor associated macrophages [11, 12] or the ratio of Foxp3+ regulatory T cells to granzyme B+ cytotoxic T cells and NK cells, have not been validated in PTLD. Predictive biomarkers for response to RIS treatment do not exist nor are biomarkers available to identify an at risk population where control of EBV-driven proliferation will fail. In this manuscript we aim to review the current knowledge of TME composition in PTLD with a focus on the different factors involved in PTLD development.
PTLD, a Complex Disorder
PTLD is a complex disorder, with various histopathological presentations, comprising a spectrum from benign (mononucleosis-like) to malignant lymphoproliferations. The different morphological lesions are thought to represent the different - and in some cases sequential - stages in pathogenesis. The WHO classification recognizes 4 categories: nondestructive, polymorphic, monomorphic and Hodgkin-type PTLD [1]. Within the nondestructive PTLDs four variants are recognized: plasmacytic hyperplasia, infectious mononucleosis-like PTLD, florid follicular hyperplasia and more recently EBV+/HHV8+ germinotropic lymphoproliferative disorder [1, 13]. They are polyclonal proliferations retaining the local tissue architecture intact. Polymorphic PTLD consists of a spectrum of EBV-transformed cells present in an abundant inflammatory stroma, containing a mixture of T cells, plasma cells, macrophages, and dendritic cells. Monomorphic PTLD represents the PT-counterpart of all possible types of non-Hodgkin lymphomas of B- or T cell origin in immunocompetent individuals [2].
The heterogeneity of this disease in subtype, anatomical localization, relation to EBV, the type of grafted organ and the variation in immunosuppressive therapy regimens complicates research. For the purpose of clarity we will first describe the impact of EBV, iatrogenic immunosuppression and chronic immune-stimulation on immune cells before summarizing what is known about the TME in the different morphological subtypes of PTLD.
Impact of EBV on the Microenvironment
EBV-positive malignancies have a distinct gene signature compared to their EBV-negative counterparts [14–16] and the lack of recurrent oncogenic karyotypic aberrations in EBV+ diffuse large B cell lymphoma (DLBCL) indicates a critical role for EBV as the driver of malignancy [17]. When investigating the role of EBV in TME composition it is important to note that EBV can strictly regulate its own viral protein expression and that different EBV-driven lymphoproliferative disorders are linked with specific combinations of viral protein expression known as latency programs [18] (Table 1). This implies that EBV+ B-cells in different malignancies will have different effects on the microenvironment. Diseases linked to a restricted latency program such as Burkitt lymphoma or plasmablastic lymphoma have limited immune cell infiltration while those with a broad latency, such as DLBCL, have a more abundant infiltration [5]. Interestingly, the main EBV oncogenic protein, latent membrane protein 1, influences both lytic viral replication and the expression of immunosuppressive markers pointing towards complex interactions between EBV latency program, lymphomagenesis and the microenvironment [19, 20]. The strict regulation of viral protein expression is however just one of the plenty of mechanisms through which EBV can influence anti-viral responses (Table 2). The myriad of EBV-related effects on the infected B cell itself and a full review of the ways in which EBV alters infected cells to promote proliferation and achieves cell immortalization is beyond the scope of this review. Excellent reviews summarizing these mechanisms have recently been published [44–47].
Table 1.
Epstein-Barr virus latency programs in different lymphoproliferative disorders
| Latency | Expressed EBV gene products | Associated disease |
|---|---|---|
| III (growth) | EBER1–2, EBNA1–6, LMP1, LMP2A-B | Post-transplant DLBCL, AIDS-related lymphoma, Acute infectious mononucleosis |
| II (default) | EBER 1–2, EBNA1, LMP1- 2A | Post-transplant DLBCL, classical Hodgkin lymphoma |
| I (low) | EBER 1–2, EBNA1 | (Post-transplant) Burkitt lymphoma, (Post-transplant) plasmablastic lymphoma |
EBV Epstein-Barr virus; EBER: EBV-encoded RNA; EBNA Epstein-Barr virus nuclear antigen; LMP latent membrane protein; DLBCL diffuse large B-cell lymphoma
Table 2.
Potential Epstein-Barr virus-related mechanism influencing the microenvironment
| Related EBV factor | Mechanism | Effect | Reference(s) |
|---|---|---|---|
| EBNA1 | CCL20 expression | Treg recruitment | [21] |
| LMP1 | IL6, IL8 and TNFα expression | Pro-inflammatory effects including neutrophil recruitment and tolerogenic effects including Tc impairment | [20, 22–25] |
| LMP1 | TGFβ expression | Tolerogenic effects including T-cell regulation | [25, 26] |
| LMP1, vIL10, EBERs | IL10 expression | Tolerogenic effects including Th1 and monocyte inhibition | [23, 27, 28] |
| LMP1 | CCL5, CCL17 and CCL22 expression | Tolerogenic effects including Th2 and Treg recruitment | [22, 29] |
| LMP1 | Extra-mitochondrial glycolysis | MDSC induction | [30] |
| LMP1 & LMP2 | Galectin-1, Galectin-9, AP-1 and PD-L1 overexpression, | Tolerogenic effects including dendritic cell induction and selective apoptosis of Th1, Th17 and Tc cells | [14, 31–36] |
| BZLF-1 | Inhibition of CIITA transcription | Disruption of antiviral Th activity | [37] |
| BZLF-1 | Interaction with RACK1 | PKC and phagocytic monocyte inhibition | [38, 39] |
| EBERs | TLR3 activation | Innate immune cell signaling | [40] |
| EBERs | IL12, MHCII and protease inhibition | Disruption of antiviral T-cell function | [41, 42] |
| EBERs | … | For a more complete review see Iwakiri et al. | [43] |
| All viral proteins | Viral protein epitope recognition | Antiviral immune response including Th1 and Tc activation | [2] |
A non-exhaustive summary of described EBV-related effects with a potential impact on the immune-cell microenvironment. The in vivo and PTLD specific effects however, are often in need of further validation
AP-1 Activator protein 1; CCL C-C motif chemokine ligand; CIITA class II, major histocompatibility complex, transactivator; EBERs Epstein-Barr virus encoded RNAs; EBNA1 EBV nuclear antigen 1; EBV Epstein-Barr virus; IL6, IL8 & IL10 Interleukin 6, 8 & 10; LMP1 & LMP2 latent membrane protein 1&2; MDSC myeloid derived suppressor cells; MHCII major histocompatibility complex class II; PD-L1 programmed death-ligand 1; PKC protein C kinase; RACK1 Receptor of Activated Protein C Kinase 1; Tc cytotoxic T cells; Th1, Th2 & Th17 T helper cells 1, 2 & 17; TGFβ Transforming Growth Factor Beta; Treg regulatory T cell; TLR Toll-like receptor 3; TNFα tumor necrosis factor alpha; vIL10 viral interleukin 10
Some changes to infected B-cells, such as upregulation of costimulatory molecules (B7, ICAM), might however be important because they potentially contribute to T cell inactivation [48]. The in vivo impact on the microenvironment is however difficult to assess since the vast majority of studies are either in vitro work on EBV+ cell lines [40, 49] or blood samples of seropositive donors with sparse in vivo validation [50]. When investigating the impact of EBV in PTLD, it is important to take into account the co-occurrence of both iatrogenic immunosuppression and chronic immune-stimulation. The current general hypothesis is that cytotoxic T cells react to EBV antigens and are attracted in greater numbers [51]. Yet one study observed a high ratio of T helper cells (CD4+) over cytotoxic T-cells (CD8+) in EBV+ PTLD [51] suggesting that the influence of immunosuppression potentially outweighs that of EBV. Despite this, one approach to gain more insight in the TME composition in PTLD is to look at other EBV-related lymphoproliferative disorders in immunocompetent patients and what it can potentially tell about the PT-counterparts of these disorders.
The Role of Specific EBV Viral Factors in Suppressing Anti-Viral Responses
The EBV-infected B cells influence anti-viral immune responses and consequently the microenvironment through a plethora of mechanisms (Table 2). Besides its primary function of ensuring that the EBV DNA is tethered to the chromatin in dividing cells, so that EBV can persist after cell division, the EBV viral protein EBNA1 inhibits downstream TGFβ signaling to promote malignant cell growth [26] and enhances the expression of CCL20, which may play a role in recruiting regulatory T cells (FoxP3+) to the tumor microenvironment [21]. In healthy seropositive individuals EBNA1 induces a CD4 positive, IFNγ producing T cell response [52]. One study by Smets et al. [53] suggested that increased EBV viral load, combined with decreased numbers of EBV-reactive T-cells based on IFNγ, could be a predictive marker of PTLD. LMP1 in turn is not only a constitutively active mimic of CD40, a costimulatory factor involved in B cell activation, but plays a critical role in creating a highly immunosuppressive microenvironment. LMP1 induces secretion of IL6, IL8, IL10, TNFα and TGFβ through the MAPK and PI3K pathways [20, 22–25]. This is further enhanced by the expression of EBV encoded viral vIL10 [27]. LMP1 is also a master regulator of lytic viral replication trough the activation of TERT (the catalytic telomerase component) via nuclear factor κB (NF-κB) and MAPK/ERK1/2 pathways, followed by inhibition of BZLF-1 expression [19, 54, 55]. Both LMP1 and LMP2A are involved in transcription of Galectin-1 with proven overexpression in EBV monomorphic PTLD [31] and a correlation with EBV latency [56]. Through NF-kB signaling LMP1 also increases the production of CCL5, CCL17 and CCL22 [22, 29]. These chemokines in turn can attract T-helper type 2 and regulatory T-cells [57–60] which inhibit cytotoxic T cells. LMP1 and LMP2 have been shown to increase PD-L1 protein dosage through upregulation of an AP-1-dependent pathway and STAT3 signaling rather than chromosome 9p24.1 amplification, as is also observed in Hodgkin lymphoma and EBV+ PT-DLBCL [14, 32–35]. Surface expression of PD-L1 which inhibits cytotoxic T cell function through interaction with the PD1 receptor has been detected in about 90% of EBV-driven lymphomas including PTLD [61–63]. LMP1 was also recently shown to induce myeloid derived suppressor cells in vitro in EBV+ nasopharyngeal carcinoma [30]. A subset of EBV-driven lymphomas also express BZLF-1 which switches on the lytic replication cycle of the virus. This is mainly observed in heavily immunodeficient population early post-hematopoietic stem cell transplantation [63]. BZLF-1 has been shown to inhibit CIITA transcription which disrupts antiviral CD4+ T cell activity by targeting the class II antigen presentation pathway [37]. BZLF-1 may also interact with RACK1 [64] which leads to inhibition of protein kinase C activity [38] and potentially reduced phagocytic activity of monocytes [39]. Not only viral proteins have immunomodulatory effects, also non-coding Epstein-Barr virus Encoded RNAs (EBERs) influence immune cells [65]. EBERs induce the expression of IL-10 in Burkitt Lymphoma cell lines [28], IL-9 in T cells [66] and innate immune signals through TLR3 [40]. EBV-related miRNAs can also interfere with antiviral CD4+ and CD8+ T cell function by targeting IL-12, MHC class II, and lysosomal proteases [41, 42]. A more complete overview of EBERs in pathogenesis was made by Iwakiri et al. [43]. In summary EBV infected B-cells can influence the microenvironment trough release of various chemokines, inflammatory cytokines and exosomes containing among other things galectin-1 and galectin-9 [47, 67]. Due to the distinct latency programs of EBV and the specific role of different EBV products in shaping the immune response (Table 2), we highly encourage sufficient EBV latency typing in future studies of the TME in PTLD.
Lessons Learnt from EBV-Related Lymphoproliferative Diseases in Immunocompetent Individuals
Infectious Mononucleosis
Research done on the TME in symptomatic EBV infection, known as infectious mononucleosis or kissing’s disease, might not be fully representative for PTLD since EBV infection in healthy individuals is ultimately controlled and does not predispose to malignancy [68]. Histologically, dispersed large EBV+ B-cells are accompanied by an abundant inflammatory T cell and macrophage rich reaction (Fig. 2) with clear morphological resemblance with infectious mononucleosis-like PTLD. Recently, there has been increased focus on the innate immune response and specifically the role of NK cells in EBV infection control [69]. The study of NK cells in the latent phase of infection as in PT-context has yet to be studied in depth [69] but Lunemann et al. [70] found a specific IFNγ producing NK-cell subset that, when added to cultures of primary B cells infected with exogenous EBV, decreased the rate of B cell transformation as compared to other NK-cell subsets.
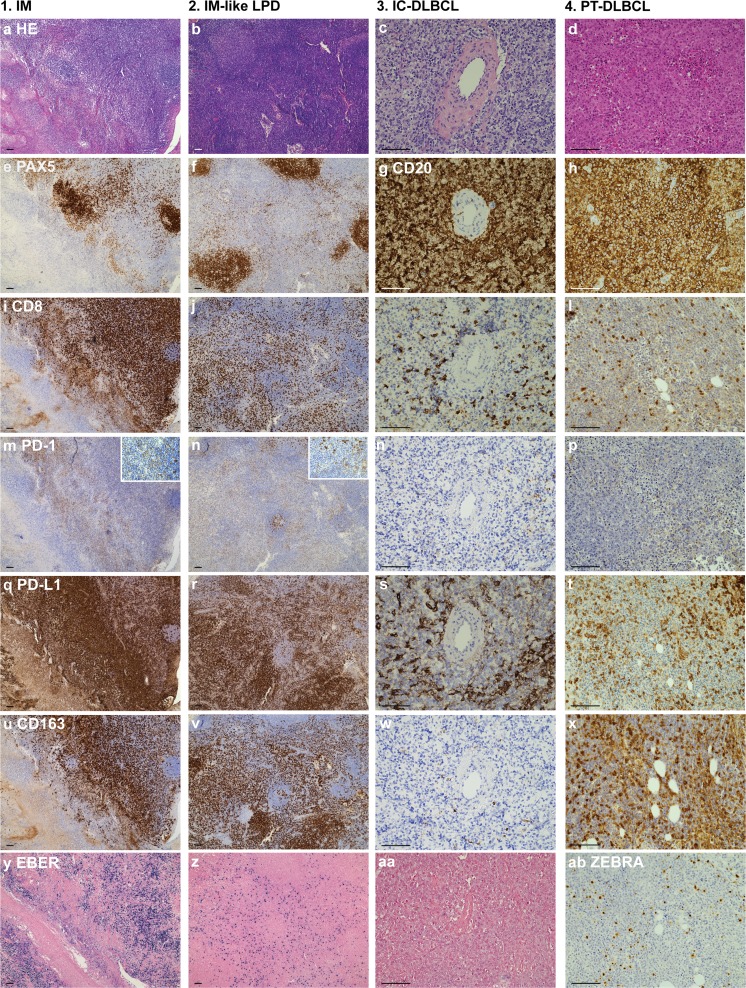
Fig. 2.
Representative cases. Representative cases of primary infectious mononucleosis in an immunocompetent young adult (column 1), infectious mononucleosis-like reactivation in a PT-patient (column 2), DLBCL in an immunocompetent patient (column 3) and PT-DLBCL highlighting the similarities between the different lesions. (a-d) hematoxylin and eosin stain; (e-f) Pax5 IHC stains and (g-h) CD20 IHC stains marking B cells; (i-l) CD8 IHC stains marking cytotoxic T cells; (m-o) PD-1 IHC stains and (q-t) PD-L1 IHC stains marking immune checkpoint therapy targets, the cutouts in (m-n) illustrate the difference in PD-1 staining intensity between cytotoxic T cells and follicular T-helper cells; (u-x) CD163 IHC stains marking macrophages; (y-aa) EBV RNA (EBER) in situ hybridization marking EBV presence; (ab) ZEBRA[BZLF] IHC stain for lytic viral replication. Abbreviations; IM: Infectious mononucleosis; LPD: lymphoproliferative disorder; IC: immune competent; DLBCL: diffuse large B-cell lymphoma; PT: post-transplant; scale bar = 100 μm; IHC, immunohistochemical
Classical Hodgkin Lymphoma
The differences in TME between EBV-positive and EBV-negative Hodgkin lymphomas (HL) in immunocompetent individuals was summarized by Murray et al. [36] and Tan et al. [71]. Since HL can develop in otherwise immunocompetent individuals these studies provide a way to observe the effect of EBV on the microenvironment without being confounded by other factors such as iatrogenic T cell depletion. In HL the presence of EBV correlates with both increased expression of cytotoxic T cell and T cell helper type 1 pathways [57]. EBV+ HL cells attract T helper type 2 and CCR4R+ T regulatory cells in vitro [72] and attracts higher numbers of cytotoxic T cells (CD8+) [16, 73] and regulatory T cells (CD25+, FoxP3+) [74, 75] together with and upregulation of markers of immunosuppression such as LAG3 and IL10 [74]. IL10 is secreted in part by an increased number of T regulatory 1 cells (ITGA2+, ITGB2+, LAG3+) [74] . LAG3 expression by cytotoxic T cells has been correlated with suppression of LMP-specific anti-EBV T cell response [76]. Natural killer cells have not been studied extensively in HL in contrast to infectious mononucleosis but a higher percentage has been reported [73]. Increased numbers of immature dendritic cells [77] (CD207+, CD83-) and macrophages [78, 79] were also reported. In PTLD these findings need to be validated but might be relevant for the morphologically similar polymorphic and Hodgkin lymphoma-like subtypes.
EBV+ Diffuse Large B Cell Lymphoma
DLBCL is the most frequent lymphoma in the general population, but infrequently associated with EBV in (mostly elderly) patients with no known immunodeficiency. EBV+ DLBCL was recognized by the WHO in 2008 as ‘EBV-positive diffuse large B-cell lymphoma of the elderly’ and renamed in 2017 as ‘EBV-positive diffuse large B-cell lymphoma, not otherwise specified’, since it occurs at all ages [80, 81]. No in depth study on the TME has been performed, but old age is related to T cell dysfunction and immunosenescence [82] and an immunotolerogenic immune state was reported in young patients with PD-L1 expression in tumor cells and presence of indoleamine 2,3-dioxygenase–positive dendritic cells/histiocytes [81]. A study on EBV+ DLBCL in the primary central nervous system lymphoma also found higher counts of PD-1+ lymphocytes in EBV+ cases vs. EBV- but no difference in FoxP3+ lymphocytes [83].
Gene expression profiling of both EBV- and EBV+ PT-DLBCL has shown an upregulation of antiviral immune signaling in EBV+ cases together with increased expression of markers indicative of an immune tolerant microenvironment, such as PD-L1, IDO1 and CD163 [2]. Immunohistochemical characterization showed an increased cytotoxic T cell infiltration in EBV+ PT-DLBCL but still lower counts compared to EBV- DLBCL in otherwise immunocompetent patients [63] illustrating the presence of an antiviral response although influences of other factors such as immunosuppressive therapy need to be taken into account.
Lessons Learnt from HIV-Associated Lymphomas
Human Immunodeficiency Virus (HIV) is a chronically persisting virus with immune state altering properties. Patients infected with HIV have an increased risk for the development of both Hodgkin and non-Hodgkin lymphomas which are often co-infected with EBV and/or HHV8. HIV-related DLBCL has become less frequent with current highly active antiretroviral therapy (HAART) therapy but has not disappeared [84]. Interestingly an increased population of dysfunctional cytotoxic T cells (CD8+, TIM3+) was observed in patients with progressive HIV-1 infection [85] but recent identification of HIV-specific resident memory CD8+ T cells illustrate how identification of HIV and consequentially EBV specific T cell responses is still underway [86]. Pantanowitz et al. [87] summarized the known changes in the TME in EBV+ HIV-related DLBCL compared to DLBCL, NOS as follows: 1) increased cytotoxic T cells (CD8+), 2) decreased regulatory T cells (Foxp3+) and 3) no significant differences in macrophage numbers but higher expression of PD-L1 on both macrophages and tumor cells.
Impact of Chronic Antigen Stimulation on Microenvironment
Antigens from the grafted organ and the EBV virus cause a continuous stimulation of the immune system by prolonged T cell receptor engagement [88]. In the case of autologous hematopoietic stem cell transplantation the novel graft-derived immune cells are stimulated by all other cells in the patient’s body. This has a major impact on the immune system and specifically on T cells. Cytotoxic T cells (CD8+) comprise a spectrum of naïve T cells, effector T cells, effector memory T cells and also dysfunctional T cells subsets such as exhausted T cells [89]. Exhausted T cells are characterized by expression of inhibitory receptors such as PD-1, LAG3 and TIM3 [89]. Both chronic infections as well as cancer can lead to a state of T cell exhaustion through chronic immune-stimulation [88]. Exhausted T-cell induction is employed by EBV in order to allow viral replication as shown in non-transplanted patients with HIV and EBV infections [90, 91]. Similar changes are observed for T helper cells (CD4+) with skewing towards T follicular helper cells (CD4+, PD-1+) and away from the antiviral T-helper type-1 (T-bet+) subset in persistent viral infection in mice [92]. This functional profile was related to antigen load in HIV-1 infected humans [93] and Moran et al. [94] found the level of PD-1 expression in cytotoxic T cells (CD8+) to be increased post-transplantation, independent of EBV viral load, suggesting that these changes in the T cell population are at least partially caused by non-viral factors. The relative weight of chronic antigen stimulation in the process of T cell exhaustion remains however artificial since EBV on itself also induces IL-10 and TGFβ which in turn cause T cell exhaustion [88]. High PD-1+ cell counts were associated with worse outcome in Asian and Latin-American DLBCL patients both EBV+ and EBV- [95] but further studies are needed to investigate the relevant PD-1+ cell types and the role of T cell exhaustion in PTLD.
Impact of Iatrogenic Immunosuppression on Microenvironment
Immunosuppressive therapy post-transplantation is designated to prevent graft rejection by depletion and prevention of T cell activation. Maintenance therapy often consists of a low level glucocorticoids, calcineurin inhibitors (cyclosporine A or tacrolimus), antimetabolic agents (azathioprine or mycophenolate mofetil) and mammalian target of rapamycin (mTOR) inhibitors (everolimus and sirolimus) [3]. Epidemiological studies of PT-patients have shown how immune surveillance is important in preventing carcinogenesis, irrespective of EBV, [96, 97] and established PTLD risk factors include intensity of induction immunosuppressive therapy and duration of maintenance therapy [3]. The efficacy of reduction in immunosuppression (RIS) in both EBV-positive and EBV-negative lesions [98] also indicates a role for immunosuppression in PTLD development independent of EBV status [3, 99]. The frequent co-treatment with different types of immunomodulatory/immunosuppressive drugs and different underlying disorders complicates the study of the effects of these drugs on the immune system [46]. Yet differences can be expected since not all drugs are associated with identical risks. There is for example strong evidence for an increased risk associated with tacrolimus and azathioprine but also strong evidence for no increased risk associated with mycophenolate [3].
A cytotoxic T cell response to primary EBV infection can still be generated during a PT-immunosuppressive drug regimen [100, 101] and cytotoxic T cell infiltration (CD3+, TIA-1+) has been associated with better overall survival in PTLD [102]. But when comparing the TME of EBV-negative PT-DLBCL with EBV-negative DLBCL in immunocompetent hosts, significantly less cytotoxic T cells (CD8+) were observed [63, 103]. The impact of immunosuppressive therapy on T cell exhaustion in PTLD remains elusive but one study found PD-1+ cells in the TME more frequently in patients after heart transplantations than other solid organ transplantations [61]. A lower amount of regulatory T-cells was also observed in PT-DLBCL vs. DLBCL in immunocompetent hosts and this might be due to immunosuppressive therapy [63, 104]. Due do the severe risk associated with graft rejection in the heart transplant population these patients receive more intensive immunosuppression therapy regimens and have a higher life-long risk for PTLD. Current hypothesis is that both diminished numbers of EBV-specific T-cells and impaired function of these cells are of importance in the pathogenesis of PTLD.
TME in the Different Morphological Subtypes of PTLD
Nondestructive PTLD
Nondestructive PTLD consists of multiple distinct histopathological lesions, but all conserve local tissue architecture. These lesions most frequently develop in tonsils and/or adenoids but other sites both nodal and extranodal can be involved as well [105]. An association with EBV is observed in almost all cases of nondestructive PTLD although some cases of florid follicular hyperplasia may be EBV negative [1]. Since most cases of nondestructive PTLD usually regresses spontaneously, or after RIS, research focusing on these lesions is limited [106, 107]. Plasmacytic hyperplasia consists of numerous polytypic plasma cells, small lymphocytes and infrequent immunoblasts; infectious mononucleosis-like PTLD has similar features as infectious mononucleosis: numerous EBV+ immunoblasts surrounded by an abundant inflammatory infiltrate of T-cells, plasma cells and macrophages [1]. The germinotropic lymphoproliferative disorder (EBV+/HHV8+) has been implicated as well and is characterized by EBV/HHV8 double positive B cells in lymph node germinal centers. Plasmacytoid dendritic cells (CD123+, BDCA-2+) play a role in the anti-viral immune response and a marked increase of these cells was observed in nondestructive PTLD vs. monomorphic-PTLD [108]. Serum concentration of human IL-10 has been proposed as a diagnostic biomarker [109, 110] but biomarkers predicting sequential development of polymorphic and /or monomorphic PTLD are currently lacking.
Polymorphic PTLD
Polymorphic PTLD consists of a heterogeneous population of EBV-transformed B-lymphocytes [3], ranging from small over intermediate to large immunoblasts and plasma cells. The lesion is characterized by the effacement of lymph node architecture or destruction of extranodal tissue and often necrosis, but does not meet the criteria of a recognized lymphoma described in an immunocompetent host [1]. There is no official criterion for the proportion of transformed cells but they are present in a mixture of non-transformed plasma cells, eosinophils, heterogeneous T-cells, histiocytes and dendritic cells. Relapse and subsequent monomorphic PTLDs have been described [111], but predictive markers are again lacking. Low numbers of FoxP3+ cells have been associated with an increased risk of progression [56], but studies focused on the TME in P-PTLD are lacking despite its abundance and heterogeneous composition. This is especially unfortunate since the composition of both large transformed B cells and smaller (EBV-) B cells makes P-PTLD an excellent model to study the B cell component of the microenvironment.
Monomorphic PTLD
Monomorphic PTLD are monoclonal proliferations that fulfill the criteria for a recognized lymphoma in otherwise immunocompetent patients [1]. Up to 65% are associated with EBV [3] and it is the most frequently diagnosed subtype (80% of PTLD) [112]. DLBCL is the most frequent subtype of monomorphic PTLD (90%) [3], but Burkitt lymphoma, plasmablastic lymphoma, primary central nervous system lymphoma, Burkitt-like lymphoma with 11q aberrations [113] and to a lesser extent lymphomas of T cell or NK-cell origin also exist [112, 114]. PT-DLBCL and DLBCL developing in immunocompetent patients have similar morphology (Fig. 2) but described differences in the microenvironment [115] warrant further investigation. The microenvironment of lymphomas in immunocompetent patients has been extensively reviewed by Scott et al. [5]. Important to note is that the amount of non-malignant immune cells in the microenvironment varies between these different entities, Burkitt lymphoma for example has a limited tumor microenvironment with just a few tingible body macrophages [5].
The main changes to the TME in the PTLD counterparts are induced by the 4 PTLD specific factors (Fig. 1). Cytotoxic T cell dysfunction is influenced by both EBV, chronic antigen stimulation and immunosuppressive therapy. EBV uses a plethora of mechanisms to achieve immune-evasion involving multiple cytokines and immune checkpoints such as PD-L1 [14].
In the PT-DLBCL the infiltration of cytotoxic T cells (CD3+, TIA-1+) and high levels of PD-1 positive lymphocytes [56] and macrophages (CD68+) [116] have been associated with better overall survival [102], while no effect on survival has been found for regulatory T cell (FoxP3+) counts [56, 102] or galectin-1+ cytotoxic T cell counts [56]. The EBV+ malignant B cell population is so dominant in monomorphic PTLD that non-malignant B cells are virtually absent and not much is known of their role in the microenvironment.
Conclusions and Future Perspectives
The important role of EBV in the majority of PTLD makes it into a viable target for specific therapies. The current therapy protocol for EBV+ PTLD of B cell origin is a combination of RIS, followed by anti-CD20 immunotherapy and/or CHOP chemotherapy and is no different from the treatment of EBV-negative PTLD. Various immunotherapies have been suggested for the treatment of EBV+ PTLD including adoptive T cell transfer [117], immune checkpoint inhibitors [118] and induction of lytic viral replication in combination with ganciclovir antiviral therapy [119].
Cytotoxic T cell dysfunction caused by EBV specific mechanisms and chronic antigen stimulation leads to an upregulation of immune checkpoint markers which could be targeted by therapy. PD-L1 expression has been reported in PTLD tumor cells and M2 macrophages [61–63]. Both EBV and chronic immune stimulation lead to increased LAG3 expression in cytotoxic T cells (CD8+). Anti-LAG3 therapy is currently undergoing phase 1 trials for multiple indications including B cell non-Hodgkin lymphomas [120]. One reason for concern however, is the possible impact on graft rejection since auto-immune effects have been observed in otherwise immunocompetent cancer patients treated with multiple immunotherapy agents [121]. In one case where PD-L1 targeting therapy (nivolumab) was used in a renal transplant patient to treat metastatic squamous-cell carcinoma the patient lost the allograft despite high-dose glucocorticoids [122]. Ex vivo adoptive cytotoxic T cells (CTL) therapy targeting EBV antigens might circumvent these problems and could be a possibility for EBV+ PTLD treatment [123]. Older pilot studies already demonstrated PTLD remission after adoptive transfer of EBV-CTLs [124, 125]. These are CTLs with a broad reactivity to a range of EBNA- and LMP-derived epitopes [125] and function of the allograft was preserved [125]. A caveat here are the effects of iatrogenic and disease-related immunosuppression which might reduce efficient T cell responses in PTLD [118]. One more positive note, the RIS also has therapeutic effects in EBV-negative PTLD highlighting that immune-modulating therapy does not need to focus on EBV-specific effects alone but could be effective in all PTLDs, including EBV-negative cases in which anti-EBV cytotoxic T cell therapy would be ineffective. Hematopoietic stem cell transplant patients are both heavily immunosuppressed and donor-derived immune cells are exposed to the abundant antigen stimulation since all cells of the host cells are allogeneic. PT-DLBCL arising in this situation is distinct for other PT-DLBCL and might be sensitive to bortezomib in combination with anti-viral therapy, since they are characterized by high endoplasmic reticulum stress and lytic EBV replication [63]. All these therapies are promising but discovery of biomarkers that can predict therapy response will be dependent on a better understanding of the TME in vivo. Especially a predictive biomarker to determine if RIS-only will lead to regression of the lesion is of interest since this could help avoid unnecessary therapies that are both a physical and financial burden for patients.
Besides the search for better therapeutic options, other questions, like how to identify PT-patients that will most likely develop PTLD and why PTLD develops in these cases remain. Answering these questions is the necessary first step in the development of preventive strategies and/or effective implementation of existing antiviral prophylaxis. Not only to prevent PTLD development but also to determine which non-destructive and polymorphic PTLD are likely to progress to monomorphic PTLD. More study of the TME in non-destructive and polymorphic PTLD-subtypes, including the innate immune cells is needed, considering the role of NK-cells in the control of EBV-reactivation [126]. Better characterization of the TME in PTLD lymphomas is crucial to find these biomarkers and decide the optimal therapeutic strategy for each patient.
Acknowledgments
We would like to recognize and thank the other members of the ‘Leuven PTLD consortium’: prof. dr. Daan Dierickx and prof. dr. Gregor Verhoef (department of Hematology) and prof. dr. Olivier Gheysens (department of Nuclear Medicine) with whom we collaborate for all PTLD focused research. The artwork used for Fig. 1 was created by Veerle Haemels.
Financial Support
TT holds a Mandate for Fundamental and Translational Research from the ‘Stichting tegen Kanker’ (2014–083).
LM is a PhD student, financially supported by KULeuven, Department of Imaging and Pathology, ‘Stefanie’s Rozen fonds’, ‘Fonds Tom Debackere’, ‘Stichting Me to You (https://www.stichtingmetoyou.be/nl/)’ and “Emmanuel van der Schueren beurs (Kom op tegen Kanker)’.
Compliance with Ethical Standards
Conflicts of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Swerdlow S (2017) Post-transplant lymphoproliferative diorders. In: Swerdlow S (ed) WHO classification of Tumours of Haematopoietic and lymphoid tissues, revised 4t. Lyon, pp 453–462
- 2.Morscio J, Dierickx D, Tousseyn T. Molecular pathogenesis of B-cell posttransplant lymphoproliferative disorder: what do we know so far? Clin Dev Immunol. 2013;2013:1–13. doi: 10.1155/2013/150835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dierickx D, Habermann T. Post-transplantation lymphoproliferative disorders in adults. N Engl J Med. 2018;378:549–562. doi: 10.1056/NEJMra1702693. [DOI] [PubMed] [Google Scholar]
- 4.Nuckols JD, Baron PW, Stenzel TT, Olatidoye BA, Tuttle-Newhall JE, Clavien PA, Howell DN. The pathology of liver-localized post-transplant lymphoproliferative disease: a report of three cases and a review of the literature. Am J Surg Pathol. 2000;24:733–741. doi: 10.1097/00000478-200005000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Scott DW, Gascoyne RD. The tumour microenvironment in B cell lymphomas. Nat Rev Cancer. 2014;14:517–534. doi: 10.1038/nrc3774. [DOI] [PubMed] [Google Scholar]
- 6.Menter T, Tzankov A. Mechanisms of immune evasion and immune modulation by lymphoma cells. Front Oncol. 2018;8:1–11. doi: 10.3389/fonc.2018.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belli C, Trapani D, Viale G, D'Amico P, Duso BA, Della Vigna P, Orsi F, Curigliano G. Targeting the microenvironment in solid tumors. Cancer Treat Rev. 2018;65:22–32. doi: 10.1016/j.ctrv.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Reshef R, Vardhanabhuti S, Luskin MR, Heitjan DF, Hadjiliadis D, Goral S, Krok KL, Goldberg LR, Porter DL, Stadtmauer EA, Tsai DE. Reduction of immunosuppression as initial therapy for posttransplantation lymphoproliferative disorder. Am J Transplant. 2011;11:336–347. doi: 10.1111/j.1600-6143.2010.03387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thorley-Lawson DA. EBV the prototypical human tumor virus - just how bad is it? J Allergy Clin Immunol. 2005;116:251–261. doi: 10.1016/j.jaci.2005.05.038. [DOI] [PubMed] [Google Scholar]
- 10.Álvaro T, Lejeune M, Salvadó MT, et al (2005) Outcome in Hodgkin ’ s Lymphoma Can Be Predicted from the Presence of Accompanying Cytotoxic and Regulatory T Cells Outcome in Hodgkin ’ s Lymphoma Can Be Predicted from the Presence of Accompanying Cytotoxic and Regulatory T Cells 11:1467–1473. 10.1158/1078-0432.CCR-04-1869 [DOI] [PubMed]
- 11.Gotti M, Nicola M, Lucioni M, Fiaccadori V, Ferretti V, Sciarra R, Costanza M, Bono E, Molo S, Maffi A, Croci GA, Varettoni M, Frigeni M, Pascutto C, Arcaini L, Bonfichi M, Paulli M, Cazzola M. Independent prognostic impact of tumour-infiltrating macrophages in early-stage Hodgkin’s lymphoma. Hematol Oncol. 2017;35:296–302. doi: 10.1002/hon.2295. [DOI] [PubMed] [Google Scholar]
- 12.Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, Delaney A, Jones SJ, Iqbal J, Weisenburger DD, Bast MA, Rosenwald A, Muller-Hermelink HK, Rimsza LM, Campo E, Delabie J, Braziel RM, Cook JR, Tubbs RR, Jaffe ES, Lenz G, Connors JM, Staudt LM, Chan WC, Gascoyne RD. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med. 2010;362:875–885. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Said J, Isaacson PG, Campo E, Harris NL, et al. HHV8-positive germinotropic lymphoproliferative disorder. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO classification of Tumours of Haematopoietic and lymphoid tissues, 2017 revis. Lyon: IARC; 2017. pp. 325–329. [Google Scholar]
- 14.Finalet Ferreiro J, Morscio J, Dierickx D, Vandenberghe P, Gheysens O, Verhoef G, Zamani M, Tousseyn T, Wlodarska I. EBV-positive and EBV-negative Posttransplant diffuse large B cell lymphomas have distinct genomic and transcriptomic features. Am J Transplant. 2016;16:414–425. doi: 10.1111/ajt.13558. [DOI] [PubMed] [Google Scholar]
- 15.Chetaille B, Bertucci F, Finetti P, Esterni B, Stamatoullas A, Picquenot JM, Copin MC, Morschhauser F, Casasnovas O, Petrella T, Molina T, Vekhoff A, Feugier P, Bouabdallah R, Birnbaum D, Olive D, Xerri L. Molecular profiling of classical Hodgkin lymphoma tissues uncovers variations in the tumor microenvironment and correlations with EBV infection and outcome. Blood. 2009;113:2765–3775. doi: 10.1182/blood-2008-07-168096. [DOI] [PubMed] [Google Scholar]
- 16.Barros MHM, Vera-Lozada G, Soares FA, Niedobitek G, Hassan R. Tumor microenvironment composition in pediatric classical Hodgkin lymphoma is modulated by age and Epstein-Barr virus infection. Int J Cancer. 2012;131:1142–1152. doi: 10.1002/ijc.27314. [DOI] [PubMed] [Google Scholar]
- 17.Finalet Ferreiro J, Rouhigharabaei L, Urbankova H, van der Krogt JA, Michaux L, Shetty S, Krenacs L, Tousseyn T, de Paepe P, Uyttebroeck A, Verhoef G, Taghon T, Vandenberghe P, Cools J, Wlodarska I. Integrative genomic and transcriptomic analysis identified candidate genes implicated in the pathogenesis of hepatosplenic T-cell lymphoma. PLoS One. 2014;9:e102977. doi: 10.1371/journal.pone.0102977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morscio J, Tousseyn T. Recent insights in the pathogenesis of post-transplantation lymphoproliferative disorders. World J Transplant. 2016;6:505. doi: 10.5500/wjt.v6.i3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giunco S, Petrara MR, Zangrossi M, Celeghin A, de Rossi A. Extra-telomeric functions of telomerase in the pathogenesis of Epstein-Barr virus-driven B-cell malignancies and potential therapeutic implications. Infect Agent Cancer. 2018;13:1–7. doi: 10.1186/s13027-018-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gandhi MK, Moll G, Smith C, Dua U, Lambley E, Ramuz O, Gill D, Marlton P, Seymour JF, Khanna R. Galectin-1 mediated suppression of Epstein-Barr virus specific T-cell immunity in classic Hodgkin lymphoma. Blood. 2007;110:1326–1329. doi: 10.1182/blood-2007-01-066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baumforth KRN, Birgersdotter A, Reynolds GM, Wei W, Kapatai G, Flavell JR, Kalk E, Piper K, Lee S, Machado L, Hadley K, Sundblad A, Sjoberg J, Bjorkholm M, Porwit AA, Yap LF, Teo S, Grundy RG, Young LS, Ernberg I, Woodman CBJ, Murray PG. Expression of the Epstein-Barr virus-encoded Epstein-Barr virus nuclear antigen 1 in Hodgkin’s lymphoma cells mediates up-regulation of CCL20 and the migration of regulatory T cells. Am J Pathol. 2008;173:195–204. doi: 10.2353/ajpath.2008.070845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vockerodt M, Morgan SL, Kuo M, Wei W, Chukwuma MB, Arrand JR, Kube D, Gordon J, Young LS, Woodman CB, Murray PG. The Epstein-Barr virus oncoprotein, latent membrane protein-1, reprograms germinal Centre B cells towards a Hodgkin’s reed-Sternberg-like phenotype. J Pathol. 2008;216:83–92. doi: 10.1002/path.2384. [DOI] [PubMed] [Google Scholar]
- 23.Lambert SL, Martinez OM. Latent membrane protein 1 of EBV activates phosphatidylinositol 3-kinase to induce production of IL-10. J Immunol. 2007;179:8225–8234. doi: 10.4049/jimmunol.179.12.8225. [DOI] [PubMed] [Google Scholar]
- 24.Eliopoulos AG, Gallagher NJ, Blake SM, et al. Activation of the p38 mitogen-activated protein kinase pathway by Epstein-Barr virus-encoded latent membrane protein 1 coregulates interleukin-6 and interleukin-8 production. J Biol Chem. 1999;274:16085–16096. doi: 10.1074/jbc.274.23.16085. [DOI] [PubMed] [Google Scholar]
- 25.Liu W, Lin Y, Xiao H, Xing S, Chen H, Chi PD, Zhang G. Epstein-Barr virus infection induces indoleamine 2,3-dioxygenase expression in human monocyte-derived macrophages through p38/mitogen-activated protein kinase and NF-κB pathways: impairment in T cell functions. J Virol. 2014;88:6660–6671. doi: 10.1128/JVI.03678-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flavell JR, Baumforth KRN, Wood VHJ, Davies GL, Wei W, Reynolds GM, Morgan S, Boyce A, Kelly GL, Young LS, Murray PG. Down-regulation of the TGF-beta target gene, PTPRK, by the Epstein-Barr virus-encoded EBNA1 contributes to the growth and survival of Hodgkin lymphoma cells. Blood. 2008;111:292–301. doi: 10.1182/blood-2006-11-059881. [DOI] [PubMed] [Google Scholar]
- 27.Jochum S, Moosmann A, Lang S et al (2012) The EBV immunoevasins vIL-10 and BNLF2a protect newly infected B cells from immune recognition and elimination. PLoS Pathog 8. 10.1371/journal.ppat.1002704 [DOI] [PMC free article] [PubMed]
- 28.Kitagawa N, Goto M, Kurozumi K, et al. Epstein - Barr virus-encoded poly ( a )- RNA supports Burkitt ’ s lymphoma growth through interleukin-10 induction. EMBO J. 2000;19:6742–6750. doi: 10.1093/emboj/19.24.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakayama T, Hieshima K, Nagakubo D, Sato E, Nakayama M, Kawa K, Yoshie O. Selective induction of Th2-attracting chemokines CCL17 and CCL22 in human B cells by latent membrane protein 1 of Epstein-Barr virus selective induction of Th2-attracting chemokines CCL17 and CCL22 in human B cells by latent membrane protein 1 of Epstein. J Virol. 2004;78:1665–1674. doi: 10.1128/JVI.78.4.1665-1674.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai TT, Ye SB, Liu YN, He J, Chen QY, Mai HQ, Zhang CX, Cui J, Zhang XS, Busson P, Zeng YX, Li J. LMP1-mediated glycolysis induces myeloid-derived suppressor cell expansion in nasopharyngeal carcinoma. PLoS Pathog. 2017;13:1–23. doi: 10.1371/journal.ppat.1006503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ouyang J, Juszczynski P, Rodig SJ, Green MR, O'Donnell E, Currie T, Armant M, Takeyama K, Monti S, Rabinovich GA, Ritz J, Kutok JL, Shipp MA. Viral induction and targeted inhibition of galectin-1 in EBV + posttransplant lymphoproliferative disorders. Blood. 2011;117:4315–4322. doi: 10.1182/blood-2010-11-320481. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto R, Nishikori M, Kitawaki T, Sakai T, Hishizawa M, Tashima M, Kondo T, Ohmori K, Kurata M, Hayashi T, Uchiyama T. PD-1 PD-1 ligand interaction contributes to immunosuppressive microenvironment of Hodgkin lymphoma. Blood. 2008;111:3220–3224. doi: 10.1182/blood-2007-05-085159. [DOI] [PubMed] [Google Scholar]
- 33.Juszczynski P, Ouyang J, Monti S, Rodig SJ, Takeyama K, Abramson J, Chen W, Kutok JL, Rabinovich GA, Shipp MA. The AP1-dependent secretion of galectin-1 by reed Sternberg cells fosters immune privilege in classical Hodgkin lymphoma. Proc Natl Acad Sci. 2007;104:13134–13139. doi: 10.1073/pnas.0706017104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green MR, Rodig S, Juszczynski P, Ouyang J, Sinha P, O'Donnell E, Neuberg D, Shipp MA. Constitutive AP-1 activity and EBV infection induce PD-l1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: implications for targeted therapy. Clin Cancer Res. 2012;18:1611–1618. doi: 10.1158/1078-0432.CCR-11-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilardini Montani MS, Santarelli R, Falcinelli L, Gonnella R, Granato M, di Renzo L, Cuomo L, Vitillo M, Faggioni A, Cirone M. EBV up-regulates PD-L1 on the surface of primary monocytes by increasing ROS and activating TLR signaling and STAT3. J Leukoc Biol. 2018;104:821–832. doi: 10.1002/JLB.2A0118-029RR. [DOI] [PubMed] [Google Scholar]
- 36.Murray P, Bell A. Contribution of the Epstein-Barr virus to the pathogenesis of Hodgkin lymphoma. In: Münz C, editor. Epstein Barr Virus Volume 1. Cham: Springer International Publishing; 2015. pp. 287–313. [DOI] [PubMed] [Google Scholar]
- 37.Li D, Qian L, Chen C, Shi M, Yu M, Hu M, Song L, Shen B, Guo N. Down-regulation of MHC class II expression through inhibition of CIITA transcription by lytic Transactivator Zta during Epstein-Barr virus reactivation. J Immunol. 2009;182:1799–1809. doi: 10.4049/jimmunol.0802686. [DOI] [PubMed] [Google Scholar]
- 38.Savard M, Gosselin J. Epstein-Barr virus immunossuppression of innate immunity mediated by phagocytes. Virus Res. 2006;119:134–145. doi: 10.1016/j.virusres.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 39.Savard M, Bélanger C, Tardif M, et al. Infection of primary human monocytes by Epstein-Barr virus. J Virol. 2000;74:2612–2619. doi: 10.1128/jvi.74.6.2612-2619.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iwakiri D, Zhou L, Samanta M, Matsumoto M, Ebihara T, Seya T, Imai S, Fujieda M, Kawa K, Takada K. Epstein-Barr virus (EBV)–encoded small RNA is released from EBV-infected cells and activates signaling from toll-like receptor 3. J Exp Med. 2009;206:2091–2099. doi: 10.1084/jem.20081761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Albanese M, Tagawa T, Bouvet M, Maliqi L, Lutter D, Hoser J, Hastreiter M, Hayes M, Sugden B, Martin L, Moosmann A, Hammerschmidt W. Epstein–Barr virus microRNAs reduce immune surveillance by virus-specific CD8 + T cells. Proc Natl Acad Sci. 2016;113:E6467–E6475. doi: 10.1073/pnas.1605884113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tagawa T, Albanese M, Bouvet M, Moosmann A, Mautner J, Heissmeyer V, Zielinski C, Lutter D, Hoser J, Hastreiter M, Hayes M, Sugden B, Hammerschmidt W. Epstein-Barr viral miRNAs inhibit antiviral CD4 + T cell responses targeting IL-12 and peptide processing. J Exp Med. 2016;213:2065–2080. doi: 10.1084/jem.20160248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iwakiri D. Epstein-Barr virus-encoded RNAs: key molecules in viral pathogenesis. Cancers (Basel) 2014;6:1615–1630. doi: 10.3390/cancers6031615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hawthorne DC, Leupold U, Allday MJ, et al (2015) Epstein Barr Virus Volume 2. Springer International Publishing, Cham
- 45.Allday MJ, Bazot Q, White RE (2015) Epstein Barr Virus Volume 2. Springer International Publishing, Cham
- 46.Martinez OM, Krams SM. The immune response to Epstein Barr virus and implications for posttransplant lymphoproliferative disorder. Transplantation. 2017;101:2009–2016. doi: 10.1097/TP.0000000000001767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dolcetti R. Cross-talk between Epstein-Barr virus and microenvironment in the pathogenesis of lymphomas. Semin Cancer Biol. 2015;34:58–69. doi: 10.1016/j.semcancer.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 48.Salek-Ardakani S, Arrand JR, Mackett M. Epstein-Barr virus encoded interleukin-10 inhibits HLA-class I, ICAM-1, and B7 expression on human monocytes: implications for immune evasion by EBV. Virology. 2002;304:342–351. doi: 10.1006/viro.2002.1716. [DOI] [PubMed] [Google Scholar]
- 49.Samanta M, Takada K. Modulation of innate immunity system by Epstein-Barr virus-encoded non-coding RNA and oncogenesis. Cancer Sci. 2010;101:29–35. doi: 10.1111/j.1349-7006.2009.01377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maini MK, Gudgeon N, Wedderburn LR, Rickinson AB, Beverley PCL. Clonal expansions in acute EBV infection are detectable in the CD8 and not the CD4 subset and persist with a variable CD45 phenotype. J Immunol. 2000;165:5729–5737. doi: 10.4049/jimmunol.165.10.5729. [DOI] [PubMed] [Google Scholar]
- 51.Perera SM, Thomas JA, Burke M, Crawford DH. Analysis of the T-cell micro-environment in Epstein-Barr virus-related post-transplantation B lymphoproliferative disease. J Pathol. 1998;184:177–184. doi: 10.1002/(SICI)1096-9896(199802)184:2<177::AID-PATH977>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 52.Heller KN, Upshaw J, Seyoum B, Zebroski H, Munz C. Distinct memory CD4+ T-cell subsets mediate immune recognition of Epstein Barr virus nuclear antigen 1 in healthy virus carriers. Blood. 2007;109:1138–1146. doi: 10.1182/blood-2006-05-023663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smets F, Latinne D, Bazin H, et al. Ratio between Epstein-Barr viral load and anti-Epstein-Barr virus specific T-cell response as a predictive marker of posttransplant lymphoproliferative disease. Transplantation. 2002;73:1603–1610. doi: 10.1097/00007890-200205270-00014. [DOI] [PubMed] [Google Scholar]
- 54.Giunco S, Dolcetti R, Keppel S, Celeghin A, Indraccolo S, Dal Col J, Mastorci K, de Rossi A. hTERT inhibition triggers Epstein-Barr virus lytic cycle and apoptosis in immortalized and transformed B cells: a basis for new therapies. Clin Cancer Res. 2013;19:2036–2047. doi: 10.1158/1078-0432.CCR-12-2537. [DOI] [PubMed] [Google Scholar]
- 55.Terrin L, Dal Col J, Rampazzo E, Zancai P, Pedrotti M, Ammirabile G, Bergamin S, Rizzo S, Dolcetti R, de Rossi A. Latent membrane protein 1 of Epstein-Barr virus activates the hTERT promoter and enhances telomerase activity in B lymphocytes. J Virol. 2008;82:10175–10187. doi: 10.1128/JVI.00321-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vase MØ, Maksten E, Bendix K, et al. Tumor microenvironmental features and outcome in post-transplant lymphoproliferative disorder. Blood. 2014;124:1617 LP–1611617. [Google Scholar]
- 57.Skinnider BF, Mak TW, Houston JP, et al. Review article the role of cytokines in classical Hodgkin lymphoma. Cytometry A. 2002;77:861–872. [Google Scholar]
- 58.Aldinucci D, Lorenzon D, Cattaruzza L, Pinto A, Gloghini A, Carbone A, Colombatti A. Expression of CCR5 receptors on reed-Sternberg cells and Hodgkin lymphoma cell lines: involvement of CCL5/Rantes in tumor cell growth and microenvironmental interactions. Int J Cancer. 2008;122:769–776. doi: 10.1002/ijc.23119. [DOI] [PubMed] [Google Scholar]
- 59.Fischer M, Juremalm M, Olsson N, Backlin C, Sundström C, Nilsson K, Enblad G, Nilsson G. Expression of CCL5/RANTES by Hodgkin and reed-Sternberg cells and its possible role in the recruitment of mast cells into lymphomatous tissue. Int J Cancer. 2003;107:197–201. doi: 10.1002/ijc.11370. [DOI] [PubMed] [Google Scholar]
- 60.van den Berg A, Visser L, Poppema S. High expression of the CC chemokine TARC in reed-Sternberg cells. Am J Pathol. 1999;154:1685–1691. doi: 10.1016/S0002-9440(10)65424-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kinch A, Sundström C, Baecklund E, Backlin C, Molin D, Enblad G (2018) Expression of PD-1, PD-L1, and PD-L2 in posttransplant lymphoproliferative disorder after solid organ transplantation. Leuk Lymphoma:1–9. 10.1080/10428194.2018.1480767 [DOI] [PubMed]
- 62.Chen BJ, Chapuy B, Ouyang J, Sun HH, Roemer MGM, Xu ML, Yu H, Fletcher CDM, Freeman GJ, Shipp MA, Rodig SJ. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19:3462–3473. doi: 10.1158/1078-0432.CCR-13-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morscio J, Finalet Ferreiro J, Vander Borght S, Bittoun E, Gheysens O, Dierickx D, Verhoef G, Wlodarska I, Tousseyn T. Identification of distinct subgroups of EBV-positive post-transplant diffuse large B-cell lymphoma. Mod Pathol. 2017;30:370–381. doi: 10.1038/modpathol.2016.199. [DOI] [PubMed] [Google Scholar]
- 64.Tardif M, Savard M, Flamand L, Gosselin J. Impaired protein kinase C activation/translocation in Epstein-Barr virus-infected monocytes. J Biol Chem. 2002;277:24148–24154. doi: 10.1074/jbc.M109036200. [DOI] [PubMed] [Google Scholar]
- 65.Nanbo A, Inoue K, Adachi-Takasawa K, Takada K. Epstein-Barr virus RNA confers resistance to interferon-alpha-induced apoptosis in Burkitt’s lymphoma. EMBO J. 2002;21:954–965. doi: 10.1093/emboj/21.5.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang L, Aozasa K, Oshimi K, Takada K (2004) Epstein-Barr virus ( EBV ) -encoded RNA promotes growth of EBV-infected T cells through Interleukin-9 induction Epstein-Barr virus ( EBV ) -encoded RNA promotes growth of EBV-infected T cells through Interleukin-9 induction. 5332–5337. 10.1158/0008-5472.CAN-04-0733 [DOI] [PubMed]
- 67.Keryer-Bibens C, Pioche-Durieu C, Villemant C, Souquère S, Nishi N, Hirashima M, Middeldorp J, Busson P. Exosomes released by EBV-infected nasopharyngeal carcinoma cells convey the viral latent membrane protein 1 and the immunomodulatory protein galectin 9. BMC Cancer. 2006;6:283. doi: 10.1186/1471-2407-6-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Münz C, Chijioke O (2017) Natural killer cells in herpesvirus infections. F1000Research 6:1231. 10.12688/f1000research.11197.1 [DOI] [PMC free article] [PubMed]
- 69.Münz C. Epstein-Barr virus-specific immune control by innate lymphocytes. Front Immunol. 2017;8:1–7. doi: 10.3389/fimmu.2017.01658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lunemann A, Vanoaica LD, Azzi T, Nadal D, Munz C. A distinct subpopulation of human NK cells restricts B cell transformation by EBV. J Immunol. 2013;191:4989–4995. doi: 10.4049/jimmunol.1301046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tan G, Visser L, Tan L, Berg A, Diepstra A. The microenvironment in Epstein–Barr virus-associated malignancies. Pathogens. 2018;7:40. doi: 10.3390/pathogens7020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ishida T, Ishii T, Inagaki A, Yano H, Komatsu H, Iida S, Inagaki H, Ueda R. Specific recruitment of CC chemokine receptor 4-positive regulatory T cells in Hodgkin lymphoma fosters immune privilege. Cancer Res. 2006;66:5716–5722. doi: 10.1158/0008-5472.CAN-06-0261. [DOI] [PubMed] [Google Scholar]
- 73.Wu R, Sattarzadeh A, Rutgers B, Diepstra A, van den Berg A, Visser L (2016) The microenvironment of classical Hodgkin lymphoma: heterogeneity by Epstein-Barr virus presence and location within the tumor. Blood Cancer J 6. 10.1038/bcj.2016.26 [DOI] [PMC free article] [PubMed]
- 74.Morales O, Mrizak D, François V, Mustapha R, Miroux C, Depil S, Decouvelaere AV, Lionne-Huyghe P, Auriault C, de Launoit Y, Pancré V, Delhem N. Epstein-Barr virus infection induces an increase of T regulatory type 1 cells in Hodgkin lymphoma patients. Br J Haematol. 2014;166:875–890. doi: 10.1111/bjh.12980. [DOI] [PubMed] [Google Scholar]
- 75.Assis MCG, Campos AHFM, Oliveira JSR, Soares FA, Silva JMK, Silva PB, Penna AD, Souza EM, Baiocchi OCG. Increased expression of CD4+CD25+FOXP3+ regulatory T cells correlates with Epstein-Barr virus and has no impact on survival in patients with classical Hodgkin lymphoma in Brazil. Med Oncol. 2012;29:3614–3619. doi: 10.1007/s12032-012-0299-4. [DOI] [PubMed] [Google Scholar]
- 76.Gandhi MK, Lambley E, Duraiswamy J, et al. Expression of LAG-3 by tumor-infiltrating lymphocytes is coincident with the suppression of latent membrane antigen-specific CD8+ T-cell function in Hodgkin lymphoma patients. Blood. 2006;108:2280–2289. doi: 10.1182/blood-2006-04-015164. [DOI] [PubMed] [Google Scholar]
- 77.Braz-Silva PH, Vitale S, Butori C, Guevara N, Santini J, Magalhães M, Hofman P, Doglio A. Specific infiltration of langerin-positive dendritic cells in EBV-infected tonsil, Hodgkin lymphoma and nasopharyngeal carcinoma. Int J Cancer. 2011;128:2501–2508. doi: 10.1002/ijc.25597. [DOI] [PubMed] [Google Scholar]
- 78.Barros MHM, Hassan R, Niedobitek G, et al. Tumor-associated macrophages in pediatric classical Hodgkin lymphoma: association with Epstein-Barr virus, lymphocyte subsets, and prognostic impact. Clin Cancer Res. 2012;18:3762–3771. doi: 10.1158/1078-0432.CCR-12-0129. [DOI] [PubMed] [Google Scholar]
- 79.Kamper P, Bendix K, Hamilton-Dutoit S, Honore B, Nyengaard JR, d'Amore F. Tumor-infiltrating macrophages correlate with adverse prognosis and Epstein-Barr virus status in classical Hodgkin’s lymphoma. Haematologica. 2011;96:269–276. doi: 10.3324/haematol.2010.031542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Swerdlow SH, Campo E, Harris NL, et al (2008) WHO classification of Tumours of Haematopoietic and lymphoid tissues, revised fourth edition, revised 4t. World Health Organization
- 81.Nicolae A, Pittaluga S, Abdullah S, Steinberg SM, Pham TA, Davies-Hill T, Xi L, Raffeld M, Jaffe ES. EBV-positive large B-cell lymphomas in young patients: a nodal lymphoma with evidence for a tolerogenic immune environment. Blood. 2015;126:863–872. doi: 10.1182/blood-2015-02-630632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pawelec G. Age and immunity: what is “immunosenescence”? Exp Gerontol. 2018;105:4–9. doi: 10.1016/j.exger.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 83.Sugita Y, Furuta T, Ohshima K, Komaki S, Miyoshi J, Morioka M, Abe H, Nozawa T, Fujii Y, Takahashi H, Kakita A. The perivascular microenvironment in Epstein-Barr virus positive primary central nervous system lymphoma: the role of programmed cell death 1 and programmed cell death ligand 1. Neuropathology. 2017;38:125–134. doi: 10.1111/neup.12435. [DOI] [PubMed] [Google Scholar]
- 84.Taylor JG, Liapis K, Gribben JG. The role of the tumor microenvironment in HIV-associated lymphomas. Biomark Med. 2015;9:473–482. doi: 10.2217/bmm.15.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, Wong JC, Satkunarajah M, Schweneker M, Chapman JM, Gyenes G, Vali B, Hyrcza MD, Yue FY, Kovacs C, Sassi A, Loutfy M, Halpenny R, Persad D, Spotts G, Hecht FM, Chun TW, McCune JM, Kaul R, Rini JM, Nixon DF, Ostrowski MA. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205:2763–2779. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Buggert M, Nguyen S, Salgado-Montes de Oca G, Bengsch B, Darko S, Ransier A, Roberts ER, del Alcazar D, Brody IB, Vella LA, Beura L, Wijeyesinghe S, Herati RS, del Rio Estrada PM, Ablanedo-Terrazas Y, Kuri-Cervantes L, Sada Japp A, Manne S, Vartanian S, Huffman A, Sandberg JK, Gostick E, Nadolski G, Silvestri G, Canaday DH, Price DA, Petrovas C, Su LF, Vahedi G, Dori Y, Frank I, Itkin MG, Wherry EJ, Deeks SG, Naji A, Reyes-Terán G, Masopust D, Douek DC, Betts MR (2018) Identification and characterization of HIV-specific resident memory CD8+ T cells in human lymphoid tissue. Sci Immunol 3. 10.1126/sciimmunol.aar4526 [DOI] [PMC free article] [PubMed]
- 87.Pantanowitz L, Carbone A, Dolcetti R. Microenvironment and HIV-related lymphomagenesis. Semin Cancer Biol. 2015;34:52–57. doi: 10.1016/j.semcancer.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 88.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Davoodzadeh Gholami M, kardar GA, Saeedi Y, et al (2017) Exhaustion of T lymphocytes in the tumor microenvironment: significance and effective mechanisms. Cell Immunol 322:1–14. 10.1016/j.cellimm.2017.10.002 [DOI] [PubMed]
- 90.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, Koup RA. PD-1 is a regulator of virus-specific CD8 + T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kaufmann DE, Walker BD (2008) Programmed death-1 as a factor in immune exhaustion and activation in HIV infection. Curr Opin HIV AIDS 3: [DOI] [PubMed]
- 92.Fahey LM, Wilson EB, Elsaesser H, Fistonich CD, McGavern DB, Brooks DG. Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. J Exp Med. 2011;208:987–999. doi: 10.1084/jem.20101773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Streeck H, Brumme ZL, Anastario M, Cohen KW, Jolin JS, Meier A, Brumme CJ, Rosenberg ES, Alter G, Allen TM, Walker BD, Altfeld M. Antigen load and viral sequence diversification determine the functional profile of HIV-1-specific CD8+ T cells. PLoS Med. 2008;5:0790–0803. doi: 10.1371/journal.pmed.0050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moran J, Dean J, De Oliveira A, et al. Increased levels of PD-1 expression on CD8 T cells in patients post-renal transplant irrespective of chronic high EBV viral load. Pediatr Transplant. 2013;17:806–814. doi: 10.1111/petr.12156. [DOI] [PubMed] [Google Scholar]
- 95.Cohen M, Vistarop AG, Huaman F, Narbaitz M, Metrebian F, de Matteo E, Preciado MV, Chabay PA. Cytotoxic response against Epstein Barr virus coexists with diffuse large B-cell lymphoma tolerogenic microenvironment: clinical features and survival impact. Sci Rep. 2017;7:10813. doi: 10.1038/s41598-017-11052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Von Boehmer L, Draenert A, Jungraithmayr W, et al. Immunosuppression and lung cancer of donor origin after bilateral lung transplantation. Lung Cancer. 2012;76:118–122. doi: 10.1016/j.lungcan.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 97.Bererhi L, Pallet N, Zuber J, Anglicheau D, Kreis H, Legendre C, Candon S. Clinical and immunological features of very long-term survivors with a single renal transplant. Transpl Int. 2012;25:545–554. doi: 10.1111/j.1432-2277.2012.01451.x. [DOI] [PubMed] [Google Scholar]
- 98.Tsai DE, Hardy CL, Tomaszewski JE, Kotloff RM, Oltoff KM, Somer BG, Schuster SJ, Porter DL, Montone KT, Stadtmauer EA. Reduction in immunosuppression as initial therapy for posttransplant lymphoproliferative disorder: analysis of prognostic variables and long-term follow-up of 42 adult patients. Transplantation. 2001;71:1076–1088. doi: 10.1097/00007890-200104270-00012. [DOI] [PubMed] [Google Scholar]
- 99.Dierickx D, Tousseyn T, Gheysens O. How I treat how I treat posttransplant lymphoproliferative disorders case reports. Blood. 2015;126:2274–2284. doi: 10.1182/blood-2015-05-615872. [DOI] [PubMed] [Google Scholar]
- 100.Pietersma FL, van Oosterom A, Ran L, Schuurman R, Meijer E, de Jonge N, van Baarle D. Adequate control of primary EBV infection and subsequent reactivations after cardiac transplantation in an EBV seronegative patient. Transpl Immunol. 2012;27:48–51. doi: 10.1016/j.trim.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 101.Falco D a, Nepomuceno RR, Krams SM, et al. Identification of Epstein-Barr virus-specific CD8+ T lymphocytes in the circulation of pediatric transplant recipients. Transplantation. 2002;74:501–510. doi: 10.1097/00007890-200208270-00012. [DOI] [PubMed] [Google Scholar]
- 102.Richendollar BG, Tsao RE, Elson P, Jin T, Steinle R, Pohlman B, Hsi ED. Predictors of outcome in post-transplant lymphoproliferative disorder: an evaluation of tumor infiltrating lymphocytes in the context of clinical factors. Leuk Lymphoma. 2009;50:2005–2012. doi: 10.3109/10428190903315713. [DOI] [PubMed] [Google Scholar]
- 103.Calvo-Turrubiartes M, Romano-Moreno S, García-Hernández M, Chevaile-Ramos JA, Layseca-Espinosa E, González-Amaro R, Portales-Pérez D. Quantitative analysis of regulatory T cells in kidney graft recipients: a relationship with calcineurin inhibitor level. Transpl Immunol. 2009;21:43–49. doi: 10.1016/j.trim.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 104.Carmona-Escamilla MA, Queipo G, García-Mosqueda LA, García-Covarrubias L, Fonseca-Sánchez MA, Villanueva-Ortega E, Prieto P, Lascurain R. Peripheral blood regulatory T cells are diminished in kidney transplant patients with chronic allograft nephropathy. Transplant Proc. 2018;50:444–448. doi: 10.1016/j.transproceed.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 105.Chadburn A, Chen JM, Hsu DT, Frizzera G, Cesarman E, Garrett TJ, Mears JG, Zangwill SD, Addonizio LJ, Michler RE, Knowles DM. The morphologic and molecular genetic categories of posttransplantation lymphoproliferative disorders are clinically relevant. Cancer. 1998;82:1978–1987. [PubMed] [Google Scholar]
- 106.Nelson BP, Wolniak KL, Evens A, Chenn A, Maddalozzo J, Proytcheva M. Early posttransplant lymphoproliferative disease. Am J Clin Pathol. 2012;138:568–578. doi: 10.1309/AJCPQYYE04AVGVYI. [DOI] [PubMed] [Google Scholar]
- 107.Vakiani E, Basso K, Klein U, Mansukhani MM, Narayan G, Smith PM, Murty VV, Dalla-Favera R, Pasqualucci L, Bhagat G. Genetic and phenotypic analysis of B-cell post-transplant lymphoproliferative disorders provides insights into disease biology. Hematol Oncol. 2008;26:199–211. doi: 10.1002/hon.859. [DOI] [PubMed] [Google Scholar]
- 108.Ibrahim HAH, Menasce L, Pomplun S, et al. Tumour infiltrating plasmacytoid dendritic cells in B cell post-transplant lymphoproliferative disorders, human immunodeficiency virus-associated B cell lymphomas and immune competent diffuse large B cell lymphomas. Histopathology. 2011;59:152–156. doi: 10.1111/j.1365-2559.2011.03872.x. [DOI] [PubMed] [Google Scholar]
- 109.Birkeland SA, Bendtzen K, Møller B, et al. Interleukin-10 and Posttransplant lymphoproliferative disorder after kidney transplantation. Immunobiology. 1999;67:876–881. doi: 10.1097/00007890-199903270-00015. [DOI] [PubMed] [Google Scholar]
- 110.Muti G, Klersy C, Baldanti F, Granata S, Oreste P, Pezzetti L, Gatti M, Gargantini L, Caramella M, Mancini V, Gerna G, Morra E, for the Co-operative Study Group on PTLDs* Epstein – Barr virus ( EBV ) load and interleukin-10 in lymphoproliferative disorders. Br J Haematol. 2003;122:927–933. doi: 10.1046/j.1365-2141.2003.04540.x. [DOI] [PubMed] [Google Scholar]
- 111.Wu TT, Swerdlow SH, Locker J, Bahler D, Randhawa P, Yunis EJ, Dickman PS, Nalesnik MA. Recurrent Epstein-Barr virus-associated lesions in organ transplant recipients. Hum Pathol. 1996;27:157–164. doi: 10.1016/s0046-8177(96)90369-x. [DOI] [PubMed] [Google Scholar]
- 112.Dierickx D, Tousseyn T, Sagaert X, Fieuws S, Wlodarska I, Morscio J, Brepoels L, Kuypers D, Vanhaecke J, Nevens F, Verleden G, van Damme-Lombaerts R, Renard M, Pirenne J, de Wolf-Peeters C, Verhoef G. Single-center analysis of biopsy-confirmed posttransplant lymphoproliferative disorder: incidence, clinicopathological characteristics and prognostic factors. Leuk Lymphoma. 2013;54:2433–2440. doi: 10.3109/10428194.2013.780655. [DOI] [PubMed] [Google Scholar]
- 113.Ferreiro JF, Morscio J, Dierickx D, Marcelis L, Verhoef G, Vandenberghe P, Tousseyn T, Wlodarska I. Post-transplant molecularly defined Burkitt lymphomas are frequently MYC-negative and characterized by the 11q-gain/loss pattern. Haematologica. 2015;100:e275–e279. doi: 10.3324/haematol.2015.124305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Herreman A, Dierickx D, Morscio J, Camps J, Bittoun E, Verhoef G, de Wolf-Peeters C, Sagaert X, Tousseyn T. Clinicopathological characteristics of posttransplant lymphoproliferative disorders of T-cell origin: single-center series of nine cases and meta-analysis of 147 reported cases. Leuk Lymphoma. 2013;54:2190–2199. doi: 10.3109/10428194.2013.775436. [DOI] [PubMed] [Google Scholar]
- 115.Morscio J, Dierickx D, Ferreiro JF, Herreman A, Van Loo P, Bittoun E, Verhoef G, Matthys P, Cools J, Wlodarska I, De Wolf-Peeters C, Sagaert X, Tousseyn T (2013) Gene expression profiling reveals clear differences between EBV-positive and EBV-negative posttransplant lymphoproliferative disorders. Am J Transplant 13(5):1305–1316 [DOI] [PubMed]
- 116.Fu L, Xie J, Lin J, Wang J, Wei N, Huang D, Wang T, Shen J, Zhou X, Wang Z. Monomorphic post-transplant lymphoproliferative disorder after kidney transplantation and hematopoietic stem cell transplantation: Clinicopathological characteristics, treatments and prognostic factors. Indian J Hematol Blood Transfus. 2017;33:492–499. doi: 10.1007/s12288-017-0799-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brody J, Kohrt H, Marabelle A, Levy R. Active and passive immunotherapy for lymphoma: proving principles and improving results. J Clin Oncol. 2011;29:1864–1875. doi: 10.1200/JCO.2010.33.4623. [DOI] [PubMed] [Google Scholar]
- 118.Barnett R, Barta VS, Jhaveri KD. Preserved renal-allograft function and the PD-1 pathway inhibitor Nivolumab. N Engl J Med. 2017;376:191–192. doi: 10.1056/NEJMc1614298. [DOI] [PubMed] [Google Scholar]
- 119.Perrine SP, Hermine O, Small T, Suarez F, O'Reilly R, Boulad F, Fingeroth J, Askin M, Levy A, Mentzer SJ, di Nicola M, Gianni AM, Klein C, Horwitz S, Faller DV. A phase 1/2 trial of arginine butyrate and ganciclovir in patients with Epstein-Barr virus-associated lymphoid malignancies. Blood. 2007;109:2571–2578. doi: 10.1182/blood-2006-01-024703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Andrews LP, Marciscano AE, Drake CG, Vignali DAA. LAG3 (CD223) as a cancer immunotherapy target. Immunol Rev. 2017;276:80–96. doi: 10.1111/imr.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Melero I, Berman DM, Aznar MA, Korman AJ, Gracia JLP, Haanen J. Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat Rev Cancer. 2015;15:457–472. doi: 10.1038/nrc3973. [DOI] [PubMed] [Google Scholar]
- 122.Lipson EJ, Bagnasco SM, Moore J, et al. Tumor regression and allograft rejection after Administration of Anti-PD-1. N Engl J Med. 2016;374:896–898. doi: 10.1056/NEJMc1509268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gottschalk S, Rooney CM. Münz C (ed) Cham: Springer International Publishing; 2015. Adoptive T-cell immunotherapy BT - Epstein Barr virus volume 2: one herpes virus: many diseases; pp. 427–454. [Google Scholar]
- 124.Heslop HE, Brenner MK, Rooney C, Krance RA, Roberts WM, Rochester R, Smith CA, Turner V, Sixbey J, Moen R, Boyett JM. Administration of neomycin-resistance-gene-marked EBV-specific cytotoxic T lymphocytes to recipients of mismatched-related or phenotypically similar unrelated donor marrow grafts. Hum Gene Ther. 1994;5:381–397. doi: 10.1089/hum.1994.5.3-381. [DOI] [PubMed] [Google Scholar]
- 125.Rooney CM, Ng CYC, Loftin S, Smith CA, Li C, Krance RA, Brenner MK, Heslop HE, Rooney CM, Brenner MK, Brenner MK, Krance RA, Heslop HE. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet. 1995;345:9–13. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- 126.Pappworth IY, Wang EC, Rowe M. The switch from latent to productive infection in Epstein-Barr virus-infected B cells is associated with sensitization to NK cell killing. J Virol. 2007;81:474–482. doi: 10.1128/JVI.01777-06. [DOI] [PMC free article] [PubMed] [Google Scholar]