Abstract
Osteoarthritis (OA) results from an imbalance of the dynamic equilibrium between the breakdown and repair of joint tissues. Previously, we reported that Runx1 enhanced chondrogenic differentiation through transcriptional induction of COL2A1, and suppressed hypertrophic differentiation. Here, we investigated the involvement of Runx1 in OA development as well as its potential underlying molecular mechanism. When we analysed OA development in Col2a1-Cre;Runx1fl/fl and Runx1fl/fl mice by surgically inducing joint instability, Cartilage degradation and osteophyte formation of Col2a1-Cre;Runx1fl/fl joints was accelerated compared with joints in Runx1fl/fl animals 8 weeks after surgery. To investigate chondrocyte regulation by Runx1, we analysed interactions with co-factors and downstream molecules. Runx1 enhanced cartilage matrix production in cooperation with Sox5, Sox6, and Sox9, and co-immunoprecipitation assays showed protein–protein binding between Runx1 and each Sox protein. Knockdown of Runx1 increased expression of a hypertrophic marker, Co10a1, in mouse articular cartilage and primary chondrocytes. This expression was accompanied by decreased expression of Bapx1, a potent suppressor of hypertrophic differentiation. Notably, Runx1-induced suppression of hypertrophic differentiation was diminished by siRNA silencing of Bapx1, whereas chondrogenic markers were unaltered. Thus, Runx1 contributes to articular cartilage maintenance by enhancing matrix production in cooperation with Sox proteins, and suppressing hypertrophic differentiation at least partly via Bapx1 induction.
Subject terms: Cartilage development, Disease model
Introduction
Development of osteoarthritis (OA) – the most common degenerative joint disorder – results not only from an imbalance in the dynamic equilibrium between breakdown and repair of joint tissues, but also ectopic endochondral ossification under mechanical loading conditions1,2. During this process, chondrocytes undergo hypertrophic differentiation characterized by secretion of type X collagen (Col10a1). Next, avascular cartilage tissue is converted into highly vascularized bone tissue via degradation of cartilage matrix and vascular invasion1,3.
Runt-related transcription factor (Runx) family proteins, including Runx1, Runx2, and Runx3, play crucial roles in skeletal development4,5. In particular, Runx2 has been well characterized in skeletal development and OA. Runx2 deletion results in a lack of ossification that impairs chondrocyte maturation6. Runx2 protein, which is highly expressed in the prehypertrophic and hypertrophic zones of limb epiphyseal cartilage, promotes hypertrophic differentiation5,7. Previously, we demonstrated suppression of OA development by Runx2 haploinsufficiency8, which was recently confirmed using a chondrocyte-specific Runx2 knockout9. Indeed, a series of studies concluded that Runx2 deficiency decelerates OA development by suppressing hypertrophic differentiation10.
In contrast to Runx2, Runx1 is involved in early chondrogenic differentiation. Runx1, which is widely expressed by chondrocyte progenitors and stimulates chondrogenesis4,11. Previously, we reported that Runx1 enhanced cartilage matrix production and induced chondrogenic transcription factors such as sex determining region Y-box (Sox) genes12,13. Mechanistically, Runx1 activates the COL2A1 promoter through specific binding to a Runx motif in the 5′-flanking region12. In addition, Runx1 suppresses hypertrophic differentiation of cultured chondrocytes12. In articular cartilage, RUNX1 expression is downregulated in patients with OA compared with healthy individuals12. Mechanical compression induces upregulation of Runx1 in cartilage tissues, which contributes to chondrocyte proliferation14. Chondrogenic compounds, such as TD-198946 and Kartogenin, have been shown to function via Runx1 induction12,15. Moreover, we recently showed that intraarticular injection of polyplex nanomicelles containing RUNX1 mRNA suppressed development of surgically-induced OA in mice16. Collectively, these data support a protective role of Runx1 with regard to articular cartilage maintenance; however, molecular mechanisms underlying enhancement of cartilage matrix production and suppression of hypertrophic differentiation by Runx1 are not well understood.
Herein, we investigated roles of Runx1 during OA development using chondrocyte-specific Runx1 knockout mice. We further examined interactions between Runx1 and other chondrogenic factors in enhancement of cartilage matrix production, as well as the function of molecules downstream of Runx1 in regulation of hypertrophic differentiation.
Results
Runx1 deficiency enhanced OA development
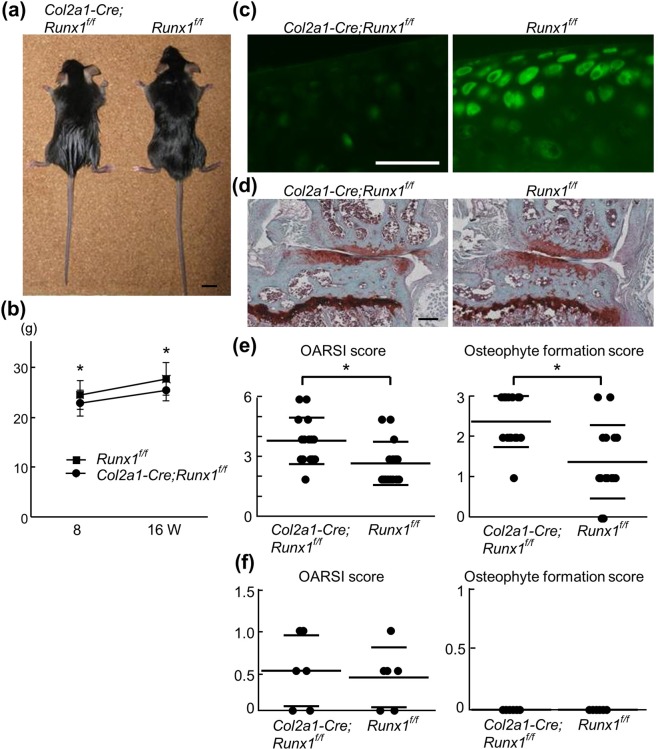
First, the involvement of Runx1 in OA development was examined. Although no abnormalities were found in skeletal morphology or patterning, chondrocyte-specific Runx1 knockout mice (Col2a1-Cre;Runx1fl/fl), showed slight dwarfism compared with their Runx1fl/fl littermates at 8 weeks of age (Fig. 1a). Moreover, body weights of Col2a1-Cre;Runx1fl/fl mice were about 10% less than that of control littermates throughout the experimental period (Fig. 1b). After confirming the efficient deletion of Runx1 in adult articular chondrocytes (Fig. 1c), we created the surgical OA model17. Cartilage degradation and osteophyte formation of Col2a1-Cre;Runx1fl/fl joints were significantly accelerated compared with Runx1fl/fl littermate joints after 8 weeks, in spite of the significantly lighter body weight of Col2a1-Cre;Runx1fl/fl mice (Fig. 1d,e). In contrast, there was no significant difference in OA progression between 16-week-old Col2a1-Cre;Runx1fl/fll and Runx1fl/fl littermates (Fig. 1f and see also Safranin-O staining in Fig. 2a). These data suggested that Runx1 can protect articular cartilages from OA-inducing stimuli.
Figure 1.
OA development in Col2a1-Cre;Runx1fl/fl and Runx1fl/fl mice. (a) Gross appearance of Col2a1-Cre;Runx1fl/fl and Runx1fl/fl littermates at 8 weeks of age. Scale bars, 10 mm. (b) Total body weight of Col2a1-Cre;Runx1fl/fl and Runx1fl/fl littermates at 8 or 16 weeks of age. Data are expressed as means (symbols) ± SD (error bars) of 15 mice per group. (c) Runx1 immunofluorescence in normal knee cartilage of Col2a1-Cre;Runx1fl/fl and Runx1fl/fl littermates at 16 weeks of age. Scale bars, 50 μm. (d) Safranin O staining of knee joints 8 weeks after OA surgery in Col2a1-Cre;Runx1fl/fl and Runx1fl/fl littermates. Scale bars, 200 μm. (e) Quantification of OA development by Osteoarthritis Research Society International (OARSI) grading system and osteophyte formation score. Data are expressed as means ± SD of 15 mice per group. *P < 0.05 vs. Runx1fl/fl. (f) Quantification of OA development by Osteoarthritis Research Society International (OARSI) grading system and osteophyte formation score in normal knee cartilage of Col2a1-Cre;Runx1fl/fl and Runx1fl/fl littermates at 16 weeks of age. Data are expressed as means ± SD of 6 mice per group.
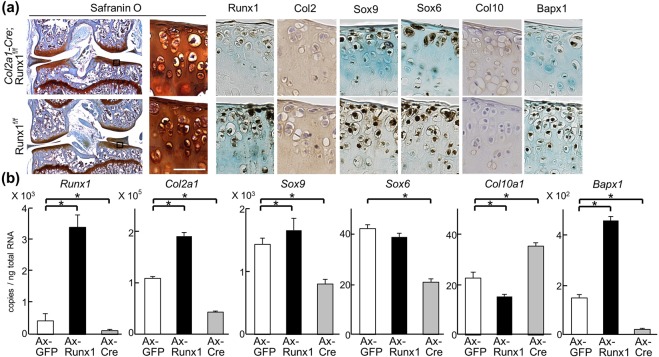
Figure 2.
Altered marker expression by Runx1. (a) Safranin O staining and immunohistochemistry with antibodies to marker proteins in articular cartilage of 16-week-old Col2a1-Cre;Runx1fl/fl and Runx1fl/fl littermates under physiological conditions. Inset boxes in Safranin O staining indicate regions shown in enlarged safranin O and immunostaining images. Scale bars, 50 μm. (b) mRNA levels of marker genes in Runx1fl/fl primary articular chondrocytes adenovirally transduced (Ax) with GFP, Runx1, or Cre after 5 days of culture. *P < 0.01 versus Ax-GFP. Data are expressed as mean ± SD of six samples per group.
Runx1 deficiency induces downregulation of chondrogenic markers and upregulation of hypertrophic markers
Expression of marker proteins was examined in Col2a1-Cre;Runx1fl/fl and Runx1fl/fl cartilage under physiological conditions without any operation. Chondrogenic factors such as Sox6 and Sox9 were decreased in Col2a1-Cre;Runx1fl/fl cartilage (Fig. 2a) as well as the expression of Runx1. In contrast, the hypertrophic marker Col10 was increased by Runx1 deletion (Fig. 2a and Supplementary Fig. S1a,b). Bapx1, a suppressive factor of chondrocyte hypertrophy, was clearly decreased in Col2a1-Cre;Runx1fl/fl cartilage (Fig. 2a). We next quantified changes in mRNA levels of marker genes induced by Runx1 overexpression or knockdown. We obtained primary articular chondrocytes from Runx1fl/fl mice, and transduced adenoviral GFP, Runx1, or Cre. Col2a1, Sox9 and Bapx1 were upregulated by Runx1 overexpression, while Sox6 was unchanged (Fig. 2b). In contrast, Runx1 deletion decreased all these markers (Fig. 2a,b). Col10a1 were downregulated by Runx1 overexpression, and upregulated by Runx1 knockdown (Fig. 2b). These data are comparable to the result of immunohistochemistry. Thus, the series of in vivo and in vitro data supports negative roles of Runx1 in chondrocyte hypertrophy as well as positive ones in early chondrogenic differentiation in articular cartilages.
Runx1 cooperates with Sox proteins to enhance cartilage matrix production
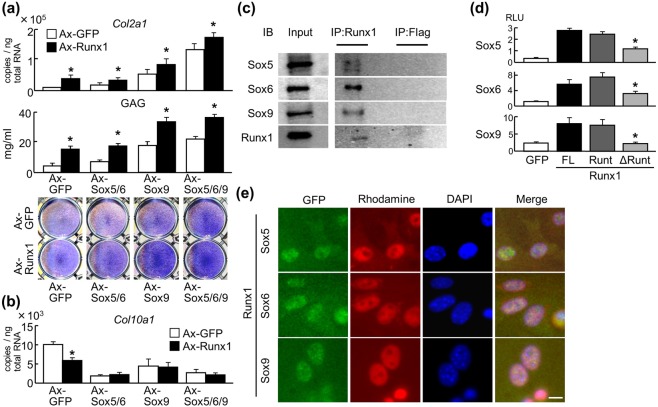
Next, we examined interactions between Runx1 and Sox proteins. Adenoviral overexpression of Runx1 and Sox was initially performed in C3H10T1/2 cells, which were examined for chondrogenic differentiation. Levels of Col2a1 mRNA, glycosaminoglycan (GAG), and Toluidine blue staining were increased by Runx1 alone, and further enhanced by co-overexpression of Sox5, Sox6, or Sox9 (Fig. 3a), indicating that Runx1 enhances cartilage matrix production in cooperation with Sox proteins. Upon transduction of these factors into ATDC5 cells and subsequent pellet culture to induce hypertrophic differentiation, Col10a1 expression was significantly decreased by Runx1 or Sox overexpression; however, it was not further suppressed by co-overexpression (Fig. 3b), indicating suppression of hypertrophic differentiation by Runx1 is not associated with Sox proteins. We next investigated protein–protein interactions between Runx1 and Sox proteins. Co-IP using human articular chondrocytes showed specific binding between endogenous Runx1 and Sox5, Sox6, or Sox9 (Fig. 3c and Supplementary Fig. S2); the interaction was also confirmed by Co-IP in HEK293 cells overexpressing those proteins (Supplementary Fig. S3). A mammalian two-hybrid assay confirmed physical interactions between these proteins, and experiments using Runx1 deletion mutants showed that the runt domain is essential for interactions with Sox proteins (Fig. 3d and Supplementary Fig. S4). Immunocytochemistry showed nuclear co-localization of endogenous Runx1 and Sox proteins (Fig. 3e). Immunohistochemistry for Sox and Runx proteins in mouse E17.5 tibial limb cartilage showed that Runx1 and Sox proteins were co-localized in periarticular and prehypertrophic chondrocytes. In contrast, Runx2 was primarily expressed in late differentiating chondrocytes in the hypertrophic zone (Supplementary Fig. S5). Taken together, these data suggest that Runx1 enhances cartilage matrix production partially through interactions with Sox5, Sox6, and Sox9.
Figure 3.
Interaction between Runx1 and Sox proteins. (a) Levels of Col2a1 mRNA, glycosaminoglycan (GAG), and Toluidine blue staining in C3H10T1/2 cells adenovirally transduced with Runx1 in combination with GFP, Sox5 + Sox6, Sox9, or Sox5 + Sox6 + Sox9. Data are expressed as means (bars) ± SD (error bars) of six samples per group *P < 0.01 versus each Ax-GFP control. (b) Levels of Col10a1 mRNA in ATDC5 cells adenovirally transduced with Runx1 in combination with GFP, Sox5 + Sox6, Sox9, or Sox5 + Sox6 + Sox9. Cells were cultured in pellets for 3 days after adenoviral transduction. Data are expressed as means (bars) ± SD (error bars) of four samples per group *P < 0.01 versus each Ax-GFP control. (c) Co-immunoprecipitation (Co-IP) assay using cell lysates of human articular chondrocytes. Cell lysates underwent IP with an antibody to Flag or Runx1, and were then immunoblotted with the other antibody. Full-length blots are presented in Supplementary Figure 2. (d) Mammalian two-hybrid assay by transfection of luciferase reporter vectors expressing GAL4–Runx1 (FL, full-length Runx1; Runt, the runt domain only; and ΔRunt, Runx1 mutant lacking the runt domain) and VP16–Sox proteins with GAL4 binding sites into HuH-7 cells. Data are expressed as means (bars) ± s.d. (error bars) of four samples per group *P < 0.01 vs. FL. (e) Immunocytochemistry of endogenous Runx1, Sox5, Sox6, and Sox9 in human articular chondrocytes were detected by secondary antibodies with red and green fluorescence, respectively. Scale bar, 10 μm.
Co-localization and induction of Bapx1 by Runx1 in articular cartilage and meniscus
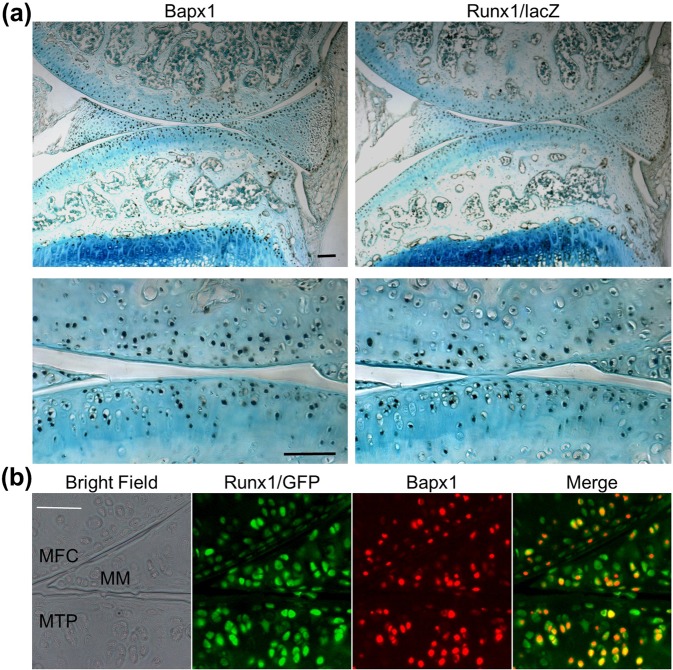
We then examined molecular mechanisms underlying suppression of hypertrophic differentiation by Runx1. Among candidate molecules demonstrating suppressive effects against hypertrophy, we focused on the transcription factor Bapx1, also known as Nkx3.2. Bapx1 is indispensable for skeletal development, as its deficiency leads to the impairment of hypertrophic differentiation and maturation of growth plate chondrocytes18. Bapx1 expression was markedly decreased in Col2a1-Cre;Runx1fl/fl cartilage (Fig. 2a). In primary chondrocyte cultures, Bapx1 was increased by Runx1 overexpression, and decreased by Runx1 knockdown (Fig. 2b). Immunohistochemistry showed that Bapx1 protein was widely localized throughout normal articular cartilage and menisci of Runx1 heterozygous knock-in mice (Runx1lz/+)11, similar to the distribution pattern of Runx1 (Fig. 4a). Indeed, upon performing co-immunofluorescence of Bapx1 and Runx1 in knee joints of Runx1-IRES-GFP knock-in mice19, Runx1 was mostly detected in Bapx1 expressed cells (Fig. 4b and Supplementary Fig. S6).
Figure 4.
Expression patterns of Runx1 and Bapx1 in articular cartilage. (a) Immunohistochemistry with antibodies to Bapx1 and β-gal in articular cartilage of Runx1 heterozygous lacZ knock-in mice (Runxlz/+) under physiological conditions. Scale bars, 100 μm for low and high magnification images. (b) Immunofluorescence with antibodies to GFP and Bapx1 in knee joints of Runx1-IRES-GFP mice under physiological condition. Scale bar, 50 μm. MFC, medial femoral condyle; MTP, medial tibial plateau; MM, medial meniscus.
Bapx1 mediates the suppression of hypertrophic differentiation by Runx1
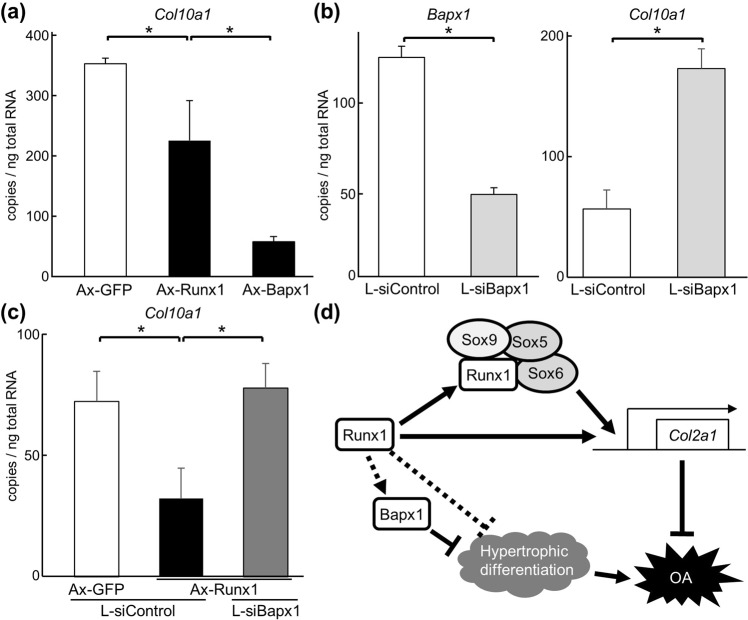
Finally, we investigated the association of Runx1 and Bapx1 in regulation of hypertrophy. When equal amounts of adenoviral vectors for Runx1 and Bapx1 were transduced into mouse primary chondrocytes, decreases in Col10a1 induced by Bapx1 were more substantial than those induced by Runx1 (Fig. 5a). To examine Bapx1 loss-of-function, lentiviral transduction of siRNA was used to prepare Bapx1-downregulated chondrocytes. Although suppression of Bapx1 was partial, Col10a1 was increased by Bapx1 suppression (Fig. 5b). Upon examining overexpression of Runx1 in Bapx1-downregulated and control cells, decreases of Col10a1 induced by Runx1 were cancelled by Bapx1 suppression (Fig. 5c). These data suggest that Bapx1 at least partly mediates suppression of hypertrophic differentiation induced by Runx1.
Figure 5.
Regulation of chondrocyte hypertrophic differentiation by Runx1 and Bapx1. (a) Levels of Col10a1 mRNA levels in primary chondrocytes adenovirally transfected with GFP, Runx1 or Bapx1 after 5 days of culture. Data are expressed as means (bars) ± s.d. (error bars) of three samples per group *P < 0.01. (b) Levels of Bapx1 and Col10a1 mRNA in primary chondrocytes lentivirally transfected with shRNAs against Bapx1(L-siBapx1) or control (L-siControl). Data are expressed as means (bars) ± s.d. (error bars) of three samples per group *P < 0.01. (c) Levels of Col10a1 mRNA in primary articular chondrocytes adenovirally transfected with GFP or Runx1, and lentivirally transfected with L-siBapx1 or L-siControl. Data are expressed as means (bars) ± SD (error bars) of three samples per group *P < 0.01. (d) Schematic of proposed mechanism for inhibitory effects of Runx1 against OA development. Runx1 induces Col2a1 expression via direct binding to its 5′-flanking region and in cooperation with Sox5, Sox6, and Sox9. Runx1 suppresses hypertrophic differentiation through induction of Bapx1.
Discussion
The present study showed, using both in vivo and in vitro experiments, that Runx1 plays an essential role in articular cartilage maintenance. Chondrocyte-specific deletion of Runx1 enhanced OA development, although the body weight of knockout mice was also less than that of control littermates. In Runx1-knockout cartilage, expression of Col2 and chondrogenic Sox proteins was decreased, while hypertrophic makers were increased. In vitro experiments revealed that Runx1 enhanced cartilage matrix production in cooperation with Sox5, Sox6, and Sox9. Meanwhile, Runx1 suppressed hypertrophic differentiation of chondrocytes through induction of Bapx1.
During endochondral ossification, Sox5, Sox6, and Sox9 are bona fide transcription factors that are indispensable for chondrogenesis20. Sox9, which is expressed in mesenchymal chondroprogenitor cells, broadly regulates chondrocyte differentiation and cartilage matrix production in conjunction with subsequently expressed Sox5 and Sox620,21. Runx1 is also expressed in mesenchymal chondroprogenitor cells and limb chondrocytes at an early stage of differentiation4. Loss of Runx1 function results in impaired skeletal growth22; however, underlying mechanisms are not fully understood. The present data show that Runx1 cooperates with Sox5, Sox6, and Sox9 (through protein–protein binding via the runt domain of Runx1) to enhance cartilage matrix production. Although we did not experimentally examine this feature, it may also bind to a high-mobility-group (HMG)-type DNA-binding domain present in Sox proteins, as the HMG domain mediates protein–protein interactions23. Meanwhile, we previously identified the responsive element of Runx1 in the 5′-flanking region of the Col2a1 promoter12. Considering that core Sox9 enhancers are localized in the first intron of the Col2a1 gene24, Runx1 may enhance Col2a1 transcription both directly and indirectly (Fig. 5d) to exert anabolic effects in chondrocytes of the developing skeleton and articular cartilage.
In the present study, we identified Bapx1 as a novel downstream molecule of Runx1. Bapx1-deficient mice exhibited lethal skeletal dysplasia, with abnormal development of the vertebral column18,25. Although the sclerotome cells of mutants appeared to migrate and condense normally into vertebral bodies, they failed to differentiate into hypertrophic chondrocytes18. Subsequent studies revealed that suppression of hypertrophic differentiation by Bapx1 is mediated by Runx226,27. Additionally, Bapx1 contributes to chondrocyte viability through Rela activation28. A recent study using human primary articular chondrocytes showed that Bapx1 suppresses hypertrophic marker genes including RUNX2, COL10A1, and alkaline phosphatase, while Bapx1 did not influence expression of the chondrogenic factors SOX9, COL2A1, or aggrecan29. These reports support a suppressive function of Bapx1 on chondrocyte hypertrophy, although some others suggest opposite ones18,25. Considering these findings and the present data, Runx1 possibly suppresses hypertrophic chondrocyte differentiation in a Bapx1-dependent manner, and also suppresses subsequent OA development. However, involvement of the Runx1-Bapx1 pathway in skeletal development has yet to be examined; we are still unable to rule out the possibility that Runx1 and Bapx1 independently act on chondrocyte hypertrophy (Fig. 5d).
In conclusion, we demonstrated that Runx1 regulates articular cartilage by enhancing matrix production and suppressing hypertrophic differentiation via Bapx1 induction and/or another distinct pathway that induces some regulators for hypertrophy (Fig. 5d). These data indicate that Runx1 is a potent factor for the maintenance of articular joints, similar to previous studies demonstrating that TD-198946 and Kartogenin exert chondroprotective effects through Runx1 induction12,15,30. Thus, the results of the present study may contribute to the elucidation of molecular mechanisms underlying articular cartilage homeostasis and OA development.
Methods
Ethics statement
We performed all animal experiments according to a protocol approved by the animal care and use committee of the University of Tokyo. We obtained samples of human articular cartilage from three individuals undergoing total knee arthroplasty with written informed consent, as approved by the Ethics Committee of the University of Tokyo. All methods were performed in accordance with the relevant guidelines and regulations.
Mice
In each experiment, we compared genotypes of littermates maintained in a C57BL/6 background with a standard diet. Col2a1-Cre mice and Runx1-flox mice were provided by Dr. Shu Takeda (Tokyo Medical and Dental University, Tokyo, Japan)13. To generate Col2a1-Cre;Runx1fl/fl mice, Runx1fl/fl mice were mated with Col2a1-Cre mice to obtain Col2a1-Cre;Runx1fl/+ mice, which were then mated with Runx1fl/fl mice. Runx1 heterozygous LacZ knock-in mice were provided by Dr. Stefano Stifani (McGill University)11. Runx1-IRES-GFP mice were provided by Dr. James Downing (St Jude Children’s Research Hospital)19.
OA experiment
The experimental OA model was performed on 8-week-old Col2a1-Cre;Runx1fl/fl and Runx1fl/fl littermate male mice, as previously described17. Briefly, under general anaesthesia, the medial collateral ligament was transected and the medial meniscus was removed using a surgical microscope. A sham operation was performed on the contralateral knee joint using the same approach, with no ligament transection or meniscectomy. All mice had their left knee joints operated on, while the right knees were subjected to sham operation. Mice were analysed 8 weeks after surgery. OA development was assessed by two blinded independent observers using the Osteoarthritis Research Society International (OARSI) scoring system31 and osteophyte formation score17.
Biochemical measurement of glycosaminoglycan (GAG)
We collected whole cell lysates from C3H10T1/2 cells using an M-PER kit (Pierce Chemical), and evaluated GAG contents using and Alcian blue-binding assay (Wieslab).
Co-immunoprecipitation (Co-IP) and mammalian two-hybrid assays
We collected whole cell lysates from human articular chondrocytes using an M-PER kit, and performed co-IP using a Catch and Release kit (Upstate Biotechnology, Lake Placid, NY) with anti-Flag (F7425, Sigma-Aldrich, St. Louis, MO), anti-Runx1 (23980), anti-Sox5 (94396), anti-Sox6 (30455) and anti-Sox9 (185230) antibodies (Abcam, Cambridge, UK). Immune complexes were eluted and subjected to SDS-PAGE. Mammalian two-hybrid assays were performed with the Checkmate Mammalian Two-Hybrid System (Promega, Madison, WI).
Histological analyses
Sections were stained with Safranin O-fast green using standard protocols32. For immunohistochemistry, sections were incubated with antibodies to Runx1 (Abcam23980), Sox6 (Abcam30455), Sox9 (Abcam185230), Col10 (Affymetrix 14-9771-80; eBioscience, Austria), Col2 (Millipore MAB8887), GFP (Abcam 290,), Bapx1 (Abcam 83288) and β-gal (Promega Z378A) diluted at 1:500 by blocking reagent, and detected with a CSAII Biotin-free Tyramide Signal Amplication System (Dako, Glostrup, Denmark). For Col10 and Col2 sections were treated with hyaluronidase [25 mg/ml in phosphate-buffered saline (PBS)] for 30 min. Sections were counterstained with methyl green or hematoxylin.
Immunocytochemistry
Human articular chondrocytes were fixed in 4% paraformaldehyde/PBS for 10 min and incubated for one hour with primary antibodies to Runx1 (mouse monoclonal, Abcam189172), Sox5 (Abcam94396), Sox6 (Abcam30455) or Sox9 (Abcam185230), which was diluted at 1:250 in blocking reagent, at room temperature. A secondary antibody conjugated with Alexa Fluor 488(anti-mouse) for Runx1 and 568 (anti-rabbit) (Molecular Probes, Eugene, OR) for Sox5, Sox6 and Sox9 were used as secondary antibodies, and the nucleus was counterstained with Hoechst 33258 (Sigma-Aldrich). Double immunofluorescence was visualized using Rabbit IgG Labeling Kit Zenon Alexa Fluor 488 (Z25302, Invitrogen, Carlsbad, CA) for detection of GFP, and Rabbit IgG Labeling Kit Zenon Alexa Fluor 647 (Z25308, Invitrogen) for detection of Bapx1.
Cell cultures
We obtained samples of human articular cartilage from three individuals undergoing total knee arthroplasty after obtaining written informed consent from the patients. Human articular chondrocytes were isolated and cultured as previously described33. C3H10T1/2, HuH-7and ATDC5 cells (Riken BRC, Tokyo, Japan) were maintained in monolayer culture as previously described34. Primary mouse costal chondrocytes were isolated and the pellet cell cultures were performed. In addition, primary mouse articular chondrocytes were isolated and cultured as previously described35 for use in functional analyses. Adenovirus vectors for GFP, Runx1, Bapx1 and Cre were prepared as previously described12. Cells were transduced with adenoviral vectors at a multiplicity of infection (MOI) of 100. Lentivirus vectors of control shRNA (pSMART Non-targeting mCMV-TurboRFP) and Bapx1 shRNA (pSMART 2.0 mCMV/turboRFP Nkx3.2) were purchased from Dharmacon (Lafayette, CO).
Real-time RT-PCR analysis
Total RNA was extracted from cells using an RNeasy Mini kit (Qiagen, Hilden, Germany). One microgram of RNA was reverse-transcribed with a QuantiTect Reverse Transcription kit (Qiagen) to produce single-stranded cDNA. Real-time RT-PCR was performed with an ABI Prism 7500 Sequence Detection System (Applied Biosystems, Foster City, CA) using FastStart Universal SYBR Green Master Mix (Roche, Tokyo, Japan) with rodent actin as the internal control. Primer sequences are shown in Supplementary Table 1.
Statistical analysis
Quantitative data were expressed as mean ± standard deviation (SD), with statistical significance evaluated using analysis of variance, Student t test or Mann-Whitney’s U Test for OARSI score, as appropriate. P values less than 0.05 were considered significant.
Supplementary information
Acknowledgements
We thank A. Kimura and S. Takeda for providing Col2a1-Cre mice and Runx1fl/fl mice, S. Stifani for Runx1lz/+ heterozygous mice and J. Downing for Runx1-IRES-GFP mice. We also thank Amy Van Deusen, of Edanz Group Japan, for editing a draft of this manuscript. This study was supported by a grant-in-aid for scientific research from the Japanese Ministry of Education, Culture, Sports, Science and Technology (#22689052, #24659665, #25713052, #15K15536 and #15K0296), the Center for NanoBio Integration (CNBI program), the Center for Medical System Innovation (CMSI program), the Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST Program) and Core-to-Core Program A (Advanced Research Networks). The sponsors had no role in study design, data collection, data analysis, data interpretation or writing of the manuscript.
Author Contributions
F.Y., S.O., T.S., S.T. and U.C. conducted the project planning; F.Y. and Y.M. performed the experiments; F.Y., S.O., Y.M., T.S., S.T., and U.C. handled data analysis; F.Y., S.O., T.S. and U.C. wrote the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-43948-3.
References
- 1.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 2.Kawaguchi H. Endochondral ossification signals in cartilage degradation during osteoarthritis progression in experimental mouse models. Molecules and cells. 2008;25:1–6. [PubMed] [Google Scholar]
- 3.Ortega N, Behonick DJ, Werb Z. Matrix remodeling during endochondral ossification. Trends in cell biology. 2004;14:86–93. doi: 10.1016/j.tcb.2003.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, et al. Runx1/AML1/Cbfa2 mediates onset of mesenchymal cell differentiation toward chondrogenesis. J Bone Miner Res. 2005;20:1624–1636. doi: 10.1359/JBMR.050516. [DOI] [PubMed] [Google Scholar]
- 5.Komori T. Regulation of skeletal development by the Runx family of transcription factors. J Cell Biochem. 2005;95:445–453. doi: 10.1002/jcb.20420. [DOI] [PubMed] [Google Scholar]
- 6.Komori T, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/S0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 7.Higashikawa A, et al. Identification of the core element responsive to runt-related transcription factor 2 in the promoter of human type X collagen gene. Arthritis Rheum. 2009;60:166–178. doi: 10.1002/art.24243. [DOI] [PubMed] [Google Scholar]
- 8.Kamekura S, et al. Contribution of runt-related transcription factor 2 to the pathogenesis of osteoarthritis in mice after induction of knee joint instability. Arthritis Rheum. 2006;54:2462–2470. doi: 10.1002/art.22041. [DOI] [PubMed] [Google Scholar]
- 9.Liao L, et al. Deletion of Runx2 in Articular Chondrocytes Decelerates the Progression of DMM-Induced Osteoarthritis in Adult Mice. Scientific reports. 2017;7:2371. doi: 10.1038/s41598-017-02490-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Kraan PM, van den Berg WB. Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration? Osteoarthritis Cartilage. 2012;20:223–232. doi: 10.1016/j.joca.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Lian JB, et al. Runx1/AML1 hematopoietic transcription factor contributes to skeletal development in vivo. J Cell Physiol. 2003;196:301–311. doi: 10.1002/jcp.10316. [DOI] [PubMed] [Google Scholar]
- 12.Yano F, et al. A novel disease-modifying osteoarthritis drug candidate targeting Runx1. Ann Rheum Dis. 2013;72:748–753. doi: 10.1136/annrheumdis-2012-201745. [DOI] [PubMed] [Google Scholar]
- 13.Kimura A, et al. Runx1 and Runx2 cooperate during sternal morphogenesis. Development. 2010;137:1159–1167. doi: 10.1242/dev.045005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LeBlanc KT, et al. Runx1 Activities in Superficial Zone Chondrocytes, Osteoarthritic Chondrocyte Clones and Response to Mechanical Loading. J Cell Physiol. 2015;230:440–448. doi: 10.1002/jcp.24727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson K, et al. A stem cell-based approach to cartilage repair. Science. 2012;336:717–721. doi: 10.1126/science.1215157. [DOI] [PubMed] [Google Scholar]
- 16.Aini H, et al. Messenger RNA delivery of a cartilage-anabolic transcription factor as a disease-modifying strategy for osteoarthritis treatment. Scientific reports. 2016;6:18743. doi: 10.1038/srep18743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamekura S, et al. Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthritis Cartilage. 2005;13:632–641. doi: 10.1016/j.joca.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Akazawa H, et al. Targeted disruption of the homeobox transcription factor Bapx1 results in lethal skeletal dysplasia with asplenia and gastroduodenal malformation. Genes Cells. 2000;5:499–513. doi: 10.1046/j.1365-2443.2000.00339.x. [DOI] [PubMed] [Google Scholar]
- 19.Lorsbach RB, et al. Role of RUNX1 in adult hematopoiesis: analysis of RUNX1-IRES-GFP knock-in mice reveals differential lineage expression. Blood. 2004;103:2522–2529. doi: 10.1182/blood-2003-07-2439. [DOI] [PubMed] [Google Scholar]
- 20.Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16:2813–2828. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lefebvre V, Behringer RR, de Crombrugghe B. L-Sox5, Sox6 and Sox9 control essential steps of the chondrocyte differentiation pathway. Osteoarthritis Cartilage. 2001;9(Suppl A):S69–75. doi: 10.1053/joca.2001.0447. [DOI] [PubMed] [Google Scholar]
- 22.Soung do Y, et al. Runx1 dose-dependently regulates endochondral ossification during skeletal development and fracture healing. J Bone Miner Res. 2012;27:1585–1597. doi: 10.1002/jbmr.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akiyama H. Control of chondrogenesis by the transcription factor Sox9. Mod Rheumatol. 2008;18:213–219. doi: 10.3109/s10165-008-0048-x. [DOI] [PubMed] [Google Scholar]
- 24.Lefebvre V, Huang W, Harley VR, Goodfellow PN, de Crombrugghe B. SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene. Mol Cell Biol. 1997;17:2336–2346. doi: 10.1128/MCB.17.4.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tribioli C, Lufkin T. The murine Bapx1 homeobox gene plays a critical role in embryonic development of the axial skeleton and spleen. Development. 1999;126:5699–5711. doi: 10.1242/dev.126.24.5699. [DOI] [PubMed] [Google Scholar]
- 26.Provot S, et al. Nkx3.2/Bapx1 acts as a negative regulator of chondrocyte maturation. Development. 2006;133:651–662. doi: 10.1242/dev.02258. [DOI] [PubMed] [Google Scholar]
- 27.Kawato Y, et al. Nkx3.2-induced suppression of Runx2 is a crucial mediator of hypoxia-dependent maintenance of chondrocyte phenotypes. Biochem Biophys Res Commun. 2011;416:205–210. doi: 10.1016/j.bbrc.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 28.Park M, et al. Constitutive RelA activation mediated by Nkx3.2 controls chondrocyte viability. Nat Cell Biol. 2007;9:287–298. doi: 10.1038/ncb1538. [DOI] [PubMed] [Google Scholar]
- 29.Caron MM, et al. BAPX-1/NKX-3.2 Acts as a Chondrocyte Hypertrophy Molecular Switch in Osteoarthritis. Arthritis &. rheumatology. 2015;67:2944–2956. doi: 10.1002/art.39293. [DOI] [PubMed] [Google Scholar]
- 30.Blanco FJ, Ruiz-Romero C. New targets for disease modifying osteoarthritis drugs: chondrogenesis and Runx1. Ann Rheum Dis. 2013;72:631–634. doi: 10.1136/annrheumdis-2012-202652. [DOI] [PubMed] [Google Scholar]
- 31.Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18(Suppl 3):S17–23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 32.Schmitz N, Laverty S, Kraus VB, Aigner T. Basic methods in histopathology of joint tissues. Osteoarthritis Cartilage. 2010;18(Suppl 3):S113–116. doi: 10.1016/j.joca.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 33.Murahashi Y, et al. Intra-articular administration of IkappaBalpha kinase inhibitor suppresses mouse knee osteoarthritis via downregulation of the NF-kappaB/HIF-2alpha axis. Scientific reports. 2018;8:16475. doi: 10.1038/s41598-018-34830-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yano F, et al. The canonical Wnt signaling pathway promotes chondrocyte differentiation in a Sox9-dependent manner. Biochem Biophys Res Commun. 2005;333:1300–1308. doi: 10.1016/j.bbrc.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 35.Gosset M, Berenbaum F, Thirion S, Jacques C. Primary culture and phenotyping of murine chondrocytes. Nat Protoc. 2008;3:1253–1260. doi: 10.1038/nprot.2008.95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.