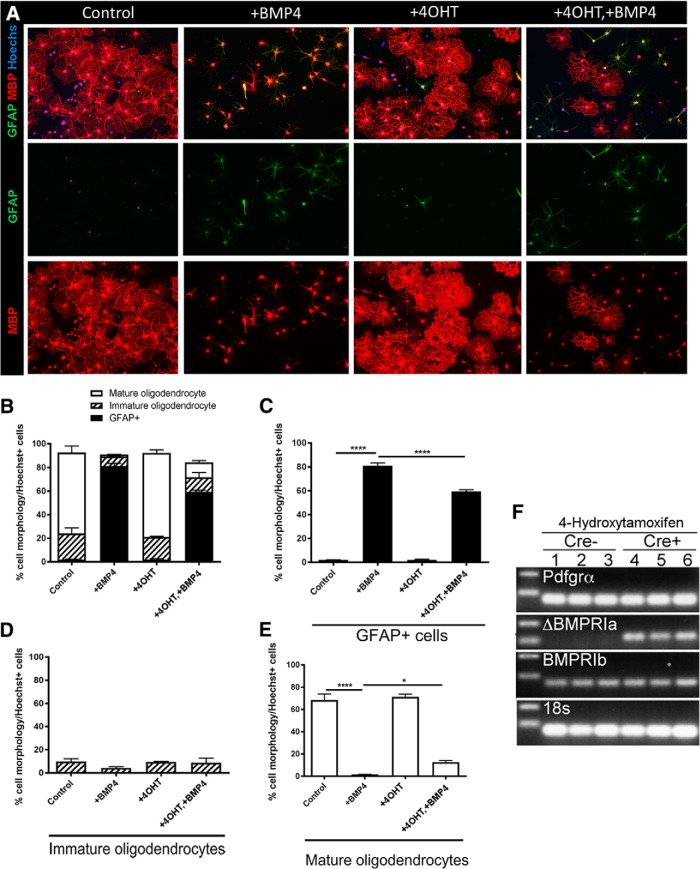
Figure 7.
BMP4 signals via BMPR1A in OPCs to potentiate oligodendrocyte differentiation and reduce astrogliogenesis in vitro. A, Representative micrographs of immunostaining of differentiated OPC cultures (isolated from Pdgfra-CreERT2::Bmpr1a fl/fl mice) for MBP and GFAP under untreated (Control) conditions, or following treatment with BMP4, 4OHT, or both BMP4 and 4OHT (+4OHT+BMP4). B, Quantification of cell-phenotypic distribution for each condition based on GFAP expression and MBP+ morphology, as described above (Fig. 4B). C, Quantification of the proportion of GFAP+ cells in the cultures. BMP4 significantly increased the proportion of GFAP+ cells compared with untreated (Control) cultures, whereas 4OHT alone exerted no significant effect. Pretreatment with 4OHT before BMP4 (+4OHT+BMP4) significantly attenuated the effect of BMP4. D, Quantification of the proportion of immature oligodendrocytes in the cultures. Treatment with BMP4, 4OHT, or both BMP4 and 4OHT (+4OHT+BMP4) exerted no significant effect. E, Quantification of the proportion of mature oligodendrocytes in the cultures. Treatment with BMP4 significantly decreased OPC differentiation, whereas 4OHT alone exerted no significant effect. Pretreatment with 4OHT before BMP4 (+4OHT+BMP4) significantly attenuated the inhibitory effect of BMP4 on OPC differentiation. F, PCR analysis of 4OHT-treated OPCs to assess Bmpr1a knockout. Pdgfra-CreERT2::Bmpr1a fl/fl and Cre[−] OPCs were isolated and treated with 4OHT for 24 h and analyzed for the transcription of a sequence corresponding to Bmpr1a-ex2, rendering the resulting protein untranscribable; N = 4 animals/group). *p < 0.05, ****p < 0.0001. Scale bar, 20 µm.