Abstract
Many patients with chronic obstructive pulmonary disease (COPD) continue to experience exacerbations despite receiving standard-of-care treatments. Novel approaches to COPD treatment focus on understanding and targeting molecular mechanisms of airway inflammation, airway obstruction, remodeling and lung destruction. Several identified phenotypes and endotypes of COPD will pave the future path for a more personalized approach to therapy. Although well known to be associated with neutrophilic inflammation, COPD may also be driven by eosinophilic inflammation both at stable states and during exacerbation. Targeting eosinophilic inflammation has been successful in managing severe eosinophilic asthma and may hold promise in certain phenotypes of COPD. The most promising biologic treatments at an advanced stage of development are agents blocking interleukin (IL)-5 or its receptor. This review examines our current understanding of the eosinophilic inflammation in COPD and the rationale for IL-5 targeting agents.
Keywords: COPD, airway inflammation, eosinophils, IL-5, mepolizumab, benralizumab
Introduction
Chronic obstructive pulmonary disease (COPD) is a common airway disease associated with significant morbidity and mortality worldwide. The Global initiative for Obstructive Lung Disease (GOLD) defines COPD as a common preventable and treatable disease, characterized by progressive airflow limitation that triggered by the response in the airways and the lung to noxious particles or fumes.1 The pathogenesis of airflow limitation in COPD is driven by airway inflammation, airway remodeling of small airways (chronic bronchitis) as well as parenchymal lung destruction (emphysema). Globally, there are about 251 million cases of COPD accounting for an estimated of more than 3 million deaths; about 5% of total deaths globally the year 2016.2 In fact, COPD accounts for most deaths from chronic lower respiratory disease and is currently the third leading cause of death in the United States.3
In the year 2017, GOLD refined the assessment tool for COPD that focused on spirometry, symptom assessment and assessment of risk of exacerbations. Management goals of COPD include improving symptoms and reducing future exacerbations. Recurrent exacerbations of COPD impose a significant burden on patient and health care cost, contributes to decline in lung function, quality of life, and is associated with negative impact on mental health and high mortality.4,5
Phenotypes and endotypes of COPD
COPD is a heterogeneous disease with varying clinical presentations (phenotypes) and underlying pathobiological mechanisms (endotypes).6–8 Identifying phenotypes and endotypes of COPD will lead to a more precise approach to this impactful disease. Quantitative cell counts in sputum in patients with symptoms of bronchitis provide a reliable (although not practical) method to assess airway inflammation. Based on the cellular inflammatory substrate, COPD phenotypes include: 1) neutrophilic, 2) eosinophilic, 3) mixed eosinophilic and neutrophilic and 4) paucigranulocytic.9 Blood biomarkers such as blood eosinophils may correlate with sputum eosinophils in patients with eosinophilic airway inflammation.9
Neutrophilic airway inflammation is the most predominant type seen in COPD and is mediated by non-Th2 (Helper T cells type 2) mechanisms and is typically steroid non-responsive. The most common etiology/trigger of this subtype includes cigarette smoking, air pollution, ozone exposure, occupational exposure and infections.
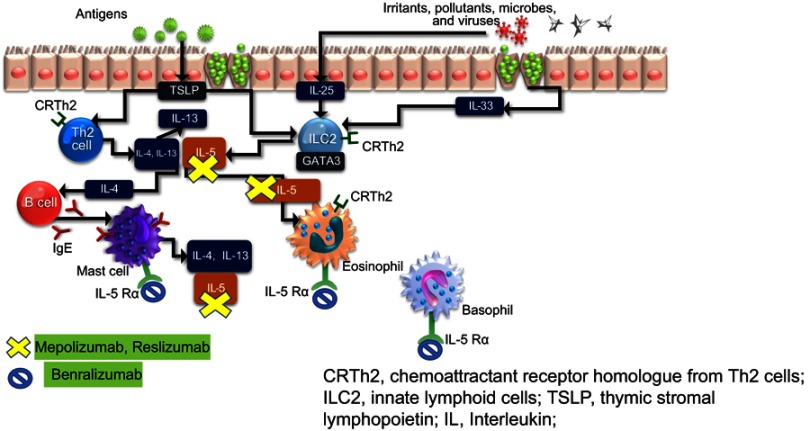
Eosinophilic airway inflammation which is most commonly encountered in asthma has been also described in some patients with COPD.10 T2 airway inflammation which encompasses eosinophilic inflammation is propagated by both the adaptive and innate immune response. Alarmins including the cytokines IL-33, IL-25 and thymic stromal lymphopoietin (TSLP) are produced by epithelial cells that have been exposed to pollutants or other environmental triggers including allergens. In the case of allergenic triggers, these cytokines initiate an adaptive immune response via dendritic cells that stimulate naïve T cells to differentiate into Th2 cells, which produce IL-5, IL-13 and IL-4. Non-allergen triggers can initiate an innate immune response potentially occurs via stimulation of type 2 innate lymphoid cells (ILC-2), which has been associated with the production of type 2 effector cytokines (IL-5 and IL-13) and eosinophilic airway inflammation (See Figure 1).
Figure 1.
T2 inflammatory pathway and the target site for anti-IL-5 agents.
Abbreviations: CRTh2, chemoattractant receptor homolog from Th2 cells; ILC2, innate lymphoid cells; TSLP, thymic stromal lymphopoietin; IL, Interleukin.
Role of eosinophils in COPD
It is estimated that eosinophilic inflammation is present in up to 40% cases of COPD.11 Elevated concentrations (>3%) found in sputum of a subset of patients with COPD.12 Furthermore, approximately 1 in 5 exacerbations is thought to be associated with an eosinophilic exacerbation.13–15 Indeed, airway biopsies and sputum taken during an acute exacerbations show an increased number of eosinophil concentrations compared with stable COPD. Eosinophils release eosinophilic cationic protein (ECP) and eosinophil peroxidase (EPO) which are toxic to bronchial epithelial cells and cause the release of cytokines which drive airway inflammation.12 Furthermore, airway eosinophilia has been shown to predict an increased risk of exacerbations11 and lung tissue and airway remodeling as well as an increased expression of interleukin (IL)-5.16 From the Genetic Epidemiology of COPD (COPDGene) study and the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study, stratified analyses confirmed that the increased exacerbation risk associated with an eosinophil count of 300 cells/µL or greater was driven by subjects with a history of frequent exacerbations.17
Targeting eosinophilic inflammation in COPD
Reducing eosinophilic airway inflammation in asthma has also been shown to reduce the risk of exacerbation.13–15 Clinically, an eosinophilic phenotype of COPD has been defined by the presence of a peripheral blood eosinophil count of 3% or more or >150–200 cells per cubic millimeter.
Roche and colleagues18 analyzed data from Effect of indacaterol Glycopyrronium vs Fluticasone Salmeterol on COPD exacerbations (FLAME) trial on the role of blood eosinophils in predicting the response of patients with COPD to inhaled corticosteroid efficacy. The FLAME trial compared ICS responsiveness to blood eosinophil percentage (2%, 3% and 5%) and absolute blood eosinophil count (<150 cells/µL, 150–300 cells/µL and >300 cells/µL). The indacaterol/glycopyrronium combination was found to be more effective than fluticasone/salmeterol in preventing acute exacerbations of COPD in all patients and in subgroups with different blood eosinophil range. However, other studies reveal contradicting results and suggest that a high baseline eosinophil count predicts response to ICS in COPD patients at high risk of exacerbation.18,19 However, Lipson et al randomized 10,355 patients with COPD, comparing 52 weeks of a once-daily fluticasone furoate (an inhaled glucocorticoid), umeclidinium (LAMA) and vilanterol (LABA) versus fluticasone furoate–vilanterol or umeclidinium–vilanterol.20 The annual rate of moderate or severe exacerbations was lower with triple therapy than with either dual-therapy, regardless of eosinophil level, although a greater reduction in the exacerbations was observed in patients with eosinophil levels of at least 150 cells/µL.
In addition to corticosteroids, other medications targeting eosinophils that are available or in development include those that block IL-5/IL-5 receptor alpha (IL-5Rα), IL-13/IL-4 receptor alpha (IL-4Rα), chemoattractant receptor–homologous molecules (DP2), IL-25, IL-33 and thymic stromal lymphopoietin (TSLP).
Targeting IL-5
Over the last decade, several monoclonal antibodies and molecular therapies have been developed to target eosinophilic inflammation and some are approved in the treatment of severe asthma.21,22
Interleukin 5 (IL-5) is a homodimer cytokine involved in eosinophil differentiation, recruitment, maturation, activation and degranulation.23 The IL-5 receptor (IL-5R) consists of an IL-5-specific alpha subunit that interacts in conformational dynamic ways with the receptors β subunit, an aggregate of domains it shares with binding sites of IL-3 and granulocyte-macrophage colony-stimulating factor. IL-5 and IL-5R driven allergic and inflammatory immune responses are characterized in numerous diseases, such as asthma, atopic dermatitis, chronic obstructive pulmonary disease, eosinophilic gastrointestinal diseases, hyper-eosinophilic syndrome, eosinophilic granulomatosis with polyangiitis (EGPA) and eosinophilic nasal polyposis. Sputum concentrations of IL-5 correlate with the degree of eosinophilia and response to glucocorticoids for patients with stable COPD and soluble IL-5Rα is increased during virus-induced COPD exacerbations.24
Molecular targets of IL-5 include monoclonal antibodies against circulating IL-5 (mepolizumab, reslizumab) or the IL-5 receptor-alpha (benralizumab). Figure 1 shows their site of action of available anti-IL-5 medications. Below, we provide details on clinical trials that have been completed with some of these agents in the eosinophilic COPD phenotype.
Mepolizumab
Mepolizumab is a humanized, IgG1 monoclonal antibody that targets circulating IL-5, which inhibits binding of IL-5 to the alpha chain of IL-5 receptor and neutralizes its effect.25,26 Mepolizumab has been approved as an-add on maintenance treatment of severe eosinophilic asthma.27
Post-hoc analysis of two trials, MENSA and DREAM in severe asthma showed that mepolizumab was associated with lower exacerbation rates (needing systemic steroids or hospitalization) than placebo among patients with severe eosinophilic asthma with clinical features of COPD.28 Furthermore, mepolizumab was evaluated in a pilot randomized clinical trial in COPD with eosinophilic bronchitis by Dasgupta et al,29 as a proof of principle, single center, randomized placebo-controlled, parallel group, double-blinded 6 month trial and 4 month follow-up. The primary objective was to determine if mepolizumab could decrease sputum eosinophil percentage in COPD patients with sputum eosinophilia. Other outcomes assessed on blood eosinophilia, lung function, exacerbation rate, symptoms and quality of life, sputum hyaluronan and versican. Eighteen patients were randomly assigned to mepolizumab (n=8) and placebo (n=10). Patients received monthly infusions of mepolizumab 750 mg or placebo in addition to their inhalers. Mepolizumab reduced sputum eosinophil counts from 11% to 0.5% at 6 months vs placebo 7% to 2% (p<0.05). Blood eosinophils decreased from 0.7+0.5 cells mm–3 to 0.03+0.05 cells mm–3 (p<0.05). There was no significant difference in pre-and post-bronchodilator spirometry measures, residual volume, total lung capacity, diffusion capacity, sputum hyaluronan, versican or radiological evidence of remodeling or patient-related outcomes at 3 and 6 months. This study also analyzed an important result that decrease in sputum and blood eosinophils were not associated with significant improvements in lung functions parameters, exacerbation rates, sputum markers of remodeling and health-related quality of scores.
Mepolizumab was evaluated for patients with eosinophilic COPD in two multicenter, Phase III randomized, controlled, clinical trials for the prevention of exacerbation. The Mepolizumab vs Placebo as Add-on Treatment for Frequently Exacerbating COPD Patients Characterized by Eosinophil Level trial (METREO) Phase III study (NCT02105961) evaluated two dosages of mepolizumab (100 mg and 300 mg, every 4 weeks) versus placebo (n=674) for 62 weeks for patients with ≥2 exacerbations or ≥1 severe exacerbations in the previous year while on triple therapy including inhaled corticosteroids and an eosinophilic phenotype (≥150 cells/μL at screening or ≥300 cells/μL during the previous year).28 The Mepolizumab vs Placebo as Add-on Treatment for Frequently Exacerbating COPD Patients trial (METREX) Phase III study (NCT02105948) compared mepolizumab 100 mg every 4 weeks with placebo (n=837) over 52 weeks for patients with COPD who had ≥2 moderate exacerbations (ie, treated with systemic glucocorticoids, antibiotics, or both in association with worsening of COPD) or ≥1 severe exacerbations (ie, leading to hospitalization) in the previous year. Patients with both eosinophilic (≥150 cells/μL at screening or ≥300 cells/μL during the previous year) and noneosinophilic (<150 cells/μL at screening and no evidence of ≥300 cells/μL in the previous year) phenotypes were included, and results were analyzed for those with baseline blood eosinophil counts ≥150 cells/μL versus <150 cells/μL. The primary endpoint for both studies was the annual rate of moderate or severe exacerbations. Secondary endpoints include time to first exacerbation, rate of exacerbations requiring emergency department visit, hospitalization or both, mean change from baseline at week 52 in St George’s Respiratory questionnaire (SGRQ-C) and COPD assessment test score (CAT score).
In the METREO study, the exacerbation rate ratios in the 100 mg and 300 mg mepolizumab groups compared with placebo were 0.80 and 0.86 (p=0.07 and p=0.14, respectively). In the METREX study, mepolizumab reduced the mean annual exacerbation rate for patients with eosinophilia (n=462) (1.40 versus 1.71 exacerbations/year; p=0.04), but no significant benefit over placebo was observed in the overall population. In both studies, there were no statistically significant differences observed between the two groups in patient-reported outcomes.
In a post-hoc meta-analysis of the eosinophilic patient populations (≥300 cells/μL at screening or during the previous year) from the combined trials demonstrated that the rate of moderate (leading to systemic glucocorticoid treatment, antibiotic treatment or both) or severe (leading to hospitalization or resulting in death) exacerbations was 23% lower for patients treated with mepolizumab 100 mg compared with placebo recipients (rate ratio, 0.77). The meta-analysis also demonstrated greater treatment effects with mepolizumab versus placebo with increasing screening blood eosinophil counts for exacerbations treated with systemic glucocorticoids but not in patients whose exacerbations were treated with antibiotics. This differential response to mepolizumab according to the trigger of exacerbation (microbial or non-microbial) is quite interesting and requires further exploration in future studies. It also remains unclear whether all exacerbations during treatment with mepolizumab require treatment with systemic steroids. No significant differences in adverse events were observed.
In May 2018, FDA did not approve mepolizumab for treatment of COPD and stated that the drug failed to demonstrate efficacy for the new indication and that studies included patients who may have had asthma-COPD overlap (ACO) which may have reflected the potential benefit of the drug in these subsets. Future mepolizumab studies for COPD are in the planning stage to address these concerns.
Benralizumab
Benralizumab is humanized afucosylated, anti–IL-5Rα monoclonal antibody that prevents IL-5 signaling by binding to the IL-5Rα receptor. It binds to natural killer cells which release proapoptotic proteins such as granzymes and perforins causing rapid and direct depletion of eosinophils and basophils via enhanced antibody-dependent cell-mediated cytotoxicity.30 Benralizumab has been approved for severe eosinophilic asthma based on two large Phase III clinical trials.31,32
Benralizumab was the first anti-IL5 to be evaluated in COPD. In a Phase IIa clinical trial, Brightling and colleagues33 conducted a randomized, double-blind, placebo-controlled study in adults 40–85 years old, with moderate-to-severe COPD, at least one acute exacerbation of COPD, and a sputum eosinophil count of 3.0% or more within the previous year (NCT01227278). Participants were randomly assigned (1:1) via computer-generated permuted block randomization (block size of four), with an interactive voice or web-response system, to receive placebo or 100 mg for 52 weeks. Benralizumab subcutaneously, every 4 weeks (three doses), then every 8 weeks (five doses) over 48 weeks. One hundred and one patients were randomized to receive Benralizumab (51) versus Placebo (50) and 88 patients completed the study. Benralizumab did not reduce annualized rate of exacerbations compared to placebo, rate of 0.95 versus 0.92 in the overall population but was associated with significant improvement in pre-bronchodilator FEV1 compared with placebo (0.13 L versus –0.06 L; p=0.014) as early as week 4. In sub-analysis, there was a 31% reduction in exacerbations with benralizumab versus placebo treatment for patients with baseline blood eosinophils ≥200 cells/μL. Patients with blood eosinophils ≥200 cells/μL also exhibited significant improvement in FEV1 (p=0.035), but patients with lower eosinophil counts did not. Adverse events were similar in the two groups.
More recently, two Phase III studies (GALATHEA and TERRANOVA, NCT02138916 and NCT02155660), randomized, double-blinded, 56-week placebo-controlled multicenter trials assessing safety and efficacy of benralizumab as an add-on therapy to dual or triple inhaled therapy compared to placebo in patients with moderate-to-severe COPD with history of exacerbation across a range of blood eosinophils. Press releases about the analysis of GALATHEA34 on May 11, 2018, and TERRANOVA35 released on May 30, 2018, revealed that these trials failed to meet primary endpoint of statistically significant reduction of exacerbations in patients with COPD.
Reslizumab
Reslizumab is a humanized anti-IL-5 monoclonal antibody approved for severe eosinophilic persistent asthma for patients aged 18 years. Like mepolizumab, this drug acts on circulating IL-5 and neutralizes its effect. However, this drug is administered intravenously and it is weight based (3 mg per kilogram every 4 weeks). Although approved in asthma, it has not been evaluated in the COPD population.
Conclusion
The fact that COPD is a heterogeneous disease with multiple phenotypes and endotypes has led to the examination of novel targets for therapy that target specific pathologic mechanisms. For such treatments to work, biomarkers are needed to identify the most appropriate patients. The use of blood eosinophil count has been used to determine a subgroup of patients with eosinophilic phenotype of COPD. Despite conflicting evidence, blood eosinophils appear to predict COPD exacerbations and response to some anti-inflammatory medications such as inhaled corticosteroids. IL-5 is an important effector cytokine in driving eosinophilic airway inflammation in both asthma and COPD. The results of the anti-IL-5/IL-5Rα clinical trials in asthma confirmed the efficacy and safety of such agents in severe eosinophilic asthma (Table 1). The efficacy and safety of two of the agents currently approved for treatment of severe eosinophilic asthma have now been examined in large clinical trials in the eosinophilic COPD population. However, preliminary data from these studies, although encouraging, have not shown consistent results. Several factors may have contributed to this inconsistency of results. The cutoff values for blood eosinophils used to enroll patients with COPD in these studies were similar to those used in asthma studies. Future studies need to be more restrictive in the inclusion criteria of the patient population enrolled to allow for better selection of patients who would respond to such treatments.
Table 1.
Agents targeting IL-5 in COPD
| Agent/Trials | Dose, frequency | Study design, duration, number of subjects | Outcomes |
|---|---|---|---|
| Mepolizumab (Anti-IL5) | |||
| Dasgupta et al,29 NCT01463644 A pilot phase II |
750 mg monthly IV | Single center, Randomized, double blind, placebo controlled 24 weeks 18 patients |
Primary: Mepolizumab reduced sputum eosinophil counts from 11% to 0.5% at 6 months vs placebo 7% to 2%(p<0.05). Blood eosinophils decreased from 0.7+0.5 cells.mm−3 to 0.03+0.05 cells mm−3(p<0.05). Secondary: No significant difference in the lung function parameters, exacerbation rates, sputum markers of remodeling, health-related quality of life scores. |
| Pavord et al,28 NCT02105948 (METREX), Phase III NCT02105961 (METREO), Phase III |
100 mg subcutaneous q 4 weeks 300 mg subcutaneous q 4 weeks |
Multicenter Randomized, double blind, placebo controlled 52 weeks 1,510 patients |
Primary: Significantly reduction in the annual exacerbation rate vs placebo for patients with eosinophilic phenotype (patients with blood eosinophil counts 150 cells/μL at screening or ≥300 cell/μL within the previous (1.40 versus 1.71; n=462; p=0.04); difference was not significant in the overall population Secondary: Significant reduction in time to first moderate/severe exacerbation in the eosinophilic population (192 versus 141 days; p=0.04); no statistically significant differences in any other endpoints between groups Primary: Rate ratios for exacerbations were 0.80 (p=0.07) and 0.86 (p=0.14) versus placebo for 100-mg and 300-mg dosages of mepolizumab, respectively Secondary: No statistical significance in any endpoints versus placebo in either group. |
| Benralizumab IL-(IL5Rα receptor antagonist) | |||
| Brightling et al,33 NCT01227278 Phase IIa |
100 mg subcutaneous q 4 weeks | Single center randomized, double blind, placebo controlled 56 weeks 101 patients |
Primary: Annualized rate of acute exacerbations of COPD: benralizumab 0.95, placebo 0.92 (no significant difference) Secondary: Significant increase in pre-bronchodilator FEV1 versus placebo (0.13 L versus −0.06 L; p=0.014); no significant differences between groups in change from baseline for mean SGRQ-C, CRQ-SAS, BODE scores; no difference in treatment- emergent adverse events between treatment groups. |
| Criner et al, GALATHEA34 Phase III Celli et al, TERRANOVA35 Phase III |
Undisclosed dosage | Multicenter Randomized, double blind, placebo controlled 56–60 weeks |
Results are not yet released, preliminary data revealed no difference in primary outcome in decreasing exacerbations in patients with COPD. |
Abbreviations: BODE, BMI, Airway Obstruction, Dyspnea, Exercise tolerance; CRQ-SAS, Chronic respiratory Questionnaire-Standardized dyspnea domain; IV, Intravenous; Q, every; SGRQ-C, St. George’s Respiratory Questionnaire-COPD.
Disclosure
DKN has no conflicts of interest to disclose in this work. NAH received honoraria for serving as consultant or on advisory boards for GSK, Astra Zeneca, Sanofi/Regeneron, Novartis, Boehringer Ingelheim, Sunovion, Mylan and Gossamer Bio. His institutions have received research grant support on his behalf from Astra Zeneca, GSK, Boehringer Ingelheim.
References
- 1.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Arch Bronconeumol. 2017;53(3):128–149. doi: 10.1016/j.arbres.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 2.https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd); 2017. Accessed February25, 2019.
- 3.Croft JB, Wheaton AG, Liu Y, et al. Urban-rural county and state differences in chronic obstructive pulmonary disease – United States, 2015. Morb Mortal Wkly Rep. 2018;67(7):205–211. doi: 10.15585/mmwr.mm6707a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi: 10.1056/NEJMoa0909883 [DOI] [PubMed] [Google Scholar]
- 6.Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182(5):598–604. doi: 10.1164/rccm.200912-1843CC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lange P, Halpin DM, O‘Donnell DE, MacNee W. Diagnosis, assessment, and phenotyping of COPD: beyond FEV(1). Int J Chron Obstruct Pulmon Dis. 2016;11(Spec Iss):3–12. doi: 10.2147/COPD.S85976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balkissoon R. New treatment options for COPD: how do we decide phenotypes, endotypes or treatable traits? Chronic Obstr Pulm Dis (Miami). 2018;5(1):72–80. doi: 10.15326/jcopdf.5.1.2018.0128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dasgupta A, Neighbour H, Nair P. Targeted therapy of bronchitis in obstructive airway diseases. Pharmacol Ther. 2013;140(3):213–222. doi: 10.1016/j.pharmthera.2013.07.001 [DOI] [PubMed] [Google Scholar]
- 10.Tashkin DP, Wechsler ME. Role of eosinophils in airway inflammation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2018;13:335–349. doi: 10.2147/COPD.S152291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh D, Kolsum U, Brightling CE, Locantore N, Agusti A, Tal-Singer R. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J. 2014;44(6):1697–1700. doi: 10.1183/09031936.00162414 [DOI] [PubMed] [Google Scholar]
- 12.Saha S, Brightling CE. Eosinophilic airway inflammation in COPD. Int J Chron Obstruct Pulmon Dis. 2006;1(1):39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siva R, Green RH, Brightling CE, et al. Eosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trial. Eur Respir J. 2007;29(5):906–913. doi: 10.1183/09031936.00146306 [DOI] [PubMed] [Google Scholar]
- 14.Couillard S, Larivee P, Courteau J, Vanasse A. Eosinophils in COPD exacerbations are associated with increased readmissions. Chest. 2017;151(2):366–373. doi: 10.1016/j.chest.2016.10.003 [DOI] [PubMed] [Google Scholar]
- 15.Saetta M, Di Stefano A, Maestrelli P, et al. Airway eosinophilia in chronic bronchitis during exacerbations. Am J Respir Crit Care Med. 1994;150(6 Pt 1):1646–1652. doi: 10.1164/ajrccm.150.6.7952628 [DOI] [PubMed] [Google Scholar]
- 16.Chung KF, Adcock IM. Multifaceted mechanisms in COPD: inflammation, immunity, and tissue repair and destruction. Eur Respir J. 2008;31(6):1334–1356. doi: 10.1183/09031936.00018908 [DOI] [PubMed] [Google Scholar]
- 17.Yun JH, Lamb A, Chase R, et al. Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2018;141(6):2037–2047.e2010. doi: 10.1016/j.jaci.2018.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roche N, Chapman KR, Vogelmeier CF, et al. Blood eosinophils and response to maintenance chronic obstructive pulmonary disease treatment. Data from the FLAME trial. Am J Respir Crit Care Med. 2017;195(9):1189–1197. doi: 10.1164/rccm.201701-0193OC [DOI] [PubMed] [Google Scholar]
- 19.Keene JD, Jacobson S, Kechris K, et al. Biomarkers predictive of exacerbations in the SPIROMICS and COPDGene cohorts. Am J Respir Crit Care Med. 2017;195(4):473–481. doi: 10.1164/rccm.201607-1330OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680. doi: 10.1056/NEJMoa1713901 [DOI] [PubMed] [Google Scholar]
- 21.Bel EH, Ten Brinke A. New anti-eosinophil drugs for asthma and COPD: targeting the trait! Chest. 2017;152(6):1276–1282. doi: 10.1016/j.chest.2017.05.019 [DOI] [PubMed] [Google Scholar]
- 22.Markham A. Benralizumab: first global approval. Drugs. 2018;78(4):505–511. doi: 10.1007/s40265-018-0876-8 [DOI] [PubMed] [Google Scholar]
- 23.Varricchi G, Bagnasco D, Borriello F, Heffler E, Canonica GW. Interleukin-5 pathway inhibition in the treatment of eosinophilic respiratory disorders: evidence and unmet needs. Curr Opin Allergy Clin Immunol. 2016;16(2):186–200. doi: 10.1097/ACI.0000000000000251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caramori G, Adcock IM, Di Stefano A, Chung KF. Cytokine inhibition in the treatment of COPD. Int J Chron Obstruct Pulmon Dis. 2014;9:397–412. doi: 10.2147/COPD.S42544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mepolizumab: 240563, anti-IL-5 monoclonal antibody – GlaxoSmithKline, anti-interleukin-5 monoclonal antibody – GlaxoSmithKline, SB 240563. Drugs R D. 2008;9(2):125–130. doi: 10.2165/00126839-200809020-00006 [DOI] [PubMed] [Google Scholar]
- 26.GSK. Nucala prescribing information. Available from: https://www.accessdata/fda.gov/drugsatfda_docs/label/2015/125526Orig1s000Lbl.pd. Accessed November 2018.
- 27.Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–1207. doi: 10.1056/NEJMoa1403290 [DOI] [PubMed] [Google Scholar]
- 28.Pavord ID, Chanez P, Criner GJ, et al. Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N Engl J Med. 2017;377(17):1613–1629. doi: 10.1056/NEJMoa1708208 [DOI] [PubMed] [Google Scholar]
- 29.Dasgupta A, Kjarsgaard M, Capaldi D, et al. A pilot randomised clinical trial of mepolizumab in COPD with eosinophilic bronchitis. Eur Respir J. 2017;49:3. doi: 10.1183/13993003.02486-2016 [DOI] [PubMed] [Google Scholar]
- 30.Kolbeck R, Kozhich A, Koike M, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125(6):1344–1353.e1342. doi: 10.1016/j.jaci.2010.04.004 [DOI] [PubMed] [Google Scholar]
- 31.FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet (London, England). 2016;388(10056):2128–2141. doi: 10.1016/S0140-6736(16)31322-8 [DOI] [PubMed] [Google Scholar]
- 32.Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet (London, England). 2016;388(10056):2115–2127. doi: 10.1016/S0140-6736(16)31324-1 [DOI] [PubMed] [Google Scholar]
- 33.Brightling CE, Bleecker ER, Panettieri RA Jr., et al. Benralizumab for chronic obstructive pulmonary disease and sputum eosinophilia: a randomised, double-blind, placebo-controlled, phase 2a study. Lancet Respir Med. 2014;2(11):891–901. doi: 10.1016/S2213-2600(14)70187-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benralizumab Efficacy in Moderate to Very Severe Chronic Obstructive Pulmonary Disease (COPD) With Exacerbation History (GALATHEA) NCT02138916; 2018.
- 35.Efficacy and Safety of Benralizumab in Moderate to Very Severe Chronic Obstructive Pulmonary Disease (COPD) With Exacerbation History (TERRANOVA) NCT02155660; 2018.