Although nontypeable Haemophilus influenzae (NTHi) is a human-specific nasopharyngeal commensal bacterium, it also causes upper respiratory tract infections in children and lower respiratory tract infections in the elderly, resulting in frequent antibiotic use. The transition from symbiotic colonizing bacterium to opportunistic pathogen is not completely understood.
KEYWORDS: Haemophilus influenzae, IgG, IgM, Neu5Ac, Neu5Gc, complement resistance, sialic acid
ABSTRACT
Although nontypeable Haemophilus influenzae (NTHi) is a human-specific nasopharyngeal commensal bacterium, it also causes upper respiratory tract infections in children and lower respiratory tract infections in the elderly, resulting in frequent antibiotic use. The transition from symbiotic colonizing bacterium to opportunistic pathogen is not completely understood. Incorporation of sialic acids into lipooligosaccharides is thought to play an important role in bacterial virulence. It has been known for more than 25 years that sialic acids increase resistance to complement-mediated killing; however, the mechanism of action has not been elucidated thus far. Here, we provide evidence that growth of NTHi in the presence of sialic acids Neu5Ac and Neu5Gc decreases complement-mediated killing through abrogating the classical pathway of complement activation by preventing mainly IgM antibody binding to the bacterial surface. Therefore, strategies that interfere with uptake or incorporation of sialic acids into the lipooligosaccharide, such as novel antibiotics and vaccines, might be worth exploring to prevent or treat NTHi infections.
INTRODUCTION
Haemophilus influenzae is a Gram-negative human-specific nasopharyngeal commensal bacterium that can be characterized by its polysaccharide capsule into serotypes A to F and unencapsulated strains, also termed nontypeable H. influenzae (NTHi). Capsulated H. influenzae, mainly serotype B, was found to cause invasive diseases, including bacteremia and meningitis, whereas NTHi is more often the etiological cause of mucosal infections, including otitis media in children and pneumonia in the elderly (1).
The transition from symbiotic colonizing bacterium to an opportunistic disease-causing pathogen is not completely understood. One of the factors possibly contributing to this phenotype switch is the incorporation of sialic acids into the lipooligosaccharide (LOS), because NTHi strains that lack this ability are avirulent in animal models (2, 3). Sialic acids are complex nine-carbon sugars abundantly expressed on human glycoconjugates (4). NTHi is not able to synthesize Neu5Ac and is dependent on uptake of sialic acid from its environment, likely from mucins that are rich in Neu5Ac (5). Sialic acids can be taken up by NTHi using a tripartite ATP-independent periplasmic (TRAP) transporter system (6) and incorporated into the variable outer core of the LOS (5). The laboratory of Micheal Apicella was the first to discover that LOS from some Haemophilus species, including NTHi, was sialylated (7). Incorporation of sialic acid renders bacteria more resistant to complement-mediated killing (8), a mechanism also observed for other mucosal pathogens, such as Neisseria meningitidis and Neisseria gonorrhoeae (9).
Activation of the complement system is an essential host response in the clearance of NTHi. In a chinchilla otitis media model, it was shown that complement was essential for clearance of NTHi from the middle ear space (2). Higher complement resistance was found for NTHi strains collected from patients with otitis media, lower respiratory tract infection, and sepsis than for colonizing strains, indicating that resistance to complement-mediated killing counteracts the clearance mechanisms of complement and contributes to its ability to cause disease (10–12). Therefore, understanding the mechanism by which incorporation of sialic acids increases resistance to complement-mediated killing is important to be able to prevent the phenotype switch from symbiotic colonizing bacterium to an opportunistic disease-causing pathogen.
In this study, we provide evidence that growth of NTHi bacteria in the presence of sialic acid increases complement resistance due to lowered binding of antibodies, mainly IgM, to the bacterial surface.
RESULTS
Uptake of sialic acid decreased serum killing of multiple NTHi strains.
Large differences between NTHi bacteria, either genetically or phenotypically, have been described. Therefore, in order to rigorously determine the role of sialic acids in serum resistance, 4 different NTHi strains collected from patients with different disease etiologies were included in this study. Strain 11P6H was isolated from sputum of a chronic obstructive pulmonary disease (COPD) patient (13), strain 3655 was isolated from a patient with acute otitis media (14), strain R2866 was isolated from blood of a patient with meningitis (15), and strain 86-028NP was isolated from a patient with chronic otitis media (16).
First, NTHi strains were grown with or without N-azidoacetylneuraminic acid (Neu5Az) in order to determine incorporation of sialic acids on the bacterial surface by flow cytometry. All NTHi strains were able to incorporate Neu5Az, although signals differed among strains, with strain NTHi 11P6H having the highest level (Fig. 1A).
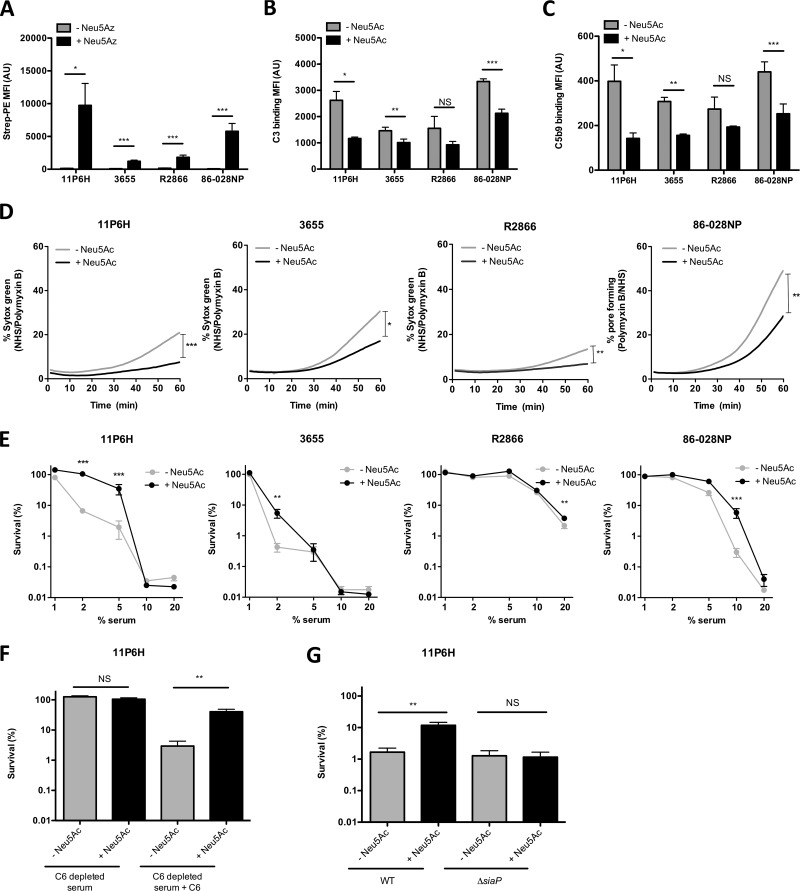
FIG 1.
Uptake of sialic acid decreases complement binding and serum killing of multiple NTHi strains. (A) Bacteria were grown with 100 μM Neu5Az, and the presence of Neu5Az on the bacterial surface was detected by flow cytometry (n = 6). Bacteria were incubated with 10% NHS in HBSS3+ for 30 min, and surface binding of complements C3 (B) and C5b9 (C) was determined by flow cytometry (n = 4). (D) Bacteria were incubated with 10% NHS, 10% HI serum, or 200 μg/ml polymyxin B in HBSS3+, and Sytox green signal was detected over a period of 60 min. Relative fluorescence intensity was determined by subtracting the fluorescence intensity of the NHS from the fluorescence intensity of the HI serum, followed by dividing it by the maximum fluorescence intensity of polymyxin B. The area under the curve was determined for statistical analysis (n = 4 or 6). (E) Bacteria were incubated with diluted NHS or diluted HI serum in HBSS3+ for 1 h, and survival was determined by dividing the CFU counts in NHS by the CFU count in HI serum after 1 h of incubation (n = 4). (F) NTHi 11P6H bacteria were incubated with 2.5% C6-depleted serum or 2.5% HI-C6-depleted serum in HBSS3+ for 1 h, and survival was determined by dividing the CFU counts in C6-depleted serum by the CFU count in HI-C6-depleted serum after 1 h of incubation (n = 6). (G) NTHi 11P6H WT or 11P6HΔsiaP mutant bacteria were incubated with 5% NHS or 5% HI serum in HBSS3+ for 1 h, and survival was determined by dividing the CFU counts in NHS by the CFU count in HI serum after 1 h of incubation (n = 3). A two-tailed paired t test (A, B, C, D, F, and G) or 2-way analysis of variance with Bonferroni posttest (E) was used for statistical analysis. *, P < 0.05; **, P < 0.01; ***, P < 0.001; NS, not significant.
NTHi strains were grown with or without the sialic acid N-acetylneuraminic acid (Neu5Ac), and complement C3 and C5b9 complex binding to the bacterial surface was determined by flow cytometry. Decreased complement C3 (Fig. 1B) and C5b9 complex (Fig. 1C) binding was observed on the surface of all strains when grown in medium supplemented with Neu5Ac, which was significant for strains 11P6H, 3655, and 86-028NP.
Pore formation by the membrane attack complex was determined by using Sytox green, which is a DNA binding fluorescent compound that is not able to pass the bacterial membrane. Upon pore formation, Sytox green enters the bacteria, resulting in an increase in fluorescence signal. All strains grown without Neu5Ac showed an increase in Sytox green signal over time, although the magnitude was strain dependent (Fig. 1D). Growth of strains with Neu5Ac significantly inhibited pore formation, which was in accordance with decreased C5b9 complex binding on the bacterial surface.
We next determined bacterial survival in different concentrations of pooled normal human serum (NHS). Survival in serum differed among the different NTHi strains, where strains 11P6H and 3655 were completely eradicated with 10% NHS and strain R2866 was most resistant to complement-mediated killing (Fig. 1E). For all strains, growth in the presence of Neu5Ac increased resistance to complement-mediated killing. Killing was dependent on the formation of a functional membrane attack complex, because killing for NTHi 11P6H was absent from C6-deficient serum and restored with reconstitution with purified C6 (Fig. 1F). Sialic acid-mediated resistance to complement killing was dependent on functional sialic acid transport, because a 11P6HΔsiaP sialic acid transporter mutant strain showed no difference in complement-mediated killing when grown with or without sialic acid, whereas this was not the case for the 11P6H wild-type (WT) strain (Fig. 1G).
Factor H was not involved in sialic acid-mediated complement resistance.
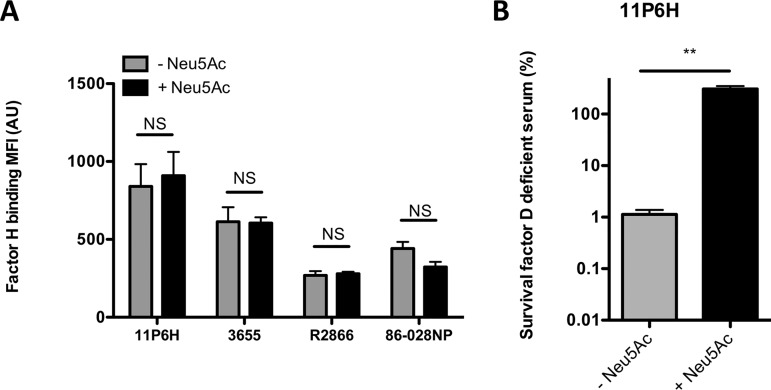
Previous studies of sialic acid incorporation by Haemophilus somnus and N. gonorrhoeae indicated that the increased serum resistance was due to binding of human factor H (fH) (17, 18). To investigate whether fH recruitment to NTHi was also involved in sialic acid-mediated complement resistance, we performed detection of fH by flow cytometry. Addition of Neu5Ac had no effect on binding of fH to the bacterial surface (Fig. 2A). The contribution of the alternative pathway of complement activation in complement-mediated killing of NTHi grown with and without sialic acid was investigated with the use of factor D-deficient serum. NTHi grown with sialic acid showed a higher survival in fD-deficient serum than bacteria grown without sialic acid (Fig. 2B), indicating that the sialic acid-mediated serum resistance was alternative pathway independent.
FIG 2.
Uptake of sialic acid does not affect factor H binding. (A) Bacteria were incubated with 10% HI serum in HBSS3+ for 30 min, and surface binding of factor H was determined by flow cytometry (n = 4). (B) NTHi 11P6H bacteria were incubated with 5% factor D-deficient serum or 5% HI-factor D-deficient serum in HBSS3+ for 1 h, and survival was determined by dividing the CFU counts in factor D-deficient serum by the CFU count in HI-factor D-deficient serum after 1 h of incubation (n = 2). A two-tailed paired t test was used for statistical analysis. **, P < 0.01; NS, not significant.
Decreased binding of CRP, IgG, and IgM to NTHi grown with sialic acid.
Based on the results described above, the alternative pathway of complement activation can be excluded. We focused on a role of the classical pathway of complement activation. The contribution of the classical pathway of complement activation in sialic acid-mediated complement resistance was determined with C1q-depleted serum. Complement-mediated killing was abrogated for NTHi 11P6H grown with or without Neu5Ac in the absence of C1q (Fig. 3A). Supplementation of C1q-depleted serum with C1q restored complement-mediated killing, and NTHi 11P6H grown with Neu5Ac was shown to be more resistant to complement-mediated killing (Fig. 3A).
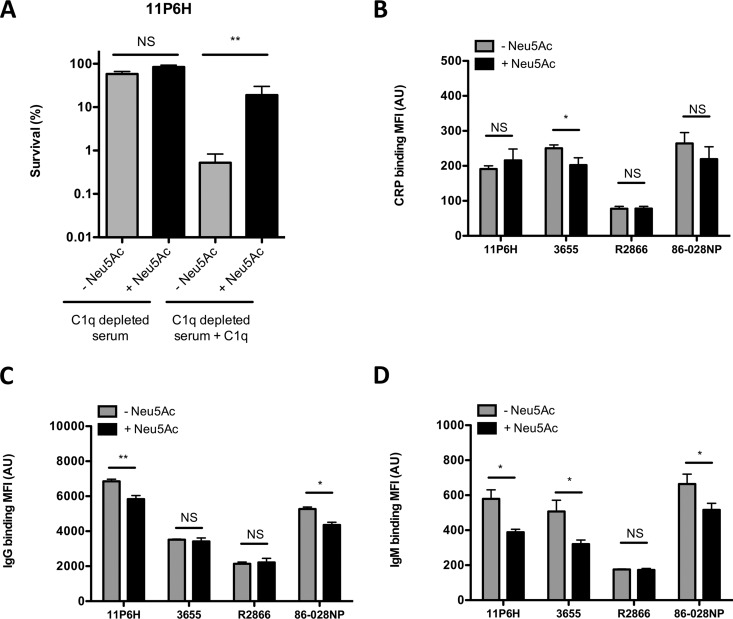
FIG 3.
Uptake of sialic acid decreases binding of CRP, IgG, and IgM to multiple NTHi bacteria. (A) NTHi 11P6H bacteria were incubated with 5% C1q-depleted serum or 5% HI-C1q-depleted serum with or without C1q supplementation in HBSS3+ for 1 h, and survival was determined by dividing the CFU counts in C1q-depleted serum by the CFU count in HI-C1q-depleted serum after 1 h of incubation (n = 4). Bacteria were incubated with 10% HI serum in HBSS3+ for 30 min, and surface binding of CRP (B), IgG (C), and IgM (D) was determined by flow cytometry (n = 4). A two-tailed paired t test was used for statistical analysis. *, P < 0.05; **, P < 0.01; NS, not significant.
Binding of C-reactive protein (CRP), IgG, and IgM has been shown to be effective in activating the classical complement pathway. Binding of CRP was not different (11P6H, R2866, and 86-028NP) or was lower (3655) for NTHi strains grown in the presence of Neu5Ac (Fig. 3B). Significantly lower binding of IgG (11P6H and 86-028NP) and IgM (11P6H, 3655, and 86-028NP) was observed when NTHi strains were grown in the presence of Neu5Ac (Fig. 3C and D).
IgM-dependent complement activation is required for complement-mediated killing of NTHi.
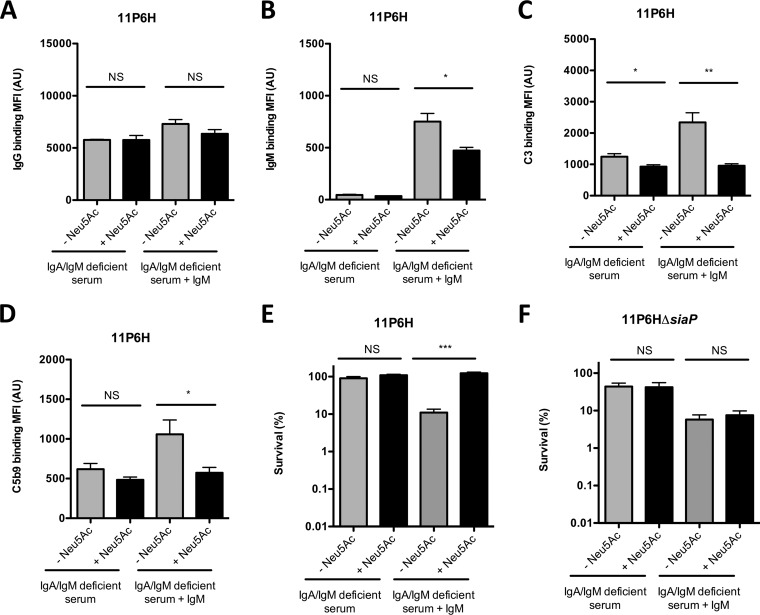
Because decreased binding of IgM was found to be most significant between NTHi strains grown with or without Neu5Ac (Fig. 3), we determined the role of IgM in complement-mediated killing of NTHi 11P6H grown with or without Neu5Ac. For these experiments, we used serum from an agammaglobulinemia patient on IgG replacement therapy, which lacks IgA and IgM. The role of IgM could then be determined by supplementing IgA/IgM-deficient serum with IgM. Binding of IgG to NTHi 11P6H grown with and without Neu5Ac was not significantly different with IgA/IgM-deficient serum with or without serum IgM supplementation (Fig. 4A). Binding of IgM was absent with IgA/IgM-deficient serum and was significantly different between NTHi 11P6H samples grown with or without Neu5Ac when supplemented with serum IgM (Fig. 4B). Binding of complement C3 and C5b9 complex was slightly lower for NTHi 11P6H grown with Neu5Ac than for NTHi 11P6H grown without Neu5Ac in IgA/IgM-deficient serum, which is likely due to the small difference in binding of IgG to the bacterial surface (Fig. 4C and D). However, the difference in complement binding between NTHi 11P6H grown with or without Neu5Ac was more evident after supplementation of serum IgM. Serum survival of NTHi 11P6H grown with and without Neu5Ac was high in IgA/IgM-deficient serum and decreased significantly for NTHi 11P6H grown without Neu5Ac with supplementation of serum IgM (Fig. 4E), whereas survival remained low for the 11P6HΔsiaP mutant grown with and without sialic acid in IgM-supplemented IgA/IgM-deficient serum, indicating that transport of sialic acid was required for increased survival (Fig. 4F). Therefore, we conclude that growth of NTHi bacteria in the presence of sialic acid increases complement resistance due to lowered binding of antibodies, mainly IgM, to the bacterial surface.
FIG 4.
Sialic acid-mediated decrease in IgM binding is required for decreased complement-mediated killing of NTHi. NTHi 11P6H bacteria were incubated with 10% HI-IgA/IgM-deficient serum (IgG/IgM) or 10% IgA/IgM-deficient serum (C3/C5b9) with or without IgM supplementation in HBSS3+ for 30 min, and surface binding of IgG (A), IgM (B), C3 (C), or C5b9 (D) was determined by flow cytometry (n = 4). (E and F) NTHi 11P6H (E) and NTHi 11P6HΔsiaP (F) bacteria were incubated with 5% IgA/IgM-deficient serum or 5% HI-IgA/IgM-deficient serum with or without IgM supplementation in HBSS3+ for 1 h, and survival was determined by dividing the CFU counts in IgA/IgM-deficient serum by the CFU counts in HI-IgA/IgM-deficient serum after 1 h of incubation (n = 4). A two-tailed paired t test was used for statistical analysis. *, P < 0.05; **, P < 0.01; ***, P < 0.001; NS, not significant.
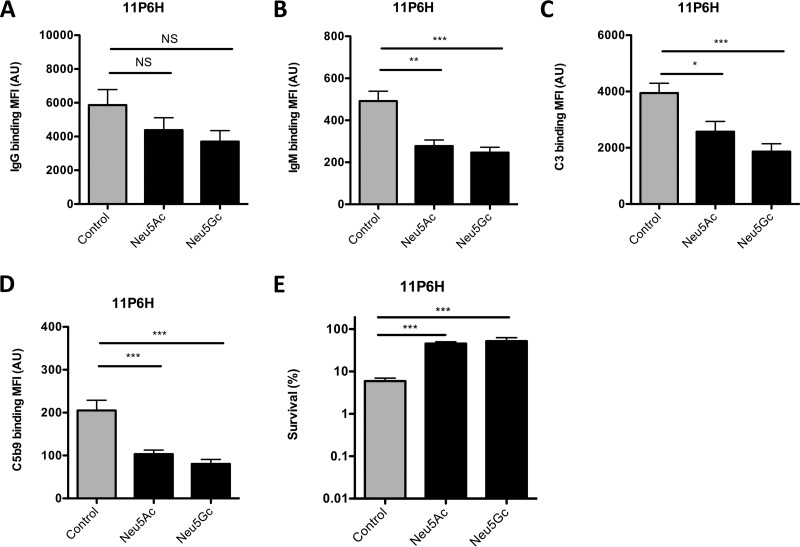
Neu5Ac and Neu5Gc increase resistance to complement-mediated killing.
In addition to Neu5Ac, NTHi is able to incorporate Neu5Gc, a sialic acid variant found in most nonhuman mammals (19). We determined the effect of growing NTHi 11P6H with Neu5Ac or Neu5Gc on recognition by antibodies and complement-mediated killing.
In accordance with our previous findings, growth of NTHi 11P6H with Neu5Ac decreased antibody binding, mainly IgM, to the bacterial surface, resulting in decreased complement deposition and complement-mediated killing (Fig. 5A to E). Similarly, growth of NTHi 11P6H with Neu5Gc decreased binding of IgG but mainly that of IgM, resulting in an even more pronounced decrease in complement deposition and increased serum survival (Fig. 5A to E).
FIG 5.
Neu5Ac and Neu5Gc decrease antibody binding, complement activation, and complement-mediated killing. NTHi 11P6H bacteria grown alone or with 1 mM Neu5Ac or 1 mM Neu5Gc were incubated with 10% HI serum (IgG/IgM) or 10% NHS (C3/C5b9) in HBSS3+ for 30 min, and surface binding of IgG (A), IgM (B), C3 (C), or C5b9 (D) was determined by flow cytometry (n = 6). (E) NTHi 11P6H bacteria grown alone or with 1 mM Neu5Ac or 1 mM Neu5Gc were incubated with 5% serum or 5% HI serum in HBSS3+ for 1 h, and survival was determined by dividing the CFU counts in serum by the CFU count in HI serum after 1 h of incubation (n = 4). One-way analysis of variance with Dunnett’s multiple-comparison test was used for statistical analysis. *, P < 0.05; **, P < 0.01; ***, P < 0.001; NS, not significant.
DISCUSSION
In this study, we investigated the mechanism underlying sialic acid-mediated complement resistance. Our findings provide compelling evidence that uptake of sialic acid has a significant effect on complement-mediated killing through decreased binding of IgM to the bacterial surface.
The magnitude of sialic acid-mediated complement resistance differed from strain to strain. Large effects were seen for strains 11P6H, 3655, and 86-028NP, whereas only a modest effect was seen for strain R2866, which might be due to its intrinsic resistance to complement-mediated killing due to other complement evasion mechanisms, such as a complex LOS structure preventing IgM antibody binding and factor H binding to outer membrane protein P5 (10, 20–22). By measuring incorporation of Neu5Az on the bacterial surface, detected Neu5Az incorporation was high for NTHi strain 11P6H, modest for 86-028NP, and lowest for 3655 and R2866, which supports the largest effect of sialic acid on complement binding and complement-mediated killing of NTHi strain 11P6H. These strain-dependent effects of sialic acid on complement resistance are in agreement with the study of Hood et al., who showed increased serum resistance with NTHi strain 477 when grown with sialic acid; however, they did not observe this effect for NTHi strain 375 (8). Deletion of the sialic acid transporter siaP abrogated the effect of sialic acid to increase resistance to complement-mediated killing. However, we were not able to complement the 11P6HΔsiaP mutant; therefore, we cannot rigorously claim that the lack of transport of sialic acids mediates the phenotype.
Flow cytometric analysis revealed no differences in binding of fH to NTHi strains grown with or without sialic acid. These data are in contrast to those of studies using N. gonorrhoeae and H. somnus, where the presence of sialic acid increases binding of fH to the bacterial surface (17, 18). Apparently, different sialic acid-mediated mechanisms have evolved to escape complement-mediated killing.
While identifying the determinants of sialic acid-mediated complement resistance, clear differences in IgM binding to the bacterial surface were detected between strains grown with or without sialic acid. The reason for this decreased binding of IgM remains elusive, but it has major effects on complement-mediated killing of the bacteria. Incorporation of sialic acid by N. gonorrhoeae was shown to decrease bactericidal antibody binding, but antibody isotypes were not determined in this study (23). In later experiments by de la Paz and coworkers, masking of LOS target sites on N. gonorrhoeae for particularly bactericidal IgM antibodies was postulated to be an important mechanism for sialic acid-mediated complement resistance (24). Unencapsulated N. meningitidis, unable to incorporate sialic acids, showed increased binding of IgM to the bacterial surface, which was associated with increased complement C3 binding and complement-mediated killing (25). In accordance with these data, we found that NTHi bacteria grown without Neu5Ac show increased binding of IgM to the bacterial surface compared to strains grown with Neu5Ac, resulting in more complement activation and complement-mediated killing.
Neu5Ac is a precursor for N-glycolylneuraminic acid (Neu5Gc), but humans are not able to produce Neu5Gc due to a mutation in the CMP-Neu5Ac hydrolase enzyme CMAH (26). NTHi was shown to incorporate Neu5Gc and induce anti-Neu5Gc antibodies in mice (19), and these antibodies have been found in the blood of humans in various levels (27). We found that both Neu5Ac and Neu5Gc decreased antibody binding to the bacterial surface, resulting in decreased complement-mediated killing. Because antibody binding to the bacterial surface was low for NTHi 11P6H grown with Neu5Gc, it is likely that the pooled NHS serum in these experiments lacks or had very low levels of anti-Neu5Gc antibodies. In individual sera that contain a decent level of anti-Neu5Gc antibodies, incorporation of Neu5Gc might increase antibody-dependent complement-mediated killing; however, we have not been able to address this in our experiments.
NTHi bacteria have evolved multiple mechanisms to prevent binding of IgM to its bacterial surface. Outer membrane protein P5, which was also seen to bind human factor H (21, 22), was shown to decrease binding of IgM by unknown mechanisms (22). Modification of the LOS was shown to decrease binding of IgM, resulting in lowered complement and opsonophagocytic killing (21, 28). The clinical relevance of NTHi recognition by IgM was demonstrated in patients with hyper-IgM syndrome. In this study, patients with hyper-IgM syndrome were shown to be more protected against NTHi colonization of the respiratory tract (29). Therefore, mechanisms that lower binding of IgM to the bacterial surface, such as the incorporation of sialic acid, might be important not only for its pathogenic potential but also to successfully colonize the human upper respiratory tract.
In conclusion, this study confirmed that uptake of sialic acid resulted in increased complement resistance. This sialic acid-mediated complement resistance was associated with decreased binding of IgM to the bacterial surface. Strategies that interfere with uptake or incorporation of sialic acids into the LOS, such as novel antibiotics and vaccines, might be useful to prevent or treat NTHi infections.
MATERIALS AND METHODS
Ethics statement.
Serum from an agammaglobulinemia patient on IgG replacement therapy and C6-deficient serum from a patient with a compound heterozygous C6 mutation were obtained for this study, which was approved by the ethics committee of the Radboud University Medical Center, Nijmegen, the Netherlands.
Neu5Gc synthesis.
Synthesis of N-glycolylneuraminic acid (Neu5Gc) was performed as described previously (30). In short, d-mannosamine hydrochloride (ManHCl) was converted into N-glycolyl-d-mannosamine (ManGc) by a reaction with acetoxyacetyl chloride under mild basic conditions. Finally, ManGc was converted into Neu5Gc using sialic acid aldolase. Spectral data were identical to those previously reported.
Bacterial strains and growth conditions.
NTHi strains 11P6H, 3655, 86-028NP, and R2866 were used in this study (Table 1). Strains were cultured overnight at 37°C in 5% CO2 on brain heart infusion (BHI) agar plates supplemented with 1 μg/ml hemin (Sigma-Aldrich) and 2 μg/ml β-NAD (Merck) (sBHI). Bacteria were grown in supplemented RPMI medium (31) with or without addition of 100 μM N-azidoacetylneuraminic acid (Neu5Az) (32), 1 mM N-acetylneuraminic acid (Neu5Ac) (Carbosynth), or 1 mM Neu5Gc at 37°C in 5% CO2 to an optical density at 620 nm (OD620) of 0.5. Aliquots with 16% glycerol were stored at −80°C for further experiments.
TABLE 1.
Characteristics of NTHi strains used in this study
| Strain | Description | Reference or source |
|---|---|---|
| 11P6H | Clinical isolate from an adult during an acute exacerbation of COPD | 13 |
| 11P6HΔsiaP | 11P6H with siaP gene replaced by spectinomycin resistance cassette | This study |
| 3655 | Clinical isolate from a 10-yr-old child with acute otitis media with effusion | 14 |
| 86-028NP | Clinical isolate from a child with chronic otitis media | 16 |
| 86-028NPΔsiaP | 86-028NP with siaP gene replaced by spectinomycin resistance cassette | 32 |
| R2866 | Clinical isolate from a young child with meningitis following acute otitis media | 15 |
Serum sources.
Pooled normal human serum (NHS) was obtained from Immucor (catalog no. PHS-N100, lot 2148U). Serum from an agammaglobulinemia patient on IgG replacement therapy was used as IgA/IgM-deficient serum (33). Purified IgM from human serum (I8260; Sigma-Aldrich) was washed with phosphate-buffered saline (PBS) on an Amicon Ultra-0.5 centrifugal filter unit column (Millipore) to remove the preservative sodium azide and suspended into PBS at a concentration of 1 mg/ml. C6-deficient serum was obtained from a patient with a compound heterozygous C6 mutation (34), and purified C6 was from Complement Technologies. Factor D-deficient serum was from a patient with two homozygous factor D mutations (35). C1q-depleted serum and purified C1q were from Complement Technologies.
Serum survival assay.
NTHi stocks were thawed, washed once with PBS, and diluted to an OD620 of 0.1 in PBS and diluted 10,000-fold in Hanks balanced salt solution plus Ca2+/Mg2+ and 0.1% gelatin (HBSS3+) to obtain a concentration of ∼200,000 CFU/ml. Fifty μl bacteria was mixed with 50 μl serum or heat-inactivated (HI) serum diluted in HBSS3+ and incubated for 1 h at 37°C. Samples were diluted 10- and 100-fold with PBS, and three droplets of 20 μl of the undiluted, 10-fold-diluted, and 100-fold-diluted bacteria were plated on sBHI plates and grown overnight at 37°C and 5% CO2. Survival was determined by dividing the CFU counts in NHS by the CFU count in HI serum after 1 h of incubation and is presented as a percentage.
Sytox assay.
NTHi stocks were thawed, washed once with HBSS3+, and diluted to an OD620 of 0.4 with HBSS3+. Bacteria were mixed 1:1 with Sytox green diluted 1:1,250. The sample next was mixed 1:1 with 20% NHS or 400 μg/ml polymyxin B in HBSS3+ to obtain a final OD620 of 0.1, 1:5,000 Sytox green, and 10% NHS or 200 μg/ml polymyxin B. Fluorescence intensity was measured for 1 h every minute with a Tecan Spark at 37°C with the following settings: gain, 30; z-axis, 18,000; excitation wavelength, 485 nm; and emission wavelength, 530 nm. Relative fluorescence intensity was determined by dividing the fluorescence intensity of the NHS by the fluorescence intensity of polymyxin B and is presented as a percentage. The area under the curve was determined for statistical analysis.
CuAAC and flow cytometry analysis.
To detect Neu5Az on the bacterial surface, bacteria were reacted for 20 min at 37°C with click buffer (250 μM CuSO4, 200 μM l-histidine, 500 μM sodium ascorbate in PBS) containing 50 μM alkyne-PEG4-biotin conjugate (Sigma-Aldrich) and stained with streptavidin-phycoerythrin (Strep-PE) for 10 min at room temperature. Bacteria without copper-catalyzed alkyne-azide cycloaddition (CuAAC) reaction were used as an antibody control. Bacteria were fixed in 2% paraformaldehyde and taken up in PBS for flow cytometry analysis. Fluorescence was determined by flow cytometry using a fluorescence-activated cell sorter (FACS) LSR II instrument (BD Biosciences) and expressed as mean fluorescence intensity (MFI) in arbitrary units (AU). Data were analyzed by using FlowJo, version 10.4.1. Representative histograms for flow cytometry are depicted in the supplemental material.
Flow cytometric analysis.
NTHi stocks were thawed, washed once with HBSS3+, and diluted to an OD620 of 0.1 with HBSS3+. Fifty μl bacteria was mixed with 50 μl HBSS3+, serum (C3 and C5b9), or HI serum (IgG, IgM, CRP, and fH) in HBSS3+ and incubated for 30 min at 37°C. Bacteria were pelleted by centrifugation at 3,200 × g and fixed for 20 min in 2% paraformaldehyde in PBS at room temperature. Bacteria were washed with PBS, and all antibody incubations were performed in PBS plus 2% bovine serum albumin (BSA) for 15 min at room temperature. Surface-bound complement C3 and C5b9 complex were detected with 1:500-diluted fluorescein isothiocyanate (FITC)-labeled polyclonal goat anti-human C3 (MP biomedicals) and 1:100-diluted C5b9-specific monoclonal (clone aE11) antibody (Santa Cruz Biotechnology), followed by staining with 1:200 PE-labeled goat anti-mouse IgG (ThermoFisher Scientific). Surface-bound IgG or IgM was detected with 1:100-diluted FITC-labeled polyclonal goat anti-human IgG (Sigma-Aldrich) or FITC-labeled polyclonal goat anti-human IgM (Sigma-Aldrich), respectively. Surface-bound CRP was detected with 1:100-diluted polyclonal goat anti-human CRP (Acris Antibodies) followed by staining with 1:200-diluted FITC-labeled polyclonal donkey anti-goat IgG (Thermo-Fisher). Surface-bound fH was detected with 1:100-diluted polyclonal sheep anti-human fH (Abcam), followed by staining with 1:200-diluted FITC-labeled polyclonal donkey anti-sheep IgG (Jackson ImmunoResearch). Bacteria incubated with HBSS3+ were used as antibody control samples. Surface binding of C3, C5b9, IgG, IgM, CRP, and fH was determined by flow cytometry using a FACS LSR II instrument (BD Biosciences) and expressed as MFI in AU. Data were analyzed by using FlowJo, version 10.4.1. Representative histograms for flow cytometry are depicted in the supplemental material.
Generation of 11P6HΔsiaP gene deletion mutants.
A directed 11P6HΔsiaP gene deletion mutant was generated by allelic exchange of the target gene with a spectinomycin resistance cassette. Spectinomycin resistance cassettes with ∼1,000-bp flanking sequences were amplified from the 86-028NPΔsiaP strain with primers R2866_0441_L1 (5′-TCAACAGAATTGACCGCACT-3′) and R2866_0441_R1 (5′-CTATGGCTTGGCAGGCTTAC-3′) (32). 11P6HΔsiaP gene deletion mutants were obtained by transformation of the PCR product by the method of Herriott et al. (36) and selected by plating on sBHI agar plates containing 150 μg/ml of spectinomycin. The gene deletion mutant was validated by PCR with primer sets R2866_0441_L1 + R2866_0441_C (5′-GGAAAGATCCTTGACCAGCTT-3′) and R2866_0441_L1 + PBMrTn9 (5′-CAATGGTTCAGATACGACGAC-3′) (37), which control for the presence of the gene or spectinomycin cassette, respectively. Gene deletions were crossed back to the WT strain by using chromosomal DNA of the mutant strains as the donor during transformation.
Statistical analysis.
Statistical analyses were performed with GraphPad Prism, version 5.03 for Windows (GraphPad Software, Inc.). For survival experiments, percentages were log10 transformed for statistical analysis. Differences were considered significant at a P value of <0.05. The specific statistical tests that were used for the various experiments are specified in the figure legends.
Supplementary Material
ACKNOWLEDGMENTS
M.M.P.O. and J.D.L. designed the experiments, performed the experiments, and interpreted the data. M.M.P.O., M.I.D.J., and J.D.L. wrote the manuscript. We declare that we have no competing financial interests.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00077-19.
REFERENCES
- 1.Van Eldere J, Slack MP, Ladhani S, Cripps AW. 2014. Non-typeable Haemophilus influenzae, an under-recognised pathogen. Lancet Infect Dis 14:1281–1292. doi: 10.1016/S1473-3099(14)70734-0. [DOI] [PubMed] [Google Scholar]
- 2.Figueira MA, Ram S, Goldstein R, Hood DW, Moxon ER, Pelton SI. 2007. Role of complement in defense of the middle ear revealed by restoring the virulence of nontypeable Haemophilus influenzae siaB mutants. Infect Immun 75:325–333. doi: 10.1128/IAI.01054-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouchet V, Hood DW, Li J, Brisson JR, Randle GA, Martin A, Li Z, Goldstein R, Schweda EK, Pelton SI, Richards JC, Moxon ER. 2003. Host-derived sialic acid is incorporated into Haemophilus influenzae lipopolysaccharide and is a major virulence factor in experimental otitis media. Proc Natl Acad Sci U S A 100:8898–8903. doi: 10.1073/pnas.1432026100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varki A, Schauer R. 2009. Sialic acids In Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME (ed), Essentials of glycobiology. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [PubMed] [Google Scholar]
- 5.Apicella MA. 2012. Nontypeable Haemophilus influenzae: the role of N-acetyl-5-neuraminic acid in biology. Front Cell Infect Microbiol 2:19. doi: 10.3389/fcimb.2012.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Severi E, Randle G, Kivlin P, Whitfield K, Young R, Moxon R, Kelly D, Hood D, Thomas GH. 2005. Sialic acid transport in Haemophilus influenzae is essential for lipopolysaccharide sialylation and serum resistance and is dependent on a novel tripartite ATP-independent periplasmic transporter. Mol Microbiol 58:1173–1185. doi: 10.1111/j.1365-2958.2005.04901.x. [DOI] [PubMed] [Google Scholar]
- 7.Mandrell RE, McLaughlin R, Aba Kwaik Y, Lesse A, Yamasaki R, Gibson B, Spinola SM, Apicella MA. 1992. Lipooligosaccharides (LOS) of some Haemophilus species mimic human glycosphingolipids, and some LOS are sialylated. Infect Immun 60:1322–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hood DW, Makepeace K, Deadman ME, Rest RF, Thibault P, Martin A, Richards JC, Moxon ER. 1999. Sialic acid in the lipopolysaccharide of Haemophilus influenzae: strain distribution, influence on serum resistance and structural characterization. Mol Microbiol 33:679–692. doi: 10.1046/j.1365-2958.1999.01509.x. [DOI] [PubMed] [Google Scholar]
- 9.Parsons NJ, Patel PV, Tan EL, Andrade JR, Nairn CA, Goldner M, Cole JA, Smith H. 1988. Cytidine 5'-monophospho-N-acetyl neuraminic acid and a low molecular weight factor from human blood cells induce lipopolysaccharide alteration in gonococci when conferring resistance to killing by human serum. Microb Pathog 5:303–309. doi: 10.1016/0882-4010(88)90103-9. [DOI] [PubMed] [Google Scholar]
- 10.Langereis JD, Stol K, Schweda EK, Twelkmeyer B, Bootsma HJ, de Vries SP, Burghout P, Diavatopoulos DA, Hermans PW. 2012. Modified lipooligosaccharide structure protects nontypeable Haemophilus influenzae from IgM-mediated complement killing in experimental otitis media. mBio 3:e00079-12. doi: 10.1128/mBio.00079-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura S, Shchepetov M, Dalia AB, Clark SE, Murphy TF, Sethi S, Gilsdorf JR, Smith AL, Weiser JN. 2011. Molecular basis of increased serum resistance among pulmonary isolates of non-typeable Haemophilus influenzae. PLoS Pathog 7:e1001247. doi: 10.1371/journal.ppat.1001247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langereis JD, Cremers AJH, Vissers M, van Beek J, Meis JF, de Jonge MI. 2018. Non-typeable Haemophilus influenzae invasive blood isolates are mainly phosphorylcholine negative and show decreased complement-mediated killing in comparison to colonizing isolates from the oropharynx, which is associated with lower binding of IgM and CRP to the bacterial surface. Infect Immun 87:e00604-18. doi: 10.1128/IAI.00604-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yi K, Sethi S, Murphy TF. 1997. Human immune response to nontypeable Haemophilus influenzae in chronic bronchitis. J Infect Dis 176:1247–1252. doi: 10.1086/514119. [DOI] [PubMed] [Google Scholar]
- 14.Musser JM, Barenkamp SJ, Granoff DM, Selander RK. 1986. Genetic relationships of serologically nontypable and serotype b strains of Haemophilus influenzae. Infect Immun 52:183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams BJ, Morlin G, Valentine N, Smith AL. 2001. Serum resistance in an invasive, nontypeable Haemophilus influenzae strain. Infect Immun 69:695–705. doi: 10.1128/IAI.69.2.695-705.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki K, Bakaletz LO. 1994. Synergistic effect of adenovirus type 1 and nontypeable Haemophilus influenzae in a chinchilla model of experimental otitis media. Infect Immun 62:1710–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ram S, Sharma AK, Simpson SD, Gulati S, McQuillen DP, Pangburn MK, Rice PA. 1998. A novel sialic acid binding site on factor H mediates serum resistance of sialylated Neisseria gonorrhoeae. J Exp Med 187:743–752. doi: 10.1084/jem.187.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inzana TJ, Balyan R, Howard MD. 2012. Decoration of Histophilus somni lipooligosaccharide with N-acetyl-5-neuraminic acid enhances bacterial binding of complement factor H and resistance to killing by serum and polymorphonuclear leukocytes. Vet Microbiol 161:113–121. doi: 10.1016/j.vetmic.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Taylor RE, Gregg CJ, Padler-Karavani V, Ghaderi D, Yu H, Huang S, Sorensen RU, Chen X, Inostroza J, Nizet V, Varki A. 2010. Novel mechanism for the generation of human xeno-autoantibodies against the nonhuman sialic acid N-glycolylneuraminic acid. J Exp Med 207:1637–1646. doi: 10.1084/jem.20100575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erwin AL, Allen S, Ho DK, Bonthius PJ, Jarisch J, Nelson KL, Tsao DL, Unrath WCT, Watson ME, Gibson BW, Apicella MA, Smith AL. 2006. Role of lgtC in resistance of nontypeable Haemophilus influenzae strain R2866 to human serum. Infect Immun 74:6226–6235. doi: 10.1128/IAI.00722-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langereis JD, de Jonge MI, Weiser JN. 2014. Binding of human factor H to outer membrane protein P5 of non-typeable Haemophilus influenzae contributes to complement resistance. Mol Microbiol 94:89–106. doi: 10.1111/mmi.12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosadini CV, Ram S, Akerley BJ. 2014. Outer membrane protein P5 is required for resistance of nontypeable Haemophilus influenzae to both the classical and alternative complement pathways. Infect Immun 82:640–649. doi: 10.1128/IAI.01224-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parsons NJ, Andrade JR, Patel PV, Cole JA, Smith H. 1989. Sialylation of lipopolysaccharide and loss of absorption of bactericidal antibody during conversion of gonococci to serum resistance by cytidine 5'-monophospho-N-acetyl neuraminic acid. Microb Pathog 7:63–72. doi: 10.1016/0882-4010(89)90112-5. [DOI] [PubMed] [Google Scholar]
- 24.de la Paz H, Cooke SJ, Heckels JE. 1995. Effect of sialylation of lipopolysaccharide of Neisseria gonorrhoeae on recognition and complement-mediated killing by monoclonal antibodies directed against different outer-membrane antigens. Microbiology 141:913–920. doi: 10.1099/13500872-141-4-913. [DOI] [PubMed] [Google Scholar]
- 25.Vogel U, Weinberger A, Frank R, Muller A, Kohl J, Atkinson JP, Frosch M. 1997. Complement factor C3 deposition and serum resistance in isogenic capsule and lipooligosaccharide sialic acid mutants of serogroup B Neisseria meningitidis. Infect Immun 65:4022–4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chou HH, Takematsu H, Diaz S, Iber J, Nickerson E, Wright KL, Muchmore EA, Nelson DL, Warren ST, Varki A. 1998. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc Natl Acad Sci U S A 95:11751–11756. doi: 10.1073/pnas.95.20.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Padler-Karavani V, Yu H, Cao H, Chokhawala H, Karp F, Varki N, Chen X, Varki A. 2008. Diversity in specificity, abundance, and composition of anti-Neu5Gc antibodies in normal humans: potential implications for disease. Glycobiology 18:818–830. doi: 10.1093/glycob/cwn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langereis JD, Weiser JN. 2014. Shielding of a lipooligosaccharide IgM epitope allows evasion of neutrophil-mediated killing of an invasive strain of nontypeable Haemophilus influenzae. mBio 5:e01478-14. doi: 10.1128/mBio.01478-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Micol R, Kayal S, Mahlaoui N, Beauté J, Brosselin P, Dudoit Y, Obenga G, Barlogis V, Aladjidi N, Kebaili K, Thomas C, Dulieu F, Monpoux F, Nové-Josserand R, Pellier I, Lambotte O, Salmon A, Masseau A, Galanaud P, Oksenhendler E, Tabone M-D, Teira P, Coignard-Biehler H, Lanternier F, Join-Lambert O, Mouillot G, Theodorou I, Lecron J-C, Alyanakian M-A, Picard C, Blanche S, Hermine O, Suarez F, Debré M, Lecuit M, Lortholary O, Durandy A, Fischer A. 2012. Protective effect of IgM against colonization of the respiratory tract by nontypeable Haemophilus influenzae in patients with hypogammaglobulinemia. J Allergy Clin Immunol 129:770–777. doi: 10.1016/j.jaci.2011.09.047. [DOI] [PubMed] [Google Scholar]
- 30.Pearce OM, Varki A. 2010. Chemo-enzymatic synthesis of the carbohydrate antigen N-glycolylneuraminic acid from glucose. Carbohydr Res 345:1225–1229. doi: 10.1016/j.carres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coleman HN, Daines DA, Jarisch J, Smith AL. 2003. Chemically defined media for growth of Haemophilus influenzae strains. J Clin Microbiol 41:4408–4410. doi: 10.1128/JCM.41.9.4408-4410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heise T, Langereis JD, Rossing E, de Jonge MI, Adema GJ, Bull C, Boltje TJ. 2018. Selective inhibition of sialic acid-based molecular mimicry in Haemophilus influenzae abrogates serum resistance. Cell Chem Biol 25:1279–1285. doi: 10.1016/j.chembiol.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 33.Langereis JD, Henriet SS, Kuipers S, Weemaes CMR, van der Burg M, de Jonge MI, van der Flier M. 2018. IgM augments complement bactericidal activity with serum from a patient with a novel CD79a mutation. J Clin Immunol 38:185–192. doi: 10.1007/s10875-017-0474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Westra D, Kurvers RA, van den Heuvel LP, Wurzner R, Hoppenreijs EP, van der Flier M, van de Kar NC, Warris A. 2014. Compound heterozygous mutations in the C6 gene of a child with recurrent infections. Mol Immunol 58:201–205. doi: 10.1016/j.molimm.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 35.Sprong T, Roos D, Weemaes C, Neeleman C, Geesing CL, Mollnes TE, van Deuren M. 2006. Deficient alternative complement pathway activation due to factor D deficiency by 2 novel mutations in the complement factor D gene in a family with meningococcal infections. Blood 107:4865–4870. doi: 10.1182/blood-2005-07-2820. [DOI] [PubMed] [Google Scholar]
- 36.Herriott RM, Meyer EM, Vogt M. 1970. Defined nongrowth media for stage II development of competence in Haemophilus influenzae. J Bacteriol 101:517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Vries SP, Burghout P, Langereis JD, Zomer A, Hermans PW, Bootsma HJ. 2013. Genetic requirements for Moraxella catarrhalis growth under iron-limiting conditions. Mol Microbiol 87:14–29. doi: 10.1111/mmi.12081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.