Plasmodium falciparum cell-traversal protein for ookinetes and sporozoites (PfCelTOS) is an advanced vaccine candidate that has a crucial role in the traversal of the malaria parasite in both mosquito and mammalian hosts. As recombinant purified proteins are normally poor immunogens, they require to be admixed with an adjuvant(s); therefore, the objective of the present study was to evaluate the capacity of different vaccine adjuvants, monophosphoryl lipid A (MPL), CpG, and Quillaja saponaria Molina fraction 21 (QS-21), alone or in combination (MCQ [MPL/CpG/QS-21]), to enhance the immunogenicity of Escherichia coli-expressed PfCelTOS in BALB/c mice.
KEYWORDS: CpG, MPL, PfCelTOS, Plasmodium falciparum, QS-21, adjuvants, malaria, vaccine
ABSTRACT
Plasmodium falciparum cell-traversal protein for ookinetes and sporozoites (PfCelTOS) is an advanced vaccine candidate that has a crucial role in the traversal of the malaria parasite in both mosquito and mammalian hosts. As recombinant purified proteins are normally poor immunogens, they require to be admixed with an adjuvant(s); therefore, the objective of the present study was to evaluate the capacity of different vaccine adjuvants, monophosphoryl lipid A (MPL), CpG, and Quillaja saponaria Molina fraction 21 (QS-21), alone or in combination (MCQ [MPL/CpG/QS-21]), to enhance the immunogenicity of Escherichia coli-expressed PfCelTOS in BALB/c mice. This goal was achieved by the assessment of anti-PfCelTOS IgG antibodies (level, titer, IgG isotype profile, avidity, and persistence) and extracellular Th1 cytokines using an enzyme-linked immunosorbent assay (ELISA) on postimmunized BALB/c mouse sera and PfCelTOS-stimulated splenocytes, respectively. Also, an assessment of the transmission-reducing activity (TRA) of anti-PfCelTOS obtained from different vaccine groups was carried out in female Anopheles stephensi mosquitoes by using a standard membrane feeding assay (SMFA). In comparison to PfCelTOS alone, administration of PfCelTOS with three distinct potent Th1 adjuvants in vaccine mouse groups showed enhancement and improvement of PfCelTOS immunogenicity that generated more bias toward a Th1 response with significantly enhanced titers and avidity of the anti-PfCelTOS responses that could impair ookinete development in A. stephensi. However, immunization of mice with PfCelTOS with MCQ mixture adjuvants resulted in the highest levels of induction of antibody titers, avidity, and inhibitory antibodies in oocyst development (88%/26.7% reductions in intensity/prevalence) in A. stephensi. It could be suggested that adjuvant combinations with different mechanisms stimulate better functional antibody responses than adjuvants individually against challenging diseases such as malaria.
INTRODUCTION
Malaria remains a significant disease in most tropical countries, and despite the application of different key interventions to control malaria, such as prompt diagnosis, effective treatment with artemisinin-based combination therapies, and the use of insecticidal bed nets, the disease is still a major threat to global health. Most deaths, particularly in sub-Saharan Africa, are caused by Plasmodium falciparum, a dangerous species of Plasmodia infecting humans (1). Malaria control and elimination programs, however, are being hampered by the resistance of parasites and mosquito vectors to drugs and insecticides, respectively (2, 3). Therefore, novel strategies are urgently needed to permanently interrupt malaria transmission, which causes hundreds of millions of clinical cases each year. In this regard, from past malaria eradication attempts, it is obvious that there is a critical need for effective malaria vaccines to achieve malaria elimination and eradication success (4). Such a vaccine should involve not only a classical transmission-blocking vaccine (TBV) (which targets antigens from gametes, zygotes, or ookinetes) but also preerythrocytic and asexual blood-stage vaccines that are capable of reducing sexual stages. With the renewed emphasis on malaria elimination and eradication, the primary focus of malaria vaccine development is now vaccines that interrupt malaria transmission (VIMT) (5).
The malaria parasite has a complex life cycle in both human and mosquito hosts, and once the parasite changes hosts, it develops motile stages that can invade human host or vector cells to complete the essential segments of a complicated life cycle. The P. falciparum cell-traversal protein of ookinetes and sporozoites (PfCelTOS) gene, as an interesting preerythrocytic target vaccine antigen, was first identified from P. falciparum genomic sequence and proteome databases, and it was recognized by volunteers immunized with radiation-attenuated P. falciparum sporozoites (6). Later on, its crucial role in the traversal of the malaria parasite in both mosquito and mammalian hosts, which is required for successful malaria infections, was reported by Kariu and coworkers (7), and its potential as a vaccine candidate antigen was then shown in a murine animal model (8). Thus, this 25-kDa microneme-secreted protein with its biological function may be an attractive target for both classical TBV and preerythrocytic (7) vaccines (VIMT). In general, targeting antigens expressed in different stages is more effective from a vaccine point of view because broad immune coverage inhibits immune escape of parasites at different stages. As the CelTOS protein is highly conserved (7), it could induce broadly protective immunity against multiple Plasmodium species in a single-subunit vaccine.
CelTOS-based vaccines have been revealed to induce potent antibody and T cell responses in experimental animal models (8–11) and could prevent the establishment of blood-stage infection in mice (7–9) and oocyst development in mosquito hosts (11). In spite of a previous report of cross-species protection in a murine animal model (8), by using transgenic Plasmodium berghei parasites expressing the Pfceltos gene, no sterile protection or delay in the time to parasitemia was observed in BALB/c mice (12, 13). Moreover, cellular interferon gamma (IFN-γ) responses against CelTOS have been detected in adults from Ghana who were naturally infected with P. falciparum, which emphasizes the potential immunological impact of this antigen (14). It has also been reported that all human volunteers who were immunized with radiation-attenuated sporozoites induced strong immune responses to CelTOS, which was correlated with protection against disease (6, 15). Although early evidence in preclinical animal studies is encouraging, the potential for efficacy induced by a PfCelTOS-based vaccine in humans remains to be realized (16). This antigen was tested in a first-in-human clinical trial; while safe and immunogenic, no protective efficacy was observed in controlled human malaria infection (CHMI) (16). Moreover, a phase Ia trial with PfCelTOS in GSK’s AS01B adjuvant (Clinicaltrials.gov identifier NCT02174978) (16) is currently ongoing, for which results are being awaited. Therefore, it seems that the PfCelTOS subunit vaccine is not able to provide absolute protection, and improvements in the immunogenicity and productivity of this antigen possibly need the potent formulation of CelTOS-based vaccines.
Adjuvants are becoming increasingly more important for the development of a new generation of malaria subunit vaccines and are capable of enhancing the immunogenicity of subunit proteins by different mechanisms (17). One of these vaccine adjuvants is CpG, a synthetic oligodeoxynucleotide (ODN) composed of unmethylated CG motifs (cytosine phosphoguanosine dinucleotides common in bacteria and viruses) that is recognized by endosomal Toll-like receptor 9 (TLR9) (18, 19). CpG motifs mediate their immunostimulatory capacity through TLR9 expressed on dendritic cells (DCs) to secrete proinflammatory (interleukin-6 [IL-6], IL-12, IL-18, and tumor necrosis factor alpha [TNF-α]) and Th1-type cytokines (20, 21). Moreover, they indirectly support the maturation and proliferation of natural killer cells, T cells, and monocytes/macrophages; hence, they have a high potential for inducing innate immunity as well as specific humoral, Th1-biased CD4+ T cell, and cytotoxic T lymphocyte (CTL) responses (22). Clinical trials have indicated that CpG ODNs are reasonably safe when administered as vaccine adjuvants (23) and could enhance the immunogenicity of subunit protein antigens and peptide-based vaccines. Moreover, in a clinical trial of the commercial hepatitis B vaccine combined with CpG, higher protective antibody titers were observed after the administration of fewer doses in both healthy and hyporesponsive individuals (24, 25). This vaccine adjuvant has also been used in combination with conventional treatments for cancer (26–28) as well as against infection (28) and allergy (29).
An additional vaccine adjuvant is monophosphoryl lipid A (MPL), which is the detoxified derivative of lipopolysaccharide (LPS) from the Gram-negative bacterium Salmonella enterica serovar Minnesota R595 and mediates immune activation by interacting with TLR4, similar to LPS (30), which triggers the production of different cytokines, such as TNF-α, IL-12, and IFN-γ, that promote Th1 responses. MPL has been approved for use as a part of vaccines against allergy (31) and stage IV melanoma (32). In malaria clinical trials, strategies of combining MPL with other adjuvants, like alum (33) and Quillaja saponaria Molina fraction 21 (QS-21), have been explored and resulted in producing adjuvant systems (ASs), such as AS04, AS02, AS01, AS01B, and AS02A (34), which are safe and well tolerated (35).
QS-21 is the most widely used adjuvant in vaccine formulations (36); it is a purified fraction of Quillaja saponaria saponin with low toxicity in animal models (37). It has the ability to stimulate both antigen-specific humoral (more specifically, IgG2a isotype) and CTL immune responses and also to promote Th1 cytokine responses (IL-2 and IFN-γ) (38) to subunit antigens (39, 40) by altering the integrity of the target immune cell membrane and inducing danger signals that augment immune responses (41). Clinical trials for the evaluation of QS-21, as an adjuvant alone or in combination with other immunostimulants (e.g., AS01 and AS02) for a vaccine against malaria, are ongoing (42, 43). Clinical trials involving breast cancer or prostate cancer patients have shown that QS-21 is a well-tolerated and immunogenic adjuvant capable of inducing antigen-specific antibody responses (40, 44, 45). Meanwhile, current efforts are being made to develop optimal combinations of QS-21 with different adjuvants (such as MPL and CpG ODN) in cancer vaccines (40, 44).
Purified recombinant subunit proteins are normally poor immunogens, thus requiring to be admixed with an adjuvant(s) to enhance their immunogenicity (46, 47). In addition, effective protection against different stages of human Plasmodium infection requires distinct types of immune responses; therefore, the role of adjuvants is of great importance. Adjuvants are critical components of many subunit malaria vaccines, and it seems that no single adjuvant is capable of inducing all the protective immune responses required in many malarial subunit vaccines. In the light of this fact, the combination adjuvant approach was considered an avenue toward designing effective vaccines against difficult pathogens such as malaria. On this basis, GlaxoSmithKline Biologicals (GSK Bio) developed a series of ASs to induce quicker and stronger, as well as prolonged and protected, immune responses to a subunit vaccine (48). Such formulations could also trigger multiple signaling pathways (36, 49) to elevate the potency, longevity, and quality of specific immune responses to target antigens. Therefore, the objective of the present study was to evaluate the capacity of three different human-use-compatible vaccine adjuvants, MPL, CpG, and QS-21, alone or in combination (MCQ [MPL/CpG/QS-21]), to improve and enhance the immunogenicity of Escherichia coli-expressed PfCelTOS in BALB/c mice. This goal was achieved by the assessment of the anti-PfCelTOS IgG antibody titer, its isotype profile, its avidity, and its persistence, as well as the production of the cytokines IFN-γ, TNF-α, IL-4, and IL-10, in the postimmunized BALB/c mouse. Finally, the induction of high levels of functional anti-PfCelTOS IgG, which were able to effectively inhibit the parasite transmission cycle in the female Anopheles stephensi mosquito vector, was assessed using a standard membrane feeding assay (SMFA).
RESULTS
Anti-PfCelTOS IgG antibody responses and persistence of IgG responses.
The PfCelTOS protein as an antigen was successfully expressed in the E. coli BL21/pET23a expression system after 16 h of induction using 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at an optical density at 600 nm (OD600) of 0.6 to 0.8. The lysate of recombinant bacteria on SDS-PAGE gels indicated an ∼20-kDa protein. Furthermore, the purity of PfCelTOS was confirmed by SDS-PAGE, as it moved as a single band (50), and Western blot analysis using anti-His antibody and sera from P. falciparum-infected patients (as the positive control) affirmed the purified PfCelTOS antigen (50). This recombinant protein was purified and desalted on a large scale for mouse immunization, and the endotoxin concentration of this purified PfCelTOS was less than 0.1 endotoxin units (EU)/ml. Thus, the injection of 200 μl of PfCelTOS/mouse indicated an acceptable amount (0.02 EU) of endotoxin in each mouse. Also, the concentration of the triple-adjuvant combination (MCQ) (5 μg/mouse of each adjuvant) was effective in enhancing antibody responses, which stimulated strong cytokine secretion and was well tolerated in mice, with a satisfactory safety profile and with no observed morbidity compared to the control mice. Female BALB/c mice (n = 160) were randomly distributed into 10 groups and immunized subcutaneously on days 0, 14, and 28 at the base of the tail with PfCelTOS (10 μg at prime and 5 μg at boost) alone, as a nonadjuvanted vaccine group (group 1), or in combination with distinct adjuvants, MPL (group 2), CpG ODN (group 3), and QS-21 (group 4) alone and as a mixture (MCQ) (group 5), as adjuvanted vaccine groups. Each control mouse received MPL (group 6), CpG ODN (group 7), or QS-21 (group 8) alone or as a mixture (MCQ) (group 9) or 1× phosphate-buffered saline (PBS) alone (group 10), as negative controls (Table 1). Serum samples were collected from the tail vein on days 10, 24, 38, and 180 after the first immunization, and by using an enzyme-linked immunosorbent assay (ELISA), specific anti-PfCelTOS IgG was detected in different immunized mouse groups (Fig. 1). A significant increase in the level of anti-PfCelTOS IgG antibodies was detected on days 24 and 38 (boost) compared to day 10 (postprime) after immunization in all mouse vaccine groups (groups 1 to 5) (P < 0.0001 by a paired-sample t test) (Fig. 1). After the second boost (on day 38 after the first immunization), the highest and lowest significant levels of anti-PfCelTOS IgG antibody were observed in mouse group 5 receiving PfCelTOS in combination with MCQ (mean OD490, 3.54 ± 0.127) (Fig. 1) and group 1 receiving PfCelTOS antigen alone (mean OD490, 1 ± 0.179), respectively (P < 0.0001 by one-way analysis of variance [ANOVA]) (Fig. 1). Furthermore, multiple comparisons among vaccine groups 2 to 4, which received the antigen with a single adjuvant (PfCelTOS/MPL, PfCelTOS/CpG, and PfCelTOS/QS-21, respectively), showed a significant difference in the levels of anti-PfCelTOS IgG in vaccine group 4 compared with those in immunized mouse groups 2 and 3 on day 38 after the first immunization (P < 0.0001 by Tukey’s honestly significant difference [HSD] post hoc test) (Fig. 1 and 2). No detectable anti-PfCelTOS IgG antibodies were found in the control groups that were immunized with only the adjuvant(s) and 1× PBS.
TABLE 1.
Mouse immunization and bleeding strategiesa
| Mouse group (n = 16/group) | Strategy |
||
|---|---|---|---|
| Prime (day 0) | Boost 1 (day 14) | Boost 2 (day 28) | |
| Vaccine groups | |||
| 1 | Ag (10 μg) | Ag (5 μg) | Ag (5 μg) |
| 2 | Ag/MPL | Ag/MPL | Ag/MPL |
| 3 | Ag/CpG | Ag/CpG | Ag/CpG |
| 4 | Ag/QS-21 | Ag/QS-21 | Ag/QS-21 |
| 5 | Ag/MCQ | Ag/MCQ | Ag/MCQ |
| Control groups | |||
| 6 | MPL | MPL | MPL |
| 7 | CpG | CpG | CpG |
| 8 | QS-21 | QS-21 | QS-21 |
| 9 | MCQ | MCQ | MCQ |
| 10 | 1× PBS | 1× PBS | 1× PBS |
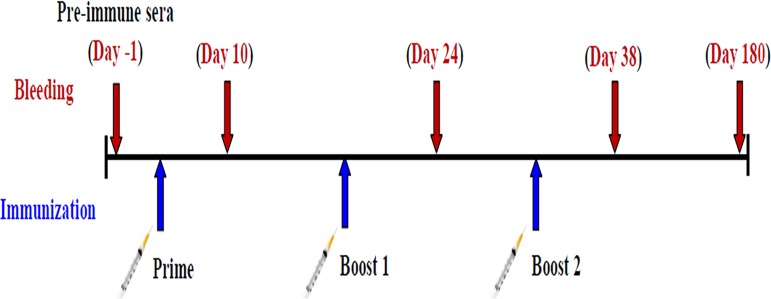 | |||
Female BALB/c mice (n = 160) were randomly distributed into 10 groups (each group containing 16 mice) and immunized subcutaneously at the base of the tail with 200 μl PfCelTOS (10 μg at prime and 5 μg at boost, based on the optimization approach) alone, as a nonadjuvanted vaccine group, or in combination with distinct adjuvants (based on the optimization approach), MPL (monophosphoryl lipid A) (10 μg/mouse), CpG ODN (synthetic oligodeoxynucleotides containing unmethylated CpG motifs) (10 μg/mouse), and QS-21 (Quillaja saponaria Molina fraction 21) (10 μg/mouse), alone and as a mixture (MCQ [MPL/CpG/QS-21]) (5 μg/mouse of each), as adjuvanted vaccine groups. Each control mouse received 1× PBS alone and MPL (10 μg/mouse), CpG ODN (10 μg/mouse), and QS-21 (10 μg/mouse) alone and as a mixture (MCQ) (5 μg/mouse of each) as negative controls. Serum samples were collected from the tail vein on days 10, 24, 38, and 180 after the first immunization. Ag, PfCelTOS antigen.
FIG 1.
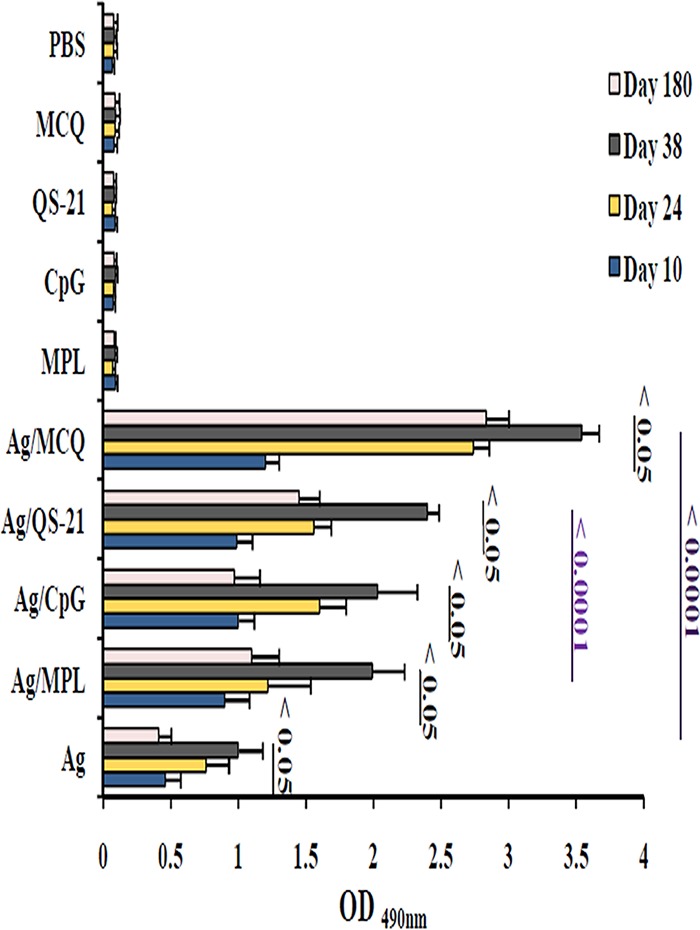
Anti-PfCelTOS IgG antibody levels in vaccine mouse sera at different time points by an ELISA. Groups of 6- to 8-week-old female BALB/c mice were immunized subcutaneously with recombinant PfCelTOS alone or formulated with different adjuvants (MPL, CpG, QS-21, and MCQ [MPL/CpG/QS-21]). Anti-PfCelTOS IgG antibody levels were compared between the sera collected from different immunized mouse groups on day 10, day 24 (10 days after the first boost), and day 38 (10 days after the second boost) after the first immunization. The individual mouse serum samples were incubated in duplicate wells with 1:200-diluted sera for 90 min. There was a significant difference in total IgG antibody levels between different immunization time points in vaccine groups (P < 0.05 by a paired-sample t test). The bars and error bars show the mean OD490 values and standard deviations (SD) for 16 individual mice in each group, respectively. The ELISA cutoffs were calculated as the mean OD490 of preimmune mouse sera (as the negative controls; n = 30) plus 3 SD. The cutoffs for IgG on days 10, 24, 38, and 180 after the first immunization were an OD490 of 0.1 each. Ag, PfCelTOS antigen.
FIG 2.
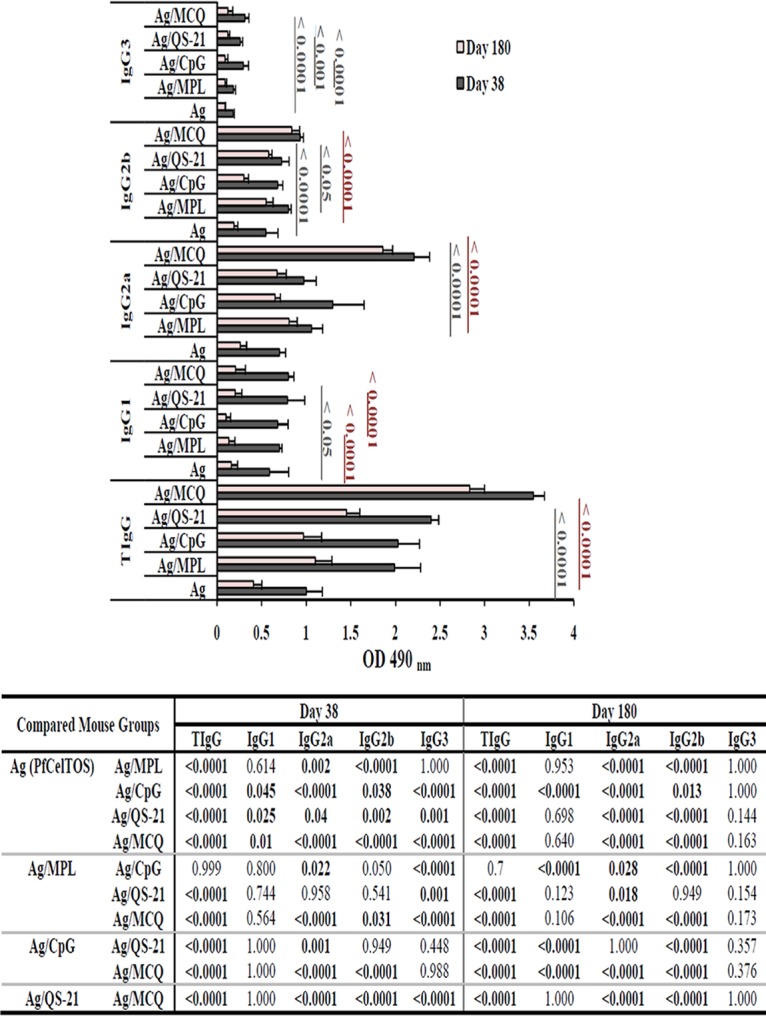
Assessment of anti-PfCelTOS IgG subclass profiles and longevity. Preimmune sera (n = 30) were used as negative controls to determine the ELISA cutoffs, which were calculated as the mean OD490 plus 3 SD. The cutoffs for total IgG (TIgG), IgG1, IgG2a, IgG2b, and IgG3 were OD490 values of 0.101, 0.064, 0.072, 0.093, and 0.091, respectively, on day 38 and 0.1, 0.083, 0.089, 0.108, and 0.068, respectively, on day 180 after the first immunization. The bars and error bars show the mean OD490 values and standard deviations (SD) for 16 individual mice (day 38) and 10 individual mice (day 180) in each group, respectively. The highest levels of total IgG, IgG2a, and IgG2b were detected in group 5, which received PfCelTOS in combination with MCQ (MPL/CpG/QS-21) adjuvants on days 38 and 180 after the first immunization (P < 0.05 by one-way ANOVA). The table shows multiple comparisons of means for anti-PfCelTOS IgG and its subclasses among the nonadjuvanted (group 1) and adjuvanted (groups 2 to 5) vaccine groups on days 38 and 180 after the first immunization, which were performed by using Tukey’s HSD post hoc test.
For determination of the longevity of anti-PfCelTOS IgG in immunized mice, sera were collected from each group on day 180 after the first immunization, and the level of IgG antibody against PfCelTOS significantly decreased relative to the responses on day 38 after the first immunization in all the vaccine groups (groups 1 to 5) (P < 0.05 by a paired-sample t test) (Fig. 1 and 2). The highest and lowest reductions of anti-PfCelTOS IgG were observed in vaccine group 1 (PfCelTOS, 59%) (Fig. 2) and group 5 (PfCelTOS/MCQ, 20%), respectively (P < 0.0001 by one-way ANOVA) (Fig. 2). Additionally, among adjuvanted vaccine groups 2 to 5, the highest decline in IgG antibody levels was observed in group 3 (PfCelTOS/CpG, 52%), and multiple comparisons among the above-mentioned groups showed a significant difference in the level of anti-PfCelTOS IgG in vaccine group 5 (P < 0.0001 by Tukey’s HSD post hoc test) (Fig. 2).
Anti-PfCelTOS IgG isotype profile and persistence.
Analysis of the anti-PfCelTOS IgG subclasses was performed using immunized mouse sera collected from mouse groups 1 to 10 on days 38 and 180 following the first immunization. As shown in Fig. 2, on days 38 and 180 following the first immunization, the predominant subclass against PfCelTOS in all vaccinated mouse groups (groups 1 to 5) was IgG2a antibody. On day 38 after the first immunization, the highest and lowest levels of the IgG2a and IgG2b isotypes were observed in mouse group 5, which received the antigen with MCQ mixture adjuvants (mean OD490, 2.21 [IgG2a] and 0.93 [IgG2b]), and group 1, which received the antigen without any adjuvant (mean OD490, 0.701 [IgG2a] and 0.548 [IgG2b]), respectively (P < 0.05 by one-way ANOVA) (Fig. 2). Comparison of the levels of specific anti-PfCelTOS IgG2a in all the vaccine groups on day 38 following the first immunization unveiled a bias, group 5 (PfCelTOS/MCQ, mean OD490 of 2.21) > group 3 (PfCelTOS/CpG, mean OD490 of 1.3) > group 2 (PfCelTOS/MPL, mean OD490 of 1.06) > group 4 (PfCelTOS/QS-21, mean OD490 of 0.974) > group 1 (PfCelTOS, mean OD490 of 0.701). However, the levels of IgG2b in different vaccine groups were as follows: group 5 (mean OD490 of 0.93) > group 2 (mean OD490 of 0.8) > group 4 (mean OD490 of 0.724) > group 3 (mean OD490 of 0.68) > group 1 (mean OD490 of 0.548). Furthermore, multiple comparisons of the adjuvanted vaccine groups (groups 2 to 4) revealed that there was a significant difference in the levels of IgG2a antibody between vaccine group 2 (PfCelTOS/MPL) and group 3 (PfCelTOS/CpG) and also between vaccine group 3 (PfCelTOS/CpG) and group 4 (PfCelTOS/QS-21) on day 38 following the first immunization (P < 0.05 by Tukey’s HSD post hoc test) (Fig. 2). Regarding IgG2b, there was no significant difference in the levels of the IgG2b subclass among vaccine groups 2, 3, and 4 on day 38 (P > 0.05 by one-way ANOVA) (Fig. 2). For IgG1 and IgG3, the highest and lowest levels of these subclasses were found in mouse group 5 (PfCelTOS/MCQ; mean OD490, 0.8 [IgG1] and 0.311 [IgG3]) and group 1 (PfCelTOS; mean OD490, 0.589 [IgG1] and 0.18 [IgG3]) on day 38 after the first immunization (Fig. 2). Multiple comparisons of the adjuvanted vaccine groups (groups 2 to 5) showed a significant difference in the levels of IgG3 antibody between vaccine group 5 and vaccine groups 2 and 4 (P < 0.0001 by Tukey’s HSD post hoc test) (Fig. 2). However, no significant difference was found in the levels of anti-PfCelTOS IgG1 among vaccine groups 2 to 5 on day 38 following the first immunization (P > 0.05 by one-way ANOVA) (Fig. 2).
Evaluation of the persistence of elicited anti-PfCelTOS IgG isotypes in the immunized mice on day 180 after the primary immunization showed that the levels of anti-PfCelTOS IgG1, IgG2a, IgG2b, and IgG3 antibodies significantly decreased in comparison to those on day 38 after the first immunization in mouse vaccine groups 2 to 4 (P < 0.05 by a paired-sample t test) (Fig. 2). However, in vaccine group 5 (PfCelTOS/MCQ), no significant difference was observed in the levels of IgG2a and IgG2b antibodies (P > 0.05 by a paired-sample t test) (Fig. 2). On day 180 following the primary immunization, the highest and lowest reductions in the IgG2a and IgG2b subclass responses to PfCelTOS were observed in vaccine group 1 (PfCelTOS alone; IgG2a = 63% and IgG2b = 65%) and group 5 (PfCelTOS/MCQ; IgG2a = 16% and IgG2b = 10%), respectively (P < 0.05 by one-way ANOVA) (Fig. 2). Additionally, among adjuvanted vaccine groups 2 to 5, the highest reductions of IgG2a and IgG2b subclass responses were observed in group 3 (PfCelTOS/CpG; 50% and 56% for IgG2a and IgG2b, respectively) (Fig. 2). The levels of the IgG2a and IgG2b subclasses in vaccine group 5 (PfCelTOS/MCQ) were significantly higher than those in adjuvanted vaccine groups 2 to 4 (P < 0.0001 by one-way ANOVA) (Fig. 2). Multiple comparisons of the adjuvanted vaccine groups (groups 2 to 4) showed a significant difference in the levels of IgG2a antibody between vaccine group 2 (PfCelTOS/MPL) and immunized mouse group 3 (PfCelTOS/CpG) and group 4 (PfCelTOS/QS-21) (P < 0.05 by Tukey’s HSD post hoc test,) (Fig. 2). Regarding IgG1 and IgG3, on day 180 after the primary immunization, the highest and lowest reductions were observed in mouse group 3 receiving PfCelTOS/CpG (IgG1 = 85% and IgG3 = 70%) and group 1 receiving PfCelTOS alone (IgG1 = 73% and IgG3 = 50%), respectively (Fig. 2). Moreover, the level of the anti-PfCelTOS IgG1 subclass response in mouse group 3 was significantly lower than those in vaccine groups 1, 2, 4, and 5 (P < 0.0001 by one-way ANOVA) (Fig. 2) on day 180 after the primary immunization.
Antibody titer and avidity of anti-PfCelTOS IgG and its subclasses.
The endpoint titers of anti-PfCelTOS total IgG, IgG2a, and IgG2b antibodies were evaluated on days 38 and 180 after the first immunization by an ELISA. Among all the vaccine groups (groups 1 to 5), the highest endpoint titers of anti-PfCelTOS total IgG, IgG2a, and IgG2b were detected in mouse group 5 receiving PfCelTOS/MCQ on days 38 and 180 following the first immunization (Fig. 3). Regarding IgG, the lowest endpoint titer, 12,800, was detected in mouse vaccine group 1 (PfCelTOS alone) on day 38, which was reduced to 6,400 on day 180 after the first immunization. However, the endpoint titer of IgG in mouse group 5 was 204,800 on day 38, which declined to 102,400 on day 180 after the first immunization. In the case of IgG2a, the mouse group that received PfCelTOS (group 1) had the lowest endpoint titer (12,800) on day 38, which was reduced to 6,400 on day 180 after the first immunization (Fig. 3). In addition, immunization with PfCelTOS/MCQ induced an endpoint titer of 204,800 for IgG2a on day 38, which was reduced to 102,400 on day 180 following the first immunization. Regarding IgG2b, immunization of mice with PfCelTOS alone (group 1) and PfCelTOS/CpG (group 3) induced the lowest endpoint titers (12,800) on day 38, which were reduced to 3,200 for vaccine group 1 and 6,400 for vaccine group 3 on day 180 after the first immunization (Fig. 3). However, mouse group 5 (PfCelTOS/MCQ) had an endpoint titer of 102,400 for IgG2b on day 38, which was reduced to 51,200 on day 180 after the first immunization (Fig. 3).
FIG 3.
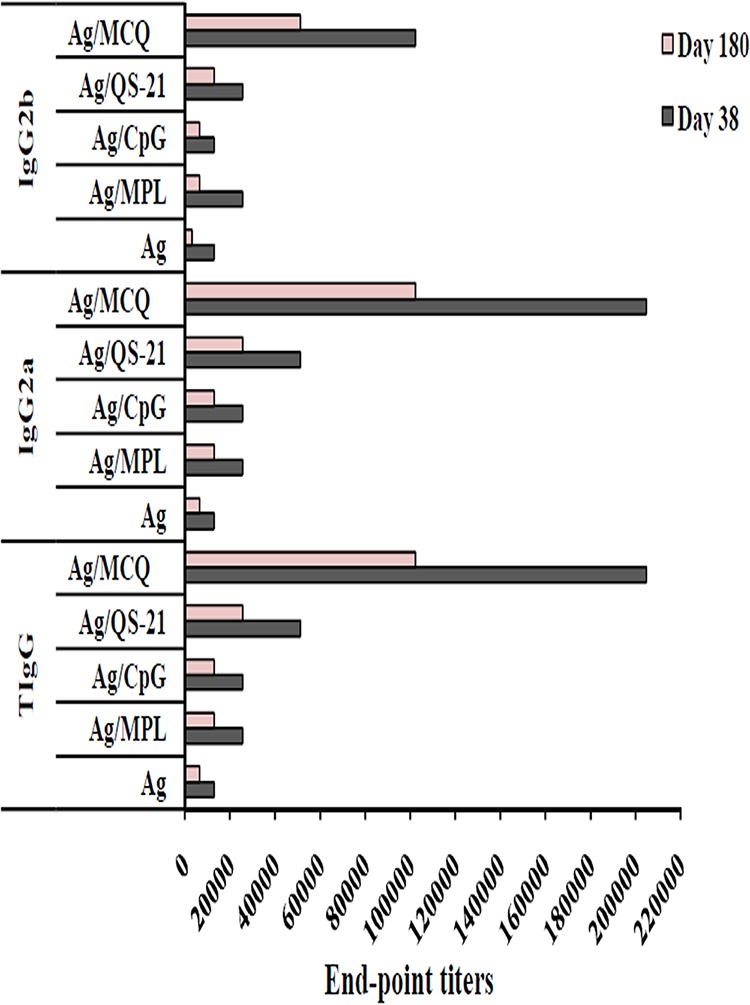
Evaluation of endpoint titers of anti-PfCelTOS total IgG, IgG2a, and IgG2b antibodies. For titration, 1:200 to 1:409,600 dilutions of mouse sera from different vaccine groups (groups 1 to 5) were analyzed using an ELISA. The bars show the last dilution of test sera in which OD490 values were above that of cutoff. Among all the vaccine groups (groups 1 to 5), the highest endpoint titers of anti-PfCelTOS total IgG, IgG2a, and IgG2b were detected in mouse group 5 receiving PfCelTOS/MCQ (MPL/CpG/QS-21) on days 38 and 180 after the first immunization. The cutoffs for total IgG, IgG2a, and IgG2b were OD490 values of 0.1, 0.072, and 0.093, respectively, on day 38 and 0.1, 0.089, and 0.108, respectively, on day 180.
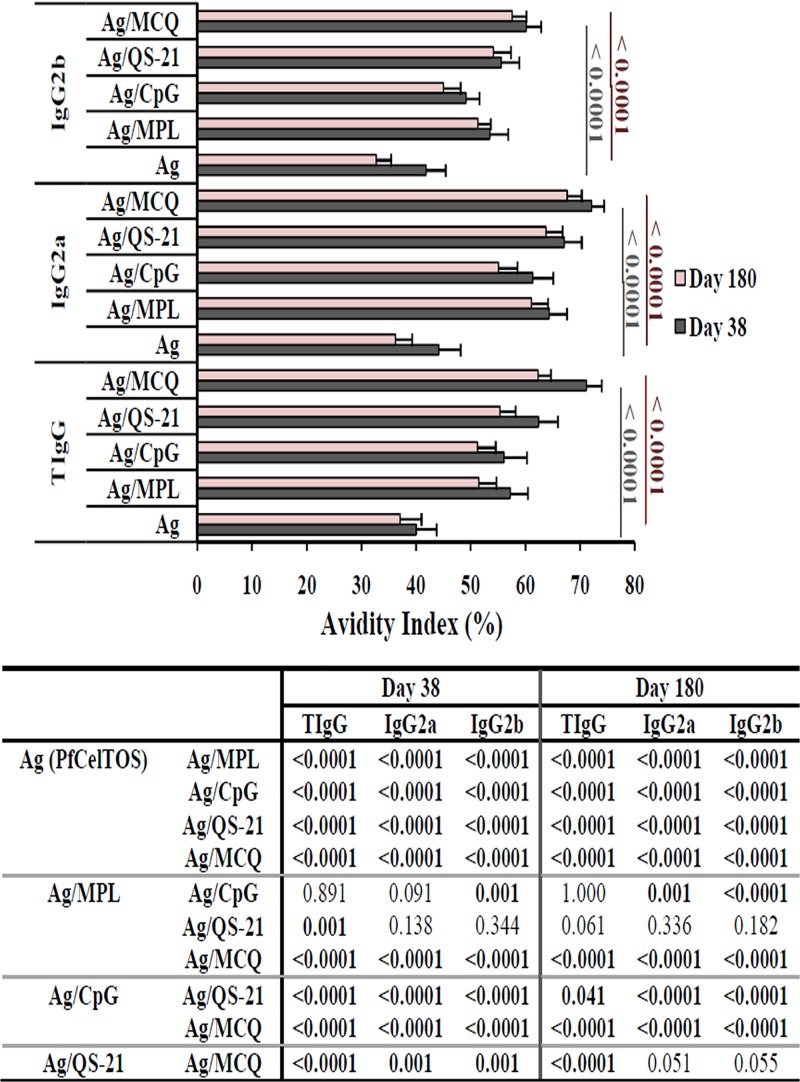
In this study, high-avidity IgG and IgG2a antibodies were induced in the adjuvanted vaccine groups (groups 2 to 5) receiving PfCelTOS antigen formulated with different adjuvants on days 38 and 180 following the primary immunization (Fig. 4). However, immunization of mice with PfCelTOS antigen without any adjuvant resulted in intermediate avidity for IgG, IgG2a, and IgG2b antibodies on days 38 and 180 after the first immunization (Fig. 4). Also, among the adjuvanted vaccine groups (groups 2 to 5), intermediate-avidity IgG2b antibodies were found only in group 3, which received PfCelTOS formulated with CpG adjuvant (Fig. 4), on days 38 and 180 after the first immunization. The highest avidity index (AI) for IgG, IgG2a, and IgG2b subclass antibodies was observed in mouse group 5 receiving PfCelTOS in MCQ mixture adjuvants on days 38 and 180 following the first immunization (Fig. 4). Multiple comparisons of AIs for IgG among adjuvanted vaccine groups 2 to 5 revealed that high-avidity IgG antibody in mouse group 5 (PfCelTOS/MCQ) was significantly different from those in mouse groups 2 to 4 (PfCelTOS/MPL, PfCelTOS/CpG, and PfCelTOS/QS-21) on days 38 and 180 following the first immunization (P < 0.0001 by Tukey’s HSD post hoc test) (Fig. 4). Multiple comparisons among adjuvanted vaccine groups 2 to 5 indicated a significant difference in the AIs of IgG2a and IgG2b in vaccine group 5 (PfCelTOS/MCQ) compared with vaccine groups 2 to 4 on day 38 and also groups 2 and 3 on day 180 following the first immunization (P < 0.05 by Tukey’s HSD post hoc test) (Fig. 4).
FIG 4.
Avidity analyses of anti-PfCelTOS IgG, IgG2a, and IgG2b antibodies by an ELISA. The avidity index (AI) was calculated as the portion of the OD value of urea-treated serum samples compared to that of untreated samples multiplied by 100. AI values of <30%, 30 to 50%, and >50% correspond to low, intermediate, and high avidity, respectively. The bars and error bars show the mean AIs and SD for 16 individual mice (day 38) and 10 individual mice (day 180) in each group, respectively. The comparisons between the groups for AIs were analyzed by one-way ANOVA, followed by Tukey’s HSD post hoc test, and a P value of <0.05 was considered statistically significant, as shown in the table. The highest AI of anti-PfCelTOS total IgG, IgG2a, and IgG2b antibodies was observed in vaccine group 5 (PfCelTOS/MCQ [MPL/CpG/QS-21]) on days 38 and 180 after the first immunization.
Cellular immune responses.
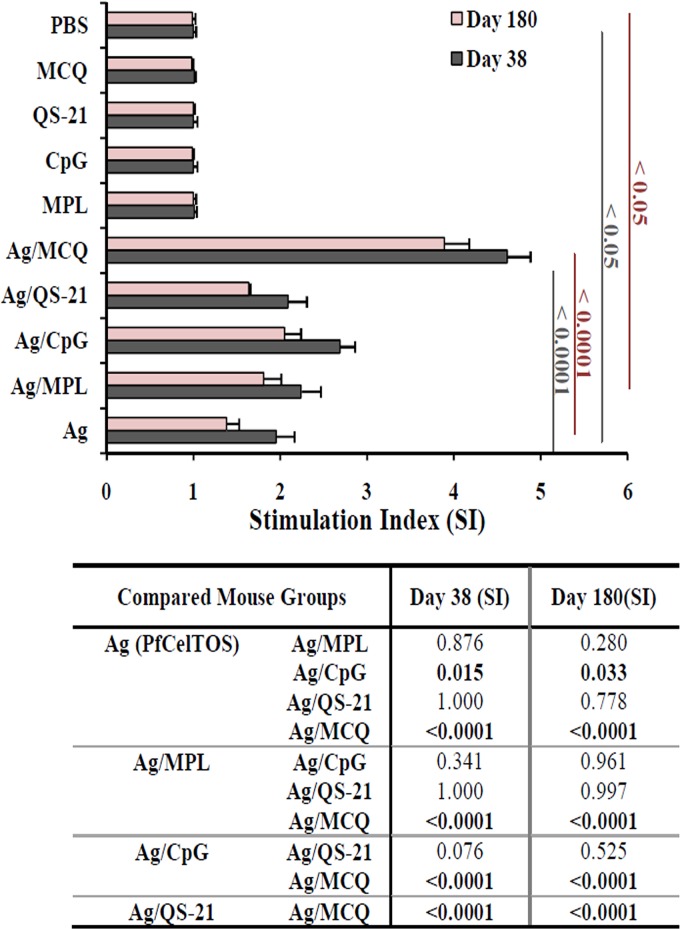
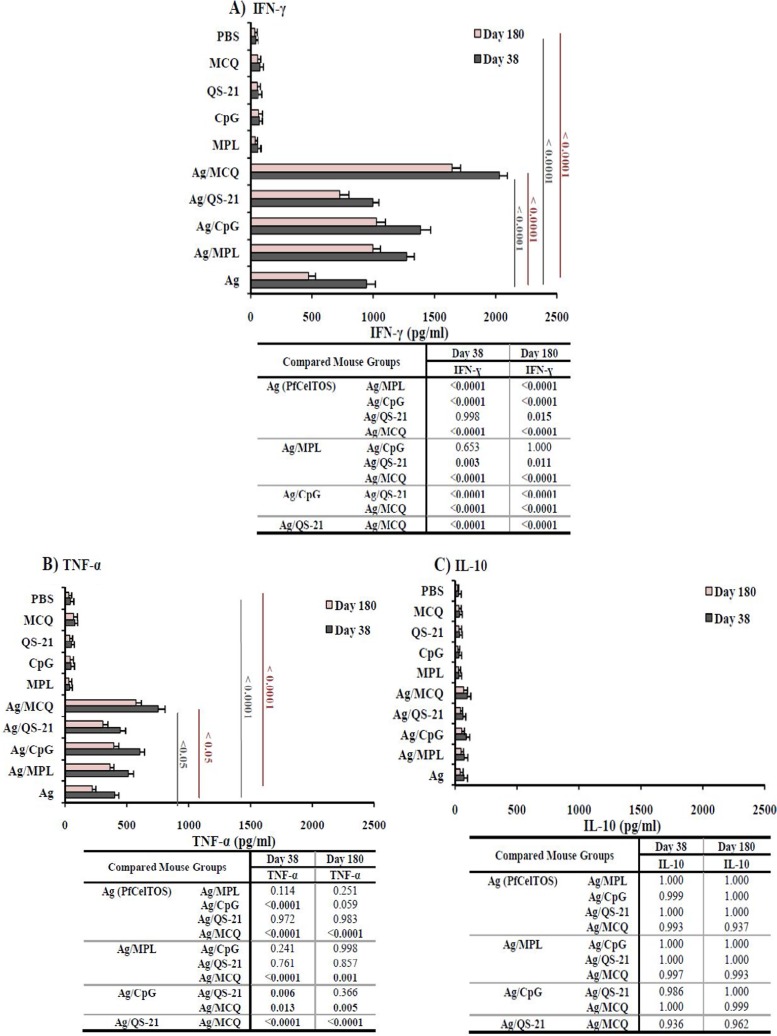
Both proliferation and cytokine production were measured in PfCelTOS-stimulated splenocytes in the mouse groups immunized with different formulations of adjuvants on days 38 and 180 following the primary immunization. Multiple comparisons of the proliferation levels among vaccine groups 1 to 5 showed a significant increase in proliferation in mouse group 5 (PfCelTOS/MCQ) compared with mouse groups 1 to 4 (PfCelTOS alone, PfCelTOS/MPL, PfCelTOS/CpG, and PfCelTOS/QS-21, respectively) (P < 0.0001 by Tukey’s HSD post hoc test) (Fig. 5). The analysis of cytokine profiles revealed that significant levels of IFN-γ were elicited in all the vaccine groups (groups 1 to 5) compared to the mouse control groups (groups 6 to 10) on days 38 and 180 following the primary immunization (P < 0.0001 by one-way ANOVA) (Fig. 6A). Among vaccine groups 1 to 5, the highest and lowest levels of IFN-γ production were detected in immunized mouse group 5 (PfCelTOS/MCQ; 2,028.2 and 1,642.8 pg/ml) and nonadjuvanted vaccine group 1 (PfCelTOS; 943.4 and 471.3 pg/ml) on days 38 and 180 following the first immunization, respectively (P < 0.0001 by one-way ANOVA) (Fig. 6A). Additionally, multiple comparisons among adjuvanted vaccine groups 2 to 5 showed that the level of IFN-γ in vaccine group 5 was significantly higher than those in vaccine groups 2 to 4 on days 38 and 180 following the first immunization (P < 0.0001 by Tukey’s HSD post hoc test) (Fig. 6A). On day 180 after the primary immunization, the level of IFN-γ significantly decreased in all the vaccine groups (groups 1 to 5) (P < 0.05 by a paired-sample t test) (Fig. 6A). A significant decline in the level of IFN-γ was observed on day 180 after the first immunization in mice immunized with PfCelTOS alone (50%). However, the lowest reduction in the level of IFN-γ was observed in mouse group 5 receiving PfCelTOS formulated with the MCQ mixture adjuvants (19%).
FIG 5.
Proliferation of lymphocytes in cultured splenocyte cells of immunized mice (groups 1 to 10) in the presence of PfCelTOS antigen in vitro. On days 38 and 180 after the first immunization, spleens from each mouse group (4 mice from each vaccine and control group) were removed, and lymphocyte proliferation was evaluated using the MTT assay. On day 180 after the primary immunization, lymphocyte proliferation was significantly reduced in all the mouse vaccine groups (P < 0.05 by a paired-sample t test). Data were analyzed using one-way ANOVA, followed by Tukey’s HSD post hoc test. MCQ, MPL/CpG/QS-21.
FIG 6.
Assessment of IFN-γ, TNF-α, and IL-10 production in vaccine groups (groups 1 to 5) and control groups (groups 6 to 10) on days 38 and 180 after the first immunization by an ELISA. (A) For immunized mice receiving PfCelTOS with different adjuvants alone or in combination, the mean IFN-γ responses in the presence of ConA (as the positive control) and no antigen (as the negative control) were in the range of 1,821 to 2,101 and 21 to 35 pg/ml, respectively. (B) Among different vaccine groups, the mean TNF-α responses in the presence of ConA (as the positive control) and no antigen (as the negative control) were in the range of 964 to 1,150 and 20 to 38 pg/ml, respectively. (C) IL-10 responses in the presence of ConA (as the positive control) and no antigen (as the negative control) were in the range of 152 to 459 and 19 to 35 pg/ml, respectively. On day 180 after primary immunization, the levels of all cytokines were significantly reduced in all the vaccine mouse groups (P < 0.05 by a paired-sample t test). The table shows multiple comparisons of IFN-γ, TNF-α, and IL-10 among the nonadjuvanted (group 1) and adjuvanted (groups 2 to 5) vaccine groups on days 38 and 180 after the first immunization, which was performed by using Tukey’s HSD post hoc test. MCQ, MPL/CpG/QS-21.
A significant difference was found in the levels of TNF-α between vaccine groups 1 to 5 and control groups 6 to 10 (P < 0.0001 by one-way ANOVA) (Fig. 6B). The highest and lowest levels of TNF-α were produced by vaccine group 5 (752.8 and 572.1 pg/ml) and group 1 (400.6 and 220.4 pg/ml) on days 38 and 180 after the first immunization, respectively (P < 0.05 by one-way ANOVA) (Fig. 6B). The level of TNF-α in mouse group 5 (PfCelTOS/MCQ) was significantly different from those in mouse groups 2 to 4 (PfCelTOS/MPL, PfCelTOS/CpG, and PfCelTOS/QS-21, respectively) on days 38 and 180 following the primary immunization (P < 0.05 by one-way ANOVA) (Fig. 6B). Comparison of the reductions of TNF-α production on day 180 in all mouse vaccine groups (groups 1 to 5) revealed the highest and lowest declines in mouse group 1 (45%) and group 5 (24%), respectively. Analysis of IL-10 (as a regulatory and Th2 cytokine) showed a low level of IL-10 production (<100 pg/ml) in all the mouse vaccine groups (groups 1 to 5) and control groups (groups 6 to 10) on days 38 and 180 following the first immunization (<100 pg/ml). There was no significant difference in the levels of IL-10 between vaccine groups 1 to 5 (mean IL-10 concentration, 40.4 to 96.1 pg/ml) and control groups 6 to 10 (mean concentration, 19.3 to 34.3 pg/ml) on days 38 and 180 following the primary immunization (P > 0.05 by one-way ANOVA) (Fig. 6C). Regarding the levels of IL-4 secretion as Th2-type responses, low levels of IL-4, with no significant differences, were detected in different immunized (groups 1 to 5) and control (groups 6 to 10) mouse groups (P > 0.05 by one-way ANOVA) (data not shown).
Induction of Th1-type immune responses by immunization of mice with PfCelTOS in different adjuvant formulations.
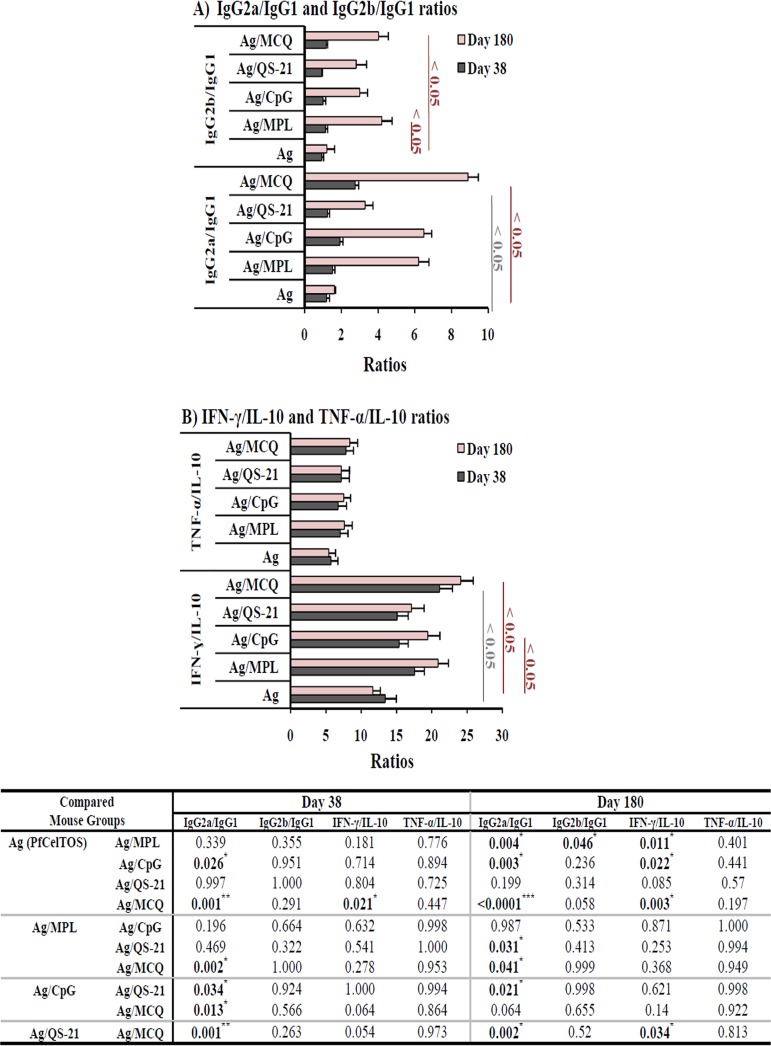
Immunization with PfCelTOS formulated with the MCQ mixture adjuvants (group 5) induced the highest IgG2a/IgG1 ratio on day 38 (2.76; P < 0.05 by one-way ANOVA) and day 180 (8.9) following the first immunization (Fig. 7A). However, the lowest IgG2a/IgG1 ratios on day 38 (1.19) and day 180 (1.62) following the first immunization were detected in vaccine group 1, which received the antigen without any adjuvant. The highest and lowest IgG2b/IgG1 ratios on day 38 (1.16 and 0.92) and day 180 (4 and 1.2) following the first immunization were detected in vaccine groups 5 and 1, respectively (Fig. 7A). Also, multiple comparisons of the IgG2b/IgG1 ratios among vaccine groups 1 and 3 to 5 showed no significant difference on days 38 and 180 following the first immunization (P > 0.05 by Tukey’s HSD post hoc test) (Fig. 7A). The mouse group immunized with PfCelTOS/MCQ (group 5) induced higher IFN-γ/IL-10 ratios (day 38, 21.1; day 180, 24.12) and TNF-α/IL-10 ratios (day 38, 7.83; day 180, 8.4) ratios than in vaccine groups 2 to 4. The IFN-γ/IL-10 ratio in vaccine group 5 was significantly higher than those in vaccine group 1 (day 38, 13.43; day 180, 11.66) and group 4 (day 180, 17.12) (P < 0.05 by Tukey's HSD post hoc test) (Fig. 7B). Additionally, the TNF-α/IL-10 ratios were not significantly different among all the vaccine groups (groups 1 to 5) on days 38 and 180 following the first immunization (P > 0.05 by one-way ANOVA) (Fig. 7B).
FIG 7.
Evaluation of Th1/Th2 ratios. Th1 (anti-PfCelTOS IgG2a and IgG2b antibodies and cytokines IFN-γ and TNF-α) and Th2 (anti-PfCelTOS IgG1 and the cytokine IL-10) responses were analyzed in different vaccine groups on days 38 and 180 after the first immunization. (A) For immunized mice receiving PfCelTOS alone or with different adjuvants (groups 1 to 5), an increase in the IgG2a/IgG1 ratio was observed in mouse group 5, which received PfCelTOS in combination with MCQ (MPL/CpG/QS-21), on day 38 (ratio, 2.76; P < 0.05 by one-way ANOVA) and day 180 (8.9). (B) In mouse group 5, which received the antigen with MCQ adjuvants, the IFN-γ/IL-10 ratios (day 38 ratio, 21.1; day 180 ratio, 24.12) were significantly higher than those in mouse group 1, which received the antigen alone (P < 0.05 by one-way ANOVA). The table shows multiple comparisons of IgG2a/IgG1, IgG2b/IgG1, IFN-γ/IL-10, and TNF-α/IL-10 (Th1/Th2) ratios among the nonadjuvanted (group 1) and adjuvanted (groups 2 to 5) vaccine groups on days 38 and 180 after the first immunization using Tukey’s HSD post hoc test.
Transmission-reducing activity (TRA) of anti-PfCelTOS in different vaccine groups.
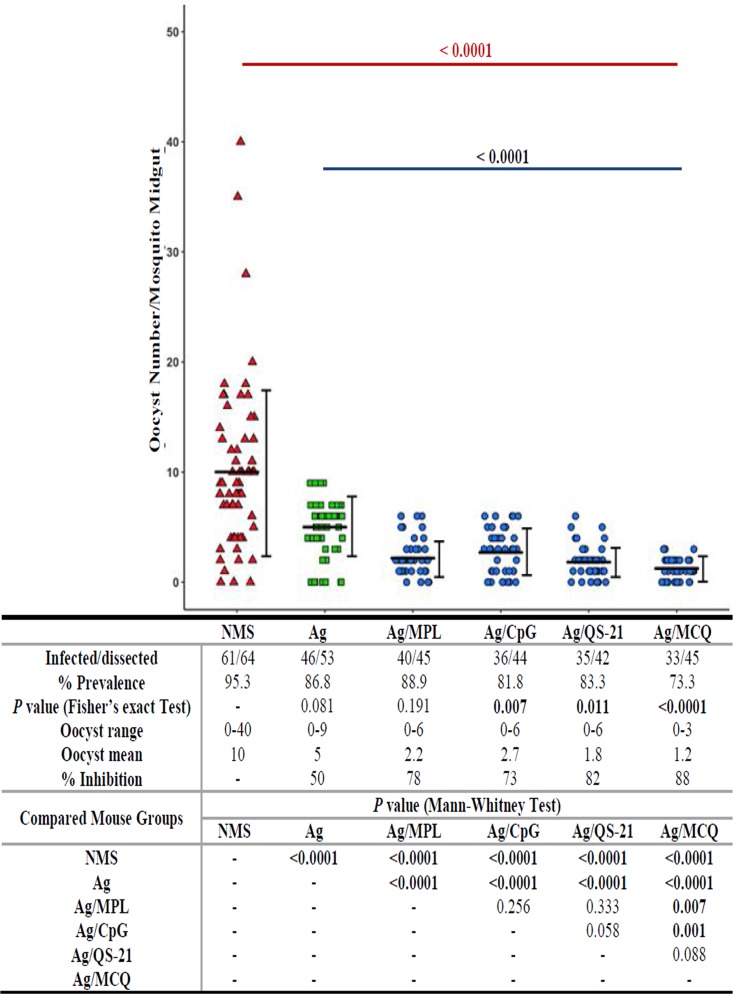
The effect of anti-PfCelTOS mouse polyclonal antibodies (groups 1 to 5) on the inhibition of P. falciparum NF54 infection in A. stephensi mosquitoes was evaluated by an SMFA. Pooled sera of vaccine groups 1 to 5 (PfCelTOS, PfCelTOS/MPL, PfCelTOS/CpG, PfCelTOS/QS21, and PfCelTOS/MCQ, respectively) and pooled sera of the nonadjuvant control group (normal mouse serum [NMS]) and adjuvanted control groups (MPL, CPG, or QS-21 alone or MCQ combination adjuvants) were used in the SMFA, and antibodies against PfCelTOS showed different levels of reduction of oocyst intensity of P. falciparum infection in A. stephensi (Fig. 8). Mean oocyst numbers per midgut in all the control groups ranged between 9.4 and 10, with no significant difference (P > 0.05 by a Mann-Whitney U test) (Table 2). The highest and lowest infection prevalences were observed in mouse group 2 receiving PfCelTOS/MPL (40 positive out of 45 mosquitoes examined; 88.9%) and group 5 that received the antigen with the MCQ mixture adjuvant (33 positive out of 45 mosquitoes examined; 73.3%), respectively (Fig. 8). There was a significant difference in the prevalences of infection of A. stephensi among adjuvanted vaccine groups 3 to 5 (ranging from 73.3 to 83.3%) in comparison with that of the NMS control group (95.3%) (P < 0.05 by Fisher’s exact test) (Fig. 8). Additionally, the anti-PfCelTOS antibodies from vaccine groups 1 to 5 significantly inhibited oocyst formation in A. stephensi relative to the NMS control group (P < 0.0001 by a Mann-Whitney U test) (Fig. 8). Anti-PfCelTOS antibodies from nonadjuvanted vaccine group 1 (PfCelTOS alone) and adjuvanted vaccine groups 2 to 5 showed different levels of inhibition of oocyst intensity (ranging from 50 to 88%) in A. stephensi. The mean oocyst intensity was significantly reduced from 10 oocysts in the NMS control group to 1.2 in vaccine group 5 (P < 0.0001 by a Mann-Whitney U test) (Fig. 8). Multiple comparisons between nonadjuvanted vaccine group 1 and adjuvanted vaccine groups 2 to 5 indicated a significant difference in oocyst inhibition (P < 0.0001 by a Mann-Whitney U test) (Fig. 8). In addition, the polyclonal anti-PfCelTOS antibodies from mouse group 5 (PfCelTOS/MCQ) showed the lowest mean number of oocysts (1.2) and the highest level of oocyst inhibition (88%) and were significantly different in comparison to those of vaccine groups 1 to 3 (P < 0.05 by a Mann-Whitney U test) but not those of group 4 (Fig. 8).
FIG 8.
Inhibition of P. falciparum NF54 parasite development by anti-PfCelTOS polyclonal antibodies in A. stephensi mosquitoes. Pooled mouse sera (n = 16) from different vaccine groups (groups 1 to 5) collected on day 38 after the first immunization were admixed with mature P. falciparum NF54 cultured gametocytes and fed to A. stephensi mosquitoes (n = 50/cup) in standard membrane feeding assays (SMFAs). Pooled normal mouse serum (NMS) (n = 30 randomly selected from 160 female BALB/c mice before immunization) was used as the negative control. On days 9 to 10 after feeding, the mosquitoes’ midguts were dissected, and oocyst counts, which revealed the successful development of P. falciparum in A. stephensi mosquitoes, were recorded. Two separate membrane feeds were done using serum from each vaccine group (groups 1 to 5), and oocyst counts were pooled for statistical analysis. The dots represent the numbers of oocysts in individual mosquitoes, and the horizontal and vertical lines indicate the arithmetic means and standard deviations (SD) of oocyst counts, respectively. The prevalence of infected mosquitoes in the vaccine groups (groups 1 to 5) and the control group (NMS), range of oocyst numbers, mean number of oocysts, percent inhibition relative to the NMS control group, and multiple comparisons of different vaccine groups and the NMS control group are indicated in the table. A two-tailed Mann-Whitney U test and Fisher’s exact test were used to estimate the differences in infection intensity and prevalence, respectively, using IBM SPSS 20.0 for Windows. MCQ, MPL/CpG/QS-21.
TABLE 2.
Effect of mouse anti-rPfCelTOS IgG antibodies on P. falciparum infectivity in A. stephensi mosquitoes determined using an SMFAa
| Group | Antibody from mouse immunization group | Arithmetic mean no. of oocysts in midgut (range) | No. of infected/no. of dissected mosquitoes (%) |
|---|---|---|---|
| Vaccine | Ag | 5 (0–9) | 46/53 (86.8) |
| Ag/MPL | 2.2 (0–6) | 40/45 (88.9) | |
| Ag/CpG | 2.7 (0–6) | 36/44 (81.8) | |
| Ag/QS-21 | 1.8 (0–6) | 35/42 (83.3) | |
| Ag/MCQ | 1.2 (0–3) | 33/45 (73.3) | |
| Control | |||
| Adjuvanted | MPL | 9.5 (0–38) | 57/61 (93.4) |
| CpG | 9.4 (0–38) | 56/60 (93.3) | |
| QS-21 | 9.4 (0–37) | 58/61 (95.1) | |
| MCQ | 9.5 (0–37) | 59/63 (94) | |
| Nonadjuvanted | NMS | 10 (0–40) | 61/64 (95.3) |
Statistical analysis of mean oocyst numbers per midgut in all different control groups (preimmune/normal mouse serum [NMS] [nonadjuvanted control group] and MPL, CpG, QS21, and MCQ [MPL/CpG/QS-21] [adjuvanted control groups]) showed no significant difference (P > 0.05 by a Mann-Whitney U test). The pooled anti-PfCelTOS antibodies from all the vaccine groups significantly inhibited oocyst formation in A. stephensi relative to the NMS control group (P < 0.0001 by a Mann-Whitney U test). Ag, PfCelTOS antigen.
DISCUSSION
One of the promising approaches to achieving malaria elimination and eradication would be subunit-based VIMT. However, a recombinant purified protein in a subunit vaccine might typically induce low or modest immune responses. This low responsiveness may be a result of purification processes (51) and/or fast degradation of the recombinant antigen in vivo. However, subunit vaccines may also preserve some of the characteristics that induce the innate immune system, but this stimulation may be insufficient to induce long-lasting immunity. Therefore, to improve subunit vaccine immunogenicity, the use of a potent and proper adjuvant(s) might be a promising strategy to improve pathogen-specific humoral, cellular, and functional responses (36). In this regard, in the present investigation, different adjuvants, including MPL, CpG ODN, and QS-21, were used alone or in combination to evaluate their ability to enhance and stimulate desired multiple immune responses to recombinant PfCelTOS (rPfCelTOS), as one of the promising multistage VIMT targets (8, 9, 11, 52, 53) that, hopefully, is capable of preventing hepatocyte invasion and impairing the development of parasites in mosquito vectors.
In this work, comparison of immune responses in all the mouse vaccine groups showed that in vaccine group 1 receiving the antigen without any adjuvant, comparable levels of both specific anti-PfCelTOS IgG1 (as an indicator of Th2 and humoral responses) and IgG2a (as an indicator of Th1 and cellular responses) were detected. This result signifies that the PfCelTOS formulation without any adjuvant skews the response toward mixed Th1/Th2 responses. However, in the immunized mouse groups that received PfCelTOS formulated with different adjuvants, predominantly IgG2a and IgG2b antibodies (Th1 response) and a smaller amount of IgG1 and IgG3 were induced. These results showed that the combination of the recombinant PfCelTOS antigen with the selected adjuvants improved its immunogenicity in the generation of a high-titer and persistent Th1 response, which is needed for the VIMT. This observation was in accordance with previous work indicating that PfCelTOS emulsified in glucopyranosyl lipid adjuvant-stable emulsion (GLA-SE) adjuvant (a stable oil-in-water emulsion combined with a TLR4 agonist) induced a Th1-biased immune response in BALB/c mice (10). Moreover, this finding is consistent with previous works suggesting that MPL (54, 55), CpG (56, 57), and QS-21 (58–60) are capable of eliciting a Th1 immune response. Note that this murine result also paralleled our previous work (50), in which Iranian malaria-exposed subjects had cytophilic anti-PfCelTOS-specific IgG1 and IgG3 (equivalent to murine antibodies IgG2a and IgG2b) as the predominant IgG isotypes. This evidence confirmed that the recombinant protein preserved the conformation of B cell epitopes; therefore, E. coli-expressed PfCelTOS is a good antigen and can be recognized by sporozoite-induced antibodies in natural infection (50). Furthermore, the comparability of IgG2a and IgG2b antibodies to human IgG1 and IgG3 (61) suggested that these isotypes are cytophilic and highly effective in mediating antibody-dependent cellular mechanisms, such as phagocyte activation and complement fixation, and have the ability to bind to protein antigens.
In subunit vaccine development, the quality of antibodies that may possibly determine protection against a certain pathogen must be considered (62). In this work, adjuvanted vaccine group 5 that received the subunit antigen with a combination of three distinct adjuvants (MCQ) reflected high-avidity specific anti-PfCelTOS IgG antibodies, indicating that the maturation of the immune response resulted in an enhanced avidity of antibodies to recombinant PfCelTOS. Moreover, a sustained and high level of antibody response to PfCelTOS antigen was detected, with a gradual decline at day 180 following the first immunization, in adjuvanted vaccine group 5, which suggests long persistence and high stability of immune responses in this vaccine group. However, in the nonadjuvanted vaccine group (group 1), a specific anti-PfCelTOS IgG antibody with intermediate avidity was detected, indicating that the adjuvants MPL, CpG, and QS-21, and in particular their combination, have a key role in the maturation and persistence of anti-PfCelTOS antibody responses with high titers and avidity, and these points can be considered in the development of subunit PfCelTOS-based vaccine.
Complex pathogens such as P. falciparum require both antibodies and cell-mediated responses, which result in the activation of effector CD4+ and/or CD8+ T cells. CD4+ T cells not only secrete cytokines that activate macrophages to destroy phagocytosed pathogens but also provide help to antibody-producing B cells to facilitate the development of high-affinity antibodies and memory B cells. In this work, an admixture of the MPL, CpG, and QS-21 adjuvants with the PfCelTOS antigen induced a significantly higher level of IFN-γ production than in the group receiving the antigen without any adjuvant as well as in the control groups. However, it should be noted that the coadministration of MCQ with PfCelTOS in the immunized mice induced significantly higher levels of IFN-γ than in those that received MPL, CpG, or QS-21 alone.
In this regard, it has been reported that MPL induces antigen-specific T cells producing IFN-γ and antibody isotype switching toward IgG2a antibodies in mice (63). Therefore, activation of TLR4 by MPL might stimulate the maturation of antigen-presenting cells and secretion of cytokines, such as IL-6, TNF-α, IL-1, IFN-γ, and IL-12, which are crucial for the activation and differentiation of B and T cells (64–66) that lead to enhanced humoral and cellular immune responses. CpG ODN stimulates IFN-γ production by the expression of IL-12 through macrophages (67) and/or indirectly activates NK cells (68, 69). QS-21 also shifts the response to a more Th1-biased response; stimulates the induction of Th1 cytokines, such as IL-2 and IFN-γ; as well as shifts specific antibodies to the IgG2a isotype to the target protein antigens (38, 59, 60). The role of the IFN-γ and TNF-α cytokines in Plasmodium infection is resistance against infection, as a previous work reported that treatment with anti-TNF-α monoclonal antibody results in a reduced level of IFN-γ and, thus, longer times for parasite clearance (70).
It has been suggested that Plasmodium infections may possibly promote the induction of the cytokine IL-10 (as an immunoregulator), which has a crucial role in inhibiting the effects of cytokines released by Th1 cells (71). Therefore, the low level of production of IL-10 (<100 pg/ml) in vaccine groups confirms and supports the notion that the MPL, CpG, and QS-21 adjuvants increase the level, avidity, and persistence of anti-PfCelTOS antibodies as a result of the enhancement and stimulation of the Th1 cytokines. Moreover, it has been shown that exogenous IL-12 (72) and IL-4 (73) promote Th1 and Th2 differentiation of Th effector cells, respectively. In this work, the induction of IFN-γ and TNF-α (which promote Th1 responses), but not IL-10 and IL-4 (which promote Th2 responses), suggests that the administration of these three adjuvants in vivo may generate a Th1 immune response by the induction of CelTOS-specific CD4+ T cells that promote the production of higher levels of proinflammatory Th1 cytokines and lower levels of T-regulatory IL-10 and IL-4 cytokines. On this basis, as the Th1 cytokines are necessary for the induction of mature and high-avidity antibodies to specific antigens, the switching of the anti-PfCelTOS IgG antibodies to IgG2a and IgG2b isotypes and the ratios of IgG2a/IgG1 (1.24 to 2.76 in groups 2 to 5) and of IgG2b/IgG1 (0.93 to 1.16 in groups 2 to 5), which were higher in the adjuvanted vaccine groups than in nonadjuvanted vaccine group 1 (IgG2a/IgG1 ratio, 1.19; IgG2b/IgG1 ratio, 0.92), provide more evidence for the ability of MPL, CpG, or QS-21 alone or, in particular, their combination to shift the immune response to Th1, which might help eliminate infection.
In general, based on previous reports (36, 74) and the support of the present results, combining different types of adjuvants with distinct mechanisms into antigen-specific formulations could induce appropriate, broadly immune responses and durable efficacy to improve vaccine formulations that will ultimately lead to protection against disease. Such an observation was comparable to those of studies that used a live-attenuated yellow fever vaccine that activates multiple DC subsets through TLR2, TLR7/8, and TLR9, which trigger the activation of different signaling pathways (e.g., MyD88 and TRIF), thus leading to a robust, balanced immune response (75). Moreover, lessons from the RTS,S (P. falciparum circumsporozoite protein) vaccine in different clinical trials suggested that AS01 (a liposomal suspension of MPL/QS-21), AS02 (oil-in-water emulsion of MPL/QS-21), and AS04 (alum/MPL) show higher efficacy than formulations containing either an antigen alone or an antigen with only one adjuvant (76, 77).
One of the most crucial bottlenecks for the development of malaria parasites in the female Anopheles host is ookinete-to-oocyst transformation in the mosquito midgut basal lamina (8). In this work, the TRAs (functional activities) of anti-PfCelTOS from the immunized mouse groups that received the antigen formulated with MPL, CpG, and QS-21 alone were comparable; however, the coadministration of the subunit antigen with these three adjuvants significantly enhanced PfCelTOS-specific antibody avidity and inhibition. In fact, the presence of such responses (anti-PfCelTOS antibodies and CelTOS-specific CD4+ T cells) in the blood meal could prevent ookinete development in the mosquito host through binding to the ookinetes and blocking their traversal, thus preventing further development into oocysts in the midgut basal lamina. Moreover, since PfCelTOS is an important protein for the traversal of the parasite in both hosts (human and mosquito), such a high-avidity anti-PfCelTOS antibody might also impair sporozoite motility and hepatocyte infectivity in the human host, as previously shown in the murine model (7–9). Thus, a PfCelTOS-based vaccine could be more effective in inhibiting immune escape of parasites at different stages.
Also, the use of different adjuvants with multiple signaling pathways may reasonably increase the quantity, quality, longevity, and functional activities of specific anti-PfCelTOS antibodies. Actually, such a formulation that combines a recombinant antigen with different adjuvants could have a larger impact on population transmission than a single-antigen-adjuvanted vaccine by increasing efficacy at lower antibody titers. This result again confirms that triggering multiple TLRs could be essential for functional antibody responses, as shown previously (78). In the present work, although the high-avidity anti-CelTOS antibodies did not absolutely block parasite development in mosquitoes, this finding could encourage further research aimed at using different potent vaccine adjuvant formulations and/or combining PfCelTOS with other potent blocking antigens (multiantigenic) to improve PfCelTOS-based vaccines. Thus, the present data confirm and provide further proof for using this protein as a multiple-stage vaccine candidate in VIMT development (8, 9, 11, 52).
In conclusion, the present work provides new approaches to the development of a PfCelTOS-based vaccine by screening adjuvants that have the ability to enhance subunit vaccine immunogenicity and to induce high-quality as well as persistent humoral and cellular immune responses with functional activity. Since PfCelTOS has a short time of exposure to the immune system with low natural boosting, an adjuvant(s) could help maintain robust memory immune responses. Thus, in the present work, it was noticeable that coadministration of MCQ adjuvants (with distinct mechanisms) with the E. coli-expressed PfCelTOS antigen enhanced and improved the immunogenicity of the recombinant antigen, which generated more bias toward a Th1 response, with significantly enhanced anti-PfCelTOS avidity and titers. Such functional responses could impair ookinete development in A. stephensi. Therefore, based on the present findings, it could be suggested that adjuvant combinations with different mechanisms stimulate better functional antibody responses than adjuvants individually against challenging diseases such as malaria.
MATERIALS AND METHODS
PfCelTOS antigen preparation for immunological study.
The sequence of the Pfceltos gene representing amino acids 25 to 182 (GenBank accession no. MF371005.1) was cloned, expressed, and purified as described previously (50). Briefly, the recombinant plasmid pET23a-PfCelTOS was transformed into E. coli BL21(DE3), and transformants were used for the expression of PfCelTOS in Terrific broth (TB) in the presence of 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG; Thermo Scientific, Waltham, MA, USA). The pellet of recombinant clones was collected 16 h after induction and kept at −80°C until use. The C-terminal His tag fusion protein was then purified with Ni-nitrilotriacetic acid (NTA) agarose (Qiagen, Hilden, Germany) under denaturing conditions. Subsequently, the eluates containing PfCelTOS were desalted with Econo-Pac 10 DG columns (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s protocol and concentrated with a concentrator (Eppendorf, Hamburg, Germany). The concentration of the purified recombinant protein was determined by a Bradford assay (79), with a spectrophotometer (DeNovix, Wilmington, DE, USA) at 595 nm. The obtained protein was analyzed by 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then confirmed by Western blot analysis using both monoclonal penta-His antibody (Qiagen) and pooled P. falciparum-infected patient sera (50). By using a Limulus amoebocyte lysate (LAL) chromogenic kit (Lonza, Walkersville, MD, USA), the level of bacterial endotoxin was measured in the Quality Control Unit of the Recombinant Protein Production Complex of the Pasteur Institute of Iran, Karaj.
Mouse immunization strategy with different vaccine formulations.
Inbred female BALB/c mice (6 to 8 weeks old) were obtained from the Department of Laboratory Animal Science at the Pasteur Institute of Iran (Karaj) and immunized with the purified recombinant protein. All the animal procedures were done according to protocols approved by the Committee of Animal Ethics of the Pasteur Institute of Iran (IR.PII.REC.1395.4). For immunization, female BALB/c mice (n = 160) were randomly distributed into 10 groups (with each group containing 16 mice) (Table 1). The mice were immunized subcutaneously on days 0, 14, and 28 at the base of the tail with a volume of 200 μl containing purified PfCelTOS (10 μg/mouse at prime and 5 μg/mouse at boost based on the optimization approach) alone (as the nonadjuvanted vaccine group) or in combination with an equal volume (10 μg/mouse based on the optimization approach) of adjuvants MPL, CpG, and QS-21 alone (all from InvivoGen, San Diego, CA, USA) and as a mixture (MCQ) (5 μg/mouse of each adjuvant based on the optimization approach) as the adjuvanted vaccine groups (Table 1). As negative controls, mice were immunized with sterile 1× PBS (pH 7.4), MPL (10 μg/mouse), CpG ODN (10 μg/mouse), and QS-21 (10 μg/mouse) alone and as a mixture (MCQ) (5 μg/mouse of each) (Table 1).
ELISA.
Mouse serum samples were collected from the tail vein before the first immunization (as preimmune serum or NMS) and on days 10, 24, 38, and 180 after the primary immunization and evaluated for anti-PfCelTOS antibody responses and their persistence using an ELISA. The optimal antigen concentrations and the dilutions of the primary and secondary antibodies were determined by a checkerboard cross-titration assay. In brief, purified PfCelTOS (20 ng/well) in 0.06 M carbonate-bicarbonate buffer (pH 9.6) was used as an antigen, and MaxiSorp flat-bottom 96-well microplates (Jet Bio-fil Co., Ltd., China) were coated with the antigen and kept at 4°C overnight. The plates were then washed three times with PBS-T buffer (1× PBS containing 0.05% Tween 20) and blocked with 1× PBS containing 2% bovine serum albumin (BSA; Roche, Switzerland) at room temperature (RT) for 2 h. After washing, duplicate wells were incubated with a 1:200 dilution of individual mouse serum samples (in PBS-T containing 0.5% BSA) at RT for 90 min. The plates were washed and incubated with 100 μl of a 1:25,000 dilution of goat anti-mouse IgG antibody conjugated with horseradish peroxidase (HRP; Sigma-Aldrich Co., St. Louis, MO, USA) at RT for 60 min. Bound IgG antibodies were visualized after adding o-phenylenediamine (OPD)–H2O2 (Sigma-Aldrich) as a substrate, the reaction was then stopped with 2 N H2SO4, and finally, the absorbance (OD490) was measured using an ELISA microplate reader (BioTek, Winooski, VT, USA). The ELISA cutoffs were measured from the average of the NMS (n = 30) plus 3 standard deviations (SD). In order to evaluate the IgG subclass responses to PfCelTOS, the test was performed as described above, and instead of goat anti-mouse IgG-HRP, 100 μl of either goat anti-mouse IgG1, IgG2a, IgG2b, or IgG3 (diluted 1:1,000 in PBS-T buffer) (Sigma-Aldrich) as a secondary antibody was added to each well and incubated at RT for 1 h. After washing, the plates were incubated with a 1:10,000 dilution of anti-goat IgG-HRP (Sigma-Aldrich) at RT for 1 h, and the reaction was then developed by using an enzyme-specific substrate, as mentioned above.
Anti-PfCelTOS antibody titration and avidity.
Antibody titration was done to assess the endpoint titers of anti-PfCelTOS raised in mice on days 38 and 180 after the first immunization by an ELISA. To this end, the ELISA was performed as mentioned above, but after antigen coating and blocking, pooled sera from each mouse group were used in serial dilutions (1:200 to 1:409,600), and the titration endpoints of anti-PfCelTOS total IgG, IgG2a, and IgG2b antibodies were obtained as the last dilution of serum with an OD490 value above the cutoff. For avidity antibodies (IgG, IgG2a, and IgG2b), individual mouse serum samples obtained on day 38 (n = 16) and day 180 (n = 10) after the first immunization were used. To do the avidity ELISA, two MaxiSorp plates (Jet Bio-fil, China) were coated with the PfCelTOS antigen (20 ng/well) and blocked with 2% BSA for 1 h. After a washing step, the individual mouse serum samples (1:200) were incubated at RT for 90 min. In the next washing step, one of the plates was washed three times with PBS-T, and the other plate was washed with dissociation buffer containing PBS-T-urea (8 M), with vigorous shaking. Incubation with secondary antibody, washing steps, and development of the enzyme reaction were performed as described above for the ELISA. The AI was estimated as the ratio between the OD value of urea-treated samples and that of the nontreated samples multiplied by 100. Low-, intermediate-, and high-avidity anti-PfCelTOS-specific antibodies were determined as AI values of ≤30%, 30% to 50%, and >50%, respectively.
Lymphocyte proliferation assay.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide thiazolyl blue (MTT) dye was used for the proliferation assay of mouse lymphocytes on days 38 and 180 after the first immunization. Mice from each group (n = 4) and at each time point were euthanized under sterile conditions. The spleens were removed, and a single-cell suspension of the spleens was prepared in RPMI 1640 medium (Gibco, Invitrogen, Scotland, UK). Next, by using ammonium chloride-potassium lysis buffer (pH 7.2), the red blood cells were lysed, and the cells were washed. The cells were then resuspended in complete culture medium containing RPMI 1640 medium (Gibco), 5% fetal calf serum (FCS; Sigma-Aldrich), 2.3 × 10−2 mM 2-mercaptoethanol, penicillin-streptomycin (100 U–100 μg/ml), and 10 mM HEPES (Sigma-Aldrich), and their viability was assessed by trypan blue dye exclusion. Next, 100 μl of the cell suspension (2 × 106 cells/ml) was cultured in a flat-bottom 96-well tissue culture plate (Orange Scientific, EU, Belgium) in triplicates in the presence of concanavalin A (ConA) (5 μg/ml) (as the positive control), PfCelTOS (10 μg/ml), and medium alone (as the negative control). All the cells were incubated in a 5% CO2 incubator in a humidified atmosphere at 37°C for 48 h. Subsequently, the supernatants were removed, the formazan crystals were dissolved in 0.04 N HCl in absolute isopropanol, and the OD was measured at 550 nm. The stimulation index (SI) was calculated by dividing the mean absorbance of the cells with the antigen by the mean absorbance of the cells without the antigen.
Cytokine assays.
Extracellular cytokine profiles of stimulated splenocytes of mice immunized with PfCelTOS were assessed using murine cytokine immunoassay kits (R&D Systems, Minneapolis, MN, USA). To perform this test, the splenocytes of each mouse group (n = 4) on days 38 and 180 after the primary immunization were cultured as described above. After optimization, the supernatants of splenocyte cultures, which were stimulated with the target antigen (10 μg/ml), were collected at 24 and 48 h (for IL-4) and at 72 h (for IL-10, TNF-α, and IFN-γ). For the quantitative estimation of cytokine levels, standard curves were created with known concentrations of recombinant mouse IL-4, IL-10, TNF-α, and IFN-γ for each experiment. All the tests were performed in duplicate, and the mean concentration (±SD) was recorded for each set of samples.
Assessment of functional transmission-reducing activity of anti-PfCelTOS antibodies.
The ability of anti-PfCelTOS IgG antibodies to inhibit the development of P. falciparum strain NF54 (a kind gift from the Pasteur Institute of Paris) in female A. stephensi mosquitoes (mysorensis; maintained at the National Insectarium of the Pasteur Institute of Iran, Karaj) was assessed by an SMFA. In brief, P. falciparum NF54 parasites were cultured continuously on human erythrocytes (RBCs) (O+ blood group; Blood Transfusion Organization, Tehran, Iran) in RPMI 1640 medium (Gibco) supplemented with 5% pooled human AB+ serum, 0.2% (wt/vol) AlbuMAX II (Gibco), 25 mM HEPES, 50 mg/liter of hypoxanthine, 1.96 g/liter of glucose, and 25 mM NaHCO3 (pH 7.2). The production of mature stage V gametocyte for mosquito feeding was performed based on a previously reported method (80), and the culture was monitored microscopically daily. Different stages of gametocytes were distinguished according to the guidelines of Carter and Miller (81). Under these conditions, a high level of mature gametocytes (stage V) with exflagellation ability will be visible on day 12. For the SMFA, female mosquitoes (4 to 5 days old; n = 50 per cup) were placed into pint-size ice cream cups 1 day before infectious blood feeding and starved overnight. For feeding female A. stephensi mosquitoes via a parafilm membrane, mature stage V P. falciparum NF54 gametocytes (with 1 to 3 exflagellating centers per ×40 field) were mixed with washed human O+ blood at 50% hematocrit in prewarmed human AB+ serum. An equal amount of human sera of either pooled sera from the negative-control groups (nonadjuvanted, NMS; adjuvanted, MPL, CpG, and QS-21 alone and the MCQ combination) or neat sera from different vaccinated groups (groups 1 to 5) was then added to the mixture, and the mixtures were placed into each membrane feeder. The mosquitoes were allowed to feed for 20 min, and all fully engorged mosquitoes were then maintained at 80% relative humidity at 26°C and provided with fresh damp cotton soaked in 10% sugar. Nine to 10 days after feeding, midguts from surviving mosquitoes were dissected and stained with 0.2% mercurochrome in 1× PBS for 10 min, the oocysts were counted under a light microscope, and the number of infected mosquitoes and the percent reduction in infection intensity were recorded relative to the control group. Two independent replicate experiments were performed, oocyst counts were determined, and finally, the data were pooled for statistical analysis. For the SMFA, the percent inhibition of mean oocyst intensity was estimated as 100 × [1 − (mean number of oocysts in the test group/mean number of oocysts in the control group)]. Also, the percent inhibition of oocyst prevalence was calculated based on the following formula: 100 × [1 − (proportion of mosquitoes with any oocysts in the test group/proportion of mosquitoes with any oocysts in the control group)].
Statistical analyses.
A database was created with IBM SPSS Statistics for Windows, version 20.0. (IBM Corp., Armonk, NY, USA). Comparisons between the mouse groups for antibody levels and cellular immune responses were assessed by one-way ANOVA, followed by Tukey’s HSD post hoc test. In addition, the paired-sample t test was used to evaluate the persistence of humoral and cellular immune responses in each group on days 38 and 180 after the primary immunization. Significant differences in the rates and intensities of infection were determined by Fisher’s exact test and a Mann-Whitney U test, respectively. For all tests, P values of <0.05 were considered statistically significant.
ACKNOWLEDGMENTS
We gratefully acknowledge the Iranian blood transfusion staff for providing blood and human serum samples. We thank Ronak Abbasi for her technical assistance with mouse immunization and Zahra Ghorbanzadeh for her technical assistance with the SMFA. We also thank M. Saffari for English editing of the manuscript.
This work was financially supported by a grant (no. 886) from the Pasteur Institute of Iran (Tehran) to S. Zakeri and by a Ph.D. scholarship to S. Pirahmadi.
We declare that we have no conflict of interest.
REFERENCES
- 1.World Health Organization. 2017. World malaria report 2017. World Health Organization, Geneva, Switzerland: https://apps.who.int/iris/bitstream/10665/259492/1/9789241565523-eng.pdf. [Google Scholar]
- 2.World Health Organization. 2012. Global plan for insecticide resistance management in malaria vectors. World Health Organization, Geneva, Switzerland: https://www.who.int/malaria/publications/atoz/gpirm/en/. [Google Scholar]
- 3.Dondorp AM, Yeung S, White L, Nguon C, Day NP, Socheat D, von Seidlein L. 2010. Artemisinin resistance: current status and scenarios for containment. Nat Rev Microbiol 8:272–280. doi: 10.1038/nrmicro2331. [DOI] [PubMed] [Google Scholar]
- 4.Greenwood BM. 2008. Control to elimination: implications for malaria research. Trends Parasitol 24:449–454. doi: 10.1016/j.pt.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 5.malERA Consultative Group on Vaccines. 2011. A research agenda for malaria eradication: vaccines. PLoS Med 8:e1000398. doi: 10.1371/journal.pmed.1000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doolan DL, Southwood S, Freilich DA, Sidney J, Graber NL, Shatney L, Bebris L, Florens L, Dobano C, Witney AA, Appella E, Hoffman SL, Yates JR III, Carucci DJ, Sette A. 2003. Identification of Plasmodium falciparum antigens by antigenic analysis of genomic and proteomic data. Proc Natl Acad Sci U S A 100:9952–9957. doi: 10.1073/pnas.1633254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kariu T, Ishino T, Yano K, Chinzei Y, Yuda M. 2006. CelTOS, a novel malarial protein that mediates transmission to mosquito and vertebrate hosts. Mol Microbiol 59:1369–1379. doi: 10.1111/j.1365-2958.2005.05024.x. [DOI] [PubMed] [Google Scholar]
- 8.Bergmann-Leitner ES, Mease RM, De la Vega P, Savranskaya T, Polhemus M, Ockenhouse C, Angov E. 2010. Immunization with pre-erythrocytic antigen CelTOS from Plasmodium falciparum elicits cross-species protection against heterologous challenge with Plasmodium berghei. PLoS One 5:e12294. doi: 10.1371/journal.pone.0012294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergmann-Leitner ES, Legler PM, Savranskaya T, Ockenhouse CF, Angov E. 2011. Cellular and humoral immune effector mechanisms required for sterile protection against sporozoite challenge induced with the novel malaria vaccine candidate CelTOS. Vaccine 29:5940–5949. doi: 10.1016/j.vaccine.2011.06.053. [DOI] [PubMed] [Google Scholar]
- 10.Fox CB, Baldwin SL, Vedvick TS, Angov E, Reed SG. 2012. Effects on immunogenicity by formulations of emulsion-based adjuvants for malaria vaccines. Clin Vaccine Immunol 19:1633–1640. doi: 10.1128/CVI.00235-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espinosa DA, Vega-Rodriguez J, Flores-Garcia Y, Noe AR, Muñoz C, Coleman R, Bruck T, Haney K, Stevens A, Retallack D, Allen J, Vedvick TS, Fox CB, Reed SG, Howard RF, Salman AM, Janse CJ, Khan SM, Zavala F, Gutierrez GM. 2017. The Plasmodium falciparum cell-traversal protein for ookinetes and sporozoites as a candidate for preerythrocytic and transmission-blocking vaccines. Infect Immun 85:e00498-16. doi: 10.1128/IAI.00498-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Longley RJ, Salman AM, Cottingham MG, Ewer K, Janse CJ, Khan SM, Spencer AJ, Hill AV. 2015. Comparative assessment of vaccine vectors encoding ten malaria antigens identifies two protective liver-stage candidates. Sci Rep 5:11820. doi: 10.1038/srep11820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ewer KJ, Sierra-Davidson K, Salman AM, Illingworth JJ, Draper SJ, Biswas S, Hill AV. 2015. Progress with viral vectored malaria vaccines: a multi stage approach involving “unnatural immunity.” Vaccine 33:7444–7451. doi: 10.1016/j.vaccine.2015.09.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anum D, Kusi KA, Ganeshan H, Hollingdale MR, Ofori MF, Koram KA, Gyan BA, Adu-Amankwah S, Badji E, Huang J, Belmonte M, Banania GJ, Kwofie TB, Villasante E, Dodoo D, Sedegah M. 2015. Measuring naturally acquired ex vivo IFN-γ responses to Plasmodium falciparum cell-traversal protein for ookinetes and sporozoites (CelTOS) in Ghanaian adults. Malar J 14:20. doi: 10.1186/s12936-014-0539-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aguiar JC, Bolton J, Wanga J, Sacci JB, Iriko H, Mazeika JK, Han ET, Limbach K, Patterson NB, Sedegah M, Cruz AM, Tsuboi T, Hoffman SL, Carucci D, Hollingdale MR, Villasante ED, Richie TL. 2015. Discovery of novel Plasmodium falciparum pre-erythrocytic antigens for vaccine development. PLoS One 10:e0136109. doi: 10.1371/journal.pone.0136109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Draper SJ, Angov E, Horii T, Miller LH, Srinivasan P, Theisen M, Biswas S. 2015. Recent advances in recombinant protein-based malaria vaccines. Vaccine 33:7433–7443. doi: 10.1016/j.vaccine.2015.09.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Awate S, Babiuk LA, Mutwiri G. 2013. Mechanisms of action of adjuvants. Front Immunol 4:114. doi: 10.3389/fimmu.2013.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krieg AM. 2002. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol 20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 19.Dasari P, Nicholson IC, Hodge G, Dandie GW, Zola H. 2005. Expression of Toll-like receptors on B lymphocytes. Cell Immunol 236:140–145. doi: 10.1016/j.cellimm.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 20.Krieg AM. 2003. CpG DNA: trigger of sepsis, mediator of protection, or both? Scand J Infect Dis 35:653–659. doi: 10.1080/00365540310015999. [DOI] [PubMed] [Google Scholar]
- 21.Klinman DM. 2004. Use of CpG oligodeoxynucleotides as immunoprotective agents. Expert Opin Biol Ther 4:937–946. doi: 10.1517/14712598.4.6.937. [DOI] [PubMed] [Google Scholar]
- 22.Harandi AM, Holmgren J. 2004. CpG DNA as a potent inducer of mucosal immunity: implications for immunoprophylaxis and immunotherapy of mucosal infections. Curr Opin Investig Drugs 5:141–145. [PubMed] [Google Scholar]
- 23.Bode C, Zhao G, Steinhagen F, Kinjo T, Klinman DM. 2011. CpG DNA as a vaccine adjuvant. Expert Rev Vaccines 10:499–511. doi: 10.1586/erv.10.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halperin SA, Dobson S, McNeil S, Langley JM, Smith B, McCall-Sani R, Levitt D, Nest GV, Gennevois D, Eiden JJ. 2006. Comparison of the safety and immunogenicity of hepatitis B virus surface antigen co-administered with an immunostimulatory phosphorothioate oligonucleotide and a licensed hepatitis B vaccine in healthy young adults. Vaccine 24:20–26. doi: 10.1016/j.vaccine.2005.08.095. [DOI] [PubMed] [Google Scholar]
- 25.Barry M, Cooper C. 2007. Review of hepatitis B surface antigen-1018 ISS adjuvant-containing vaccine safety and efficacy. Expert Opin Biol Ther 7:1731–1737. doi: 10.1517/14712598.7.11.1731. [DOI] [PubMed] [Google Scholar]
- 26.Krieg AM. 2004. Antitumor applications of stimulating Toll-like receptor 9 with CpG oligodeoxynucleotides. Curr Oncol Rep 6:88–95. doi: 10.1007/s11912-004-0019-0. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt C. 2007. Clinical setbacks for Toll-like receptor 9 agonists in cancer. Nat Biotechnol 25:825–826. doi: 10.1038/nbt0807-825. [DOI] [PubMed] [Google Scholar]
- 28.Scheiermann J, Klinman DM. 2014. Clinical evaluation of CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases and cancer. Vaccine 32:6377–6389. doi: 10.1016/j.vaccine.2014.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta GK, Agrawal DK. 2010. CpG oligodeoxynucleotides as TLR9 agonists: therapeutic application in allergy and asthma. BioDrugs 24:225–235. doi: 10.2165/11536140-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 30.Duthie MS, Windish HP, Fox CB, Reed SG. 2011. Use of defined TLR ligands as adjuvants within human vaccines. Immunol Rev 239:178–196. doi: 10.1111/j.1600-065X.2010.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hopkins M, Lees BG, Richardson DG, Woroniecki SR, Wheeler AW. 2001. Standardisation of glutaraldehyde-modified tyrosine-adsorbed tree pollen vaccines containing the Th1-inducing adjuvant, monophosphoryl lipid A (MPL). Allergol Immunopathol (Madr) 29:245–254. doi: 10.1016/S0301-0546(01)79066-0. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell MS. 1998. Perspective on allogeneic melanoma lysates in active specific immunotherapy. Semin Oncol l25:623–635. [PubMed] [Google Scholar]
- 33.Abdulla S, Salim N, Machera F, Kamata R, Juma O, Shomari M, Kubhoja S, Mohammed A, Mwangoka G, Aebi T, Mshinda H, Schellenberg D, Carter T, Villafana T, Dubois MC, Leach A, Lievens M, Vekemans J, Cohen J, Ballou WR, Tanner M. 2013. Randomized, controlled trial of the long term safety, immunogenicity and efficacy of RTS,S/AS02(D) malaria vaccine in infants living in a malaria-endemic region. Malar J 12:11. doi: 10.1186/1475-2875-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mata E, Salvador A, Igartua M, Hernández RM, Pedraz JL. 2013. Malaria vaccine adjuvants: latest update and challenges in preclinical and clinical research. Biomed Res Int 2013:282913. doi: 10.1155/2013/282913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garçon N, Di Pasquale A. 2017. From discovery to licensure, the adjuvant system story. Hum Vaccin Immunother 13:19–33. doi: 10.1080/21645515.2016.1225635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee S, Nguyen MT. 2015. Recent advances of vaccine adjuvants for infectious diseases. Immune Netw 15:51–57. doi: 10.4110/in.2015.15.2.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fleck JD, Kauffmann C, Spilki F, Lencina CL, Roehe PM, Gosmann G. 2006. Adjuvant activity of Quillaja brasiliensis saponins on the immune responses to bovine herpes virus type 1 in mice. Vaccine 24:7129–7134. doi: 10.1016/j.vaccine.2006.06.059. [DOI] [PubMed] [Google Scholar]
- 38.Kensil CR, Kammer R. 1998. QS-21: a water-soluble triterpene glycoside adjuvant. Expert Opin Investig Drugs 7:1475–1482. doi: 10.1517/13543784.7.9.1475. [DOI] [PubMed] [Google Scholar]
- 39.Rajput ZI, Hu SH, Xiao CW, Arijo AG. 2007. Adjuvant effects of saponins on animal immune responses. J Zhejiang Univ Sci B 8:153–161. doi: 10.1631/jzus.2007.B0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gin DY, Slovin SF. 2011. Enhancing immunogenicity of cancer vaccines: QS-21 as an immune adjuvant. Curr Drug Ther 6:207–212. doi: 10.2174/157488511796391988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.den Brok MH, Büll C, Wassink M, de Graaf AM, Wagenaars JA, Minderman M, Thakur M, Amigorena S, Rijke EO, Schrier CC, Adema GJ. 2016. Saponin-based adjuvants induce cross-presentation in dendritic cells by intracellular lipid body formation. Nat Commun 7:13324. doi: 10.1038/ncomms13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu D, Tuo W. 2016. QS-21: a potent vaccine adjuvant. Nat Prod Chem Res 3:e113. doi: 10.4172/2329-6836.1000e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ogise J, Mumo R, Nyachieo A, Mutiso J, Kamau J, Onkoba N. 2016. Adjuvants in malaria vaccine development strategies: a review. Pathog Infect Dis 2:e1259. [Google Scholar]
- 44.Kim SK, Ragupathi G, Cappello S, Kagan E, Livingston PO. 2000. Effect of immunological adjuvant combinations on the antibody and T-cell response to vaccination with MUC1-KLH and GD3-KLH conjugates. Vaccine 19:530–537. doi: 10.1016/S0264-410X(00)00195-X. [DOI] [PubMed] [Google Scholar]
- 45.Slovin SF, Ragupathi G, Musselli C, Fernandez C, Diani M, Verbel D, Danishefsky S, Livingston P, Scher HI. 2005. Thomsen-Friedenreich (TF) antigen as a target for prostate cancer vaccine: clinical trial results with TF cluster (c)-KLH plus QS21 conjugate vaccine in patients with biochemically relapsed prostate cancer. Cancer Immunol Immunother 54:694–702. doi: 10.1007/s00262-004-0598-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burke RL, Goldbeck C, Ng P, Stanberry L, Ott G, Van Nest G. 1994. The influence of adjuvant on the therapeutic efficacy of a recombinant genital herpes vaccine. J Infect Dis 170:1110–1119. doi: 10.1093/infdis/170.5.1110. [DOI] [PubMed] [Google Scholar]
- 47.Pashine A, Valiante NM, Ulmer JB. 2005. Targeting the innate immune response with improved vaccine adjuvants. Nat Med 11:S63–S68. doi: 10.1038/nm1210. [DOI] [PubMed] [Google Scholar]
- 48.Vandepapelière P, Horsmans Y, Moris P, Van Mechelen M, Janssens M, Koutsoukos M, Van Belle P, Clement F, Hanon E, Wettendorff M, Garçon N, Leroux-Roels G. 2008. Vaccine adjuvant systems containing monophosphoryl lipid A and QS21 induce strong and persistent humoral and T cell responses against hepatitis B surface antigen in healthy adult volunteers. Vaccine 26:1375–1386. doi: 10.1016/j.vaccine.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 49.Querec T, Bennouna S, Alkan S, Laouar Y, Gorden K, Flavell R, Akira S, Ahmed R, Pulendran B. 2006. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J Exp Med 203:413–424. doi: 10.1084/jem.20051720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pirahmadi S, Zakeri S, Mehrizi AA, Karimi L, Djadid ND. 2019. Heterogeneity in the acquisition of naturally acquired antibodies to cell-traversal protein for ookinetes and sporozoites (CelTOS) and thrombospondin-related adhesion protein (TRAP) of Plasmodium falciparum in naturally infected patients from unstable malaria areas in Iran. Acta Trop 190:365–374. doi: 10.1016/j.actatropica.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 51.Garçon N, Leroux-Roels G, Cheng WF. 2011. Vaccine adjuvants. Perspect Vaccinol 1:89–113. doi: 10.1016/j.pervac.2011.05.004. [DOI] [Google Scholar]
- 52.Bergmann-Leitner ES, Hosie H, Trichilo J, Deriso E, Ranallo RT, Alefantis T, Savranskaya T, Grewal P, Ockenhouse CF, Venkatesan MM, Delvecchio VG, Angov E. 2013. Self-adjuvanting bacterial vectors expressing pre-erythrocytic antigens induce sterile protection against malaria. Front Immunol 4:176. doi: 10.3389/fimmu.2013.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bergmann-Leitner ES, Chaudhury S, Steers NJ, Sabato M, Delvecchio V, Wallqvist AS, Ockenhouse CF, Angov E. 2013. Computational and experimental validation of B and T-cell epitopes of the in vivo immune response to a novel malarial antigen. PLoS One 8:e71610. doi: 10.1371/journal.pone.0071610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fransen F, Boog CJ, van Putten JP, van der Ley P. 2007. Agonists of Toll-like receptors 3, 4, 7, and 9 are candidates for use as adjuvants in an outer membrane vaccine against Neisseria meningitidis serogroup B. Infect Immun 75:5939–5946. doi: 10.1128/IAI.00846-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rhee EG, Kelley RP, Agarwal I, Lynch DM, La Porte A, Simmons NL, Clark SL, Barouch DH. 2010. TLR4 ligands augment antigen-specific CD8+ T lymphocyte responses elicited by a viral vaccine vector. J Virol 84:10413–10419. doi: 10.1128/JVI.00928-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sugai T, Mori M, Nakazawa M, Ichino M, Naruto T, Kobayashi N, Kobayashi Y, Minami M, Yokota S. 2005. A CpG-containing oligodeoxynucleotide as an efficient adjuvant counterbalancing the Th1/Th2 immune response in diphtheria-tetanus-pertussis vaccine. Vaccine 23:5450–5456. doi: 10.1016/j.vaccine.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 57.Fogg CN, Americo JL, Lustig S, Huggins JW, Smith SK, Damon I, Resch W, Earl PL, Klinman DM, Moss B. 2007. Adjuvant-enhanced antibody responses to recombinant proteins correlates with protection of mice and monkeys to orthopox virus challenges. Vaccine 25:2787–2799. doi: 10.1016/j.vaccine.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moore A, McCarthy L, Mills KH. 1999. The adjuvant combination monophosphoryl lipid A and QS21 switches T cell responses induced with a soluble recombinant HIV protein from Th2 to Th1. Vaccine 17:2517–2527. doi: 10.1016/S0264-410X(99)00062-6. [DOI] [PubMed] [Google Scholar]
- 59.Singh M, O’Hagan DT. 2003. Recent advances in veterinary vaccine adjuvants. Int J Parasitol 33:469–478. doi: 10.1016/S0020-7519(03)00053-5. [DOI] [PubMed] [Google Scholar]
- 60.Sun HX, Xie Y, Ye YP. 2009. Advances in saponin-based adjuvants. Vaccine 27:1787–1796. doi: 10.1016/j.vaccine.2009.01.091. [DOI] [PubMed] [Google Scholar]
- 61.Hussain R, Dawood G, Abrar N, Toossi Z, Minai A, Dojki M, Ellner JJ. 1995. Selective increases in antibody isotypes and immunoglobulin G subclass responses to secreted antigens in tuberculosis patients and healthy household contacts of the patients. Clin Diagn Lab Immunol 2:726–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lambert P-H, Liu M, Siegrist C-A. 2005. Can successful vaccines teach us how to induce efficient protective immune responses? Nat Med 11:S54–S62. doi: 10.1038/nm1216. [DOI] [PubMed] [Google Scholar]
- 63.Casella CR, Mitchell TC. 2008. Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell Mol Life Sci 65:3231–3240. doi: 10.1007/s00018-008-8228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ulrich JT, Myers KR. 1995. Monophosphoryl lipid A as an adjuvant. Past experiences and new directions. Pharm Biotechnol 6:495–524. doi: 10.1007/978-1-4615-1823-5_21. [DOI] [PubMed] [Google Scholar]
- 65.Baldridge JR, McGowan P, Evans JT, Cluff C, Mossman S, Johnson D, Persing D. 2004. Taking a Toll on human disease: Toll-like receptor 4 agonists as vaccine adjuvants and monotherapeutic agents. Expert Opin Biol Ther 4:1129–1138. doi: 10.1517/14712598.4.7.1129. [DOI] [PubMed] [Google Scholar]
- 66.De Becker G, Moulin V, Pajak B, Bruck C, Francotte M, Thiriart C, Urbain J, Moser M. 2000. The adjuvant monophosphoryl lipid A increases the function of antigen-presenting cells. Int Immunol 12:807–815. doi: 10.1093/intimm/12.6.807. [DOI] [PubMed] [Google Scholar]
- 67.Chace JH, Hooker NA, Mildenstein KL, Krieg AM, Cowdery JS. 1997. Bacterial DNA-induced NK cell IFN-gamma production is dependent on macrophage secretion of IL-12. Clin Immunol Immunopathol 84:185–193. doi: 10.1006/clin.1997.4380. [DOI] [PubMed] [Google Scholar]
- 68.Ballas ZK, Rasmussen WL, Krieg AM. 1996. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J Immunol 157:1840–1845. [PubMed] [Google Scholar]
- 69.Cowdery JS, Chace JH, Yi AK, Krieg AM. 1996. Bacterial DNA induces NK cells to produce IFN-gamma in vivo and increases the toxicity of lipopolysaccharides. J Immunol 156:4570–4575. [PubMed] [Google Scholar]
- 70.Looareesuwan S, Sjostrom L, Krudsood S, Wilairatana P, Porter RS, Hills F, Warrell DA. 1999. Polyclonal anti-tumor necrosis factor-alpha Fab used as an ancillary treatment for severe malaria. Am J Trop Med Hyg 61:26–33. doi: 10.4269/ajtmh.1999.61.26. [DOI] [PubMed] [Google Scholar]
- 71.Cooper CL, Ahluwalia NK, Efler SM, Vollmer J, Krieg AM, Davis HL. 2008. Immunostimulatory effects of three classes of CpG oligodeoxynucleotides on PBMC from HCV chronic carriers. J Immune Based Ther Vaccines 6:3. doi: 10.1186/1476-8518-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Amsen D, Spilianakis CG, Flavell RA. 2009. How are T(H)1 and T(H)2 effector cells made? Curr Opin Immunol 21:153–160. doi: 10.1016/j.coi.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ansel KM, Djuretic I, Tanasa B, Rao A. 2006. Regulation of Th2 differentiation and Il4 locus accessibility. Annu Rev Immunol 24:607–656. doi: 10.1146/annurev.immunol.23.021704.115821. [DOI] [PubMed] [Google Scholar]
- 74.Schijns VE, Lavelle EC. 2011. Trends in vaccine adjuvants. Expert Rev Vaccines 10:539–550. doi: 10.1586/erv.11.21. [DOI] [PubMed] [Google Scholar]
- 75.Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, Villinger F, Murthy N, Steel J, Jacob J, Hogan RJ, García-Sastre A, Compans R, Pulendran B. 2011. Programming the magnitude and persistence of antibody responses with innate immunity. Nature 470:543–547. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garçon N, Chomez P, Van Mechelen M. 2007. GlaxoSmithKline adjuvant systems in vaccines: concepts, achievements and perspectives. Expert Rev Vaccines 6:723–739. doi: 10.1586/14760584.6.5.723. [DOI] [PubMed] [Google Scholar]
- 77.Hoffman SL, Vekemans J, Richie TL, Duffy PE. 2015. The march toward malaria vaccines. Vaccine 33:D13–D23. doi: 10.1016/j.vaccine.2015.07.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pasare C, Medzhitov R. 2005. Control of B-cell responses by Toll-like receptors. Nature 438:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 79.Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 80.Fivelman QL, McRobert L, Sharp S, Taylor CJ, Saeed M, Swales CA, Sutherland CJ, Baker DA. 2007. Improved synchronous production of Plasmodium falciparum gametocytes in vitro. Mol Biochem Parasitol 154:119–123. doi: 10.1016/j.molbiopara.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 81.Carter R, Miller LH. 1979. Evidence for environmental modulation of gametocytogenesis in Plasmodium falciparum in continuous culture. Bull World Health Organ 57:37–52. [PMC free article] [PubMed] [Google Scholar]