In the progression of the life cycle of Plasmodium falciparum, a small proportion of asexual parasites differentiate into male or female sexual forms called gametocytes. Just like their asexual counterparts, gametocytes are contained within the infected host’s erythrocytes (RBCs).
KEYWORDS: gametocytes, Plasmodium falciparum, transmission blocking, malaria
ABSTRACT
In the progression of the life cycle of Plasmodium falciparum, a small proportion of asexual parasites differentiate into male or female sexual forms called gametocytes. Just like their asexual counterparts, gametocytes are contained within the infected host’s erythrocytes (RBCs). However, unlike their asexual partners, they do not exit the RBC until they are taken up in a blood meal by a mosquito. In the mosquito midgut, they are stimulated to emerge from the RBC, undergo fertilization, and ultimately produce tens of thousands of sporozoites that are infectious to humans. This transmission cycle can be blocked by antibodies targeting proteins exposed on the parasite surface in the mosquito midgut, a process that has led to the development of candidate transmission-blocking vaccines (TBV), including some that are in clinical trials. Here we review the leading TBV antigens and highlight the ongoing search for additional gametocyte/gamete surface antigens, as well as antigens on the surfaces of gametocyte-infected erythrocytes, which can potentially become a new group of TBV candidates.
INTRODUCTION
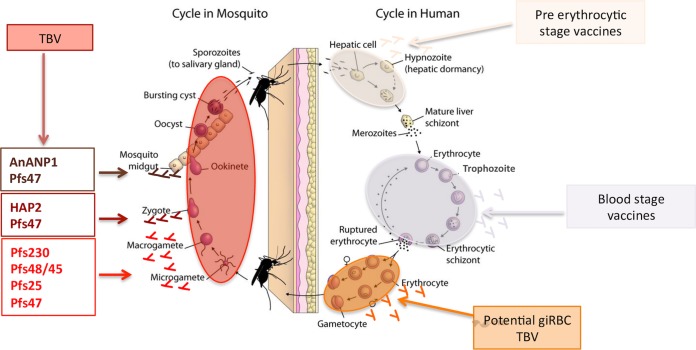
Malaria control challenges arising from a combination of antimalarial-drug resistance and insecticide resistance (1) have reignited interest in the development of effective malaria vaccines and drugs that target multiple stages of the parasite’s life cycle. Consequently, there has been increased focus on characterizing the sexual-stage parasite in order to gain an in-depth understanding of essential processes such as metabolism, sequestration, and infectiousness to the mosquito (2–5). The sexual-stage parasite begins its life cycle as sexually committed (sc) merozoites contained within an sc schizont in an infected red blood cell (RBC) (Fig. 1). When this sc schizont matures, it bursts the RBC and releases the sc merozoites, which subsequently invade surrounding RBCs. Within the RBC, sc ring-stage parasites form and then differentiate into mature transmissible gametocytes. For most Plasmodium parasites, the times required for gametocyte and asexual maturation are similar, and all erythrocytic stages continue to circulate in the peripheal blood. However, in the case of the most virulent human malaria parasite, Plasmodium falciparum, which is the primary focus of this review, an sc ring develops through five morphologically distinct developmental phases (stages I to V) over the course of 10 to 12 days to become a mature male or female gametocyte (6). Immature gametocytes (stages I to IV) are not observed in peripheral blood, and early histological studies, as well as recent molecular studies, have identified the bone marrow as a major sequestration site (7, 8). Apart from the bone marrow, immature gametocytes have been identified in other organs, such as the spleen, gut, brain, and heart (9). Following maturation to stage V, P. falciparum gametocytes are released and circulate in the blood for 4 to 6 days before dying. Continuous production with each asexual cycle ensures that mature stage V gametocyte-infected RBCs (giRBCs) circulate continuously for several weeks (5, 10), making them accessible for uptake when a female mosquito takes a blood meal. The transcriptomes and proteomes of gametocytes have been found to contain sexual-stage-specific gene transcripts and proteins, some of which are expressed only in either immature or mature gametocytes (11–16).
FIG 1.
Life cycle of P. falciparum. Pre-erythrocytic-stage malaria vaccines target the sporozoites that are released from an infected mosquito into the human host and infected liver cells. Blood-stage vaccines target the merozoites released from the liver schizont as well as preventing the development of the asexual-stage-infected erythrocyte and the resulting merozoites produced from the erythrocytic schizont. Potential giRBC vaccines will target male and female gametocytes that develop within the erythrocyte. Transmission-blocking vaccines prevent the eventual development of oocysts and sporozoites within the mosquito. (Adapted from an Open Courseware image from the Johns Hopkins Bloomberg School of Public Health [188].)
Once in the mosquito midgut, mature male and female gametocytes of all Plasmodium species egress out of the RBC and differentiate into male and female gametes, respectively. Subsequently, mating (fertilization) occurs, resulting in the production of a motile ookinete within 24 h. The ookinete migrates through the midgut epithelium and forms an oocyst, where sporozoites mature over the course of 10 to 12 days before they are released and migrate to the mosquito’s salivary glands. The infectous sporozoites are then released into a new host when the infected mosquito takes a blood meal.
The majority of current first-line antimalarial drugs are schizonticides that target the asexual parasite; a few of these, such as chloroquine and artemisinin, have some level of efficacy against early/young gametocytes (stages I, II, and III) (17), but not against late-stage gametocytes (stages IV and V) (18–20). The ineffectiveness of first-line antimalarials against transmissible mature gametocytes allows malaria transmission to continue despite the effective clearance of asexual parasites. Given the prolonged gametocyte maturation period in P. falciparum, a patient can remain infectious for more than a week after drug treatment (19, 20). It is thus imperative to develop new tools, such as transmission-blocking (TB) drugs and transmission-blocking vaccines (TBV), to effectively clear all gametocytes. A reduction in gametocyte carriage can result in reduced malaria transmission and the eventual eradication of the disease. This review focuses on P. falciparum malaria and covers well-known TBV candidate antigens as well as exploring a new class of gametocyte antigens, which may have the potential to elicit TB antibodies. The lack of an in vitro culture system for Plasmodium vivax, the other major cause of human malaria, has hindered de novo vaccine discovery. An effective alternative strategy has been to target the P. vivax homologs of P. falciparum TB candidates (21).
TRANSMISSION-BLOCKING VACCINE DEVELOPMENT
P. falciparum gametocyte and gamete surface antigens.
Emergence from the RBC within minutes of mosquito feeding effectively exposes all the proteins that were present on the gametocyte membrane to the contents of the blood meal, including antibodies and complement, which remain active in the midgut (22, 23). P230 and P48/45 are two of the best-characterized gametocyte membrane proteins that are exposed following gamete emergence from the RBC. The mosquito midgut environment also triggers the expression of additional gamete surface proteins from mRNA stored in the parasite’s cytoplasm, such as P25 (24) and HAP2 (25). In contrast to gametocyte membrane proteins, these gamete-specific proteins are not exposed to the immune response of the human host, and thus, antibodies are not expected to be naturally acquired but can be induced by vaccination. Antibodies recognizing either type of gamete surface antigen have been shown to block/prevent the completion of the sporogonic life cycle of the parasite within the mosquito and to result in the development of transmission-reducing (TR) immunity or complete TB immunity (TBI) upon vaccination (26–31). Another set of antibodies that have been found to prevent the completion of the sporogonic life cycle of the malaria parasite target mosquito midgut proteins, including Anopheles alanyl aminopeptidase N (AnAPN1) (32).
Parasite proteins expressed on the RBC surface during gametocyte development in the human host could be another type of TBI target antigen. It is well established that intraerythrocytic asexual parasites export proteins to the RBC surface, but evidence for this in giRBCs has been more elusive and represents an opportunity to identify novel vaccine candidates. Potential giRBC surface proteins are discussed in detail under “Potential giRBC surface antigens” below.
Transmission-blocking antibodies.
Early work using avian and murine Plasmodium species clearly demonstrated that anti-gamete antibodies bind to the surfaces of gametes and prevent the progression of parasite development within the mosquito midgut (33–35). The establishment of in vitro culture for the human malaria agent P. falciparum allowed the isolation of gametes that were used to produce murine monoclonal antibodies (MAbs) that recognized the gamete surface and also blocked oocyst production (35–38) in an experimental membrane-feeding assay. This assay allows mosquitoes to feed on gametocyte-infected red blood cells that have been mixed with test antibodies and is the gold standard for measuring TB activity (TBA) (39–41). Monoclonal antibodies with TBA or transmission-reducing activity (TRA) were then used to identify specific target proteins on the gamete surface: first Pfs48/45, Pfs230, and Pfs25, and later Pfs28 (Table 1). Pfs48/45, Pfs230, and Pfs25 are still being developed as TBV candidates and are discussed in detail below, while antibodies against Pfs28 were not found to be as as effective but did enhance the TBA of antibodies against Pfs25 (42). Polyclonal antibodies targeting the male gamete protein HAP2 and a MAb targeting female-specific Pfs47, a paralog of Pfs48/45, have been shown to reduce transmission significantly (43, 44) and are included in the discussion. An Anopheles midgut antigen, AnAPN1, with the potential to block the transmission of malaria in a parasite strain- and species-transcending manner (45), has also been identified and is discussed below.
TABLE 1.
Characteristics of some important sexual-stage antigens
| Gene (protein) | TMDa | Localization | Reference(s) |
|---|---|---|---|
| PF3D7_0209000 (Pfs230) | 0 | Low-level protein expression begins in stage IIb gametocytes and peaks in stage III gametocytes. Gene expression persists from early gametocytes through macrogametes to zygotes. | 2, 35, 36, 66, 159 |
| PF3D7_1031000 (Pfs25) | 1 | Surfaces of emerged (extracellular) macrogametes through to ookinetes. As with Pfs28, gene expression begins in stage V gametocytes but remains in a translationally repressed state until gametocyte egress from the giRBC. | 35, 100, 159, 160 |
| PF3D7_1346700 (Pfs48/45) | 1 | As with Pfs230, low-level protein expression begins in stage IIb gametocytes and increases in stage III gametocytes. Gene expression persists from male and female gametocytes through sporogonic macrogametes to the zygote. | 2, 35, 37, 71, 159 |
| PF3D7_1346800 (Pfs47) | 2 | Protein expressed on the surfaces of extracellular female gametocytes and gametes through to ookinetes. Low levels of gene transcripts are found in early stage II/III gametocytes, and levels are increased in stage IV/V. | 42, 79, 91 |
| Pf3D7_1014200 (HAP2) | 2 | Surfaces of male gametocytes and microgametes. | 44 |
Sera from Plasmodium-infected individuals have also been shown to have TRA (30, 31, 41, 46–48), which has been associated with high antibody titers against gametocyte surface antigens Pfs230 and/or Pfs48/45 in some studies (46, 47, 49). A recent study of plasma from adults living in an area of malaria endemicity confirmed that naturally acquired antibodies targeting recombinant Pfs230 region CMB (amino acids [aa] 444 to 730) and Pfs48/45 region 10C (aa 159 to 428) had TRA. However, the study also demonstrated that the antibodies that remained after immunodepletion of anti-CMB and 10C-reactive antibodies had TRA and recognized the surfaces of intact gametes lacking both Pfs48/45 and Pfs230. Together, the data suggest the presence of gamete surface antigens in addition to Pfs230 and Pfs48/45 that could be new TB targets (50). To identify potential candidates, a protein microarray was used to compare the reactivity profiles of plasma from two samples, one with ≥90% TRA and the other with <10% TRA. Antibody reactivity was higher against Pfs230, Pfs48/45, and 43 other gametocyte proteins in the plasma with high TRA. This set of proteins provides candidates that need to be further evaluated for their ability to induce antibodies that block transmission in the mosquito midgut and are discussed in detail under “Current transmission-blocking vaccine candidates” below.
In contrast to antigens expressed in both gametocytes and gametes, naturally induced antibody responses to Pfs25 and Pfs28 (Table 1) have not been reported (51, 52), which is expected, since the antigen is expressed only after gametocyte egress from the erythrocyte within the mosquito. This lack of exposure during a natural malaria infection has raised concerns about the ability of a vaccine targeting an antigen expressed only in the mosquito midgut to be boosted by a natural malaria infection. To overcome this lack of natural stimulation, repeat immunizations may be necessary. Antibodies that target mosquito midgut antigens are similarly not primed in the human host during an infection and will need repeated immunizations. The attractiveness of this class of antibodies is the potential to disrupt the sporogonic life cycle of all malaria parasites (45).
An alternative TB strategy is to target parasite antigens exposed on the surfaces of giRBCs that are accessible to antibodies. In contrast to the finding of the robust immune responses against antigens on the surfaces of erythrocytes infected with asexual-stage parasites (iRBCs) (53), few studies have identified natural immune responses against antigens on the surfaces of giRBCs (54). One study involving Gambian children found that antibodies from gametocyte carriers recognized the surfaces of mature gametocytes produced from the P. falciparum parasite strain 3D7 (55). Although the targets of the gametocyte-recognizing antibodies were not determined, the study supported the existence of gametocyte-specific antigens on the surfaces of giRBCs. If present, parasite-produced antigens on the giRBC surface would be good targets for TBV candidates (53), since the clearance of gametocytes from the circulation would result in the interruption of malaria transmission.
Transmission-blocking immunity.
The identification of MAbs that effectively block parasite transmission in a standard membrane-feeding assay (SMFA) clearly demonstrates the importance of antibodies in TBI, but the roles of other immune effector mechanisms are less well studied. There is no major histocompatibility complex class I (MHC-I) expression on the host RBCs, and therefore, gametocyte antigens cannot be presented directly to CD8+ T cells, suggesting a minimal role for CD8+ T-cell responses. However, in contrast to asexual parasites, unless mature intraerythrocytic stage V gametocytes are taken up by a mosquito, they die after circulating in the human host for 4 to 6 days and are cleared from the body. This provides antigen-presenting cells (APCs) the opportunity to take up gametocyte antigens and load them onto MHC-II molecules for presentation to CD4+ T cells. Once activated, CD4+ T cells could enhance antibody production and memory B-cell formation. Effector CD4+ T cells are also important sources of cytokines that activate macrophages and other immune effector cells. Although the role of CD4+ T cells in the induction of antibodies against asexual-stage P. falciparum parasites has been studied extensively (56), their role in inducing gametocyte-specific antibodies is not well established (57–59). In general, antibody responses against gametocyte antigens tend to be lower than anti-asexual-antigen responses, but the intensity and breadth of the response increases over the season, suggesting boosting during reexposure (60). The reduced response to gametocytes could be due to the low number of gametocytes produced during an infection, as well as to decreased CD4+ T-cell help. There is some evidence that titers of antibodies against some gametocyte antigens decline quickly (60, 61), but this decline is antigen and epitope specific, suggesting that it is not due to a general suppression of CD4+ T-cell help. The marked epitope specificity indicates that the interplay between antigens, B cells, APCs, and T cells needs to be tested for each candidate vaccine in order to determine the optimal immunization strategy.
The cytokines gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) are secreted from a number of cell types (including NK cells, γδ T cells, and macrophages) as well as Plasmodium-activated CD4+ T cells and have been identified as factors that inactivate gametocytes in symptomatic malaria infections (62). However, whether cytokine levels are altered by giRBC exposure remains an open question. Complement-mediated lysis of giRBCs has also been suggested (63), as well as nonopsonic phagocytosis of young (stage I and II) giRBCs (64). Immune components of the host’s blood, including antibodies, white blood cells, cytokines, reactive nitrogen species, and complement proteins, among others, are available to target all the various sporogonic parasite forms, as they remain active in the mosquito midgut hours after ingestion (65). The human complement system has been implicated in reducing transmission in the mosquito midgut, since the TR activity of antibodies against some antigens, such as Pfs230, requires complement (66, 67).
Current transmission-blocking vaccine candidates.
Initially, the four P. falciparum sexual-stage antigens identified as targets of transmission-blocking monoclonal antibodies were advanced as transmission-blocking malaria vaccine candidates: two (Pfs230 and Pfs48/45) are antigens whose expression begins in intracellular gametocytes within the human host, and the other two (Pfs25 and Pfs28) are antigens whose expression begins in extracellular gametocytes within the mosquito. Since then, three additional candidates have been discovered: two expressed by the parasite, HAP2 (32) and Pfs47 (68), and one mosquito midgut protein, AnAPN1 (69) (Fig. 2), which is discussed below. Homologs of all these antigens are also expressed by P. vivax, the other major cause of human malaria, and to date, all but HAP2 have also been reported to be targets of TRA antibodies (70, 71).
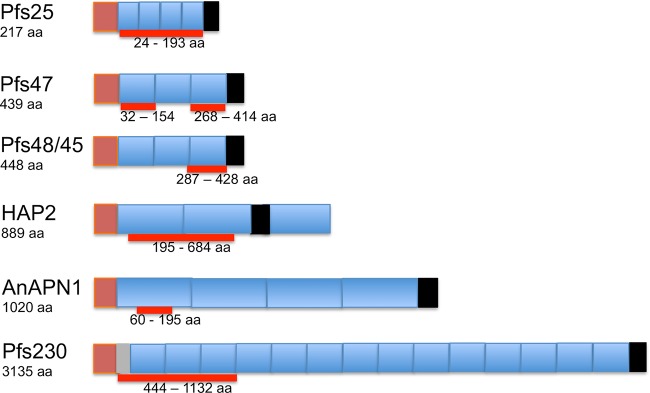
FIG 2.
Schematic of TBV gene structures. Brown segments represent the signal peptide motif; the gray segment represents the prodomain; blue segments represent the main structural domains; black segments represent the GPI motif or, for HAP2, the transmembrane domain. Thick red lines represent the regions containing the active epitopes.
GAMETOCYTE/GAMETE ANTIGENS Pfs230, Pfs48/45, Pfs47, AND HAP2
Pfs230, Pfs48/45, and Pfs47 belong to the 6-Cys s48/45 family of proteins (72). Some essential features of this family of proteins include their surface localization on exposed, extracelluar gametes (Table 1) and the presence of at least one s48/45 domain comprising 6 positionally conserved cysteine residues (73, 74). It was initially thought that the family comprised 10 members (72); however, 5 additional members have been identified (74). Although the functions of only a few family members are known (74), five members—Pfs230 and Pfs48/45, their respective paralogs Pf230p and Pfs47, and the putative secreted ookinete protein 12 (PSOP12)—are expressed on gametocytes, and their expression persists through the gamete to the zygote stage of the sexual life cycle of P. falciparum (36, 73, 74). Until recently, only Pfs230 and Pfs48/45 had been shown to be TB targets and had been very well characterized (37, 75–82). The monoclonal antibodies initially available against Pfs47 did not exhibit TB activity (83), and therefore, Pfs47 had not been the focus of much previous analysis. However, the transmission-blocking potential of Pfs47 has been demonstrated recently (43).
Pfs230.
Pfs230 is a 3,135-amino-acid protein encoded by a 9.4-kb gene and is predicted to be 363 kDa long (77). It is, however, truncated to 300 kDa as the gametes emerge from the red blood cells (80). Gene disruption studies showed that Pfs230 is retained on the surface of the parasite plasma membrane through interactions with Pfs48/45 and is critical for the formation of exflagellation centers in the male gametocyte (82). Pfs230 contains 14 s48/45 6-Cys domains (67), which is the maximum number that has been found among members of the 6-Cys s48/45 family (74). The numerous cysteine motif domains of the protein have made expression of full-length, correctly folded, soluble protein difficult, but fragment expression has been achieved (85–87). Through the expression of various maltose binding protein (MBP)-tagged fragments of Pfs230 in Escherichia coli, antibodies generated against a region designated “C” (aa 443 to 1132) were found to reduce transmissibility to mosquitoes by as much as 80% (88). Studies on various fragments of this region, expressed in a wheat germ cell-free expression system, also indicated that the N-terminal subdomain (aa 443 to 588) was sufficient to induce transmission-blocking activity (87). Higher concentrations of antibodies against this subdomain were found to correlate positively with the age of the host in a preliminary study and were not affected by deletion of one of the two “YGE” tripeptides (85). Optimization using the Pichia pastoris expression system identified recombinant Pfs230 aa 443 to 730 (Pfs230D1M) as a strong transmission-blocking vaccine candidate, and two formulations (Pfs230D1M conjugated to exoprotein A [Pfs230D1M-EPA]), one with aluminum hydroxide (Alhydrogel) (ClinicalTrials registration no. NCT02334462) and one with AS01 (ClinicalTrials registration no. NCT02942277), are currently in phase I clinical trials (67).
Pfs48/45.
Pfs48/45 is a 448-aa protein that is encoded by a 1.3-kb gene and is predicted to be 51 kDa long. It is a glycosylphosphatidylinositol (GPI)-anchored protein that interacts with Pfs230 and anchors it to the plasma membrane of the parasite (76). Pfs48/45 has been identified as a vital antigen required for male fertility (75). It contains three s48/45 domains and has three main epitopes. Antibodies against epitope I have the highest level of TB activity and are independently able to reduce gametocyte infectivity to mosquitoes in a complement-independent manner (89). Antibodies against epitopes II and III exhibit negligible TB activity independently (89). Epitope I has few polymorphisms, and antibody titers against epitope I have been found to be higher in adults than in younger people (85, 90), making it a suitable region for the production of a transmission-blocking vaccine candidate. A major challenge to the production of correctly folded, functional fragments of Pfs48/45 has been the generation of a construct with precise pairing of cysteine residues during disulfide bond formation, due to the presence of numerous cysteine residues. Initial success was achieved by using E. coli to produce the full-length protein without the secretory signal sequence (aa 27 to 427) (81), as well as constructs comprising aa 28 to 428 (16C) or aa 159 to 428 (10C) as chimeras, both individually fused to MBP and referred to as M-Pfs16C and M-Pfs10C, respectively (91). However, neither of these constructs could be scaled up using good manufacturing practices (GMP), due to incorrect protein folding (92). Subsequently, the 10C fragment (aa 159 to 428), which contains epitopes I and II, was fused to the nonrepititive region (R0) of P. falciparum glutamate-rich protein (GLURP; aa 26 to 500) and expressed in Lactococcus lactis with much better success at GMP than that for M-Pfs10C, although the yield of correctly folded protein was low, and extensive in vitro refolding and purification was required to increase it (92). In order to overcome the yield-associated challenges, the 10C fragment was further reduced to a 6C fragment (aa 287 to 428) that contained only epitope I, still fused to R0 (93). In order to obtain a purified 6C fragment, without the R0 fusion partner, a tobacco etch virus (TEV) protease site was inserted between R0 and the 6C fragment to obtain pure, correctly folded Pfs48/45 6C protein (85). Correctly folded full-length Pfs48/45 has recently been produced with high yields in insect cells (Drosophila S2 cells) and has been found to be the target for highly potent transmission-blocking antibodies (94). No construct has reached phase I clinical trials yet, although a number are in preclinical development (68).
Pfs47.
Pfs47 is a 439-amino-acid protein encoded by a 1.32-kb gene and predicted to be 50.8 kDa long. It is a GPI-anchored protein that is expressed only by female gametocytes and is retained on the surfaces of female gametes (83) through fertilization and development into ookinetes (95). Earlier studies of this antigen suggested a minimal role and functional redundancy, since gene disruption and monoclonal antibodies against this antigen did not lead to reduced mosquito infection, undermining its potential as a vaccine candidate (83). Subsequent genetic linkage and functional genomic studies, however, identified this antigen as the crucial antigen that protects the parasite from the mosquito’s hemolymph complement-like immune system (96). The antigen downregulates JNK signaling in mosquito midgut cells, which targets the invading ookinete for subsequent complement-like removal (97, 98). The gene encoding this antigen has been found to be highly polymorphic (with 42 haplotypes) (84), especially in the region encoding the second of the three 6-Cys domains, which shows high geographic diversity. It has been reported that Pfs47 mediates immune evasion in different mosquito species in a haplotype-dependent manner (99). A recent study demonstrated that the full-length protein expressed in E. coli as a chimera with thioredoxin protein was immunogenic but did not induce TB immunity. Monoclonal antibodies generated against the full-length protein did not have transmission-blocking activity and were found to bind s48/45 domain 1 or 3. Domain 2 was not tested initially, because it could not be generated in E. coli due to toxicity. This problem was overcome by expressing a section of domain 2 (aa 178 to 267) in which the two cysteines were replaced with alanines (mD2). The original MAbs generated against full-length protein did not recognize the recombinant, so mD2 was used to generate additional MAbs, some of which were found to have significant TRA, inhibiting fertilization and subsequent ookinete formation (43). Further analysis of domain 2 identified a smaller, relatively conserved 52-aa region (aa 178 to 229) that elicited TRA antibodies in mice, and this is currently a prospective transmission-blocking vaccine candidate. This antigen is undergoing preclinical development (100).
HAP2.
P. falciparum HAP2 (PfHAP2) is an 889-amino-acid protein encoded by a 1-intron, 2,816-bp gene. PfHAP2 belongs to the HAP2 family of proteins, which are class II viral fusion proteins with a cysteine-rich extracellular region (101). PfHAP2 is expressed only in male gametocytes and activated male gametes and therefore is referred to as the male gamete protein (44). The HAP2 gene is an essential gene, which enables the male microgamete to fuse with and fertilize the female macrogamete (25). The conserved “fusion/cd loop” region (aa 174 to 205 in Plasmodium berghei and aa 178 to 207 in P. falciparum) has been found to be essential for HAP2 function (102). Subsequently, a study showed that antibodies against aa 355 to 609 of P. berghei HAP2 inhibited oocyst formation in the mosquito in vivo, although this inhibition was reversed at low antibody dilutions (44). Another study produced a fragment of PfHAP2 (aa 195 to 684) representing the ortholog of P. berghei aa 355 to 604 in the wheat germ cell-free expression system (101). Purified IgG from sera obtained from mice immunized with PfHAP2 strongly inhibited (by 97%) oocyst formation in Anopheles mosquitoes in the presence of complement (32). Additional studies have shown that antibodies targeting the “cd loop” region (aa 178 to 195 in P. falciparum and aa 174 to 191 in P. berghei) possess transmission-blocking activity (101). No construct has reached phase I clinical trials yet.
Pfs25.
Pfs25 is a 217-aa protein encoded by a 0.65-kb gene and predicted to be a 24-kDa, GPI-anchored protein that belongs to a 13-member P25 family of proteins (103). This family of proteins is similar to the s48/45 family by virtue of having a relatively complex tertiary structure containing a large number of disulfide bridges (103). The protein is not expressed on the giRBC surface but rather is expressed after egress of the sexual-stage parasite from the giRBC (104). Vaccina virus was the first recombinant expression system found to generate correctly folded recombinant Pfs25 that bound to specific TB monoclonal antibodies (105, 106). Since then, expression in a variety of recombinant systems, including yeast (105, 107), plants (108), and algae (109), have been successful (Table 2). Monoclonal antibodies raised against correctly folded recombinant Pfs25 antigens, such as the highly effective 4B7, have been found to achieve potent TBA at high concentrations (105, 110), and MAb 4B7 is used as a reference for mosquito-feeding assays (40, 111, 112). Pfs25 is among the most advanced antigens in terms of TBV development (100); however, the lack of natural boosting within the human host continues to be a concern. A number of yeast- and plant-expressed Pfs25 products are in phase I clinical development; these include Pfs25-EPA formulated in Alhydrogel (ClinicalTrials registration no. NCT01434381, NCT01867463, and NCT02334462) or AS01 (ClinicalTrials registration no. NCT02942277) and chimeric Pfs25 fused in frame to the alfalfa mosaic virus coat protein and produced as a virus-like particle (VLP) formulated in Alhydrogel (ClinicalTrials registration no. NCT02013687). The initial trials using Alhydrogel formulations of Pfs-EPA or Pfs25-VLP showed that they were safe but did not stimulate robust TBA titers, and these results have led to the testing of alternative adjuvants, including AS01 (113–115).
TABLE 2.
Heterologous expression of some important sexual-stage antigens
| Antigen | Fragment/domain | Expression system(s)a | TBA (%)b | Hu. comp.c | Reference(s) |
|---|---|---|---|---|---|
| Pfs48/45 | Full length (aa 1–448) | Sf9 insect cells | ND | NA | 170 |
| aa 27–427 | Drosophila melanogaster | Yes (>96) | Yes | 94 | |
| M-Pfs10C (aa 159–428) | E. coli | Yes (>90) | Yes | 91 | |
| aa 118–218 | E. coli | ND | NA | 170 | |
| Full length (M-Pfs16C) | E. coli | ND | NA | 91 | |
| CH-rPfs48/45 (full length) | E. coli | Yes (>97) | Yes | 171 | |
| Epitope I (aa 287–428) | L. lactis | ND | NA | 85 | |
| Epitope I (aa 291–428) | L. lactis | Yes (>90) | No | 93 | |
| Epitope I+II (aa 159–428) | L. lactis | Yes (>90) | Yes | 92 | |
| C-terminal (domain III) | Chlamydomonas reinhardtii | ND | NA | 172 | |
| 16C (full length) | DNA based | Yes (>90) | Yes | 173 | |
| Pfs48F1 (aa 28–401) | Nicotiana benthamiana | ND | NA | 174 | |
| Full length | N. benthamiana | ND | NA | 175 | |
| Pfs48/45+NGln (aa 28–427) | MVA, ChAd63 | Yes (45.5) | Yes | 118 | |
| Pfs48/45-NGln (aa 28–427) | MVA, ChAd63 | No | Yes | 118 | |
| Pfs230 | Prodomain (aa 443–588) | L. lactis | ND | NA | 85 |
| Prodomain, C0 (aa 443–588) | WGCF system | Yes (≤82)d | Yes | 87 | |
| Region C (aa 443–1132) | WGCF system | Yes (≤99)d | Yes | 87 | |
| Pfs230C1 (aa 443–715) | WGCF system | Yes (≤91)d | Yes | 87 | |
| Pfs230C2 (aa 443–915) | WGCF system | Yes (≤79)d | Yes | 87 | |
| Region C (aa 443–1132) | E. coli | Yes (≤76) | Yes | 67 | |
| Pfs230D1–2 (aa 443–915) | E. coli | Yes (≤65) | Yes | 176 | |
| Region C (aa 443–1132) | DNA based | Yes (94.4) | Yes | 177 | |
| D1M (aa 542–736) | P. pastoris | Yes (≤100) | Yes | 176 | |
| Pfs230D1H (aa 443–736) | P. pastoris | Yes (≤100) | Yes | 176 | |
| 230CMB (aa 443–730) | Plant | Yes (≤100) | Yes | 86 | |
| Region C (aa 443–1132) | HEK293 cells | Yes (100) | Yes | 118 | |
| Pfs230C1 (aa 443–731) | Baculovirus | Yes (99.5) | Yes | 178 | |
| Pfs25 | Pfs25B (aa 22–190) | Yeast | Yes (≤100) | No | 105 |
| aa 22–193 | P. pastoris | Yes (≤100) | No | 179 | |
| TBV25H (aa 22–193) | Saccharomyces cerevisiae | Yes (≤100) | No | 180 | |
| aa 18–202 | DNA based | Yes (≤99) | Yes | 181 | |
| Full length | DNA based | Yes (≤99) | Yes | 173 | |
| aa 18–202 | WGCF system | Yes (≤100) | No | 182 | |
| aa 1–217 | Cell-free system | Yes (≤100) | No | 183 | |
| aa 24–193 | Cell-free system | Yes (≤32) | 183 | ||
| Pfs25-FhCMB (aa 23–193) | Plant | Yes (≤99) | No | 49 | |
| a-Pfs25 (aa 22–193) | Algae | Yes (≤100) | No | 184 | |
| CHrPfs25 (full length) | E. coli | Yes (≤100) | No | 185 | |
| Full length | E. coli | Yes (≤62)e | No | 186 | |
| Full length (aa 1–217) | Adenovirus | Yes (≤82.5) | No | 187 | |
| 4B7 and 1D2 (aa 122–134) | Adenovirus | Yes (≤78.1) | No | 187 | |
| aa 22–192 | MVA, ChAd63 | Yes (≤99) | Yes | 118 | |
| aa 22–193 | Baculovirus | Yes (≥98) | No | 179 | |
| Pfs47 | Full length (aa 28–414) | Insect cells | ND | No | 43 |
| Full length (aa 28–414) | E. coli | No | No | 43 | |
| Pfs47-mD2-Del1 (aa 178–267) | E. coli | Yes (87)f | Yes/no | 43 | |
| Pfs47-mD2-Del2 (aa 178–229) | E. coli | Yes (97)f | Yes/no | 43 | |
| Pfs47-mD2-Del3 (aa 155–181) | E. coli | No | Yes/no | 43 | |
| HAP2 | aa 195–684 | WGCF system | Yes (97) | NS | 32, 112 |
| AgAPN1 | aa 60–195 | E. coli | Yes (≤100) | Yes | 45 |
| aa 60–195 | E. coli | Yes (≤100) | Yes | 117 | |
| aa 60–195 | Bacteria | Yes (≤87) | NS | 69 | |
| rAnAPN160-942 | D. melanogaster | ND | NA | 45 | |
| AgAPN1 Nterm (aa 61–195) | MVA, ChAd63 | Yes (48)f | Yes/no | 118 | |
| AgAPN1 DomI (aa 20–303) | MVA, ChAd63 | No | Yes | 118 | |
| AgAPN1 (aa 20–998) | MVA, ChAd63 | No | Yes | 118 |
WGCF, wheat germ cell free; MVA, modified vaccinia virus Ankara; ChAd63, chimpanzee adenovirus 63.
ND, not determined.
Hu. comp., human complement; NA, not applicable; NS, not stated.
Reduced TBA in the absence of complement.
At low IgG concentrations.
Not affected by the presence or absence of complement.
AnAPN1.
The most advanced mosquito-based malaria transmission-blocking vacine candidate is the Anopheles alanyl aminopeptidase N (AnAPN1) (116), a member of the M1 family of metallopeptidases (69). AnAP1 is a 1,020-aa residue GPI-anchored protein predicted to be 113.5 kDa long. The gene is composed of four domains, designated domains I to IV, the N-terminal domain I (aa 57 to 270), domain II (aa 271 to 523), domain III (aa 524 to 613), and the C-terminal domain IV (aa 614 to 942). AnAPN1 is found on the apical surfaces of both sugar-fed and blood-fed Anopheles gambiae midguts and is a ligand for both murine and human Plasmodium ookinetes (69). Antibodies against a 135-aa fragment of the N-terminal domain I (aa 60 to 195) are capable of inhibiting the completion of the sporogonic life cycles of both P. falciparum and P. berghei in the mosquito (69, 117). Recent epitope mapping of AnAPN1 has identified aa 98 to 123 and aa 173 to 194 as targets of antibodies that block the binding of AnAPNI to the ookinete micronemal pore-forming protein (116). AnAPN1 has been expressed in a variety of expression systems, including E. coli, viral vectors (recombinant chimpanzee adenovirus 63 [ChAd63] and modified vaccina virus Ankara [MVA]) (118), and HEK293 human embryonic kidney cells, as either full-length protein or fragments including all or part of the N-terminal domain I. Antibodies against full-length AnAPN1 expressed in viral vectors did not exhibit TBA (118), while those expressed in bacteria exhibited 66% to 68% inhibition of P. falciparum oocyte development (69). Antibodies against the entire N-terminal domain I expressed in viral vectors similarly lacked TBA; however, antibodies against the 135-aa fragment of domain I (aa 61 to 196) expressed in viral vectors exhibited 45% TBA only at low antibody concentrations (118). Other studies, however, have demonstrated TBA as high as 100% for antibodies raised against the same 135-aa fragment of N-terminal domain I expressed in E. coli (45, 117). This antigen has not yet advanced to phase I clinical development.
NOVEL GAMETOCYTE AND GAMETE SURFACE ANTIGENS
A recent study aimed to identify novel TBV candidates by probing a protein microarray containing 315 P. falciparum gametocyte-related proteins with human plasma from an area of malaria endemicity (50). The antibody responses of plasma samples with high (≥90%) and low (<10%) TRA were compared, and 43 proteins, in addition to Pfs230 and Pfs48/45, showed were significantly higher levels in plasma samples with high TRA. Thirteen of these 43 proteins were highlighted as possible TBV candidates based on enriched expression in gametocytes and the presence of at least one predicted transmembrane domain (TMD) or a secretory signal sequence. Two of the 13 antigens, PF3D7_1021100 and PF3D7_1324600, were specifically predicted by go_cell_comp annotation to be surface exposed, and another two, Pf11-1 (PF3D7_1038400) and gamete surface and sporozoite traversal protein/GEST (PF3D7_1449000), have previously been functionally associated with gamete emergence in the mosquito midgut (50). Except for Pf11-1, the TBA of antibodies specific for any of these antigens has not been reported. Antibodies did recognize Pf11-1 on the gamete surface during emergence but did not exhibit potent TB responses (119). Given the large size of Pf11-1 and the recent demonstration of the marked specificity of the TB epitope in Pfs47, it may be worthwhile to reassess specific protein domains in Pf11-1 as TB antibody targets. Additional work is needed to carefully characterize these 13 antigens in order to validate their potential as TBV candidates.
PARASITE-SPECIFIC INFECTED-ERYTHROCYTE SURFACE ANTIGENS
After successful RBC invasion, P. falciparum develops and resides in the parasitophorous vacuole (PV), which separates it from the RBC cytoplasm. As the parasite grows, it actively enlarges the PV membrane (PVM) and exports proteins across the PVM to modify both the RBC cytoplasm and the plasma membrane (13, 120–122). A number of these exported, parasite-produced proteins are exposed on the RBC surface during asexual intracellular development. Many of these surface-exposed parasite proteins belong to large multigene families, notably the surface-associated interspersed protein (SURFIN) (123), the subtelomeric variable open reading frame (STEVOR) (124), Maurer’s cleft two-transmembrane domain (PfMC-2TM) (124), type A and B repetitive interspersed family (RIFIN) (125, 126), and P. falciparum erythrocyte membrane protein 1 (PfEMP1), which is encoded by var genes (127, 128). These multigene families are known to be highly polymorphic and thus, as a group, are simply called variant suface antigens (VSA). Erythrocyte surface exposure allows these genes to mediate the interaction of iRBCs with other cells, including uninfected RBCs and endothelial cells, and to serve as targets for immune responses. Antibodies against specific PfEMP1 family members are thought to be associated with allele-specific protection against malaria. However, the expression of only 1 of the 60 distinct PfEMP1 proteins encoded in the genome at a time, coupled with the extreme polymorphism in the population, allows immune evasion through the continual emergnce of parasites expressing distinct PfEMP1 proteins (129, 130). PfEMP1 also mediates endothelial cell adhesion, allowing iRBCs to be sequestered and thus avoid circulation (53) and clearance in the spleen. The roles of the other iRBC surface protein families are less well defined, especially for the SURFIN family, and can differ between family members. RIFIN and STEVOR family members have been identified that bind glycoproteins on the uninfected RBC surface and play roles in merozoite invasion and adhesion and iRBC sequestration (124, 131–134). Some members of the SURFIN family of proteins have also been suggested to be involved in merozoite invasion (123); however, more studies are needed to confirm this.
None of these protein families have been well characterized in giRBCs, but if exposed on the surface, they could be targets for transmission-blocking immunity, as seen with anti-PfEMP antibodies against asexual parasites. Initally, the sequestration of immature gametocytes until they mature to stage V was thought to be due to the presence of adhesins on the surfaces of immature giRBCs that are lost/degraded upon maturity (135). The inability to identify PfEMP1 family members on immature gametocytes (13), as well as the negligible binding of giRBCs to a variety of bone marrow-derived endothelial cells (135–138) and C32 melanoma cells (139), has given rise to an alternative theory, that sequestration is not dependent on adhesins. This alternative theory suggests that the rigidity of immature gametocytes keeps them embedded within the bone marrow until they mature to stage V, regain elasticity, and return to the circulation (135, 140). However, transcripts for members of the SURFIN, STEVOR, and RIFIN families have been found in gametocytes, and both indirect and live immunofluorescence assays (IFA) indicate that the proteins are expressed on the gametocyte surface (141) (Table 3). Recent proteomic data have identified a number of proteins encoded by conserved single-copy-number genes exposed on the asexual iRBC surface that may function as blood-stage malaria vaccines (127). To identify additional gametocyte-specific giRBC proteins, a similar, careful protemic analysis of giRBCs is needed.
TABLE 3.
Selected potential RBC surface proteins with transcripts in gametocytes
| Antigen | PlasmoDB ID | Expression profilea
|
TMD | |
|---|---|---|---|---|
| Gametocyte | Asexual | |||
| SURFIN | ||||
| SURFIN 1.1 | Pf3D7_0113100 | II/V | All (low) | Yes |
| SURFIN 1.3 | Pf3D7_0113600 | II/V | All (low) | No |
| SURFIN 8.2 | Pf3D7_0830800 | V | All (low) | Yes |
| SURFIN 13.1 | Pf3D7_1301800 | V | All (low) | Yes |
| STEVOR | ||||
| STEVOR | Pf3D7_0617600 | II | R, LT | Yes |
| STEVOR | Pf3D7_1040200 | II | R, ET, LT, Sc | Yes |
| RIFIN | ||||
| Type B | PF3D7_0425700 | II | R | Yes |
| Type A | PF3D7_0114700 | II (low) | R | Yes |
| Type B | PF3D7_0115200 | II (low) | R | Yes |
| Type B | Pf3D7_0222700 | V/II | R | Yes |
| Type NDb | PF3D7_0632300 | II/V | R | Yes |
| Type B | PF3D7_0900300 | II (low)/V (low) | R/Sc | Yes |
| Type B | PF3D7_0900500 | V | All (low) | Yes |
| Type A | PF3D7_0901000 | II (low)/V (low) | R/ET | Yes |
| Type B | PF3D7_1300600 | V/II | LT (low) | Yes |
| Type A | PF3D7_0115600 | II/V | R | Yes |
| Type A | PF3D7_0324800 | II/V | R, ET, LT | Yes |
| Other | ||||
| Conserved protein | PF3D7_1021100 | V | All | Yes |
| Export protein (EP) | PF3D7_1149100 | II/V | ET | No |
| EP (PHISTc) | PF3D7_0936800 | II/V | R | Yes |
| FIKK4.1 | PF3D7_0424500 | II/V | R | Yes |
| MESA | PF3D7_0500800 | II | ET/LT | No |
Roman numerals refer to stages: II, early gametocytes; V, mature gametocytes. All, all asexual-parasite stages; R, ring-stage asexual parasites; ET, early trophozoites; LT, late trophozoites; Sc, schizonts; low, low expression levels. The expression data are from Lopez-Barragan et al. (14) as published in PlasmoDB (142).
POTENTIAL giRBC SURFACE ANTIGENS
stevor/STEVOR.
There are 226 members of the subtelomeric variable open reading frame (STEVOR) protein family (142). They range from about 27-kDa to 35-kDa proteins and have been strongly predicted as possible gametocyte surface antigens (141, 143). STEVOR proteins have been identified on the surfaces of late-trophozoite- and schizont-infected erythrocytes (53), and some studies have also identified STEVOR proteins on the membranes of stage III to V giRBCs (141); however, limitations such as the availability of variant- and domain-specific reagents to ascertain the actual localization of the proteins weaken the report. Plasmodium falciparum is able to coexpress multiple stevor genes in a single parasite (53). Although the surface exposure of STEVOR proteins on giRBCs might suggest that STEVOR proteins play a role in gametocyte sequestration, the absence of STEVOR proteins on sequestrable early-stage (stage I and II) gametocytes (141) suggests that gametocyte sequestration is unlikely STEVOR dependent.
rif/RIFIN.
The repetitive interspersed (RIFIN) proteins belong to a family of 222 variable genes (142) that code for proteins ranging between 30 and 45 kDa (125, 144). These proteins, like the STEVOR proteins, have been identified on the surfaces of parasite-infected erythrocytes, merozoites, sporozoites, and gametocyes (15, 126, 145). RIFIN proteins have been suggested to be trafficked through the Maurer’s cleft (MC) (126, 146) to the surface of the parasitized RBC (pRBC) (125, 144). All RIFIN proteins were initially thought to possess two putative TMDs (147). However, it has recently been confirmed that although some variants possess two TMDs, others have one (148). The RIFIN proteins can be classified, based on sequence diversity, architecture, and cellular localization, into two subgroups, RIFIN-A (RIF_A) and RIFIN-B (RIF_B) (126, 148), which are most often coexpressed in a single parasite (149).
surf/SURFIN.
The surface-associated interspersed gene (surf) family encodes 13 high-molecular-weight proteins (about 280 to 300 kDa) known as SURFIN proteins (PlasmoDB). SURFIN proteins have structural similarities with other exported and surface-exposed Plasmodium proteins, such as an N-terminal signal sequence similar to that of P. vivax VIR (PvVIR) and tryptophan-rich domains (WRD) similar to those of PfEMP1, both of which are surface-exposed proteins (123). Indirect IFA on iRBCs were used to identify the localization of some surfins. SURFIN 4.2 was found to colocalize with PfEMP1 on the iRBC membrane (123), and SURFIN 4.1 was found within the PV (150) and the iRBC cytosol (151). In contrast to the observations for rifin and stevor genes, coexpression of multiple surf variants in a single isolate has not yet been reported, although several copy number variants have been identified (150). Just as with stevor and rifin genes, surf genes are generally differentially transcribed in different stages of the intraerythrocytic parasite (14). A few family members, such as surf 1.3, surf 4.2, and surf 8.3, are detected in all the various asexual intraerythrocytic stages of the parasite (150), as opposed to surf 8.2, which is preferentially expressed in mature stage V gametocytes, and surf 4.1, which is preferentially expressed in schizonts (14). As opposed to rifin and stevor genes, where all family members have Plasmodium export element (PEXEL) motifs, some surfins, such as surf 8.2, are PEXEL-negative exported proteins (PNEPs). Although surf 4.2 has two PEXEL motifs, translocation to the iRBC surface was found to be independent of either motifs (152). The high levels of surf 8.2 transcripts in gametocytes (14) and the presence of a TMD and a PNEP sequence suggest that SURFIN 8.2 is exposed on the surfaces of gametocyte-infected RBCs; however, this assumption has yet to be validated.
Insights/perspectives.
Additional effective transmission-blocking interventions are needed to complement current global malaria control and elimination efforts. A key process in the life cycle of P. falciparum parasites that enhances both disease and transmission is sequestration. Sequestration, which is thought to decrease recognition and clearance of the parasite by the spleen, has been extensively studied in the asexual parasite (127, 128, 153–157). Sequestration in the sexual-stage parasite has not gained as much attention (136), although it is assumed to be responsible for gametocyte development to maturity within the host without splenic clearance, which increases the likelihood of malaria transmission. A humanized mouse model with a human bone marrow transplant has recently been made available for the study of gametocyte sequestration (158) and should enable us to better understand its mechanisms. Although there is some indirect evidence of gametocyte-specific giRBC surface antigens (55, 159, 160), no specific antigens have been identified. Enhancing immune recognition of giRBCs by using vaccine-induced antibodies to target a gametocyte-specific giRBC surface antigen could reduce the prevalence of mature and infective gametocytes, leading to a reduction in malaria transmission. A few studies have identified antibody responses that are specific to gametocyte carriage (60, 161); however, the localization of these antigens on the giRBC or gamete is not currently known. Parasite diversity is a major challenge to malaria vaccine design (162). It has been observed that the licensed pre-erythrocytic-stage circumsporozoite protein (CSP) vaccine RTS,S is more effective against an infection with parasites whose genetic backbones are similar to that of the 3D7 vaccine strain than against genetically diverse parasites (163). This finding suggests that TBVs based on a single genetic backbone will likely be more effective against vaccine-like parasites. However, diversity in sexual-stage parasites is limited relative to that of asexual- and pre-erythocytic-stage antigens (164, 165), so TBVs based on sexual-stage parasites likely will be effective against more strains.
More efforts are needed to identify and characterize giRBCs as well as to continue to explore the gamete surface and mosquito midgut in order to aid in the development of effective TB interventions. It is also important to extend the analysis of candidates identified in Plasmodium falciparum to P. vivax, the most prevalent form of the malaria parasite circulating in Asia (166–168).
Conclusion.
Antigens on the surfaces of gametocytes and antigens exported by gametocytes to the surfaces of giRBCs have the potential to reduce and technically prevent malaria transmission. Apart from the well-described antigens Pfs25, Pfs230, and Pfs48/45, and the more recent demonstrations that HAP2, AnAPN1, and a region of Pfs47 may elicit TB antibodies, the presence of other gametocyte antigens that elicit TBA has yet to be discovered and/or validated. Identification of additional essential TB antigens could be important for the design and construction of effective TB vaccines.
ACKNOWLEDGMENTS
F.K.A. and J.A. are recipients of WACCBIP postgraduate fellowships, and K.C.W. and L.E.A. are supported by NIAID grant AI069314.
REFERENCES
- 1.Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Mao S, Sam B, Sopha C, Chuor CM, Nguon C, Sovannaroth S, Pukrittayakamee S, Jittamala P, Chotivanich K, Chutasmit K, Suchatsoonthorn C, Runcharoen R, Hien TT, Thuy-Nhien NT, Thanh NV, Phu NH, Htut Y, Han K-T, Aye KH, Mokuolu OA, Olaosebikan RR, Folaranmi OO, Mayxay M, Khanthavong M, Hongvanthong B, Newton PN, Onyamboko MA, Fanello CI, Tshefu AK, Mishra N, Valecha N, Phyo AP, Nosten F, Yi P, Tripura R, Borrmann S, Bashraheil M, Peshu J, Faiz MA, Ghose A, Hossain MA, Samad R, et al. 2014. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 371:411–423. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ngwa CJ, Rosa TF, Pradel G. 2016. The biology of malaria gametocytes, p 117–144. In Rodriguez-Morales AJ. (ed), Current topics in malaria. IntechOpen, London, United Kingdom. [Google Scholar]
- 3.Josling GA, Williamson KC, Llinas M. 2018. Regulation of sexual commitment and gametocytogenesis in malaria parasites. Annu Rev Microbiol 72:501–519. doi: 10.1146/annurev-micro-090817-062712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sinden RE. 2015. The cell biology of malaria infection of mosquito: advances and opportunities. Cell Microbiol 17:451–466. doi: 10.1111/cmi.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bousema T, Drakeley C. 2011. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin Microbiol Rev 24:377–410. doi: 10.1128/CMR.00051-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hawking F, Wilson ME, Gammage K. 1971. Evidence for cyclic development and short-lived maturity in the gametocytes of Plasmodium falciparum. Trans R Soc Trop Med Hyg 65:549–559. doi: 10.1016/0035-9203(71)90036-8. [DOI] [PubMed] [Google Scholar]
- 7.Aguilar R, Magallon-Tejada A, Achtman AH, Moraleda C, Joice R, Cisteró P, Li Wai Suen CSN, Nhabomba A, Macete E, Mueller I, Marti M, Alonso PL, Menéndez C, Schofield L, Mayor A. 2014. Molecular evidence for the localization of Plasmodium falciparum immature gametocytes in bone marrow. Blood 123:959–966. doi: 10.1182/blood-2013-08-520767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farfour E, Charlotte F, Settegrana C, Miyara M, Buffet P. 2012. The extravascular compartment of the bone marrow: a niche for Plasmodium falciparum gametocyte maturation? Malar J 11:285. doi: 10.1186/1475-2875-11-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joice R, Nilsson SK, Montgomery J, Dankwa S, Egan E, Morahan B, Seydel KB, Bertuccini L, Alano P, Williamson KC, Duraisingh MT, Taylor TE, Milner DA, Marti M. 2014. Plasmodium falciparum transmission stages accumulate in the human bone marrow. Sci Transl Med 6:244re5. doi: 10.1126/scitranslmed.3008882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bousema T, Okell L, Shekalaghe S, Griffin JT, Omar S, Sawa P, Sutherland C, Sauerwein R, Ghani AC, Drakeley C. 2010. Revisiting the circulation time of Plasmodium falciparum gametocytes: molecular detection methods to estimate the duration of gametocyte carriage and the effect of gametocytocidal drugs. Malar J 9:136. doi: 10.1186/1475-2875-9-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lasonder E, Ishihama Y, Andersen JS, Vermunt AM, Pain A, Sauerwein RW, Eling WM, Hall N, Waters AP, Stunnenberg HG, Mann M. 2002. Analysis of the Plasmodium falciparum proteome by high-accuracy mass spectrometry. Nature 419:537–542. doi: 10.1038/nature01111. [DOI] [PubMed] [Google Scholar]
- 12.Rothen J, Murie C, Carnes J, Anupama A, Abdulla S, Chemba M, Mpina M, Tanner M, Lee Sim BK, Hoffman SL, Gottardo R, Daubenberger C, Stuart K. 2018. Whole blood transcriptome changes following controlled human malaria infection in malaria pre-exposed volunteers correlate with parasite prepatent period. PLoS One 13:e0199392. doi: 10.1371/journal.pone.0199392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silvestrini F, Lasonder E, Olivieri A, Camarda G, van Schaijk B, Sanchez M, Younis Younis S, Sauerwein R, Alano P. 2010. Protein export marks the early phase of gametocytogenesis of the human malaria parasite Plasmodium falciparum. Mol Cell Proteomics 9:1437–1448. doi: 10.1074/mcp.M900479-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez-Barragan MJ, Lemieux J, Quinones M, Williamson KC, Molina-Cruz A, Cui K, Barillas-Mury C, Zhao K, Su XZ. 2011. Directional gene expression and antisense transcripts in sexual and asexual stages of Plasmodium falciparum. BMC Genomics 12:587. doi: 10.1186/1471-2164-12-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Florens L, Washburn MP, Raine JD, Anthony RM, Grainger M, Haynes JD, Moch JK, Muster N, Sacci JB, Tabb DL, Witney AA, Wolters D, Wu Y, Gardner MJ, Holder AA, Sinden RE, Yates JR, Carucci DJ. 2002. A proteomic view of the Plasmodium falciparum life cycle. Nature 419:520–526. doi: 10.1038/nature01107. [DOI] [PubMed] [Google Scholar]
- 16.Young JA, Fivelman QL, Blair PL, de la Vega P, Le Roch KG, Zhou Y, Carucci DJ, Baker DA, Winzeler EA. 2005. The Plasmodium falciparum sexual development transcriptome: a microarray analysis using ontology-based pattern identification. Mol Biochem Parasitol 143:67–79. doi: 10.1016/j.molbiopara.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Lucantoni L, Duffy S, Adjalley SH, Fidock DA, Avery VM. 2013. Identification of MMV malaria box inhibitors of Plasmodium falciparum early-stage gametocytes using a luciferase-based high-throughput assay. Antimicrob Agents Chemother 57:6050–6062. doi: 10.1128/AAC.00870-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butterworth AS, Skinner-Adams TS, Gardiner DL, Trenholme KR. 2013. Plasmodium falciparum gametocytes: with a view to a kill. Parasitology 140:1718–1734. doi: 10.1017/S0031182013001236. [DOI] [PubMed] [Google Scholar]
- 19.Okell LC, Drakeley CJ, Ghani AC, Bousema T, Sutherland CJ. 2008. Reduction of transmission from malaria patients by artemisinin combination therapies: a pooled analysis of six randomized trials. Malar J 7:125. doi: 10.1186/1475-2875-7-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen PQ, Li GQ, Guo XB, He KR, Fu YX, Fu LC, Song YZ. 1994. The infectivity of gametocytes of Plasmodium falciparum from patients treated with artemisinin. Chin Med J (Engl) 107:709–711. [PubMed] [Google Scholar]
- 21.Mueller I, Shakri AR, Chitnis CE. 2015. Development of vaccines for Plasmodium vivax malaria. Vaccine 33:7489–7495. doi: 10.1016/j.vaccine.2015.09.060. [DOI] [PubMed] [Google Scholar]
- 22.Sinden RE. 1982. Gametocytogenesis of Plasmodium falciparum in vitro: an electron microscopic study. Parasitology 84:1–11. doi: 10.1017/S003118200005160X. [DOI] [PubMed] [Google Scholar]
- 23.Aikawa M, Carter R, Ito Y, Nijhout MM. 1984. New observations on gametogenesis, fertilization, and zygote transformation in Plasmodium gallinaceum. J Protozool 31:403–413. doi: 10.1111/j.1550-7408.1984.tb02987.x. [DOI] [PubMed] [Google Scholar]
- 24.Mair GR, Braks JA, Garver LS, Wiegant JC, Hall N, Dirks RW, Khan SM, Dimopoulos G, Janse CJ, Waters AP. 2006. Regulation of sexual development of Plasmodium by translational repression. Science 313:667–669. doi: 10.1126/science.1125129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Tewari R, Ning J, Blagborough AM, Garbom S, Pei J, Grishin NV, Steele RE, Sinden RE, Snell WJ, Billker O. 2008. The conserved plant sterility gene HAP2 functions after attachment of fusogenic membranes in Chlamydomonas and Plasmodium gametes. Genes Dev 22:1051–1068. doi: 10.1101/gad.1656508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graves PM, Wirtz RA, Carter R, Burkot TR, Looker M, Targett GA. 1988. Naturally occurring antibodies to an epitope on Plasmodium falciparum gametes detected by monoclonal antibody-based competitive enzyme-linked immunosorbent assay. Infect Immun 56:2818–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roeffen W, Geeraedts F, Eling W, Beckers P, Wizel B, Kumar N, Lensen T, Sauerwein R. 1995. Transmission blockade of Plasmodium falciparum malaria by anti-Pfs230-specific antibodies is isotype dependent. Infect Immun 63:467–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roeffen W, Mulder B, Teelen K, Bolmer M, Eling W, Targett GA, Beckers PJ, Sauerwein R. 1996. Association between anti-Pfs48/45 reactivity and P. falciparum transmission-blocking activity in sera from Cameroon. Parasite Immunol 18:103–109. doi: 10.1046/j.1365-3024.1996.d01-54.x. [DOI] [PubMed] [Google Scholar]
- 29.Boudin C, Olivier M, Molez JF, Chiron JP, Ambroise-Thomas P. 1993. High human malarial infectivity to laboratory-bred Anopheles gambiae in a village in Burkina Faso. Am J Trop Med Hyg 48:700–706. doi: 10.4269/ajtmh.1993.48.700. [DOI] [PubMed] [Google Scholar]
- 30.Drakeley CJ, Akim NI, Sauerwein RW, Greenwood BM, Targett GA. 2000. Estimates of the infectious reservoir of Plasmodium falciparum malaria in The Gambia and in Tanzania. Trans R Soc Trop Med Hyg 94:472–476. doi: 10.1016/S0035-9203(00)90056-7. [DOI] [PubMed] [Google Scholar]
- 31.Mendis K, Munesinghe Y, De Silva Y, Keragalla I, Carter R. 1987. Malaria transmission-blocking immunity induced by natural infections of Plasmodium vivax in humans. Infect Immun 55:369–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miura K, Takashima E, Deng B, Tullo G, Diouf A, Moretz SE, Nikolaeva D, Diakite M, Fairhurst RM, Fay MP, Long CA, Tsuboi T. 2013. Functional comparison of Plasmodium falciparum transmission-blocking vaccine candidates by the standard membrane-feeding assay. Infect Immun 81:4377–4382. doi: 10.1128/IAI.01056-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gwadz RW, Carter R, Green I. 1979. Gamete vaccines and transmission-blocking immunity in malaria. Bull World Health Organ 57(Suppl 1):175–180. [PMC free article] [PubMed] [Google Scholar]
- 34.Mendis KN, Targett GA. 1979. Immunisation against gametes and asexual erythrocytic stages of a rodent malaria parasite. Nature 277:389–391. doi: 10.1038/277389a0. [DOI] [PubMed] [Google Scholar]
- 35.Kaushal DC, Carter R, Rener J, Grotendorst CA, Miller LH, Howard RJ. 1983. Monoclonal antibodies against surface determinants on gametes of Plasmodium gallinaceum block transmission of malaria parasites to mosquitoes. J Immunol 131:2557–2562. [PubMed] [Google Scholar]
- 36.Vermeulen AN, Ponnudurai T, Beckers PJ, Verhave JP, Smits MA, Meuwissen JH. 1985. Sequential expression of antigens on sexual stages of Plasmodium falciparum accessible to transmission-blocking antibodies in the mosquito. J Exp Med 162:1460–1476. doi: 10.1084/jem.162.5.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quakyi IA, Carter R, Rener J, Kumar N, Good MF, Miller LH. 1987. The 230-kDa gamete surface protein of Plasmodium falciparum is also a target for transmission-blocking antibodies. J Immunol 139:4213–4217. [PubMed] [Google Scholar]
- 38.Rener J, Graves PM, Carter R, Williams JL, Burkot TR. 1983. Target antigens of transmission-blocking immunity on gametes of Plasmodium falciparum. J Exp Med 158:976–981. doi: 10.1084/jem.158.3.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miura K, Swihart BJ, Deng B, Zhou L, Pham TP, Diouf A, Burton T, Fay MP, Long CA. 2016. Transmission-blocking activity is determined by transmission-reducing activity and number of control oocysts in Plasmodium falciparum standard membrane-feeding assay. Vaccine 34:4145–4151. doi: 10.1016/j.vaccine.2016.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miura K, Stone WJ, Koolen KM, Deng B, Zhou L, van Gemert GJ, Locke E, Morin M, Bousema T, Sauerwein RW, Long CA, Dechering KJ. 2016. An inter-laboratory comparison of standard membrane-feeding assays for evaluation of malaria transmission-blocking vaccines. Malar J 15:463. doi: 10.1186/s12936-016-1515-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bousema T, Dinglasan RR, Morlais I, Gouagna LC, van Warmerdam T, Awono-Ambene PH, Bonnet S, Diallo M, Coulibaly M, Tchuinkam T, Mulder B, Targett G, Drakeley C, Sutherland C, Robert V, Doumbo O, Toure Y, Graves PM, Roeffen W, Sauerwein R, Birkett A, Locke E, Morin M, Wu Y, Churcher TS. 2012. Mosquito feeding assays to determine the infectiousness of naturally infected Plasmodium falciparum gametocyte carriers. PLoS One 7:e42821. doi: 10.1371/journal.pone.0042821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duffy PE, Kaslow DC. 1997. A novel malaria protein, Pfs28, and Pfs25 are genetically linked and synergistic as falciparum malaria transmission-blocking vaccines. Infect Immun 65:1109–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Canepa GE, Molina-Cruz A, Yenkoidiok-Douti L, Calvo E, Williams AE, Burkhardt M, Peng F, Narum D, Boulanger MJ, Valenzuela JG, Barillas-Mury C. 2018. Antibody targeting of a specific region of Pfs47 blocks Plasmodium falciparum malaria transmission. NPJ Vaccines 3:26. doi: 10.1038/s41541-018-0065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blagborough AM, Sinden RE. 2009. Plasmodium berghei HAP2 induces strong malaria transmission-blocking immunity in vivo and in vitro. Vaccine 27:5187–5194. doi: 10.1016/j.vaccine.2009.06.069. [DOI] [PubMed] [Google Scholar]
- 45.Armistead JS, Morlais I, Mathias DK, Jardim JG, Joy J, Fridman A, Finnefrock AC, Bagchi A, Plebanski M, Scorpio DG, Churcher TS, Borg NA, Sattabongkot J, Dinglasan RR. 2014. Antibodies to a single, conserved epitope in Anopheles APN1 inhibit universal transmission of Plasmodium falciparum and Plasmodium vivax malaria. Infect Immun 82:818–829. doi: 10.1128/IAI.01222-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Drakeley CJ, Bousema JT, Akim NI, Teelen K, Roeffen W, Lensen AH, Bolmer M, Eling W, Sauerwein RW. 2006. Transmission-reducing immunity is inversely related to age in Plasmodium falciparum gametocyte carriers. Parasite Immunol 28:185–190. doi: 10.1111/j.1365-3024.2005.00818.x. [DOI] [PubMed] [Google Scholar]
- 47.Drakeley CJ, Eling W, Teelen K, Bousema JT, Sauerwein R, Greenwood BM, Targett GA. 2004. Parasite infectivity and immunity to Plasmodium falciparum gametocytes in Gambian children. Parasite Immunol 26:159–165. doi: 10.1111/j.0141-9838.2004.00696.x. [DOI] [PubMed] [Google Scholar]
- 48.Lin Ouedraogo A, Goncalves BP, Gneme A, Wenger EA, Guelbeogo MW, Ouedraogo A, Gerardin J, Bever CA, Lyons H, Pitroipa X, Verhave JP, Eckhoff PA, Drakeley C, Sauerwein R, Luty AJ, Kouyate B, Bousema T. 2016. Dynamics of the human infectious reservoir for malaria determined by mosquito feeding assays and ultrasensitive malaria diagnosis in Burkina Faso. J Infect Dis 213:90–99. doi: 10.1093/infdis/jiv370. [DOI] [PubMed] [Google Scholar]
- 49.Jones S, Grignard L, Nebie I, Chilongola J, Dodoo D, Sauerwein R, Theisen M, Roeffen W, Singh SK, Singh RK, Singh S, Kyei-Baafour E, Tetteh K, Drakeley C, Bousema T. 2015. Naturally acquired antibody responses to recombinant Pfs230 and Pfs48/45 transmission blocking vaccine candidates. J Infect 71:117–127. doi: 10.1016/j.jinf.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 50.Stone WJR, Campo JJ, Ouedraogo AL, Meerstein-Kessel L, Morlais I, Da D, Cohuet A, Nsango S, Sutherland CJ, van de Vegte-Bolmer M, Siebelink-Stoter R, van Gemert GJ, Graumans W, Lanke K, Shandling AD, Pablo JV, Teng AA, Jones S, de Jong RM, Fabra-Garcia A, Bradley J, Roeffen W, Lasonder E, Gremo G, Schwarzer E, Janse CJ, Singh SK, Theisen M, Felgner P, Marti M, Drakeley C, Sauerwein R, Bousema T, Jore MM. 2018. Unravelling the immune signature of Plasmodium falciparum transmission-reducing immunity. Nat Commun 9:558. doi: 10.1038/s41467-017-02646-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoffman SL, Vekemans J, Richie TL, Duffy PE. 2015. The march toward malaria vaccines. Am J Prev Med 49:S319–S333. doi: 10.1016/j.amepre.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carter R, Graves PM, Quakyi IA, Good MF. 1989. Restricted or absent immune responses in human populations to Plasmodium falciparum gamete antigens that are targets of malaria transmission-blocking antibodies. J Exp Med 169:135–147. doi: 10.1084/jem.169.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chan JA, Fowkes FJ, Beeson JG. 2014. Surface antigens of Plasmodium falciparum-infected erythrocytes as immune targets and malaria vaccine candidates. Cell Mol Life Sci 71:3633–3657. doi: 10.1007/s00018-014-1614-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baird JK, Jones TR, Purnomo Masbar S, Ratiwayanto S, Leksana B. 1991. Evidence for specific suppression of gametocytemia by Plasmodium falciparum in residents of hyperendemic Irian Jaya. Am J Trop Med Hyg 44:183–190. doi: 10.4269/ajtmh.1991.44.183. [DOI] [PubMed] [Google Scholar]
- 55.Saeed M, Roeffen W, Alexander N, Drakeley CJ, Targett GA, Sutherland CJ. 2008. Plasmodium falciparum antigens on the surface of the gametocyte-infected erythrocyte. PLoS One 3:e2280. doi: 10.1371/journal.pone.0002280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stevenson MM, Ing R, Berretta F, Miu J. 2011. Regulating the adaptive immune response to blood-stage malaria: role of dendritic cells and CD4+ Foxp3+ regulatory T cells. Int J Biol Sci 7:1311–1322. doi: 10.7150/ijbs.7.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu F, Liu T, Zhao C, Lu X, Zhang J, Xu W. 2017. Whole-killed blood-stage vaccine-induced immunity suppresses the development of malaria parasites in mosquitoes. J Immunol 198:300–307. doi: 10.4049/jimmunol.1600979. [DOI] [PubMed] [Google Scholar]
- 58.Targett GA, Harte PG, Eida S, Rogers NC, Ong CS. 1990. Plasmodium falciparum sexual stage antigens: immunogenicity and cell-mediated responses. Immunol Lett 25:77–81. doi: 10.1016/0165-2478(90)90095-8. [DOI] [PubMed] [Google Scholar]
- 59.Harte PG, Rogers NC, Targett GA. 1985. Role of T cells in preventing transmission of rodent malaria. Immunology 56:1–7. [PMC free article] [PubMed] [Google Scholar]
- 60.Skinner J, Huang CY, Waisberg M, Felgner PL, Doumbo OK, Ongoiba A, Kayentao K, Traore B, Crompton PD, Williamson KC. 2015. Plasmodium falciparum gametocyte-specific antibody profiling reveals boosting through natural infection and identifies potential markers of gametocyte exposure. Infect Immun 83:4229–4236. doi: 10.1128/IAI.00644-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Graves PM, Carter R, Burkot TR, Quakyi IA, Kumar N. 1988. Antibodies to Plasmodium falciparum gamete surface antigens in Papua New Guinea sera. Parasite Immunol 10:209–218. doi: 10.1111/j.1365-3024.1988.tb00215.x. [DOI] [PubMed] [Google Scholar]
- 62.Naotunne TS, Karunaweera ND, Mendis KN, Carter R. 1993. Cytokine-mediated inactivation of malarial gametocytes is dependent on the presence of white blood cells and involves reactive nitrogen intermediates. Immunology 78:555–562. [PMC free article] [PubMed] [Google Scholar]
- 63.Belachew EB. 2018. Immune response and evasion mechanisms of Plasmodium falciparum parasites. J Immunol Res 2018:6529681. doi: 10.1155/2018/6529681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith TG, Serghides L, Patel SN, Febbraio M, Silverstein RL, Kain KC. 2003. CD36-mediated nonopsonic phagocytosis of erythrocytes infected with stage I and IIA gametocytes of Plasmodium falciparum. Infect Immun 71:393–400. doi: 10.1128/IAI.71.1.393-400.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith RC, Vega-Rodríguez J, Jacobs-Lorena M. 2014. The Plasmodium bottleneck: malaria parasite losses in the mosquito vector. Mem Inst Oswaldo Cruz 109:644–661. doi: 10.1590/0074-0276130597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Read D, Lensen AH, Begarnie S, Haley S, Raza A, Carter R. 1994. Transmission-blocking antibodies against multiple, non-variant target epitopes of the Plasmodium falciparum gamete surface antigen Pfs230 are all complement-fixing. Parasite Immunol 16:511–519. doi: 10.1111/j.1365-3024.1994.tb00305.x. [DOI] [PubMed] [Google Scholar]
- 67.Williamson KC, Criscio MD, Kaslow DC. 1993. Cloning and expression of the gene for Plasmodium falciparum transmission-blocking target antigen, Pfs230. Mol Biochem Parasitol 58:355–358. doi: 10.1016/0166-6851(93)90058-6. [DOI] [PubMed] [Google Scholar]
- 68.Doumbo OK, Niaré K, Healy SA, Sagara I, Duffy PE. 2018. Malaria transmission-blocking vaccines: present status and future perspectives, p 288–298. In Manguin S, Dev V (ed), Towards malaria elimination: a leap forward. IntechOpen, London, United Kingdom. [Google Scholar]
- 69.Dinglasan RR, Kalume DE, Kanzok SM, Ghosh AK, Muratova O, Pandey A, Jacobs-Lorena M. 2007. Disruption of Plasmodium falciparum development by antibodies against a conserved mosquito midgut antigen. Proc Natl Acad Sci U S A 104:13461–13466. doi: 10.1073/pnas.0702239104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tachibana M, Suwanabun N, Kaneko O, Iriko H, Otsuki H, Sattabongkot J, Kaneko A, Herrera S, Torii M, Tsuboi T. 2015. Plasmodium vivax gametocyte proteins, Pvs48/45 and Pvs47, induce transmission-reducing antibodies by DNA immunization. Vaccine 33:1901–1908. doi: 10.1016/j.vaccine.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 71.Tachibana M, Sato C, Otsuki H, Sattabongkot J, Kaneko O, Torii M, Tsuboi T. 2012. Plasmodium vivax gametocyte protein Pvs230 is a transmission-blocking vaccine candidate. Vaccine 30:1807–1812. doi: 10.1016/j.vaccine.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 72.Templeton TJ, Kaslow DC. 1999. Identification of additional members define a Plasmodium falciparum gene superfamily which includes Pfs48/45 and Pfs230. Mol Biochem Parasitol 101:223–227. doi: 10.1016/S0166-6851(99)00066-3. [DOI] [PubMed] [Google Scholar]
- 73.van Dijk MR, van Schaijk BC, Khan SM, van Dooren MW, Ramesar J, Kaczanowski S, van Gemert GJ, Kroeze H, Stunnenberg HG, Eling WM, Sauerwein RW, Waters AP, Janse CJ. 2010. Three members of the 6-Cys protein family of Plasmodium play a role in gamete fertility. PLoS Pathog 6:e1000853. doi: 10.1371/journal.ppat.1000853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arredondo SA, Kappe S. 2017. The s48/45 six-cysteine proteins: mediators of interaction throughout the Plasmodium life cycle. Int J Parasitol 47:409–423. doi: 10.1016/j.ijpara.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Dijk MR, Janse CJ, Thompson J, Waters AP, Braks JA, Dodemont HJ, Stunnenberg HG, van Gemert GJ, Sauerwein RW, Eling W. 2001. A central role for P48/45 in malaria parasite male gamete fertility. Cell 104:153–164. doi: 10.1016/S0092-8674(01)00199-4. [DOI] [PubMed] [Google Scholar]
- 76.Kumar N. 1987. Target antigens of malaria transmission blocking immunity exist as a stable membrane bound complex. Parasite Immunol 9:321–335. doi: 10.1111/j.1365-3024.1987.tb00511.x. [DOI] [PubMed] [Google Scholar]
- 77.Williamson KC, Kaslow DC. 1993. Strain polymorphism of Plasmodium falciparum transmission-blocking target antigen Pfs230. Mol Biochem Parasitol 62:125–127. doi: 10.1016/0166-6851(93)90186-2. [DOI] [PubMed] [Google Scholar]
- 78.Williamson KC, Fujioka H, Aikawa M, Kaslow DC. 1996. Stage-specific processing of Pfs230, a Plasmodium falciparum transmission-blocking vaccine candidate. Mol Biochem Parasitol 78:161–169. doi: 10.1016/S0166-6851(96)02621-7. [DOI] [PubMed] [Google Scholar]
- 79.Bustamante PJ, Woodruff DC, Oh J, Keister DB, Muratova O, Williamson KC. 2000. Differential ability of specific regions of Plasmodium falciparum sexual-stage antigen, Pfs230, to induce malaria transmission-blocking immunity. Parasite Immunol 22:373–380. doi: 10.1046/j.1365-3024.2000.00315.x. [DOI] [PubMed] [Google Scholar]
- 80.Williamson KC. 2003. Pfs230: from malaria transmission-blocking vaccine candidate toward function. Parasite Immunol 25:351–359. doi: 10.1046/j.1365-3024.2003.00643.x. [DOI] [PubMed] [Google Scholar]
- 81.Outchkourov N, Vermunt A, Jansen J, Kaan A, Roeffen W, Teelen K, Lasonder E, Braks A, van de Vegte-Bolmer M, Qiu LY, Sauerwein R, Stunnenberg HG. 2007. Epitope analysis of the malaria surface antigen Pfs48/45 identifies a subdomain that elicits transmission blocking antibodies. J Biol Chem 282:17148–17156. doi: 10.1074/jbc.M700948200. [DOI] [PubMed] [Google Scholar]
- 82.Eksi S, Czesny B, Van Gemert GJ, Sauerwein RW, Eling W, Williamson KC. 2006. Malaria transmission‐blocking antigen, Pfs230, mediates human red blood cell binding to exflagellating male parasites and oocyst production. Mol Microbiol 61:991–998. doi: 10.1111/j.1365-2958.2006.05284.x. [DOI] [PubMed] [Google Scholar]
- 83.van Schaijk BC, van Dijk MR, van de Vegte-Bolmer M, van Gemert GJ, van Dooren MW, Eksi S, Roeffen WF, Janse CJ, Waters AP, Sauerwein RW. 2006. Pfs47, paralog of the male fertility factor Pfs48/45, is a female specific surface protein in Plasmodium falciparum. Mol Biochem Parasitol 149:216–222. doi: 10.1016/j.molbiopara.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 84.Anthony TG, Polley SD, Vogler AP, Conway DJ. 2007. Evidence of non-neutral polymorphism in Plasmodium falciparum gamete surface protein genes Pfs47 and Pfs48/45. Mol Biochem Parasitol 156:117–123. doi: 10.1016/j.molbiopara.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 85.Acquah FK, Obboh EK, Asare K, Boampong JN, Nuvor SV, Singh SK, Theisen M, Williamson KC, Amoah LE. 2017. Antibody responses to two new Lactococcus lactis-produced recombinant Pfs48/45 and Pfs230 proteins increase with age in malaria patients living in the Central Region of Ghana. Malar J 16:306. doi: 10.1186/s12936-017-1955-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Farrance CE, Rhee A, Jones RM, Musiychuk K, Shamloul M, Sharma S, Mett V, Chichester JA, Streatfield SJ, Roeffen W, van de Vegte-Bolmer M, Sauerwein RW, Tsuboi T, Muratova OV, Wu Y, Yusibov V. 2011. A plant-produced Pfs230 vaccine candidate blocks transmission of Plasmodium falciparum. Clin Vaccine Immunol 18:1351–1357. doi: 10.1128/CVI.05105-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tachibana M, Wu Y, Iriko H, Muratova O, MacDonald NJ, Sattabongkot J, Takeo S, Otsuki H, Torii M, Tsuboi T. 2011. N-terminal prodomain of Pfs230 synthesized using a cell-free system is sufficient to induce complement-dependent malaria transmission-blocking activity. Clin Vaccine Immunol 18:1343–1350. doi: 10.1128/CVI.05104-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Williamson KC, Keister DB, Muratova O, Kaslow DC. 1995. Recombinant Pfs230, a Plasmodium falciparum gametocyte protein, induces antisera that reduce the infectivity of Plasmodium falciparum to mosquitoes. Mol Biochem Parasitol 75:33–42. doi: 10.1016/0166-6851(95)02507-3. [DOI] [PubMed] [Google Scholar]
- 89.Carter R, Graves P, Keister D, Quakyi I. 1990. Properties of epitopes of Pfs 48/45, a target of transmission blocking monoclonal antibodies, on gametes of different isolates of Plasmodium falciparum. Parasite Immunol 12:587–603. doi: 10.1111/j.1365-3024.1990.tb00990.x. [DOI] [PubMed] [Google Scholar]
- 90.Kocken CH, Milek RL, Lensen TH, Kaslow DC, Schoenmakers JG, Konings RN. 1995. Minimal variation in the transmission-blocking vaccine candidate Pfs48/45 of the human malaria parasite Plasmodium falciparum. Mol Biochem Parasitol 69:115–118. doi: 10.1016/0166-6851(94)00193-Q. [DOI] [PubMed] [Google Scholar]
- 91.Outchkourov NS, Roeffen W, Kaan A, Jansen J, Luty A, Schuiffel D, van Gemert GJ, van de Vegte-Bolmer M, Sauerwein RW, Stunnenberg HG. 2008. Correctly folded Pfs48/45 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in mice. Proc Natl Acad Sci U S A 105:4301–4305. doi: 10.1073/pnas.0800459105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Theisen M, Roeffen W, Singh SK, Andersen G, Amoah L, van de Vegte-Bolmer M, Arens T, Tiendrebeogo RW, Jones S, Bousema T, Adu B, Dziegiel MH, Christiansen M, Sauerwein R. 2014. A multi-stage malaria vaccine candidate targeting both transmission and asexual parasite life-cycle stages. Vaccine 32:2623–2630. doi: 10.1016/j.vaccine.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 93.Singh SK, Roeffen W, Andersen G, Bousema T, Christiansen M, Sauerwein R, Theisen M. 2015. A Plasmodium falciparum 48/45 single epitope R0.6C subunit protein elicits high levels of transmission blocking antibodies. Vaccine 33:1981–1986. doi: 10.1016/j.vaccine.2015.02.040. [DOI] [PubMed] [Google Scholar]
- 94.Lennartz F, Brod F, Dabbs R, Miura K, Mekhaiel D, Marini A, Jore MM, Sogaard MM, Jorgensen T, de Jongh WA, Sauerwein RW, Long CA, Biswas S, Higgins MK. 2018. Structural basis for recognition of the malaria vaccine candidate Pfs48/45 by a transmission blocking antibody. Nat Commun 9:3822. doi: 10.1038/s41467-018-06340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Molina-Cruz A, Canepa GE, Barillas-Mury C. 2017. Plasmodium P47: a key gene for malaria transmission by mosquito vectors. Curr Opin Microbiol 40:168–174. doi: 10.1016/j.mib.2017.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Molina-Cruz A, Garver LS, Alabaster A, Bangiolo L, Haile A, Winikor J, Ortega C, van Schaijk BC, Sauerwein RW, Taylor-Salmon E, Barillas-Mury C. 2013. The human malaria parasite Pfs47 gene mediates evasion of the mosquito immune system. Science 340:984–987. doi: 10.1126/science.1235264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ramphul UN, Garver LS, Molina-Cruz A, Canepa GE, Barillas-Mury C. 2015. Plasmodium falciparum evades mosquito immunity by disrupting JNK-mediated apoptosis of invaded midgut cells. Proc Natl Acad Sci U S A 112:1273–1280. doi: 10.1073/pnas.1423586112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Smith RC, Jacobs-Lorena M. 2015. Malaria parasite Pfs47 disrupts JNK signaling to escape mosquito immunity. Proc Natl Acad Sci U S A 112:1250–1251. doi: 10.1073/pnas.1424227112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Molina-Cruz A, Canepa GE, Kamath N, Pavlovic NV, Mu J, Ramphul UN, Ramirez JL, Barillas-Mury C. 2015. Plasmodium evasion of mosquito immunity and global malaria transmission: the lock-and-key theory. Proc Natl Acad Sci U S A 112:15178–15183. doi: 10.1073/pnas.1520426112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Coelho CH, Doritchamou JYA, Zaidi I, Duffy PE. 2017. Advances in malaria vaccine development: report from the 2017 malaria vaccine symposium. NPJ Vaccines 2:34. doi: 10.1038/s41541-017-0035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Angrisano F, Sala KA, Da DF, Liu Y, Pei J, Grishin NV, Snell WJ, Blagborough AM. 2017. Targeting the conserved fusion loop of HAP2 inhibits the transmission of Plasmodium berghei and falciparum. Cell Rep 21:2868–2878. doi: 10.1016/j.celrep.2017.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fedry J, Liu Y, Pehau-Arnaudet G, Pei J, Li W, Tortorici MA, Traincard F, Meola A, Bricogne G, Grishin NV, Snell WJ, Rey FA, Krey T. 2017. The ancient gamete fusogen HAP2 is a eukaryotic class II fusion protein. Cell 168:904–915.e10. doi: 10.1016/j.cell.2017.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kaslow DC, Quakyi IA, Syin C, Raum MG, Keister DB, Coligan JE, McCutchan TF, Miller LH. 1988. A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature 333:74–76. doi: 10.1038/333074a0. [DOI] [PubMed] [Google Scholar]
- 104.Vermeulen AN, van Deursen J, Brakenhoff RH, Lensen TH, Ponnudurai T, Meuwissen JH. 1986. Characterization of Plasmodium falciparum sexual stage antigens and their biosynthesis in synchronised gametocyte cultures. Mol Biochem Parasitol 20:155–163. doi: 10.1016/0166-6851(86)90027-7. [DOI] [PubMed] [Google Scholar]
- 105.Barr PJ, Green KM, Gibson HL, Bathurst IC, Quakyi IA, Kaslow DC. 1991. Recombinant Pfs25 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in experimental animals. J Exp Med 174:1203–1208. doi: 10.1084/jem.174.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kaslow DC, Isaacs SN, Quakyi IA, Gwadz RW, Moss B, Keister DB. 1991. Induction of Plasmodium falciparum transmission-blocking antibodies by recombinant vaccinia virus. Science 252:1310–1313. doi: 10.1126/science.1925544. [DOI] [PubMed] [Google Scholar]
- 107.Stowers AW, Keister DB, Muratova O, Kaslow DC. 2000. A region of Plasmodium falciparum antigen Pfs25 that is the target of highly potent transmission-blocking antibodies. Infect Immun 68:5530–5538. doi: 10.1128/IAI.68.10.5530-5538.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Farrance CE, Chichester JA, Musiychuk K, Shamloul M, Rhee A, Manceva SD, Jones RM, Mamedov T, Sharma S, Mett V, Streatfield SJ, Roeffen W, van de Vegte-Bolmer M, Sauerwein RW, Wu Y, Muratova O, Miller L, Duffy P, Sinden R, Yusibov V. 2011. Antibodies to plant-produced Plasmodium falciparum sexual stage protein Pfs25 exhibit transmission blocking activity. Hum Vaccin 7(Suppl):191–198. doi: 10.4161/hv.7.0.14588. [DOI] [PubMed] [Google Scholar]
- 109.Patra KP, Li F, Carter D, Gregory JA, Baga S, Reed SG, Mayfield SP, Vinetz JM. 2015. Alga-produced malaria transmission-blocking vaccine candidate Pfs25 formulated with a human use-compatible potent adjuvant induces high-affinity antibodies that block Plasmodium falciparum infection of mosquitoes. Infect Immun 83:1799–1808. doi: 10.1128/IAI.02980-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Miura K, Keister DB, Muratova OV, Sattabongkot J, Long CA, Saul A. 2007. Transmission-blocking activity induced by malaria vaccine candidates Pfs25/Pvs25 is a direct and predictable function of antibody titer. Malar J 6:107. doi: 10.1186/1475-2875-6-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li Y, Leneghan DB, Miura K, Nikolaeva D, Brian IJ, Dicks MD, Fyfe AJ, Zakutansky SE, de Cassan S, Long CA, Draper SJ, Hill AV, Hill F, Biswas S. 2016. Enhancing immunogenicity and transmission-blocking activity of malaria vaccines by fusing Pfs25 to IMX313 multimerization technology. Sci Rep 6:18848. doi: 10.1038/srep18848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Miura K, Deng B, Tullo G, Diouf A, Moretz S, Locke E, Morin E, Fay MP, Long C. 2013. Qualification of standard membrane-feeding assay with Plasmodium falciaprum malaria and potential improvements for future assays. PLoS One 8:e57909. doi: 10.1371/journal.pone.0057909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sagara I, Healy SA, Assadou MH, Gabriel EE, Kone M, Sissoko K, Tembine I, Guindo MA, Doucoure M, Niare K, Dolo A, Rausch KM, Narum DL, Jones DL, MacDonald NJ, Zhu D, Mohan R, Muratova O, Baber I, Coulibaly MB, Fay MP, Anderson C, Wu Y, Traore SF, Doumbo OK, Duffy PE. 2018. Safety and immunogenicity of Pfs25H-EPA/Alhydrogel, a transmission-blocking vaccine against Plasmodium falciparum: a randomised, double-blind, comparator-controlled, dose-escalation study in healthy Malian adults. Lancet Infect Dis 18:969–982. doi: 10.1016/S1473-3099(18)30344-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chichester JA, Green BJ, Jones RM, Shoji Y, Miura K, Long CA, Lee CK, Ockenhouse CF, Morin MJ, Streatfield SJ, Yusibov V. 2018. Safety and immunogenicity of a plant-produced Pfs25 virus-like particle as a transmission blocking vaccine against malaria: a phase 1 dose-escalation study in healthy adults. Vaccine 36:5865–5871. doi: 10.1016/j.vaccine.2018.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Talaat KR, Ellis RD, Hurd J, Hentrich A, Gabriel E, Hynes NA, Rausch KM, Zhu D, Muratova O, Herrera R, Anderson C, Jones D, Aebig J, Brockley S, MacDonald NJ, Wang X, Fay MP, Healy SA, Durbin AP, Narum DL, Wu Y, Duffy PE. 2016. Safety and immunogenicity of Pfs25-EPA/Alhydrogel, a transmission blocking vaccine against Plasmodium falciparum: an open label study in malaria naive adults. PLoS One 11:e0163144. doi: 10.1371/journal.pone.0163144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Atkinson SC, Armistead JS, Mathias DK, Sandeu MM, Tao D, Borhani-Dizaji N, Tarimo BB, Morlais I, Dinglasan RR, Borg NA. 2015. The Anopheles-midgut APN1 structure reveals a new malaria transmission-blocking vaccine epitope. Nat Struct Mol Biol 22:532–539. doi: 10.1038/nsmb.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mathias DK, Plieskatt JL, Armistead JS, Bethony JM, Abdul-Majid KB, McMillan A, Angov E, Aryee MJ, Zhan B, Gillespie P, Keegan B, Jariwala AR, Rezende W, Bottazzi ME, Scorpio DG, Hotez PJ, Dinglasan RR. 2012. Expression, immunogenicity, histopathology, and potency of a mosquito-based malaria transmission-blocking recombinant vaccine. Infect Immun 80:1606–1614. doi: 10.1128/IAI.06212-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kapulu MC, Da DF, Miura K, Li Y, Blagborough AM, Churcher TS, Nikolaeva D, Williams AR, Goodman AL, Sangare I, Turner AV, Cottingham MG, Nicosia A, Straschil U, Tsuboi T, Gilbert SC, Long CA, Sinden RE, Draper SJ, Hill AV, Cohuet A, Biswas S. 2015. Comparative assessment of transmission-blocking vaccine candidates against Plasmodium falciparum. Sci Rep 5:11193. doi: 10.1038/srep11193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Feng Z, Hoffmann RN, Nussenzweig RS, Tsuji M, Fujioka H, Aikawa M, Lensen TH, Ponnudurai T, Pologe LG. 1993. Pfs2400 can mediate antibody-dependent malaria transmission inhibition and may be the Plasmodium falciparum 11.1 gene product. J Exp Med 177:273–281. doi: 10.1084/jem.177.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gruring C, Heiber A, Kruse F, Ungefehr J, Gilberger TW, Spielmann T. 2011. Development and host cell modifications of Plasmodium falciparum blood stages in four dimensions. Nat Commun 2:165. doi: 10.1038/ncomms1169. [DOI] [PubMed] [Google Scholar]
- 121.Marti M, Good RT, Rug M, Knuepfer E, Cowman AF. 2004. Targeting malaria virulence and remodeling proteins to the host erythrocyte. Science 306:1930–1933. doi: 10.1126/science.1102452. [DOI] [PubMed] [Google Scholar]
- 122.Spielmann T, Gilberger T-W. 2010. Protein export in malaria parasites: do multiple export motifs add up to multiple export pathways? Trends Parasitol 26:6–10. doi: 10.1016/j.pt.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 123.Winter G, Kawai S, Haeggstrom M, Kaneko O, von Euler A, Kawazu S, Palm D, Fernandez V, Wahlgren M. 2005. SURFIN is a polymorphic antigen expressed on Plasmodium falciparum merozoites and infected erythrocytes. J Exp Med 201:1853–1863. doi: 10.1084/jem.20041392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bachmann A, Scholz JAM, Janßen M, Klinkert M-Q, Tannich E, Bruchhaus I, Petter M. 2015. A comparative study of the localization and membrane topology of members of the RIFIN, STEVOR and PfMC-2TM protein families in Plasmodium falciparum-infected erythrocytes. Malar J 14:274. doi: 10.1186/s12936-015-0784-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fernandez V, Hommel M, Chen Q, Hagblom P, Wahlgren M. 1999. Small, clonally variant antigens expressed on the surface of the Plasmodium falciparum-infected erythrocyte are encoded by the rif gene family and are the target of human immune responses. J Exp Med 190:1393–1404. doi: 10.1084/jem.190.10.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Petter M, Haeggstrom M, Khattab A, Fernandez V, Klinkert MQ, Wahlgren M. 2007. Variant proteins of the Plasmodium falciparum RIFIN family show distinct subcellular localization and developmental expression patterns. Mol Biochem Parasitol 156:51–61. doi: 10.1016/j.molbiopara.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 127.Nilsson Bark SK, Ahmad R, Dantzler K, Lukens AK, De Niz M, Szucs MJ, Jin X, Cotton J, Hoffmann D, Bric-Furlong E, Oomen R, Parrington M, Milner D, Neafsey DE, Carr SA, Wirth DF, Marti M. 2018. Quantitative proteomic profiling reveals novel Plasmodium falciparum surface antigens and possible vaccine candidates. Mol Cell Proteomics 17:43–60. doi: 10.1074/mcp.RA117.000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wahlgren M, Goel S, Akhouri RR. 2017. Variant surface antigens of Plasmodium falciparum and their roles in severe malaria. Nat Rev Microbiol 15:479–491. doi: 10.1038/nrmicro.2017.47. [DOI] [PubMed] [Google Scholar]
- 129.Baruch DI, Pasloske BL, Singh HB, Bi X, Ma XC, Feldman M, Taraschi TF, Howard RJ. 1995. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell 82:77–87. doi: 10.1016/0092-8674(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 130.Smith JD, Chitnis CE, Craig AG, Roberts DJ, Hudson-Taylor DE, Peterson DS, Pinches R, Newbold CI, Miller LH. 1995. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell 82:101–110. doi: 10.1016/0092-8674(95)90056-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Khattab A, Bonow I, Schreiber N, Petter M, Schmetz C, Klinkert MQ. 2008. Plasmodium falciparum variant STEVOR antigens are expressed in merozoites and possibly associated with erythrocyte invasion. Malar J 7:137. doi: 10.1186/1475-2875-7-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Niang M, Yan Yam X, Preiser PR. 2009. The Plasmodium falciparum STEVOR multigene family mediates antigenic variation of the infected erythrocyte. PLoS Pathog 5:e1000307. doi: 10.1371/journal.ppat.1000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Goel S, Palmkvist M, Moll K, Joannin N, Lara P, Akhouri RR, Moradi N, Öjemalm K, Westman M, Angeletti D, Kjellin H, Lehtiö J, Blixt O, Ideström L, Gahmberg CG, Storry JR, Hult AK, Olsson ML, von Heijne G, Nilsson I, Wahlgren M. 2015. RIFINs are adhesins implicated in severe Plasmodium falciparum malaria. Nat Med 21:314. doi: 10.1038/nm.3812. [DOI] [PubMed] [Google Scholar]
- 134.Mwakalinga SB, Wang CW, Bengtsson DC, Turner L, Dinko B, Lusingu JP, Arnot DE, Sutherland CJ, Theander TG, Lavstsen T. 2012. Expression of a type B RIFIN in Plasmodium falciparum merozoites and gametes. Malar J 11:429. doi: 10.1186/1475-2875-11-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tiburcio M, Silvestrini F, Bertuccini L, Sander AF, Turner L, Lavstsen T, Alano P. 2013. Early gametocytes of the malaria parasite Plasmodium falciparum specifically remodel the adhesive properties of infected erythrocyte surface. Cell Microbiol 15:647–659. doi: 10.1111/cmi.12062. [DOI] [PubMed] [Google Scholar]
- 136.Silvestrini F, Tiburcio M, Bertuccini L, Alano P. 2012. Differential adhesive properties of sequestered asexual and sexual stages of Plasmodium falciparum on human endothelial cells are tissue independent. PLoS One 7:e31567. doi: 10.1371/journal.pone.0031567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rogers NJ, Hall BS, Obiero J, Targett GA, Sutherland CJ. 2000. A model for sequestration of the transmission stages of Plasmodium falciparum: adhesion of gametocyte-infected erythrocytes to human bone marrow cells. Infect Immun 68:3455–3462. doi: 10.1128/IAI.68.6.3455-3462.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Day KP, Hayward RE, Smith D, Culvenor JG. 1998. CD36-dependent adhesion and knob expression of the transmission stages of Plasmodium falciparum is stage specific. Mol Biochem Parasitol 93:167–177. doi: 10.1016/S0166-6851(98)00040-1. [DOI] [PubMed] [Google Scholar]
- 139.Hayward RE, Tiwari B, Piper KP, Baruch DI, Day KP. 1999. Virulence and transmission success of the malarial parasite Plasmodium falciparum. Proc Natl Acad Sci U S A 96:4563–4568. doi: 10.1073/pnas.96.8.4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tiburcio M, Niang M, Deplaine G, Perrot S, Bischoff E, Ndour PA, Silvestrini F, Khattab A, Milon G, David PH, Hardeman M, Vernick KD, Sauerwein RW, Preiser PR, Mercereau-Puijalon O, Buffet P, Alano P, Lavazec C. 2012. A switch in infected erythrocyte deformability at the maturation and blood circulation of Plasmodium falciparum transmission stages. Blood 119:e172–e180. doi: 10.1182/blood-2012-03-414557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.McRobert L, Preiser P, Sharp S, Jarra W, Kaviratne M, Taylor MC, Renia L, Sutherland CJ. 2004. Distinct trafficking and localization of STEVOR proteins in three stages of the Plasmodium falciparum life cycle. Infect Immun 72:6597–6602. doi: 10.1128/IAI.72.11.6597-6602.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Aurrecoechea C, Brestelli J, Brunk BP, Dommer J, Fischer S, Gajria B, Gao X, Gingle A, Grant G, Harb OS, Heiges M, Innamorato F, Iodice J, Kissinger JC, Kraemer E, Li W, Miller JA, Nayak V, Pennington C, Pinney DF, Roos DS, Ross C, Stoeckert CJ Jr, Treatman C, Wang H. 2009. PlasmoDB: a functional genomic database for malaria parasites. Nucleic Acids Res 37:D539–D543. doi: 10.1093/nar/gkn814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sutherland CJ. 2001. Stevor transcripts from Plasmodium falciparum gametocytes encode truncated polypeptides. Mol Biochem Parasitol 113:331–335. doi: 10.1016/S0166-6851(01)00225-0. [DOI] [PubMed] [Google Scholar]
- 144.Kyes SA, Rowe JA, Kriek N, Newbold CI. 1999. Rifins: a second family of clonally variant proteins expressed on the surface of red cells infected with Plasmodium falciparum. Proc Natl Acad Sci U S A 96:9333–9338. doi: 10.1073/pnas.96.16.9333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang CW, Mwakalinga SB, Sutherland CJ, Schwank S, Sharp S, Hermsen CC, Sauerwein RW, Theander TG, Lavstsen T. 2010. Identification of a major rif transcript common to gametocytes and sporozoites of Plasmodium falciparum. Malar J 9:147. doi: 10.1186/1475-2875-9-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Haeggström M, Kironde F, Berzins K, Chen Q, Wahlgren M, Fernandez V. 2004. Common trafficking pathway for variant antigens destined for the surface of the Plasmodium falciparum-infected erythrocyte. Mol Biochem Parasitol 133:1–14. doi: 10.1016/j.molbiopara.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 147.Horrocks P, Muhia D. 2005. Pexel/VTS: a protein-export motif in erythrocytes infected with malaria parasites. Trends Parasitol 21:396–399. doi: 10.1016/j.pt.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 148.Bultrini E, Brick K, Mukherjee S, Zhang Y, Silvestrini F, Alano P, Pizzi E. 2009. Revisiting the Plasmodium falciparum RIFIN family: from comparative genomics to 3D-model prediction. BMC Genomics 10:445. doi: 10.1186/1471-2164-10-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Joannin N, Abhiman S, Sonnhammer EL, Wahlgren M. 2008. Sub-grouping and sub-functionalization of the RIFIN multi-copy protein family. BMC Genomics 9:19. doi: 10.1186/1471-2164-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mphande FA, Ribacke U, Kaneko O, Kironde F, Winter G, Wahlgren M. 2008. SURFIN4.1, a schizont-merozoite associated protein in the SURFIN family of Plasmodium falciparum. Malar J 7:116. doi: 10.1186/1475-2875-7-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhu X, Yahata K, Alexandre JS, Tsuboi T, Kaneko O. 2013. The N-terminal segment of Plasmodium falciparum SURFIN4.1 is required for its trafficking to the red blood cell cytosol through the endoplasmic reticulum. Parasitol Int 62:215–229. doi: 10.1016/j.parint.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 152.Alexandre JS, Yahata K, Kawai S, Torii M, Kaneko O. 2011. PEXEL-independent trafficking of Plasmodium falciparum SURFIN4.2 to the parasite-infected red blood cell and Maurer's clefts. Parasitol Int 60:313–320. doi: 10.1016/j.parint.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 153.van der Heyde HC, Nolan J, Combes V, Gramaglia I, Grau GE. 2006. A unified hypothesis for the genesis of cerebral malaria: sequestration, inflammation and hemostasis leading to microcirculatory dysfunction. Trends Parasitol 22:503–508. doi: 10.1016/j.pt.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 154.Smith JD, Gamain B, Baruch DI, Kyes S. 2001. Decoding the language of var genes and Plasmodium falciparum sequestration. Trends Parasitol 17:538–545. doi: 10.1016/S1471-4922(01)02079-7. [DOI] [PubMed] [Google Scholar]
- 155.Sherman IW, Eda S, Winograd E. 2003. Cytoadherence and sequestration in Plasmodium falciparum: defining the ties that bind. Microbes Infect 5:897–909. doi: 10.1016/S1286-4579(03)00162-X. [DOI] [PubMed] [Google Scholar]
- 156.Rogerson SJ. 2003. Sequestration: causes and consequences. Redox Rep 8:295–299. doi: 10.1179/135100003225002970. [DOI] [PubMed] [Google Scholar]
- 157.David PH, Hommel M, Miller LH, Udeinya IJ, Oligino LD. 1983. Parasite sequestration in Plasmodium falciparum malaria: spleen and antibody modulation of cytoadherence of infected erythrocytes. Proc Natl Acad Sci U S A 80:5075–5079. doi: 10.1073/pnas.80.16.5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Duffier Y, Lorthiois A, Cistero P, Dupuy F, Jouvion G, Fiette L, Mazier D, Mayor A, Lavazec C, Moreno Sabater A. 2016. A humanized mouse model for sequestration of Plasmodium falciparum sexual stages and in vivo evaluation of gametocytidal drugs. Sci Rep 6:35025. doi: 10.1038/srep35025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sutherland CJ. 2009. Surface antigens of Plasmodium falciparum gametocytes—a new class of transmission-blocking vaccine targets? Mol Biochem Parasitol 166:93–98. doi: 10.1016/j.molbiopara.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 160.Dinko B, King E, Targett GA, Sutherland CJ. 2016. Antibody responses to surface antigens of Plasmodium falciparum gametocyte-infected erythrocytes and their relation to gametocytaemia. Parasite Immunol 38:352–364. doi: 10.1111/pim.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Meerstein-Kessel L, Bousema T, Stone W. 2018. Detecting gametocytes: how sensitive is sensible? J Infect Dis 217:1011–1012. doi: 10.1093/infdis/jix672. [DOI] [PubMed] [Google Scholar]
- 162.Kwiatkowski D, Marsh K. 1997. Development of a malaria vaccine. Lancet 350:1696–1701. doi: 10.1016/S0140-6736(97)03256-X. [DOI] [PubMed] [Google Scholar]
- 163.Juliano JJ, Parobek CM, Brazeau NF, Ngasala B, Randrianarivelojosia M, Lon C, Mwandagalirwa K, Tshefu A, Dhar R, Das BK, Hoffman I, Martinson F, Martensson A, Saunders DL, Kumar N, Meshnick SR. 2016. Pooled amplicon deep sequencing of candidate Plasmodium falciparum transmission-blocking vaccine antigens. Am J Trop Med Hyg 94:143–146. doi: 10.4269/ajtmh.15-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Patel P, Bharti PK, Bansal D, Raman RK, Mohapatra PK, Sehgal R, Mahanta J, Sultan AA, Singh N. 2017. Genetic diversity and antibody responses against Plasmodium falciparum vaccine candidate genes from Chhattisgarh, Central India: implication for vaccine development. PLoS One 12:e0182674. doi: 10.1371/journal.pone.0182674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Niederwieser I, Felger I, Beck HP. 2001. Limited polymorphism in Plasmodium falciparum sexual-stage antigens. Am J Trop Med Hyg 64:9–11. doi: 10.4269/ajtmh.2001.64.9. [DOI] [PubMed] [Google Scholar]
- 166.World Health Organization. 2018. World malaria report 2018. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 167.Hisaeda H, Stowers AW, Tsuboi T, Collins WE, Sattabongkot JS, Suwanabun N, Torii M, Kaslow DC. 2000. Antibodies to malaria vaccine candidates Pvs25 and Pvs28 completely block the ability of Plasmodium vivax to infect mosquitoes. Infect Immun 68:6618–6623. doi: 10.1128/IAI.68.12.6618-6623.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ramjanee S, Robertson JS, Franke-Fayard B, Sinha R, Waters AP, Janse CJ, Wu Y, Blagborough AM, Saul A, Sinden RE. 2007. The use of transgenic Plasmodium berghei expressing the Plasmodium vivax antigen P25 to determine the transmission-blocking activity of sera from malaria vaccine trials. Vaccine 25:886–894. doi: 10.1016/j.vaccine.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 169.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 170.Kocken CH, Jansen J, Kaan AM, Beckers PJ, Ponnudurai T, Kaslow DC, Konings RN, Schoenmakers JG. 1993. Cloning and expression of the gene coding for the transmission blocking target antigen Pfs48/45 of Plasmodium falciparum. Mol Biochem Parasitol 61:59–68. doi: 10.1016/0166-6851(93)90158-T. [DOI] [PubMed] [Google Scholar]
- 171.Chowdhury DR, Angov E, Kariuki T, Kumar N. 2009. A potent malaria transmission blocking vaccine based on codon harmonized full length Pfs48/45 expressed in Escherichia coli. PLoS One 4:e6352. doi: 10.1371/journal.pone.0006352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jones CS, Luong T, Hannon M, Tran M, Gregory JA, Shen Z, Briggs SP, Mayfield SP. 2013. Heterologous expression of the C-terminal antigenic domain of the malaria vaccine candidate Pfs48/45 in the green algae Chlamydomonas reinhardtii. Appl Microbiol Biotechnol 97:1987–1995. doi: 10.1007/s00253-012-4071-7. [DOI] [PubMed] [Google Scholar]
- 173.Datta D, Bansal GP, Grasperge B, Martin DS, Philipp M, Gerloff D, Ellefsen B, Hannaman D, Kumar N. 2017. Comparative functional potency of DNA vaccines encoding Plasmodium falciparum transmission blocking target antigens Pfs48/45 and Pfs25 administered alone or in combination by in vivo electroporation in rhesus macaques. Vaccine 35:7049–7056. doi: 10.1016/j.vaccine.2017.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mamedov T, Ghosh A, Jones RM, Mett V, Farrance CE, Musiychuk K, Horsey A, Yusibov V. 2012. Production of non-glycosylated recombinant proteins in Nicotiana benthamiana plants by co-expressing bacterial PNGase F. Plant Biotechnol J 10:773–782. doi: 10.1111/j.1467-7652.2012.00694.x. [DOI] [PubMed] [Google Scholar]
- 175.Prokhnevsky A, Mamedov T, Leffet B, Rahimova R, Ghosh A, Mett V, Yusibov V. 2015. Development of a single-replicon miniBYV vector for co-expression of heterologous proteins. Mol Biotechnol 57:101–110. doi: 10.1007/s12033-014-9806-5. [DOI] [PubMed] [Google Scholar]
- 176.MacDonald NJ, Nguyen V, Shimp R, Reiter K, Herrera R, Burkhardt M, Muratova O, Kumar K, Aebig J, Rausch K, Lambert L, Dawson N, Sattabongkot J, Ambroggio X, Duffy PE, Wu Y, Narum DL. 2016. Structural and immunological characterization of recombinant 6-cysteine domains of the Plasmodium falciparum sexual stage protein Pfs230. J Biol Chem 291:19913–19922. doi: 10.1074/jbc.M116.732305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fanning SL, Czesny B, Sedegah M, Carucci DJ, van Gemert GJ, Eling W, Williamson KC. 2003. A glycosylphosphatidylinositol anchor signal sequence enhances the immunogenicity of a DNA vaccine encoding Plasmodium falciparum sexual-stage antigen, Pfs230. Vaccine 21:3228–3235. doi: 10.1016/S0264-410X(03)00265-2. [DOI] [PubMed] [Google Scholar]
- 178.Lee SM, Wu CK, Plieskatt JL, Miura K, Hickey JM, King CR. 2017. An N-terminal Pfs230 domain produced in baculovirus as a biological active transmission-blocking vaccine candidate. Clin Vaccine Immunol 24:e00140-17. doi: 10.1128/CVI.00140-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lee SM, Wu CK, Plieskatt J, McAdams DH, Miura K, Ockenhouse C, King CR. 2016. Assessment of Pfs25 expressed from multiple soluble expression platforms for use as transmission-blocking vaccine candidates. Malar J 15:405. doi: 10.1186/s12936-016-1464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kaslow DC, Shiloach J. 1994. Production, purification and immunogenicity of a malaria transmission-blocking vaccine candidate: TBV25H expressed in yeast and purified using nickel-NTA agarose. Nat Biotechnol 12:494–499. doi: 10.1038/nbt0594-494. [DOI] [PubMed] [Google Scholar]
- 181.Lobo CA, Dhar R, Kumar N. 1999. Immunization of mice with DNA-based Pfs25 elicits potent malaria transmission-blocking antibodies. Infect Immun 67:1688–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tsuboi T, Takeo S, Iriko H, Jin L, Tsuchimochi M, Matsuda S, Han ET, Otsuki H, Kaneko O, Sattabongkot J, Udomsangpetch R, Sawasaki T, Torii M, Endo Y. 2008. Wheat germ cell-free system-based production of malaria proteins for discovery of novel vaccine candidates. Infect Immun 76:1702–1708. doi: 10.1128/IAI.01539-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kapoor N, Vanjak I, Rozzelle J, Berges A, Chan W, Yin G, Tran C, Sato AK, Steiner AR, Pham TP, Birkett AJ, Long CA, Fairman J, Miura K. 2018. Malaria derived glycosylphosphatidylinositol anchor enhances anti-Pfs25 functional antibodies that block malaria transmission. Biochemistry 57:516–519. doi: 10.1021/acs.biochem.7b01099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gregory JA, Li F, Tomosada LM, Cox CJ, Topol AB, Vinetz JM, Mayfield S. 2012. Algae-produced Pfs25 elicits antibodies that inhibit malaria transmission. PLoS One 7:e37179. doi: 10.1371/journal.pone.0037179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kumar R, Angov E, Kumar N. 2014. Potent malaria transmission-blocking antibody responses elicited by Plasmodium falciparum Pfs25 expressed in Escherichia coli after successful protein refolding. Infect Immun 82:1453–1459. doi: 10.1128/IAI.01438-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Parzych EM, Miura K, Ramanathan A, Long CA, Burns JM Jr. 2018. Evaluation of a Plasmodium-specific carrier protein to enhance production of recombinant Pfs25, a leading transmission-blocking vaccine candidate. Infect Immun 86:e00486-17. doi: 10.1128/IAI.00486-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.McGuire KA, Miura K, Wiethoff CM, Williamson KC. 2017. New adenovirus-based vaccine vectors targeting Pfs25 elicit antibodies that inhibit Plasmodium falciparum transmission. Malar J 16:254. doi: 10.1186/s12936-017-1896-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Johns Hopkins Bloomberg School of Public Health. “Life cycle of the malaria parasite” from Epidemiology of Infectious Diseases. Available at http://ocw.jhsph.edu. Copyright © Johns Hopkins Bloomberg School of Public Health. Creative Commons BY-NC-SA.