Abstract
A series of HZSM-5 catalysts with different Si/Al ratio were prepared and characterized with various characteristic methods XRD, BET, NH3-TPD, FT-IR spectra of adsorbed pyridine, etc. The effect of Si/Al ratio of HZSM-5 catalysts on the prins condensation of isobutylene and formaldehyde to isoprene was investigated in detail. It is resulted that the catalytic activities are dependent on the acid strength, decreasing with the ratio of Si/Al increases. The optimized catalyst was HZSM-5 (600) with 8.8% of isobutene conversion and 90.2% of selectivity to isoprene, indicating that the higher Si/Al ratio was termed as appropriate for prins condensation of isobutylene and formaldehyde to isoprene. On the contrary, the low Si/Al ratio may lead to the formation of by-products and coke deposits, which leads the catalysts deactivation.
Keywords: Organic chemistry, Materials chemistry
1. Introduction
Increasing attention is pained on the prins condensation of olefins and aldehydes to prepare unsaturated alcohols, glycols, acetals and isoprene. Especially the isoprene, is considered to be a substantial monomer to manufacture substitutes for natural rubbers, and also utilized for the production of paint resins, adhesives, motor oil viscosity improvers, etc [1, 2]. As of today, the vapour-phase one-stage mechanism, involving the use of solid catalyst, appears to be quite an appealing approach owing to its straightforward as well as ecological process [3, 4]. Therefore, searching for a suitable solid catalyst is an important issue in this area.
Until today, the mostly solid catalysts used for the condensation of isobutene and formaldehyde include oxides, sulphates, phosphates, zeolites, and heteropoly acids. Dang et al. [5, 6], used CuSO4-MOx/SiO2 and SbxOy/SiO2 as the catalysts for the condensation of isobutylene and formaldehyde, and revealed that the synergetic effect between acidic sites and basic sites was the key factor for isoprene generation. Krzywicki et al. [3] used H3PO4/Al2O3 with different contents of phosphoric acid as catalyst for isobutene condensation using formaldehyde and emphasized the strong acidic sites accelerated the formation of isoprene. Notably, varied of phosphate catalysts provide improved findings for the study of condensation. Ivanova et al. [7] worked out that the Brønsted acid sites are the active sites for the formation of isoprene. And the prevalence of water in the reaction is likely to perform a major function for the stability of the catalytic operation, above of stream, 57% of isoprene could be consistently generated up to 30 h. Our team [8] used SiO2 supported silicotungstic acids as the solid catalysts for the Prins condensation of isobutylene with formaldehyde for the production of isoprene. The best catalytic ability was obtained over HSiW-5, and an adequate quantity of weak Lewis acid sites is beneficial for prins condensation was concluded. Dumitriu et al. [9, 10] performed the study of the catalytic yield of isobutylene with formaldehyde for forming the isoprene across a sequence of zeolites, indicating that the catalytic activity and selectivity was dependent on the strength and density of the acid sites. Hence, the prins condensation gave preference to the reasonably strong acid sites. It is worth to say that aforementioned findings are based on the measurement which was carried out in a pulse catalytic reactor, the concluded laws might be different from that attained in continuous flow systems.
This paper aims at selecting a suitable Si/Al ratio of HZSM-5 catalysts for isoprene production in continuous flow systems, together with studying the effect of the acidity of catalysts on the prins condensation. For this purpose, a series of HZSM-5 catalysts with different Si/Al ratio have been prepared and used in the prins condensation reaction of isobutene and formaldehyde to isoprene. Moreover, acidity of the catalysts has been elucidated by NH3-TPD and FTIR of pyridine adsorption spectroscopy.
2. Experimental
2.1. Catalyst preparation
The preparation of the ZSM-5 zeolites with varied Si/Al ratios (200–800) was in accordance with the methods in the literature [11]. Usually, a slow dripping of an aqueous solution of H2SO4 was conducted into a solution that contained 34.0 g Na2SiO3•9H2O, measured quantity of NaAlO2 and 3.2 g tetrapropyl ammonium bromide satisfying stirring. The pH value stands approximately 8.0. Subsequent to stirring the mix at room temperature for 5 h, the mixture gel was shifted into an autoclave, followed by crystallization at 453 K for two days. The as-synthesized zeolite was calcined at 453 K for 6 h in air to remove the template, Na-ZSM-5 was obtained. Then the type of Na-ZSM-5 was ion exchange with 5 wt% NH4NO3 solutions to obtain NH4ZSM-5, which was calcined 823 K for 3 h to get the HZSM-5 catalyst.
2.2. Catalyst characterization
Recording of the Powder X-ray diffraction (XRD) patterns was carried out with a Shimadzu Lab XRD-6000 X-ray Diffractometer using nickel-filtered Cu Kα radiation. Determination of the Si/Al ratio was performed with the use of inductively coupled plasma (ICP) on a Thermo IRIS Intrepid ⅡXSP atomic emission spectrometer that followed the dissolution of the specimens in HF solution. N2 was put to use as an adsorbate in an ASAP 2010 system of Micromeritics under liquid-N2 temperature for the purpose of measuring the samples' areas of BET particular surface. NH3-TPD analysis was carried out on a self-developed tool. The measurement mechanism was carried out in accordance with the former literature reports [12]. Pyridine adsorption fourier transform infrared (FTIR) spectrum was attained in accordance with the literature as well [13]. Testing of the TG-DSC profiles was carried out on TG-8120 tool, in an air stream at a heating rate of 20 K min-1 from 306 to 1073 K.
2.3. Catalytic activity
The active test of catalyst was carried out in a steady flow fixed-bed reactor catering to the conditions as hereunder: in every experiment, use of 0.7 g catalyst was made, and the reaction was carried out following 100 kPa, isobutene/formaldehyde molar ratio that was 7, the reaction temperature stood at 573 K, in addition to the weight hourly space velocity (WHSV) of formaldehyde as well as isobutene being 3 g/(g h). The use of Formalin having 37 wt% of formaldehyde was made as formaldehyde source. A syringe pump was utilized to deliver liquid formalin while the supply of isobutene came from the mass flow meter. The analysis of the effluents of the reaction was carried out with the on-line gas chromatography (Shimadzu GC-14A), equipped with a flame detector and SE-54 capillary column.
3. Results and discussion
3.1. Structural characterization of the HZSM-5 zeolites
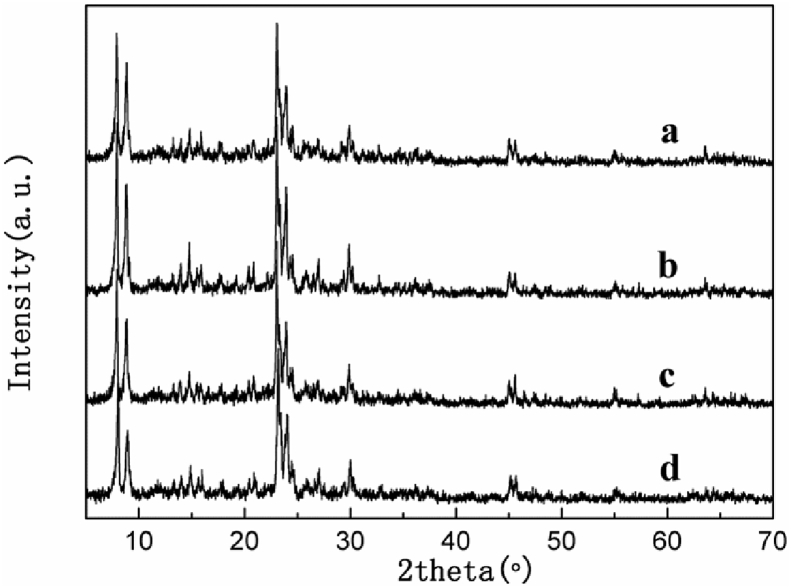
The XRD patterns of all the HZSM-5 catalysts have been presented in Fig. 1. Distinct diffraction peaks were detected in the 2θ ranges of 7–10 as well as 20–25, which is consistent with the reports in literature, indicating that all zeolites exhibit MFI topology with high crystalline purity [14, 15, 16, 17].
Fig. 1.
XRD patterns of HZSM-5 catalysts with different Si/Al ratio. (a) HZSM-5(200); (b) HZSM-5(400); (c) HZSM-5(600); (d) HZSM-5(800).
The results of the physical and chemical characterization of the samples are summarized in Table 1. The chemical compositions of the samples were measured by ICP. The measurements of surface areas and micropore volumes of these HZSM-5 catalysts were characterized with the BET methodology. All the zeolite catalysts possessed relatively high surface area falling in the range of 324–423 m2g-1, which indicated that they were of good quality.
Table 1.
Physicochemical property of HZSM-5 catalysts with different Si/Al ratio.
| Sample | Si/Al ratioa | Surface areab (m2g−1) | Pore volumec (cm3g−1) | Tpeak(K) |
Acid sites from NH3-TPD (mmolg−1)d |
|||
|---|---|---|---|---|---|---|---|---|
| LT | HT | Total | Weak | Strong | ||||
| HZSM-5(200) | 191 | 407 | 0.26 | 473 | 679 | 0.52 | 0.23 | 0.29 |
| HZSM-5(400) | 367 | 423 | 0.37 | 459 | 671 | 0.24 | 0.11 | 0.13 |
| HZSM-5(600) | 550 | 370 | 0.22 | 456 | 660 | 0.17 | 0.07 | 0.10 |
| HZSM-5(800) | 738 | 324 | 0.19 | 447 | 656 | 0.13 | 0.06 | 0.07 |
Molar ratio determined by ICP.
BET method.
Volume adsorbed at p/p0 = 0.99.
Calculated with Gaussian function fit.
3.2. Acidity of HZSM-5 catalysts
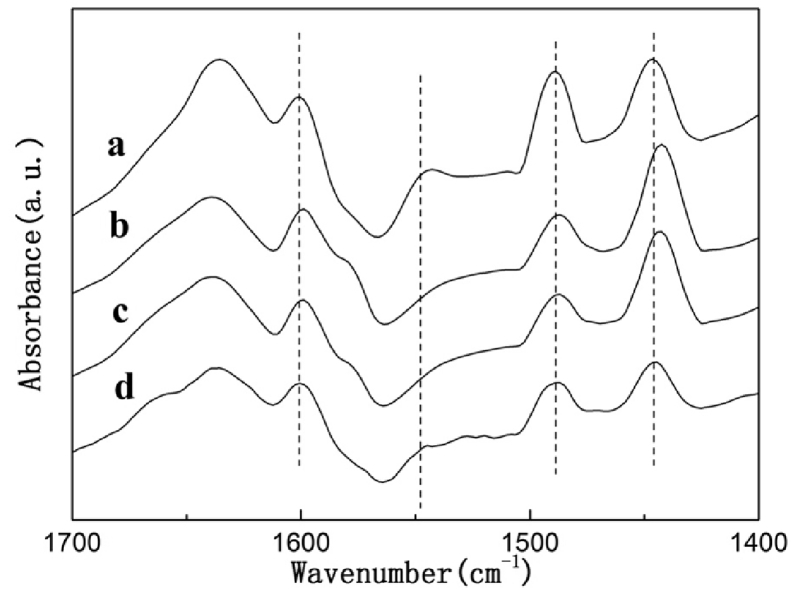
Pyridine adsorption was carried out for conducting investigation of the nature acidic sites over the surface of HZSM-5 with various Si/Al ratios. Moreover, the FTIR spectra have been presented in Fig. 2. In accordance with the published research work [18, 19, 20], the spectra of pyridine adsorbed on several catalysts portraying bands approximately 1597 cm−1 and 1445 cm−1 are designated to pyridine coordinated to Lewis acid sites. The band at 1540 cm−1 is owed to a combination band that has links with Brønsted acid sites, while the band at 1480 cm−1 can be ascribed to Lewis and Brønsted acid sites. As evident from the Fig. 2, the key acid type was the Lewis acid site together with the coexistence of Brønsted acid. Both the Lewis acid and Brønsted acid exhibited a reduction with the increase in the ratio of Si/Al [21].
Fig. 2.
Py-IR spectra of HZSM-5 catalysts with different Si/Al ratio. (a) HZSM-5(200); (b) HZSM-5(400); (c) HZSM-5(600); (d) HZSM-5(800).
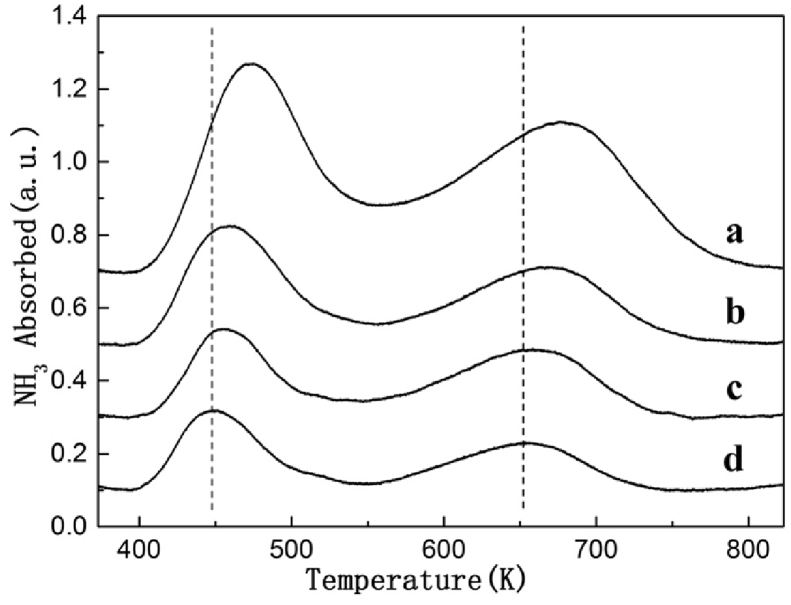
The acid properties of different HZSM-5 samples was also measured by temperature-programmed desorption (NH3-TPD). The acid sites distribution and acid amount of these catalysts are summarized in Table 1 and Fig. 3. A high temperature desorption peak (HT peak) observed at 656 K–679 K attributed to the desorption of NH3 molecule from strong acid sites. A low temperature peak (LT peak) observed at 447 K–473 K was attributed to the desorption of NH3 molecule from weak acid sites. These results reveal good agreement with the reported data [22, 23, 24]. In general, the strong acid sites could be owned to the bridging hydroxyl groups (Si–OH–Al) on the zeolite, the weak acid sites could be owned to the silanol groups or extra framework aluminum species [22]. As shown in Table 1, both the two desorption peaks slightly shifts towards lower temperature, and the peak area decreases as the Si/Al ratio increased. Obviously, both the density and the strength of acid sites was affected by Si/Al ratio.
Fig. 3.
NH3-TPD profiles of HZSM-5 catalysts with different Si/Al ratio. (a) HZSM-5(200); (b) HZSM-5(400); (c) HZSM-5(600); (d) HZSM-5(800).
3.3. Catalytic performance
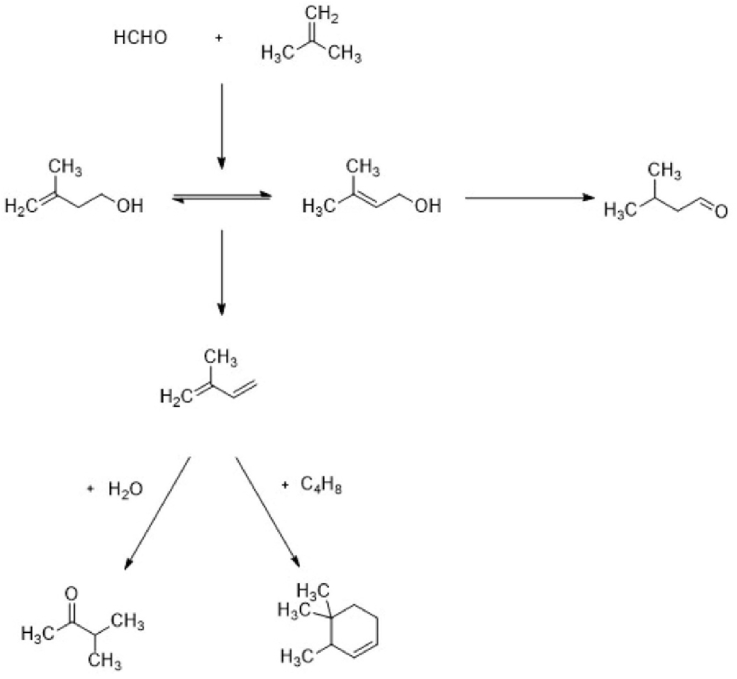
The Prins condensation was carried out in order to produce isoprene over the HZSM-5 catalysts, the catalytic properties and product distributions has been presented in Table 2, and the key by-products received were 3-Methylbuten-1-ols, 3-Methyl-1-butanal, 3-methyl-2-butanone as well as 3,4,4-trimethylcyclohexene. The reaction network has been presented in Fig. 4. The key reaction route involves the condensation of formaldehyde with isobutene resulting into 3-methylbuten-1-ols, which can be further turned into the aimed reaction of product-isoprene with the use of dehydration. 3-Methyl-2-buten-1-ol can be translated into 3-metyl-1-butanal. Furthermore, the isoprene may be converted into 3-methyl-2-butanone through the interaction with H2O, or 3, 4, 4-trimethylcyclohexene through the interaction with isobutene for the purpose of producing 7.
Table 2.
Catalytic performance for the vapour phase condensation of isoprene and formaldehyde over HZSM-5 catalysts.
| Catalyst | Coversion of isobutylene (%) | Selectivity (%) |
Yield of isoprene (%) | ||||
|---|---|---|---|---|---|---|---|
| isoprene | 3-Methylbuten-1-ols | 3-Methyl-1-butanal | 3-Methyl-2-butanone | 3,4,4-Thimethyl cyclohexene | |||
| HZSM-5(200) | 9.8 | 78.6 | 0.1 | 1.0 | 3.3 | 2.1 | 7.7 |
| HZSM-5(400) | 9.2 | 83.8 | 0.1 | 1.0 | 2.5 | 1.5 | 7.7 |
| HZSM-5(600) | 8.8 | 90.2 | 0.1 | 0.8 | 1.4 | 0.5 | 7.9 |
| HZSM-5(800) | 8.6 | 89.9 | 0.2 | 0.8 | 1.3 | 0.4 | 7.7 |
Fig. 4.
Reaction network.
As evident from the Table 2, HZSM-5(200) exhibited 9.8% of isobutene conversion, with 78.6% selectivity to isoprene. With the ratio of Si/Al increases, the selectivity for isoprene increased. However, the isobutene conversion and by-products selectivity decreased. The selectivity to isoprene reached maximum (90.2%) on HZSM-5(600). With the ratio of Si/Al further increased, the catalytic performance began to decrease slightly.
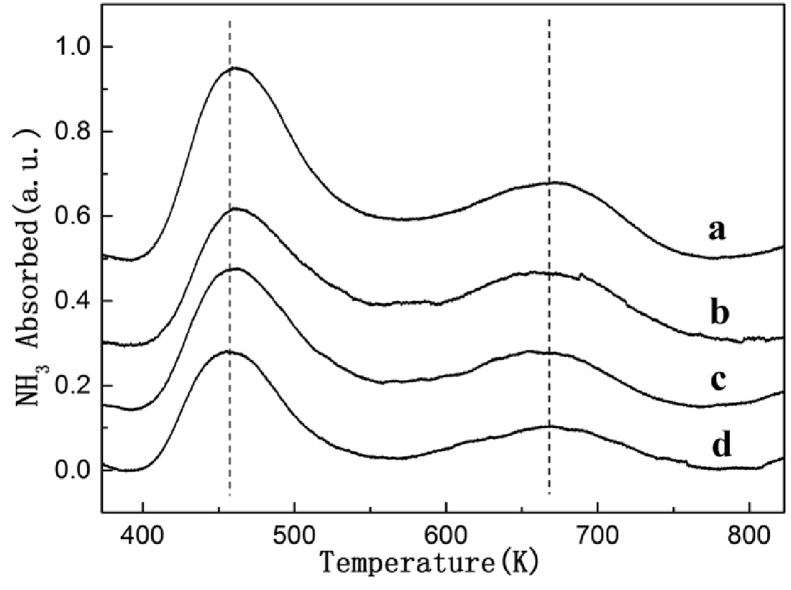
In order to further probe into the catalytic active sites of HZSM-5 catalysts, NH3-TPD profiles of expended HZSM-5 catalysts were accumulated (Fig. 5). Subsequent to 120 minutes of reaction, the amount of strong acid sites on HZSM-5 catalysts decreases, this result was observed obviously at HZSM-5 with low Si/Al ratio. According to the experimental result of our former research work [8], the concentration and strength of acid sites could affect the catalytic properties. A proper quantity of weak Lewis acid sites was the active site for the condensation of isobutene with formaldehyde. Furthermore, the moderately strong acid sites can give birth to by-products, in addition to coke deposits. Therefore, we held the belief that the low Si/Al ratio with strong acid centres has the capacity to result to the formation of coke and by-products during the reaction, which would encompass the strong acid centres in turn. An appropriate amount of weak acid sites with suitable Si/Al ratio is propitious to isoprene formation.
Fig. 5.
NH3-TPD profiles of spent HZSM-5 catalysts with different Si/Al ratio. (a) HZSM-5(200); (b) HZSM-5(400); (c) HZSM-5(600); (d) HZSM-5(800).
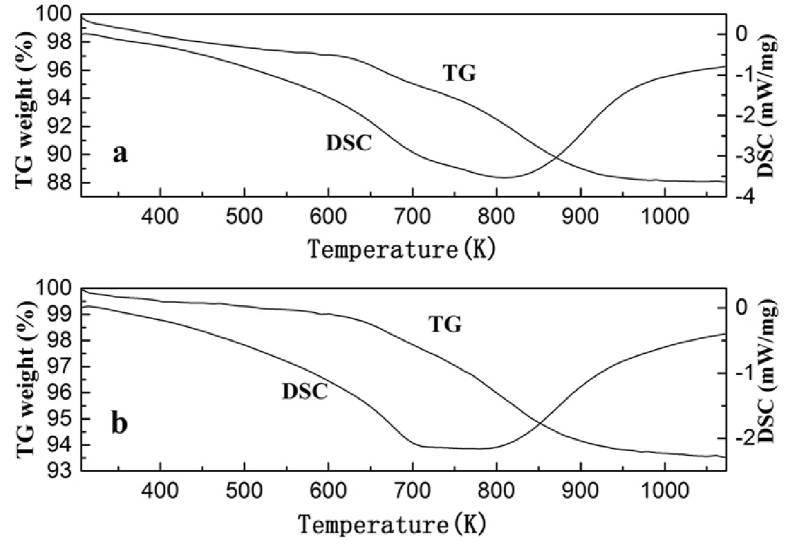
For the purpose of researching the impact of Si/Al ratio for carbon deposition on the catalysts, TG-DSC analysis of the spent HZSM-5 catalysts having varied Si/Al ratio was carried out (Fig. 6). It become evident that, following the low temperature range (i.e., <597 K), the mass losses of two samples approximately amount to be 0.9% and 2% that match with the loss of chemically adsorbed water, possessing loose bond with the surface [25]. Subjected to the temperatures above 597 K, HZSM-5(200) exhibits a mass loss of 9 percent that is ascribed to the decomposition of the cokes [26]. It is noteworthy that the mass loss at high temperature was considerably lowered with the increase of Si/Al ratio. These findings further confirmed that the carbon deposition is easily formed on the ZSM-5 zeolite with low Si/Al ratio, and a high Si/Al ratio can be an indication of the carbon deposition formation.
Fig. 6.
TG-DSC profiles of spent HZSM-5(200) and HZSM-5(600) catalysts.
4. Conclusions
A series of HZSM-5 zeolites with the Si/Al ratios falling in the ranges from 200 to 800 was studied for the prins condensation of isobutylene and formaldehyde to isoprene. The Si/Al ratio of the HZSM-5 zeolites exerted a substantial impact on the structure and the acidity of the HZSM-5 zeolite, further the catalytic performance in the isoprene production. The acid density and the acid strength decreased with the increasing of Si/Al ratio. HZSM-5 (600) is discovered to be the optimized catalyst owing to the appropriate acid density and acid strength of the catalyst. The catalysts with low Si/Al ratio have strong acid sites, leading to the coke deposits and the side reactions happen.
Declarations
Author contribution statement
Xue Yu: Performed the experiments; Analyzed and interpreted the data.
Bin Liu: Contributed reagents, materials, analysis tools or data.
Yuewei Zhang: Conceived and designed the experiments; Wrote the paper.
Funding statement
This work was supported by the Technology Institute of Shanghai Huayi (Group) Enterprise, Science and Technology Innovation and Development Plan Project in Jilin City (201750231), Science and Technology Research Plan in Jilin Province (crucial scientific research program) (No: 20150204020GX), the open subject in National Key Laboratory of Inorganic Synthesis and Preparative Chemistry (No: 2017-30), the Youth Foundation of Jilin Province (No: 20160520130JH) and Research projects in Jilin Institute of Chemical Technology (2016004, 2018030).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Taalman R.D.F.M. Isoprene: background and issues. Toxicology. 1996;113:242–246. doi: 10.1016/0300-483x(96)03452-x. [DOI] [PubMed] [Google Scholar]
- 2.Dumitriu E., Hulea V., Chelaru C., Hulea T. Selective synthesis of isoprene by prins condensation using molecular sieves. Microporous Mesoporous Mater. 1994;84:1997–2004. [Google Scholar]
- 3.Krzywicki A., Wilanowicz T., Malinowski S. Catalytic and physicochemical properties of the Al2O3-H3PO4 system, I. vapor phase condensation of isobutylene and formaldehyde the prins reaction. React. Kinet. Mech. Catal. 1979;11:399–403. [Google Scholar]
- 4.Dumitriu E., Gongescu D., Hulea V. Contribution to the study of isobutene condensation with formaldehyde catalyzed by zeolites. Heterog. Catal. Fine Chem. III. 1993:669–676. [Google Scholar]
- 5.Dang Z., Gu J., Yu L., Zhang C. Vapor-phase synthesis of isoprene from formaldehyde and isobutylene over CuSO4-MOx/SiO2 catalysts. React. Kinet. Mech. Catal. 1991;43:495–500. [Google Scholar]
- 6.Dang Z., Ding S. Investigation of the surface characteristics and catalystic activity of SbxOy/SiO2 catalysts for vapor-phase synthesis of isoprene from isobutylene and formaldehyde. J. Mol. Catal. 1987;3:146–152. in chinese. [Google Scholar]
- 7.Sushkevich V.L., Ordomsky V.V., Ivanova I.I. Investigation of the surface characteristics and catalystic activity of SbxOy/SiO2 catalysts for vapor-phase synthesis of isoprene from isobutylene and formaldehyde. Appl. Catal. A. 2012;441–442:21–29. [Google Scholar]
- 8.Yu X., Zhu W., Zhai S., Bao Q., Cheng D., Xia Y., Wang Z., Zhang W. Prins condensation for the synthesis of isoprene from isobutylene and formaldehyde over sillica-supported H3SiW12O40 catalysts. React. Kinet. Mech. Catal. 2016;117:761–771. [Google Scholar]
- 9.Dumitriu E., On D.T., Kaliaguine S. Isoprene by prins condensation over acidic molecular sieves. J. Catal. 1997;170:150–160. [Google Scholar]
- 10.Dumitriu E., Hulea V., Fechete I., Catrinescu C., Auroux A., Lacaze J.F., Guimon C. Prins condensation of isobutylene and formaldehyde over Fe-silicates of MFI structure. Appl. Catal., A. 1999;181:15–28. [Google Scholar]
- 11.Ding J., Wang M., Peng L., Xue N., Wang Y., He M.Y. Combined desilication and phosphorus modification for high-silica ZSM-5 zeolite with related study of hydrocarbon cracking performance. Appl. Catal., A. 2015;503:147–155. [Google Scholar]
- 12.Yu X., Zhai S., Zhu W., Gao S., Yan J., Yuan H., Chen L., Luo J., Zhang W., Wang Z. The direct transformation of ethanol to ethyl acetate over Cu/SiO2 catalysts that contain copper phyllosilicate. J. Chem. Sci. 2014;126:1013–1020. [Google Scholar]
- 13.Zhu X., Li X., Jia M., Liu G., Zhang W., Jiang D. Vapour-phase selective O-methylation of catechol with methanol over Ti-containing aluminium phosphate catalysts. Appl. Catal., A. 2005;282:155–161. [Google Scholar]
- 14.Bayat A., Sadrameli S.M., Towfighi J. Production of green aromatics via catalytic cracking of Canola Oil Methyl Ester (CME) using HZSM-5 catalyst with different Si/Al ratios. Fuel. 2016;180:244–255. [Google Scholar]
- 15.Gao Y., Zheng B., Wu G., Ma F., Liu C. Effect of the Si/Al ratio on the performance of hierarchical ZSM-5 zeolites for methanol aromatization. RSC Adv. 2016;6:83581–83588. [Google Scholar]
- 16.Aboul-Fotouh S.M.K., Ali L.I., Naghmash M.A., Aboul-Gheit N.A.K. Effect of the Si/Al ratio of HZSM-5 zeolite on the production of dimethyl ether before and after ultrasonication. J. Fuel Chem. Technol. 2017;45:581–588. [Google Scholar]
- 17.Ghanbari B., Zangeneh F.K., Rizi Z.T., Aghaei E. Highly efficient production of benzene-free aromatics from methanol over low-Si/Al-ratio alkali-modified Fe/Zn/HZSM-5. ACS Omega. 2018;3:18821–18835. doi: 10.1021/acsomega.8b01380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X., Han D., Wang H., Liu G., Wang B., Li Z., Wu J. Propene oligomerization to high-quality liquid fuels over Ni/HZSM-5. Fuel. 2015;144:9–14. [Google Scholar]
- 19.Jia Y., Wang J., Zhang K., Feng W., Liu S., Ding C., Liu P. Nanocrystallite self-assembled hierarchical ZSM-5 zeolite microsphere for methanol to aromatics. Microporous Mesoporous Mater. 2017;247:103–115. [Google Scholar]
- 20.Wu J., Zhu H., Wu Z., Qin Z., Yan L., Du B., Fan W., Wang J. High Si/Al ratio HZSM-5 zeolite: an efficient catalyst for the synthesis of polyoxymethylene dimethyl ethers from dimethoxymethane and trioxymethylene. Green Chem. 2015;17:2353–2357. [Google Scholar]
- 21.Meng T., Mao D., Guo Q., Ma Z. Effect of the Si/Al ratios of nanocrystalline HZSM-5 zeolite on the performance in catalytic conversion of ethanol to propylene. J. Nanosci. Nanotechnol. 2017;17:3779–3785. [Google Scholar]
- 22.Lü J., Zhou S., Ma K., Meng M., Tian Y. The effect of P modification on the acidity of HZSM-5 and P-HZSM-5/CuO-ZnO-Al2O3 mixed catalysts for hydrogen production by dimethyl ether steam reforming. Chin. J. Catal. 2015;36:1295–1303. [Google Scholar]
- 23.Weber R.W., Möller K.P., O'Connor C.T. The chemical vapour and liquid deposition of tetraethoxysilane on ZSM-5, mordenite and beta. Microporous Mesoporous Mater. 2000;35–36:533–543. [Google Scholar]
- 24.Chu S., Yang L.n., Guo X., Dong L., Chen X., Li Y., Mu X. The influence of pore structure and Si/Al ratio of HZSM-5 zeolites on the product distributions of α-cellulose hydrolysis. Mol. Catal. 2018;445:240–247. [Google Scholar]
- 25.Jaumain D., Su B.L. Direct catalytic conversion of chloromethane to higher hydrocarbons over a series of ZSM-5 zeolites exchanged with alkali cations. J. Mol. Catal. A Chem. 2003;197:263–273. [Google Scholar]
- 26.Tao L., Chen L., Yin S.F., Luo S.L., Ren Y.Q., Li W.S., Zhou X.P., Au C.T. Catalytic conversion of CH3Br to aromatics over PbO-modified HZSM-5. Appl. Catal., A. 2009;367:99–107. [Google Scholar]