Abstract
Background
Identification and assessment of therapeutic potential of natural products derived from medicinal plants have led to the discovery of innovative and economical drugs to treat several diseases, including chronic wounds. In vitro cell based scratch assay is an appropriate and inexpensive method for initial understanding of wound healing potential of medicinal plant extracts. The current study was aimed at investigating the wound healing capacity of Aristolochia saccata leaf extract by using scratch assay as a primary model, where proliferative and migratory capabilities of test compounds could be monitored through microscopy studies. A. saccata is an evergreen climbing shrub belongs to the family Aristolochiaceae.
Methods
Methanolic extraction of the plant material was done using Soxhlet apparatus and the cytotoxicity of the extract on L929 cells was studied by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. L929 is a human fibroblast cell line. In vitro scratch assay was performed to evaluate the wound healing properties of A. saccata leaf extract and possible mechanism of action was analyzed by flow cytometric expression studies of an extracellular matrix (ECM) factor, collagen type-1.
Results
MTT assay revealed that A. saccata leaf extract had no cytotoxic effect on the cells and at higher concentrations, the extract showed mild toxicity resulting in the death of just 2.88% cells. Scratch assay showed 34.05%, 70.00%, 93.52% wound closure at 12hrs, 24hrs and 48hrs of incubation respectively. These results were similar compared to positive control which showed 37.60, 56.41 and 99.05% of wound closure. Further, flow cytometry-based studies revealed that the A. saccata leaf extract induced the expression of ECM remodelling factor collagen-1.
Conclusion
Our study revealed the wound healing capabilities of A. saccata In vitro. Hence, A. saccata could be recommended as a potential source of wound healing agents.
Keywords: Plant biology, Cell biology, Pharmaceutical science, Molecular biology, Biotechnology, Biochemistry
1. Introduction
Skin, the largest organ of human body, protects visceral organs from infection by microbes and injury. Wound healing mechanism is obligatory to regain the lost tissue and maintain tissue homeostasis. New tissue formation is a complex process, which involves multiple steps such as inflammation, angiogenesis, granulation tissue formation, re-epithelialization, and ECM reconstruction [1]. Upon injury in the skin, cells such as fibroblasts, keratinocytes, macrophages, and other immune cells rapidly proliferate and migrate towards the wound and initiate the complex healing process. Hence, migration of cells towards wound is one of the key phases of wound healing process and in general, it is governed by various stimulatory factors of tissue microenvironment [2].
Fibroblasts are most abundant cells in skin tissue and the major functions of these cells during wound healing include, rupturing of fibrin clots, generation of extracellular matrix (ECM) components and collagen structures that support the tissue homeostasis[3, 4]. Collagen synthesis and granulation tissue formation play critical role in wound contraction. For this reason, contemporary wound healing research is focused on the identification of new therapeutic agents, which has a stimulatory effect on the activation and modification of collagen producing fibroblasts [5].
Several In vitro and animal models are available to screen the wound healing nature of new therapeutic agents. Among them, fibroblast cell-based scratch assay is an inexpensive and well-established model, which supports the initial understanding of wound healing efficacy of new therapeutic agents [6].
Natural extracts have been playing fundamental role in the acceleration of wound healing process. However, the scientific evidence of their efficacy is limited. Hence, efforts to identify the bioactive compounds of medicinally important herbal extracts and their mechanism of action has always tremendous importance in the medical research [7]. A. saccata is an evergreen climbing shrub belonging to the family Aristolochiaceae. Over 500 species of this family have been identified [8] and the extracts of some of the species have been used as medicine for various diseases, though the scientific evidences are limited. Aristolochiaceae plants are known to treat arthritis, snake bites, wounds and skin diseases. They are also known to possess anti-cancer, anti-inflammatory [9], anti-feedant [10], muscle relaxant [11] properties. Although these medicinal properties of the plant are known traditionally, these properties have not been scientifically validated through In vitro or In vivo experiments. Specific medicinal properties of this plant, as evidenced by cellular and molecular biology experiments, are not reported. Therefore, we strived to validate the traditional claims and checked for the wound healing potency of the plant extract.
In the present study, the wound-healing efficacy of methanolic extract of A. saccata leaf was determined by In vitro scratch assay and its role in the stimulation of collagen-1 expression in L929 cells was studied by flow cytometry.
2. Results
2.1. Cytotoxicity effect of A. saccata leaf extract
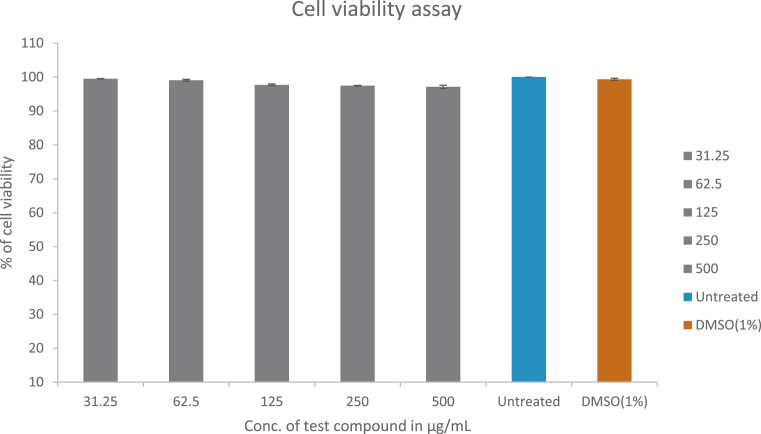
Although plant extracts have been long studied for their medicinal properties, the cytotoxic effects of such extracts on the cell type of interest is sometimes neglected. However, in the recent times, there has been a growing trend in testing this critical component [12, 13]. Cytotoxic effect of A. saccata leaf extract on L929 cells was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The cells were exposed to different concentrations of test compound for 48hrs and the cytotoxic effect of the extract was evaluated. The percentage viability of L929 cells at the highest treated concentration of A. saccata leaf extract was observed to be 97.12 ± 0.65. The concentrations of A. saccata leaf extract used for treatment and their corresponding percentage cell viability were tabulated in Table 1 and represented in Fig. 1 and 2. These results indicated that the extract was not cytotoxic and could be assessed for their medicinal properties.
Table- 1.
Cell viability effects of A. saccata extract in L929 cell line.
| Culture conditions | % of cell viability |
|---|---|
| Vehicle Control | 99.33 ± 0.42 |
| 31.25 | 99.49 ± 0.10 |
| 62.5 | 99.06 ± 0.34 |
| 125 | 97.70 ± 0.31 |
| 250 | 97.44 ± 0.14 |
| 500 | 97.12 ± 0.65 |
Fig. 1.
The effect of A. saccata on L929 cell line viability was determined by MTT assay method. Each bar graph represents % viability of L929 cells against 31.25 to 500 μg/mL concentrations of A. saccata extract after 48 hrs exposure. Untreated cells were negative control and 1% Dimethyl Sulfoxide (DMSO) as vehicle control. Experiments were performed in triplicates and data was shown as mean ± SD.
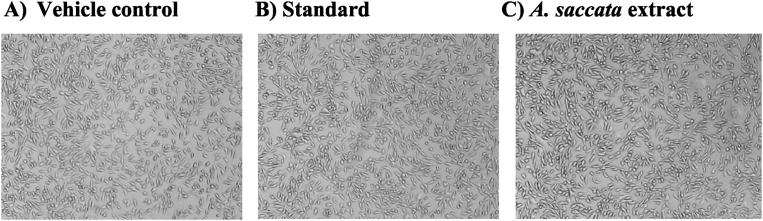
Fig. 2.
Images of L929 cell line in inverted light microscopy after the exposure to A. saccata extract. From ‘A’ to ‘C’ where A) vehicle control (1% DMSO) B) 5 μg of standard drug Cipladine and C) 500 μg/mL concentration of A. saccata extract. After incubation of 48 hours A. saccata extract displayed no toxicity.
2.2. Fibroblast cell migration was induced by A. saccata leaf extract
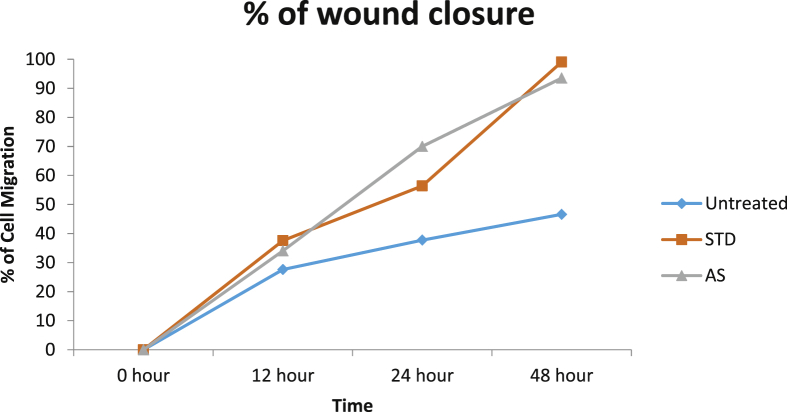
Activation, proliferation and migration of fibroblasts are the primary steps in wound healing, where multiple cell types and other micro environmental factors are involved. Scratch assay is a widely applied In vitro technique for understanding the wound healing capabilities of medicinally important compounds [14]. In the current study, L929 cells were treated with 125 μg/mL of A. saccata extract for 48hrs. Cell migration at 0, 12, 24, 48 h were captured and wound closure distance was calculated by Image J software. The results indicated that A. saccata leaf extract, at 125 μg/mL, closed the gap created by the scratch by 93.525% in 48 h. Percentage wound closure at different time intervals in untreated, extract treated and control drug-treated cells have been represented in Fig. 3. A. saccata leaf extract induced the migration of L929 cells resulting in wound closure. In the standard-drug treated cells, 99.05% of the gap was closed at 48 h. Fig. 4 shows the microscopic images of untreated, standard drug-treated and extract-treated L929 cells. The photographs show increased cell migration in the control drug-treated cells and extract treated cells
Fig. 3.
Percentage of cells migrated towards the wound and involved in wound closure. Migration of cells in the absence or presence of A. saccata leaf extract. Blue: cells with culture medium alone; Orange: 5 μg/mL of standard drug Cipladine; Grey: 125 μg/mL of A. saccata leaf extract.
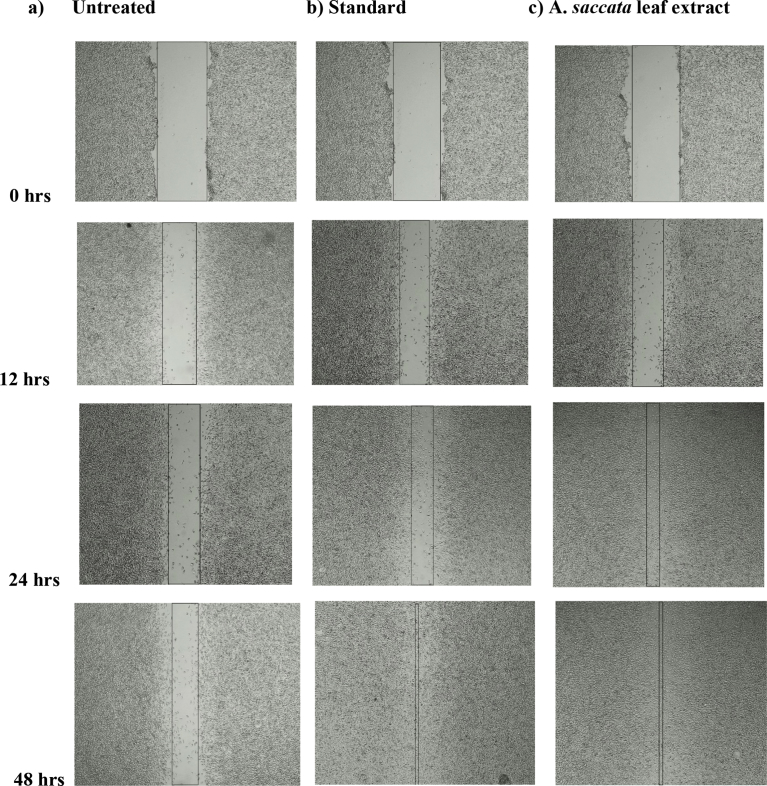
Fig. 4.
Microscopical images representing the In vitro wound healing nature of methanolic extract of A. saccata leaf: L929 cells were incubated in presence or absence of A. saccata leaf extract and standard drug Cipladine and images were captured at 0, 12, 24 and 48 hrs. (a) Negative control, (b) 5μg of positive control Cipladine (c) 125 μg/ml of A. saccata leaf extract. The boundaries of the scratched wounds were determined by the dark lines.
2.3. Collagen type 1 expression was increased dose dependently
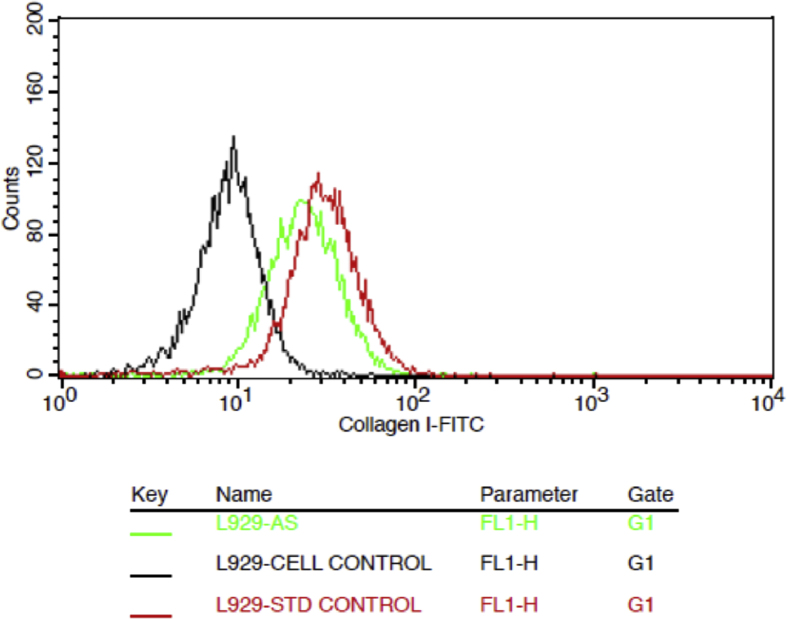
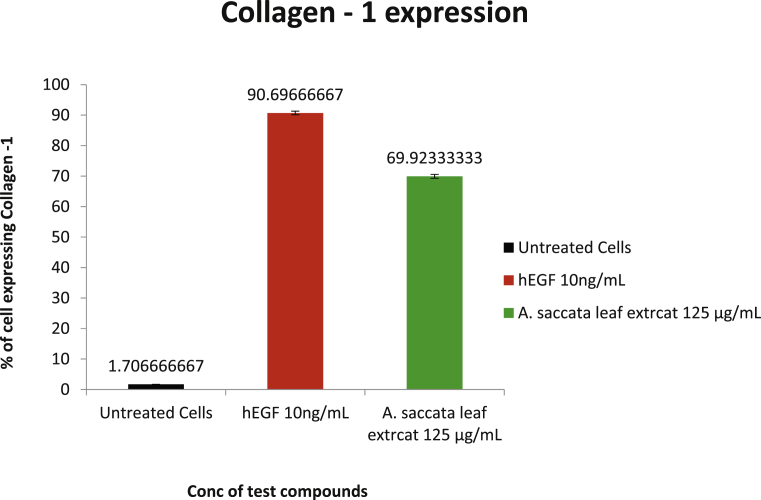
Collagen-1 is the major protein of the extracellular matrix (ECM) and is not only involved in the formation of ECM during the wound healing process, but also enhances cellular proliferation, migration, differentiation and synthesis of other essential proteins from the surrounding cells [15, 16]. In the current study, the expression of Collagen type 1 was analysed by flow cytometry after 48 h of treatment of L929 cells with 125 μg/mL of A. saccata leaf extract or 10 ng/mL of human Epidermal Growth Factor (hEGF). Experimental results showed increased expression of collagen type 1 in A. saccata leaf extract treated and hEGF treated cells. The percentage of cells that expressed collagen type 1 in the extract treated cells was 69.92 % and in hEGF treated cells was 90.69 % of cells. The flow cytometric analysis of Collagen type 1 expression and the quantification of the expressing cells in untreated, extract treated and hEGF treated cells have been represented in Fig. 5 and 6, respectively. These results indicate a clear increase in expression of Collagen type 1 in extract-treated cells compared to the untreated control indicating that the extract enhances Collagen type 1 expression in L929 cells, possibly thereby enabling the wound healing process.
Fig. 5.
Flow cytometry based expression studies of collagen-1. L929 cells were exposed to 125 μg/ml and 10 ng/ml of A. saccata leaf extract and hEGF respectively for 48hrs. a) black-untreated cells; b) green – A. saccata leaf extract; c) red-hEGF.
Fig. 6.
Bar graphs represent the % of L929 cells expressing collagen-1 upon treatment with 125 μg/ml of A. saccata leaf extract and 10 ng/ml of hEGF for 48 hrs a) black-untreated cells; b) green – A. saccata leaf extract; c) red-hEGF. The studies were conducted in triplicates and 10000 events were measured for each flow cytometry run.
3. Discussion
Wound healing is a complex mechanism and a variety of plants used traditionally in folk medicine have been ethnopharmacologically validated for their wound healing properties. A number of In vitro studies have been conducted with the crude plant extracts or isolated secondary metabolites to understand their extended use in wound healing [17]. Several medicinal properties like arthritis, snake bites, wound healing and skin diseases, anticancer, anti-inflammatory [9], antifeedant [10], muscle relaxant [11] properties of plants belonging to the Aristolochiaceae family have been reported both in traditional and modern medical research deeming the plants to be medicinally relevant and important. Although these medicinal properties of the plant are known traditionally, these properties have not been scientifically validated. Therefore, in this study, we evaluated the wound healing properties of A. saccata by In vitro assays and expression studies. A typical wound healing process encompasses complex cellular changes that include inflammation, angiogenesis, re-epithelialization, granulation tissue formation, and remodeling of extracellular matrix [2]. In the initial stages of wound healing, fibroblasts play a vital role by actively proliferating, migrating to wound area and inducing the synthesis of new extracellular matrix (ECM), and thick actin myofibroblasts [18]. Fibroblast cell lines like TIG119 [19], NHDF [20], HDF-D [20], L929 [21, 22] etc. have been employed to assess the wound healing potencies of several compounds In vitro. Besides plant extracts, herbal formulations made of a combination of plant extracts have also been tested using these cell lines, particularly the L929 cells [21]. In other words, the migratory and proliferative abilities of the fibroblasts are pivotal to wound healing. In order to assess the wound healing potency of the methanolic extract of A. saccata leaves, we performed scratch assay, a widely used In vitro assay in wound healing studies [14], on the fibroblast cell line L929. In our study, we observed that L929 cells migrated better toward the artificially created wound when treated with the A. saccata leaf extract. This suggests that the extract accelerates wound healing by inducing the migration of fibroblasts. A similar study on another species of the same family, A. bracteolate, reported that its methanolic extract stimulated the migration of fibroblasts and kerationcyctes and enhanced the expression of wound healing related genes [23].
Further, fibroblasts synthesize ECM components and growth factors like Collagen, FGF, EGF, TGF-β etc., which form the key regulators of wound healing process and are involved in the regeneration of injured ECM [4]. Of these, collagen is a key component of the ECM and its synthesis is proportional to the amount of hydroxyproline synthesized 4,18,24−27. Collagen type 1 is a crucial factor and is imperative to induce major cellular events like extracellular matrix remodeling during the wound healing process [24, 25, 26, 27] and angiogenesis [28]. Moreover, the strength and integrity of the newly formed blood vessels is directly linked to the amount of collagen synthesized by the fibroblasts [18]. Studies focused on plants for wound healing properties have also demonstrated their ability to increase collagen production. For example, the oral and topical administrations of Centella asiatica extract not only showed increased collagen synthesis but also better maturation and crosslinking of collagen in rat models [29]. Also, Shirwaikar et. al. reported that the use of A. bracteolate increased the rate of wound contraction and there was a significant increase in the hydroxyproline content, which is an indication of collagen levels [30]. Adams et. al. reported a few native plants of Australia that differentially induce Collagen I and Collagen III In vitro upholding them as a useful source of wound healing compounds. In this report, the bioactive compounds that bring about the induction of collagen were also elucidated, suggesting that plants induce Collagen expression through their bioactive compounds [31]. Therefore, we studied the expression of Collagen type 1 in the untreated, A. saccata extract treated and control molecule-treated L929 cells. In our study, the expression of Collagen type 1 was found to be upregulated on treating L929 cells with the A. saccata extract, suggesting that A. saccata possibly enhances the expression of Collagen type 1, thereby initiating the migration of fibroblasts bringing about wound healing. This indicates that A. saccata has potential wound healing properties and can be used to extract lead molecules in the discovery of wound healing agents. The phytochemical analysis of other well-known wound healing plants reveal the possible role of these phytoconstituents especially flavonoids and triterpenoids in wound healing. These phytochemicals have been documented to possess astringent, free radical scavenging and antioxidant properties, which are known to aid wound healing process [32, 33]. Another possible mechanism is that the plant extracts increase the proliferation of fibroblasts cells and in turn increase the production of collagen in the affected area. This was observed through increase in DNA, total protein and total collagen content of granulation tissues in wounded rat models treated with plant extracts [34].
The extract was also tested for its cytotoxicity using MTT assay. This cytotoxicity assay is based on the idea that early screening of any biological material for toxicity may help in the evaluating its biological and therapeutic relevance. Assessing the cytotoxic effects of the plant extract on the cells or an in vivo model is critical as some plant metabolites might have toxic effects on the cells because of their intermolecular interactions in the cells. This is indicated by a measure of the half maximal inhibitory concentration (IC50) value. A high IC50 value is representative of the fact that high concentration of the extract is essential to cause detrimental effects on the cell, whereas a low IC50 value is indicative of the cytotoxic ability of the extract at smaller doses [35]. Previous literature suggests that an IC50 value of 100 μg/mL may be possibly toxic to cells [36]. Further, American National Cancer Institute (NCI) has set the IC50 limit of 30 μg/mL concentration for the extract to be considered toxic to the cells [37]. In our study, L929 cells were treated with extract concentrations much higher than these recommended thresholds and the percentage viability of the cells was not affected much. This supports our claim that A. saccata extracts can be used in for wound healing without the fear of toxicity. Nevertheless, the scope of these claims is limited to the highest concentration of extract and the cell line type used in the study. A future study with an in vivo model, a different cell type and/or with higher concentrations may prove to be different. However, our study is a step forward in adding ethnopharmacolgical validation to the use of A. saccata in wound healing cases.
4. Materials & methods
4.1. Chemicals and reagents
Dulbecco's Modified Eagle's medium (DMEM) (#AL219A, Himedia), Fetal Bovine Serum (#RM10432, Himedia), Delbucco's Phosphate Buffered Saline (DPBS) (#TL1006, Himedia), Mouse Anti-Human collagen I- Fluorescein isothiocyanate (FITC) antibody (#FCMAB412F, Merck), Cipladine (Cipla Ltd), hEGF (#11376454001, Roche) MTT Reagent (# 4060 Himedia), DMSO (#PHR1309, Sigma), Fluorescent Activated Cell Sorter (FACS) Calibur (BD Biosciences, INDIA), Microplate reader (#EC800, Biotek).
4.2. Methanolic extraction of leaves
Fresh leaves of A. saccata were shade dried, finely powdered using a mortar and pestle, and 100 g of the powder was used for methanolic extraction. The powder was mixed in 200 mL of methanol and was continuously agitated for 24 h using a magnetic stirrer. The extract was then filtered through Whatman no 1 filter paper and filtrate was concentrated in rotary evaporator at 40 °C. The extract was stored in dry tubes until further use. A stock solution of the extract was prepared in DMSO and working concentrations were prepared by diluting the stock in DMEM with 10% Fetal Bovine Serum.
4.3. Cell culture
The Mouse fibroblast cell line L929 was procured form NCCS, Pune and cultured in DMEM, premixed with 10% Fetal Bovine Serum and antibiotics (streptomycin 100 U/mL and penicillin 100 U/mL). The cells were maintained at 37 °C with 5% CO2 in a humidified incubator and were passaged when they reached 80% confluency. Cells were counted using a Hemocytometer. Viability was calculated to seed the cells at appropriate densities, to perform the assays.
4.4. Cytotoxicity studies
The cytotoxicity of A. saccata leaf extract on L929 cells was evaluated by MTT assay [30]. Briefly, L929 cells were seeded in a 96-well plate at an initial seeding density of 2*104 cells/well/200μL of DMEM and were cultured for 12 h. The cells were then treated with different concentrations of A. saccata leaf extract (31.25, 62.5, 125, 250, and 500 μg/ml), and were incubated for 48 h at 37 °C and 5% CO2. Post incubation, the spent medium was removed and 20 μL of 5 mg/mL of MTT reagent was added to the cells and incubated for 2 h in the CO2 incubator. The formazan crystals were solubilized with 100 μL of DMSO and absorbance at 570nm was determined using a microplate reader. The cells treated with DMEM alone were considered as negative control and 100% viable. The percentage of cell viability was calculated using the formula:
Percentage of cell viability was plotted against concentrations of test samples. Three sets of experiments were performed in triplicate in and the data were presented as mean ± SD (n = 3).
4.5. Scratch assay
The wound healing capabilities of the A. saccata leaf extract was assayed by performing In vitro cell migration studies on L929 cells by a previously described method [14]. Briefly, 2*105 cells/mL were seeded in 6-well plates and were cultured overnight. Cells were then washed with Delbucco's Phosphate Buffered Saline (DPBS) and a scratch was made with a sterile 200μL tip. The detached cells and other cellular debris were removed by washing the cells with DPBS. The cells were treated with 125 μg/mL of A. saccata leaf extract and 5 μg/mL of positive control, Cipladine [17] and incubated for 24 h. Cipladine is a standard drug that is used in wound healing [17, 32]. Untreated cells were negative control. The cell migration and morphological changes of cells were observed in the images taken by inverted microscope, equipped with digital camera. The experiments were performed in triplicate (n = 3). The width of the scratch and wound closure at different time intervals (0, 12, 24 and 48hrs) was analyzed by Image J software.
4.6. Flow cytometry
L929 cells were seeded in 6 well plate at an initial density of 2*105 cells/ml and were treated with 125 μg/ml of A. saccata leaf extract or 10 ng/mL of hEGF. After 48 h of incubation, cells were trypsinized and washed with DPBS. Cells were fixed and permeabilized with 70% ice cold methanol at -20 °C and stained with Mouse Anti-Mouse Collagen I-FITC antibody by incubating at room temperature in dark for 30 min. FACS Calibur was used to evaluate the expression of collagen-1 and data was analysed by CellQuest Pro software.
All studies were conducted in triplicates and results expressed as mean ± SD (n = 3).
4.7. Statistical analysis
All the experiments were conducted in triplicates, and the results are expressed as mean percentage inhibition ± standard deviation (SD) (n = 3). Statistical significance was determined by one-way analysis of variance, followed by Bonferroni post hoc test for multiple comparisons, and p < 0.05 was considered statistically significant. All statistical analyses and IC50 values determination were carried out in GraphPad Prism (version 3.1) software, GraphPad Software, 2365 Northside Dr. Suite 560, San Diego, CA 92108.
5. Conclusion
In conclusion, the methanolic extract of A. saccata leaf enhanced wound closure in L929 cells and expressed higher levels of Collagen type 1. Further, the extract was found to have no cytotoxic effect. These data suggest that A. saccate has possible wound healing properties and can be a plausible source for the extraction of natural wound healing compounds.
Declarations
Author contribution statement
Srinivasa Rao Bolla: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Janardhana Papayya Balakrishna, Shiva Shankar Reddy Gollapalli: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Abeer Mohammed Al-Subaiei, Reem Yousuf Al-Jindan, Vishnu Priya Veeraraghavan, Padma Kanchi Ravi, Joel Palpath Joseph, Aruthra Arumugam Pillai: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Surapaneni Krishna Mohan: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Martin P. Wound healing – aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 2.Guo S., Dipietro L.A. Factors affecting wound healing. J. Dent. Res. 2010;89:219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bainbridge P. Wound healing and the role of fibroblasts. J. Wound Care. 2013;22:407–412. doi: 10.12968/jowc.2013.22.8.407. [DOI] [PubMed] [Google Scholar]
- 4.Schafer M., Werner S. Transcriptional control of wound repair. Annu. Rev. Cell Dev. Biol. 2007;23:69–92. doi: 10.1146/annurev.cellbio.23.090506.123609. [DOI] [PubMed] [Google Scholar]
- 5.Ehrlich H.P., Hunt T.K. Collagen organization critical role in wound contraction. Adv. Wound Care. 2012;1:3–9. doi: 10.1089/wound.2011.0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hulkower K.I., Herber R.L. Cell migration and invasion assays as tools for drug discovery. Pharmaceutics. 2011;3:107–124. doi: 10.3390/pharmaceutics3010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prakash Nagori Badri, Solanki Renu. Role of medicinal plants in wound healing. Res. J. Med. Plant. 2011;5:392–405. [Google Scholar]
- 8.Sarma Bhaskar, Tanti Bhaben. Karyomorphology of three species of Aristolochia – rare and endemic medicinal plants of Assam, India. Caryologia. 2015;68:154–158. [Google Scholar]
- 9.Das R., Kausik A., Pal T.K. Anti-inflammatory activity study of antidote Aristolochia indica to the venom of Heteropneustes fossilis in rats. J. Chem. Pharm. Res. 2010;2:554–562. [Google Scholar]
- 10.Lajide L., Escoubas P., Mizutani J. Antifeedant activity of metabolites of Aristolochia albida against the Tobacco cutworm Spodoptera litura. J. Agric. Food Chem. 1993;41:669–673. [Google Scholar]
- 11.Lemos V.S., Thomas G., Barbosa-Filho J.M. Pharmacological studies on Aristolochia papillaris mast (Aristolochiaceae) J. Ethnopharmacol. 1993;40:141–145. doi: 10.1016/0378-8741(93)90060-i. [DOI] [PubMed] [Google Scholar]
- 12.Makhafola Tshepiso J., McGaw Lyndy J., Eloff Jacobus N. In vitro cytotoxicity and genotoxicity of five Ochna species (Ochnaceae) with excellent antibacterial activity. South Afr. J. Bot. 2014;91:9–13. [Google Scholar]
- 13.Mongalo Nkoana Ishmael. Securidaca longipedunculata Fresen (Polygalaceae): a review of its ethnomedicinal uses, phytochemistry, pharmacological properties and toxicology. J. Ethnopharmacol. 2015;165:215–226. doi: 10.1016/j.jep.2015.02.041. [DOI] [PubMed] [Google Scholar]
- 14.Liang Chun-Chi, Park Ann Y., Guan Jun-Lin. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 15.Rangaraj Aravindan, Harding Keith, Leaper David. Role of Collagen in Wound Management. Wounds. 2011:7. [Google Scholar]
- 16.DiCosmo Frank. Edge effect: the role of collagen in wound healing. Adv. Skin Wound Care. 2009;22:12–15. [Google Scholar]
- 17.Kumar B., Vijayakumar M., Govindarajan R., Pushpangadan P. Ethnopharmacological approaches to wound healing—exploring medicinal plants of India. J. Ethnopharmacol. 2007;114(2):103–113. doi: 10.1016/j.jep.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Li W., Fan J.H., Chen M., Guan S.X., Sawcer D., Bokoch G.M., Woodley D.T. Mechanism of human dermal fibroblast migration driven by type I collagen and platelet-derived growth factor-BB. Mol. Biol. Cell. 2004;15:294–309. doi: 10.1091/mbc.E03-05-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harishkumar M., Masatoshi Y., Hiroshi S. Revealing the mechanism of in vitro wound healing properties of citrus tamurana extract. BioMed Res. Int. 2013:2013. doi: 10.1155/2013/963457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muniandy K., Gothai S., Tan W.S. In Vitro wound healing potential of stem extract of Alternanthera sessilis. Evid. Based Complement Altern. Med. 2018;2018 doi: 10.1155/2018/3142073. Published 2018 Feb 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Talekar Yogesh, G. Apte Kishori, Paygude Shubhangi, R. Tondare Prasad, Parab Pradeep. Studies on wound healing potential of polyherbal formulation using in vitro and in vivo assays. J. Ayurveda Integr. Med. 2017;8:73–81. doi: 10.1016/j.jaim.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pitz Hda S., Pereira A., Blasius M.B. In Vitro evaluation of the antioxidant activity and wound healing properties of jaboticaba (plinia peruviana) fruit peel hydroalcoholic extract. Oxid. Med. Cell Longev. 2016;2016 doi: 10.1155/2016/3403586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Girija D.M., Kalachaveedu M., Subbarayan R., Jenifer P., Rao S.R. Aristolochia bracteolata enhances wound healing in vitro through anti-inflammatory and proliferative effect on human dermal fibroblasts and keratinocytes. Phcog. J. 2017;9(6s) [Google Scholar]
- 24.Schreier T., Degen E., Baschong W. Fibroblast migration and proliferation during in-vitro wound-healing – a quantitative comparison between various growth-factors and a low-molecular-weight blood dialysate used in the clinic to normalize impaired wound-healing. Res. Exp. Med. 1993;193:195–205. doi: 10.1007/BF02576227. [DOI] [PubMed] [Google Scholar]
- 25.Xue M., Jackson C.J. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv. Wound Care. 2015;4:119–136. doi: 10.1089/wound.2013.0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harjanto D., Zaman M.H. Modeling extracellular matrix reorganization in 3D environments. PLoS One. 2013;8 doi: 10.1371/journal.pone.0052509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kular Jaspreet K. The extracellular matrix: structure, composition, age-related differences, tools for analysis and applications for tissue engineering. J. Tissue Eng. 2014 doi: 10.1177/2041731414557112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corpechot C., Barbu V., Wendum D., Kinnman N., Rey C., Poupon R., Housset C., Rosmorduc O. Hypoxia-induced VEGF and collagen I expressions are associated with angiogenesis and fibrogenesis in experimental cirrhosis. Hepatology. 2003;35:1010–1021. doi: 10.1053/jhep.2002.32524. [DOI] [PubMed] [Google Scholar]
- 29.Suguna L., Singh S., Sivakumar P., Sampath P., Chandrakasan G. Influence of Terminalia chebula on dermal wound healing in rats. Phytother Res. 2002;16(3):227–231. doi: 10.1002/ptr.827. [DOI] [PubMed] [Google Scholar]
- 30.Shirwaikar A., Somashekar A.P., Udupa A.L., Udupa S.L., Somashekar S. Wound healing studies of Aristolochia bracteolata Lam. with supportive action of antioxidant enzymes. Phytomedicine. 2003;10(6-7):558–562. doi: 10.1078/094471103322331548. [DOI] [PubMed] [Google Scholar]
- 31.Adams D.H., Shou Q., Wohlmuth H., Cowin A.J. Native Australian plant extracts differentially induce Collagen I and Collagen III in vitro and could be important targets for the development of new wound healing therapies. Fitoterapia. 2016 Mar 1;109:45–51. doi: 10.1016/j.fitote.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 32.James O., Friday E.T. Phytochemical composition, bioactivity and wound healing potential of Euphorbia heterophylla (Euphorbiaceae) leaf extract. Int. J. Pharm. Biomed. Res. 2010;1(1):54–63. [Google Scholar]
- 33.Shenoy C., Patil M.B., Kumar R., Patil S. Preliminary phytochemical investigation and wound healing activity of Allium cepa Linn (Liliaceae) Int. J. Pharm. Pharm. Sci. 2009;2(2):167–175. [Google Scholar]
- 34.Bhaskar A., Nithya V. Evaluation of the wound-healing activity of Hibiscus rosa sinensis L (Malvaceae) in Wistar albino rats. Indian J. Pharmacol. 2012;44(6):694. doi: 10.4103/0253-7613.103252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okeleye Benjamin I., Mkwetshana Noxolo T., Ndip Roland N. Evaluation of the antibacterial and antifungal potential of Peltophorum africanum: toxicological effect on human chang liver cell line. Sci. World J. 2013;2013 doi: 10.1155/2013/878735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nibret Endalkachew. Screening of some Tanzanian medicinal plants for their trypanocidal and cytotoxic activities. Phytother. Res. 2010;24:945–947. doi: 10.1002/ptr.3066. [DOI] [PubMed] [Google Scholar]
- 37.Hasibuan Poppy Anjelisa Z. 2014. Cytotoxic Effect of N-Hexane, Ethylacetate and Ethanol Extracts of Plectranthus amboinicus,(Lour.) Spreng.) on HeLa and Vero Cells Lines. [Google Scholar]