Abstract
Small bowel capsule endoscopy can detect subtle mucosal lesions in pediatric patients with Crohn’s disease, and our aim was to evaluate its application in established ileocolonic Crohn’s disease. Colonic inflammation was evaluated with the colonic Simple Endoscopic Score for Crohn’s Disease (SES-CD) (excluding the score of the terminal ileum). Small bowel inflammation was evaluated with the Lewis score and/or Capsule Endoscopy Crohn’s Disease Activity Index (CECDAI). A Lewis score <135 was defined as small bowel inactive. A colonic SES-CD of 0 (colonic inactive group) was observed in 22/42 procedures (52.4%), and active small bowel lesions were observed in 11/22 procedures (50.0%). The Lewis score was lower in the colonic inactive group compared to the colonic active group. Correlations between the colonic SES-CD, the Lewis score and CECDAI were weak. The Lewis score and CECDAI in the colonic inactive group had significant correlation with fecal calprotectin levels. We suggest that Crohn’s disease patients without both colonic active lesions and elevation of fecal calprotectin levels may not need to receive small bowel capsule endoscopy due to low incidence of lesions in small bowel.
Keywords: capsule endoscopy, fecal calprotectin, pediatric Crohn’s disease, Lewis score
Introduction
Crohn’s disease (CD) is a chronic idiopathic inflammatory bowel disease that can affect any segment of the gastrointestinal tract. Small bowel (SB) lesions occur in approximately 60–90% of pediatric patients with CD, but the presence of SB lesion activity does not often correlate with clinical symptom severity.(1–4) Most patients with CD have SB lesions located in the terminal ileum accessible by an ileocolonoscopy (ICS).(3,5–7) Currently, however, the advent of small bowel capsule endoscopy (SBCE) and cross-sectional imaging [particularly computed tomography enterography (CTE) and magnetic resonance enterography (MRE)] have markedly improved the accuracy of SB lesion and jejunum evaluation. Thus, these current modalities are recommended for the evaluation of SB lesions in CD.(3,8–11)
SBCE is a noninvasive procedure that can detect subtle SB mucosal lesions without radiation exposure in adults. In addition, the diagnostic yield of SBCE during the evaluation of established non-stricturing small bowel CD is superior to small-bowel follow-through (SBFT), CTE, and MRE.(12,13) SBCE for patients with established CD has a risk of capsule retention. However, the use of a patency capsule (COVIDIEN Ltd., Dublin, Ileland) in patients at risk of capsule retention has proven useful in reducing the retention risk.(14–16)
The risk of capsule retention with a patency capsule in pediatric patients with CD is equivalent to the adult risk.(14,15) SBCE has been recommended for evaluating the activity of SB lesions in pediatric patients with CD because of the safety and non-invasiveness of the procedure.(8,9) The Food & Drug Administration (FDA) recommends SBCE for patients aged older than 2 years and invasive esophagogastroduodenoscopic assist for patients with dysphagia. In addition, since SBCE is expensive in some countries,(17) the European Society of Gastrointestinal Endoscopy (ESGE) recommends careful selection of patients with suspected or established CD for the SBCE procedure.(9,18)
The use of SBCE is generally determined by a patient’s medical history and/or biomarker levels of CD.(9,18) However, the validity of this approach is not known, and the appropriate biomarkers of SB lesion activity in established CD have not been determined to date.(19–25) We consider that established ileocolonic CD may be detected by ICS and, if the colonic endoscopic findings correlate with small bowel endoscopic findings, small intestinal endoscopic findings may be predicted. However, there is no previous study that reported a correlation between the endoscopic findings of the small bowel and colon in patients with established ileocolonic CD. The aim of this study is to determine the potential application of SBCE in pediatric patients with established CD.
Materials and Methods
Patient demographics and clinical data
We examined all records of SBCE and ICS performed on pediatric patients with established ileocolonic CD (aged <19 years) in Osaka Medical College Hospital from August 2012 to August 2017. The interval period between SBCE and ICS was within one month. The exclusion criteria were as follows: An initial diagnosis of CD and use of nonsteroidal anti-inflammatory drugs (NSAIDs) and/or corticosteroids in the last month, a postoperative status, and SBCE was inadequate to evaluate the entire area of the small intestine. Blood tests, including evaluation of the levels of albumin (Alb), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), fecal hemoglobin, and fecal calprotectin [determined by colloidal gold aggregation (CGA) assay],(26) were performed within one week after the SBCE procedure. Clinical activity was evaluated by the Pediatric Crohn’s Disease Activity Index (PCDAI).(27) PCDAI is often used for pediatric patients with CD, and a PCDAI <10 is defined as clinical remission. The study protocol was approved by the ethics committee of Osaka Medical College (code no. 2240).
Small bowel capsule endoscopy
All SBCE procedures were performed using the Pill Cam SB2 plus/SB3 and RAPID software (COVIDIEN). All patients received a patency capsule (COVIDIEN) before SBCE to avoid retention and were instructed to prepare for the SBCE procedure with magnesium citrate or polyethylene glycol 2–8 h before the procedure, along with the prokinetic Domperidone and/or mosapride citrate. After the SBCE procedure, we calculated the Lewis score and Capsule Endoscopy Crohn’s Disease Activity Index (CECDAI).(28–30) The Lewis score is the most widely used scoring system for small intestine capsule endoscopic findings for CD and was calculated based on the assessment of villous edema, ulceration, and stenosis for each small bowel tertile divided into equal thirds based on the transit time of the capsule. Following the method in previous literature,(27) we defined a Lewis score <135 as SB inactive and ≥135 as SB active. CECDAI was also used for the assessment of SB CD activity, and inflammation, extent of disease, and presence of strictures were calculated by summation of the proximal and distal scores divided into equal seconds based on the transit time.(29,30) In CECDAI, aphtha and erosions were counted as a score.
Ileocolonoscopy
Endoscopic findings of the colon were assessed and scored by the Simple Endoscopic Score for Crohn’s Disease (SES-CD).(31) In brief, the colonic SES-CD was calculated based on the ulcer size, ulcerated surface, affected surface, and stenosis for each bowel segment from the rectum to the terminal ileum. A colonic SES-CD = 0 was defined as “colonic inactive”, and a colonic SES-CD ≥1 was defined as “colonic active”.(25)
Statistical analyses
Descriptive statistics were presented as the mean ± SD for normal distributions. Non-normal distributions were expressed as the median [interquartile range (IQR)]. Group differences were evaluated using the Chi-square test or Fisher’s exact test for qualitative variables. The Mann-Whitney U test was used when the data did not follow a normal distribution. A p<0.05 was considered statistically significant. Spearman’s rank correlation coefficient was used to assess the correlation between the Lewis score, CECDAI, and colonic SES-CD. Statistical analyses were performed using SPSS ver. 23 (IBM Ltd., Armonk, NY).
Results
Clinical and demographic characteristics
A total of 42 procedures were performed, and 22 pediatric patients with ileocolonic CD were identified and included in the study (Table 1). The median age was 15.1 (IQR 12.7–17.4) years. Growth retardation was mild (growth height z-score, mean −0.7 ± 2.6), and the PCDAI was not very high (PCDAI, median 5.0, IQR 0–15.0). 5-Aminosalicylate, thiopurine, and infliximab were administered for 34/42 (81.0%) patients, 22/42 (52.4%) patients, and 10/42 (23.8%) patients, respectively. Neither patency capsule retention nor SBCE retention was found in the current study.
Table 1.
Clinical and demographic characteristics of patients
| Procedure | 42 (22 patients) | |
|---|---|---|
| Age (years) | median (IQR) | 15.1 (12.7–17.4) |
| Male/Female | 33/9 | |
| Height (cm) | mean ± SD | 159.3 ± 11.3 |
| Body weight (kg) | mean ± SD | 50.5 ± 10.7 |
| Body mass index (kg/m2) | mean ± SD | 19.7 ± 3.0 |
| Growth height z-score | mean ± SD | –0.7 ± 2.6 |
| PCDAI | median (IQR) | 5.0 (0–15.0) |
| Treatment | ||
| 5-ASA | 81.0% (34/42) | |
| Azathiopurine (or 6-mercaptopurine) | 52.4% (22/42) | |
| Prednisolone | 0% (0/42) | |
| Infliximab | 23.8% (10/42) | |
| Others (no medication or herbal medicine) | 14.3% (6/42) |
Abbreviations are referred following. PCDAI, Pediatric Crohn’s Disease Activity Index (clinical remission; <10); 5-ASA, 5-aminosalicylic acid.
The proportion of active lesions
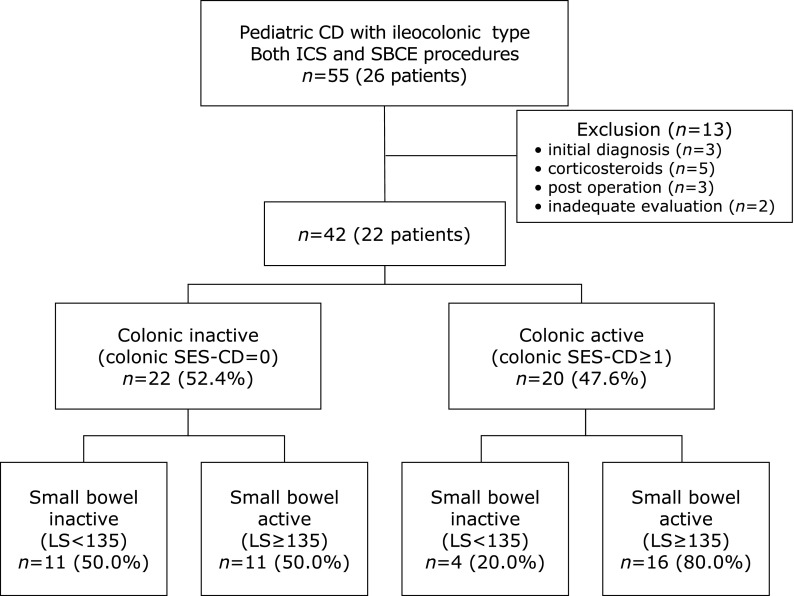
A total of 22 (52.4%) procedures were performed in the colonic inactive group (colonic SES-CD = 0), and 20 (47.6%) procedures were performed in the colonic active group (colonic SES-CD ≥1) (Fig. 1). In the colonic inactive group, 11/22 procedures (50.0%) were performed on SB active (Lewis score ≥135) patients.
Fig. 1.
Summary of the small bowel and colonic endoscopic findings. CD, Crohn’s disease; ICS, ileocolonoscopy; SBCE, small bowel capsule endoscopy; SES-CD, Simple Endoscopic Score for Crohn’s Disease; LS, Lewis score.
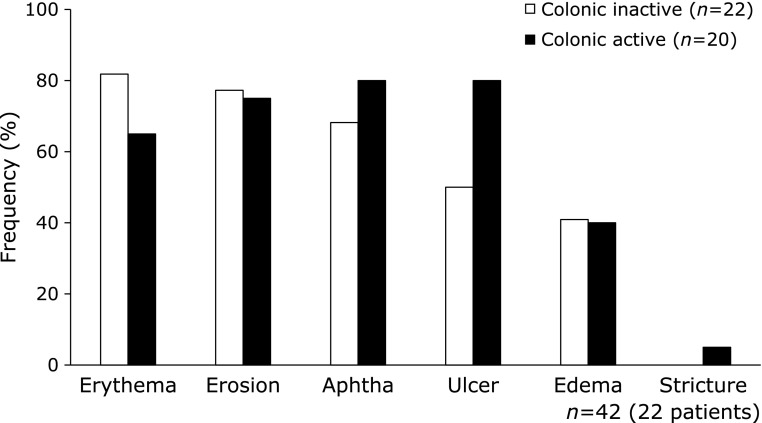
Frequency differences of SB lesions by type in colonic mucosal activity
The frequency of SB lesions by type (Fig. 2) was evaluated between the colonic inactive group and colonic active group (Fig. 3). The frequency of SB lesions with ulcers was higher in the colonic active group compared to the colonic inactive group (p = 0.058).
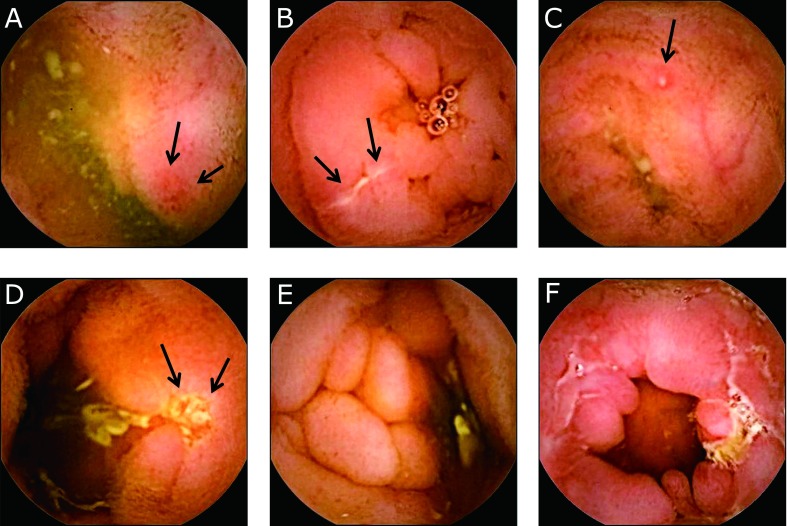
Fig. 2.
Active lesion of Crohn’s disease in small bowel capsule endoscopy. (A) erythema (arrow), (B) erosion (arrow), (C) aphtha (arrow), (D) ulcer (arrow), (E) edematous villi, and (F) stricture.
Fig. 3.
Comparison of active lesion rates in the small bowel between the colonic inactive group and colonic active group. The frequency of active lesions had no statistically significant difference between the colonic inactive group and colonic active group. Statistical analysis was evaluated by Fisher’s exact test.
Comparison of SB mucosal activity and colonic mucosal activity
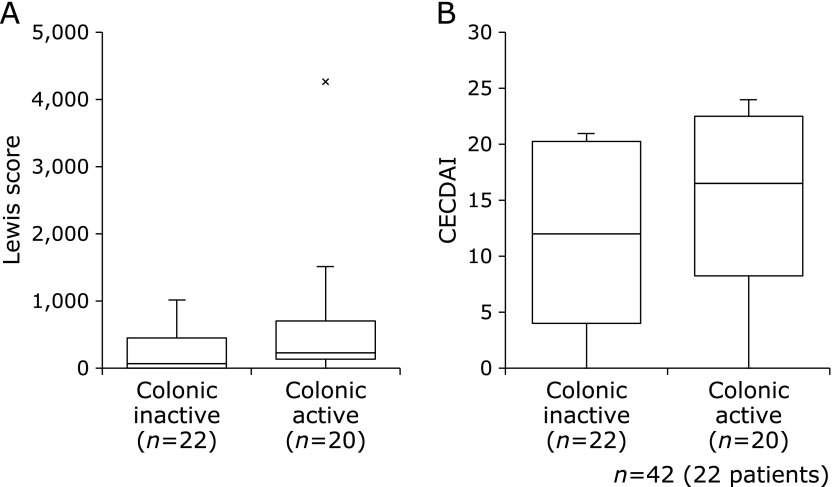
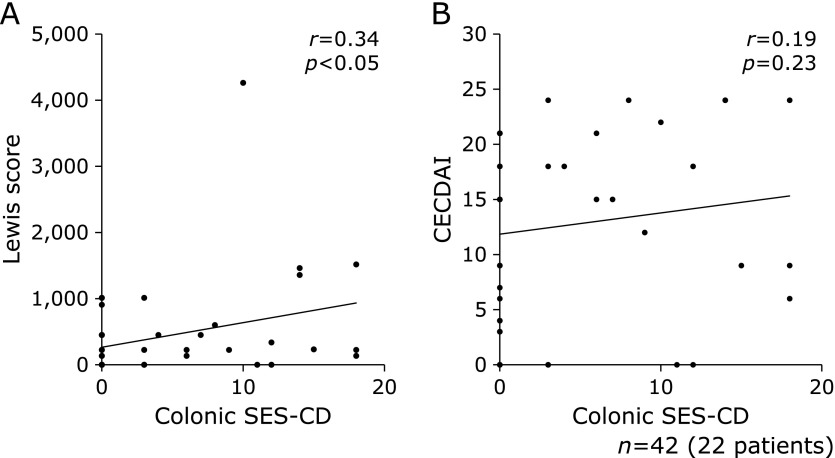
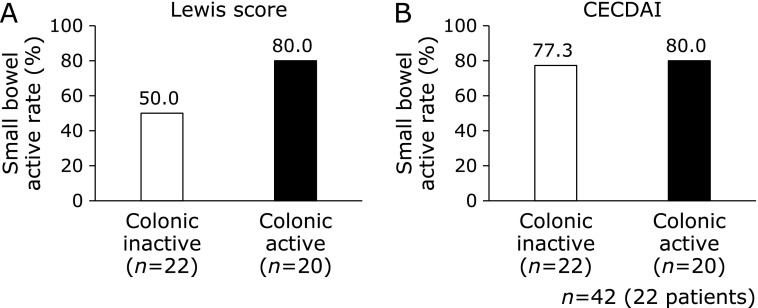
The Lewis score in the colonic active group was higher compared to the colonic inactive group (p = 0.056), but the CECDAI had no statistically significant difference between both groups (p = 0.248) (Fig. 4). Alternatively, there was weak correlation between colonic SES-CD and the Lewis score (Fig. 5a; r = 0.34, p<0.05), and CECDAI was not significantly correlated with colonic SES-CD (Fig. 5b; r = 0.19, p = 0.23). There was no statistically significant difference in the frequency of active SB lesions by the Lewis score or CECDAI between both groups (Fig. 6).
Fig. 4.
Comparison of small bowel activity between the colonic inactive group and colonic active group. (A) Lewis score and (B) CECDAI. Data were presented as the median. The Lewis score and CECDAI had no statistically significant difference between both groups (Lewis score: p = 0.056, CECDAI: p = 0.248). Statistical differences were assessed using Mann-Whitney U test.
Fig. 5.
Correlation of small bowel activity with the Lewis score or CECDAI. (A) Lewis score and (B) CECDAI. The correlation between the Lewis score and colonic SES-CD was weak, and CECDAI was not significantly correlated with colonic SES-CD (Lewis score: r = 0.337, p = 0.029, CECDAI: r = 0.190, p = 0.229). Statistical differences were assessed using Spearman's rank correlation coefficient.
Fig. 6.
Comparison of small bowel activity between the colonic inactive group and active group. (A) Lewis score and (B) CECDAI. Small bowel inactive was defined as a Lewis score ≥135 or CECDAI ≥3.8. The Lewis score and CECDAI had no statistically significant difference between both groups (Lewis score: p = 0.058, CECDAI: p = 1.000). Statistical analysis was evaluated by Fisher’s exact test.
Indicative biomarkers of SB activity
In the colonic inactive group, we compared several factors between the SB inactive and SB active groups. Age and Alb in the SB inactive group were significantly higher compared with those in the SB active group (Table 2; p = 0.006, p = 0.019, respectively), whereas CRP, ESR, and fecal calprotectin levels in the SB inactive group were lower than those in the SB active group (Table 2; p = 0.007, p = 0.003, p = 0.001, respectively). The proportion of infliximab in the SB inactive group was higher than that in the SB active group (Table 2; p = 0.005).
Table 2.
Comparison small bowel inactive and active in colonic inactive group
| Small bowel inactive (n = 11) | Small bowel active (n = 11) | p value | ||
|---|---|---|---|---|
| Age (years) | median (IQR) | 16.3 (15.8–18.3) | 12.8 (11.2–15.1) | 0.006** |
| Male/Female | 11/0 | 11/0 | 0.317 | |
| Body mass index (kg/m2) | mean ± SD | 20.2 ± 2.2 | 20.8 ± 3.7 | 0.922 |
| Growth height z-score | mean ± SD | –1.4 ± 2.2 | –2.1 ± 2.0 | 0.577 |
| C-reactive protein (mg/dl) | median (IQR) | 0.03 (0.02–0.05) | 0.09 (0.07–0.39) | 0.007** |
| ESR (mm/1 h) | median (IQR) | 3.0 (2.0–5.0) | 8.0 (4.0–11.0) | 0.003** |
| Albumin (g/dl) | mean ± SD | 4.7 ± 0.4 | 4.3 ± 0.3 | 0.019* |
| Fecal hemoglobin (ng/ml) | mean ± SD | 0.0 ± 0.0 | 81.6 ± 258.0 | 0.294 |
| Fecal calprotectin (µg/g) | median (IQR) | 98 (47–224) | 826 (488–1,235) | 0.001** |
| PCDAI | median (IQR) | 5.0 (0.0–5.0) | 0 (0–20.0) | 0.777 |
| Treatment | ||||
| 5-ASA | 100% (11/11) | 72.7% (8/11) | 0.069 | |
| Azathioprine (or 6-mercaptopurine) | 45.5% (5/11) | 63.6% (7/11) | 0.403 | |
| Infliximab | 54.5% (6/11) | 0% (0/11) | 0.005** | |
| Others (no medication or herbal medicine) | 0% (0/11) | 27.3% (3/11) | 0.069 |
Asterisks represent significant difference and abbreviations are referred following. ESR, erythrocyte sedimentation rate; PCDAI, Pediatric Crohn’s Disease Activity Index (clinical remission; <10); 5-ASA, 5-aminosalicylic acid.
In the colonic inactive group, the Lewis score had a statistically significant correlation with CRP, ESR, and Alb (Table 3A; r = 0.551, p = 0.008 vs r = 0.649, p = 0.001 vs r = −0.462, p = 0.031). Moreover, there was a strong correlation between the Lewis score and fecal calprotectin levels (Table 3A; r = 0.827, p = 0.00008). The correlation of CECDAI with biomarkers followed a similar pattern to that of the Lewis score (Table 3B).
Table 3.
Correlation between the scores of CD and biomarkers in colonic inactive group
| A. Lewis score | |||
|---|---|---|---|
| n | r | p | |
| C-reactive protein (mg/dl) | 22 | 0.551 | 0.008** |
| Erythrocyte sedimentation rate (mm/1 h) | 22 | 0.649 | 0.001** |
| Albumin (g/dl) | 22 | –0.462 | 0.031* |
| Fecal hemoglobin (ng/ml) | 21 | 0.14 | 0.545 |
| Fecal calprotectin (µg/g) | 16 | 0.827 | 0.00008** |
| B. CECDAI | |||
|---|---|---|---|
| n | r | p | |
| C-reactive protein (mg/dl) | 22 | 0.487 | 0.022* |
| Erythrocyte sedimentation rate (mm/1 h) | 22 | 0.453 | 0.034* |
| Albumin (g/dl) | 22 | –0.469 | 0.028* |
| Fecal hemoglobin (ng/ml) | 21 | 0.299 | 0.189 |
| Fecal calprotectin (µg/g) | 16 | 0.796 | 0.0002** |
Asterisks represent significant difference and abbreviations are referred following. CD, Crohn’s Disease; CECDAI, Capsule endoscopy Crohn’s disease activity index.
In the colonic active group, the Lewis score had a statistically significant correlation with CRP, Alb, and fecal hemoglobin level (Table 4A; r = 0.634, p = 0.003 vs r = −0.598, p = 0.005 vs r = 0.733, p = 0.002). However, there was no correlation between the Lewis score and fecal calprotectin levels (Table 4A; r = 0.162, p = 0.615). The correlation of CECDAI with biomarkers followed a similar pattern to that of the Lewis score (Table 4B).
Table 4.
Correlation between the scores of CD and biomarkers in colonic active group
| A. Lewis score | |||
|---|---|---|---|
| n | r | p | |
| C-reactive protein (mg/dl) | 20 | 0.634 | 0.003** |
| Erythrocyte sedimentation rate (mm/1 h) | 20 | 0.347 | 0.134 |
| Albumin (g/dl) | 20 | –0.598 | 0.005** |
| Fecal hemoglobin (ng/ml) | 20 | 0.733 | 0.002** |
| Fecal calprotectin (µg/g) | 12 | 0.162 | 0.615 |
| B. CECDAI | |||
|---|---|---|---|
| n | r | p | |
| C-reactive protein (mg/dl) | 20 | 0.61 | 0.004** |
| Erythrocyte sedimentation rate (mm/1 h) | 20 | 0.173 | 0.466 |
| Albumin (g/dl) | 20 | –0.526 | 0.017* |
| Fecal hemoglobin (ng/ml) | 20 | 0.494 | 0.027* |
| Fecal calprotectin (µg/g) | 12 | 0.139 | 0.666 |
Asterisks represent significant difference and abbreviations are referred following. CD, Crohn’s Disease; CECDAI, Capsule endoscopy Crohn’s disease activity index.
Discussion
This is the first study to report the correlation between small bowel and colon endoscopic findings in pediatric ileocolonic CD. Previously, SBFT or ICS was performed to assess SB lesions. However, the SBFT procedure has a lower diagnostic ability than SBCE, and SBCE is particularly superior at the assessment of subtle mucosal inflammation.(32) In recent years, SBCE has been recommended as one of the modalities to assess SB lesions in suspected CD without colonic lesions.(10) In contrast, the usefulness of ICS for the assessment of SB lesions is limited, although subtle lesions may be assessed by it.(5) In the current study, there was no correlation between the mucosal activity of SB and colon in pediatric patients with established ileocolonic CD. Therefore, it may be difficult to evaluate SB activity by ICS alone. However, fecal calprotectin levels were significantly correlated with SB mucosal activity in ileocolonic CD without colonic inflammation. Thus, we conclude that SBCE more effectively assesses the whole small intestine and prevents missing SB ulcers, considering that ICS alone found 50.0% of the SB ulcers in the colonic inactive group.
Colonic mucosal healing in CD has been well discussed;(33) however, there are very few reports on SB mucosal healing in CD.(19) There are some reports on the availability of SBCE;(12,13) however, the prognosis in SB active CD patients must be elucidated. Several studies have established that SBCE findings of SB activity are observed in CD patients even though they have clinical or biomarker remission and that SBCE contributes to determining how patients with CD are managed.(19,20,34) However, determining whether mild SBCE findings affect the management and prognosis of patients with CD remains controversial. We should pay careful attention to the interpretation of SBCE findings, because positive subtle mucosal findings are reported in 80% of the healthy patients.(35) Thus, further investigation will be needed to clarify if finding mild SB lesions in ileocolonic CD contributes to the management strategies or prognosis of patients with CD.
Several studies reported the correlation between SBCE findings and biomarkers including CRP, ESR, and fecal calprotectin levels.(19–25,36–43) The current study indicated that the Lewis score and CECDAI had significant correlations with CRP, ESR, Alb, and fecal calprotectin levels in colonic inactive group. In addition, there was a strong correlation between fecal calprotectin levels and the Lewis score in the colonic inactive group. However, the medians of CRP, ESR, and Alb were almost within normal values, and there was little difference between both groups. It may be difficult to evaluate the results and apply the findings in clinical practice. The fecal calprotectin level differences between the SB inactive and active groups without colonic inflammation were statistically significant, and the fecal calprotectin levels had a strong correlation with the Lewis score and CECDAI. In the current study, there were different strengths of the correlation between fecal calprotectin levels and the scoring systems including the Lewis score and CECDAI (p = 0.00008 vs p = 0.0002). This difference may be a result of the different scoring mechanisms, because the Lewis score evaluates mainly ulcerated lesions.
The SBCE procedure has some problems including the high cost, burden of the SBCE interpretation, difficulty of cleansing the small intestine, and SBCE swallowing particularly in pediatric patients. The most successful swallowing age for SBCE without using an esophagogastroduodenoscopy is considered to be ≥8 years old,(44) although the FDA approved SBCE for patients ≥2 years old. Modalities other than SBCE, including CTE, MRE, and ultrasound are generally performed for young children.(45) However, it has been reported that SBCE in combination with ICS improves diagnostic accuracy.(13) Based on our findings, fecal calprotectin level may be the most useful biomarker to determine whether SBCE should be performed in pediatric patients with ileocolonic CD. On the other hand, the patients with active colonic lesions are likely to benefit from SBCE, because the Lewis score and the CECDAI in the patients with active colonic lesions were higher than those in the patients without such lesions in the current study. Therefore, both the patients with colonic active lesions and those having no colonic active lesions with elevated calprotectin levels are candidates for SBCE. Alternatively, we consider that it may be unnecessary for CD patients without both colonic active lesions and elevation of calprotectin to receive SBCE due to low incidence of lesions in small bowel.
There are several limitations in the present study. First, the patients’ background of the medication history was different, and any subjects received more than one procedure. This suggests a possibility that the results depend on the type of treatment. Moreover, determining the cut-off value for calprotectin levels were difficult because the number of procedures in the current study was small. Therefore, a larger-scale study will be required to clarify this issue. Second, the present study included less severe cases in the colonic inactive group. There was no significant difference between the SB active and SB inactive without colonic lesion groups regarding PCDAI and growth height z-score. It is contraindicated to perform SBCE for severe stricture CD patients and, therefore, the findings may be biased. We conclude that it is better to perform cross-sectional imaging including CTE and MRE for these severe cases. Third, fecal calprotectin levels in colonic active CD may not be a useful marker for the application of SBCE, because they are affected by colonic active lesions regardless of the presence or absence of SB lesions.
In conclusion, assessment of both colonic mucosal activity by ICS and SB mucosal activity by SBCE in pediatric patients with established ileocolonic CD is important, because there is no correlation between colonic mucosal activity and SB mucosal activity. Therefore, we suggest that CD patients without both colonic active lesions and elevation of calprotectin levels may not need to receive SBCE due to low incidence of lesions in small bowel. Further investigation will be required to clarify this issue.
Author Contributions
TO, AY and TA designed the study; TO, AY, TA, EK, KI and MA performed procedures; TO collected and analyzed data; TO, AY, TA and KT wrote the manuscript; AY, KT, and TA gave technical support and conceptual advice. HT reviewed and edited the manuscript. All authors read and approved the final manuscript.
Abbreviations
- Alb
albumin
- CD
Crohn’s disease
- CECDAI
Capsule Endoscopy Crohn’s Disease Activity Index
- CGA
colloidal gold aggregation
- CRP
C-reactive protein
- CTE
computed tomography enterography
- ESGE
European Society of Gastrointestinal Endoscopy
- ESR
erythrocyte sedimentation rate
- FDA
Food & Drug Administration
- ICS
ileocolonoscopy
- MRE
magnetic resonance enterography
- NSAIDs
nonsteroidal anti-inflammatory drugs
- PCDAI
Pediatric Crohn’s Disease Activity Index
- SB
small bowel
- SBCE
small bowel capsule endoscopy
- SBFT
small bowel follow-through
- SES-CD
Simple Endoscopic Score for Crohn’s Disease
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Chouraki V, Savoye G, Dauchet L, et al. . The changing pattern of Crohn’s disease incidence in northern France: a continuing increase in the 10- to 19-year-old age bracket (1988–2007). Aliment Pharmacol Ther 2011; 33: 1133–1142. [DOI] [PubMed] [Google Scholar]
- 2.Ishige T, Tomomasa T, Takebayashi T, et al. . Inflammatory bowel disease in children: epidemiological analysis of the nationwide IBD registry in Japan. J Gastroenterol 2010; 45: 911–917. [DOI] [PubMed] [Google Scholar]
- 3.Gollop JH, Phillips SF, Melton LJ 3rd, Zinsmeister AR. Epidemiologic aspects of Crohn’s disease: a population based study in Olmsted County, Minnesota, 1943–1982. Gut 1988; 29: 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park SK, Ye BD, Kim KO, et al. . Guidelines for video capsule endoscopy: emphasis on Crohn’s disease. Clin Endosc 2015; 48: 128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flamant M, Trang C, Maillard O, et al. . The prevalence and outcome of jejunal lesions visualized by small bowel capsule endoscopy in Crohn’s disease. Inflamm Bowel Dis 2013; 19: 1390–1396. [DOI] [PubMed] [Google Scholar]
- 6.Mehdizadeh S, Chen GC, Barkodar L, et al. . Capsule endoscopy in patients with Crohn’s disease: diagnostic yield and safety. Gastrointest Endosc 2010; 71: 121–127. [DOI] [PubMed] [Google Scholar]
- 7.Soon SY, Ansari A, Sanderson JD. Small-bowel barium follow-through is rarely required in patients with a normal ileoscopy and terminal ileal biopsy and a normal or unremarkable colonoscopy. Scand J Gastroenterol 2004; 39: 1293–1295. [DOI] [PubMed] [Google Scholar]
- 8.Arguelles-Arias F, Donat E, Fernandez-Urien I, et al. . Guideline for wireless capsule endoscopy in children and adolescents: a consensus document by the SEGHNP (Spanish Society for Pediatric Gastroenterology, Hepatology, and Nutrition) and the SEPD (Spanish Society for Digestive Diseases). Rev Esp Enferm Dig 2015; 107: 714–731. [DOI] [PubMed] [Google Scholar]
- 9.Pennazio M, Spada C, Eliakim R, et al. . Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2015; 47: 352–376. [DOI] [PubMed] [Google Scholar]
- 10.Levine A, Koletzko S, Turner D, et al. . ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr 2014; 58: 795–806. [DOI] [PubMed] [Google Scholar]
- 11.Enns RA, Hookey L, Armstrong D, et al. . Clinical practice guidelines for the use of video capsule endoscopy. Gastroenterology 2017; 152: 497–514. [DOI] [PubMed] [Google Scholar]
- 12.Dionisio PM, Gurudu SR, Leighton JA, et al. . Capsule endoscopy has a significantly higher diagnostic yield in patients with suspected and established small-bowel Crohn’s disease: a meta-analysis. Am J Gastroenterol 2010; 105: 1240–1248. [DOI] [PubMed] [Google Scholar]
- 13.Leighton JA, Gralnek IM, Cohen SA, et al. . Capsule endoscopy is superior to small-bowel follow-through and equivalent to ileocolonoscopy in suspected Crohn’s disease. Clin Gastroenterol Hepatol 2014; 12: 609–615. [DOI] [PubMed] [Google Scholar]
- 14.Cohen SA, Gralnek IM, Ephrath H, Stallworth A, Wakhisi T. The use of patency capsule in pediatric Crohn’s disease: a prospective evaluation. Dig Dis Sci 2011; 56: 860–865. [DOI] [PubMed] [Google Scholar]
- 15.Cohen SA. The potential applications of capsule endoscopy in pediatric patients compared with adult patients. Gastroenterol Hepatol (N Y) 2013; 9: 92–97. [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang W, Han ZL, Cheng Y, et al. . Value of the patency capsule in pre-evaluation for capsule endoscopy in cases of intestinal obstruction. J Dig Dis 2014; 15: 345–351. [DOI] [PubMed] [Google Scholar]
- 17.Maconi G, Magro F. Comparing techniques to achieve high accuracy and low cost: how should we first diagnose Crohn’s disease? J Comp Eff Res 2015; 4: 75–78. [DOI] [PubMed] [Google Scholar]
- 18.Annese V, Daperno M, Rutter MD, et al. . European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis 2013; 7: 982–1018. [DOI] [PubMed] [Google Scholar]
- 19.Kopylov U, Yablecovitch D, Lahat A, et al. . Detection of small bowel mucosal healing and deep remission in patients with known small bowel Crohn’s disease using biomarkers, capsule endoscopy, and imaging. Am J Gastroenterol 2015; 110: 1316–1323. [DOI] [PubMed] [Google Scholar]
- 20.Kopylov U, Nemeth A, Koulaouzidis A, et al. . Small bowel capsule endoscopy in the management of established Crohn’s disease: clinical impact, safety, and correlation with inflammatory biomarkers. Inflamm Bowel Dis 2015; 21: 93–100. [DOI] [PubMed] [Google Scholar]
- 21.Höög CM, Bark LÅ, Broström O, Sjöqvist U. Capsule endoscopic findings correlate with fecal calprotectin and C-reactive protein in patients with suspected small-bowel Crohn’s disease. Scand J Gastroenterol 2014; 49: 1084–1090. [DOI] [PubMed] [Google Scholar]
- 22.Yang L, Ge ZZ, Gao YJ, et al. . Assessment of capsule endoscopy scoring index, clinical disease activity, and C-reactive protein in small bowel Crohn’s disease. J Gastroenterol Hepatol 2013; 28: 829–833. [DOI] [PubMed] [Google Scholar]
- 23.Gurudu SR, Leighton JA. Correlation of two capsule endoscopy scoring systems with fecal calprotectin: does it really matter? Dig Dis Sci 2012; 57: 827–829. [DOI] [PubMed] [Google Scholar]
- 24.Aggarwal V, Day AS, Connor SJ, Leach ST, Grimm MC, Craig PI. Capsule endoscopy findings in small bowel Crohn’s disease patients in clinical remission: correlation with the Crohn’s disease activity index, faecal calprotectin and S100A12. Gastroenterology 2010; 138: S-114. [Google Scholar]
- 25.Stawczyk-Eder K, Eder P, Lykowska-Szuber L, et al. . Is faecal calprotectin equally useful in all Crohn’s disease location? A prospective, comparative study. Arch Med Sci 2015; 11: 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inoue K, Aomatsu T, Yoden A, Okuhira T, Kaji E, Tamai H. Usefulness of a novel and rapid assay system for fecal calprotectin in pediatric patients with inflammatory bowel disease. J Gastroenterol Hepatol 2014; 29: 1406–1412. [DOI] [PubMed] [Google Scholar]
- 27.Hyams JS, Ferry GD, Mandel FS, et al. . Development and validation of a pediatric Crohn’s disease activity index. J Pediatr Gastroenterol Nutr 1991; 12: 439–447. [PubMed] [Google Scholar]
- 28.Gralnek IM, Defranchis R, Seidman E, Leighton JA, Legnani P, Lewis BS. Development of a capsule endoscopy scoring index for small bowel mucosal inflammatory change. Aliment Pharmacol Ther 2008; 27: 146–154. [DOI] [PubMed] [Google Scholar]
- 29.Gal E, Geller A, Fraser G, Levi Z, Niv Y. Assessment and validation of the new capsule endoscopy Crohn’s disease activity index (CECDAI). Dig Dis Sci 2008; 53: 1933–1937. [DOI] [PubMed] [Google Scholar]
- 30.Niv Y, Ilani S, Levi Z, et al. . Validation of the Capsule Endoscopy Crohn’s Disease Activity Index (CECDAI or Niv score): a multicenter prospective study. Endoscopy 2012; 44: 21–26. [DOI] [PubMed] [Google Scholar]
- 31.Daperno M, D’Haens G, Van Assche G, et al. . Development and validation of a new, simplified endoscopic findings score for Crohn’s disease: the SES-CD. Gastrointest Endosc 2004; 60: 505–512. [DOI] [PubMed] [Google Scholar]
- 32.Dionisio PM, Gurudu SR, Leighton JA, et al. . Capsule endoscopy has a significantly higher diagnostic yield in patients with suspected and established small-bowel Crohn’s disease: a meta-analysis. Am J Gastroenterol 2010; 105: 1240–1248. [DOI] [PubMed] [Google Scholar]
- 33.De Cruz P, Kamm MA, Prideaux L, Allen PB, Moore G. Mucosal healing in Crohn's disease: a systematic review. Inflamm Bowel Dis 2013; 19: 429–444. [DOI] [PubMed] [Google Scholar]
- 34.Cotter J, Dias de Castro F, Moreira MJ, Rosa B. Tailoring Crohn’s disease treatment: the impact of small bowel capsule endoscopy. J Crohns Colitis 2014; 8: 1610–1615. [DOI] [PubMed] [Google Scholar]
- 35.Tachecí I, Bradna P, Douda T, et al. . Wireless capsule enteroscopy in healthy volunteers. Acta Medica (Hradec Kralove) 2016; 59: 79–83. [DOI] [PubMed] [Google Scholar]
- 36.Olsen PA, Fossmark R, Qvigstad G. Fecal calprotectin in patients with suspected small bowel disease--a selection tool for small bowel capsule endoscopy? Scand J Gastroenterol 2015; 50: 272–277. [DOI] [PubMed] [Google Scholar]
- 37.Tsibouris P, Periklis A, Chrissostomos K, et al. . When Crohn’s disease is in remission, more patients complete capsule endoscopy study but less lesions are identified. Saudi J Gastroenterol 2013; 19: 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arieira C, Dias de Castro F, Rosa B, Moreira MJ, Firmino-Machado J, Cotter J. Can we rely on inflammatory biomarkers for the diagnosis and monitoring Crohn’s disease activity? Rev Esp Enferm Dig 2017; 109: 828–833. [DOI] [PubMed] [Google Scholar]
- 39.He C, Zhang J, Chen Z, et al. . Relationships of capsule endoscopy Lewis score with clinical disease activity indices, C-reactive protein, and small bowel transit time in pediatric and adult patients with small bowel Crohn’s disease. Medicine (Baltimore) 2017; 96: e7780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aggarwal V, Day AS, Connor S, et al. . Role of capsule endoscopy and fecal biomarkers in small-bowel Crohn’s disease to assess remission and predict relapse. Gastrointest Endosc 2017; 86: 1070–1078. [DOI] [PubMed] [Google Scholar]
- 41.Yablecovitch D, Lahat A, Neuman S, et al. . The Lewis score or the capsule endoscopy Crohn’s disease activity index: which one is better for the assessment of small bowel inflammation in established Crohn’s disease? Therap Adv Gastroenterol 2018; 11: 1756283X17747780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitselos IV, Katsanos KH, Tatsioni A, et al. . Association of clinical and inflammatory markers with small bowel capsule endoscopy findings in Crohn’s disease. Eur J Gastroenterol Hepatol 2018; 30: 861–867. [DOI] [PubMed] [Google Scholar]
- 43.Klang E, Kopylov U, Eliakim R, et al. . Diffusion-weighted imaging in quiescent Crohn’s disease: correlation with inflammatory biomarkers and video capsule endoscopy. Clin Radiol 2017; 72: 798.e7–798.e13. [DOI] [PubMed] [Google Scholar]
- 44.Fritscher-Ravens A, Scherbakov P, Bufler P, et al. . The feasibility of wireless capsule endoscopy in detecting small intestinal pathology in children under the age of 8 years: a multicentre European study. Gut 2009; 58: 1467–1472. [DOI] [PubMed] [Google Scholar]
- 45.Carter D, Katz LH, Bardan E, et al. . The accuracy of intestinal ultrasound compared with small bowel capsule endoscopy in assessment of suspected Crohn’s disease in patients with negative ileocolonoscopy. Therap Adv Gastroenterol 2018; 11: 1756284818765908. [DOI] [PMC free article] [PubMed] [Google Scholar]