1. Introduction
Hemophagocytic lymphohistiocytosis (HLH) is associated to high mortality and morbidity. A condition often underrecognized, HLH should be in the differential diagnosis of patients presenting with fever, cytopenia, hypertriglyceridemia and high ferritin levels (>500 μg/L). Acquired or secondary HLH in adults is a heterogenous disease triggered by infectious, autoimmune, or neoplastic conditions. Infection associated HLH (IAHS) have been described in association to immunodeficiency and secondary to infections with endemic pathogens. Extensive investigation with microbiology, serology, urine antigens and biopsies of the bone marrow, lung or lymph nodes are often necessary. We present a series of three adult cases of HLH in patients admitted to the intensive care unit with a presumptive diagnosis of sepsis. Early recognition and treatment of this infection sometimes with concomitant administration of immunosuppressant therapy could impact the high mortality associated to this overlap syndrome.
2. Case: 1
A 28-year-old healthy Caucasian man presented with one week history of fever, chills, generalized musculoskeletal pain, nausea, vomiting, diarrhea and headache. One week prior to symptoms onset he was hiking at the Roanoke River in Virginia. On physical examination his blood pressure was 123/69 mmHg, pulse 96/min, temperature 103 F (39.4 °C), respiratory rate 36/min. He was in moderate distress. He had conjunctivae injection, enlarged cervical lymphadenopathy, clear lungs and normal heart sounds on auscultation. He had bilateral petechiae in his legs. Laboratory revealed WBC 0.9 (normal 4.0–10.0 × 10/L), platelets 17 (normal 150–450 × 10/L), transaminitis AST-457, ALT- 96 (AST normal 10–42 IU/L, ALT normal 10–60 IU/L), CK- 8663 (normal 26–308 IU/L), fibrinogen 162 (normal 204–475 mg/dL), triglycerides 249 (normal <150 mg/dL) and ferritin 61,437 (normal 23.9–336.2ng/mL). HIV test was negative. Autoimmune workup included ANA with reflux, ANCA and rheumatoid factor were all negative. CT scan of the abdomen did not show organomegaly.
He was admitted to the intensive care unit (ICU) for sepsis with multiorgan dysfunction and was empirically started on vancomycin, cefepime and doxycycline. Bone marrow biopsy for evaluation of cytopenia's demonstrated presence of histiocytes with intracellular RBC and WBC's (Fig. 1). Further workup was negative for malignancies. Serology was positive for IgG EBV with EBV DNA PCR viral load of 2096 (normal < 200 copies/ml). DNA PCR Ehrlichia chaffeensis was positive. He received a dose of Etoposide and Dexamethasone. He completed total of 14 days of doxycycline and remained asymptomatic one month after hospital discharge.
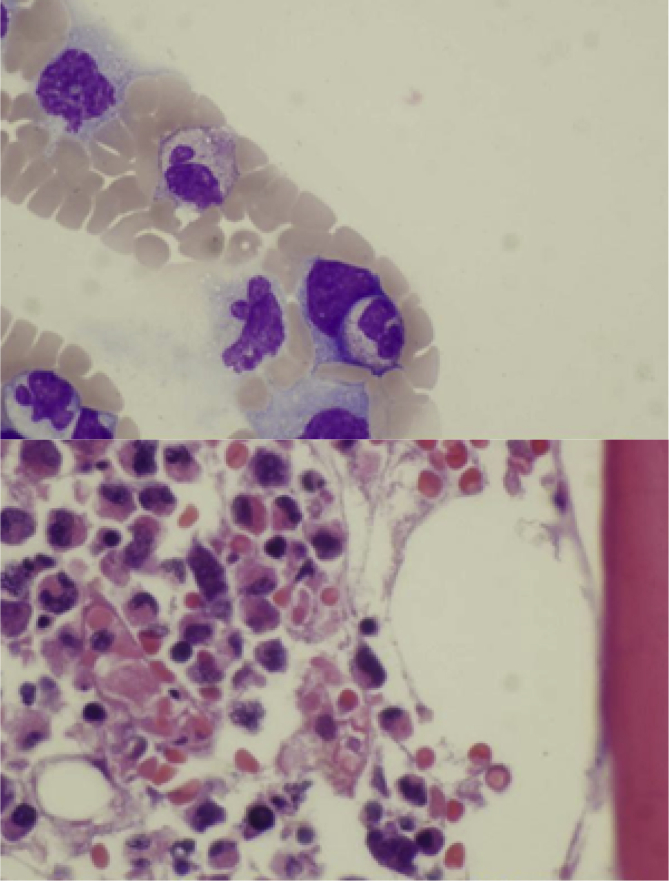
Fig. 1.
Histiocytes with intracytoplasmic RBC and WBC.
3. Case: 2
A 53 year-old Caucasian woman with past medical history of fibromyalgia, hypothyroidism and left carotid stenosis presented with one week history of headache, myalgia, high grade fever, arthralgia, nauseas and vomiting. She was treated empirically with cefdinir and prednisone for possible pharyngitis; however her symptoms did not improve. She was brought to the hospital with confusion. She endorsed tick exposure two weeks prior to the presentation with a tick found on her abdomen.
Physical examination revealed temperature 104 F (40 °C), pulse 120/min, respiratory rate 30/min, and blood pressure 110/63. She had diffuse petechial rash on her abdomen and extremities. She had severe leukopenia 0.8 (normal 4.0–10.0 × 10/L), thrombocytopenia 26 (normal 150–450 × 10/L), lactic acidosis 5.1 (normal 0.5–2.0 mmoL/L), transaminitis AST-373, ALT-84 (AST normal 10–42 IU/L, ALT normal 10–60 IU/L), triglyceride 452, and ferritin 47,547 (normal 23.9–336.2ng/mL). She also had AKI with creatinine 1.35 and CPK 909 (normal 26–308 IU/L). Interleukin 2 receptor level was elevated 10,668 (normal 532–1891 pg/mL). HIV test was negative. Autoimmune workup included ANA with reflux, ANCA and rheumatoid factor were all negative. Peripheral blood smear revealed morulae indicating a tick-borne infection. CT scan of the abdomen showed mild hepatomegaly and the spleen was normal in size.
She was admitted to the ICU for suspected sepsis with multiorgan failure. Ehrlichiosis was suspected on the basis of a tick bite and the presence of morulae in the cytoplasm of a monocyte (Fig. 2). She was treated with IV doxycycline 100 mg every 12 hours. Her hospital course was also complicated by acute encephalopathy requiring invasive mechanical ventilation for airway protection. CSF analysis revealed few inclusions within histiocytes. Bone marrow biopsy confirmed hemophagocytosis. DNA PCR for Ehrlichia chaffeensis was positive. DNA PCR for EBV and CMV DNA was negative. HIV and RMSF were also negative.
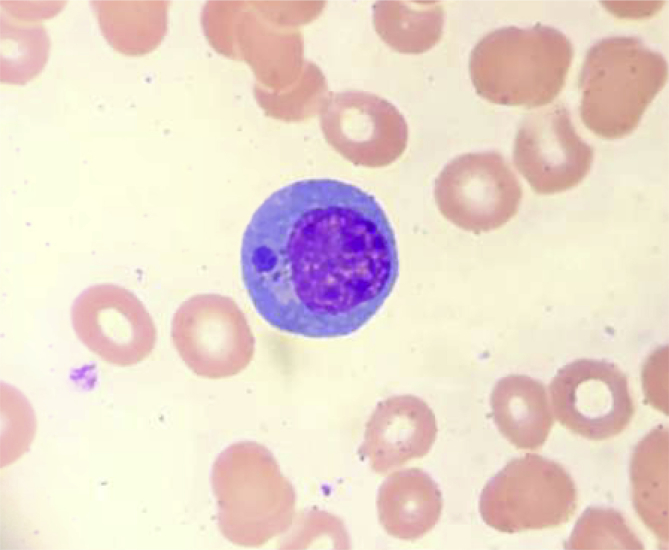
Fig. 2.
Intracytoplasmic inclusions in the histiocytic appearing cells suspicious for Ehrlichiosis.
Her H-score was 294, with probability of diagnosis of HLH to be 100%. She was started on HLH-94 protocol including IV dexamethasone and etoposide. She completed 2 weeks of doxycycline and received 4 doses of etoposide along with dexamethasone and was discharged on dexamethasone taper with etoposide in a stable condition with hematology follow up.
4. Case: 3
A 40 year-old Guatemalan man with HIV/AIDS (CD4 count 26) not on antiretroviral therapy for the last eight months presented with ten days history of weight loss, vomiting, cough and night sweats. On examination he looked cachectic and frail. The temperature was 103F (39.4 °C), the blood pressure 106/93 mm Hg, the pulse 121/minute, the respiratory rate 27/minute, and the oxygen saturation 100% while he was breathing ambient air. He had generalized weakness and anasarca. A diffuse macular rash was present as well as tender splenomegaly and inguinal lymphadenopathy.
A CT scan of the chest, abdomen and pelvis revealed lower lobe pulmonary nodules, splenomegaly (22cm), enlarged axillary, inguinal and mesenteric lymph nodes. He was transferred to the ICU for suspected severe sepsis and disseminated intravascular coagulation (INR was 3, D-dimer 15 mcg/mL and fibrinogen 100 mg/dL). On complete blood count analysis he had a WBC 2.0 (normal 4.0–10.0 × 10/L), hemoglobin 7 (normal 14–18 g/dL), and platelets 30 (normal 150–450 × 10/L). His ferritin level was 4200 (normal 23.9–336.2ng/mL) and lactate dehydrogenase 1390 (normal 140–271 U/L). The level of interleukin 2 (IL-2) receptor alpha was elevated at 1314 (normal range 223–710 U/mL). Serum triglycerides level was normal. Routine blood culture showed no bacterial or fungal growth. Interferon-gamma release assay for tuberculosis was negative. Hepatitis A, B and C serologic tests were negative. Autoimmune workup included ANA, ANCA and rheumatoid factor were all negative. Cryptococcal Ag, Francisella tularensis IgG, Rickettsia rickettsii IgG and IgM, Trypanosoma cruzi IgM and Toxoplasma gondii IgM were all negative. Serology was positive to CMV IgG and PCR, but no baseline test was available to compare. Positive EBV IgG and EBV nuclear Ag IgG was consistent with past infection or a reactivated EBV infection. Acid-fast bacilli stain was negative and cultures remained negative for other common and opportunistic infections (PCP, CMV, HSV were all negative). Buffy coat fungal culture and stain was consistent with histoplasma. Bronchoscopy with BAL was positive for intracellular organisms with morphology consistent with histoplasmosis on Grocott–Gomori's methenamine silver stain.
Liposomal amphotericin B was added to the empiric antibacterials immediately after the buffy coat test was reported positive. Atovaquone was started later and he continued on Azithromycin for chemoprophylaxis. Acyclovir was added for suspected CMV infection. HAART therapy (Darunavir, Dolutegravir, Emtricitab-Tenofovir) was started for his uncontrolled HIV infection one month after initiation of antifungals.
A bone marrow biopsy was performed for evaluation of pancytopenia. The biopsy showed pancytopenia, increased iron stores, extensive intracytoplasmic fungi consistent with histoplasmosis and hemophagocytosis (Fig. 3). Cultures from the bone marrow aspirate confirmed disseminated Histoplasma capsulatum.
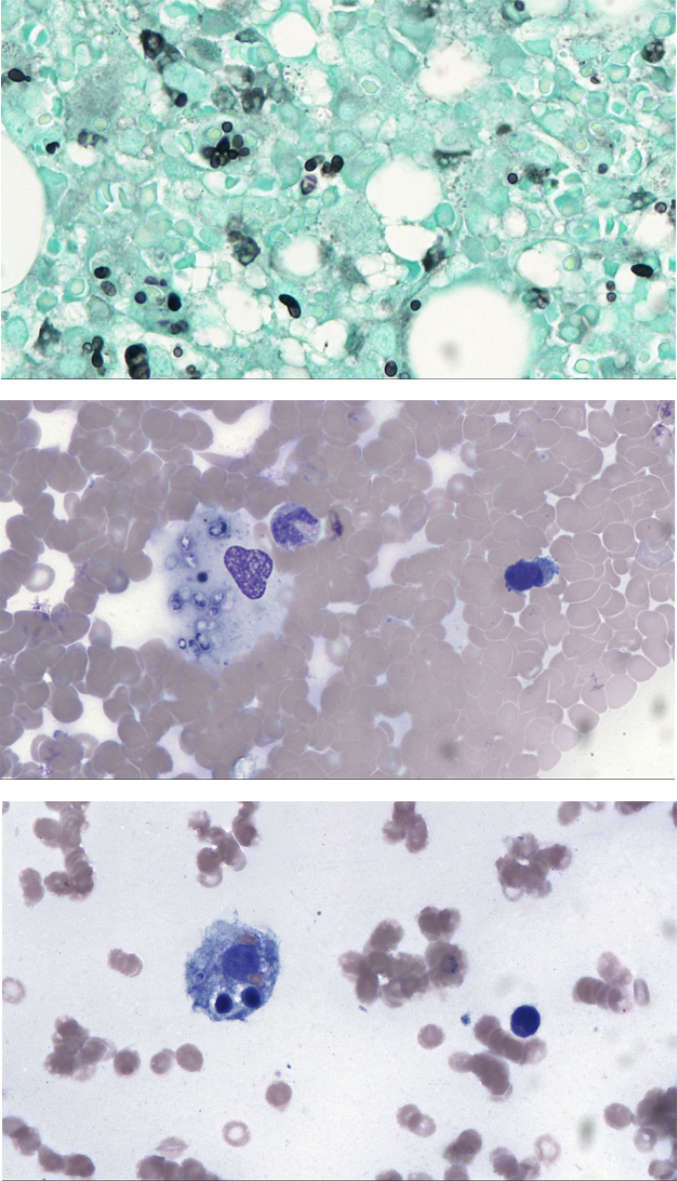
Fig. 3.
(A, B, C)- Bone Marrow biopsy GMS 40x: Special stain for GMS confirmed the presence of intracellular fungi consistent with Histoplasma capsulatum (A). Bone Marrow Aspiration 40x: A macrophage with intracellular fungi consistent with Histoplasma capsulatum (B). Histiocyte containing erythrocytes, lymphocytes, and a microorganism consistent with Histoplasma capsulatum (C).
Dexamethasone was started sixteen days after initiation of HAART therapy when the patient developed acute changes in his mental status. Although he initially improved, few days later his condition deteriorated. He developed septic shock with multiorgan failure secondary to MRSA pneumonia and disseminated Candida lusitaniae infection. Two and a half months after the diagnosis of histoplasmosis-induced hemophagocytic syndrome, supportive care was withdrawn and he succumbed to his illness due to HLH overlap with severe sepsis syndrome with multi-organ failure.
5. Discussion
HLH is a life-threatening disorder characterized by unregulated hyperimmune response to an antigen which results in phagocytosis of white cells, RBCs and platelets by mononuclear macrophages. It was first described in 1952 by two Scottish pediatricians, Farquhar and Claireaux at University of Edinburgh. There are 7 types of familial HLH from genetic mutations in proteins encoding lymphocyte granule mediated pathways [1]. Secondary causes of HLH are seen in adults with autoimmune diseases, infections and malignancies. Viral infections are the most common triggers amongst themare EBV, CMV, and HIV infections.
About 50% of acquired HLH in adults are from malignancy. Most common tumors triggering HLH are hematological neoplasms especially T and NK cell lymphomas or leukemias. In a retrospective study of 20 patients from a single center, incidence of malignancy HLH (M-HLH) was 1.2% [2]. Diagnosis of M-HLH is challenging as symptoms and signs are nonspecific and they overlap with other conditions like sepsis and chemotherapy [3].
In one of the largest series of adults with HLH, Parikh et al. described 62 cases of which 52% were diagnosed with tumor-associated HLH and 34% with infection-associated HLH (4 cases of disseminated histoplasmosis). Fever, hemophagocytosis in bone marrow or lymph nodes, hyperferritinemia, and cytopenias were the most common findings irrespective of the causes of HLH. One interesting finding was a lower platelet count in patients with malignancy-associated HLH compared with those without a tumor [7].
Order of references need to be adjusted as this paragraph was moved up.
Infection with Histoplasma capsulatum commonly occurs from inhaling soil enriched with bird and bat feces. In a review of cases with HIV infection-associated HLH, Subedee et al. have found that the majority of AIDS patients with disseminated histoplasmosis developed HLH if the CD4 count was less than 70 cells/mm. Their reported mortality was around 44% and the two main risk factors for death were not getting antifungal treatment and having Histoplasma in blood [4].
Bacterial causes include tuberculosis and rare cases from Ehrlichiosis. E. chaffeensis is transmitted by the Lone Star tick (Amblyommaamericanum). Ehrlichia can be identified by PCR from peripheral blood and serology.
Add more information about Ehrlichia HLH (use your reference) for example the very high level of ferritin that is seen in Ehrlihicha HLH, etc …
6. Diagnosis
The diagnosis of HLH is often missed especially in the early phase of the disease when not all the diagnostic criteria are present. The clinical presentation and nonspecific laboratory findings are commonly shared with patients admitted to the Intensive care unit for severe sepsis. When the full array of clinical findings is present at the peak of the disease, it is too late and the disease is frequently fatal if appropriate antimicrobials have not been already started.
Patients present with nonspecific symptoms like fever, chills, myalgia, abdominal pain, and cytopenias. Diagnosis of HLH is met if one of the following was fulfilled: a molecular diagnosis consistent with HLH, or meeting five of the following eight criteria (Table 1):
Table 2.
Summary of clinical findings.
| Diagnostic Criteria for Hemophagocytic Lymphohistiocytosis | |||
|---|---|---|---|
| Criterion | Patient 1 | Patient 2 | Patient 3 |
| Fever (Temperature>38.5C for >7 days) | Yes | Yes | Yes |
| Splenomegaly | no | No | Yes |
| Cytopenias involving two or more lines | yes | Yes | Yes |
| Hypertriglyceridemia and/or hypofibrinogenemia (fasting TG's levels>2mmol/liter; fibrinogen<1.5g/liter) | Yes | Yes | Yes |
| (hypofibrinogenemia) | (both) | (hypofibrinogenemia) | |
| Ferritin level>500mcg/L | Yes | Yes | Yes |
| Soluble CD25 level>2400 U/mL (ie, sIL2R) | Not measured | Yes | Not measured |
| Decreased or absent natural killer cell activity | Not measured | Yes | Not measured |
| Hemophagocytosis in bone marrow, central nervous system, or lymph nodes | Yes | Yes | Yes |
Table 1.
At least five of the eight criteria must be present for a diagnosis to be established.
| Diagnostic Criteria for Hemophagocytic Lymphohistiocytosis | |
|---|---|
| Criterion | Present in This Patient |
| Fever (Temperature >38.5C for >7 days) | Yes |
| Splenomegaly | Yes |
| Cytopenias involving 2 or more lines | Yes |
| Hypertriglyceridemia and/or hypofibrinogenemia (fasting triglyceride levels >3 mmoL/liter; fibrinogen <1.5g/liter) | Yes (hypofibrinogenemia) |
| Ferritin level >500mcg/L | Yes |
| Soluble CD25 level>2400 U/mL | No |
| Decreased or absent natural killer cell activity | Not measured |
| Hemophagocytosis in bone marrow, central nervous system, or lymph nodes | Yes |
Elevated ferritin >10,000 μg/L has been demonstrated to be 90% sensitive and 96% specific for HLH. If left untreated, mortality is high with median survival less than 2 months [5]. Coagulopathy is a prominent feature of HLH, as low fibrinogen is found in most patients. Further coagulation studies demonstrated normal factor V and VIII levels and an absent fibrin split product. These findings provide evidence against disseminated intravascular coagulation, a diagnosis that may overlap with HLH due to the shared findings of thrombocytopenia and hypofibrinogenemia [5].
Diagnosis of HLH in the ICU setting is extremely challenging considering the overlap of symptoms and signs with sepsis and multiorgan failure. Tang et al. in their study in 24 pediatric patients with M-HLH showed increased INF-gamma, IL-10 and slightly increase in IL-6 compared to bacterial sepsis patients. Bacterial sepsis was associated with borderline increase in INF gamma, IL-10 and significant increase in IL-6. IL-10 level >2000 ng/ml was associated with poor HLH treatment response and outcome [6].
Bone marrow might be normal or show cytopenias in early cases of secondary HLH. In patients with high clinical suspicion, repeat bone marrow biopsy may be needed. Primary HLH is usually treated per HLH-2004 protocol. HLH-1994 treatment protocol recommended an 8-week induction with IV dexamethasone and etoposide, and HLH-2004 protocol added cyclosporine to help prevent relapse.
7. Treatment
In pediatric cases of inherited HLH, the first line of treatment is chemotherapy with IV dexamethasone plus etoposide +/- intrathecal methotrexate (if presence of neurologic symptoms) and cyclosporine to prevent relapses (HLH-94 or 2004 modified protocol). Allogeneic stem cell transplant have been shown to decrease mortality in children with familial HLH.
In adults with infection triggered-HLH, antimicrobials are considered the first line therapy. In these cases the role of chemotherapy and immunosuppression is not clear. Systemic steroids are sometimes added to antimicrobials but its benefits are unknown due to lack of data. Moreover, in a case series of eleven patients with acquired-HLH and disseminated histoplasmosis (nine patients had HIV/AIDS), all three patients that received steroids died [8]. Whether the higher mortality in this group that received steroids compared to the patients who only received antifungals reflects treatment bias of sicker individuals is unknown. Except in cases of EBV-HLH where early use of etoposide has shown to improve long-term survival [9], no prospective study or randomized clinical trials have explored the benefit of immunosuppression in infection-triggered HLH.
In Ehrlichiosis induced HLH, most patients respond to early initiation of doxycycline 100 mg twice daily. This therapy can be continued 4–5 days after clinically resolution of symptoms. If refractory, chemotherapeutic agents like etoposide, cyclosporine, intrathecal methotrexate, and systemic steroids can be used. Early initiation of etoposide with cyclosporine, within 4 weeks of diagnosis, has been shown to improve long-term survival specifically in cases of EBV-HLH (90.2% +/- 6.9% in patient receiving early etoposide vs 56.5% +/- 12.6% in patient who did not receive treatment or delayed treatment; P < 0.01) due to its ability to hinder lymphocytes activation by EBV [10]. In the three cases described, only the patients with Ehrlichia associated HLH received etoposide in conjunction with dexamethasone. In case 2, only one dose of etoposide with dexamethasone was administered, as he responded very well to IV doxycycline.
Salvage therapies include IVIG, INF gamma inhibitor (infliximab), alemtuzumab (humanized anti-CD52 monoclonal antibody) which are bridge to allogenic stem cell transplantation [11,12]. Currently there are 22 clinical trials recruiting HLH patients looking into various diagnostic and treatment options excluding 12 trials which already completed the study and results are yet to be announced.
In conclusion, the majority of HLH cases in adults are secondary to an underlying trigger. Searching for the underlying cause requires an extensive evaluation and a detailed history including exposure to outdoor activities. Early institution of appropriate antimicrobials with or without addition of immunosuppressants is crucial to halt the deadly progression of the disease. The optimal treatment of infection-related HLH in adults is not clear and it may vary from patient to patient.
Learning points:
-
1.
High index of suspicion is required for HLH in patients presenting with fever, cytopenia's, hepatosplenomegaly and high ferritin levels (particularly > 10,000)
-
2.
Rule out acquired cause of HLH including malignancy, infections and autoimmune diseases
-
3.
Early treatment of underlying cause with antibiotics and immunosuppressive agents is required
-
4.
For patients with EBV reactivated HLH, early immunosuppression should be considered
-
5.
Salvage therapy include IVIG, anti-CD52 monoclonal antibody and infliximab
-
6.
Allogeneic bone marrow transplantation should be considered in refractory cases of primary HLH
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmcr.2019.100854.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zaher K. Ehrlichia-induced hemophagocytic lymphohistiocytosis: a case series. Blood. 2014;124:4105. [Google Scholar]; Ehrlichia-Induced Hemophagocytic Lymphohistiocytosis: A Case Series. Zaher K et al,. Blood 2014 124:4105
- 2.Strenger Volker. Malignancy and chemotherapy induced haemophagocytic lymphohistiocytosis in children and adolescents—a single centre experience of 20 years. Ann. Hematol. 2018;97(6):989–998. doi: 10.1007/s00277-018-3254-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; Volker Strenger et al, Malignancy and chemotherapy induced haemophagocyticlymphohistiocytosis in children and adolescents-a single centre experience of 20 years. doi: 10.1007/s00277-018-3254-4.
- 3.Wang Hongluan. A systematic review of malignancy-associated hemophagocytic lymphohistiocytosis that needs more attentions. Oncotarget. 2017;8(35):59977. doi: 10.18632/oncotarget.19230. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hongluan Wang et al, A systematic review of malignancy-associated hemophagocytic lymphohistiocytosis that needs more attentions. [DOI] [PMC free article] [PubMed]
- 4.Subedee Hemophagocytic syndrome in the setting of AIDS and disseminated histoplasmosis: case report and a review of literature. J. Int. Assoc. Provid. AIDS Care (JIAPAC) 2015 Sep-Oct;14(5):391–397. doi: 10.1177/2325957415570740. [DOI] [PubMed] [Google Scholar]; Subedeeet al, Hemophagocytic Syndrome in the Setting of AIDS and Disseminated Histoplasmosis: Case Report and a Review of Literature. 2015 Sep-Oct; 14(5):391-397 [DOI] [PubMed]
- 5.George Melissa R. Hemophagocytic lymphohistiocytosis: review of etiologies and management. J. Blood Med. 2014;5:69–86. doi: 10.2147/JBM.S46255. Published online 2014 Jun 12. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hemophagocytic lymphohistiocytosis: review of etiologies and management. Melissa R George. J Blood Med. 2014; 5: 69-86. Published online 2014 Jun 12. doi: 10.2147/JBM.S46255 [DOI] [PMC free article] [PubMed]
- 6.Tang Early diagnostic and prognostic significance of a specific Th1/Th2 cytokine pattern in children with haemophagocytic syndrome. Br. J. Haematol. 2008;143(1):84–91. doi: 10.1111/j.1365-2141.2008.07298.x. [DOI] [PubMed] [Google Scholar]; Tang et al, Early diagnostic and prognostic significance of a specific Th1/Th2 cytokine pattern in children with haemophagocytic syndrome. [DOI] [PubMed]
- 7.Parikh S.A., Kapoor P., Letendre L., Kumar S., Wolanskyj A.P. Prognostic factors and outcomes of adults with hemophagocytic lymphohistiocytosis. Mayo Clin. Proc. 2014;89(4):484–492. doi: 10.1016/j.mayocp.2013.12.012. [DOI] [PubMed] [Google Scholar]; Parikh SA, Kapoor P, Letendre L, Kumar S, Wolanskyj AP: Prognostic factors and outcomes of adults with hemophagocytic lymphohistiocytosis. Mayo Clinic proceedings 2014, 89(4):484-492 [DOI] [PubMed]
- 8.Townsend J.L., Shanbhag Histoplasmosis-induced hemophagocytic syndrome: a case series and review of the literature. Open Forum Infect. Dis. 2015 Apr 15;2(2):ofv055. doi: 10.1093/ofid/ofv055. [DOI] [PMC free article] [PubMed] [Google Scholar]; Townsend JL,Shanbhag et al. Histoplasmosis-Induced Hemophagocytic Syndrome: A Case Series and Review of the Literature.Open Forum Infect Dis. 2015 Apr 15; 2(2):ofv055 [DOI] [PMC free article] [PubMed]
- 9.Tothova Hemophagocytic syndrome and critical illness: new insights into diagnosis and management. J. Intensive Care Med. 2015 Oct;30(7):401–412. doi: 10.1177/0885066613517076. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tothova. Hemophagocytic Syndrome and Critical Illness: New Insights into Diagnosis and Management.2015 Oct; 30(7):401-412. doi: 10.1177/0885066613517076. Epub 2014 Jan 8. [DOI] [PMC free article] [PubMed]
- 10.Requirement for etoposide in the treatment of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis. Imashuku Set al. J. Clin. Oncol. 2001 May 15;19(10):2665–2673. doi: 10.1200/JCO.2001.19.10.2665. [DOI] [PubMed] [Google Scholar]; Requirement for etoposide in the treatment of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis. Imashuku Set al. J Clin Oncol. 2001 May 15;19(10):2665-2673. [DOI] [PubMed]
- 11.Marsh R.A. Salvage therapy of refractory hemophagocytic lymphohistiocytosis with alemtuzumab. Pediatr. Blood Cancer. 2013;60(1):101–109. doi: 10.1002/pbc.24188. Epub 2012 Apr 22. [DOI] [PMC free article] [PubMed] [Google Scholar]; Marsh RA et al, Salvage therapy of refractory hemophagocytic lymphohistiocytosis with alemtuzumab. doi: 10.1002/pbc.24188. Epub 2012 Apr 22.. [DOI] [PMC free article] [PubMed]
- 12.Strout M.P. Alemtuzumab as a bridge to allogeneic sct in atypical hemophagocytic lymphohistiocytosis. Nature reviews Clinical oncology. 2010;7(7):415. doi: 10.1038/nrclinonc.2010.40. Epub 2010 Apr 20. [DOI] [PubMed] [Google Scholar]; Strout MP et al, Alemtuzumab as a bridge to allogeneic SCT in atypical hemophagocytic lymphohistiocytosis. doi: 10.1038/nrclinonc.2010.40. Epub 2010 Apr 20.. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.