Abstract
Aim
To assess the potential biological differences between vitamin D2 and D3 using urinary metabolite profiles in response to vitamin D3 or D2 supplementation.
Method
Subjects consisted of 29 subjects with impaired fasting glucose and/or impaired glucose tolerance. Subjects were randomized into two groups, vitamin D2 (20,000 IU weekly, n = 14) or vitamin D3 (15,000 IU weekly, n = 15). Urine and serum samples were taken at two different time points for each subject (at baseline and at 12 weeks). Urinary metabolite profiling was performed by liquid chromatography electrospray ionization quadrupole time-of-flight mass spectrometry (LC-ESI-QTOF-MS). Serum calcium was analyzed on an automated biochemical analyzer and serum intact parathyroid hormone was determined by electrochemiluminescence immunoassay.
Results
At baseline, there was no statistically significant difference in clinical characteristics including age, gender, body mass index, waist circumference and 25-hydroxyvitamin D (25(OH)D) levels between the 2 groups. Weekly administration of 20,000 U D2 for 12 weeks resulted in comparable 25(OH)D concentrations as compared to weekly 15,000 U D3 supplementation (97.8 ± 305 vs. 96.8 ± 3.4 nmol/L, p = 0.84). No difference in serum calcium (2.3 ± 0.03 vs. 2.2 ± 0.03 nmol/L, p = 0.52) or intact parathyroid hormone (5.3 ± 0.3 vs. 4.9 ± 0.5 pmol/L, p = 0.54) at 12 weeks was found. Principle component analysis did not reveal apparent segregation of metabolites according to D2 or D3 supplementation. Moreover, using partial least square regression, no apparent separation between the D2 and the D3 group was found. No important metabolite influencing the separation of the D2 from the D3 group was found using variables importance on projection analysis.
Conclusions
At comparable circulating 25(OH)D concentrations, vitamin D2 or D3 supplementation does not appear to result in different urinary metabolite profiles. Our finding does not support a biological difference between vitamin D2 and D3.
Keywords: Metabolomics, Vitamin D2, Vitamin D3, Prediabetes
Introduction
It is well established that vitamin D affects calcium and bone metabolism. Since vitamin D receptors are ubiquitous in the body, other biological function of vitamin D have been explored and relationships between vitamin D and non-skeletal disorders have been demonstrated [1], [2], [3]. Vitamin D deficiency has been link to prediabetes. The risk of developing diabetes in prediabetic subjects with low 25(OH)D level has been reported to be greater than those who had high 25(OH)D level [4], [5]. Vitamin D supplementation during the prediabetic stage has been shown to be effective in preventing or reduce the rate of progression toward diabetes [6], [7].
The two commonly available forms of vitamin D supplements are vitamin D2 and vitamin D3. Vitamin D3 increases circulating 25(OH)D levels more effectively than vitamin D2 [8]. However, whether the biological effects of vitamin D3 versus vitamin D2 at similar circulating concentrations are similar is unclear. Metabolomics is an unbiased comprehensive study of metabolites in the body simultaneously [9]. Metabolomic approach is considered to be complementary to other omics approach but is more proximal to the condition or disease of interest. It is likely that unexplored or as yet undiscovered physiologic or biochemical influence of vitamin D are likely to exist. Exploring such effects of vitamin D in an unbiased manner using metabolomic approach is likely to be helpful and may be able to uncover novel physiological effects of vitamin D. In the present study, urinary metabolite profiling in response to vitamin D3 or D2 supplementation were used to assess the potential biological difference between vitamin D3 and D2.
Materials and methods
Subjects
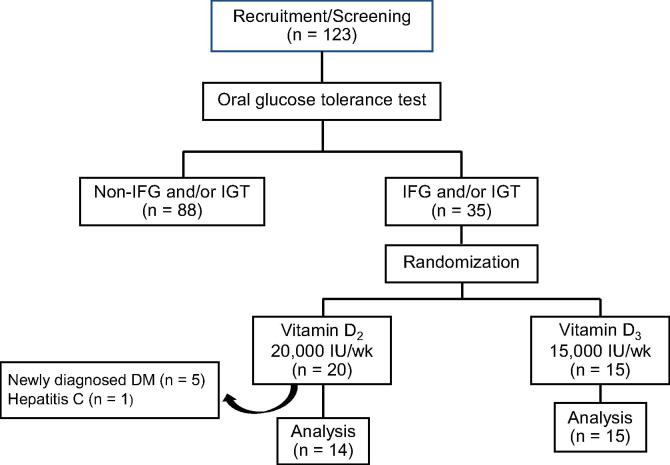
The study was conducted between July and November 2012 in healthy adults from general population of Bangkok, Thailand by advertisement for the screening of type 2 diabetes. Exclusion criteria were previous diagnosis of type 2 diabetes mellitus and pregnant women. Total 123 subjects were recruited and they were aged between 35 and 75 years. Bangkok is located in central of Thailand at the latitude of 13°45′N. The average duration of sunlight during the study is around 3.5–5.1 h of sunlight a day (Thai Meteorological Department, 2012). There is little seasonal variation in the peak sunlight. The minimum and maximum temperature ranged from 23.7 °C to 36.5 °C (Thai Meteorological Department, 2012). A 75 g oral glucose tolerance test was performed in the morning after an 8-hour overnight fast to recruit subject with impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT) according to American Diabetes Association Criteria [10]. Other inclusion criteria were adults with normal renal function, hepatic function and calcium level. Exclusion criteria were adults who have been taking vitamin D supplement over 400 IU/day and/or receiving medication that alter vitamin D metabolites. Thirty-five subjects with IFG and/or IGT were included in this study. Anthropometric variables including weight, height, and waist circumference were measured using standard technique. Body mass index was derived by weight (kg)/height (m)2. The experiment was approved by the Ethical Committee of Ramathibodi Hospital. Written informed consent was obtained from each participant before the initiation of the study.
Previous study has showed that serum 25(OH)D increase by approximately 1.45 and 0.95 nmol/L per 100 IU after daily vitamin D2 and D3 supplemented, respectively [11]. In this study, we aimed to raise total 25(OH)D levels to comparable levels with vitamin D2 or D3. Therefore, different weekly dosage of vitamin D2 (20,000 IU) or vitamin D3 (15,000 IU) were used. Subjects were randomly assigned to receive vitamin D2 (20,000 IU weekly, n = 20) or vitamin D3 (15,000 IU weekly, n = 15) for 12 months. We chose 12 weeks as the supplementation period in this study because Vieth et al. [12] has demonstrated that it takes about 3 months for serum 25(OH)D levels to reach steady state after a change in vitamin D intake. Six subjects of vitamin D2 group were subsequently excluded from the study, five subjects were newly diagnosed with diabetes within three months of the study period and one subjects had hepatitis C viral. Eventually, data from 29 subjects were included in the final study (Fig. 1). Compliance was assessed by tablet counting at 3-month. All subjects had over 90% compliance for vitamin D2 and vitamin D3.
Fig. 1.
Overall study design flowchart. IFG = impaired fasting glucose, IGT = impaired glucose tolerance, DM = diabetes mellitus.
Biochemical measurement:
Urine, plasma and serum samples were taken at two different time points for each subject (at baseline and at 12 weeks). Serum 25(OH)D2 and 25(OH)D3 were analyzed by LC-MS/MS with an Agilent 1200 Infinity liquid chromatograph (Agilent Technologies, Waldbronn, Germany) coupled to a QTRAP® 5500 tandem mass spectrometer (AB SCIEX, Foster City, CA, USA) using a MassChrom® 25-OH-Vitamin D3/D2 diagnostics kit (ChromSystems, Munich, Germany). The summation of serum 25(OH)D2 and 25(OH)D3 was used to reflect vitamin D status. Serum calcium was analyzed on an automated biochemical analyzer (Dimension ExL, Siemens Healthcare Diagnostics Co. Ltd., USA). Hemoglobin A1C (HbA1C) was assayed using turbidimetric inhibition immunoassay (Tina-quant Hemoglobin A1c Gen.3 kit) on a cobas c502 modules (Roche Diagnostics GmbH, Mannheim, Germany). Serum intact parathyroid hormone (PTH) and insulin were measured by chemiluminescence immunoassay on a Cobas E411 immunoassay analyzer (Roche Diagnostics GmbH, Mannheim, Germany). Computer-based homeostatic model assessment index of insulin resistance (HOMA-IR) were calculated from pairs of fasting glucose and insulin levels using homeostasis model assessment-2 (HOMA-2) calculator (www.dtu.ox.ac.uk/homa) [13]. Urinary metabolic profiling was performed by using liquid chromatography electrospray ionization quadrupole time-of-flight mass spectrometry (LC-ESI-QTOF-MS) on an Agilent 1260 Infinity liquid chromatography system couple to an Agilent 6540 UHD accurate-mass quadrupole time-of-flight mass spectrometry (Agilent Technologies, CA, USA). The system was operated both in positive and negative ion mode. Each sample was analyzed with repeated injections five times.
Quantification and identification of urine metabolites
The Masshunter Data Analysis Software (Agilent Technologies, USA) was used to collect the results. The resulting data file was cleaned of background noise and unrelated ions by the Molecular Feature Extraction (MFE) tool in the Masshunter Qualitative Analysis Software (Agilent Technologies, USA). The MFE then creates a listing of all possible components as represented by the full TOF mass spectral data. Finally, the Masshunter Mass Profiler Professional Software B.12.6.1 (Agilent Technologies, USA) was used to perform a non-targeted metabolomic analysis of the extracted features. Compounds from different samples were aligned using a RT window of 0.1% ± 0.15 min and a mass window of 5.0 ppm ± 2.0 mDa. Only common features (found in at least 75% of the samples of the same condition) were analyzed, correcting for individual bias. Accurate masses of features representing significant differences were searched against the METLIN [14] database. Principle component analysis (PCA) and partial least square (PLS) regression analysis were conducted to evaluate the changes of metabolites patterns in the vitamin D2 and D3 supplementation groups
Statistical analysis
Baseline characteristic: continuous data were expressed as median and range. Differences between two independent groups were assessed by Mann-Whitney U-test. Chi-square test was used for testing the equality of proportions between 2 groups. All analyses above were performed using SPSS statistical software package, version 20.0 (SPSS Inc., Chicago IL, USA). Metabolic Profiles: PCA with an unsupervised technique was performed to find trajectories and clustering of the data. Then PLS discriminant analysis was performed to identify differences in the metabolite profiles. Both PCA and PLS were performed using the ropls R-package version 3.8 [15] A p value less than 0.05 was considered statistically significant.
Results
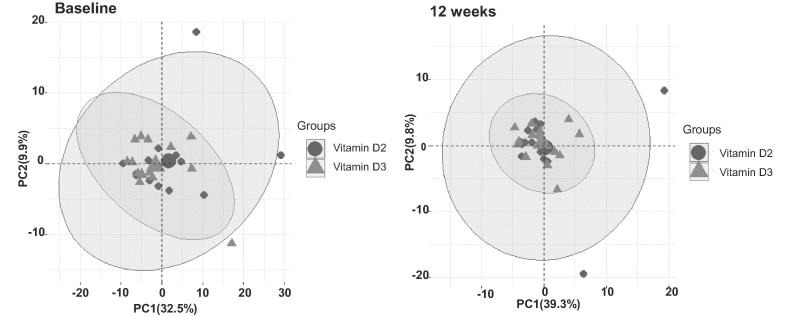
All subjects in this study were non-smokers and did not drink alcoholic beverages except one subjects in the vitamin D3 supplementation group who drank only socially. At baseline, there was no statistically significant difference in clinical characteristics including age, gender, body mass index, waist circumference and 25(OH)D levels (Table 1). Weekly administration of 20,000 U vitamin D2 for 12 weeks resulted in comparable 25(OH)D concentrations as compared to weekly 15,000 U vitamin D3 supplementation No difference in serum calcium or intact parathyroid hormone at 12 weeks was found (Table 2). Mass spectrometry revealed 371 urinary metabolites which were subsequently identified using the METLIN database. Principle component analysis did not reveal apparent segregation of metabolites according to vitamin D2 or D3 supplementation (Fig. 2). Moreover, using partial least square regression, no apparent separation between the vitamin D2 and the vitamin D3 group was found. No important metabolite influencing the separation of the vitamin D2 from the vitamin D3 group was found using variables importance on projection analysis.
Table 1.
Baseline characteristic of the prediabetic subjects.
| Characteristics | Vitamin D2 20,000 IU/week (n = 14) | Vitamin D3 15,000 IU/week (n = 15) | P value |
|---|---|---|---|
| Age (years) | 61 (49–73) | 60 (34–79) | 0.965 |
| Gender (Female/Male) | 12/2 | 13/2 | 0.942 |
| Body mass index (kg/m2) | 27.5 (23.1–29.4) | 27.2 (20.1–32.1) | 0.827 |
| Waist circumference (cm) | 95 (84.0–110) | 93 (71–117) | 0.585 |
| Hemoglobin A1c (%) | 6.0 (5.0–6.6) | 6.1 (5.2–6.5) | 0.326 |
| Serum insulin (pmol/L) | 70.7 (31.8–10.9.9) | 74.6 (15.1–1.0) | 0.631 |
| HOMA-IR | 1.6 (0.71–2.41) | 1.6 (0.34–3.8) | 0.631 |
| Serum 25-hydroxyvitamin D (nmol/L) | 66.3 (44.9–97.3) | 70.7 (31.5–90.4) | 0.662 |
| Serum calcium (mmol/L) | 2.3 (2.1–2.5) | 2.2 (2.1–2.3) | 0.517 |
Data are median (range) or proportion.
HOMA-IR: homeostatic model assessment index of insulin resistance.
Differences in continuous variabels between two groups were assessed by Mann-Whitney U-test.
Differences in gender between two groups were assessed by chi-square test.
Table 2.
Changes of serum 25(OH)D, calcium and intact parrathyroid hormone ater weekly administration of 20,000 U vitamin D2 for 12 weeks as compared to weekly 15,000 U vitamin D3 supplementation in prediabetic subjects.
| Variables | Vitamin D2 20,000 IU/week (n = 14) | Vitamin D3 15,000 IU/week (n = 15) | P value |
|---|---|---|---|
| Serum 25-hydroxyvitamin D (nmol/L) | 98.0 ± 13.0 | 97.0 ± 14.1 | 0.81 |
| Serum calcium (mmol/L) | 2.3 ± 0.03 | 2.2 ± 0.03 | 0.52 |
| Serum intact parathyroid hormone (pmol/L) | 5.0 ± 1.1 | 4.9 ± 1.5 | 0.65 |
Differences between two groups were assessed by Mann-Whitney U-test.
Fig. 2.
Principle component analysis (PCA) of metabolites according to vitamin D2 (circle black color) or D3 (triangle gray color) supplementation at baseline and at 12 weeks in prediabetic subjects.
Discussion
Prediabetes are associated with low vitamin D levels [16], [17] and the risk of developing diabetes is much greater for prediabetes who are vitamin D deficient [5], [18]. Vitamin D supplement is therefore widely used to improve vitamin D status. The two commonly available forms of vitamin D supplements are vitamin D2 and vitamin D3. Supplementation with vitamin D3 has been shown in some studies to reduce the rates of progression to diabetes [6]. Guidelines for vitamin D supplementation by a number of recommending bodies suggest that vitamin D2 and vitamin D3 are equivalent and can be used interchangeably. However, it has been demonstrated that vitamin D3 is more potent than vitamin D2 in raising circulating 25(OH)D [19], [20], [21], [22]. Our study also showed similar finding in that after supplementation 3 months with vitamin D3 at 15,000 IU, weekly and vitamin D2 at 20,000 IU, weekly were comparable in raising blood levels of 25(OH)D. Nevertheless, it is unclear if the potency in terms of biological effects, both skeletal and non-skeletal, of vitamin D3 and D2 are similar. In the present study, after achieving comparable circulating 25(OH)D levels after supplementation with vitamin D2 or vitamin D3, we could not demonstrate any difference in serum calcium and PTH between vitamin D2 and D3. The findings suggest that the skeletal effects of vitamin D2 and D3 are likely to be similar.
Evaluation of endogenous metabolites has played an important role in understanding naturally occurring biochemical pathway and bodily changes. A number of studies have utilized metabolite profiling to help further understanding of various aspects of vitamin D effects and metabolism [23], [24], [25], [26], [27]. Understanding whether or not vitamins D2 is really biosimilar to vitamin D3 is important to ensure proper public health advice in preventing or correcting vitamin D as vitamin D2 is the major compound used in some countries whereas vitamin D3 is used more in others. To our knowledge, there has been no previous study of the metabolite profile after vitamin D3 or D2 supplementation. In the present study, we have demonstrated that at the similar concentration of serum 25(OH)D after weekly supplementation of vitamin D2 or vitamin D3, there was no difference in urinary metabolomics profiles between these two supplementation. This finding suggested the possibility that vitamin D2 and vitamin D3 have similar non-skeletal effect in humans at least at the urinary metabolome level.
Our study has some limitations. The sample size was small which can limit the statistical power to detect less apparent effects. Moreover, lifestyle habits, except for smoking and drinking, and dietary intake were not recorded. However, our previous studies found that daily calcium intakes in Thais was less than 400 mg [28], [29], [30] which is much less than 1000–1200 mg/day according to some recommendations [31]. In addition, vitamin D intake among Thais is generally low because few natural vitamin D-rich food sources are found in Thailand, and foods are not fortified with vitamin D. Furthermore, we assessed only urinary metabolites in the present study. However, urine is the most commonly used biological matrices for metabolomics researchers because urine contains most of the body’s metabolic end products. In addition, its sampling is noninvasive, easy to obtain in large volumes and largely free from interfering proteins or lipids and chemically complex. However, the major disadvantage of using urine is that it is a less regulated fluid and the volume can vary substantially with water intake or other factors.
Conclusions
Although vitamin D2 and D3 may possess different pharmacokinetic characteristics, at comparable circulating 25(OH)D concentrations, vitamin D2 or D3 supplementation does not appear to result in different urinary metabolic profile. Our finding does not support a biological difference between vitamin D2 and D3.
Declaration of Competing Interest
The authors declare that they have no conflicts of interest.
Acknowledgement
This study was supported by the National Science and Development Agency, Thailand.
Contributor Information
Laor Chailurkit, Email: laor.cha@mahidol.ac.th.
Boonsong Ongphiphadhanakul, Email: boonsong.ong@mahidol.ac.th.
References
- 1.Al Nozha O.M. Vitamin D and extra-skeletal health: causality or consequence. Int J Health Sci (Qassim) 2016;10(3):443–452. [PMC free article] [PubMed] [Google Scholar]; Al Nozha O M. Vitamin D and extra-skeletal health: causality or consequence, Int J Health Sci (Qassim) 2016;10(3):443-52. [PMC free article] [PubMed]
- 2.Bouvard B., Annweiler C., Salle A. Extraskeletal effects of vitamin D: facts, uncertainties, and controversies. Joint Bone Spine. 2011;78(1):10–16. doi: 10.1016/j.jbspin.2010.10.011. [DOI] [PubMed] [Google Scholar]; Bouvard B, Annweiler C, Salle A, et al. Extraskeletal effects of vitamin D: facts, uncertainties, and controversies, Joint Bone Spine 2011;78(1):10-6. [DOI] [PubMed]
- 3.Pludowski P., Holick M.F., Pilz S. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence. Autoimmun Rev. 2013;12(10):976–989. doi: 10.1016/j.autrev.2013.02.004. [DOI] [PubMed] [Google Scholar]; Pludowski P, Holick M F, Pilz S, et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence, Autoimmun Rev 2013;12(10):976-89. [DOI] [PubMed]
- 4.Forouhi N.G., Menon R.K., Sharp S.J. Effects of vitamin D2 or D3 supplementation on glycaemic control and cardiometabolic risk among people at risk of type 2 diabetes: results of a randomized double-blind placebo-controlled trial. Diabetes Obes Metab. 2016;18(4):392–400. doi: 10.1111/dom.12625. [DOI] [PMC free article] [PubMed] [Google Scholar]; Forouhi N G, Menon R K, Sharp S J, et al. Effects of vitamin D2 or D3 supplementation on glycaemic control and cardiometabolic risk among people at risk of type 2 diabetes: results of a randomized double-blind placebo-controlled trial, Diabetes Obes Metab 2016;18(4):392-400. [DOI] [PMC free article] [PubMed]
- 5.Deleskog A., Hilding A., Brismar K. Low serum 25-hydroxyvitamin D level predicts progression to type 2 diabetes in individuals with prediabetes but not with normal glucose tolerance. Diabetologia. 2012;55(6):1668–1678. doi: 10.1007/s00125-012-2529-x. [DOI] [PubMed] [Google Scholar]; Deleskog A, Hilding A, Brismar K, et al. Low serum 25-hydroxyvitamin D level predicts progression to type 2 diabetes in individuals with prediabetes but not with normal glucose tolerance, Diabetologia 2012;55(6):1668-78. [DOI] [PubMed]
- 6.Niroomand M., Fotouhi A., Irannejad Nand Hosseinpanah F. Diabetes Res Clin Pract; 2019. Does high-dose vitamin D supplementation impact insulin resistance and risk of development of diabetes in patients with pre-diabetes? A double-blind randomized clinical trial; pp. 1481–1489. [DOI] [PubMed] [Google Scholar]; Niroomand M, Fotouhi A, Irannejad NandHosseinpanah F. Does high-dose vitamin D supplementation impact insulin resistance and risk of development of diabetes in patients with pre-diabetes? A double-blind randomized clinical trial, Diabetes Res Clin Pract 2019;1481-9. [DOI] [PubMed]
- 7.Mirhosseini N., Vatanparast H., Mazidi MandKimball S.M. Vitamin D Supplementation glycemic control, and insulin resistance in prediabetics: a meta-analysis. J Endocr Soc. 2018;2(7):687–709. doi: 10.1210/js.2017-00472. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mirhosseini N, Vatanparast H, Mazidi MandKimball S M. Vitamin D Supplementation, Glycemic Control, and Insulin Resistance in Prediabetics: A Meta-Analysis, J Endocr Soc 2018;2(7):687-709. [DOI] [PMC free article] [PubMed]
- 8.Tripkovic L., Lambert H., Hart K. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr. 2012;95(6):1357–1364. doi: 10.3945/ajcn.111.031070. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tripkovic L, Lambert H, Hart K, et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis, Am J Clin Nutr 2012;95(6):1357-64. [DOI] [PMC free article] [PubMed]
- 9.Nicholson J.K., Lindon J., CandHolmes E. 'Metabonomics': understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica. 1999;29(11):1181–1189. doi: 10.1080/004982599238047. [DOI] [PubMed] [Google Scholar]; Nicholson J K, Lindon J CandHolmes E. 'Metabonomics': understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data, Xenobiotica 1999;29(11):1181-9. [DOI] [PubMed]
- 10.American Diabetes Association. (2) Classification and diagnosis of diabetes, Diabetes Care 2015;38 SupplS8-s16. [DOI] [PubMed]
- 11.Binkley N., Gemar D., Engelke J. Evaluation of ergocalciferol or cholecalciferol dosing, 1600 IU daily or 50,000 IU monthly in older adults. J Clin Endocrinol Metab. 2011;96(4):981–988. doi: 10.1210/jc.2010-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]; Binkley N, Gemar D, Engelke J, et al. Evaluation of ergocalciferol or cholecalciferol dosing, 1,600 IU daily or 50,000 IU monthly in older adults, J Clin Endocrinol Metab 2011;96(4):981-8. [DOI] [PMC free article] [PubMed]
- 12.Vieth R., Chan P.C., MacFarlane G.D. Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am J Clin Nutr. 2001;73(2):288–294. doi: 10.1093/ajcn/73.2.288. [DOI] [PubMed] [Google Scholar]; Vieth R, Chan P CandMacFarlane G D. Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level, Am J Clin Nutr 2001;73(2):288-94. [DOI] [PubMed]
- 13.Levy J.C., Matthews D.R., Hermans M.P. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21(12):2191–2192. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]; Levy J C, Matthews D RandHermans M P. Correct homeostasis model assessment (HOMA) evaluation uses the computer program, Diabetes Care 1998;21(12):2191-2. [DOI] [PubMed]
- 14.Smith C.A., O'Maille G., Want E.J. METLIN: a metabolite mass spectral database. Ther Drug Monit. 2005;27(6):747–751. doi: 10.1097/01.ftd.0000179845.53213.39. [DOI] [PubMed] [Google Scholar]; Smith C A, O'Maille G, Want E J, et al. METLIN: a metabolite mass spectral database, Ther Drug Monit 2005;27(6):747-51. [DOI] [PubMed]
- 15.Thevenot E.A., Roux A., Xu Y., Ezan EandJunot C. Analysis of the human Adult urinary metabolome variations with Age, Body Mass Index, and gender by implementing a comprehensive workflow for univariate and OPLS Statistical Analyses. J Proteome Res. 2015;14(8):3322–3335. doi: 10.1021/acs.jproteome.5b00354. [DOI] [PubMed] [Google Scholar]; Thevenot E A, Roux A, Xu Y, Ezan EandJunot C. Analysis of the Human Adult Urinary Metabolome Variations with Age, Body Mass Index, and Gender by Implementing a Comprehensive Workflow for Univariate and OPLS Statistical Analyses, J Proteome Res 2015;14(8):3322-35. [DOI] [PubMed]
- 16.Gupta A.K., Brashear M.M., Johnson W.D. Prediabetes and prehypertension in healthy adults are associated with low vitamin D levels. Diabetes Care. 2011;34(3):658–660. doi: 10.2337/dc10-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gupta A K, Brashear M MandJohnson W D. Prediabetes and prehypertension in healthy adults are associated with low vitamin D levels, Diabetes Care 2011;34(3):658-60. [DOI] [PMC free article] [PubMed]
- 17.Shankar A., Sabanayagam C., Kalidindi S. Serum 25-hydroxyvitamin d levels and prediabetes among subjects free of diabetes. Diabetes Care. 2011;34(5):1114–1119. doi: 10.2337/dc10-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shankar A, Sabanayagam CandKalidindi S. Serum 25-hydroxyvitamin d levels and prediabetes among subjects free of diabetes, Diabetes Care 2011;34(5):1114-9. [DOI] [PMC free article] [PubMed]
- 18.Pittas A.G., Nelson J., Mitri J. Plasma 25-hydroxyvitamin D and progression to diabetes in patients at risk for diabetes: an ancillary analysis in the Diabetes Prevention Program. Diabetes Care. 2012;35(3):565–573. doi: 10.2337/dc11-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]; Pittas A G, Nelson J, Mitri J, et al. Plasma 25-hydroxyvitamin D and progression to diabetes in patients at risk for diabetes: an ancillary analysis in the Diabetes Prevention Program, Diabetes Care 2012;35(3):565-73. [DOI] [PMC free article] [PubMed]
- 19.Trang H.M., Cole D.E., Rubin L.A. Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am J Clin Nutr. 1998;68(4):854–858. doi: 10.1093/ajcn/68.4.854. [DOI] [PubMed] [Google Scholar]; Trang H M, Cole D E, Rubin L A, et al. Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2, Am J Clin Nutr 1998;68(4):854-8. [DOI] [PubMed]
- 20.Romagnoli E., Mascia M.L., Cipriani C. Short and long-term variations in serum calciotropic hormones after a single very large dose of ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3) in the elderly. J Clin Endocrinol Metab. 2008;93(8):3015–3020. doi: 10.1210/jc.2008-0350. [DOI] [PubMed] [Google Scholar]; Romagnoli E, Mascia M L, Cipriani C, et al. Short and long-term variations in serum calciotropic hormones after a single very large dose of ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3) in the elderly, J Clin Endocrinol Metab 2008;93(8):3015-20. [DOI] [PubMed]
- 21.Heaney R.P., Recker R.R., Grote J., Horst R.L., Armas L.A. Vitamin D(3) is more potent than vitamin D(2) in humans. J Clin Endocrinol Metab. 2011;96(3):E447–E452. doi: 10.1210/jc.2010-2230. [DOI] [PubMed] [Google Scholar]; Heaney R P, Recker R R, Grote J, Horst R LandArmas L A. Vitamin D(3) is more potent than vitamin D(2) in humans, J Clin Endocrinol Metab 2011;96(3):E447-52. [DOI] [PubMed]
- 22.Lehmann U., Hirche F., Stangl G.I. Bioavailability of vitamin D(2) and D(3) in healthy volunteers, a randomized placebo-controlled trial. J Clin Endocrinol Metab. 2013;98(11):4339–4345. doi: 10.1210/jc.2012-4287. [DOI] [PubMed] [Google Scholar]; Lehmann U, Hirche F, Stangl G I, et al. Bioavailability of vitamin D(2) and D(3) in healthy volunteers, a randomized placebo-controlled trial, J Clin Endocrinol Metab 2013;98(11):4339-45. [DOI] [PubMed]
- 23.Blighe K., Chawes B.L., Kelly R.S. Vitamin D prenatal programming of childhood metabolomics profiles at age 3 y. Am J Clin Nutr. 2017;106(4):1092–1099. doi: 10.3945/ajcn.117.158220. [DOI] [PMC free article] [PubMed] [Google Scholar]; Blighe K, Chawes B L, Kelly R S, et al. Vitamin D prenatal programming of childhood metabolomics profiles at age 3 y, Am J Clin Nutr 2017;106(4):1092-9. [DOI] [PMC free article] [PubMed]
- 24.Elnenaei M.O., Chandra R., Mangion TandMoniz C. Genomic and metabolomic patterns segregate with responses to calcium and vitamin D supplementation. Br J Nutr. 2011;105(1):71–79. doi: 10.1017/S0007114510003065. [DOI] [PubMed] [Google Scholar]; Elnenaei M O, Chandra R, Mangion TandMoniz C. Genomic and metabolomic patterns segregate with responses to calcium and vitamin D supplementation, Br J Nutr 2011;105(1):71-9. [DOI] [PubMed]
- 25.Finkelstein J.L., Pressman E.K., Cooper E.M. Vitamin D status affects serum metabolomic profiles in pregnant adolescents. Reprod Sci. 2015;22(6):685–695. doi: 10.1177/1933719114556477. [DOI] [PMC free article] [PubMed] [Google Scholar]; Finkelstein J L, Pressman E K, Cooper E M, et al. Vitamin D Status Affects Serum Metabolomic Profiles in Pregnant Adolescents, Reprod Sci 2015;22(6):685-95. [DOI] [PMC free article] [PubMed]
- 26.Luque-Cordoba D., Luque de Castro M.D. Metabolomics: a potential way to know the role of vitamin D on multiple sclerosis. J Pharm Biomed Anal. 2017:13622–13631. doi: 10.1016/j.jpba.2016.12.023. [DOI] [PubMed] [Google Scholar]; Luque-Cordoba DandLuque de Castro M D. Metabolomics: A potential way to know the role of vitamin D on multiple sclerosis, J Pharm Biomed Anal 2017;13622-31. [DOI] [PubMed]
- 27.O'Sullivan A., Gibney M.J., Connor A.O. Biochemical and metabolomic phenotyping in the identification of a vitamin D responsive metabotype for markers of the metabolic syndrome. Mol Nutr Food Res. 2011;55(5):679–690. doi: 10.1002/mnfr.201000458. [DOI] [PubMed] [Google Scholar]; O'Sullivan A, Gibney M J, Connor A O, et al. Biochemical and metabolomic phenotyping in the identification of a vitamin D responsive metabotype for markers of the metabolic syndrome, Mol Nutr Food Res 2011;55(5):679-90. [DOI] [PubMed]
- 28.Chailurkit L.O., Kruavit AandRajatanavin R. Vitamin D status and bone health in healthy Thai elderly women. Nutrition. 2011;27(2):160–164. doi: 10.1016/j.nut.2009.12.001. [DOI] [PubMed] [Google Scholar]; Chailurkit L O, Kruavit AandRajatanavin R. Vitamin D status and bone health in healthy Thai elderly women, Nutrition 2011;27(2):160-4. [DOI] [PubMed]
- 29.Rajatanavin R., Chailurkit L., Saetung S., Thakkinstian A., Nimitphong H. The efficacy of calcium supplementation alone in elderly Thai women over a 2-year period: a randomized controlled trial. Osteoporos Int. 2013;24(11):2871–2877. doi: 10.1007/s00198-013-2387-5. [DOI] [PubMed] [Google Scholar]; Rajatanavin R, Chailurkit L, Saetung S, Thakkinstian AandNimitphong H. The efficacy of calcium supplementation alone in elderly Thai women over a 2-year period: a randomized controlled trial, Osteoporos Int 2013;24(11):2871-7. [DOI] [PubMed]
- 30.Piaseu N., Komindr S., Chailurkit L.O. Differences in bone mineral density and lifestyle factors of postmenopausal women living in Bangkok and other provinces. J Med Assoc Thai. 2001;84(6):772–781. [PubMed] [Google Scholar]; Piaseu N, Komindr S, Chailurkit L O, et al. Differences in bone mineral density and lifestyle factors of postmenopausal women living in Bangkok and other provinces, J Med Assoc Thai 2001;84(6):772-81. [PubMed]
- 31.Institute of Medicine (US). Committee to review dietary reference intakes for vitamin D and calcium. Dietary reference intakes for calcium and vitamin D (edited by Ross AC, Taylor C, Yaktine AL, Del Valle HB. National Academics Press, Washinton, DC., 2011. [PubMed]