Highlights
-
•
Shrimp wastewater is a rich source of P- and N-compounds suitable for cyanobacterial growth.
-
•
Phosphate in shrimp wastewater can be efficiently removed by Synechocystis ΔSphU.
-
•
ΔSphU accumulates high PHB with commercial value when shrimp wastewater contains low nitrate level.
-
•
Shrimp wastewater can be used for biodegradable plastic production by cyanobacterial cell.
Keywords: Shrimp wastewater, Batch photobioreactor, Synechocystis sp. PCC 6803 strain ΔSphU, Poly-β-hydroxybutryate, Nutrient remediation
Abstract
The wastewater discharge from the intensive shrimp aquaculture contains high concentration of nutrients, which can lead to eutrophication. This study aimed to reuse the shrimp wastewater for low cost cyanobacterial cultivation to produce biodegradable plastic poly-β-hydroxybutyrate (PHB). The Synechocystis sp. PCC 6803 (ΔSphU) lacking phosphate regulator (SphU) could utilize nutrients in shrimp wastewater for promoting biomass yield of 500 mg L−1 after 14 days. The ΔSphU showed the highest phosphate uptake rate of 20.16 mggDw−1d−1 at the first day of photobioreactor running. In addition, the nutrient removal efficiencies were 96.99% for phosphate, 80.10% for nitrate, 67.90% for nitrite and 98.07% for ammonium. The reduction of nitrate in shrimp wastewater due to nitrogen assimilation could induce PHB accumulation in ΔSphU. The highest PHB content was 32.48% (w/w) DW, with the maximum PHB productivity of 12.73 mg L−1d−1. The produced PHB of ΔSphU had material properties similar to those of the commercial PHB.
1. Introduction
Shrimp farming can be operated in either a marine or freshwater environment for human consumption. The world aquaculture production for crustaceans has increased over 60% from year 2006 to 2015 [1]. China, Norway, Vietnam, USA and Thailand are the top five countries for both aquaculture production and exportation of fishery commodities from 2013 to 2015 [1]. However, the continuous development of intensive shrimp aquaculture also brought some problems, especially the wastewater disposal. Every single tonne of typical shrimp aquaculture production can be translated into 5345–7157 m3 of effluent discharge [2], which usually contains concentrated organic matter and nutrients (mostly phosphorus and nitrogen) as a result of metabolic waste from the food supplied to shrimp aquaculture system [3]. Therefore, wastewater generated in shrimp aquaculture needs treatment prior to its reuse or release in environment to avoid the eutrophication. To date several chemical and biological treatments have been successfully used to obtain a quality of shrimp aquaculture effluent such as the chemical precipitation using ferrous chloride to remove phosphorus and the common biological nitrification/denitrification processes to remove nitrogen. Although these processes are effective, they are less environmentally friendly due to the generation of chemical waste or sludge as by product during the processes [4].
Cyanobacteria (blue green algae) are O2-evolving photosynthesizing prokaryotes that can be cultivated using aquaculture wastewater as sources of nutrient for biomass production [29]. The cultivation of cyanobacteria in aquaculture wastewater has double advantages, i.e. to reduce the cost of wastewater treatment as well as to produce valuable biomass which can be subsequently utilized for biofuels and feed application. However, there are scarce studies reporting the utilization of wastewater for biodegradable plastic production, most previous studies mainly focused on cyanobacterial cultivation for biomass and nutrient removal [29]. Poly-β-hydroxybutyrate (PHB) is a biopolymer plastic consisting of β-hydroxybutyric acid monomers linked through ester bonds, which can be biosynthesized by several cyanobacterial strains [5]. PHB has the important properties of thermoplasticity, biodegradability, non-toxic and biocompatibility making it suitable for application in agricultural and biomedical fields [6]. In this present study, the cyanobacterium Synechocystis sp. PCC 6803 mutant strain ΔSphU was used to investigate the efficiency of PHB production in a photobioreactor cultivation using shrimp wastewater as growth medium. The Synechocystis sp. PCC 6803 is a unicellular cyanobacterium, which is one of the most extensively studied species to be developed as a phototrophic cell factory for biofuels, biomaterials and commodity chemicals including PHB production (granules up to 27 mg L−1) [7,8]. The mutant strain ΔSphU is Synechocystis sp. PCC 6803 with the deletion of its phosphate regulator gene (SphU). This strain efficiently removed phosphate in wastewater down to less than 0.5 mg L−1 [9]. Therefore, the purpose of this study was to reuse the discharge wastewater from shrimp pond for low cost cultivation of cyanobacteria and production of biodegradable PHB. The obtained PHB was also analyzed for its polymer structure and material properties.
2. Materials and methods
2.1. Wastewater collection and analysis
The shrimp wastewater was collected from discharge water of shrimp pond, Samut Songkhram, Thailand in 40 L containers and brought to laboratory immediately after collection. The collected discharge water was filtered through a membrane filter (0.45 μm) to remove organic load and stored at 4 °C for further utilization. The physiochemical water qualities; color, pH, conductivity, temperature, salinity, total suspended solid (TSS), total dissolved solid (TDS), nitrate (NO3−), nitrite (NO2−), ammonium (NH4+), phosphate (PO43−) and sulfate (SO42−) were analyzed in triplicate according to standard method APHA [10]. The heavy metals detection was performed as described previously by Ansari et al. [11].
2.2. Cyanobacterial stock and growth condition
The cyanobacterium Synechocystis sp. PCC 6803 strain ΔSphU was used for the cultivation in shrimp wastewater. The cells were grown in BG11 medium containing 30 μgmL−1 chloramphenicol and incubated aerobically under continuous illumination of 40 μEm-2s−1 on a rotatory shaker at 160 rpm and 30 °C until reaching log phase. The cells were harvested by centrifugation (2790 × g, 10 min) and washed twice with BG11 without antibiotic before inoculation into a photobioreactor with initial cell concentration of 250 mg L−1.
2.3. Photobioreactor system operation
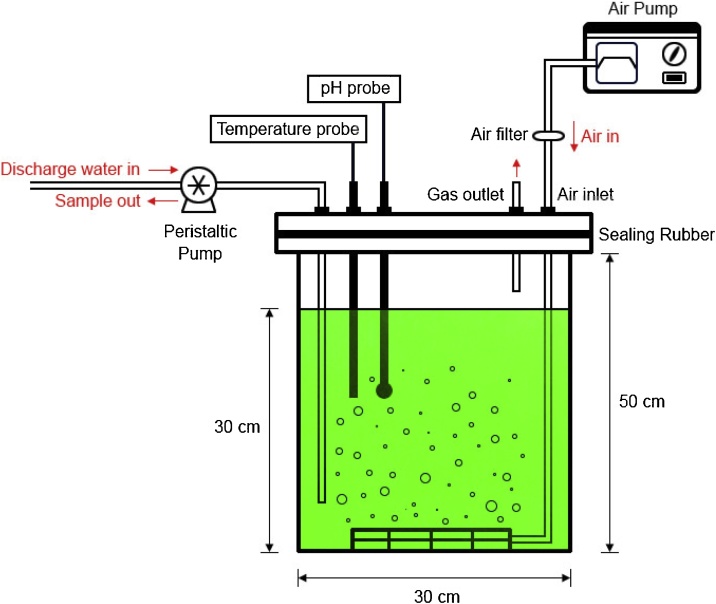
The flat-plate photobioreactor made from acrylic plastic had internal dimensions of 30 cm (length) x10 cm (width) x50 cm (height) as shown in Fig. 1. The reactor was filled to a depth of 30 cm for effective volume. A steel plate was equipped with cool white fluorescent lamps placed at a distance of 2.5 cm from the 30 cm wall of the reactor. The light intensity was 40 μEm-2s−1 on the wall of photobioreactor. The 10 L volume of shrimp wastewater was supplied as a culture medium for bath photobioreactor cultivation. The reactor was continuously purged with atmospheric air (filtered through 0.22 μm cellulose acetate membrane) supplied from the bottom of the reactor. The flow rate of supplied air was 1.0 L min−1. Temperature was maintained under ambient condition in the range of 27–30 °C and the pH was in the range of 7.0–9.0. The experiment was run for 14 days after cell inoculation.
Fig. 1.
Schematic diagram of the flat-plate photobioreactor for batch microalgal cultivation using discharge wastewater from shrimp pond.
2.4. Fluorescence microscopy
PHB granules in Synechocystis sp. PCC 6803 strain ΔSphU were visualized by staining with the fluorescent dye Nile red. The 20 μL of cell culture was mixed in 100 μL of Nile red solution containing NaCl (0.9% w/v) and Nile red dye (3 ng mL−1) and incubated overnight under darkness before observation under fluorescence microscope equipped with a digital camera (Olympus BX60, Japan), using a filter cube with 559 excitation wavelengths.
2.5. Analytical methods
2.5.1. Biomass and specific growth rate determination
Cyanobacterial growth was monitored at time intervals by measuring optical density at 730 nm (OD730) using a spectrophotometer. Total amount of chlorophyll a was measured spectrophotometrically at 665 nm in 90% (v/v) methanol extracts. Biomass dry weight (DW) was performed by separating cells from culture using 0.45 μm glass microfiber filter and dried in a 60 °C oven until a constant weight was obtained. The specific growth rate (μ), biomass yield and biomass productivity were calculated as follows:
| (1) |
Where N1 and N2 are the OD730 at the beginning (t1) and at the time of t2, respectively.
| Biomass yield (mg L−1) = X2 - X1 | (2) |
| (3) |
Where X1 and X2 are the biomass dry weight (DW) at the beginning (t1) and at the time of t2, respectively.
2.5.2. Nutrient removal efficiency analysis
Nutrient uptake rate which refers to the nutrient taken up by unit mass of cells and nutrient removal efficiency were calculated using the following equations:
| (4) |
| (5) |
Where C0 and Ct are the initial and final concentrations (mg L−1) of nutrient and V is the volume of culture (L).
2.5.3. Quantitative analysis of PHB production by HPLC
The quantitative PHB was analyzed by high performance liquid chromatography (HPLC) essentially as described by Khetkorn et al. [12]. Briefly, biomass dry weight was boiled in concentrated H2SO4 for 1 h to hydrolyze PHB polymer into crotonic acid, and then 25-fold diluted with water and filtered through a 0.45 μm polypropylene membrane filter to remove cell debris. The crotonic acid content was determined by HPLC using adipic acid as an internal HPLC standard. Commercial PHB (Sigma-Aldrich, USA) was analyzed in parallel, where 84.6 ± 4.0% (w/w) conversion of PHB to crotonic acid was obtained. The PHB productivity was calculated according to Eq. (6):
| (6) |
Where PHB content is in percentage per biomass dry weight (% w/w DW)
2.5.4. Photosynthesis efficiency and PHB synthase activity analysis
The photosynthesis efficiency was analyzed in terms of O2-evolving ability of cells (10 μg chl a mL−1) under saturating white light (500 μE m-2 s−1) with a Clark-type O2-electrode (Hansatech instruments, UK) at 25 ○C as described by [12]. The PHB synthase activity was determined as previously described by [13]. The reaction (1 mL) contained 0.2 mg of crude protein, 1.5 mM of β-hydroxybutyryl-CoA substrate and 0.5 mM of 2-nitrobenzoic acid in 25 mM Tris-HCl buffer pH 7.5 containing 5% (v/v) glycerol. The reaction was initiated by adding β-hydroxybutyryl-CoA and incubated at 30 °C for 10 min. The thiobenzoate anion resulted from the reaction was measured spectrophotometrically at 412 nm. Protein content was estimated using the Lowry method.
2.5.5. PHB extraction, polymer structure and material properties analysis
Total biomass after 14 days of cultivation was harvested and centrifuged (2790 × g, 10 min) before freeze dried using lyophilizer (OPERON, Korea). The dried cells were soaked in methanol to remove pigments before extracting PHB using hot chloroform and precipitated with diethyl ether according to Yellore and Desai [14]. The chemical structure of the polymer was analyzed using 1H and 13C nuclear magnetic resonance (NMR). The PHB 2 mg mL−1 in deuterochloroform (CDCl3) was analyzed at 25 °C using a FT-NMR spectrometer 500 MHz (Bruker Avance III HD, Germany). For thermal properties, 10 mg of PHB was analyzed using a differential scanning calorimetry (Netzsch DSC-204 F1 Phoenix, Germany) and thermogravimetric analyzer (Netzsch 209 F3 Tarsus, Germany). For mechanical analysis, the polymer film was cut into rectangles (according to the standard ASTM) and analyzed at 25 °C using a material testing machine (Hounsfield H10KM, UK). The commercial PHB (Sigma-Aldrich, USA) was used to compare their properties with extracted polymer.
2.6. Statistical analysis
All experiments were performed with three biological replicates. Data were analyzed by using one-way analysis of variance (ANOVA). The differences in means values were identified by Duncan's multiple range tests to determine whether significant difference (P < 0.05) existed among different treatments. All the statistical analyses were carried out using SPSS software version 15.0.
3. Results and discussion
3.1. Shrimp wastewater characterization
Shrimp wastewater collected from discharge water of shrimp pond was characterized for the presence of nutrients required for cyanobacterial cultivation. The physiological water qualities are shown in Table 1. The shrimp wastewater initially contained 35–60 mg L−1 of suspended solids; however, after filtration through a membrane filter it contained colored organic compounds lower than 30 mg L−1 with no negative effect on the growth of cyanobacteria due to the decrease of light penetration reaching the cells inside the culture. The nitrogen and phosphorus compounds are the most important nutrients for cyanobacterial growth and their metabolism [15]. This shrimp wastewater has high content of nitrogen and phosphorus compounds present in various inorganic forms which can be taken up by cells such as nitrate (55.28 mg L−1), nitrite (3.71 mg L−1), ammonium (2.77 mg L−1) and phosphate (8.22 mgL−1). The pH of the collected wastewater was 8.51. Moreover, the essential micronutrients such as calcium, magnesium, iron, sulfur, zinc, copper required for various physiological activities of cyanobacteria were available in this wastewater (Table 1).
Table 1.
Physiological water qualities of shrimp wastewater used for ΔSphU cultivation in a photobioreactor.
| Parameters | Initial cultivation | Unit |
|---|---|---|
| Conductivity | 12.34 ± 0.32 | mScm−1 |
| Salinity | 6.09 ± 0.09 | ppt |
| pH | 8.51 ± 0.01 | – |
| Temperature | 28.0 ± 0.05 | ºC |
| Total suspended solids; TSS | 19.37 ± 0.07 | mgL−1 |
| Total dissolved solids; TDS | 8.13 ± 0.42 | ppt |
| Calcium | 144 ± 0.45 | mgL−1 |
| Magnesium | 259 ± 0.33 | mgL−1 |
| Iron | 2.06 ± 0.22 | mgL−1 |
| Manganese | 0.15 ± 0.02 | mgL−1 |
| Copper | 0.10 ± 0.01 | mgL−1 |
| Zinc | 0.03 ± 0.05 | mgL−1 |
| Sulfate; SO42− | 318 ± 5.29 | mgL−1 |
| phosphate; PO43− | 8.22 ± 0.13 | mgL−1 |
| Nitrate; NO3− | 55.28 ± 0.49 | mgL−1 |
| Nitrite; NO2− | 3.71 ± 0.08 | mgL−1 |
| Ammonium; NH4+ | 2.77 ± 0.06 | mgL−1 |
| Total phosphorus | 11.46 ± 0.12 | mgL−1 |
3.2. Synechocystis strain ΔSphU cultivated in shrimp wastewater
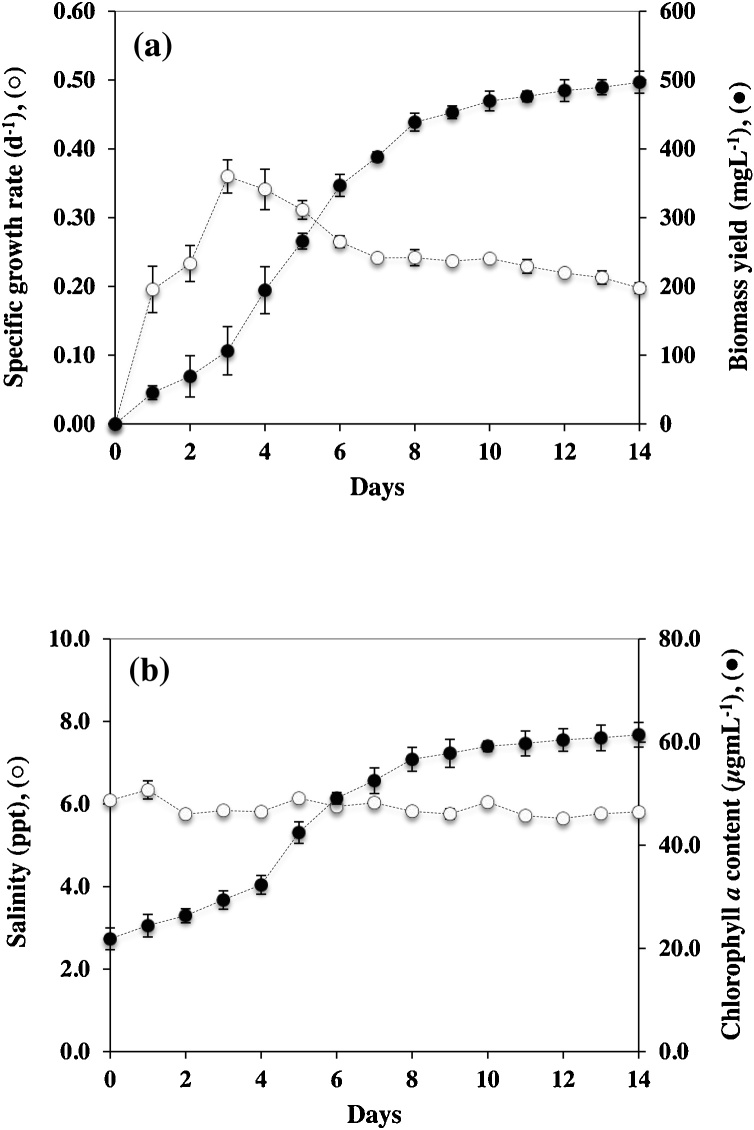
To utilize shrimp wastewater for cyanobacterial growth, Synechocystis sp. PCC 6803 strain ΔSphU was cultured with shrimp wastewater as growth medium in a batch photobioreactor (Fig. 1). The changes in physiological shrimp wastewater characteristics such as pH, temperature and salinity were determined every day during cultivation as shown in Fig. 2. The biomass yield of ΔSphU strain was continuously increased during the first week of cultivation in shrimp wastewater and then the culture entered the stationary phase. After 14 days of cultivation, ΔSphU strain showed the maximum biomass yield of approximately 500 mg L−1 (Fig. 2a), and the highest biomass productivity was 57.80 mg L−1d−1 after 7 days of cultivation (Table 2). Previously, the cyanobacterium Spirulina platensis was shown to grow well in aquaculture wastewater with a specific growth rate of 0.623 day−1 at day 3 of cultivation [16], indicating that shrimp wastewater can be reused for cyanobacterial cultivation. Moreover, the changes of pH in culture was an important effector of the cyanobacterial CO2 concentrating mechanism since the amount of dissolved inorganic carbon depends on pH, temperature and salinity [17]. The results of this study showed that temperature and salinity were not significantly changed irrespective of cultivation times, but pH of the culture was apparently increased from 8.5 to 9.9 within 3 days of cultivation (data not shown). The pH is a main factor influencing the abundance of inorganic carbon. Under alkaline condition, the HCO3− is a major inorganic carbon leading to the reduced level of CO2 and causing the growth inhibition of cyanobacteria [15,17]. Interestingly, the ΔSphU cells showed no negative effect of high pH on their growth, the highest specific growth rate was 0.36 day−1 at day 3 (Fig. 2a). Low inorganic carbonate concentration has been reported to activate CO2 concentrating mechanism of Synechocystis sp. PCC 6803 by induction of a highly efficient bicarbonate transporter for taking up HCO3- into Synechocystis cell [8,18]. This might explain why Synechocystis sp. PCC 6803 strain ΔSphU has the capability to grow under low CO2 concentrations. In addition, the increase of chlorophyll a content in Fig. 2b revealed that a high salinity (5.75–6.34 ppt) in shrimp wastewater had no influence on the photosynthesis of ΔSphU strain.
Fig. 2.
Specific growth rate and biomass yield (a) and effect of salinity variation on chlorophyll a content (b) of Synechocystis sp. PCC 6803 strain ΔSphU in batch photobioreactor using shrimp wastewater as growth medium for 14 days. The error bars represent standard deviations of means (means ± S.D., n = 3).
Table 2.
The efficiency of nutrient uptake, nutrient removal, biomass and PHB productivities of ΔSphU grown in shrimp wastewater at various times. Means ± S.D. (n = 3).
| Time | Nutrient uptake rate (mg gDW−1 d−1) |
Nutrient removal (%) |
Biomass productivity | PHB productivity | ||
|---|---|---|---|---|---|---|
| PO43− | NO3− | PO43− | NO3− | (mgL−1d−1) | (mgL−1d−1) | |
| Day 1 | 20.16 ± 0.6 | 64.92 ± 5.8 | 71.17 ± 3.5 | 35.55 ± 3.1 | 45.65 ± 9.9 | 0.13 ± 0.03 |
| Day 7 | 1.79 ± 0.01 | 9.74 ± 0.4 | 95.91 ± 0.5 | 79.11 ± 1.0 | 57.80 ± 1.0 | 5.16 ± 0.6 |
| Day 11 | 1.01 ± 0.02 | 5.53 ± 0.2 | 96.76 ± 0.3 | 79.71 ± 4.5 | 43.23 ± 0.7 | 12.73 ± 1.2 |
| Day 14 | 0.77 ± 0.03 | 4.23 ± 0.09 | 96.99 ± 0.5 | 80.10 ± 1.8 | 35.49 ± 1.1 | 11.49 ± 0.7 |
3.3. The efficiency of nutrient removal by ΔSphU strain under batch photobioreactor
With regard to water qualities, the discharge of shrimp pond wastewater containing high level of nutrients such as nitrogen and phosphorus is one of the main causes of eutrophication. Fig. 2a shows that Synechocystis strain ΔSphU utilized the nutrients present in the shrimp wastewater for promoting its growth which has an advantage in reducing the cost of wastewater treatment. However, the nutrient removal efficiencies depend on the cyanobacterial strain applied and the initial concentration of nutrients in the wastewater [19]. The nutrient removal efficiency of ΔSphU was determined as shown in Table 2. After 14 days cultivation, the removal efficiencies were found to be 96.99% for phosphate, 80.10% for nitrate, 67.90% for nitrite and 98.07% for ammonium. It should be noted that ΔSphU has a high efficiency to remove phosphate and ammonium from shrimp wastewater. Similarly, Chlorella sorokiniana and Scenedesmus obliguus also had high removal efficiency (over 95%) of both phosphate and ammonium present in aquaculture wastewater [11]. Surprisingly, ΔSphU strain effectively decreased phosphate concentration down to 2.37 mg L−1 with as high as 71.16% of removal efficiency occurring within the first day of batch photobioreactor running where the maximum phosphate uptake rate was 20.16 mg g−1d−1 (Table 2). Shrimp wastewater is plentiful with various forms of inorganic phosphorus arising from shrimp feed medium. Total phosphorus concentration in shrimp effluent was initially analyzed as 11.46 mg L−1 (Table 1). After cultivation, the total phosphorus in the photobioreator was reduced to 0.27 mg L−1 (data not shown). According to the regulations for pollution control in many countries including Thailand, the total phosphorus in wastewater from aquaculture ponds before its release into the environment must not exceed 0.4 mg L-1. The ΔSphU is therefore a promising cyanobacterium for use as a phosphorus remover. Interestingly, the ΔSphU could take up phosphate with the rate higher than those from a cyanobacterium Anabaena sp. PCC 7120 and a green microalga Chlorella sp. GD under aquaculture wastewater utilization [20,21]. The ΔSphU is a Synechocystis mutant strain lacking phosphate regulator gene (sphU). This strain was capable of accumulating phosphate inside the cell at higher concentration than that of the wild type [9], indicating that the deletion of phosphate regulator gene enabled the ΔSphU strain to remove phosphate from shrimp wastewater at high efficiency.
3.4. PHB accumulation under low concentration of nitrate in shrimp wastewater
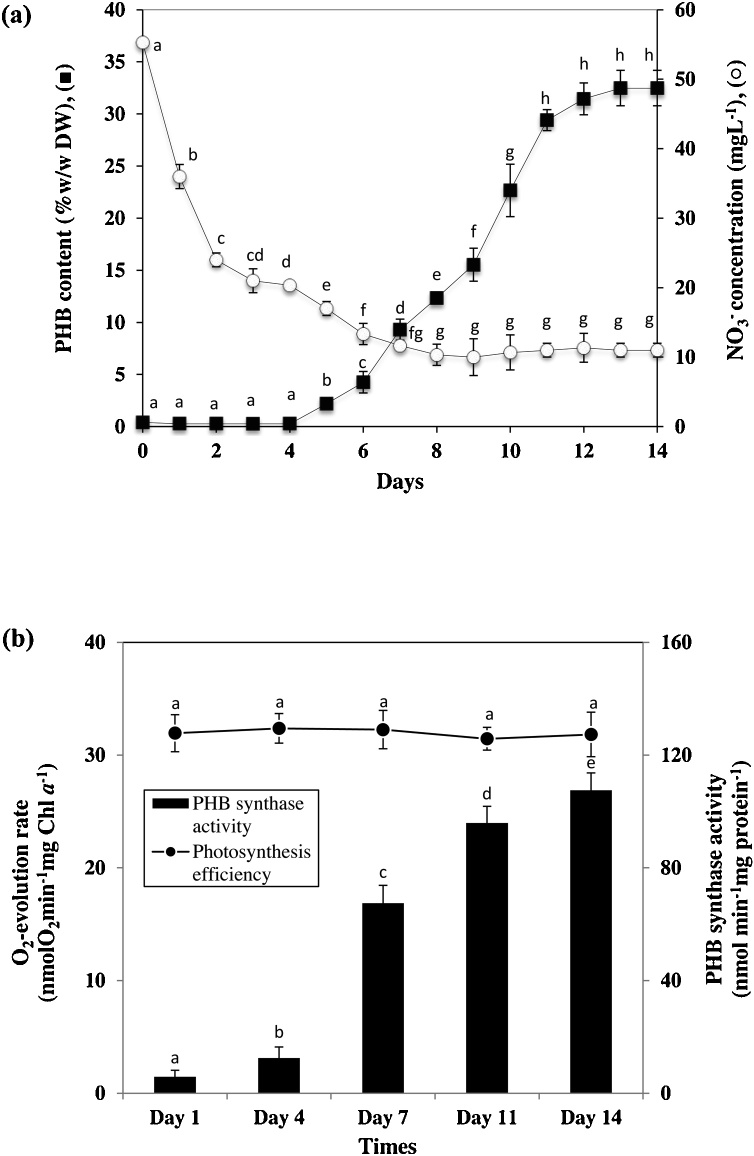
The PHB production has been widely investigated in Synechocystis strain, but the previous studies focused on optimum conditions for enhanced PHB accumulation in cells grown in algal medium [22,23]. Therefore, this study firstly reported the utilization of shrimp wastewater for inducing PHB production in Synechocystis cells. In this batch photobioreactor, Synechocystis strain ΔSphU was harvested for analysis of PHB accumulation as shown in Fig. 3a. Low levels of PHB were detected in cells during the growth promoting phase (0–4 days of cultivation), after which the levels of PHB rapidly increased from 0.29 to 29.40% (w/w) before reaching a constant level. The highest PHB content was 32.48% (w/w), with the maximum PHB productivity of 12.73 mg L−1d−1 after 11 days cultivation (Table 2). It should be noted that Synechocystis strain ΔSphU had lower PHB content when cultivated in BG11 medium as compared to that in shrimp wastewater (Table 4). The limitation of nitrogen was reported to have high impact on PHB induction in several cyanobacteria when compared to other nutrient factors [5]. In addition, the low level of nitrate in BG11 medium under photoautotrophic condition could induce the accumulation of PHB in Synechocystis sp. PCC 6803 wild type [6,24]. The Fig. 3a shows that PHB content was significantly increased when the concentration of nitrate in shrimp wastewater was lower than 20 mg L−1 caused nitrate assimilation of cyanobacteria. However, when cells were cultured longer than 7 days there were no significant changes in nitrate levels with the average level of 10.76 mg L−1. The cell biomass was also constant during the second week of cultivation (Fig. 2a), suggesting that the limited availability of nitrate in shrimp wastewater is one of important factors affecting the growth of cyanobacterial cells. In contrast, the O2-evolution of cells under saturated light exposure was not significantly different under nitrogen limited condition (Fig. 3b), indicating that the photosynthesis was still active and cells continued to fix CO2. Therefore, the PHB with high content in ΔSphU cells might serve as a storage compound under stress condition which correlated well with higher PHB synthase activity (Fig. 3b) and increased PHB granules inside the cells (Supplementary Fig. S1).
Fig. 3.
The effect of nitrate limitation on PHB content (a) and the PHB synthase activity relative with photosynthesis efficiency (b) of ΔSphU in a photobioreactor (a) when cells were cultured in shrimp wastewater for various times (means ± S.D., n = 3). The different letters within the same parameter represent significant difference according to Duncan’s test (p < 0.05).
Table 4.
Comparison of PHB content of cyanobacterial strains grown on synthetic medium and wastewaters under photobioreactor.
| Cyanobacterial strains | Growth media | Conditions of PHB production | PHB contents (% w/w DW) | References |
|---|---|---|---|---|
| Synechocystis sp. PCC 6714 | BG11 | Jacketed glass photobioreactor (1 L), under nitrogen and phosphorus limitation for 15 days | 16.4 ± 2.0 | [25] |
| Synechocystis sp. CCALA192 | BG11 | Tubular photobioreactor (200 L), under complete BG11 medium cultivation for 20 days | 12.7 | [26] |
| Synechocystis sp. PCC 6803 strain ∆SphU | BG11 | Flat-plate photobioreactor (10 L), under complete BG11 medium cultivation for 13 days (control condition) | 6.42 ± 1.8 | This study |
| Synechocystis sp. PCC 6803 strain ∆SphU | Shrimp wastewater | Flat-plate photobioreactor (10 L), under shrimp wastewater cultivation for 14 days | 32.48 ± 1.7 | This study |
| Synechocystis salina | Low-solid digestate from urban wastewater | Tubular photobioreacter (200 L), under digestate supernatant (1/3) cultivation for 40 days | 5.5 ± 0.3 | [27] |
3.5. The material properties of PHB extracted from ΔSphU grown in shrimp wastewater
Dried cells as shown in Supplementary Fig. S2 were collected to extract intracellular polymer before analyzing chemical structure. The 1H and 13C NMR spectra of polymer extracted from ΔSphU cells matched the respective spectra of the commercial PHB (Supplementary Fig. S3, S4). The thermal and mechanical properties of extracted PHB were comparable to those of the commercial PHB (Table 3). Unfortunately, the extracted PHB from ΔSphU grown in shrimp wastewater had a weaker tensile strength than the commercial PHB. In contrast, the PHB from other cyanobacteria such as Nostoc muscorum and Calothrix scytonemicola had a greater tensile strength than the commercial PHB [28].
Table 3.
Material properties of PHB produced by ΔSphU compared to commercial PHB.
| Sources of PHB | Thermal properties |
Mechanical properties |
||||
|---|---|---|---|---|---|---|
| Tm (°C) | Tg (°C) | Td (°C) | Elongation at break (%) | Tensile strength (MPa) | Young’s modulus (MPa) | |
| Commercial PHB | 172.5 | 3.5 | 276.1 | 5.8 | 26.9 | 820 |
| ΔSphUa | 179.4 | 3.2 | 279.8 | 5.9 | 15.4 | 679 |
ΔSphU cells were cultured in shrimp wastewater for 14 days before cells were collected to extract PHB. Tm, melting temperature: Tg, glass transition temperature: Td, decomposition temperature.
4. Conclusion
In conclusion, the cells of Synechocystis strain ΔSphU could utilize the nutrients present in the discharge of shrimp pond for supporting their growth, which has double advantages in reducing the cost of wastewater treatment and obtaining algal feedstock. The maximum biomass yield was approximately 500 mg L−1. The lack of phosphate regulator (SphU) in Synechocystis sp. PCC 6803 enabled the cells to efficiently remove phosphate present in the shrimp wastewater with the rate of 20.16 mg g−1d−1 at the first cultivating day. In addition, the limitation of nitrate in shrimp wastewater due to nitrogen assimilation could induce PHB accumulation 32.48% (w/w) DW in ΔSphU cells. The obtained PHB polymer showed material properties comparable to those of the commercial PHB. Until now, a few studies focusing on bath photoautotrophic cultivation of PHB in large-scale, optimizing the nutrients mainly has been done in flask scale. Table 4 represents the comparison of PHB content of cyanobacteria grown under bath photobioreacter from previous study. Batch synthetic medium cultivation of Synechocystis sp. PCC 6714 under nitrogen and phosphorus limitation, showed 16.4% (w/w) DW [25]. While, the maximum PHB content of 5.5% (w/w) DW was obtained for low-solid digestate from urban wastewater cultivation of Synechocystis sp. CCALA192 in a 200 L tubular photobioreacter [27]. Therefore, photobioreactor systems are more flexible depending on the cultivation process and the desired species. However, our study has firstly revealed that shrimp wastewater can be used for biodegradable plastic production by Synechocystis strain ΔSphU under large scale photobioreacter.
Declaration of interest
The authors declare that there are no conflicts of interest.
Acknowledgements
This work was supported by the Royal Golden Jubilee Advanced Programme 2016 [RAP59K0013] and RMUTT research foundation scholarship 2016. W. Khetkorn thanks Dr. Surachet Burut-Archanai at Center of Excellence for Marine Biotechnology, Chulalongkorn University for providing ΔSphU strain.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2019.e00345.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.FAO . Food and Agriculture Organization of the United Nations; Room. Italy: 2017. Fisheries and Aquaculture Statics. FAO Fishery and Aquaculture Statistics Yearbook. [Google Scholar]
- 2.Anh P.T., Kroeze C., Bush S.R., Mol A.P.J. Water pollution by intensive brackish shrimp farming in south-east Vietnam: causes and options for control. Agric. Water Manag. 2010;97(6):872–882. [Google Scholar]
- 3.Crab R., Avnimelech Y., Defoirdt T., Bossier P., Verstraete W. Nitrogen removal techniques in aquaculture for a sustainable production. Aquaculture. 2007;270:1–14. [Google Scholar]
- 4.Mook W.T., Chakrabarti M.H., Aroua M.K., Khan G.M.A., Ali B.S., Islam M.S., Abu Hassan M.A. Removal of total ammonia nitrogen (TAN), nitrate and total organic carbon (TOC) from aquaculture wastewater using electrochemical technology: a review. Desalination. 2012;285:1–13. [Google Scholar]
- 5.Balaji S., Gopi K., Muthuvelan B. A review on production of poly β hydroxybutyrates from cyanobacteria for the production of bioplastics. Algal Res. 2013;2:278–285. [Google Scholar]
- 6.Carpine R., Raganati F., Olivieri G., Hellingwerf K.J., Pollio A., Salatino P., Marzocchella A. Poly-β-hydroxybutyrate (PHB) production by Synechocystis PCC 6803 from CO2: model development. Algal Res. 2018;29:49–60. [Google Scholar]
- 7.Wu G.F., Wu Q.Y., Shen Z.Y. Accumulation of poly-β-hydroxybutyrate in cyanobacterium Synechocystis sp. PCC6803. Bioresour. Technol. 2001;76(2):85–90. doi: 10.1016/s0960-8524(00)00099-7. [DOI] [PubMed] [Google Scholar]
- 8.Yu Y., You L., Liu D., Hollinshead W., Tang Y.J., Zhang F. Development of Synechocystis sp. PCC 6803 as a phototrophic cell factory. Mar. Drugs. 2013;11:2894–2916. doi: 10.3390/md11082894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burut-Archanai S., Eaton-Rye J.J., Incharoensakdi A., Powtongsook S. Phosphorus removal in a closed recirculating aquaculture system using the cyanobacterium Synechocystis sp. PCC 6803 strain lacking the SphU regulator of the Pho regulon. Biochem. Eng. J. 2013;74:69–75. [Google Scholar]
- 10.APHA . 22 nd. American Public Health Association; Washington DC: 2012. Standard Methods for the Examination of Water and Wastewater. [Google Scholar]
- 11.Ansari F.A., Singh P., Guldhe A., Bux F. Microalgal cultivation using aquaculture wastewater: integrated biomass generation and nutrient remediation. Algal Res. 2017;21:169–177. [Google Scholar]
- 12.Khetkorn W., Incharoensakdi A., Lindblad P., Jantaro S. Enhancement of poly-3-hydroxybutyrate production in Synechocystis sp. PCC 6803 by overexpression of its native biosynthetic genes. Bioresour. Technol. 2016;214:761–768. doi: 10.1016/j.biortech.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Monshupanee T., Nimdach P., Incharoensakdi A. Two-stage (photoautotrophy and heterotrophy) cultivation enables efficient production of bioplastic poly-3-hydroxybutyrate in auto-sedimenting cyanobacterium. Sci. Rep. 2016;6:37121. doi: 10.1038/srep37121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yellore V., Desai A. Production of poly-3-hydroxybutyrate from lactose and whey by Methylobacterium sp. ZP24. Lett. Appl. Microbiol. 1998;26:391–394. doi: 10.1046/j.1472-765x.1998.00362.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhu J., Rong J., Zong B. Factors in mass cultivation of microalgae for biodiesel. Chinese J. Catal. 2013;34:80–100. [Google Scholar]
- 16.Wuang S.C., Khin M.C., Chua P.Q.D., Luo Y.D. Use of Spirulina biomass produced from treatment of aquaculture wastewater as agricultural fertilizers. Algal Res. 2016;15:59–64. [Google Scholar]
- 17.Markou G., Vandamme D., Muylaert K. Microalgal and cyanobacterial cultivation: the supply of nutrients. Water Res. 2014;65:186–202. doi: 10.1016/j.watres.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 18.Hasunuma T., Kikuyama F., Matsuda M., Aikawa S., Izumi Y., Kondo A. Dynamic metabolic profiling of cyanobacterial glycogen biosynthesis under conditions of nitrate depletion. J. Exp. Bot. 2013;64:2943–2954. doi: 10.1093/jxb/ert134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guldhe A., Ansari F.A., Singh P., Bux F. Heterotrophic cultivation of microalgae using aquaculture wastewater: a biorefinery concept for biomass production and nutrient remediation. Ecol. Eng. 2017;99:47–53. [Google Scholar]
- 20.Burut-Archanai S., Powtongsook S. Identification of negative regulator for phosphate-sensing system in Anabaena sp. PCC 7120: a target gene for developing phosphorus removal. Biochem. Eng. J. 2017;125:129–134. [Google Scholar]
- 21.Kuo C.M., Jian J.F., Lin T.H., Chang Y.B., Wan X.H., Lai J.T., Chang J.S., Lin C.S. Simultaneous microalgal biomass production and CO2 fixation by cultivating Chlorella sp. GD with aquaculture wastewater and boiler flue gas. Bioresour. Technol. 2016;221:241–250. doi: 10.1016/j.biortech.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Monshupanee T., Incharoensakdi A. Enhanced accumulation of glycogen, lipids and polyhydroxybutyrate under optimal nutrients and light intensities in the cyanobacterium Synechocystis sp. PCC 6803. J. Appl. Microbiol. 2014;116(4):830–838. doi: 10.1111/jam.12409. [DOI] [PubMed] [Google Scholar]
- 23.Panda B., Mallick N. Enhanced poly-beta-hydroxybutyrate accumulation in a unicellular cyanobacterium, Synechocystis sp. PCC 6803. Lett. Appl. Microbiol. 2007;44(2):194–198. doi: 10.1111/j.1472-765X.2006.02048.x. [DOI] [PubMed] [Google Scholar]
- 24.Khetkorn W., Suphan S., Pongswat S. Suitable cell age for enhanced poly-ß-hydroxybutyrate accumulation under photoautotrophic nutrient deprivation of Synechocystis sp. PCC 6803. Sci&Tech RMUTT J. 2016;6:29–36. [Google Scholar]
- 25.Kamravamanesh D., Pflugl S., Nischkauer W., Limbeck A., Lackner M., Herwig C. Photosynthetic poly-beta-hydroxybutyrate accumulation in unicellular cyano bacterium Synechocystis sp. PCC 6714. AMB Express. 2017;7:143. doi: 10.1186/s13568-017-0443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Troschl C., Meixner K., Fritz I., Leitner K., Romero A.P., Kovalcik A., Sedlacek P., Drosg B. Pilot-scale production of poly-β-hydroxybutyrate with the cyanobacterium Synechocytis sp. CCALA192 in a non-sterile tubular photobioreactor. Algal Res. 2018;34:116–125. [Google Scholar]
- 27.Meixner K., Fritz I., Daffert C., Markl K., Fuchs W., Drosg B. Processing recommendations for using low-solids digestate as nutrient solution for poly-ß-hydroxybutyrate production with Synechocystis salina. J. Biotechnol. 2016;240:61–67. doi: 10.1016/j.jbiotec.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 28.Bhati R., Samantaray S., Sharma L., Mallick N. Poly-beta-hydroxybutyrate accumulation in cyanobacteria under photoautotrophy. Biotechnol. J. 2010;5:1181–1185. doi: 10.1002/biot.201000252. [DOI] [PubMed] [Google Scholar]
- 29.Wang R., Peng B., Huang K. The research progress of CO2 sequestration by algal bio-fertilizer in China. J. CO2 Utilization. 11 2015:67–70. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.