Abstract
Background: Oxaliplatin (OXA) resistance is a main obstacle to the chemotherapy of colorectal cancer (CRC). Epithelial-mesenchymal transition (EMT), which is mainly regulated by TGF-β/Smad signaling pathway, has gradually been recognized as an important mechanism for tumor chemoresistance. Studies have shown that curcumin regulated EMT processes in many human cancers. However, whether curcumin could regulate OXA resistance in CRC through modulating TGF-β/Smad signaling-mediated EMT remains unclear.
Methods: In an attempt to investigate the effect of curcumin on OXA resistance in CRC, OXA-resistant cell line HCT116/OXA was established firstly. The effect of curcumin on cell proliferation was evaluated by MTT assay and Ki67 immunofluorescence staining, respectively. Cell apoptosis was evaluated by flow cytometry. In addition, transwell assay was used to detect the effect of curcumin on cell invasion and the activation of TGF-β/Smad signaling was examined by immunofluorescence and Western blot. Moreover, the therapeutic potential of curcumin was further examined in vivo using a CRC animal model.
Results: The OXA-resistant cell line HCT116/OXA was successfully established, and combination of OXA with curcumin reduced OXA resistance in vitro. Besides, the combination treatment inhibited the expressions of p-p65 and Bcl-2, but increased the level of active-caspase3. In addition, curcumin inhibited EMT via regulation of TGF-β/Smad2/3 signaling pathway. Moreover, in vivo study confirmed curcumin could reverse OXA resistance in CRC.
Conclusion: Our study indicated that curcumin could reserve OXA resistance in CRC through dampening TGF-β/Smads signaling in vitro and in vivo.
Keywords: colorectal cancer, oxaliplatin resistance, curcumin, epithelial-mesenchymal transition, TGF-β/Smad signaling pathway
Introduction
The 2018 American Cancer Society (ACS) report shows that there are more than 1 million new cases of colorectal cancer (CRC) annually, with a mortality rate close to 33%.1 The age-standardized incidence rate of CRC in China is 27/100,000, and that of women is 23/100,000; the incidence rate is increasing at an annual rate of 4.2%.1 The National Comprehensive Cancer Network (NCCN) guidelines recommend that patients with advanced colon cancer take a combination of surgery-based and chemotherapy-based treatments. The 2017 NCCN Guidelines established the FOLFOX and CapeOX regimens containing oxaliplatin (OXA) as first-line chemotherapy. However, studies have shown that the median disease-free survival and overall survival of advanced patients with standardized treatment regimens were only 10.3 months and 23.7 months, respectively.2 The main reason for the poor prognosis is the acquired chemoresistance of tumor cells, which occurs about half of patients with late-stage CRC receiving chemotherapy.3 In addition, neuropathy is a major limitation to the continued administration of OXA. Therefore, identifying key molecules responsible for tumor chemoresistance is of great importance for improving the efficacy of chemotherapy drugs for patients with CRC.
Curcumin is a naturally occurring polyphenolic substance extracted from the curcumaceae plant Curcuma longa and is often used as flavoring agent or pigment.4 Curcumin has been proven to have anti-inflammatory and anti-tumor effects as a traditional Chinese medicine extract.4 The anticancer effect of curcumin is mainly achieved by its negative regulation of various inflammatory cytokines, growth factors, transcription factors, protein kinases and other carcinogenic molecules.5–7 Recently, curcumin is reported to have the ability in reducing the chemoresistance of tumor cells.8,9 However, the detailed mechanisms underlying the effect of curcumin on chemoresistance remain poorly understood.
TGF-β belongs to transforming growth factor-β (TGF-β) superfamily which contains many growth factors, growth differentiation factor (Growth and differentiation factors, GDFs) and bone morphogenetic proteins (BMPs).10 Receptor dimers composed of TGF-βRI and TGF-βRII then lead to the phosphorylation of R-Smads proteins (Smad1, 2, 3, 5, 8), which enter into nucleus together with Smad4 and leading to the transcription of downstream genes.11 In recent years, TGF-β has been found to be an important inducer of Epithelial-mesenchymal transition (EMT).12 Recent studies have reported that curcumin downregulated TGF-β/Smads signaling in many human diseases.13–15 Therefore, we hypothesized that inhibition of TGF-β/Smad2/3 signaling pathway may be an important mechanism for curcumin to reverse OXA resistance in CRC.
Materials and methods
Cell culture and establishment of OXA-resistant cell lines
Human CRC cell lines HCT116 and SW480 were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in Roswell Park Memorial Institute1640 medium (RPMI1640, Invitrogen, Gaithersburg, MD, USA) with 10% fetal bovine serum (FBS, Invitrogen), 1% penicillin (Invitrogen) and streptomycin (Invitrogen) at 37°C, 5% CO2. For establishment of stable OXA-resistant cells, cells were treated with gradually increased concentration of OXA (Pharmacia, New Jersey, USA). The initial concentration of OXA was 0.5 μM. When the cell density reached about 80%, the cells were passaged. The stable OXA-resistant cell line HCT116/OXA was established in house (OXA maintained at a concentration of 5 μM). TGF-β was provided by R&D (Minneapolis, MN, USA).
3-(4,5)-dimethylthiahiazo (-z-y1)-3,5-di-phenytetrazoliumromide (MTT) assay
To explore the effect of OXA on the growth of HCT116 and HCT116/OXA cells, HCT116 and HCT116/OXA cells were cultured in RPMI1640 containing 0.5, 1, 2, 4, 8, 16 or 32 μM OXA for 48 hrs. To explore the effect of curcumin on HCT116/OXA cell growth, the cells were cultured with 1, 2, 4, 8, 16, 32 or 64 μM curcumin (MedChemExpress, Monmouth Junction, NJ, USA) for 48 hrs. The cytotoxicity of OXA, curcumin and combination of treatment were assessed by using cell growth determination kit (MTT based) (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer’s instruction. In brief, HCT116 and HCT116/OXA cells (in the logarithmic growth phase) were seeded into 96-well plate with 3.0×103 cells per well. MTT reagent was added into each well and incubated for 4 hrs. Then, 150 μl dimethyl sulfoxide (DMSO, Invitrogen, Carlsbad, CA, USA) was added into each well for 10 mins. The absorbance at 570 nm (A570) was measured by Thermo Fisher Multiskan FC (Thermo Fischer Scientific, Waltham, MA, USA).
Trypan blue staining
HCT116/OXA cells were centrifuged for 15 mins at 1000 g and resuspended in PBS. After that, cell suspension (90 μl) was mixed with 0.4% trypan blue staining buffer (10 μl) for 5 mins. Then, the number of cells was counted with a microscope. Living cells were colorless, while dead cells were stained blue.
Cell apoptosis assay
To detect cell apoptosis, Annexin V-FITC apoptosis detection kit (Beyotime, Jiangsu, China) was used according to the protocol. Cells were harvested and stained with annexin V-FITC (5 μL) and propidium iodide (PI, 5 μl). Then, the cells were incubated in dark at room temperature for 20 mins. Cell apoptosis rate was assessed by flow cytometry (Becton, Dickinson and Company, Franklin Lake, NJ, USA).
Immunofluorescence staining assay
Cells were seeded into 12-well plates with 2.0×106 cells/well. The cells were treated with 0.5% Triton-10 for 20 mins, following fixed with 4% paraformaldehyde for 15 mins. Then, the cells were blocked with 10% goat serum for 30 mins at room temperature, and incubated with primary antibodies, anti-Ki67 (1:500 dilution, Cell Signaling Technology, MA, USA) and anti-Smad4 (1:500 dilution, Cell Signaling Technology), at 4°C overnight, followed by incubation with secondary antibodies (goat anti-rabbit IgG antibody, 1:2000 dilution, Cell Signaling) at 37°C for 1 hr. Stained the nuclei with DAPI for 5 mins, finally, the cells were observed under a fluorescence microscope (Nikon, Tokyo, Japan).
Transwell assay
For cell invasion analysis, transwell assay was performed in this study. The upper chamber is pre-treated with 100 μl of Matrigel and exposed to UV light for 2 hrs. Cells were seeded into the upper chamber in serum-free medium, and the density was adjusted to about 1.0×106 cells per chamber. RPMI1640 medium with 10% FBS was added in the lower chamber. Then, these chambers were incubated for 48 hrs at 37°C, and stained the lower chamber with 0.1% crystal violet and counted the cell images under a microscope (LEICADMLB2, Frankfurt, Germany).
Western blotting assay
Total protein was extracted using RIPA plus buffer. The protein concentration was measured using BCA Protein Assay Kit (Takara, Dalian, China) as manufacturer’s instruction. 30 μg proteins were electrophoresed on 10% SDS-PAGE, and transferred to PVDF membrane (Sigma-Aldrich). The PVDF membranes were blocked with 5% non-fat milk at room temperature for 1 hr, and incubated with the primary antibodies at 4°C overnight. Following by incubation with goat anti-rabbit IgG antibody (1:10000 dilutions, Cell Signaling Technology) marked with horseradish peroxidase at room temperature for 2 hrs. Membranes were scanned by using an Odyssey Imaging System and analyzed with Odyssey v2.0 software (LICOR Biosciences, Lincoln, NE, USA). The primary antibodies used in this study are as follows: anti-phosphorylated p65 (1:1000 dilution, Cell Signaling Technology), anti-p65 (1:1000 dilution, Cell Signaling Technology), anti-Bcl-2 (1:1000 dilution, Cell Signaling Technology), anti-active-caspase3 (1:1000 dilution, Abcam Cambridge, MA, USA), anti-phosphorylated Smad2 (1:1000 dilution, Cell Signaling Technology), anti-phosphorylated Smad3 (1:1000 dilution, Cell Signaling Technology), anti-E-cadherin (1:1000 dilution, Cell Signaling Technology), anti-N-cadherin (1:1000 dilution, Cell Signaling Technology) and anti-β-actin (1:1000 dilution, Cell Signaling Technology).
Animal study
BALB/c nude mice (6–8 weeks old, 20±2 g weight) were obtained Vital River (Beijing, China). HCT116/OXA cells were transplanted subcutaneously into the right flank in each mouse according to the previous research.16 The mice were housed in pathogen-free conditions until the tumor volume reached ~200 mm3. National Institutes of Health guide for the care and use of laboratory animals was followed by us. Then, the mice were divided into four groups (six mice per group) and daily administrated by i.p. injection with vehicle [PEG400: ethanol: dextrose 5% in water (D5W)=4: 1: 5, to dissolve curcumin, vehicle group], daily; i.p. injection with 10 mg/kg OXA weekly (OXA alone group); i.p. injection with 60 mg/kg curcumin (curcumin alone group) daily; i.p. injection with 10 mg/kg OXA and 60 mg/kg curcumin (OXA + curcumin alone group) for 3 consecutive weeks. The tumor volume was calculated weekly according to the reference.17 Mice were sacrificed in 3 weeks using CO2, and the tumor weight was calculated. These animal experiments were performed following a protocol approved by the Ethics Committees of Shanghai Luodian Hospital.
Statistical analysis
SPSS 22.0 statistical software (Version 22.0 SPSS, Chicago, IL; USA) was performed for statistical analysis. Data were represented as mean ± standard deviation (SD). All experiments were repeated at least in three times. The comparison between two groups was analyzed by Student’s t-test. Comparisons among multiple groups were made with one-way analysis of variance (ANOVA) followed by Dunnett’s test. P<0.05 was accepted as a statistically significant difference.
Results
Establishment of HCT116/OXA cell line and optimization of combination strategy
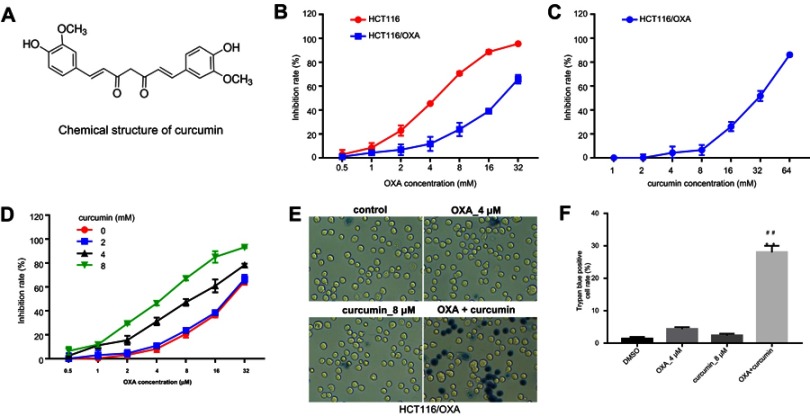
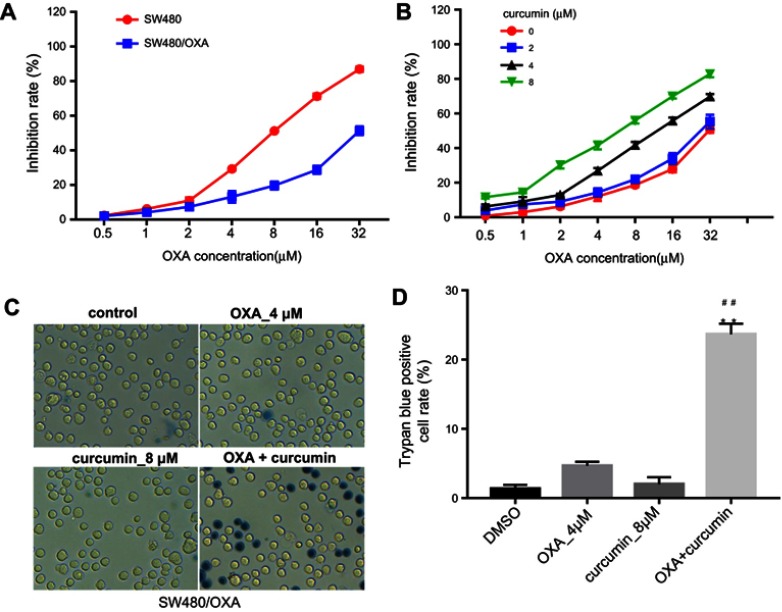
The chemical structure of curcumin was shown in Figure 1A. To explore the effect of OXA or/and curcumin on CRC cell growth, MTT assay was performed. The results indicated that HCT116/OXA was more resistant to OXA than parental HCT116 cells (Figure 1B). The IC50 values of OXA were about 4.47±0.19 μM and 20.5±1.96 μM in parental HCT116 cells and in HCT116/OXA cells, respectively. On the other hand, curcumin showed negligible toxicity to HCT116/OXA cells when its concentration is less than 8 μM (Figure 1C), and thus this concentration was used in the following experiments unless otherwise stated. Notably, curcumin dose-dependently enhanced OXA-induced growth inhibition in HCT116/OXA cells (Figure 1D, Table 1). Moreover, the result of trypan blue staining indicated 8 μM curcumin combined with 4 μM OXA significantly induced HCT116/OXA cell death, compared with 4 μM OXA alone (Figure 1E and F). Additionally, the results obtained from SW480 cells were consistent with that of HCT116 cells (Figure S1A–D). All these data suggested curcumin reduced the chemoresistance of CRC cells to OXA.
Figure 1.
Establishment of HCT116/OXA cell line and optimization of combination strategy. (A) Chemical structure of curcumin. (B) MTT assay was performed to calculate the growth inhibition rate of HCT116 and HCT116/OXA cells treated with 0.5, 1, 2, 4, 8, 16 or 32 μM OXA for 48 hrs. (C) The growth inhibition rate of HCT116/OXA cells treated with 1, 2, 4, 8, 16, 32 or 64 μM curcumin for 48 hrs. (D) The growth inhibition rate of HCT116/OXA cells treated with the combination of OXA and curcumin. (E) HCT116/OXA cell death was detected with trypan blue staining. (F) Trypan blue positive cell rate in each group was calculated. Each experiment was repeated three times. **P<0.01 compared to control group; ##P<0.01, compared to OXA alone group.
Table 1.
The IC50 of OXA on HCT116/OXA cells with or without curcumin
| Combination strategy | IC5 values (μM) |
|---|---|
| OXA+0 µM curcumin | 20.5 |
| OXA+2 µM curcumin | 19.59 |
| HBO+4 µM curcumin | 10.06 |
| HBO+8 µM curcumin | 4.588 |
Figure S1.
Curcumin reverses oxaliplatin resistance in SW480 cells. (A) MTT assay was performed to calculate the growth inhibition rate of SW480 and SW480/OXA cells treated with 0.5, 1, 2, 4, 8, 16 or 32 μM OXA for 48 hrs. (B) The growth inhibition rate of SW480/OXA cells treated with the combination of OXA and curcumin. (C) SW480/OXA cell death was detected with trypan blue staining. (D) Trypan blue positive cell rate in each group was calculated. Each experiment was repeated three times. **P<0.01 compared to control group; ##P<0.01, compared to OXA alone group.
Curcumin increased OXA-induced growth inhibition in HCT116/OXA in vitro
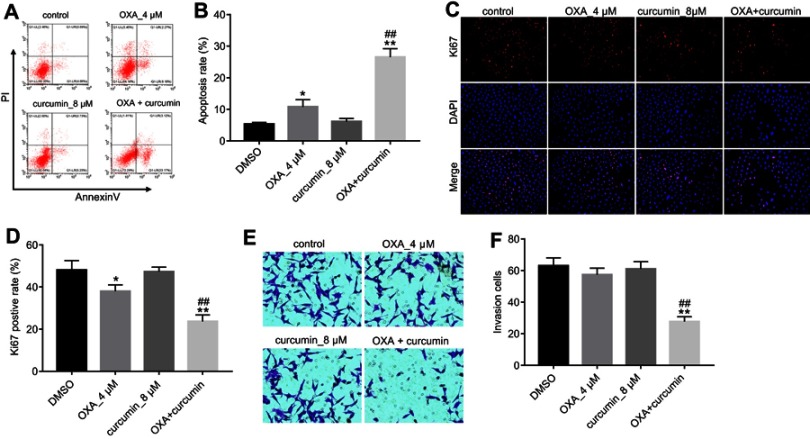
Next, we explored if curcumin could reverse the resistance of HCT116/OXA cells to OXA. Flow cytometry results showed that although OXA treatment only induced moderate cell apoptosis, combination treatment significantly enhanced the sensitivity of HCT116/OXA cells to OXA. In addition, curcumin alone did not cause obvious cell apoptosis (Figure 2A and B). In Ki67 immunofluorescence staining assay, OXA-induced growth inhibition was also reversed by curcumin (Figure 2C and D). Furthermore, combination of curcumin with OXA notably decreased the migratory ability of HCT116/OXA cells (Figure 2E and F). Collectively, these results indicated that curcumin sensitized HCT116 cells to OXA treatment.
Figure 2.
Curcumin increased OXA-induced growth inhibition in HCT116/OXA in vitro. HCT116/OXA cells were treated with OXA or/and curcumin for 48 hrs. (A) Cell apoptosis was detected with Annexin V/PI staining. (B) Cell apoptosis rate was subsequently calculated with flow cytometry. (C/D) Cell proliferation ability was analyzed by Ki67 immunofluorescence staining assay. (E/F) Cell invasion ability was analyzed by Transwell assay. Each experiment was repeated three times. *P<0.05, **P<0.01 compared to control group; ##P<0.01, compared to OXA alone group.
Curcumin downregulated the expressions of p-p65 and Bcl-2 and upregulated the level of active-caspase3
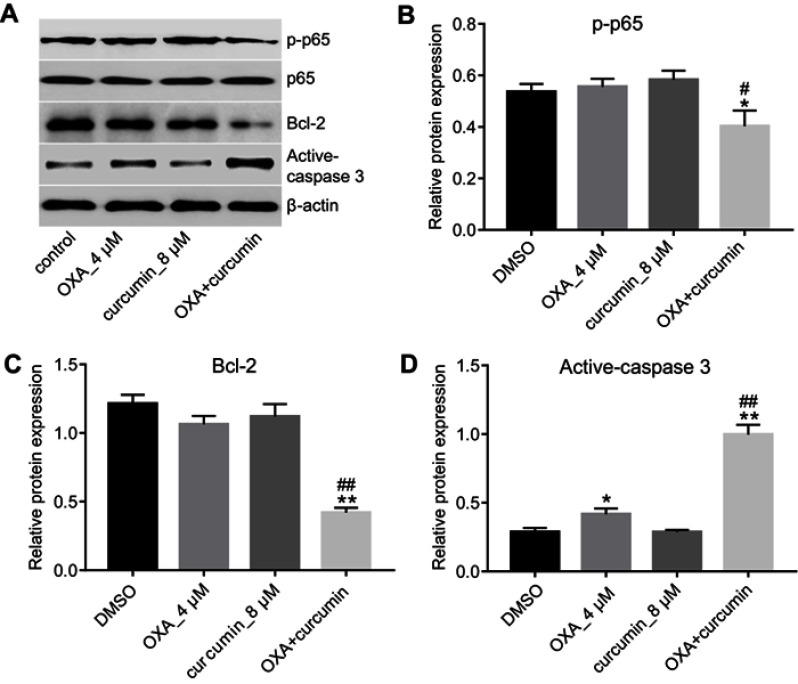
Previous reference reported that curcumin reverses the resistance of CRC to OXA by inhibiting NF-κB pathway.18 Indeed, as shown in Figure 3A and B, OXA plus curcumin treatment significantly downregulated the level of p-p65 in HCT116/OXA cells. Although the expression of apoptosis-related proteins Bcl-2 and active-caspase3 were not obviously changed in OXA-treated HCT116/OXA cells, their levels were significantly reduced by the combination treatment. In contrast, the level of cleaved-caspase3 in HCT116/OXA cells was significantly increased by OXA plus curcumin. However, curcumin alone did not have an obvious effect on the levels of p-p65, Bcl-2 and cleaved-caspase3 (Figure 3A–D). Therefore, we suggested combination of OXA with curcumin notably inhibited the pro-survival signaling in HCT116/OXA cells.
Figure 3.
Curcumin downregulated the expressions of p-p65 and Bcl-2 and upregulated the level of active-caspase3. HCT116/OXA cells were treated with OXA or/and curcumin for 48 hrs. (A–D) The relative protein levels of p-p65, p65, Bcl-2 and active-caspase3 in cells were assessed by Western blotting assay. Each experiment was repeated three times. *P<0.05, **P<0.01 compared to control group; #P<0.05, ##P<0.01, compared to OXA alone group.
Curcumin reversed EMT through dampening TGF-β/Smad2/3 pathway
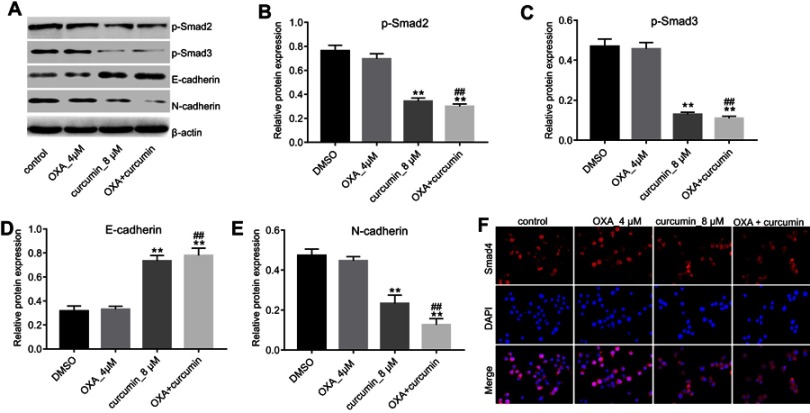
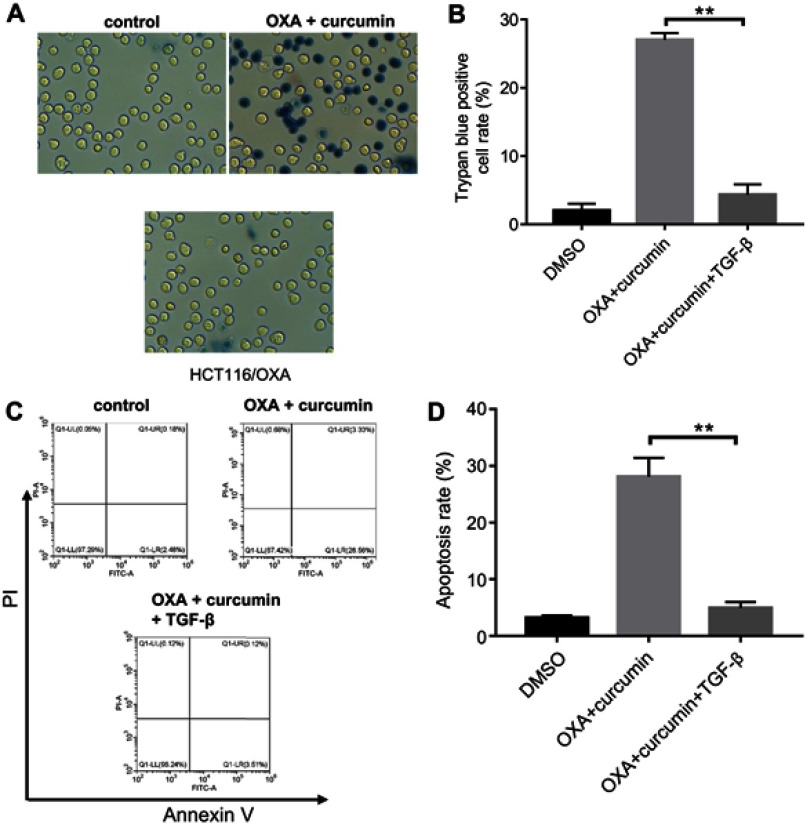
To explore the impact of curcumin on EMT, the levels of phosphorylated Smad2 (p-Smad2), phosphorylated Smad3 (p-Smad3), E-cadherin and N-cadherin in cells were measured. As shown in Figure 4A–E, the levels of p-Smad2, p-Smad3 and N-cadherin were markedly decreased, while E-cadherin level was increased by curcumin (alone or in combination with OXA). Furthermore, immunofluorescence staining indicated that Smad4 was evenly distributed throughout the cytoplasm and nucleus in control and OXA-treated HCT116/OXA cells. However, curcumin treatment induced apparent cytoplasmic localization of Smad4, indicating an inhibition of TGF-β signaling (Figure 4F). Meanwhile, TGF-β was used to confirm the impact of curcumin on TGF-β pathway. As indicated in Figure 5A and B, the inhibitory effect of curcumin plus OXA on the growth of HCT116/OXA cells was completely abolished in the presence of TGF-β. The data of apoptosis assay were consistent with trypan blue staining (Figure 5C and D). All these data suggested that curcumin could reverse EMT through downregulating TGF-β/Smad2/3 signaling pathway in HCT116/OXA cells.
Figure 4.
Curcumin inhibited EMT in CRC through inhibiting TGF-β/Smad2/3 pathway. HCT116/OXA cells were treated with OXA or/and curcumin for 48 hrs. (A–E) The relative protein levels of p-Smad2, p-Smad3, E-cadherin and N-cadherin in cells were measured by Western blotting assay. (F) The distribution of Smad4 in cells was detected by immunofluorescence staining assay. Each experiment was repeated three times. **P<0.01 compared to control group; ##P<0.01, compared to OXA alone group.
Figure 5.
The inhibitory effect of curcumin plus OXA on the growth of HCT116/OXA cells was completely abolished by TGF-β. HCT116/OXA cells were treated with HCT116/OXA cells were treated with 4 μM OXA plus 8 μM curcumin or 2 ng/mL TGF-β for 48 hrs. (A) HCT116/OXA cell death was detected with trypan blue staining. (B) Trypan blue positive cell rate in each group was calculated. (C) Cell apoptosis was detected with Annexin V/PI staining. (D) Cell apoptosis rate was subsequently calculated with flow cytometry.
Curcumin reduced OXA chemoresistance in CRC by inhibiting Smad2/3 pathway in vivo
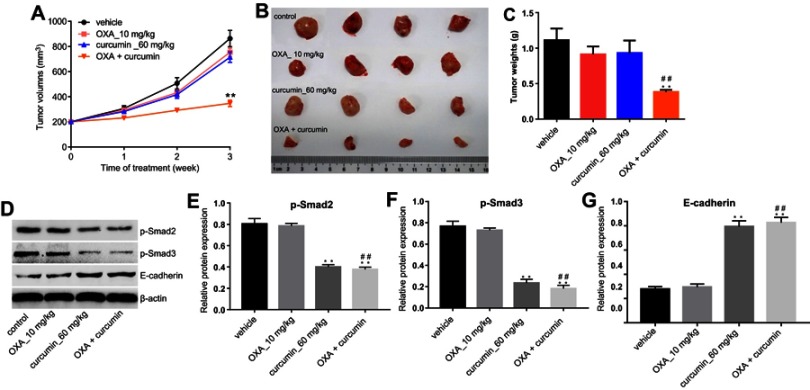
Finally, we investigated the effects of curcumin on OXA chemoresistance in CRC by using HCT116/OXA xenograft mode in vivo. As shown in Figure 6A–C, the tumor volumes and weights were significantly reduced in mice administered with OXA plus curcumin than those administered with vehicle, OXA or curcumin alone. Consistent with this finding, the levels of p-Smad2 and p-Smad3 in tumor tissues were reduced in mice administered with OXA plus curcumin, compared with those administered with OXA alone (Figure 6D–G). Collectively, curcumin significantly sensitized CRC to OXA treatment by inhibiting Smad2/3 pathway in vivo.
Figure 6.
Curcumin reduced OXA chemoresistance in CRC by inhibiting TGF-β/Smad2/3 pathway in vivo. HCT116/OXA cells were subcutaneously implanted into nude mice. Mice were divided into four groups: vehicle group, OXA alone group, curcumin alone group and OXA plus curcumin group. (A) Tumor volume of xenograft was measured weekly. (B) The mice were sacrificed in 3 weeks, and the tumors were isolated (C) Quantification of tumor weights in each group. (D–G) The relative protein levels of p-Smad2, p-Smad3 and E-cadherin in tumor tissues were measured by Western blot. Each experiment was repeated three times. **P<0.01 compared to control group; ##P<0.01, compared to OXA alone group.
Discussion
Tumor cell resistance to chemotherapeutic agents is a major cause of chemotherapy failure.19 Drug reversal of tumor multidrug resistance is an important strategy to improve the sensitivity of chemotherapy drugs, but many reversal agents such as cyclosporine A and verapamil have obvious adverse events which largely restrain their clinical application.20–22 Curcumin has the advantages of less adverse reactions, regulation and improvement of the body’s immune function, more targets, long-term application without obvious adverse events and reversal of multidrug resistance of tumors.23 However, the mechanisms by which curcumin reversed the resistance of CRC to OXA remain unclear.
Curcumin is a major active ingredient such as turmeric and medlar, and has a wide range of anti-cancer effects. Previous studies have found that curcumin can enhance the killing effect of various chemotherapy drugs on tumor cells.24 In lung cancer, curcumin combined with Sulfinosine can induce apoptosis of drug-resistant non-small cell lung cancer cell lines more effectively.25 Another study showed that curcumin can enhance the chemotherapy effect of 5-fluorouracil (5-FU) and OXA on CRC cell lines HTC116 and HT29 by inhibiting the expression of epidermal growth factor receptor and insulin-like growth factor receptor.26 In gliomas, curcumin combined with cisplatin and doxorubicin is beneficial to enhance the death of meningioma cells.27 In addition, curcumin has the property of reversing multidrug resistance of tumors. Wang et al applied curcumin to paclitaxel-resistant breast cancer cell line MCF-7/ADR, and found that curcumin can increase the accumulation of paclitaxel-targeted tumor cells, thereby promoting paclitaxel-induced MCF-7/ADR apoptosis.28 Our results showed that combination of OXA with curcumin significantly inhibited proliferation and invasion, while promoted apoptosis in HCT116/OXA cells. The results of this study suggested that curcumin could reduce OXA resistance in CRC cells. Compared with the conventional reversal agents, curcumin has the advantages of small adverse reactions, low toxicity, good safety, low price and easy availability. However, its main limitation is rapid system elimination, decreased viability at alkaline pH and limited oral bioavailability.29 Therefore, in order to overcome these limitations, how to increase its water solubility, stability, oral bioavailability and control and targeted administration will be further studied.
EMT has gradually been recognized as a new and important mechanism of tumor resistance. Tumor cells have a tendency to be interstitial in the process of producing acquired drug resistance, and tumor cells with their mesenchymal differentiation state are often characterized by primary drug resistance.30 EMT phenomenon was observed in cisplatin-resistant ovarian cancer cell lines/pancreatic cancer cell lines, adriamycin-resistant breast cancer cell lines and OXA-resistant CRC cell lines.31–33 Therefore, EMT may be a useful molecular marker and a new therapeutic target for patients with CRC who are resistant to neoadjuvant chemotherapy. Studies have shown that curcumin can regulate EMT processes in liver cancer, oral cancer, breast cancer and pancreatic cancer through different mechanisms.14,34–36 Activation of the NF-κB signaling pathway can induce the development of EMT and promote the invasion and metastasis of tumor cells. Previous studies have reported that curcumin reversed OXA resistance in CRC by inhibiting p-p65, a key molecule in the NF-κB signaling pathway.18 Similar to this study, we also found NF-κB signaling was inhibited in the presence of curcumin. In addition to NF-κB signaling, the results of current study demonstrated that curcumin treatment inhibited OXA resistance in CRC by inhibiting TGF-β/Smad/EMT pathway in vitro and vivo.
In the process of tumorigenesis and development, TGF-β plays a two-way role in initial tumor suppression and tumor promotion in the middle and late stages.37 In recent years, TGF-β has been found to be an important inducer of EMT, and EMT is regulated by Smad and non-Smad signaling pathways.12 TGF-β signaling pathway is involved in the development of chemotherapy drug resistance in CRC. The study found that TGF-β signaling pathway plays a key role in adriamycin-resistant colon cancer cells, and inhibition of TGF-β reverses EMT, thereby enhancing the sensitivity of colon cancer cell HCT116 to doxorubicin.38 Smad4 plays an important role in TGF-β-induced EMT. Studies have shown that Smad4 as a central component of EMT transition in human CRC to down-regulate E-cadherin expression.39 Similar to these findings our data indicated that curcumin significantly inhibited Smad4 entry into the nucleus.
In conclusion, based on cell studies and a mouse model, this study provides a scientific evidence that combination of OXA with curcumin could be an appropriate strategy to inhibit OXA resistance in CRC. The novelty of current study is the finding curcumin reversed OXA-acquired resistance by inhibiting Smad/EMT pathway. This study provides a scientific basis for revealing the target of curcumin in reversing the drug resistance of CRC to OXA.
Highlights
Combination of OXA with curcumin inhibited OXA resistance in CRC cells.
Combination of OXA with curcumin inhibited the expression of p-p65 and Bcl-2, but promoted active-caspase3 level in CRC cells.
Curcumin inhibited EMT via downregulation of TGF-β/Smad2/3 pathway in CRC cells.
Combination of OXA with curcumin inhibited OXA resistance by inhibiting TGF-β/Smad2/3 pathway in CRC in vivo.
Abbreviations list
5-FU, 5-fluorouracil; A570, absorbance at 570 nm; ACS, American Cancer Society; ATCC, American Type Culture Collection; BMPs, bone morphogenetic proteins; CRC, colorectal cancer; EMT, epithelial-mesenchymal transition; FBS, fetal bovine serum; GDFs, growth and differentiation factor; MTT, 3-(4,5)-dimethylthiahiazo (-z-y1)-3,5-di-phenytetrazoliumromide; NCCN, National Comprehensive Cancer Network; OXA, oxaliplatin; PI, propidiumlodide; p-p65, phosphorylated p65; p-Smad2, phosphorylated Smad2; p-Smad3, phosphorylated Smad3; RPMI1640, Roswell Park Memorial Institute1640 medium; SD, standard deviation; TGF-β, transforming growth factor-β.
Disclosure
The authors report no conflicts of interest in this work.
Supplementary material
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Petrelli F, Coinu A, Ghilardi M, Cabiddu M, Zaniboni A, Barni S. Efficacy of oxaliplatin-based chemotherapy + bevacizumab as first-line treatment for advanced colorectal cancer: a systematic review and pooled analysis of published trials. Am J Clin Oncol. 2015;38(2):227–233. doi: 10.1097/COC.0b013e3182a2d7b8 [DOI] [PubMed] [Google Scholar]
- 3.Kopetz S, Hoff PM, Morris JS, et al. Phase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. J Clin Oncol. 2010;28(3):453–459. doi: 10.1200/JCO.2009.24.8252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esatbeyoglu T, Huebbe P, Ernst IM, et al. Curcumin – from molecule to biological function. Angew Chem Int Ed Engl. 2012;51(22):5308–5332. doi: 10.1002/anie.201107724 [DOI] [PubMed] [Google Scholar]
- 5.Chen QH. Curcumin-based anti-prostate cancer agents. Anticancer Agents Med Chem. 2015;15(2):138–156. doi: 10.2174/1871520615666150116102442 [DOI] [PubMed] [Google Scholar]
- 6.Batra H, Pawar S, Bahl D. Curcumin in combination with anti-cancer drugs: a nanomedicine review. Pharmacol Res. 2019;139:91–105. [DOI] [PubMed] [Google Scholar]
- 7.Farhood B, Mortezaee K, Goradel NH, et al. Curcumin as an anti-inflammatory agent: implications to radiotherapy and chemotherapy. J Cell Physiol. 2019;234(5):5728–5740. [DOI] [PubMed] [Google Scholar]
- 8.Lopes-Rodrigues V, Oliveira A, Correia-da-Silva M, et al. A novel curcumin derivative which inhibits P-glycoprotein, arrests cell cycle and induces apoptosis in multidrug resistance cells. Bioorg Med Chem. 2017;25(2):581–596. doi: 10.1016/j.bmc.2016.11.023 [DOI] [PubMed] [Google Scholar]
- 9.Turrini E, Ferruzzi L, Fimognari C. Natural compounds to overcome cancer chemoresistance: toxicological and clinical issues. Expert Opin Drug Metab Toxicol. 2014;10(12):1677–1690. doi: 10.1517/17425255.2014.972933 [DOI] [PubMed] [Google Scholar]
- 10.Jung B, Staudacher JJ, Beauchamp D. Transforming growth factor beta superfamily signaling in development of colorectal cancer. Gastroenterology. 2017;152(1):36–52. doi: 10.1053/j.gastro.2016.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyazono K. Transforming growth factor-beta signaling in epithelial-mesenchymal transition and progression of cancer. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85(8):314–323. doi: 10.2183/pjab.85.314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katsuno Y, Lamouille S, Derynck R. TGF-beta signaling and epithelial-mesenchymal transition in cancer progression. Curr Opin Oncol. 2013;25(1):76–84. doi: 10.1097/CCO.0b013e32835b6371 [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Cheng X, Gao Y, et al. Curcumin inhibits metastasis in human papillary thyroid carcinoma BCPAP cells via down-regulation of the TGF-beta/Smad2/3 signaling pathway. Exp Cell Res. 2016;341(2):157–165. doi: 10.1016/j.yexcr.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 14.Kong D, Zhang F, Shao J, et al. Curcumin inhibits cobalt chloride-induced epithelial-to-mesenchymal transition associated with interference with TGF-beta/Smad signaling in hepatocytes. Lab Invest. 2015;95(11):1234–1245. doi: 10.1038/labinvest.2015.107 [DOI] [PubMed] [Google Scholar]
- 15.Liu D, Gong L, Zhu H, et al. Curcumin inhibits transforming growth factor beta induced differentiation of mouse lung fibroblasts to myofibroblasts. Front Pharmacol. 2016;7:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiong X, Fu L, Wang L, et al. Antitumor activity of a novel EGFR tyrosine kinase inhibitor against human lung carcinoma in vitro and in vivo. Invest New Drugs. 2009;27(1):1–11. doi: 10.1007/s10637-008-9132-5 [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Xie H, Chang P, et al. Glycoprotein M6B interacts with TbetaRI to activate TGF-beta-Smad2/3 signaling and promote smooth muscle cell differentiation. Stem Cells. 2019;37(2):190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruiz de Porras V, Bystrup S, Martinez-Cardus A, et al. Curcumin mediates oxaliplatin-acquired resistance reversion in colorectal cancer cell lines through modulation of CXC-Chemokine/NF-kappaB signalling pathway. Sci Rep. 2016;6:24675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Q, Yang Z, Nie Y, Shi Y, Fan D. Multi-drug resistance in cancer chemotherapeutics: mechanisms and lab approaches. Cancer Lett. 2014;347(2):159–166. doi: 10.1016/j.canlet.2014.03.013 [DOI] [PubMed] [Google Scholar]
- 20.Yuan R, Hou Y, Sun W, et al. Natural products to prevent drug resistance in cancer chemotherapy: a review. Ann N Y Acad Sci. 2017;1401(1):19–27. doi: 10.1111/nyas.13387 [DOI] [PubMed] [Google Scholar]
- 21.Sarisozen C, Vural I, Levchenko T, Hincal AA, Torchilin VP. PEG-PE-based micelles co-loaded with paclitaxel and cyclosporine A or loaded with paclitaxel and targeted by anticancer antibody overcome drug resistance in cancer cells. Drug Deliv. 2012;19(4):169–176. doi: 10.3109/10717544.2012.674163 [DOI] [PubMed] [Google Scholar]
- 22.Woodland C, Koren G, Wainer IW, Batist G, Ito S. Verapamil metabolites: potential P-glycoprotein-mediated multidrug resistance reversal agents. Can J Physiol Pharmacol. 2003;81(8):800–805. doi: 10.1139/y03-073 [DOI] [PubMed] [Google Scholar]
- 23.Marchiani A, Rozzo C, Fadda A, Delogu G, Ruzza P. Curcumin and curcumin-like molecules: from spice to drugs. Curr Med Chem. 2013;21(2):204–222. doi: 10.2174/092986732102131206115810 [DOI] [PubMed] [Google Scholar]
- 24.Adiwidjaja J, McLachlan AJ, Boddy AV. Curcumin as a clinically-promising anti-cancer agent: pharmacokinetics and drug interactions. Expert Opin Drug Metab Toxicol. 2017;13(9):953–972. doi: 10.1080/17425255.2017.1360279 [DOI] [PubMed] [Google Scholar]
- 25.Andjelkovic T, Pesic M, Bankovic J, Tanic N, Markovic ID, Ruzdijic S. Synergistic effects of the purine analog sulfinosine and curcumin on the multidrug resistant human non-small cell lung carcinoma cell line (NCI-H460/R). Cancer Biol Ther. 2008;7(7):1024–1032. doi: 10.4161/cbt.7.7.6036 [DOI] [PubMed] [Google Scholar]
- 26.Patel BB, Sengupta R, Qazi S, et al. Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in mediating growth inhibition of colon cancer cells by modulating EGFR and IGF-1R. Int J Cancer. 2008;122(2):267–273. doi: 10.1002/ijc.23097 [DOI] [PubMed] [Google Scholar]
- 27.Zanotto-Filho A, Braganhol E, Edelweiss MI, et al. The curry spice curcumin selectively inhibits cancer cells growth in vitro and in preclinical model of glioblastoma. J Nutr Biochem. 2012;23(6):591–601. doi: 10.1016/j.jnutbio.2011.02.015 [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Wang F, Li F, et al. A multifunctional poly(curcumin) nanomedicine for dual-modal targeted delivery, intracellular responsive release, dual-drug treatment and imaging of multidrug resistant cancer cells. J Mater Chem B. 2016;4(17):2954–2962. doi: 10.1039/C5TB02450A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson KM, Dahlin JL, Bisson J, et al. The essential medicinal chemistry of curcumin. J Med Chem. 2017;60(5):1620–1637. doi: 10.1021/acs.jmedchem.7b00407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14(10):611–629. doi: 10.1038/nrclinonc.2017.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu S, Sun J, Cai B, et al. NANOG regulates epithelial-mesenchymal transition and chemoresistance through activation of the STAT3 pathway in epithelial ovarian cancer. Tumour Biol. 2016;37(7):9671–9680. doi: 10.1007/s13277-016-4848-x [DOI] [PubMed] [Google Scholar]
- 32.Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD, Wang L-H. Twist transcriptionally up-regulates AKT2 in breast cancer cells leading to increased migration, invasion, and resistance to paclitaxel. Cancer Res. 2007;67(5):1979–1987. doi: 10.1158/0008-5472.CAN-06-1479 [DOI] [PubMed] [Google Scholar]
- 33.Yang AD, Fan F, Camp ER, et al. Chronic oxaliplatin resistance induces epithelial-to-mesenchymal transition in colorectal cancer cell lines. Clin Cancer Res. 2006;12(14 Pt 1):4147–4153. [DOI] [PubMed] [Google Scholar]
- 34.Lee AY, Fan CC, Chen YA, et al. Curcumin inhibits invasiveness and epithelial-mesenchymal transition in oral squamous cell carcinoma through reducing matrix metalloproteinase 2, 9 and modulating p53-E-cadherin pathway. Integr Cancer Ther. 2015;14(5):484–490. doi: 10.1177/1534735415588930 [DOI] [PubMed] [Google Scholar]
- 35.Chen WC, Lai YA, Lin YC, et al. Curcumin suppresses doxorubicin-induced epithelial-mesenchymal transition via the inhibition of TGF-beta and PI3K/AKT signaling pathways in triple-negative breast cancer cells. J Agric Food Chem. 2013;61(48):11817–11824. doi: 10.1021/jf404092f [DOI] [PubMed] [Google Scholar]
- 36.Sun XD, Liu XE, Huang DS. Curcumin reverses the epithelial-mesenchymal transition of pancreatic cancer cells by inhibiting the Hedgehog signaling pathway. Oncol Rep. 2013;29(6):2401–2407. doi: 10.3892/or.2013.2385 [DOI] [PubMed] [Google Scholar]
- 37.Shen L, Qu X, Ma Y, et al. Tumor suppressor NDRG2 tips the balance of oncogenic TGF-beta via EMT inhibition in colorectal cancer. Oncogenesis. 2014;3:e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J, Liu H, Yu J, Yu H. Chemoresistance to doxorubicin induces epithelial-mesenchymal transition via upregulation of transforming growth factor beta signaling in HCT116 colon cancer cells. Mol Med Rep. 2015;12(1):192–198. doi: 10.3892/mmr.2015.3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ioannou M, Kouvaras E, Papamichali R, Samara M, Chiotoglou I, Koukoulis G. Smad4 and epithelial-mesenchymal transition proteins in colorectal carcinoma: an immunohistochemical study. J Mol Histol. 2018;49(3):235–244. doi: 10.1007/s10735-018-9763-6 [DOI] [PubMed] [Google Scholar]