Abstract
In this study, a potent uricase producing organism was isolated by a thorough screening and identified as Bacillus subtilis strain SP6 by using 16s rDNA sequencing. Response surface methodological optimization was employed for the enhanced production of uricase from newly isolated Bacillus subtilis strain SP6. In media optimization studies, Plackett Burman (PB) design was used for the selection of the critical media components; which were further optimized using central composite design (CCD). Lactose, soya peptone, uric acid and FeSO4.7H2O were found to be the critical factors influencing the enzyme production. Optimum uricase production with these factors was deduced using central composite design. Significant level of the factors were 12.2 g/L of lactose, 12.79 g/L of soya peptone, 2.55 g/L of uric acid and 0.00325 g/L FeSO4.7H2O. Use of statistical optimization upsurges uricase yield from 1.2 U/ml to 15.87 U/ml enhancing the overall production by 13.23 fold; which confirms that the model is effective for process optimization.
Keywords: Biotechnology, Microbiology
1. Introduction
Uricase (Urate oxidase EC 1.7.3.3) is an enzyme of purine degradation pathway which also catalyses the oxidative degradation of insoluble uric acid to completely soluble allantoin with generation of carbon dioxide and hydrogen peroxide. The enzymatic reaction is as follows;
| Uric acid + O2 +H2O → Allantoin + H2O2 +CO2 | (1) |
Uricase play a vital role in the nitrogen metabolism. It has been most predominantly exploited in clinical biochemistry as a diagnostic reagent for measurement of uric acid in blood and other biological fluids [1]. Uricase enzyme is absent in human beings; as the gene responsible for uricase formation was lost during the early primate evolution. Uric acid acts as a powerful antioxidant, which can be useful in body to prevent the oxidative stress; hence this might be the reason for deletion of uricase genes from mammals [2]. Despite the fact of uric acid behaving as an antioxidant; it's over accumulation in the body is responsible for disease like hyperuricemia and its progressive forms like gout [3], tophi [4] gouty nephropathy [5] and tumor lysis syndrome [6]. Now, the body does not have the system for uric acid metabolism so research has been directed on the treatment of hyperuricemia and related diseases with the external administration of uricase enzyme isolated from different sources. Uricase has been previously isolated from various sources like microorganisms [7, 8], plants [9] and animals [10, 11]. An uricase from Aspergillus flavus (Uricozyme) is in the market for the treatment of tumor lysis syndrome, hyperuricemia and renal failure [12]. Currently, a new and prominent source of recombinant uricase came in the market (Rasburicase) which has higher tolerance and faster mechanism of action.
Till date, uricase has been isolated and purified from many sources. Plant sources like chickpea (Cicer arietimum L.), broad bean (Vicia faba major L.), wheat (Triticum aestivum L.) and animal sources like porcine and fish were studied for isolation and purification of uricase enzyme [9, 10, 11]. Uricases has also been purified from several microbial sources like Gliocladium viride [13] and Streptomyces [14] and Microbacteium spp. [15]. Bacillus fastidious [16]. Among all these sources bacterial sources are more useful and robust ones as they have limited requirements for the growth, faster growth rates and simple purification processes. All these pragmatic benefits facilitate the higher production of uricase enzyme in less time. Unlike the other sources have slower growth rates and their complex system requires costly purification processes hence can't be considered useful for large scale productions [17]. Though the uricase enzyme has been isolated from many organisms; there is a scope to uncover newer sources of uricases which are cost effective as well as having more specificity.
Process economics for any enzyme production is an important factor, especially for the industrially useful enzymes. The conventional procedures have certain limitations like they are time consuming, labor intensive and it fails to identify the interactions between critical factors affecting enzyme yield [18]. To overcome the disadvantages of traditional processes, superior technique like response surface methodology (RSM) is an outstanding approach by all means. RSM works on the principles of both the statistics and mathematics [17]. Its objective is to examine the interactive effects among all the variables and to optimize any kind of production. There were several reports on uricase optimization carried out using RSM. Optimization of uricase enzyme was previously reported from Pseudomonas [19], licheniformis [20]. Therefore current study focuses on isolation, screening and identification of the potent uricase producer. This is followed by response surface methodological optimization of production medium for uricase enzyme.
2. Materials and methods
2.1. Isolation of strain
For the isolation of potent uricase producer, 1 gm of poultry waste sample was added to the minimal medium containing 0.25 g/L K2HPO4, 17 g/L NH4NO3, 0.25 g/L MgSO4.7H2O, 0.02 g/L NaCl, 0.02 g/L FeSO4.7H2O and 2.5 g/L uric acid and kept in shaking incubator at 37 °C for 48 hrs [21]. To isolate the pure culture of uricase producing microorganism, turbid broth was streak plated on uric acid agar plates containing uric acid 2.5 g/L and agar-agar 28 g/L; the plates were incubated at 37 °C for 48 hrs. Discrete colonies having zone of clearance were uricase producers, which were stored and used for further study.
2.2. DNA isolation, identification and phylogenetic analysis of strain
Isolated strain was identified by 16S rDNA sequencing method. DNA extraction of potent uricolytic strain was performed by genomic DNA isolation by precipitation method. The isolated DNA was amplified by PCR. Conserved gene of 16s was amplified by using RDB 1 (5′-AGTTTGATCCTGGCTCAG-3′) as forward primer and RDB 2 (5′-AGGCCCGGGAACGTATTCTTC-3′) as reverse primer. The PCR reaction mixture containing 34.6 μl of nuclease free water, 2 μl of each primer (15μM), 1 μl of 10 mM dNTP's, 5 μl of 10X Taq buffer, 0.4 μl of 1U Taq DNA polymerase and 5 μl of DNA template (100 ng), bringing the total volume to 50 μl. This mixture was run on the PCR with following conditions, an initial denaturation at 94 °C for 5 mins, followed by 40 cycles of denaturation at 94 °C for 1 min, 55 °C for 1 min, 72 °C for 2 mins and final extension at 72 °C for 10 min. PCR amplified product was purified using GenEluteTM PCR Clean-Up kit (Sigma aldrich, USA). Amplified gene was sequenced in both directions in Xcelris laboratories, Gujarat. Analysis of the sequence data was done by using BLASTn algorithm (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The resultant sequence deposited in NCBI gene bank. Phylogenetic analysis was carried out by using MEGA 7 software. Maximum likelihood method was used for construction of phylogeny.
2.3. Uric acid plate assay
Uric acid plate assay was used for the qualitative analysis of the organisms having uricase activity [22] in which, the loopful colonies of the bacterial strain were spot inoculated on the uric acid agar plate. These plates were incubated at 37 °C for 24 hrs. The strain having uricolytic ability will show clear zone around the colonies after complete incubation. Zone of clearance indicates uricase activity.
2.4. Enzyme assay
Enzyme activity of uricase can be measured either by formation of hydrogen peroxide or disappearance of uric acid. The disappearance of uric acid was measured at 293 nm as described by Mahler [23]. The reaction mixture contains, 3 ml of 20 mM sodium borate buffer of pH 9.0 containing 100 μl of 3.57 mM uric acid solution. The reaction was started by adding 50 μl of crude enzyme to the reaction mixture and incubated at 25 °C for 10 min. The absorbance was measured at 293 nm by using UV visible spectrophotometer. Reduction in the uric acid concentration is measured by the difference between the absorbance of test and blank.1 unit (U) of enzyme activity was defined as, amount of uricase required to convert 1 μmol of uric acid into allantoin per minute at 25 °C and pH 9.0, considering the millimolar extinction coefficient of uric acid (έ) at 293 nm as 12.6 mM−1 cm−1 [14].
Uricase Calculation formula:
Where,
ΔA = Absorbance
B = Total volume of reaction mixture
df = Dilution factor
12.6 = Molar extinction coefficient of uric acid at 293 nm.
C = Volume of enzyme.
2.5. Statistical experimental design for media optimization
Media optimization is essential for increased enzyme production. Initially efficacy of various carbon and nitrogen sources was checked classically for the enhancement of enzyme production. Design Expert STAT Ease software version 11 (Minneapolis, USA) was used for statistical optimization studies. The critical components affecting uricase enzyme production were evaluated using Plackett Burman design [24]. Most significant factors identified by PB design were further optimized with the help of central composite design. All experimental sets were conducted in triplicates and data presented as mean value with ±SD.
2.6. Classical media optimization by one factor at a time method (OFAT)
Initially fermentation medium was optimized conventionally by one factor at a time method (OFAT). Carbon and nitrogen sources were screened to check their efficacy on the uricase enzyme yield. 1% w/v of various carbon sources (dextrose, fructose, lactose, CM cellulose, starch) as well as inorganic nitrogen sources (ammonium chloride, ammonium sulphate, urea, glycine, sodium nitrate) and organic nitrogen sources (soya peptone, yeast extract, beef extract, peptone) were used to check their effect on uricase production.
2.7. Placket Burman design for effective constituents
Statistical media optimization considered as a most important technique to optimize the production media for particular organisms producing specific products. For the screening of essential media components, effective carbon and nitrogen sources along with media components were selected. In present study, 9 variables were taken for Plackett Burman analysis which were as follows; lactose, soya peptone, uric acid, K2HPO4, NH4NO3, MgSO4.7H2O, NaCl and FeSO4.7H2O and inoculum size. These factors were used at three different levels as centre point (0), low level (−1) and high level (+1) as shown in Table 1. 24 hour old culture of Bacillus subtilis Strain SP6 was inoculated to the production media which was further incubated at 37 °C for 24 hrs. Total 13 trials were conducted and its composition is given in Table 2. Plackett–Burman experimental design is based on the first order model, which is as follows:
| Y = βo + ΣβiXi. | (2) |
Table 1.
Variables and levels for Plackett Burman experiment.
| Sr. No. | Media components | Coded values | −1 | 0 | +1 |
|---|---|---|---|---|---|
| 1 | Lactose (g/L) | A | 5 | 10 | 15 |
| 2 | Soya peptone (g/L) | B | 5 | 10 | 15 |
| 3 | Uric acid (g/L) | C | 1.5 | 2.25 | 3.0 |
| 4 | K2HPO4 (g/L) | D | 1.5 | 2.5 | 3.5 |
| 5 | NH4NO3 (g/L) | E | 8 | 17 | 27 |
| 6 | MgSO4.7H2O (g/L) | F | 1.5 | 2.5 | 3.5 |
| 7 | NaCl (g/L) | G | 0.001 | 0.002 | 0.003 |
| 8 | FeSO4.7H2O (g/L) | H | 0.001 | 0.002 | 0.003 |
| 9 | Inoculum size (ml) | I | 1 | 2 | 3 |
Table 2.
Media composition in Plackett–Burman design.
| Std | Lactose gL | Soya peptone g/L | Uric acid g/L | K2HPO4 g/L | NH4NO3 g/L | MgSO4. 7H2O g/L |
NaCl g/L | FeSO4. 7H2O g/L |
Inoculum size ml | Response U/ml |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | −1 | +1 | +1 | −1 | +1 | +1 | +1 | −1 | −1 | 5.77 |
| 2 | −1 | +1 | +1 | +1 | −1 | −1 | −1 | +1 | −1 | 5.08 |
| 3 | +1 | −1 | +1 | +1 | −1 | +1 | +1 | +1 | −1 | 4.61 |
| 4 | +1 | −1 | −1 | −1 | +1 | −1 | +1 | +1 | −1 | 2.9 |
| 5 | −1 | +1 | −1 | +1 | +1 | −1 | +1 | +1 | +1 | 3.25 |
| 6 | −1 | −1 | +1 | −1 | +1 | +1 | −1 | +1 | +1 | 2.66 |
| 7 | +1 | +1 | −1 | −1 | −1 | +1 | −1 | +1 | +1 | 4.58 |
| 8 | +1 | +1 | −1 | +1 | +1 | +1 | −1 | −1 | −1 | 5.11 |
| 9 | −1 | −1 | −1 | +1 | −1 | +1 | +1 | −1 | +1 | 1.6 |
| 10 | +1 | +1 | +1 | +1 | +1 | −1 | −1 | −1 | +1 | 5.55 |
| 11 | +1 | −1 | +1 | −1 | −1 | −1 | +1 | −1 | +1 | 8.95 |
| 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5.43 |
| 13 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | 1.35 |
Where, Y is the response i.e. enzyme activity of uricase enzyme, βo is the model intercept and βi is the linear coefficient, and Xi is the level of the independent variable. Regression analysis determined the most significant variables with significant p value (<0.05) affecting uricase yield, which were further optimized using central composite design.
2.8. Central composite design for significant factors
Plackett Burman design identified lactose, soya peptone, uric acid and FeSO4.7H2O as critical components, which were affecting the uricase yield. These four variables were studied at five different levels (−α, −1, 0, +1, +α) as presented in Table 3. A trial of 30 experiments was constructed using design matrix which has runs consisting 16 random points, 6 center points and 8 axial points (Table 4). After performing experiments, the results were analyzed and a second order polynomial equation was fitted to the data carrying out multiple regression analysis (Eq. 3).
| Y = βo + β1A + β2B + β3C + β4D + β1β1A2 + β2β2 B2 + β3β3C2 + β4β4D2 + β1β2AB + β1β3AC + β2 β3BC + β1β4AD + β2 β4BD +β3β4CD | (3) |
Table 3.
Critical components for CCD with various levels concentration.
| Independent variables | Coded values | −α | −1 | 0 | +1 | +α |
|---|---|---|---|---|---|---|
| Lactose | A | 2.5 | 5.06 | 11.25 | 17.43 | 20 |
| Soya peptone | B | 2.5 | 5.06 | 11.25 | 17.43 | 20 |
| Uric acid | C | 1.0 | 1.36 | 2.25 | 3.13 | 3.5 |
| FeSO4.7H2O | D | 0.001 | 0.0015 | 0.003 | 0.004 | 0.005 |
Table 4.
CCD Experimental design developed using Design Experts STAT EASE Software.
| Std | A | B | C | D | Uricase activity (U/ml) |
|
|---|---|---|---|---|---|---|
| Observed | Predicted | |||||
| 1 | −1 | −1 | −1 | −1 | 15.83 | 15.23 |
| 2 | +1 | −1 | −1 | −1 | 6.99 | 6.57 |
| 3 | −1 | +1 | −1 | −1 | 15.68 | 15.23 |
| 4 | +1 | +1 | −1 | −1 | 10.69 | 10.22 |
| 5 | −1 | −1 | +1 | −1 | 11.17 | 10.66 |
| 6 | +1 | +1 | +1 | −1 | 6.85 | 7.25 |
| 7 | −1 | +1 | +1 | −1 | 5.2 | 5.33 |
| 8 | +1 | −1 | +1 | −1 | 15.45 | 15.23 |
| 9 | −1 | +1 | −1 | +1 | 10.85 | 10.13 |
| 10 | +1 | +1 | −1 | +1 | 6.11 | 6.38 |
| 11 | −1 | −1 | −1 | +1 | 10.98 | 12.19 |
| 12 | +1 | +1 | −1 | +1 | 7.35 | 6.71 |
| 13 | −1 | −1 | +1 | +1 | 15.63 | 15.23 |
| 14 | +1 | −1 | +1 | +1 | 15.75 | 15.23 |
| 15 | −1 | +1 | +1 | +1 | 13.9 | 14.15 |
| 16 | +1 | +1 | +1 | +1 | 10.72 | 12.09 |
| 17 | −1.68 | 0 | 0 | 0 | 14.14 | 14.59 |
| 18 | +1.68 | 0 | 0 | 0 | 5.6 | 5.94 |
| 19 | 0 | −1.68 | 0 | 0 | 11.24 | 11.16 |
| 20 | 0 | +1.68 | 0 | 0 | 4.16 | 4.10 |
| 21 | 0 | 0 | −1.68 | 0 | 10.11 | 9.44 |
| 22 | 0 | 0 | +1.68 | 0 | 9.5 | 9.09 |
| 23 | 0 | 0 | 0 | −1.68 | 14.42 | 15.23 |
| 24 | 0 | 0 | 0 | +1.68 | 4.73 | 5.24 |
| 25 | 0 | 0 | 0 | 0 | 7.35 | 7.86 |
| 26 | 0 | 0 | 0 | 0 | 5.38 | 5.84 |
| 27 | 0 | 0 | 0 | 0 | 9.84 | 9.17 |
| 28 | 0 | 0 | 0 | 0 | 10.83 | 11.95 |
| 29 | 0 | 0 | 0 | 0 | 7.33 | 6.71 |
| 30 | 0 | 0 | 0 | 0 | 12.125 | 11.78 |
Where, Y is the response of uricase yield in units; A, B, C, D are the coded independent variables; β1, β2, β3 and β4 were linear coefficients; βo was the intercept term; β1β1, β2β2, β3β3 and β4β4 are the quadratic coefficients; β1β2, β1β3, β2β3, β1β4, β2β4, β3β4 are the interactive coefficients.
3. Results and discussion
3.1. Isolation and screening of strain
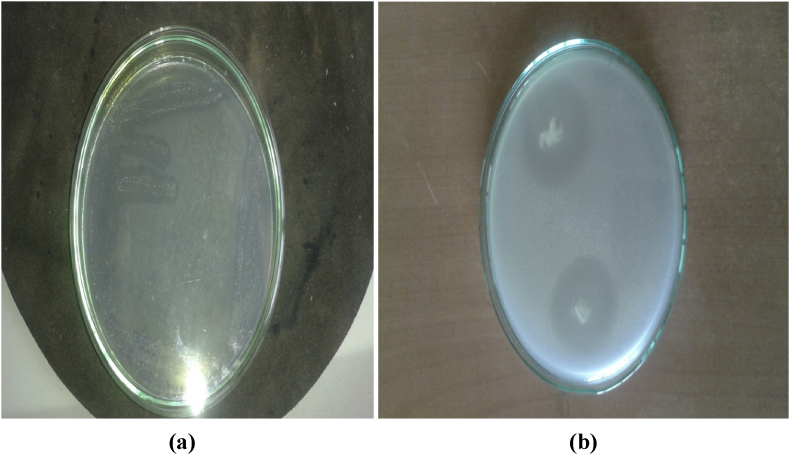
The uricase producing organisms were isolated using streak plate technique as shown in Fig. 1(a) 25 uricase producing strains were isolated from uric acid agar plates. Out of those 25 strains, most potent strain was selected on the basis of uric acid plate assay method, most significant production of uricase was observed for SP6 organism which showed bigger zone of clearance in minimum time on the area of spot inoculation Fig. 1(b).
Fig. 1.
Uricase producing bacterial isolate. (a) Clear zone around the isolated colonies obtained by four quadrant method (b) Zone of clearance by the selected isolate.
3.2. Identification of strain and phylogenetic analysis
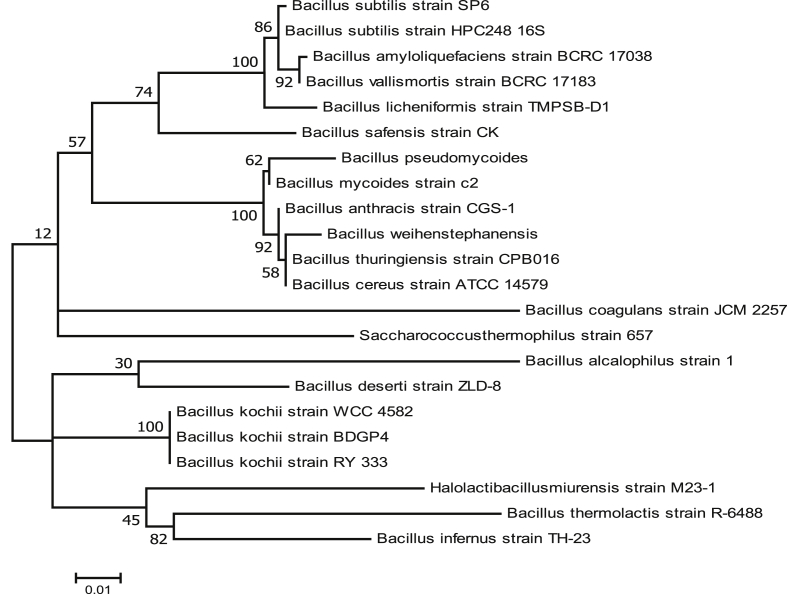
Most potent uricase producer was identified by 16S rDNA sequencing method and identified as Bacillus subtilis strain SP6 which showed 99% sequence similarity with Bacillus subtilis. The sequence was deposited in GenBank with accession no. MG661743. Phylogenetic analysis was carried using MEGA 7 software and molecular phylogeny was constructed by using maximum likelihood method as shown in Fig. 2.
Fig. 2.
Molecular Phylogenetic analysis by maximum likelihood method based on 16S rDNA sequence. Phylogeny showing relationship between SP6 strain and other species of Bacillus genus. Phylogeny was constructed by using MEGA 7.
3.3. Media optimization by one factor at a time method
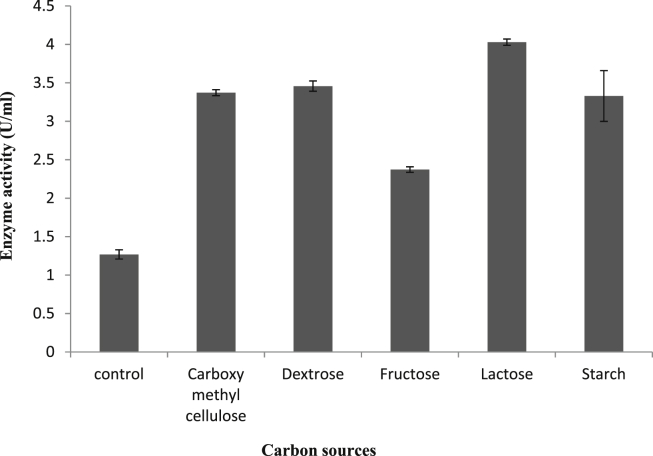
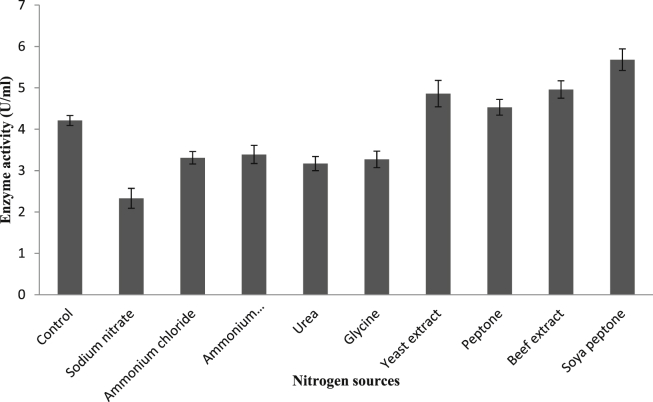
Most potent carbon and nitrogen sources were selected by one factor at a time method. Each and every carbon sources tested, enhanced the uricase production, where lactose exhibited higher uricase yield (5.5 U/ml) followed by dextrose (4.8 U/ml) as shown in Fig. 3. Similarly lactose was found to be a good enhancer for uricase production by Sphingobacterium thalpophilum VITPCB5 [25]. While in case of various nitrogen sources tested at 1% level in the production medium, most of the organic sources significantly enhanced the uricase production whereas, nearly all inorganic sources moderately decreased the uricase production. Soya peptone acted as a best nitrogen source (Fig. 4) followed by beef extract while sodium nitrate, urea, and glycine drastically decreased the enzyme production. In contrast to this, urea acted as a best nitrogen source and enhanced the uricase production in case of Sphingobacterium thalpophilum VITPCB5 whereas; in case of uricase production by Gliocladium viride, yeast extract was found to be the best nitrogen source [13]. Bacillus subtilis strain SP6 showed remarkable enhancement in the uricase yield with the help of organic nitrogen sources rather than inorganic one.
Fig. 3.
Effect of various carbon sources on uricase production.
Fig. 4.
Effect of different nitrogen sources on uricase production.
3.4. Screening of significant factors by Plackett Burman design
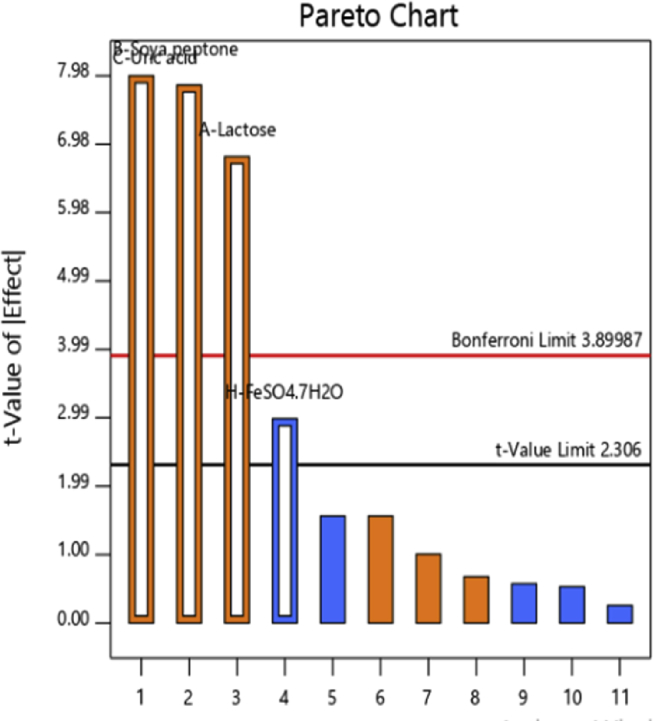
On the basis of previous reports as well as experiments carried out under this study, total nine variables were selected and their effect was checked on uricase production by using PB design (Table 1). Table 2 represents the yield of uricase production for each experimental design. The Statistical analysis using PB design (Table 5) indicated that lactose (A), soya peptone (B), uric acid (C) and FeSO4.7H2O (D) significantly affected uricase production with p values less than significance level, whereas the other components like K2HPO4, NH4 NO3, MgSO4.7H2O, NaCl were found insignificant with p values above 0.05 (Fig. 5). First order polynomial equation was derived by using regression analysis as follows (Eq. 3),
| R1 = 4.32 + 1.01 A + 1.15 B + 1.18 C − 0.4125 H | (4) |
Table 5.
ANOVA for selected factors in Plackett Burman design.
| Source | Sum of square | d.f | Mean square | F-value | p-value |
|---|---|---|---|---|---|
| Model | 46.71 | 4 | 11.68 | 30.88 | <0.0001 |
| A- Lactose | 11.98 | 1 | 11.98 | 31.68 | 0.0005 |
| B-Soya peptone | 16.50 | 1 | 16.50 | 43.62 | 0.0002 |
| C-Uric acid | 15.94 | 1 | 15.94 | 42.15 | 0.0002 |
| D-FeSO4.7H2O | 2.30 | 1 | 2.30 | 6.07 | 0.0390 |
| Residual | 3.03 | 8 | 0.3782 | ||
| Corrected total | 49.74 | 12 |
*Significant p_values at P ≤ 0.05.
Fig. 5.
Pareto chart showing four critical components affecting uricase yield.
It represents uricase production as a function of independent variables. The model F value of 30.88 emphasizes the model as significant, where there was only 0.01% chance, a model F value this large could occur due to noise. The p value <0.05 denotes that the model terms are significant. Interdependence of significant variables possibly cannot be illustrated by first order equation; so further investigation was conducted through a second order model in RSM experiment.
3.5. Medium optimization by RSM
Significant components obtained from PB design were further optimized by central composite design. The experimental design of CCD was developed by using four factors at two level factorials. Table no. 3 represents high and low levels of the components. Experimental design matrix and their results of CCD analysis were given in Table 4. The obtained regression equation after ANOVA exhibited the level of uricase production as a function of different variables, such as lactose, soya peptone, uric acid and FeSO4.7H2O. The CCD results demonstrated the following second order polynomial equation on the basis of quadratic regression analysis.
| Uricase activity = 15.23 + 0.5428A + 0.9354B + 1.90C + 0.1537D − 0.0684AB + 0.1753AC − 0.1328AD + 0.3053BC − 0.1728BD − 0.1566CD − 1.90A2 − 2.23B2 − 2.98C2 + 0.4288D2 | (5) |
Collaborative effect of media components demonstrated by standard analysis of variance (ANOVA), regression coefficient, F values, and p values of variables were examined, and are illustrated in Table 6. The model F value of 38.87 indicates that the proposed model is significant and there is only 0.01% chance that model F value, this large could occur due to noise. The values of “prob F” less than 0.05 showed model terms (A, B, C, D, AB, BD, A2, B2, C2, D2) are significant and values greater than 0.1 indicates that the model terms are not significant. The pre-determined R2 0.8723 was in reasonable agreement with the adjusted R2 0.9481 which depicted adequacy of model to predict response. Adequate precision measures signal-to-noise ratio, precision ratio greater than 4.0 is desirable, and the ratio is 18.5284. Therefore, model can be used to navigate the design space. 3.42 is “Lack of Fit F value” which implies that the lack of fit is not significant relative to the pure error. Non-significant lack of fit is good which confirmed that the model equation was adequate to predict the uricase yield. The value of coefficient of variation (CV% = 8.33) revealed the precision and reliability of the model. Fig. 6 showed the interaction among the components.
Table 6.
ANOVA for quadratic model in CCD model.
| Source | Sum of square | d.f | Mean square | F-value | p-value |
|---|---|---|---|---|---|
| Model | 392.60 | 14 | 28.04 | 38.87 | <0.0001 |
| A-Lactose | 5.89 | 1 | 5.89 | 8.17 | 0.0120 |
| B-Soya peptone | 17.50 | 1 | 17.35 | 24.26 | 0.0002 |
| C-Uric acid | 72.26 | 1 | 72.26 | 100.16 | <0.0001 |
| D-FeSO4.7H2O | 0.4726 | 1 | 0.4726 | 0.6551 | 0.4310 |
| AB | 0.0749 | 1 | 0.0749 | 0.1039 | 0.7517 |
| AC | 0.4918 | 1 | 0.4918 | 0.6816 | 0.4220 |
| AD | 0.2822 | 1 | 0.2822 | 0.3912 | 0.5411 |
| BC | 1.49 | 1 | 1.49 | 2.07 | 0.1710 |
| BD | 0.4778 | 1 | 0.4778 | 0.6623 | 0.4285 |
| CD | 0.3922 | 1 | 0.3922 | 0.5436 | 0.4723 |
| A2 | 33.74 | 1 | 33.74 | 46.77 | <0.0001 |
| B2 | 46.47 | 1 | 46.47 | 64.41 | <0.0001 |
| C2 | 83.10 | 1 | 83.10 | 115.18 | <0.0001 |
| D2 | 1.72 | 1 | 1.72 | 2.38 | 0.1438 |
| Residual | 10.82 | 15 | 0.7214 | ||
| Lack of fit | 9.44 | 10 | 0.9441 | 3.42 | 0.0935 |
| Pure error | 1.38 | 5 | 0.2760 | ||
| Corrected total | 403.42 | 29 |
*Significant p_values at P ≤ 0.05.
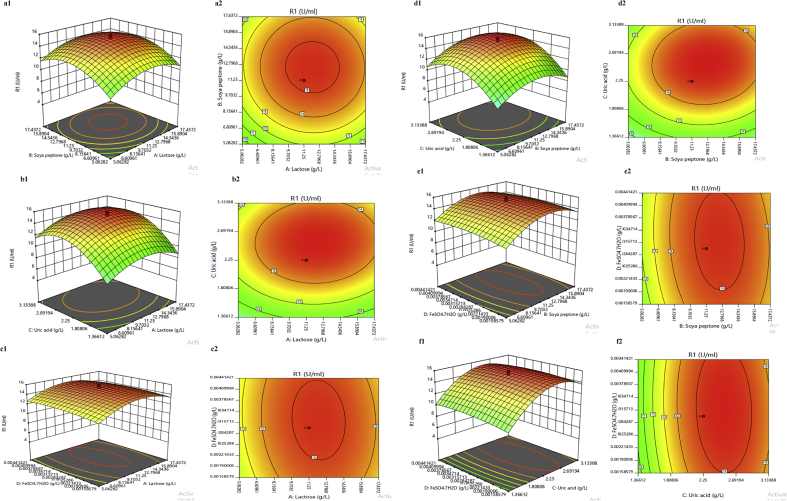
Fig. 6.
Representing the 3D surface as well as contour plots; fig a1a2 revealed non significant interaction between lactose and soya peptone, fig b1b2 illustrated moderate significant interaction of lactose and uric acid. fig c1c2 indicated moderate significant interaction among lactose and FeSO4.7H2O. fig d1 d2 revealed significant interaction between soya peptone and uric acid; fig e1 e2 illustrated significant interactions between soya peptone and FeSO4.7H2O; fig f1 f2 revealed highly significant interactions among uric acid and FeSO4.7H2O.
3.6. Interaction among the variables
Interactions between the significant variables for uricase enzyme production graphically studied by three dimensional (3D) plots and two-dimensional (2D) contour plots [26]. Out of 4variables, 2 kept at optimum level while two kept at zero level, to evaluate the yield of uricase enzyme. Three dimensional (3D) plots and two-dimensional (2D) contour plot are simple and very easy to understand. Significance or non significance of the 3D plot and 2D contour plots depends on the circular or elliptical shape of the contour plots. Circular order shows non-significant interactions whereas elliptical order specifies significant interactions [27, 28]. Interaction between the lactose and soya peptone Fig. 6 (a1 a2) was found to be circular suggesting insignificant interaction in between them. The interaction among lactose and uric acid Fig. 6 (b1 b2) was nearly elliptical showing moderate effect on uricase production whereas lactose and FeSO4.7H2O Fig. 6 (c1 c2) showed elliptical 2D contour plots, suggesting significant interaction in between them. From the subfigure of Fig. 6 (d1 d2) interaction of uric acid and soya peptone moderately influencing the uricase yield. Fig. 6 (e1 e2) and (f1 f2) represent the interaction of soya peptone with FeSO4.7H2O and uric acid with FeSO4.7H2O respectively. Contour plots of these interactions found elliptical in nature, hence suggesting significant interactions with each other.
3.7. Experimental model validation
Proposed concentration to be used for higher uricase enzyme production are 12.2 g/L of lactose, 12.79 g/L of soya peptone, 2.55 g/L of uric acid and 0.00325 g/L FeSO4.7H2O. Optimized medium showed uricase production in much higher amounts than the un-optimized media. Bacillus subtilis strain SP6 was found to be a potent uricase producer having uricase production of 15.87 U/ml which is way better than the earlier reported Pseudomonas aeruginosa 7.1 U/ml [19] and Bacillus licheniformis (0.616 U/ml) [20] however it is lower than the uricases from Rhizopus stolonifer (26.70 U/ml) [29]. Streptomyces rochei (47.49 U/ml) [13] and Gliocladium viride (84.92 U/ml) [14].
4. Conclusion
Present study describes the isolation of potent uricase producing bacterium as well as its optimization of uricase production. This newly isolated bacterium was subsequently identified as Bacillus subtilis strain SP6 on the basis of 16s rDNA sequencing. Lactose, soya peptone, uric acid and FeSO4.7H2O were the critical factors identified by PB design and were further optimized using CCD. Response surface methodologically optimized medium with simple carbon and nitrogen sources showed significant increase in the production of uricase enzyme. The yield of uricase was enhanced up to 13.23 fold in optimized medium as compared to initial production medium. To the best of our knowledge isolate Bacillus subtilis strain SP6 is the most potent uricase producing bacterium till date having ability to produce 15.87 U/ml of uricase enzyme which is higher than any bacterium earlier reported and close to some fungal uricase producers. Extension of this work will be chromatographic separation and purification of the uricase enzyme along with its complete biochemical and biophysical characterization.
Declarations
Author contribution statement
Sneha O. Pustake: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Prashant K. Bhagwat: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Padma B. Dandge: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by Grant no F.No.41-1282/2012 (SR) awarded by MRP-UGC, India, a Departmental Research Fellowship awarded to Sneha O. Pustake by the Department of Biochemistry, Shivaji University Kolhapur, and the BSR fellowship for doctoral research awarded to Prashant K. Bhagwat by the UGC, Govt. of India.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Adamek V., Kralova B., Suchova M., Valentova O., Demnerova K. Purification of microbial uricase. J. Chromatogr. 1989;497:268–275. doi: 10.1016/0378-4347(89)80028-3. [DOI] [PubMed] [Google Scholar]
- 2.Wu X.W., Lee C.C., Muzny D.M., Caskey C.T. Urate oxidase: primary structure and evolutionary implications. Proc. Natl. Acad. Sci. USA. 1989;86:9412–9416. doi: 10.1073/pnas.86.23.9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chou C.T., Lai J.S. The epidemiology of hyperuricemia and gout in Taiwan aborigines. Br. J. Rheumatol. 1998;7:258–262. doi: 10.1093/rheumatology/37.3.258. [DOI] [PubMed] [Google Scholar]
- 4.Dalbeth N., Pool B., Gamble G.D., Smith T., Callon K.E., McQueen F.M., Cornish J. Cellular characterization of the gouty tophus a quantitative analysis. Arthritis Rheum. 2010;62(5):1549–1556. doi: 10.1002/art.27356. [DOI] [PubMed] [Google Scholar]
- 5.Nickeleit V., Mihatsch M.J. Uric acid nephropathy and end-stage renal disease — review of a non-disease. Nephrol. Dial. Transplant. 1997;12:1832–1838. doi: 10.1093/ndt/12.9.1832. [DOI] [PubMed] [Google Scholar]
- 6.Patte C., Sakiroglu C., Ansoborlo S., Baruchel A., Plouvier E., Pacquement H., Babin-Boilletot A. Urate-oxidase in the prevention and treatment of metabolic complications in patients with B-cell lymphoma and leukemia, treated in the Société Française d’Oncologie Pédiatrique LMB89 protocol. Ann. Oncol. 2001;(13):789–795. doi: 10.1093/annonc/mdf134. [DOI] [PubMed] [Google Scholar]
- 7.Farley P.C., Santosa S. Regulation of expression of the Rhizopus oryzae uricase and urease enzymes. Can. J. Microbiol. 2002;48:1104–1108. doi: 10.1139/w02-103. [DOI] [PubMed] [Google Scholar]
- 8.Abdollah G.A., Moradpour Z., Baniasad M., Ghasemi Y. Isolation, molecular identification and characterization of the culture conditions for extracellular uricase production by a new strain of Pseudomonas sp. J. Pure Appl. Microbio. 2015;9:2813–2821. [Google Scholar]
- 9.Montalbini P., Redondo J., Caballero J.L., Ca´rdenas J., Pineda M. Uricase from leaves: its purification and characterization from three different higher plants. Planta. 1997;202:277–283. [Google Scholar]
- 10.Conley T.G., Priest D.G. Purification of uricase from mammalian tissue. Prep. Biochem. Biotechnol. 1979;9(2):197–203. doi: 10.1080/00327487908061683. [DOI] [PubMed] [Google Scholar]
- 11.Kinsella E., German B., Shetty J. Uricase from fish liver: isolation and some properties. Comp. Biochem. Physiol. 1985;82(4):621–624. doi: 10.1016/0305-0491(85)90498-5. [DOI] [PubMed] [Google Scholar]
- 12.Pui C.H., Relling M.V., Lascombes F., Harrison P.L., Struxiano A., Mondesir J.M., Ribeiro R.C., Sandlund J.T., Rivera G.K., Evans W.E., Mahmoud H.H. Urate oxidase in prevention and treatment of hyperuricemia associated with lymphoid malignancies. Leukemia. 1997;11:1813–1816. doi: 10.1038/sj.leu.2400850. [DOI] [PubMed] [Google Scholar]
- 13.Noura El-Ahmady El-Naggar Isolation, screening and identification of actinobacteria with uricase activity: statistical optimization of fermentation conditions for improved production of uricase by Streptomyces rochei NEAE-25. Int. J. Pharmacol. 2015;11:644–658. [Google Scholar]
- 14.Nanda P., Babu J.P., Fernandes J., Hazarika P., Dhabre R.R. Studies on production, optimization and purification of uricase from Gliocladium viride. Res. Biotechnol. 2012;3(4):35–46. [Google Scholar]
- 15.Zhou X.L., Ma X.H., Sun G.Q., Li X., Guo K.P. Isolation of a thermostable uricase-producing bacterium and study on its enzyme production conditions. Process Biochem. 2005;40:3749–3753. [Google Scholar]
- 16.Tan Qunyou, Zhang Jingqing, Wang Na, Li Xiaoling, Xiong Huarong, Teng Yongzhen, He Dan, Wu Jianyong, Zhao Chunjing, Yin Huafeng, Zhang Liangke. Uricase from Bacillus fastidious loaded in alkaline enzymosomes: enhanced biochemical and pharmacological characteristics in hypouricemic rats. Eur. J. Pharm. Biopharm. 2012;82:43–48. doi: 10.1016/j.ejpb.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Jhample S.B., Bhagwat P.K., Dandge P.B. Statistical media optimization for enhanced production of fibrinolytic enzyme from newly isolated Proteus penneri SP-20. Biocatal. Agri. Biotechnol. 2015;4:370–379. [Google Scholar]
- 18.Bhagwat P.K., Jhample S.B., Dandge P.B. Statistical medium optimization for production of collagenolytic protease by Pseudomonas sp. SUK using response surface methodology. An. Microbiol. 2015;84:520–530. [Google Scholar]
- 19.Abdel-Fattah Y.R., Saeed H.M., Gohar Y.M., El-Baz M.A. Improved production of Pseudomonas aeruginosa uricase by optimization of process parameters through statistical experimental designs. Process Biochem. 2005;40:1707–1714. [Google Scholar]
- 20.Pawar S.V., Rathod V.K. Optimization of novel and greener approach for the co-production of uricase and alkaline protease in Bacillus licheniformis by Box Behnken Model. Prep. Biochem. Biotechnol. 2017;48(1):24–33. doi: 10.1080/10826068.2017.1381623. [DOI] [PubMed] [Google Scholar]
- 21.Kasabe P.J., Mali G.T., Dandge P.B. Assessment of alkaline cholesterol oxidase purified from Rhodococcus sp. PKPD-CL for its halo tolerance, detergent and organic solvent stability. Protein Expr. Purif. 2015;116:30–41. doi: 10.1016/j.pep.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Poovizh T., Gajalakshmi P., Jayalakshmi S. Production of uricase a therapeutic enzyme from Pseudomonas putida isolated from poultry waste. Int. J. Adv. Res. 2014;2:34–40. [Google Scholar]
- 23.Mahler H.R., Hubscher G., Baum R. Studies on uricase. I. Preparation, purification, and properties of a cuproprotein. J. Biol. Chem. 1955;216:625–641. [PubMed] [Google Scholar]
- 24.Plackett R.L., Burman J.P. The design of optimum multifactorial experiments. Biometrika. 1946;33:305–325. [Google Scholar]
- 25.Ravichandran R., Hemaasri S., Cameotra S.S., Jayaprakash N.S. Purification and characterization of an extracellular uricase from a new isolate of Sphingobacterium thalpophilum (VITPCB5) Protein Expr. Purif. 2015;114:136–142. doi: 10.1016/j.pep.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 26.Gajdhane S.B., Bhagwat P.K., Dandge P.B. Response surface methodology-based optimization of production media and purification of a-galactosidase in solid-state fermentation by Fusarium moniliforme NCIM 1099. 3 Biotech. 2016;6:1–14. doi: 10.1007/s13205-016-0575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhagwat P.K., Bhise K.K., Bhuimbar M.V., Dandge P.B. Use of statistical experimental methods for optimization of collagenolytic protease production by Bacillus cereus strain SUK grown on fish scales. Environ. Sci. Pollut. Res. 2018;25:28226–28236. doi: 10.1007/s11356-018-2859-4. [DOI] [PubMed] [Google Scholar]
- 28.Muralidhar R.V., Chirumamila R., Marchant R., Nigam P. A response surface approach for the comparison of lipase production by Candida cylindracea using two different carbon sources. Biochem. Eng. J. 2001;9:17–23. [Google Scholar]
- 29.Geweely N.S., Nawar L.S. Production, optimization, purification and properties of uricase isolated from some fungal flora in Saudi Arabian soil. Aust. J. Basic and Appl. Sci. 2011;5:220–230. [Google Scholar]