Abstract
Background and purpose
The goal of this study was to investigate the rate and associated factors of Transient Ischemic Attack (TIA) misdiagnosis.
Methods
We retrospectively analyzed consecutive patients with an initial diagnosis of TIA in the emergency department (ED) in a 9-month period. All hospitalized TIA patients were evaluated by a neurologist within 24 h and had at least one hospital discharge follow-up visit within three months. Patients' clinical data and neuroimaging were reviewed. The final diagnosis was independently verified by two stroke neurologists.
Results
Out of 276 patients with the initial diagnosis of TIA, 254 patients (mean age 68.7 ± 15.4 years, 40.9% male, 25.2% final diagnosis of TIA) were included in the analysis. Twenty-four patients (9.4%) were referred to our rapid-access TIA clinic. The rate of TIA misdiagnosis among TIA clinic referred patients was 45.8%. Among the 230 patients in inpatient setting, the rate of TIA misdiagnosis was 60.0%. A hospital discharge diagnosis of TIA was observed in 54.3% of hospitalized patients; however, only 24.8% had the final diagnosis of TIA. Among hospitalized patients, the univariate analysis suggests a significant difference (P < .05) between the two groups (correctly versus misdiagnosed patients) in terms of hospital discharge diagnosis, final diagnosis, history of diabetes mellitus, and coronary artery disease. In regression model hospital discharge diagnosis (P < .001), final diagnosis (P < .001), and diabetes mellitus (P = .018) retained independent association with TIA misdiagnosis.
Conclusion
Our study indicates a high rate of TIA misdiagnosis in the emergency department, hospital, and outpatient clinics.
Keywords: Transient ischemic attack, Misdiagnosis, Disease management, Follow-up study, TIA clinic
Highlights
-
•
We observed a high rate of TIA misdiagnosis in the ED, hospital, and outpatient clinics.
-
•
We did not observe any differences between correctly diagnosed and misdiagnosed TIAs in terms of ED presenting symptoms.
-
•
Small number of hospitalized TIA patients required an intervention or had a diagnosis that could justify hospitalization.
-
•
A rapid-access TIA clinic can relocate the care for low/medium-risk TIA patients from inpatient to outpatient setting.
1. Introduction
A diagnosis of transient ischemic attack (TIA) is heavily dependent on the patient's history and risk factors. TIA is described as a transient focal neurologic dysfunction that caused by ischemic etiology in brain, spinal cord or retina; in the absence of acute infarction [1]. However, confirming or ruling out the diagnosis of a TIA can be challenging due to the subjective nature of findings in most patients [2]. Resolution of TIA signs and symptoms by the time of emergency department (ED) evaluation creates a substantial diagnosis obstacle [3].
The ABCD2 scoring system has been introduced as a useful clinical tool to risk stratify TIA patients and identify patients who have a higher risk for a subsequent stroke [4]. However, many in-hospital clinical providers do not regularly apply this scoring system and request a complete work-up for every patient with a suspicious diagnosis of TIA [5]. Accurate diagnosis or suspicious of TIA has become essential in stroke prevention due to the correlation between a TIA event and subsequent stroke [6]. However, TIA overdiagnosis may expose a higher number of patients to unnecessary diagnostic and therapeutic measures such as CT angiography, carotid endarterectomy, and antithrombotic agents [7]. Given a high estimated incidence rate of TIA in the United States, ranging from 200,000 to 500,000 patients per year [8], a high rate of misdiagnosis can turn into a health system challenge [9]. Furthermore, It has been shown that TIA may reduce patient survival rate up to 4% in the first year after index event and also up to 20% in a long-term 9 years follow up course [10].
While other studies have described signs and symptoms which may correlate with misdiagnosis of stroke [11], there is limited knowledge regarding the actual rate of TIA misdiagnosis and associated clinical factors. In this study, we aimed to investigate the rate and associated factors of TIA misdiagnosis in our health system.
2. Materials and methods
We retrospectively analyzed consecutive patients who received an initial diagnosis of TIA in the ED in one of our three tertiary stroke centers or our single TIA clinic in central and northeast Pennsylvania during a 9-month period. Our integrated healthcare system includes an extensive network of hospitals and clinics which serves a large population of around 90% white ethnicity with over one million active patients in central and northeast Pennsylvania.
We included patients who were hospitalized with the admission diagnosis of TIA in one of our three tertiary stroke centers or patients who were referred to our dedicated TIA clinic with the referral diagnosis of TIA. Per our TIA protocol, every patient who presentes to the emergency room with TIA-like symptoms should be admitted for at least 24 h observation. Medically stable patients with ABCD2 score of 3 or less without any acute imaging findings as well as patients who had their onset of symptoms >72 h before the hospital or primary care presentaion could be referred to our rapid access TIA clinic.
Every patient had an MRI (magnetic resonance imaging), including DWI (diffusion-weighted imaging), within 3 h to 48 h after symptoms onset, that was negative for an acute or subacute stroke or other acute central nervous system lesions. Each patient had presented with a transient focal neurological deficit that lasted <24 h. All hospitalized TIA patients were initially evaluated by an ED physician and subsequently by a general neurologist within 24 h. None of the neurologists who initially visited TIA patients in the ED were involved in the final diagnosis making process. Every patient had at least one hospital discharge follow-up visit with a board-certified neurologist or vascular neurologist within three months. The majority of the patients had a follow-up visit at our hospital-discharge stroke clinic; however, several patients had follow-ups in general neurology or primary care offices. Patients who did not have brain MRI or an outpatient follow-up visit were excluded from this study. We manually reviewed all the patients' baseline characteristics including demographics, initial presenting symptoms, vascular risk factors, as well as clinical work-up. We also reviewed DWI, FLAIR, T2*-weighted gradient recalled echo (GRE) sequences and CT scan.
We recorded the hospital discharge diagnosis and final diagnosis for every suspected TIA patient who was admitted to the hospital. The hospital discharge diagnosis were recorded as “TIA Mimics”, “possible TIA” and “TIA” based on the patients' discharge summary, neurology consultation notes, and problem list. The hospital discharge diagnosis was recorded as “TIA Mimics” for cases where the inpatient neurology provider ruled out the diagnosis of TIA or considered that as unlikely due to other convincing diagnoses. Furthermore, the hospital discharge diagnosis was recorded as “possible TIA” when the diagnosis of TIA could not be ruled out and other possible differential diagnoses (e.g., seizure, migraine headache) were equally considered.
The final diagnosis was made independent of the hospital discharge diagnosis. Among patients who had a hospital discharge follow-up visit outside of our hospital-discharge stroke clinic, the final diagnosis was made by consensus between our stroke research fellow (AS), and one of our vascular neurologists (NE) who reviewed the cases independently. For remaining patients who were seen in our hospital-discharge stroke clinic by one of our vascular neurologists, the final diagnosis was indepedently verified by our stroke research fellow based on all clinical information. In either situation when there was not a consensus a second vascular neurologist (RZ) reviewed the case and acted as a tiebreaker. When the final diagnosis of TIA could not fully be excluded, the diagnosis was marked as “possible”. We acknowledge that TIA does not have reliable clinical biomarkers and it is a clinical diagnosis. Therefore, we extensively reviewed every patient's demographic, past medical history, neuroimaging, and other clinical data in order to make or verify the final diagnosis. We also carefully reviewed the patients initial symptoms, sequence of the evenets, duration of symptoms, the nature of symptoms (focal vs. non-focal), corresponding vascular territory, anatomy of symptoms, associated symptoms, and other possible diffrenetial diagnoses to verify the final diagnosis (Table 1). Correctly diagnosed patients included patients in whom the final diagnosis was consistent with discharge diagnosis. Otherwise the patient was labeled as misdiagnosed. The collected data underwent a quality review by the corresponding author (RZ). Correctly diagnosed patients included patients in whom the final diagnosis was consistent with discharge diagnosis. Otherwise the patient was labeled as misdiagnosed (Table 2). The Institutional Review Board of Geisinger approved this study; written informed consent was waived.
Table 1.
Clinical and imaging elements considered for TIA diagnosis.
| Clinical and imaging elements considered for TIA diagnosis |
| Age |
| Vascular risk factors (hypertension, elevated lipids, etc) |
| Onset (sudden/gradual/stuttering) |
| Duration of symptoms (minutes to hours) |
| Focal symptoms |
| Global symptoms (loss of consciousness, confusion) |
| Single versus multiple events (interval, last event) |
| Stereotyped versus variable |
| Vascular territory |
| Other medical history (seizure, migraine headache, atrial fibrillation etc.) |
| Associated symptoms |
| MRI brain (previous ischemic lesion, microangiopathy, cerebral microbleeds) |
| Cerebral vascular imaging |
| Echocardiogram |
| Heart monitoring |
Table 2.
Definition of specific terms.
| Terms | Definition |
|---|---|
| Admission diagnosis | The initial diagnosis of admitted patients from emergency department - Every patient in this study including patients who referred to rapid access TIA clinic had the admission diagnosis of TIA |
| Discharge diagnosis | The diagnosis of patients' discharge form |
| Final diagnosis | The diagnosis made after patients' follow-up course in hospital-discharge stroke clinic and also following extensive review of patients' neuroimaging results and clinical findings by our stroke team |
| Correctly diagnosed patients | Patients in whom the final diagnosis was consistent with discharge diagnosis |
| Misdiagnosed patients | Patients in whom the final diagnosis was different from discharge diagnosis |
2.1. Statistical analysis
We summarized all continuous variables as mean ± SD (normal distribution) and as median with IQR (skewed distribution). We summarized all categorical variables as percentages with their corresponding 95% CIs. We performed statistical comparisons between two groups using the χ2 test or, in the case of small expected frequencies, Fisher's exact test. We compared continuous variables using the unpaired two-sample t-test. P˂0.05 was considered statistically significant. We used a multiple logistic regression model to evaluate potential associations between the TIA misdiagnosis (a binary dependent variable) and associated factors. We used a cut-off of P < .2 for selecting variables for the regression model. We also performed a stepwise regression analysis using all collected clinical and investigation data. We used SPSS 24.0 (Chicago, Ill., USA) for all our statistical analysis.
3. Results
Out of 276 patients who were initially evaluated for this study, 22 patients with unavailable outpatient follow-up data or brain MRI were excluded from the study. There was no significant difference between included and excluded patients in terms of age, gender, and racial distribution. A total of 254 patients (mean age: 68.7 ± 15.4 years old, 40.9% men, 95.7% Caucasians, 25.2% final diagnosis of TIA) with the admission diagnosis of TIA were included in our statistical analysis (Table 3).
Table 3.
Demographic and characteristics of studied cohort.
| Patients, no (%) | 254 |
| Gender, male, no (%) | 104 (40.9%) |
| Age, mean ± SD | 68.75 ± 15.42 |
| Race | |
| White | 243 (95.7%) |
| Black or African-American | 6 (2.4%) |
| Hispanic | 4 (1.6%) |
| Unavailable | 1 (0.4%) |
| Outpatients, no (%) | 24 (9.45%) |
| Inpatients, no (%) | 230 (90.55%) |
| Final diagnosis of TIA, no (%) | 64 (25.2%) |
| Misdiagnosis (Outpatient), no (%) | 11 (45.8%) |
| Misdiagnosis (Inpatient), no (%) | 138 (60.0%) |
Out of 254 patients, 164 patients (71.3%) were evaluated or had a hospital discharge follow-up in our hospital-discharge stroke clinic. The remaining (28.7%) had a hospital discharge follow-up either in general neurology clinic or a primary care office. The inter-rater agreement for the final diagnosis of TIA was 80.9% (κ = 0.62).
3.1. Misdiagnosis among patients referred directly to our outpatient rapid access TIA clinic
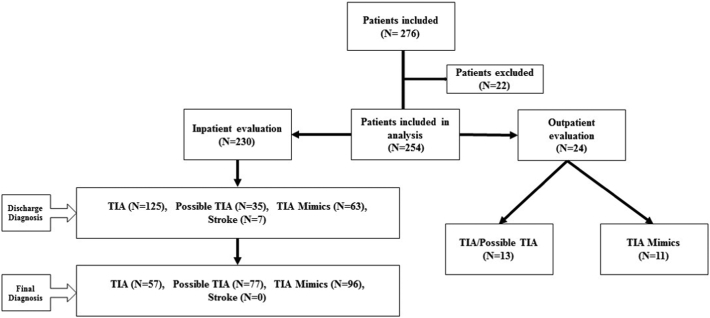
Out of 254 patients, 24 patients (9.4%) were directly referred by primary care physicians to our TIA clinic. Of them, 13 (54.2%) patients had the final diagnosis of TIA or possible TIA. The rest of the patient were diagnosed with TIA mimics (Fig. 1).
Fig. 1.
Hospital discharge diagnosis and final diagnosis for our patients.
3.2. Misdiagnosis among hospitalized TIA patients
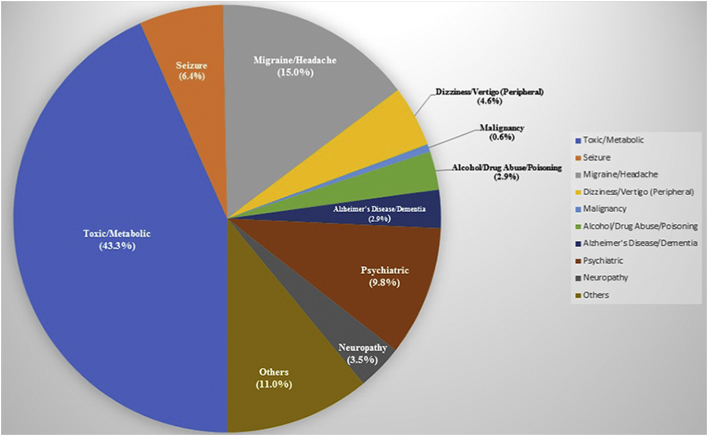
Among 230 patients who were hospitalized with an admission diagnosis of TIA, 125 (54.3%) patients were discharged with a diagnosis of TIA (discharge diagnosis). However, only 57 (24.8%) retained this diagnosis after being seen in hospital-discharge stroke clinic (final diagnosis) (Fig. 1). Only 92 (40.0%) patients had a final diagnosis that was the same as the discharge diagnosis. That refers to a misdiagnosis rate of 60.0%. Overall 96 (37.8%) patients had the final diagnosis of TIA mimics. Fig. 2 represents main alternative discharge diagnoses for all studied inpatients and outpatients in our cohort.
Fig. 2.
Main alternative discharge diagnoses for all studied inpatients and outpatients in our cohort.
Of the 230 hospitalized patients only seven patients required an intervention (cerebral angiography, cardiac catheterization, gastro-esophageal endoscopy, carotid stent placement, and carotid endarterectomy) that justified a hospitalization. None of the procedures were done urgently (within 24-h). None of the patients had an acute proximal cerebral large vessel occlusion. Four patients were found to have atrial fibrillation on the heart monitor during the hospitalization. We also observed that 25.0% of patients in whom the diagnosis of TIA was excluded were still carrying the TIA diagnosis in their problem list at the time of this study.
3.3. Associated factors with TIA misdiagnosis
Among hospitalized patients (N = 230), the univariate analysis suggests a significant difference (P < .05) between the two groups (correctly diagnosed versus misdiagnosed patients) in terms of hospital discharge diagnosis, final diagnosis, history of diabetes mellitus, and coronary artery disease (Table 4).
Table 4.
Univariate analysis comparing correctly diagnosed and misdiagnosed TIA patients.
| Correctly diagnosed (N = 92) | Misdiagnosed (N = 138) | Total (N = 230) | P-value | |
|---|---|---|---|---|
| Final TIA diagnosis | ||||
| TIA mimics | 41 (44.6%) | 55 (39.8%) | 96 | ˂0.0001⁎ |
| Possible TIA | 8 (8.7%) | 69 (50.0%) | 77 | |
| TIA | 43 (46.7%) | 14 (10.1%) | 57 | |
| aABCD2 (score > 3) | 55 (59.8%) | 77 (55.8%) | 132 | 0.549 |
| Gender (male) | 38 (41.3%) | 56 (40.6%) | 94 | 0.913 |
| Race | ||||
| White | 87 (94.6%) | 133 (96.4%) | 220 | 0.472 |
| Black or African American | 4 (4.3%) | 2 (1.4%) | 6 | |
| Hispanic | 1 (1.1%) | 2 (1.4%) | 3 | |
| Unavailable | 0 (0.0%) | 1 (0.8%) | 1 | |
| Length of hospital residence | ||||
| 1 day | 36 (39.1%) | 57 (41.3%) | 93 | 0.737 |
| 2 days | 31 (33.7%) | 50 (36.2%) | 81 | |
| ≥3 days | 24 (26.1%) | 30 (21.7%) | 54 | |
| Social history | ||||
| Tobacco use | 24 (26.1%) | 31 (22.5%) | 55 | 0.597 |
| Alcohol use | 31 (33.7%) | 39 (28.3%) | 70 | 0.380 |
| Diabetes mellitus | 36 (39.1%) | 32 (23.2%) | 68 | 0.009⁎ |
| Hypertension | 76 (82.6%) | 102 (73.9%) | 178 | 0.122 |
| Atrial Fibrillation, PAFb, flutter | 16 (17.4%) | 17 (12.3%) | 33 | 0.282 |
| Hyperlipidemia | 74 (80.4%) | 107 (77.5%) | 181 | 0.599 |
| Previous history of stroke event | 26 (28.3%) | 28 (20.3%) | 54 | 0.157 |
| Previous history of TIA event | 23 (25.0%) | 34 (24.6%) | 57 | 0.913 |
| Coronary artery disease | 46 (50.0%) | 45 (32.6%) | 91 | 0.007⁎ |
| Peripheral vascular disease | 11 (11.9%) | 16 (11.6%) | 27 | 0.910 |
| Carotid disease | ||||
| <50% Stenosis | 42 (45.6%) | 66 (47.8%) | 108 | 0.307 |
| 50–70% Stenosis | 10 (10.9%) | 15 (10.9%) | 25 | |
| >70% Stenosis | 5 (5.4%) | 2 (1.4%) | 7 | |
| Seizure disorder | 7 (7.6%) | 7 (5.1%) | 14 | 0.431 |
| History of migraine | 9 (9.8%) | 21 (15.2%) | 30 | 0.231 |
| COPD | 19 (20.6%) | 24 (17.4%) | 43 | 0.534 |
| Autoimmune disease | 2 (2.2%) | 2 (1.4%) | 4 | 0.680 |
| Coagulation disorders | 3 (3.3%) | 6 (4.3%) | 9 | 0.669 |
| History of cancer | 23 (25.0%) | 23 (16.6%) | 46 | 0.167 |
| Psychiatric illness | 34 (36.9%) | 59 (42.7%) | 93 | 0.380 |
| Kidney disease | 19 (20.6%) | 32 (23.2%) | 51 | 0.650 |
| Currently on dialysis | 0 (0.0%) | 1 (100%) | 1 | 0.413 |
| Antiplatelet (pre-hospitalization) | 55 (59.8%) | 75 (54.3%) | 130 | 0.363 |
| Oral anticoagulant (pre-hospitalization) | 13 (14.1%) | 9 (6.5%) | 22 | 0.055 |
| Altered mental status | 19 (20.6%) | 29 (21.0%) | 48 | 0.980 |
| Headache | 22 (23.9%) | 27 (19.6%) | 49 | 0.430 |
| Loss of consciousness | 1 (1.1%) | 2 (1.4%) | 3 | 0.812 |
| aABCD2 duration | ||||
| ˂10 Minutes | 13 (14.1%) | 17 (12.3%) | 30 | 0.909 |
| 10–59 Minutes | 20 (21.7%) | 32 (23.2%) | 52 | |
| ≥60 Minutes | 59 (64.1%) | 89 (64.5%) | 148 | |
| Seizure like activity | 2 (2.2%) | 3 (2.2%) | 5 | 1.000 |
P˂0.05 was considered statistically significant.
ABCD2: (Age, Blood pressure, Clinical, Duration, Diabetes).
PAF: Paroxysmal Atrial Fibrillation.
Correctly diagnosed patients included patients in whom the final diagnosis was consistent with discharge diagnosis. Otherwise the patient was labeled as misdiagnosed. We did not observe any significant differences between the two groups regarding the primary presenting TIA symptoms (unilateral arm weakness, unilateral leg weakness, aphasia, dysarthria, facial droop, unilateral arm numbness, unilateral leg numbness, facial numbness, sudden true vertigo, diplopia, ataxia, hemianopia visual disturbance, and mono-ocular blindness).
The following variables were selected for inclusion in the multiple logistic regression model based on their significance (P < .20) on univariable analysis: discharge diagnosis, final diagnosis, diabetes mellitus disease, hypertension, history of stroke, cancer, and coronary arterial disease, as well as taking oral anticoagulants before the event. In our regression model three variables including discharge diagnosis (P < .001), final diagnosis (P < .001), and diabetes mellitus disease (P = .018) retained their independent association with TIA misdiagnosis.
We also performed a stepwise regression analysis using all collected clinical and investigation data. The results of our stepwise regression were similar to the multiple logistic regression model however the latter selected the history of coronary arterial disease (P = .008). The model excluded all the initial TIA symptoms.
4. Discussion
Our study suggests a high rate of TIA misdiagnosis in inpatient or outpatient setting. In our cohort, only 25% of patients had the final diagnosis of TIA. <50% of hospitalized TIA patients who were discharged with the diagnosis of TIA, had the same final diagnosis. This high rate of misdiagnosis could be attributed to various factors. Former studies have shown a relatively low rate of consensus on TIA diagnosis among physicians [2]. This low level of agreement might be related to physicians' different level of clinical experience, subjective nature of TIA diagnosis in the absence of any reliable biomarkers, as well as differences in TIA definition [12]. TIA diagnosis relies heavily on the patients' history of present illness which is not always easy to obtain thoroughly specially in the emergency setting. At the same time, not all the TIA related work-up results are available before the hospital discharge; therefore, outpatient follow-up in a designated stroke/TIA clinic might result in a different diagnosis. The combination of the above and the fact that TIA patients have a higher risk of future cerebral ischemic events [13], contribute to TIA misdiagnosis. Although the costs of TIA misdiagnosis are asymmetrical, since misdiagnosis of a TIA patient as non-TIA has a significantly different consequence than incorrectly hospitalizing a relatively healthy patient, misdiagnosis of TIA is associated with unnecessary admission and diagnostic procedures. It also causes more anxiety and stress for patients and their families. Similar to this study, we previously showed that there is a relatively high rate of stroke misdiagnosis in the emergency department [14,15] that can be associated with significant cost burden [16].
A well-organized rapid access TIA clinic can relocate the care for low to medium-risk TIA patients from an inpatient hospital setting to an outpatient setting. There has been evidence that this practice is safe, cost-effective [[17], [18], [19], [20], [21], [22], [23]], and may improve the outcome [24]. Our results also indicated that only a small number of patients (4.3%) required an intervention or had a diagnosis (atrial fibrillation while on a heart monitor) that could have justified an inpatient hospitalization. Nevertheless, none of the procedures were done urgently, and probably the same plan of care could have been arranged in an outpatient setting. Our experience from our new TIA clinic in Geisinger has indicated that this practice is feasible. Establishment of a TIA clinic may help to reduce the rate of TIA misdiagnosis and improve the outcome as the patients are evaluated urgently by a TIA expert team and will have a rapid diagnostics and early initiation of treatment and secondary prevention. This should be emphasized that many of the patients with the referral diagnosis of TIA will have a different final diagnosis and the timely recognition of alternative diagnosis can also be important.
We observed that the problem list had not been corrected in 25% of patients. The way that the patient problem list is documented in the medical record is of high importance. Generally, the problem list is a determinant of patient's active health concerns and provides physicians with a clinical clue for the planning of accurate treatment steps [25]. Former studies have emphasized the value of patient problem list for promotion of patient health care [26,27] and its practical usage in the prevention of erroneous therapeutic plans [28].
Although other studies have reported that TIA symptoms might be helpful in differentiation of TIA from non-TIA lesions [29,30], we did not observe any differences between correctly diagnosed patients and patients in misdiagnosis group in terms of emergency department presenting symptoms. Similar to the previous report [7], we did not find statistically meaningful differences considering ABCD2 score between correctly diagnosed patients and patients in misdiagnosis group. The latter finding supports the idea that such clinical risk definition tools should be applied to correctly selected patients instead of a general tool for screening all patients in emergency departments.
In our study, the final diagnosis was the most significant factor associated with misdiagnosis of TIA. Patients with the final diagnosis of TIA had a significantly lower rate of misdiagnosis. The overdiagnosis of TIA is the main reason for this observation. It is also evident that the rate of misdiagnosis depends on the disease classification and definition. For example, if we combine the two diagnoses (possible TIA and TIA) the misdiagnosis rate among hospitalized patients in our study will drop to 35.7%. In any case, obtaining a detailed history and appropriate work-up while considering other risk factors for a TIA or TIA mimics can reduce the rate of TIA misdiagnosis.
Our study had several limitations. First, this study had a retrospective design; although, the clinical and neuroimaging data were collected prospectively. Therefore, there is a potential risk for selection bias. Second, while our study provides the largest cohort on this topic so far, it is a single-system study. Although we studied a large inpatient cohort, the number of patients whom were primarily evaluated in our TIA clinic was small. We were not able to reliably extract patients whom might have had primary presenting TIA symptoms but were missed in initial admission evaluation, as well.
In conclusion, our study indicates a high rate of TIA misdiagnosis in the emergency department, hospital, and outpatient primary clinics. Patients with the final diagnosis of TIA had a significantly lower rate of misdiagnosis.
Statement of ethics
The study protocol has been approved by the research institute's committee on human research.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Acknowledgements
This research work was part of a larger research study which was funded through Bucknell Geisinger Research Initiative (BGRI).
Contributor Information
Alireza Sadighi, Email: alireza.sadighi@gmail.com.
Alia Stanciu, Email: acs023@bucknell.edu.
Mihai Banciu, Email: mmb018@bucknell.edu.
Vida Abedi, Email: vabedi@geisinger.edu.
Nada El Andary, Email: nelandary@geisinger.edu.
Neil Holland, Email: nholland1@geisinger.edu.
Ramin Zand, Email: ramin.zand@gmail.com.
References
- 1.Sorensen A.G., Ay H. Transient ischemic attack: definition, diagnosis, and risk stratification. Neuroimaging Clin. N. Am. 2011;21(2):303–313. doi: 10.1016/j.nic.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sorensen AG, Ay H. Transient Ischemic Attack: Definition, Diagnosis, and Risk Stratification. Neuroimaging Clin N Am. 2011;21(2):303-313. doi:10.1016/j.nic.2011.01.013 [DOI] [PMC free article] [PubMed]
- 2.Castle J., Mlynash M., Lee K. Agreement regarding diagnosis of transient ischemic attack fairly low among stroke-trained neurologists. Stroke. 2010;41(7):1367–1370. doi: 10.1161/STROKEAHA.109.577650. [DOI] [PubMed] [Google Scholar]; Castle J, Mlynash M, Lee K, et al. Agreement regarding diagnosis of transient ischemic attack fairly low among stroke-trained neurologists. Stroke. 2010;41(7):1367-1370. doi:10.1161/STROKEAHA.109.577650 [DOI] [PubMed]
- 3.Sheehan O.C., Merwick A., Kelly L.A. Diagnostic usefulness of the ABCD2 score to distinguish transient ischemic attack and minor ischemic stroke from noncerebrovascular events: the North Dublin TIA study. Stroke. 2009;40(11):3449–3454. doi: 10.1161/STROKEAHA.109.557074. [DOI] [PubMed] [Google Scholar]; Sheehan OC, Merwick A, Kelly LA, et al. Diagnostic usefulness of the ABCD2 score to distinguish transient ischemic attack and minor ischemic stroke from noncerebrovascular events: the North Dublin TIA Study. Stroke. 2009;40(11):3449-3454. doi:10.1161/STROKEAHA.109.557074 [DOI] [PubMed]
- 4.Johnston S.C., Rothwell P.M., Nguyen-Huynh M.N. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet (London, England) 2007;369(9558):283–292. doi: 10.1016/S0140-6736(07)60150-0. [DOI] [PubMed] [Google Scholar]; Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet (London, England). 2007;369(9558):283-292. doi:10.1016/S0140-6736(07)60150-0 [DOI] [PubMed]
- 5.Nadarajan V., Perry R.J., Johnson J., Werring D.J. Transient ischaemic attacks: mimics and chameleons. Pract. Neurol. 2014;14(1):23–31. doi: 10.1136/practneurol-2013-000782. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nadarajan V, Perry RJ, Johnson J, Werring DJ. Transient ischaemic attacks: mimics and chameleons. Pract Neurol. 2014;14(1):23-31. doi:10.1136/practneurol-2013-000782 [DOI] [PMC free article] [PubMed]
- 6.Chandratheva A., Mehta Z., Geraghty O.C., Marquardt L., Rothwell P.M. Oxford vascular study. Population-based study of risk and predictors of stroke in the first few hours after a TIA. Neurology. 2009;72(22):1941–1947. doi: 10.1212/WNL.0b013e3181a826ad. [DOI] [PMC free article] [PubMed] [Google Scholar]; Chandratheva A, Mehta Z, Geraghty OC, Marquardt L, Rothwell PM, Oxford Vascular Study. Population-based study of risk and predictors of stroke in the first few hours after a TIA. Neurology. 2009;72(22):1941-1947. doi:10.1212/WNL.0b013e3181a826ad [DOI] [PMC free article] [PubMed]
- 7.Prabhakaran S., Silver A.J., Warrior L., McClenathan B., Lee V.H. Misdiagnosis of transient ischemic attacks in the emergency room. Cerebrovasc. Dis. 2008;26(6):630–635. doi: 10.1159/000166839. [DOI] [PubMed] [Google Scholar]; Prabhakaran S, Silver AJ, Warrior L, McClenathan B, Lee VH. Misdiagnosis of Transient Ischemic Attacks in the Emergency Room. Cerebrovasc Dis. 2008;26(6):630-635. doi:10.1159/000166839 [DOI] [PubMed]
- 8.Roger V.L., Go A.S., Lloyd-Jones D.M. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]; Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2-e220. doi:10.1161/CIR.0b013e31823ac046 [DOI] [PMC free article] [PubMed]
- 9.Benjamin E.J., Virani S.S., Callaway C.W. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]; Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137(12):e67-e492. doi:10.1161/CIR.0000000000000558 [DOI] [PubMed]
- 10.Gattellari M., Goumas C., Garden F., Worthington J.M. Relative survival after transient ischaemic attack: results from the program of research informing stroke management (PRISM) study. Stroke. 2012;43(1):79–85. doi: 10.1161/STROKEAHA.111.636233. [DOI] [PubMed] [Google Scholar]; Gattellari M, Goumas C, Garden F, Worthington JM. Relative survival after transient ischaemic attack: results from the Program of Research Informing Stroke Management (PRISM) study. Stroke. 2012;43(1):79-85. doi:10.1161/STROKEAHA.111.636233 [DOI] [PubMed]
- 11.Tarnutzer A.A., Lee S.-H., Robinson K.A., Wang Z., Edlow J.A., Newman-Toker D.E. ED misdiagnosis of cerebrovascular events in the era of modern neuroimaging: a meta-analysis. Neurology. 2017;88(15):1468–1477. doi: 10.1212/WNL.0000000000003814. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tarnutzer AA, Lee S-H, Robinson KA, Wang Z, Edlow JA, Newman-Toker DE. ED misdiagnosis of cerebrovascular events in the era of modern neuroimaging: A meta-analysis. Neurology. 2017;88(15):1468-1477. doi:10.1212/WNL.0000000000003814 [DOI] [PMC free article] [PubMed]
- 12.Ferro J.M., Falcão I., Rodrigues G. Diagnosis of transient ischemic attack by the nonneurologist. A validation study. Stroke. 1996;27(12):2225–2229. doi: 10.1161/01.str.27.12.2225. [DOI] [PubMed] [Google Scholar]; Ferro JM, Falcão I, Rodrigues G, et al. Diagnosis of transient ischemic attack by the nonneurologist. A validation study. Stroke. 1996;27(12):2225-2229. doi:10.1161/01.STR.27.12.2225 [DOI] [PubMed]
- 13.Lee W., Frayne J. Transient ischaemic attack clinic: an evaluation of diagnoses and clinical decision making. J. Clin. Neurosci. 2015;22(4):645–648. doi: 10.1016/j.jocn.2014.09.020. [DOI] [PubMed] [Google Scholar]; Lee W, Frayne J. Transient ischaemic attack clinic: An evaluation of diagnoses and clinical decision making. J Clin Neurosci. 2015;22(4):645-648. doi:10.1016/j.jocn.2014.09.020 [DOI] [PubMed]
- 14.Giraldo E.A., Khalid A., Zand R. Safety of intravenous thrombolysis within 4.5 h of symptom onset in patients with negative post-treatment stroke imaging for cerebral infarction. Neurocrit. Care. 2011;15(1):76–79. doi: 10.1007/s12028-011-9523-x. [DOI] [PubMed] [Google Scholar]; Giraldo E a., Khalid A, Zand R. Safety of intravenous thrombolysis within 4.5 h of symptom onset in patients with negative post-treatment stroke imaging for cerebral infarction. Neurocrit Care. 2011;15(1):76-79. doi:10.1007/s12028-011-9523-x [DOI] [PubMed]
- 15.Zand R., Jacewicz M., Al-Wafai A. Misdiagnosis of stroke among IV tPA-treated patients: a two-year experience in a primary stroke center and satellite hospitals. Stroke. 2012;43:A2869. [Google Scholar]; Zand R, Jacewicz M, Al-Wafai A. Misdiagnosis of Stroke among IV tPA-treated Patients: A Two-year Experience in a Primary Stroke Center and Satellite Hospitals. Stroke. 2012;43:A2869.
- 16.Goyal N., Male S., Al Wafai A., Bellamkonda S., Zand R. Cost burden of stroke mimics and transient ischemic attack after intravenous tissue plasminogen activator treatment. J. Stroke Cerebrovasc. Dis. 2015;24(4):828–833. doi: 10.1016/j.jstrokecerebrovasdis.2014.11.023. [DOI] [PubMed] [Google Scholar]; Goyal N, Male S, Al Wafai A, Bellamkonda S, Zand R. Cost Burden of Stroke Mimics and Transient Ischemic Attack after Intravenous Tissue Plasminogen Activator Treatment. J Stroke Cerebrovasc Dis. 2015;24(4):828-833. doi:10.1016/j.jstrokecerebrovasdis.2014.11.023 [DOI] [PubMed]
- 17.Martínez-Martínez M.M., Martínez-Sánchez P., Fuentes B. Transient ischaemic attacks clinics provide equivalent and more efficient care than early in-hospital assessment. Eur. J. Neurol. 2013;20(2):338–343. doi: 10.1111/j.1468-1331.2012.03858.x. [DOI] [PubMed] [Google Scholar]; Martínez-Martínez MM, Martínez-Sánchez P, Fuentes B, et al. Transient ischaemic attacks clinics provide equivalent and more efficient care than early in-hospital assessment. Eur J Neurol. 2013;20(2):338-343. doi:10.1111/j.1468-1331.2012.03858.x [DOI] [PubMed]
- 18.Yaghi S., Willey J.Z., Khatri P. Minor ischemic stroke. Neurol. Clin. Pract. 2016;6(2):157–163. doi: 10.1212/CPJ.0000000000000234. [DOI] [PMC free article] [PubMed] [Google Scholar]; Yaghi S, Willey JZ, Khatri P. mMinor ischemic stroke. Neurol Clin Pract. 2016;6(2):157-163. doi:10.1212/CPJ.0000000000000234 [DOI] [PMC free article] [PubMed]
- 19.Joshi J.K., Ouyang B., Prabhakaran S. Should TIA patients be hospitalized or referred to a same-day clinic?: a decision analysis. Neurology. 2011;77(24):2082–2088. doi: 10.1212/WNL.0b013e31823d763f. [DOI] [PubMed] [Google Scholar]; Joshi JK, Ouyang B, Prabhakaran S. Should TIA patients be hospitalized or referred to a same-day clinic?: a decision analysis. Neurology. 2011;77(24):2082-2088. doi:10.1212/WNL.0b013e31823d763f [DOI] [PubMed]
- 20.Amarenco P., Lavallée P.C., Labreuche J. One-year risk of stroke after transient ischemic attack or minor stroke. N. Engl. J. Med. 2016;374(16):1533–1542. doi: 10.1056/NEJMoa1412981. [DOI] [PubMed] [Google Scholar]; Amarenco P, Lavallée PC, Labreuche J, et al. One-Year Risk of Stroke after Transient Ischemic Attack or Minor Stroke. N Engl J Med. 2016;374(16):1533-1542. doi:10.1056/NEJMoa1412981 [DOI] [PubMed]
- 21.Majidi S., Guerrero C.R.L., Burger K.M., Rothrock F. Inpatient versus outpatient management of TIA or minor stroke : clinical outcome. J. Vasc. Interv. Neurol. 2017;9(4):49–53. [PMC free article] [PubMed] [Google Scholar]; Majidi S, Guerrero CRL, Burger KM, Rothrock F. Inpatient versus Outpatient Management of TIA or Minor Stroke : Clinical Outcome. J Vasc Interv Neurol. 2017;9(4):49-53. [PMC free article] [PubMed]
- 22.Luengo-Fernandez R., Gray A.M., Rothwell P.M. Effect of urgent treatment for transient ischaemic attack and minor stroke on disability and hospital costs (EXPRESS study): a prospective population-based sequential comparison. Lancet Neurol. 2009;8(3):235–243. doi: 10.1016/S1474-4422(09)70019-5. [DOI] [PubMed] [Google Scholar]; Luengo-Fernandez R, Gray AM, Rothwell PM. Effect of urgent treatment for transient ischaemic attack and minor stroke on disability and hospital costs (EXPRESS study): a prospective population-based sequential comparison. Lancet Neurol. 2009;8(3):235-243. doi:10.1016/S1474-4422(09)70019-5 [DOI] [PubMed]
- 23.Rothwell P.M., Giles M.F., Chandratheva A. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370(9596):1432–1442. doi: 10.1016/S0140-6736(07)61448-2. [DOI] [PubMed] [Google Scholar]; Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370(9596):1432-1442. doi:10.1016/S0140-6736(07)61448-2 [DOI] [PubMed]
- 24.Hermanson S., Vora N., Brown W., DeVoe S.J., Edwards A., Isenberg N. Transient ischemic attack (TIA) rapid-access clinic: improving outcomes and reducing costs. Neurology. 2017;88(16 Supplement) P6.266. [Google Scholar]; Hermanson S, Vora N, Brown W, DeVoe SJ, Edwards A, Isenberg N. Transient Ischemic Attack (TIA) Rapid-Access Clinic: Improving outcomes and reducing costs. Neurology. 2017;88(16 Supplement):P6.266.
- 25.Holmes C., Brown M., Hilaire D.S., Wright A. Healthcare provider attitudes towards the problem list in an electronic health record: a mixed-methods qualitative study. BMC Med. Inform. Decis. Mak. 2012;12 doi: 10.1186/1472-6947-12-127. [DOI] [PMC free article] [PubMed] [Google Scholar]; Holmes C, Brown M, Hilaire DS, Wright A. Healthcare provider attitudes towards the problem list in an electronic health record: a mixed-methods qualitative study. BMC Med Inform Decis Mak. 2012;12:127. doi:10.1186/1472-6947-12-127 [DOI] [PMC free article] [PubMed]
- 26.Hartung D.M., Hunt J., Siemienczuk J., Miller H., Touchette D.R. Clinical implications of an accurate problem list on heart failure treatment. J. Gen. Intern. Med. 2005;20(2):143–147. doi: 10.1111/j.1525-1497.2005.40206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hartung DM, Hunt J, Siemienczuk J, Miller H, Touchette DR. Clinical implications of an accurate problem list on heart failure treatment. J Gen Intern Med. 2005;20(2):143-147. doi:10.1111/j.1525-1497.2005.40206.x [DOI] [PMC free article] [PubMed]
- 27.Simborg D.W., Starfield B.H., Horn S.D., Yourtee S.A. Information factors affecting problem follow-up in ambulatory care. Med. Care. 1976;14(10):848–856. doi: 10.1097/00005650-197610000-00005. http://www.ncbi.nlm.nih.gov/pubmed/1085849 [DOI] [PubMed] [Google Scholar]; Simborg DW, Starfield BH, Horn SD, Yourtee SA. Information factors affecting problem follow-up in ambulatory care. Med Care. 1976;14(10):848-856. http://www.ncbi.nlm.nih.gov/pubmed/1085849. [DOI] [PubMed]
- 28.Wright A., Goldberg H., Hongsermeier T., Middleton B. A description and functional taxonomy of rule-based decision support content at a large integrated delivery network. J. Am. Med. Inform. Assoc. 2007;14(4):489–496. doi: 10.1197/jamia.M2364. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wright A, Goldberg H, Hongsermeier T, Middleton B. A description and functional taxonomy of rule-based decision support content at a large integrated delivery network. J Am Med Inform Assoc. 2007;14(4):489-496. doi:10.1197/jamia.M2364 [DOI] [PMC free article] [PubMed]
- 29.Wijman C.A., Wolf P.A., Kase C.S., Kelly-Hayes M., Beiser A.S. Migrainous visual accompaniments are not rare in late life: the Framingham study. Stroke. 1998;29(8):1539–1543. doi: 10.1161/01.str.29.8.1539. http://www.ncbi.nlm.nih.gov/pubmed/9707189 [DOI] [PubMed] [Google Scholar]; Wijman CA, Wolf PA, Kase CS, Kelly-Hayes M, Beiser AS. Migrainous visual accompaniments are not rare in late life: the Framingham Study. Stroke. 1998;29(8):1539-1543. http://www.ncbi.nlm.nih.gov/pubmed/9707189. [DOI] [PubMed]
- 30.Johnston S.C., Sidney S., Bernstein A.L., Gress D.R. A comparison of risk factors for recurrent TIA and stroke in patients diagnosed with TIA. Neurology. 2003;60(2):280–285. doi: 10.1212/01.wnl.0000042780.64786.ef. [DOI] [PubMed] [Google Scholar]; Johnston SC, Sidney S, Bernstein AL, Gress DR. A comparison of risk factors for recurrent TIA and stroke in patients diagnosed with TIA. Neurology. 2003;60(2):280-285. doi:10.1212/01.WNL.0000042780.64786.EF [DOI] [PubMed]