Abstract
Background
Non-surgical topical therapies have been assessed in the treatment of precancerous lesions of the cervix. Their use can offer logistical and feasibility advantages in low-resource settings. Antiviral AV2® is a mixture of natural essential oils (eugenol, carvone, nerolidol, geraniol) in olive oil, and has a broad spectrum anti-viral activity. In a phase II randomized controlled trial (RCT), AV2® proved effective in reducing the size of cervical lesions associated with human papillomavirus (HPV). The purpose of the present study was to further evaluate the efficacy of AV2 over placebo in the topical treatment of HPV-associated cervical lesions.
Methods
Women aged 25 years and older were included in this phase 3 RCT. Cytology screening, HPV testing and visual inspection of the cervix with 5% acetic acid (VIA) were performed on all participants. VIA-positive women were randomized to one of two groups to receive treatment by either AV2® or placebo. The treatment consisted of 2 puffs of spray of the investigational drug directed to the cervix. Participants were subjected to repeat examinations two months and six months later for assessment of outcomes. The primary outcome was the change of lesions on VIA at 2 months after application of the investigational drug. Secondary outcomes were: HPV clearance and cytologic regression at 2 months and 6 months, and number of participants with AEs.
Results
A total 327 VIA positive women were randomized in two groups (168 in AV2 group and 159 in placebo group). Women in the 2 groups were similar with respect to baseline demographics and clinical characteristics. At 2 months, regression of lesions on VIA was observed in 127 (89.4%) out of 142 women in AV2 group compared to 120 (91.6%) out of 131 women in placebo group (P = 0.7). On cytology, regression of lesions occurred in 14 (56%) out of 25 women in the AV2 arm and in 13 (48.1) out of 27 women in the placebo arm (p = 0.7), and HPV clearance rates were 34.1% and 35% in AV2 group and placebo group respectively (p = 0.8). At 6 months cytologic regression was observed in 64.7% of women in AV2 group and 45.8% in placebo group (p = 0.2), while HPV clearance occurred in 11 (51.9%) out of 17 women in AV2 arm versus 11 (34.4%) in placebo arm (p = 0.3).
Some local side effects (burning, itching, irritation) were similarly noted in the 2 groups (p-values = 0.169, 0.623 and 0.172 respectively) but they were mild and transitory.
Conclusion
A topical application of AV2 onto the cervix can induce the regression of cervical precancerous lesions, but its efficacy does not significantly differ with that of placebo. The discrepancy between the expected and the recorded sample size as well as the huge number of lost to follow-up probably impeded the power of analyses, which could be one of the reasons for the lack of difference seen between AV2 and placebo. Further evaluation of the effects of AV2 with different diagnostic methods and treatment regimen and arms is warranted.
Clinical trial registration
NCT02346227 registered on November 8, 2014;
Keywords: Human papillomavirus, Cervical precancerous lesions, Antiviral drug, Randomized clinical trial, Democratic Republic of the Congo
1. Introduction
Cervical cancer (CC) is a major health challenge as it is the most frequent female genital tract cancer worldwide. Annually, about 530.000 new cases are diagnosed worldwide with over 80% presenting with advanced stages of disease. Sub-Saharan Africa (SSA) shares a high burden of this disease [1,2]. The main etiologic agent for CC is infection by Human Papillomavirus (HPV), which is mostly transmitted sexually. The biological explanation of association between HPV infection and CC is persistence of infection for at least more than 6 months [3]. Cervical cancer is basically a preventable disease and several countries have utilized this opportunity to reduce its burden. In general, the preventive strategies can be broadly divided into primary prevention (HPV vaccination), secondary prevention (screening and treatment of precancerous lesions) and tertiary prevention (early treatment of cervical cancer). Secondary level prevention remains the mainstay of prevention in SSA where vaccination is not fully available. It aims to detect and treat asymptomatic women with precancerous lesions. Secondary prevention here relate to the prevention of cervical cancer by means of identification and treatment of HPV-related precancerous lesions which, according to their natural history, can progress gradually through several years into invasive cervical cancer if left untreated. Cytology screening (or Papanicolaou smear) has been the method of choice with high sensitivity and moderate specificity. Western countries have used Pap smear to drastically reduce the burden of CC during the last five decades [4]. But Low-and Middle-Income Countries (LMICs) have not been able to implement it on an organized basis due to several factors such as the scarcity of cytotechnicians and the lack of infrastructure. Thus, visual inspection of cervix with acetic acid (VIA) has been recommended by the World Health Organization (WHO) as an alternative screening strategy for LMICs where feasibility of cytology is limited [5]. VIA is cheap and easy to perform procedure, not requiring highly specialized skills. Its sensitivity is comparable with that of cytology, with a high negative predictive value [6]. According to the screen and treat (SAT) approach, lesions detected on VIA can be treated during the same visit. Several procedures (conization, electroexcision, laser vaporization, and cryotherapy) have been used to surgically remove cervical precancerous lesions. These procedures have been associated with worse surgical (excessive bleeding) and obstetrical outcomes such as cervical insufficiency, preterm birth with associated perinatal morbidity [7]. Cryotherapy is the procedure recommended in low-resource settings [8,9]. Unfortunately, it is frequently hampered by the unavailability of refrigerant gas (carbone dioxide or nitrous oxygen). Consequently, an interest has been put on alternative topical drugs which can be applied onto the cervix to induce regression of precancerous lesions without resorting to surgical methods. These drugs include 5-Fluorouracil, imiquimod, cidofovir, retinoic acid, beta and alpha-Interferon [[10], [11], [12], [13], [14], [15]]. These substances may play a tremendous role in secondary prevention of cervical cancer. Recently, an antiviral drug named AV2® (Cesa Alliance, Luxembourg), has been proposed for the same purpose. It is a mixture of FDA-approved natural essential oil components (carvone, eugenol, geraniol, nerolidol) in equal volumes diluted 50% in olive oil (Olea europea). It is postulated that, with a broad-spectrum antiviral activity, AV2® deactivates the virus outside the cell by preventing endocytosis. In effect, cervical Infection by papillomaviruses requires that virus particles (virions) gain access to the epithelial basal layer and enter basal cells. The assembly and release of new infectious virions takes place in the epithelial surface, possibly causing lesions. The antiviral drug AV2 is thought to interrupt this process, causing rapid disruption of the viral assembly, preventing permanent production of infectious virions, resulting in the rapid clearing of the cervical lesions. It could get rid of the virus and the underlying precancerous cells. Cervical lesions may then regress due to deactivation of the virus [16]. In a phase 2 randomized controlled trial, Martinez et al. concluded that AV2 was effective in inducing the regression of cervical lesions. In effect, their results showed that the application of AV2 yielded a reduction of more than 50% of the lesion size at colposcopy for 21 out of 28 (75%) patients who received the active treatment versus a 0% for the comparable placebo group (p < 0.001) [17]. This small-scaled study provided the rationale for further research on the true impact of AV2. Therefore, the present study was planned with the main objective of assessing the efficacy of AV2® in the treatment of HPV-associated precancerous lesions of the cervix.
2. Subjects and methods
The study design and methods for the present randomized, double-blind, placebo-controlled phase 3 trial (KINVAV study) have been described in detail previously [16]. The Institutional Ethical Committees of both the University of Kinshasa School of Public Health and the Antwerp University Hospital (Antwerp, Belgium) reviewed and approved the protocol. The study was registered in ClinicalTrials.gov (NCT02346227).
2.1. Participants
The study population consisted of women attending a cervical cancer screening programme at Mont-Amba Health Centre, in Kinshasa, Democratic Republic of (DRC) between July 2015 and July 2017. The centre, located at the southern part of Kinshasa, is surrounded by suburban areas where the health condition is poor with lack of organized cervical cancer screening programme.
2.2. Eligibility criteria
The target population comprised all women aged 25 years old and over, with an intact uterus, regardless of their screening history. A written informed consent was mandatory to enter the programme. There was no upper age limit because cervical cancer is often seen at older ages and this was the first opportunity for women in this community to benefit from a screening. These women were of different background and locations allowing a representability sufficient to extrapolate the results to the population.
Virgin women, pregnant and breast-feeding women, women with an antecedent of hysterectomy, women with known or suspected cervical cancer, women with debilitating diseases (such as HIV/AIDS, tuberculosis, hepatitis, cardiopathy), women who were taking an antiviral drug for any other condition, women with known or suspected allergic or adverse response to the investigational product or its components, and women unable to follow the study protocol were not enrolled.
2.3. Interventions
Prior to the start of the study, the involved doctors were trained using the IARC Manuals in the collection of cervical cells for HPV testing and cytologic testing and in performing VIA and cryotherapy [18,19]. The study personnel also followed a Good Clinical Practice (GCP) course to enhance the quality of assessments.
A community health worker instructed the women about the causes, signs, prevention, and treatment of cervical cancer. The procedure and eventual treatments of the present study were also explained to the women. Once a written informed consent was obtained, a nurse interviewed the participant with respect to sociodemographic and reproductive characteristics, using a structured questionnaire. Each participant was then examined by a trained medical doctor. While lying in the lithotomy position, a bivalve speculum was inserted into the vagina to expose the cervix. The examiner first collected a liquid-based cytology (LBC) sample using a dedicated brush (Cervex-Brush® Combi, Rovers® Medical Devices). The LBC sample was stored in Preservcyt® solution (Hologic, Marlborough, USA) at room temperature between 15° Celsius and 30° Celsius.
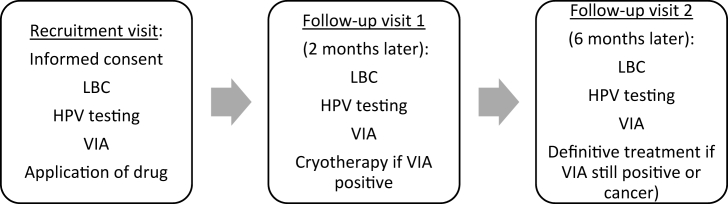
VIA was immediately performed following the collection of LBC sample. According to the WHO criteria, positive VIA was defined as the finding of a thick, well-defined acetowhite cervical lesion adjacent to or contiguous with the squamocolumnar junction [18,19,21]. VIA results served for randomization. VIA-positive women were randomly allocated in one of the two treatment groups and received either AV2® or placebo. The composition of the investigational drug has been described in the study protocol [16]. All randomized women were recalled in two months for the first follow-up visit and in six months for the final follow-up visits. Reminders consisted of phone calls or home visit, depending on the preference of the participant. LBC, HPV testing, and VIA were performed at each follow-up visit. If VIA was still positive, a standard treatment by cryotherapy was offered to the woman. In case of suspected cancer, the woman was referred for proper management according to standard protocols and local resources (Fig. 1). LBC sample served for both cytology and HPV testing. These analyses were performed at Algemen Medical Laboratory (AML, BVBA, Antwerp, Belgium) which is part of HPV national reference Centre in Belgium. HPV testing was processed using the Riatol qPCR test (AML, BVBA, Antwerp, Belgium), a HPV polymerase chain reaction test. Cytology results were reported according to the 2001 Bethesda system [20]. Results for women with atypical squamous cells of undetermined significance (ASCUS), atypical glandular cells (AGC), low-grade squamous intraepithelial lesions (LSIL), atypical squamous cells cannot exclude HSIL (ASCH), high-grade squamous intraepithelial lesions (HSIL), adenocarcinoma and squamous cell carcinoma were colloquially defined as positive cytology. Cytology and HPV results were not available at the time of randomization and served for further analyses at the end of the study.
Fig. 1.
Scheduling of the trial.
2.4. Quality assurance
Quality of assessments was monitored by medical supervision and by regular refresher courses intended to maintain the performance of health care provider. Internal and external quality-control measures were set for HPV testing and cytological analysis.
2.5. Sample size
Assuming a lesion regression rate of 50% in the non-interventional group and 70% in the antiviral drug group at 2 months post-treatment, a minimum sample size of 163 patients was needed to show with 95% power a difference between both groups. To allow for up to 10% drop-outs or unevaluable patients, we planned to recruit at least 190 patients in each arm. We expected 20% of the women to be HPV positive, of whom 10% with and 10% without lesions, indicating that over 1900 women had to be enrolled to obtain the required sample size.
2.6. Randomization and blinding
The therapeutic units were allocated by randomization in a block wise manner, without stratification, using a computer program generating series of numbers.
Participants, study personnel and outcomes assessors were blinded to the treatment arm allocation. To ensure blinding, glasses containing either active drug or placebo were identical in appearance and scent. The randomization codes should not be revealed until all participants had completed their follow-up, or extremely in case of a suspected serious AE.
2.7. Outcomes
The main outcome was the change of lesions on VIA 2 months after application of the drug. Change of lesions was defined as regression (partial or complete) or persistence/progression of the lesion seen on VIA. Complete regression, also called remission, is defined as a total disappearance or improvement of the lesion.
Secondary outcomes were: HPV clearance at 2 months and at 6 months post-randomization, regression of lesions on cytology at 2 months and at 6 months, and number of participants with AEs.
On cytology, regression is defined as improvement from any lesion to normal. Cytology results at follow-up visits were split into a dichotomous variable indicating regression of lesion versus persistence or progression of lesion.
HPV clearance (also termed as virologic regression) is defined as improvement from HPV positive to HPV negative.
2.8. Statistical considerations
Data were double-entered and cleaned in Epidata software (The “EpiData Association”, Odense, Denmark). Proportions of all categorical variables were compared between the active and the placebo groups using the chi-square test or the Fisher exact test when required.
The link between the outcomes and group status (active vs placebo) was determined using the chi-square test or the Fisher exact test (when required). For all analyses, p-values ≤ 0.05 were considered statistically significant. The analyses were completed according to the intention-to-treat (ITT) principle. They were performed including the total number of patients with available information for each specific end point. All statistical analyses were carried out under Stata SE version 15 (Stata Corp, Lakeway, College Station, Texas, USA).
3. Results
3.1. Participants flow
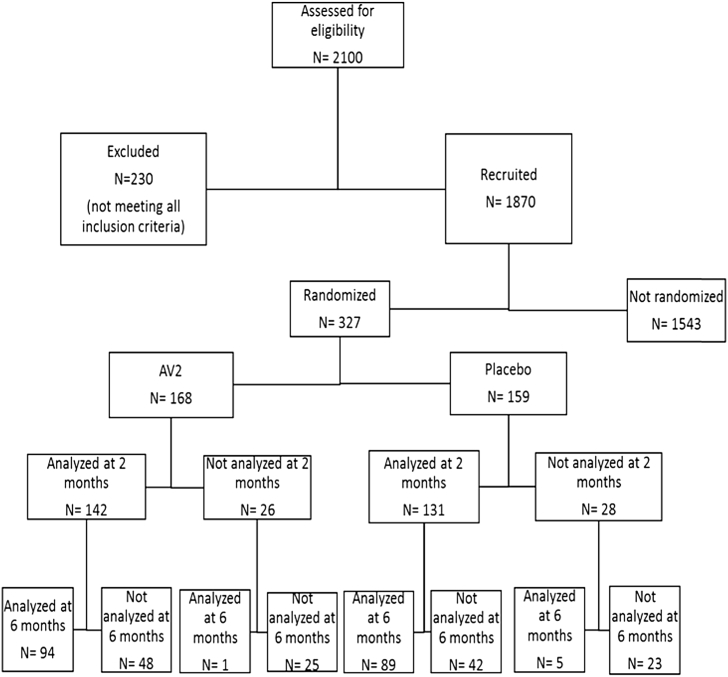
Of the 1870 eligible participants received throughout the study, 327 were randomized (168 allocated in the AV2 group and 158 in the placebo group). Out of the 327 participants who had data at baseline, 273 (83.4%) and 189 (58%) had data at 2 months and at 6 months respectively and were included in the analysis.
At the 2-month stage, 54 participants (26 in AV2 group vs 28 in placebo group) were not assessed (6 got pregnant, 6 went on a trip away from the city mainly for professional purposes, and 42 have voluntarily dropped out the study for multiple unstated reasons and cultural influences despite reminders and counselling by the study personnel). At the 6-month stage, 138 participants (73 in AV2 group vs 65 in placebo group) were lost to follow-up (2 were pregnant, 62 voluntarily withdrawn from the study and 74 were unreachable). (Fig. 2).
Fig. 2.
Flowchart showing the progress through the study.
3.2. Participants characteristics
Baseline demographics and characteristics of participants in each randomization group are summarized in Table 1. There were no statistically significant differences between the AV2 and placebo groups. This demonstrates that the randomization procedure had successfully led to comparability between trial groups. The median age of participants was 42 years in both groups (P = 0.53). The groups were comparable in regard to the following characteristics related to HPV acquisition or CIN development: marital status, menopausal status, parity, age at first intercourse, number of lifetime sexual partners, oral contraceptives use and vaginal use of traditional herbs.
Table 1.
Characteristics of participants.
| Demographics | Randomization group |
|||||
|---|---|---|---|---|---|---|
| Active |
Placebo |
P value |
||||
| n = 168 | % | n = 159 | % | |||
| Age | ||||||
| 25–29 | 27 | 51.9 | 25 | 48.1 | 0.53 | |
| 30–39 | 48 | 52.7 | 43 | 47.3 | ||
| 40–49 | 50 | 48.5 | 53 | 51.5 | ||
| 50–59 | 36 | 58.1 | 26 | 41.9 | ||
| 60+ | 7 | 36.8 | 12 | 63.2 | ||
| Mean age | 41.6 (±10.6) | 42.4 (±11.1) | ||||
| Marital status | ||||||
| Single | 39 | 48.1 | 42 | 51.9 | 0.13 | |
| Married | 110 | 55.6 | 88 | 44.4 | ||
| Separated | 9 | 50.0 | 9 | 50.0 | ||
| Widowed | 10 | 33.3 | 20 | 66.7 | ||
| Education | ||||||
| None | 7 | 58.3 | 5 | 41.7 | 0.52 | |
| Primary | 20 | 44.4 | 25 | 55.6 | ||
| Secondary | 97 | 54.5 | 81 | 45.5 | ||
| Superior | 44 | 47.8 | 48 | 52.2 | ||
| Profession | ||||||
| None | 61 | 57.5 | 45 | 42.5 | 0.68 | |
| Remunerated | 70 | 53.8 | 60 | 46.2 | ||
| Non-remunerated | 37 | 52.1 | 34 | 47.9 | ||
| Gynecologic history | ||||||
| Menopausal status | ||||||
| No | 124 | 53.9 | 106 | 46.1 | 0.16 | |
| Yes | 44 | 45.4 | 53 | 54.6 | ||
| Pregnancies | ||||||
| 0 | 6 | 30.0 | 14 | 70.0 | 0.05 | |
| 1 | 19 | 40.4 | 28 | 59.6 | ||
| 2–4 | 53 | 58.2 | 38 | 41.8 | ||
| 5+ | 90 | 53.3 | 79 | 46.7 | ||
| Parity | ||||||
| 0 | 24 | 43.6 | 31 | 56.4 | 0.05 | |
| 1 | 19 | 38.8 | 30 | 61.2 | ||
| 2–4 | 60 | 60.6 | 39 | 39.4 | ||
| 5+ | 65 | 52.4 | 59 | 47.6 | ||
| Abortions | ||||||
| 0 | 85 | 52.5 | 77 | 47.5 | 0.93 | |
| 1 | 55 | 50.5 | 54 | 49.5 | ||
| 2+ | 28 | 50.0 | 28 | 50.0 | ||
| Miscarriages | ||||||
| 0 | 104 | 50.7 | 101 | 49.3 | 0.12 | |
| 1 | 35 | 45.5 | 42 | 54.5 | ||
| 2+ | 29 | 64.4 | 16 | 35.6 | ||
| Sexual history | ||||||
| Age at first intercourse | ||||||
| 10–19 | 125 | 53.0 | 111 | 47.0 | 0.57 | |
| 20–29 | 40 | 46.5 | 46 | 53.5 | ||
| 30–39 | 3 | 60.0 | 2 | 40.0 | ||
| Lifetime sexual partners | ||||||
| 1 | 46 | 48.4 | 49 | 51.6 | 0.51 | |
| 2–5 | 106 | 52.7 | 95 | 47.3 | ||
| 6–10 | 15 | 55.6 | 12 | 44.4 | ||
| 11–19 | 0 | 0.0 | 2 | 100.0 | ||
| 20–29 | 1 | 100.0 | 0 | 0.0 | ||
| 40+ | 0 | 0.0 | 1 | 100,0 | ||
| Oral contraceptives use | ||||||
| No | 135 | 51.5 | 127 | 48.5 | 0.91 | |
| Yes | 33 | 50.8 | 32 | 49.2 | ||
| Condoms use | ||||||
| No | 94 | 54.7 | 78 | 45.3 | 0.21 | |
| Yes | 74 | 47.7 | 81 | 52.3 | ||
| Use of traditional herbs | ||||||
| No | 75 | 51.0 | 72 | 49.0 | 0.91 | |
| Yes | 93 | 51.7 | 87 | 48.3 | ||
3.3. Overall results
3.3.1. Baseline results
At baseline, VIA was positive in 327 women (168 in AV2 group and 159 in Placebo group). Among them, 104 (31.8%) were positive for HPV (52 in AV2 group and 52 in Placebo group) and only 67 (20.4%) had demonstrated a positive cytology. On cytology, ASCUS was found in 31 (9.4%) women (16 in AV2 group vs 15 in placebo group), ASCH in 4 (1.2%) women (2 in AV2 group vs 2 in placebo group), AGC in 1 (0.3%), LSIL in 9 (2.7%) women (5 in AV2 group vs 4 in placebo group), ASCH in 4 (1.2%) women (2 in AV2 group vs 2 in placebo group), HSIL in 19 (5.8%) women (9 in AV2 group vs 10 in placebo group), adenocarcinoma in 1 woman (0.3%), and squamous cell carcinoma in 2 women (0.6%). Among the 67 women with positive cytology, 38 (56.7%) were HPV positive while 65 (26.3%) women with normal cytology were also HPV positive.
The patterns of loss to follow-up was similar in the two treatment groups (26 women in AV2 group versus 28 women in placebo group at 2 months and 73 women in AV2 group versus 65 women in placebo group at 6 months). These results are summarized in Table 2.
Table 2.
VIA, Cytology and HPV results at baseline, 2 months and 6 months of participants enrolled in a RCT of AV2 versus placebo in Kinshasa, DRC.
| Baseline |
2 months |
6 months |
|||||
|---|---|---|---|---|---|---|---|
| Variable | AV2 | Placebo | AV2 | Placebo | AV2 | Placebo | |
| VIA | n = 168 (%) | n = 159 (%) | n = 142 (%) | n = 131 (%) | n = 95 (%) | n = 94 (%) | |
| Positive | 168 (51.4) | 159 (48.6) | 27 (19) | 25 (19.1) | 7 (7.4) | 9 (9.6) | |
| Negative | – | – | 115 (81) | 106 (80.9) | 88 (92.6) | 85 (90.4) | |
| Cytology | n = 168 | n = 159 | n = 142 | n = 131 | n = 95 | n = 94 | |
| NILM | 131 (77.9) | 116 (72.9) | 104 (73.2) | 94 (71.7) | 67 (70.5) | 60 (63.8) | |
| ASCUS | 16 (9.5) | 15 (9.4) | 5 (3.5) | 7 (5.3) | 5 (5.2) | 13 (13.8) | |
| AGC | 0 (0.0) | 1 (0.6) | 2 (1.4) | 0 (0.0) | 0 (0.0) | 2 (2.1) | |
| LSIL | 5 (2.9) | 4 (2.5) | 10 (7.0) | 9 (6.8) | 7 (7.3) | 4 (4.2) | |
| ASCH | 2 (1.1) | 2 (1.2) | 5 (3.5) | 5 (3.8) | 6 (6.3) | 2 (2.1) | |
| HSIL | 9 (5.3) | 10 (6.2) | 7 (4.9) | 8 (6.1) | 7 (7.3) | 9 (9.5) | |
| ADENO | 0 (0.0) | 1 (0.6) | 1 (0.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| SCC | 1 (0.6) | 1 (0.6) | 1 (0.7) | 3 (2.2) | 1 (1.0) | 2 (2.1) | |
| Uknown | 4 (2.3) | 9 (5.6) | 7 (4.9) | 5 (3.8) | 2 (2.1) | 2 (2.1) | |
| HPV result | n = 168 | n = 159 | n = 142 | n = 131 | n = 95 | n = 94 | |
| Positive | 52 (31) | 52 (32.7) | 36 (25.4) | 36 (27.5) | 22 (23.2) | 33 (35.1) | |
| Negative | 116 (69) | 107 (67.3) | 106 (74.6) | 95 (72.5) | 73 (76.8) | 61 (64.9) | |
3.3.2. Two months outcomes
-
-
Change of lesions on VIA
According to Table 3, 127 women (89.4%) out of 142 participants in AV2 group and 120 (91.6%) women out of 131 participants in placebo group had regression of lesion size at the 2-month follow-up. There was no statistically significant difference in regression rates between the two groups (p = 0.7).
-
-
Cytologic regression
Table 3.
Regression rates of cervical lesions and HPV clearance rate at 2 months and 6 months for participants enrolled in a RCT of AV2 versus placebo in Kinshasa, DRC.
| 2 Months |
6 Months |
|||||
|---|---|---|---|---|---|---|
| AV2 | Placebo | AV2 | Placebo | |||
| VIA | n = 142 (%) | n = 131 (%) | P | n = 95 (%) | n = 94 (%) | P |
| Partial regression | 12 (8.5) | 14 (10.7) | 0.7 | 4 (4.2) | 4 (4.3) | 0.7 |
| Complete regression | 115 (80.9) | 106 (80.9) | 88 (92.6) | 85 (90.4) | ||
| Unchanged or progression | 15 (10.6) | 11 (8.4) | 3 (3.2) | 5 (5.3) | ||
| Cytology | n = 25 (%) | n = 27 (%) | n = 17 (%) | n = 24 (%) | ||
| Regression | 14 (56) | 13 (48.1) | 0.7 | 11 (64.7) | 11 (45.8) | 0.3 |
| Unchanged or progression | 11 (44) | 14 (51.9) | 6 (35.3) | 13 (54.2) | ||
| HPV infection | n = 41 (%) | n = 40 (%) | n = 27 (%) | n = 32 (%) | ||
| Complete clearance | 11 (26.8) | 14 (35) | 0.8 | 10 (37) | 11 (34.4) | 0.2 |
| Partial clearance | 3 (7.3) | 0 (0.0) | 4 (14.8) | 0 (0.0) | ||
| Persistence or new appearance | 27 (65.9) | 26 (65) | 13 (48.1) | 22 (68.8) | ||
Out of 52 women with positive cytology at baseline, only 27 (52%) returned to normal. The cytologic regression rate was 56% in AV2 group compared to 48.1% in placebo group (p = 0.7).
-
-
Evolution of HPV infection
Virologic clearance occurred in 11 out of 41 women (34.1%) in AV2 group compared to 14 out of 40 women (35%) in placebo group (p = 0.8). HPV infection persisted in 27 women (66%) in AV2 group and in 26 women (65%) in placebo group.
3.3.3. Six months outcomes
-
-
Change of lesions on VIA
At 6 months, the regression rates were 96.8% and 94.7% in AV2 group and in placebo group respectively (P = 0.7).
-
-
Change of lesions on cytology
Cytologic regression was evident for 11 out of 17 women (64.7%) in AV2 group versus 11 out of 24 women (45.8%) in placebo group (p = 0.3).
-
-
HPV clearance
Virologic regression was seen in 14 (51.9%) out of 27 women who were HPV positive at baseline in the AV2 group and in 11 (34.4%) out of 32 women in the placebo group (p = 0.2). Rates of HPV persistence were high in both groups (48.1% vs 62.5%).
3.3.4. Adverse events
No severe adverse event was reported during the trial. Some participants experienced a burning/itching/irritation sensation which are common local side effects described after application of the topical drug. They are reported in Table 4. For the occurrence of each of the local side effects, there was no significant difference between the two groups of treatment.
Table 4.
Adverse local events.
| AV2 |
Placebo |
Total |
P-value |
|
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Burning: | 0.169 | |||
| No | 106 (48.8) | 111(51.2) | 217(100) | |
| Yes | 62 (56.4) | 48(43.6) | 110(100) | |
| Itching: | 0.623 | |||
| No | 161(51.1) | 154(48.9) | 315(100) | |
| Yes | 7(58.3) | 5(41.7) | 12(100) | |
| Irritation: | 0.172 | |||
| No | 93(55.0) | 76(45.0) | 169(100) | |
| Yes | 75(47.5) | 83(52.5) | 158(100) |
4. Discussion
Our study aimed at assessing the efficacy of AV2 over placebo in the regression of HPV-associated lesions of the cervix. It is the first in SSA to assess easy-to-apply topical biological agent in the treatment of HPV-associated cervical lesions.
A total of 327 women were included: 168 in the AV2 group and 159 in the placebo group. Originally, we planned to recruit at least 190 participants in each group. But we have not been able to attain these numbers due to unexpected very slow rates of recruitment, especially after the first six months of the study. We also faced a high proportion of lost to follow-up. One must recognize that in low-resource settings, implementation and conduct of clinical trials is a major challenge [[22], [23], [24], [25]]. In general, weak health systems, poverty, low literacy rates, religious and cultural beliefs, lack of inter-sectoral collaboration, war and civil strife, shortage of trained health care professionals constitute real barriers to cutting-edge clinical research in LMICs. It is very difficult to maintain sustainable contact with people for a long time when they need to be followed-up on a regular basis. For these reasons, the WHO recommended the see-and treat (SAT) approach for cervical cancer prevention in LMICs. The SAT approach involves that cervical lesions detected in a woman during a screening visit should be treated during the same visit.
Nonetheless, with 327 participants we were approximately near the target number and the external validity of our trial findings was improved. Our sample size was by far above that of most of the studies conducted in western countries with the same purpose of evaluating the efficacy of topical drugs in the treatment of precancerous lesions of the cervix [[10], [11], [12],15].
AV2 proved effective in resolving cervical lesions. The results were similar in both groups at all stages of the study. Statistically, the regressions rates were not significantly different from that observed in the placebo group. There are some possible reasons for the higher response rate to placebo. Firstly, the possible spontaneous regression of lesions (placebo effect); secondly, its ingredients (citrus Limon and citrus aurantifolia) may also have been biologically active. Considering these, the efficacy of AV2 should not be compromised because of similarity with placebo. The very similar regression patterns in both groups may suggest that the 2 products (active and placebo) were efficacious in clearing cervical lesions. This observation was also found in the study by Van Pachterbeke et al., a placebo-controlled trial evaluating a topical treatment for cervical intraepithelial neoplasia 2 and 3 using cidofovir [15].
As previously reported in the study by Martinez et al., the application of AV2 yielded a reduction of more than 50% for 21 out of 28 (75%) patients who received the active treatment versus 0% for the comparable placebo group. That small-scaled preliminary study did not provide any convincing proof for efficacy of AV2 [17]. it is a little disappointing that in a larger study, AV2 is not found superior to placebo. This may suggest that the regression of lesions in the present study could only have been the result of spontaneous regression of cervical lesions and has not been under the influence of the drug. On the other hand, active drug as well as placebo could have been active. In the study by Martinez et al., the drug was applied two times (day 1 and day 4 post-randomization) whereas in the present study we applied the drug only once at the time of randomization. In all other studies where a significant difference was found between active drug and placebo, the treatment consisted of multiple doses or applications of the topical drug. The difference in the treatment regimen (one-time application in the present study) could have constituted a bias.
One puff of the investigational drug delivers 100 μL (μl) of AV2. In our study we applied two puffs (200 μl) of AV2 once at the time of randomization. The effective dosage as well as the schedule have possibly been underestimated not leading to convincing results. Moreover, immediately after the application of two puffs of the spray, the woman stood up and was discharged. Was there an immediate leakage of spray mixed to cervical and vaginal fluids not permitting a sufficient contact with the diseased area of the cervix neither a longer period of action of the drug? This is another question that requires proper explanation. This leads to comprehend why in the study by Rahangdale et al., after the FluoroUracil cream was inserted into the vagina proximal to the cervix, participants placed a tampon per vagina overnight to keep the cream at the cervix. This procedure could have prevented the spillage of the product and then augmented the duration of action [13].
Another limitation of our study consisted in randomizing women based on a subjective test (VIA) although it has been proved effective in detecting precancerous lesions [21,26,27]. Unfortunately, we have not been able to enroll women based on more precise tests such as a positive HPV test or histological diagnosis of CIN. At the time of recruitment, we have not been able to perform HPV testing with immediate results due to lack of infrastructure. We only collected samples and sent them to external laboratory for cytological analyses and HPV testing. Availability of HPV results could have improved the results.
According to the pharmacist, once manufactured, the investigational drug can be stored for long period of time at room temperature. Therefore, denaturation of the product should be excluded as one of the reasons to explain probable inefficacy.
Timing for assessment of our primary outcome was short (possible bias), compared to other studies where primary outcome (CIN regression) was assessed at 3 months or beyond. For instance, in a RCT conducted by Meskens et al. in 1994, 32 cases (43%) of moderate dysplasia demonstrated complete histologic regression in the all-trans retinoic acid arm compared with 18 cases (27%) in the placebo arm (P = 0.041) at 27 months after study initiation [12].
On another note, HPV results at follow-up visits demonstrated a high proportion of persistent infections. Condoms use or abstinence from sexual activity during the follow-up period was not counselled to women. This lack resulted in bias concerning the dynamics of HPV infection since reinfections could not be avoided after a previous successful HPV clearance.
No serious AE was reported during our trial. A small proportion of women experienced local side effects. Sensations of burning (56% vs 44%), itching (58% vs 42%) and irritation (42.5% vs 57.5%) were not statistically different between the two groups (Table 4). It is important to evoke that the application of 5% acetic acid on the cervix may also provoke on some women the burning/itching/irritation sensation which is also transitory. It is then cumbersome to the distinguish with the feelings occasioned by the application of the investigational drug performed immediately after VIA. The combined effects of both 5% acetic acid and the investigational drug could explain the high proportion of women who experienced these side effects, since they could also be related to VIA.
As in the previous study by Martinez et al., AV2 and placebo were safely administered, well-tolerated and no participant reported serious adverse events [17]. Made of natural essential oils, AV2 is safe and at this point of view it presents an advantage over other topical drugs such as 5-Fluorouracil or Imiquimod.
5. Conclusion
AV2 has been proposed for topical treatment of HPV-associated cervical lesions, but it should be further evaluated seen all its advantages and the role it may play in cervical cancer prevention for low-resource settings. The discrepancy between the expected and the recorded sample size as well as the huge number of LTFU at 2 months and 6 months probably impeded the power of analyses, which could be one of the reasons for the lack of difference seen between AV2 and placebo. A large non-inferiority prospective randomized trial with 3 arms (AV2, placebo, and cryotherapy as standard treatment) or a larger study with 2 arms (AV2 & observation), using baseline HPV and cytology results for randomization, multiple doses of the topical drug, and longer follow-up period could help shed light on the true activity of AV2. In that perspective, the pharmacist manufacturer should be put in contribution in reviewing, if necessary, the concentrations of different compounds in both AV2 and placebo to enhance their respective specific activity without any interference.
Funding
This study was funded by a grant from the VLIR-UOS, Belgium (Grant reference ZRDC2014MP083).
Disclosure
The authors disclose no conflict of interest.
ABBREVIATIONS LIST
- AIDS
Acquired Immuno-Deficiency Syndrome
- AE
Adverse Effect
- AV2
Antiviral 2
- CC
Cervical Cancer
- CIN
Cervical Intraepithelial Neoplasia
- DRC
Democratic Republic of the Congo
- FDA
Food and Drug Administration
- GCP
Good Clinical Practice
- HIV
Human Immunodeficiency Virus
- HPV
Human Papilloma Virus
- HrHPV
High-risk HPV
- IEC
Institutional Ethics Committee
- IRB
Institutional Review Board
- LBC
Liquid-based cytology
- LMICs
Low and Middle-income countries
- LrHPV
Low-risk HPV
- RCT
Randomized Clinical Trial
- SAT
See-and-Treat
- SSA
Sub-Saharan Africa
- VIA
Visual inspection with 5% Acetic Acid
- WHO
World Health Organization
- VLIR
Vlaamse Interuniversitaire Raad (Flemish Interuniversity Council)
- Vs
versus
References
- 1.Ferlay J., Soerjomataram I., Dikshit R. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Parkin D.M., Bray F., Ferlay J., Jemal A. Cancer in Africa 2012. Cancer Epidemiol. Biomark. Prev. 2014;23:953–966. doi: 10.1158/1055-9965.EPI-14-0281. [DOI] [PubMed] [Google Scholar]
- 3.Wallin K.L., Wiklund F., Angstrom T. Type-specific persistence of human papillomavirus DNA before the development of invasive cervical cancer. N. Engl. J. Med. 1999;341:1633–1638. doi: 10.1056/NEJM199911253412201. [DOI] [PubMed] [Google Scholar]
- 4.Mathew A., George P.S. Trends in incidence and mortality rates of squamous cell carcinoma and adenocarcinoma of cervix--worldwide. Asian Pac. J. Cancer Prev. APJCP. 2009;10:645–650. [PubMed] [Google Scholar]
- 5.Organization W.H. 2013. WHO Guidelines for Screening and Treatment of Precancerous Lesions for Cervical Cancer Prevention. Geneva. [PubMed] [Google Scholar]
- 6.Zhao F.H., Lewkowitz A.K., Chen F. Pooled analysis of a self-sampling HPV DNA Test as a cervical cancer primary screening method. J. Natl. Cancer Inst. 2012;104:178–188. doi: 10.1093/jnci/djr532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arbyn M., Kyrgiou M., Simoens C. Perinatal mortality and other severe adverse pregnancy outcomes associated with treatment of cervical intraepithelial neoplasia: meta-analysis. BMJ. 2008;337:a1284. doi: 10.1136/bmj.a1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C., Yang Z., Li Z., Li L. Accuracy of several cervical screening strategies for early detection of cervical cancer: a meta-analysis. Int. J. Gynecol. Cancer. 2012;22:908–921. doi: 10.1097/IGC.0b013e318256e5e4. [DOI] [PubMed] [Google Scholar]
- 9.Luciani S., Gonzales M., Munoz S. Effectiveness of cryotherapy treatment for cervical intraepithelial neoplasia. Int. J. Gynaecol. Obstet. 2008;101:172–177. doi: 10.1016/j.ijgo.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Cinel A., Wittenberg L., Minucci D. Beta-interferon topical treatment in low and high risk cervical lesions. Clin. Exp. Obstet. Gynecol. 1991;18:91–97. [PubMed] [Google Scholar]
- 11.Grimm C., Polterauer S., Natter C. Treatment of cervical intraepithelial neoplasia with topical imiquimod: a randomized controlled trial. Obstet. Gynecol. 2012;120:152–159. doi: 10.1097/AOG.0b013e31825bc6e8. [DOI] [PubMed] [Google Scholar]
- 12.Meyskens F.L., Jr., Surwit E., Moon T.E. Enhancement of regression of cervical intraepithelial neoplasia II (moderate dysplasia) with topically applied all-trans-retinoic acid: a randomized trial. J. Natl. Cancer Inst. 1994;86:539–543. doi: 10.1093/jnci/86.7.539. [DOI] [PubMed] [Google Scholar]
- 13.Rahangdale L., Lippmann Q.K., Garcia K. Topical 5-fluorouracil for treatment of cervical intraepithelial neoplasia 2: a randomized controlled trial. Am. J. Obstet. Gynecol. 2014;210:314–318. doi: 10.1016/j.ajog.2013.12.042. e311. [DOI] [PubMed] [Google Scholar]
- 14.Ruffin M.T., Bailey J.M., Normolle D.P. Low-dose topical delivery of all-trans retinoic acid for cervical intraepithelial neoplasia II and III. Cancer Epidemiol. Biomark. Prev. 2004;13:2148–2152. [PubMed] [Google Scholar]
- 15.Van Pachterbeke C., Bucella D., Rozenberg S. Topical treatment of CIN 2+ by cidofovir: results of a phase II, double-blind, prospective, placebo-controlled study. Gynecol. Oncol. 2009;115:69–74. doi: 10.1016/j.ygyno.2009.06.042. [DOI] [PubMed] [Google Scholar]
- 16.Mutombo A.B., Tozin R., Simoens C. Efficacy of antiviral drug AV2 in the treatment of human papillomavirus-associated precancerous lesions of the uterine cervix: a randomized placebo-controlled clinical trial in Kinshasa, DR Congo. (KINVAV study) Contemp Clin Trials Commun. 2017;8:135–139. doi: 10.1016/j.conctc.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez G., Mutombo B.A., Fernandez V. A preliminary study for the treatment of cervical colposcopic lesions with the biological compound AV2. Eur. J. Gynaecol. Oncol. 2017;38:342–345. [PubMed] [Google Scholar]
- 18.Sankaranarayanan R.W.R. IARC Press; Lyon, France: 2003. A Practical Manual on Visual Screening for Cervical Neoplasia. [Google Scholar]
- 19.Sellors J.W.R.S.R. International Agency for Research on Cancer; Lyon, France: 2003. Colposcopy and Treatment of Cervical Intraepithelial Neoplasia: A Beginners' Manual. [Google Scholar]
- 20.Solomon D., Davey D., Kurman R. The 2001 Bethesda System: terminology for reporting results of cervical cytology. J. Am. Med. Assoc. 2002;287:2114–2119. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 21.Sankaranarayanan R., Nessa A., Esmy P.O., Dangou J.M. Visual inspection methods for cervical cancer prevention. Best Pract. Res. Clin. Obstet. Gynaecol. 2012;26:221–232. doi: 10.1016/j.bpobgyn.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Bonsu J.M., Frasso R., Curry A.E. Lessons from the field: the conduct of randomized controlled trials in Botswana. Trials. 2017;18:503. doi: 10.1186/s13063-017-2237-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeGregorio G., Manga S., Kiyang E. Implementing a fee-for-service cervical cancer screening and treatment program in Cameroon: challenges and opportunities. Oncol. 2017;22:850–859. doi: 10.1634/theoncologist.2016-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Randall T.C., Ghebre R. Challenges in prevention and care delivery for women with cervical cancer in sub-saharan Africa. Front Oncol. 2016;6:160. doi: 10.3389/fonc.2016.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang S.M., Qiao Y.L. Implementation of cervical cancer screening and prevention in China--challenges and reality. Jpn. J. Clin. Oncol. 2015;45:7–11. doi: 10.1093/jjco/hyu188. [DOI] [PubMed] [Google Scholar]
- 26.Sankaranarayanan R., Basu P., Wesley R.S. Accuracy of visual screening for cervical neoplasia: results from an IARC multicentre study in India and Africa. Int. J. Cancer. 2004;110:907–913. doi: 10.1002/ijc.20190. [DOI] [PubMed] [Google Scholar]
- 27.Sauvaget C., Fayette J.M., Muwonge R. Accuracy of visual inspection with acetic acid for cervical cancer screening. Int. J. Gynaecol. Obstet. 2011;113:14–24. doi: 10.1016/j.ijgo.2010.10.012. [DOI] [PubMed] [Google Scholar]