Abstract
Clinical trials using anti-vascular endothelial growth factor /(VEGF) molecules induce a modest improvement in overall survival, measurable in weeks to just a few months, and tumors respond differently to these agents. In this review article, we have exposed some tumor characteristics and processes that may impair the effectiveness of anti-angiogenic approaches, including genotypic changes on endothelial cells, the vascular normalization phenomenon, and the vasculogenic mimicry. The usage of anti-angiogenic molecules leads to hypoxic tumor microenvironment which enhances tumor invasiveness. The role of tumor-infiltrating cells, including tumor associated macrophages and fibroblasts (TAMs and TAFs) in the therapeutic response to anti-angiogenic settings was also highlighted. Finally, among the new therapeutic approaches to target tumor vasculature, anti-PD-1 or anti-PD-L1 therapy sensitizing and prolonging the efficacy of anti-angiogenic therapy, have been discussed.
Anti-Angiogenesis in Clinical Use
Anti-angiogenesis therapy started in 2004 (Table 1), with the approval by Food and Drug Administration (FDA) of the first anti-angiogenic drug, bevacizumab (Avastin), a humanized monoclonal antibody anti- VEGF-A, approved for the treatment of previously untreated metastatic colorectal cancer in combination with chemotherapy [1]. Bevacizumab can be given safely for long periods, with manageable toxicity when added to chemotherapy, even if it induces severe side effects, including lethal hemoptysis and intestinal perforations [2], [3]. The small molecule tyrosine kinase inhibitors (TKIs), including sunitinib, sorafenib, and pazopanib, are another class of anti-angiogenic drugs, able to interfere with vascular endothelial growth factor receptors-1 and -2 (VEGFR-1 and VEGFR-2), platelet derived growth factor receptor (PDGFR), fibroblast growth factor receptors (FGFRs), and Tie signaling (Table 2) [4]. VEGFR-trap protein aflibercept, formed by fusion of the VEGF binding domain of VEGFR-1 and VEGFR-2 has been approved for metastatic colorectal cancer [5].
Table 1.
Approved anti-angiogenic agents for cancer and their indications
| Monoclonal antibodies | Bevacizumab | - metastatic colorectal - cancer - non-small cell lung cancer - glioblastoma multiforme - renal cell carcinoma - epithelial ovarian cancer - fallopian tube cancer - primary peritoneal cancer - cervical cancer |
| Ramucirumab | - metastatic colorectal cancer - non-small cell lung cancer - gastric or gastroesophageal junction adenocarcinoma |
|
| Tyrosine kinase inhibitors (See Table 2) | ||
| Fusion protein | Ziv-aflibercept | - metastatic colorectal cancer |
Table 2.
Anti-angiogenic tyrosine kinase inhibitors in clinical development
| Agent | Target | Clinical activity and/or study |
|---|---|---|
| Sunitinib (SU11248; Sutent) | • VEGFR-1, -2, -3 • PDGFR • KIT • FLT3 • CSF-1R • RET |
Kidney, breast, prostate, lung, liver, ovarian, colorectal, thyroid, head and neck, gastric, bladder, cervical and pancreatic cancer, GIST, melanoma, glioblastoma, myeloma, lymphoma |
| Sorafenib (BAY439006; Nexavar) | • VEGFR-2, -3 • PDGFR • Raf • KIT |
Kidney, liver, breast, prostate, lung, ovarian, colorectal, thyroid, head and neck, gastric and pancreatic cancer, GIST, melanoma, glioblastoma, lymphoma, leukemia |
| Pazopanib (GW786034; Votrient) | • VEGFR-1, -2, -3 • PDGFR • KIT |
Kidney, breast, lung, cervical, liver, thyroid, prostate and colorectal cancer, melanoma, glioblastoma |
| Vandetanib (ZD6474; Zactima) | • VEGFR-2 • EGFR • KIT • RET |
Lung, kidney, thyroid, head and neck, prostate, ovarian, breast and colorectal cancer, glioma, neuroblastoma |
| Axitinib (AG013736) | • VEGFR-1, -2, -3 • PDGFR-β • KIT |
Kidney, lung, thyroid, pancreatic, colorectal and breast cancer, melanoma |
| Cediranib (AZD2171; Recentin) | • VEGFR-1, -2, -3 • PDGFR-β • KIT |
Kidney, breast, lung, liver, ovarian, head and neck, prostate and colorectal cancer, GIST, glioblastoma, melanoma |
| Vatalanib (PTK787; ZK222584) | • VEGFR-1, -2, -3 • PDGFR-β • KIT |
Prostate, colorectal, kidney and pancreatic cancer, melanoma, lymphoma, leukemia |
| Motesanib (AMG706) | • VEGFR-1, -2, -3 • PDGFR • KIT • RET |
Lung, thyroid, gallbladder, breast and colorectal cancer, GIST |
CSF-1R colony stimulating factor-1 receptor, EGFR epidermal growth factor receptor, FLT3 fms-related tyrosine kinase 3, GIST gastro-intestinal stromal tumor, PDGFR platelet-derived growth factor receptor, VEGFR vascular endothelial growth factor receptor, RET, rearranged during treatment, RAF, rapid accelerated fibrosarcoma
Therapeutic inhibition of angiogenesis has been associated with increased local invasiveness and distant metastasis, as shown for the first time in 2009 [6], [7]. Sunitinib and the anti VEGFR-2 antibody DC101 stimulated the invasive behavior of tumor cells despite their inhibition of tumor growth [6], [7]. Increased invasiveness might result from enhanced expression of angiogenic cytokines induced by the treatment, including VEGF and placental growth factor (PlGF), or recruitment of endothelial progenitor cells (EPCs), that promote the formation of a pre-metastatic niche [8]. Inherent or acquired resistance to anti-VEGF molecules can occur leading to a lack of response and to disease recurrence, although discontinuation of the therapy is the principal factor limiting the effectiveness of anti-angiogenic therapies [9]. Moreover, anti-angiogenic treatment reduces drugs delivery to tumors, restricting their efficacy [10]. Tumors use multiple pathways for recruiting vessels and blocking VEGF alone has incomplete effects on tumor vasculature, and tumors may switch from one mechanism to another. Breast cancer cells express several angiogenic factors including VEGF, transforming growth factor beta (TGFβ), PlGF and platelet derived growth factor (PDGF) [11]. Other factors involved in compensatory angiogenic signaling are FGF-2, hepatocyte growth factor/scatter factor (HGF/SF), angiopoietins (Angs), and interleukin-8 (IL-8) [12], [13], [14], [15], [16].
Long-Term Anti-Angiogenic Therapy Leads to Tumor Hypoxia
Tumor hypoxia is a consequence of inadequate oxygen supply caused by extensive abnormalities in the vascular network, including elongated and tortuous vessels, aberrant vessel diameters, and vessel wall abnormalities (i.e., incomplete endothelial lining, interrupted basement membrane, and lack of pericytes and contractile vessel wall components) [17].
Anti-angiogenesis is responsible for a temporary decrease in tumor hypoxia, which parallels the maturation of the vasculature. VEGF inhibition could temporarily normalize the function of tumor-associated vasculature, decreasing vascular permeability in conjunction with restoration of sustained pressure gradients, thereby enhancing systemic delivery of oxygen or perfusion of cytotoxic agents to intratumoral sites [18]. In combination with chemotherapy, such vascular normalization strategies can improve drug delivery and cancer growth control [19].
The window of vascular normalization occurs transiently during the first days of treatment. When the tumor blood vessels lose their maturation [20] hypoxia re-increases and prolonged VEGF inhibition further increases local hypoxia [21], which in turn induces systemic secretion of other angiogenic cytokines [7], [22], [23], [24]. Hypoxia within tumor increases during treatment with an anti-angiogenic agent, inducing pH drop and consequent acidosis [19]. Hypoxic conditions could also select more malignant cells, i.e. those able to grow in hypoxic conditions [25]. Moreover, hypoxia, produces a pressure mechanism that selects tumor cells with increased aggressiveness and lower sensitivity to anti-angiogenic therapy [23]. In fact, blood supply to the tumor remains heterogeneous and hypoxia increases while the permeability of tumor blood vessels is reduced, favoring disappointing results of clinical trials where anti-angiogenic therapies have been combined with systemic delivery of chemotherapeutic agents.
Hypoxia promotes the differentiation of tumor infiltrating myeloid cells to M2-pro-angiogenic tumor associated macrophages (TAMs) [26], and neovascularization through the mobilization of bone marrow-derived endothelial precursor cells [27]. Hypoxia also promotes genetic instability in tumor endothelial cells [28], and induces the selection of more invasive metastatic clones of the cancer cells that are resistant to anti-angiogenic agents [29], through the production of pro-migratory proteins, such as stromal cell derived factor 1 alpha (SDF1-α) and HGF/SF and pro-invasive extracellular matrix proteins [30], [31], which may stimulate mobilization and recruitment of EPCs and other bone marrow-derived cells [32], [33].
Genotype Alterations
The cells of the tumor vasculature are more genetically stable than tumor cells, express specific antigens [34] and are more accessible for therapeutic molecules and immune cells, even if gene and chromosomal abnormalities have been documented in subpopulations of tumor endothelial cells, including aneuploidy, abnormal multiple chromosomes, and aberrant chromosomse [35].
Colorectal cancer endothelial cells overexpress specific transcripts as a result of qualitative differences in gene profiling compared with endothelial cells of the normal colorectal mucosa [36]. A distinct gene expression pattern related to extracellular matrix and surface proteins characteristic of proliferating and migrating endothelial cells has been demonstrated in glioma and invasive breast carcinoma [37], [38].
Tumor endothelial cells isolated from human renal cell carcinoma are resistant to apoptotic stimuli with enhanced Akt activation and decreased expression of the tumor suppressor phosphatase and tensin homolog deleted from chromosome 10 (PTEN) [39]. Cytogenetic abnormalities are an expression of genetic instability and could explain the resistance of tumor endothelial cells to chemotherapeutic agents, including vincristine [39], 5-fluorouracil, adriamycin, and paclitaxel [40], [41].
Cells of the Microenvironment
TAMs have been considered as primary cause of resistance to anti-VEGF agents [42]. PlGF mediated recruitment of pro-angiogenic TAMs might be a mechanism for tumor resistance [43]. Refractoriness to anti-angiogenic therapies in glioblastoma multiforme patient is associated with higher number of CD68+ TAMs and CD11b+ myeloid cells [44]. Blocking the recruitment of TAMs may be a mechanism to overcome resistance to anti-angiogenic therapy. VEGFR-2 inhibition in RIP1-Tag2 pancreatic neuroendocrine tumors upregulates Ang-2, enhances infiltration of Tie2 expressing macrophages, and suppresses revascularization and tumor progression [45].
Tumor associated fibroblasts (TAFs) secrete several angiogenic growth factors, including epidermal growth factor (EGF), HGF, insulin-like growth factor, and FGF-2 [46]. TAFs are involved in resistance to anti-angiogenic therapy. In particular, CAFs generated PDGF-C, which is a key factor in sustaining angiogenesis and tumor growth under anti-VEGF treatment [13]. Pericytes can protect endothelial cells from VEGF withdrawal by activating compensatory pro-angiogenic pathway in anti-VEGF therapy [47].
Cancer Stem cells (CSCs) generate angiogenic factors to stimulate tumor angiogenesis, and tumor vasculature, in turn, supports CSC self-renewal and maintaining [48]. Moreover, CSCs recruit endothelial precursors for revascularization and tumor re-growth [49], and may be involved in tumor resistance as a consequence of their capability to produce much higher levels of VEGF in both anoxic and hypoxic environments than non-CSC population [50].
Bone marrow derived cells (BMDCs) are a major reserve of EPCs, which play an important role in tumor angiogenesis independent of VEGF [51]. The immature myeloid cell [52] and EPCs [53] infiltrate the tumor and mediate the resistance by incorporating themselves into vessels or by releasing pro-angiogenic growth factors [54].
Tumor Blood Vessels Normalization (Table 3)
Table 3.
Events in tumor blood vessels normalizaton
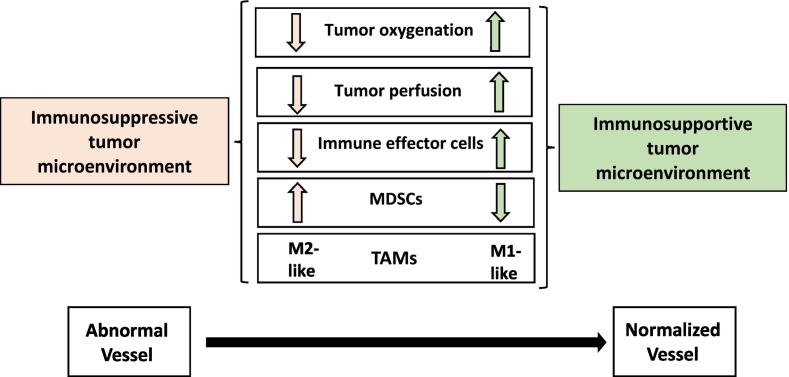
Tumor vascular normalization is accompanied by increased pericyte coverage, while pericyte deficiency could be partly responsible for vessel abnormalities in tumor blood vessels [55] and partial dissociation of pericytes contribute to increased tumor vascular permeability [56]. Pericyte coverage promotes resistance through direct support or paracrine interactions with endothelial cells and tumor vessels covered by pericytes are less sensitive to VEGF blockade [57].
Vascular normalization improves T cell extravasation and promotes the conversion of pro-angiogenic (M2-like) into angiostatic (M1-like) TAMs [58]. TAMs usually exhibit M2-like phenotype, secreting immunosuppressive cytokines, such as IL-10, CCL17 and CCL22 and producing pro-angiogenic and tissue remodeling factors, such as VEGF, PlGF and matrix metalloproteinase-9 (MMP9) [59]. The increase of oxygenation in histidine-rich glycoprotein (HRG)-positive tumors caused by vascular normalization seems to provide a stimulus for polarizing TAMs away from M2-like type, which could further sustain the normalized vasculature, leading to decreased tumor growth and metastasis [60].
In the case of cerebral tumors, the process of normalization may induce a re-establishment of the low permeability characteristics of normal brain microvasculature, preventing the delivery of chemotherapeutics [61]. In the meantime, vascular normalization induces a drop in interstitial fluid pressure and reduction in hypoxia, which provide an improvement of the penetration and activity of cytotoxic drugs [62].
The state of vascular normalization generally is transient and in this context tumor is more receptive to delivery of chemotherapeutic drug and immune cell populations [63]. However, there appears to be a high degree of variation in temporal window of vascular normalization for various anti-angiogenic agents [64].
Alternative Mechanisms of Formation of Tumor Vasculature
Other modes of tumor vascularization may be less sensitive to anti-angiogenic therapies. Intussusceptive microvascular growth (IMG) (“intussusception, known also as or non-sprouting or splitting angiogenesis”) is a concept of microvascular growth relevant for many vascular systems, which constitutes an additional and alternative mechanism to endothelial sprouting [65]. In IMG, the capillary network increased its complexity and vascular surface by insertion of a multitude of transcapillary pillars, a process they called “intussusception” (meaning “in-itself” growth) [65]. IMG generates vessels more rapidly with a less metabolic demand as compared to sprouting angiogenesis and is a strategy that tumors can use for rapid adaptation to milieu changes [65]. IMG has been observed in several human tumors, including melanoma, colon, mammary carcinomas, B-cell non-Hodgkin's lymphoma and glioblastoma [65].
In the course of the so called “vasculogenic mimicry,” blood vessels are generated without the participation of endothelial cells through a differentiation of tumor cells into endothelial-like cells, and independent of classical angiogenic factors, including FGF-2 and VEGF [66]. Vasculogenic mimicry has been observed in different human malignant tumors, including breast, melanoma, bladder, kidney, glioblastoma, prostate, ovarian, lung, sarcomas, cell renal cell carcinoma and astrocytoma [67]. An increase in vasculogenic mimicry has been demonstrated after anti-angiogenic treatment with Bevacizumab [68].
Vascular co-option occurs in site of metastases or in densely vascularized organs. Tumor cells co-opt and grow as cuffs around adjacent vessels [69]. The prevalence of vessel co-option in in liver metastasis of breast and colorectal cancer [70] may explain why anti-angiogenic therapies are poorly effective in these pathological conditions. Vessel co-option could explain the onset of resistance to anti-angiogenic regimens in glioblastoma multiforme, hepatocellular carcinoma, and in metastasis to lung [71], [72], [73]. Moreover, anti-VEGF antibody treatment is responsible of an increased vessel co-option [73]. Simultaneous inhibition of angiogenesis and vessel co-option may represent a further improvement of the therapeutic approach [70].
Therapeutic Vaccines Promoting Immune Targeting of Tumor Vascular Cells
The programmed death protein 1 (PD-1), its ligand the programmed death ligand 1 (PD-L1) and the cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) are negative regulators of T-cell immune function. Direct stimulation of the immune system with immune check-point inhibitors, such as antibody against PD-1/PD-L1 and CTLA-4 has been reported in multiple cancers. Antibodies against CTLA-4 and PD-1/PDL-1 have proven efficacy in different tumor types and five drugs of this class have been approved by the FDA [74].
The production of angiogenic molecules by tumor cells inhibits the expression of adhesion molecules involved in leukocyte interactions with blood vessels, including intercellular cell adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), E-selectin and CD34, preventing adhesion and extravasation of effector T cells into the tumor [63]. The anergic phenotype of tumor endothelial cells can be reversed by anti-angiogenic therapy, which upregulates the expression of endothelial adhesion molecules in the tumor vasculature [75]. Abrogating VEGF signaling thus looks like an immunotherapeutic strategy.
Vaccination based on tumor blood vessel associated antigens (TBVA) normalizes the vasculature, induces T cell effector response, and inhibits tumor growth in murine models [76]. Two major types of vaccines are being developed, namely vaccines against defined angiogenesis-associated antigens, and whole endothelial cell vaccines, expressing numerous angiogenic antigens, using whole endothelial cells or isolated proteins from endothelial cell membranes [77]. Moreover, VEGF decreased in patients with metastatic melanoma responding to sequential anti-CTLA-4 and anti-PD-1 therapy, but increased in non-responders, indicating a mechanism of therapeutic resistance [78].
A clinical study of a combination therapy using antibody-anti-CTLA-4 with bevacizumab reported efficacy in patients with metastatic melanoma with a median overall survival of more than 2 years [79].
Anti-PD-1 or anti-PD-L1 therapy sensitized and prolonged the efficacy of anti-angiogenic therapy and improved anti-PD-L1 favoring vascular changes such as vascular normalization that facilitate enhanced cytotoxic T cell infiltration [80]. Moreover, CD4+ T cell activation by immune-checkpoint blockade increased vessel normalization, as indicated by increased pericyte coverage, improved tumor vessel perfusion and reduced vascular permeability [81].
Concluding Remarks and Perspectives
Different mechanisms are involved in anti-angiogenic therapy resistance, in both pre-clinical and clinical setting (Table 4). The fact that tumors may grow without angiogenesis, through alternative mode of vasculature neo-formation, make them less likely to respond to anti-angiogenic drugs.
Table 4.
Mechanisms are involved in anti-angiogenic therapy resistance.
| Redundancy in growth factor signaling | |
| Recruitment of bone marrow-derived cells | |
| Stromal cells | |
| Intussusceptive microvascular growth, vasculogenic mimicry, and vessel co-option | |
| Redundancy in growth factor signaling | |
| Increased invasiveness and metastasis | |
| Endothelial heterogeneity |
Alternative therapeutic strategies may be used to overcome resistance to anti-angiogenic therapy, including the association of multiple anti-angiogenic compounds or a combination of anti-angiogenic drugs with other treatment regimens. Effectiveness of the combination therapy should be monitored during disease progression with the aim of optimize the therapy and counteract the development of further resistance.
Acknowledgements
This work has been supported by Fellowship FIRC-AIRC one-year fellowship “Laura Bassi” id. 20,879 to TA.
References
- 1.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 2.Keedy VL, Sandler AB. Inhibition of angiogenesis in the treatment of non-small cell lung cancer. Cancer Sci. 2007;98:1825–1830. doi: 10.1111/j.1349-7006.2007.00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saif MW, Elfiky A, Salem RR. Gastrointestinal perforation due to bevacizumab in colorectal cancer. Ann Surg Oncol. 2007;14:1860–1869. doi: 10.1245/s10434-006-9337-9. [DOI] [PubMed] [Google Scholar]
- 4.Hartmann J, Haap M, Kopp H-G, Lipp H-P. Tyrosine Kinase Inhibitors – A Review on Pharmacology, Metabolism and Side Effects. Curr Drug Metab. 2009;10:470–481. doi: 10.2174/138920009788897975. [DOI] [PubMed] [Google Scholar]
- 5.Gaya A, Tse V. A preclinical and clinical review of aflibercept for the management of cancer. Cancer Treat Rev. 2012;38:484–493. doi: 10.1016/j.ctrv.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Ebos JML, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated Metastasis after Short-Term Treatment with a Potent Inhibitor of Tumor Angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic Therapy Elicits Malignant Progression of Tumors to Increased Local Invasion and Distant Metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebos JM, Lee CR, Christensen JG, Mutsaers AJ, Kerbel RS. Multiple circulating proangiogenic factors induced by sunitinib malate are tumor-independent and correlate with antitumor efficacy. Proc Natl Acad Sci U S A. 2007;104:17069–17074. doi: 10.1073/pnas.0708148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Beijnum JR, Nowak-Sliwinska P, Huijbers EJM, Thijssen VL, Griffioen AW. The Great Escape; the Hallmarks of Resistance to Antiangiogenic Therapy. Pharmacol Rev. 2015;67:441–461. doi: 10.1124/pr.114.010215. [DOI] [PubMed] [Google Scholar]
- 10.Van der Veldt Astrid AM, Lubberink M, Bahce I, Walraven M, de Boer Michiel P, Greuter Henri NJM, Hendrikse NH, Eriksson J, Windhorst Albert D, Postmus Pieter E. Rapid Decrease in Delivery of Chemotherapy to Tumors after Anti-VEGF Therapy: Implications for Scheduling of Anti-Angiogenic Drugs. Cancer Cell. 2012;21:82–91. doi: 10.1016/j.ccr.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 11.Relf M, LeJeune S, Scott PA, Fox S, Smith K, Leek R, Moghaddam A, Whitehouse R, Bicknell R, Harris AL. Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor beta-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res. 1997;57:963–969. [PubMed] [Google Scholar]
- 12.Brooks AN, Kilgour E, Smith PD. Molecular Pathways: Fibroblast Growth Factor Signaling: A New Therapeutic Opportunity in Cancer. Clin Cancer Res. 2012;18:1855–1862. doi: 10.1158/1078-0432.CCR-11-0699. [DOI] [PubMed] [Google Scholar]
- 13.Crawford Y, Kasman I, Yu L, Zhong C, Wu X, Modrusan Z, Kaminker J, Ferrara N. PDGF-C Mediates the Angiogenic and Tumorigenic Properties of Fibroblasts Associated with Tumors Refractory to Anti-VEGF Treatment. Cancer Cell. 2009;15:21–34. doi: 10.1016/j.ccr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Eklund L, Saharinen P. Angiopoietin signaling in the vasculature. Exp Cell Res. 2013;319:1271–1280. doi: 10.1016/j.yexcr.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 15.Huang D, Ding Y, Zhou M, Rini BI, Petillo D, Qian C-N, Kahnoski R, Futreal PA, Furge KA, Teh BT. Interleukin-8 Mediates Resistance to Antiangiogenic Agent Sunitinib in Renal Cell Carcinoma. Cancer Res. 2010;70:1063–1071. doi: 10.1158/0008-5472.CAN-09-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shojaei F, Lee JH, Simmons BH, Wong A, Esparza CO, Plumlee PA, Feng J, Stewart AE, Hu-Lowe DD, Christensen JG. HGF/c-Met Acts as an Alternative Angiogenic Pathway in Sunitinib-Resistant Tumors. Cancer Res. 2010;70:10090–10100. doi: 10.1158/0008-5472.CAN-10-0489. [DOI] [PubMed] [Google Scholar]
- 17.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–6465. [PubMed] [Google Scholar]
- 18.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: A new paradigm for combination therapy. Nat Med. 2001;7:987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 19.Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26:605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng F, Xu Z, Wang J, Chen Y, Li Q, Zuo Y, Chen J, Hu X, Zhou Q, Wang Y. Recombinant human endostatin normalizes tumor vasculature and enhances radiation response in xenografted human nasopharyngeal carcinoma models. PloS one. 2012;7 doi: 10.1371/journal.pone.0034646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, Garkavtsev I, Xu L, Hicklin DJ, Fukumura D, di Tomaso E. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 22.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Ribatti D. Antiangiogenic therapy accelerates tumor metastasis. Leuk Res. 2011;35:24–26. doi: 10.1016/j.leukres.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 24.Loges S, Mazzone M, Hohensinner P, Carmeliet P. Silencing or fueling metastasis with VEGF inhibitors: antiangiogenesis revisited. Cancer Cell. 2009;15:167–170. doi: 10.1016/j.ccr.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 25.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11:393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 26.Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancer. 2014;6:1670–1690. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao D, Nolan D, McDonnell K, Vahdat L, Benezra R, Altorki N, Mittal V. Bone marrow-derived endothelial progenitor cells contribute to the angiogenic switch in tumor growth and metastatic progression. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 2009;1796:33–40. doi: 10.1016/j.bbcan.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim YW, Byzova TV. Oxidative stress in angiogenesis and vascular disease. Blood. 2014;123:625–631. doi: 10.1182/blood-2013-09-512749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci. 2012;33:207–214. doi: 10.1016/j.tips.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finger EC, Giaccia AJ. Hypoxia, inflammation, and the tumor microenvironment in metastatic disease. Cancer Metastasis Rev. 2010;29:285–293. doi: 10.1007/s10555-010-9224-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Semenza GL. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol. 2014;9:47–71. doi: 10.1146/annurev-pathol-012513-104720. [DOI] [PubMed] [Google Scholar]
- 32.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 33.Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegué E, Song H, VandenBerg S, Johnson RS, Werb Z. HIF1α Induces the Recruitment of Bone Marrow-Derived Vascular Modulatory Cells to Regulate Tumor Angiogenesis and Invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nanda A, St Croix B. Tumor endothelial markers: new targets for cancer therapy. Curr Opin Oncol. 2004;16:44–49. doi: 10.1097/00001622-200401000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Hida K, Maishi N, Torii C, Hida Y. Tumor angiogenesis--characteristics of tumor endothelial cells. Int J Clin Oncol. 2016;21:206–212. doi: 10.1007/s10147-016-0957-1. [DOI] [PubMed] [Google Scholar]
- 36.St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B. Genes expressed in human tumor endothelium. Science. 2000;289:1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- 37.Madden SL, Cook BP, Nacht M, Weber WD, Callahan MR, Jiang Y, Dufault MR, Zhang X, Zhang W, Walter-Yohrling J. Vascular gene expression in nonneoplastic and malignant brain. Am J Pathol. 2004;165:601–608. doi: 10.1016/s0002-9440(10)63324-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parker BS, Argani P, Cook BP, Liangfeng H, Chartrand SD, Zhang M, Saha S, Bardelli A, Jiang Y, St Martin TB. Alterations in vascular gene expression in invasive breast carcinoma. Cancer Res. 2004;64:7857–7866. doi: 10.1158/0008-5472.CAN-04-1976. [DOI] [PubMed] [Google Scholar]
- 39.Bussolati B, Deambrosis I, Russo S, Deregibus MC, Camussi G. Altered angiogenesis and survival in human tumor-derived endothelial cells. FASEB J. 2003;17:1159–1161. doi: 10.1096/fj.02-0557fje. [DOI] [PubMed] [Google Scholar]
- 40.Akiyama K, Ohga N, Hida Y, Kawamoto T, Sadamoto Y, Ishikawa S, Maishi N, Akino T, Kondoh M, Matsuda A. Tumor Endothelial Cells Acquire Drug Resistance by MDR1 Up-Regulation via VEGF Signaling in Tumor Microenvironment. Am J Pathol. 2012;180:1283–1293. doi: 10.1016/j.ajpath.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 41.Xiong YQ, Sun HC, Zhang W, Zhu XD, Zhuang PY, Zhang JB, Wang L, Wu WZ, Qin LX, Tang ZY. Human hepatocellular carcinoma tumor-derived endothelial cells manifest increased angiogenesis capability and drug resistance compared with normal endothelial cells. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:4838–4846. doi: 10.1158/1078-0432.CCR-08-2780. [DOI] [PubMed] [Google Scholar]
- 42.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fischer C, Jonckx B, Mazzone M, Zacchigna S, Loges S, Pattarini L, Chorianopoulos E, Liesenborghs L, Koch M, De Mol M. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131:463–475. doi: 10.1016/j.cell.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 44.Lu-Emerson C, Snuderl M, Kirkpatrick ND, Goveia J, Davidson C, Huang Y, Riedemann L, Taylor J, Ivy P, Duda DG. Increase in tumor-associated macrophages after antiangiogenic therapy is associated with poor survival among patients with recurrent glioblastoma. Neuro Oncol. 2013;15:1079–1087. doi: 10.1093/neuonc/not082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rigamonti N, Kadioglu E, Keklikoglou I, Wyser Rmili C, Ching C, Leow M De Palma. Role of Angiopoietin-2 in Adaptive Tumor Resistance to VEGF Signaling Blockade. Cell Rep. 2014;8:696–706. doi: 10.1016/j.celrep.2014.06.059. [DOI] [PubMed] [Google Scholar]
- 46.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song N, Huang Y, Shi H, Yuan S, Ding Y, Song X, Fu Y, Luo Y. Overexpression of platelet-derived growth factor-BB increases tumor pericyte content via stromal-derived factor-1alpha/CXCR4 axis. Cancer Res. 2009;69:6057–6064. doi: 10.1158/0008-5472.CAN-08-2007. [DOI] [PubMed] [Google Scholar]
- 48.Ribatti D. Cancer stem cells and tumor angiogenesis. Cancer Lett. 2012;321:13–17. doi: 10.1016/j.canlet.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 49.Folkins C, Shaked Y, Man S, Tang T, Lee CR, Zhu Z, Hoffman RM, Kerbel RS. Glioma tumor stem-like cells promote tumor angiogenesis and vasculogenesis via vascular endothelial growth factor and stromal-derived factor 1. Cancer Res. 2009;69:7243–7251. doi: 10.1158/0008-5472.CAN-09-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carnero A, Lleonart M. The hypoxic microenvironment: A determinant of cancer stem cell evolution. Bioessays. 2016;38(Suppl 1):S65–S74. doi: 10.1002/bies.201670911. [DOI] [PubMed] [Google Scholar]
- 51.Ferrara N. Role of myeloid cells in vascular endothelial growth factor-independent tumor angiogenesis. Curr Opin Hematol. 2010;17:219–224. doi: 10.1097/MOH.0b013e3283386660. [DOI] [PubMed] [Google Scholar]
- 52.Chung AS, Wu X, Zhuang G, Ngu H, Kasman I, Zhang J, Vernes JM, Jiang Z, Meng YG, Peale FV. An interleukin-17-mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nat Med. 2013;19:1114–1123. doi: 10.1038/nm.3291. [DOI] [PubMed] [Google Scholar]
- 53.Shaked Y, Ciarrocchi A, Franco M, Lee CR, Man S, Cheung AM, Hicklin DJ, Chaplin D, Foster FS, Benezra R. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science. 2006;313:1785–1787. doi: 10.1126/science.1127592. [DOI] [PubMed] [Google Scholar]
- 54.Shojaei F, Wu X, Zhong C, Yu L, Liang XH, Yao J, Blanchard D, Bais C, Peale FV, van Bruggen N. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature. 2007;450:825–831. doi: 10.1038/nature06348. [DOI] [PubMed] [Google Scholar]
- 55.Gerhardt H, Semb H. Pericytes: gatekeepers in tumour cell metastasis? J Mol Med. 2008;86:135–144. doi: 10.1007/s00109-007-0258-2. [DOI] [PubMed] [Google Scholar]
- 56.Hashizume H, Baluk P, Morikawa S, McLean JW, Thurston G, Roberge S, Jain RK, McDonald DM. Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol. 2000;156:1363–1380. doi: 10.1016/S0002-9440(10)65006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ribatti D, Nico B, Crivellato E. The role of pericytes in angiogenesis. Int J Dev Biol. 2011;55:261–268. doi: 10.1387/ijdb.103167dr. [DOI] [PubMed] [Google Scholar]
- 58.Huang Y, Goel S, Duda DG, Fukumura D, Jain RK. Vascular Normalization as an Emerging Strategy to Enhance Cancer Immunotherapy. Cancer Res. 2013;73:2943–2948. doi: 10.1158/0008-5472.CAN-12-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 60.Rolny C, Mazzone M, Tugues S, Laoui D, Johansson I, Coulon C, Squadrito ML, Segura I, Li X, Knevels E. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell. 2011;19:31–44. doi: 10.1016/j.ccr.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 61.Martin V, Liu D, Gomez-Manzano C. Encountering and advancing through antiangiogenesis therapy for gliomas. Curr Pharm Des. 2009;15:353–364. doi: 10.2174/138161209787315819. [DOI] [PubMed] [Google Scholar]
- 62.Batchelor TT, Sorensen AG, di Tomaso E, Zhang W-T, Duda Dan G, Cohen KS, Kozak KR, Cahill DP, Chen P-J, Zhu M. AZD2171, a Pan-VEGF Receptor Tyrosine Kinase Inhibitor, Normalizes Tumor Vasculature and Alleviates Edema in Glioblastoma Patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Griffioen AW. Anti-angiogenesis: making the tumor vulnerable to the immune system. Cancer immunology, immunotherapy : CII. 2008;57:1553–1558. doi: 10.1007/s00262-008-0524-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hernandez-Agudo E, Mondejar T, Soto-Montenegro ML, Megias D, Mouron S, Sanchez J, Hidalgo M, Lopez-Casas PP, Mulero F, Desco M. Monitoring vascular normalization induced by antiangiogenic treatment with (18)F-fluoromisonidazole-PET. Mol Oncol. 2016;10:704–718. doi: 10.1016/j.molonc.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ribatti D, Djonov V. Intussusceptive microvascular growth in tumors. Cancer Lett. 2012;316:126–131. doi: 10.1016/j.canlet.2011.10.040. [DOI] [PubMed] [Google Scholar]
- 66.Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe'er J, Trent JM, Meltzer PS, Hendrix MJ. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155:739–752. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kirschmann DA, Seftor EA, Hardy KM, Seftor RE, Hendrix MJ. Molecular pathways: vasculogenic mimicry in tumor cells: diagnostic and therapeutic implications. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:2726–2732. doi: 10.1158/1078-0432.CCR-11-3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu Y, Li Q, Li XY, Yang QY, Xu WW, Liu GL. Short-term anti-vascular endothelial growth factor treatment elicits vasculogenic mimicry formation of tumors to accelerate metastasis. J Exp Clin Cancer Res. 2012;31:16. doi: 10.1186/1756-9966-31-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, Yancopoulos GD, Wiegand SJ. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 70.Frentzas S, Simoneau E, Bridgeman VL, Vermeulen PB, Foo S, Kostaras E, Nathan M, Wotherspoon A, Gao ZH, Shi Y. Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nat Med. 2016;22:1294–1302. doi: 10.1038/nm.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bridgeman VL, Vermeulen PB, Foo S, Bilecz A, Daley F, Kostaras E, Nathan MR, Wan E, Frentzas S, Schweiger T. Vessel co-option is common in human lung metastases and mediates resistance to anti-angiogenic therapy in preclinical lung metastasis models. J Pathol. 2017;241:362–374. doi: 10.1002/path.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kuczynski EA, Yin M, Bar-Zion A, Lee CR, Butz H, Man S, Daley F, Vermeulen PB, Yousef GM, Foster FS. Co-option of Liver Vessels and Not Sprouting Angiogenesis Drives Acquired Sorafenib Resistance in Hepatocellular Carcinoma. J Natl Cancer Inst. 2016;108 doi: 10.1093/jnci/djw030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rubenstein JL, Kim J, Ozawa T, Zhang M, Westphal M, Deen DF, Shuman MA. Anti-VEGF Antibody Treatment of Glioblastoma Prolongs Survival But Results in Increased Vascular Cooption. Neoplasia. 2000;2:306–314. doi: 10.1038/sj.neo.7900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yoest JM. Clinical features, predictive correlates, and pathophysiology of immune-related adverse events in immune checkpoint inhibitor treatments in cancer: a short review. ImmunoTargets and therapy. 2017;6:73–82. doi: 10.2147/ITT.S126227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Griffioen AW, Damen CA, Mayo KH, Barendsz-Janson AF, Martinotti S, Blijham GH, Groenewegen G. Angiogenesis inhibitors overcome tumor induced endothelial cell anergy. Int J Cancer. 1999;80:315–319. doi: 10.1002/(sici)1097-0215(19990118)80:2<315::aid-ijc23>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 76.Ishizaki H, Tsunoda T, Wada S, Yamauchi M, Shibuya M, Tahara H. Inhibition of tumor growth with antiangiogenic cancer vaccine using epitope peptides derived from human vascular endothelial growth factor receptor 1. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12:5841–5849. doi: 10.1158/1078-0432.CCR-06-0750. [DOI] [PubMed] [Google Scholar]
- 77.Wagner SC, Ichim TE, Ma H, Szymanski J, Perez JA, Lopez J, Bogin V, Patel AN, Marincola FM, Kesari S. Cancer anti-angiogenesis vaccines: Is the tumor vasculature antigenically unique? J Transl Med. 2015;13:340. doi: 10.1186/s12967-015-0688-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen PL, Roh W, Reuben A, Cooper ZA, Spencer CN, Prieto PA, Miller JP, Bassett RL, Gopalakrishnan V, Wani K. Analysis of Immune Signatures in Longitudinal Tumor Samples Yields Insight into Biomarkers of Response and Mechanisms of Resistance to Immune Checkpoint Blockade. Cancer Discov. 2016;6:827–837. doi: 10.1158/2159-8290.CD-15-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hodi FS, Lawrence D, Lezcano C, Wu X, Zhou J, Sasada T, Zeng W, Giobbie-Hurder A, Atkins MB, Ibrahim N. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol Res. 2014;2:632–642. doi: 10.1158/2326-6066.CIR-14-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Allen E, Jabouille A, Rivera LB, Lodewijckx I, Missiaen R, Steri V, Feyen K, Tawney J, Hanahan D, Michael IP. Combined antiangiogenic and anti–PD-L1 therapy stimulates tumor immunity through HEV formation. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aak9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tian L, Goldstein A, Wang H, Ching Lo H, Sun Kim I, Welte T, Sheng K, Dobrolecki LE, Zhang X, Putluri N. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature. 2017;544:250–254. doi: 10.1038/nature21724. [DOI] [PMC free article] [PubMed] [Google Scholar]