Abstract
Objective
There is no consensus on the treatment of progressive multifocal leukoencephalopathy (PML) occurring in multiple sclerosis (MS) patients treated with natalizumab (Nz). We report novel immune activating treatment with filgrastim of Nz‐associated PML in MS patients treated at Rush University Medical Center.
Methods
We retrospectively analyzed 17 Nz‐PML patients treated at this single tertiary referral center between 2010 and 2017. We reviewed the clinical symptoms, diagnostic methods, survival, outcome and MS modifying therapy (MSMT) after Nz‐PML.
Results
PML occurred after an average of 49 Nz infusions. To facilitate JCV elimination by accelerating immune reconstitution inflammatory syndrome (IRIS), all patients received subcutaneous filgrastim upon PML diagnosis and discontinuation of Nz; eight received plasma exchange (PLEX). Earlier than previously published, PML‐IRIS occurred in 15 of 17 (88.2%) patients within a mean of 57.4 days (SD 21.20) after the last Nz infusion. Seven patients recovered to or near baseline. There were no PML/IRIS–related fatalities but one patient committed suicide 2.5 years later. PLEX had no impact on PML outcome. Of 17 patients, 3 (18%) had MS relapses within 1 year after PML, and 5 (29%) beyond 1 year of PML onset, which is lower than expected in highly active MS patients. Eight patients started MSMTs after Nz‐PML on an average of 26 months after Nz withdrawal.
Interpretation
Our findings indicate that immunoactivation with filgrastim during PML and careful management of subsequent IRIS is likely beneficial in patients with Nz‐PML, without worsening MS. The clinical course of MS may be ameliorated by PML.
Introduction
Relapsing–remitting multiple sclerosis (RRMS) is characterized by recurrent episodes of multifocal autoimmune inflammatory demyelination of the central nervous system often leading to neurologic disabilites.1 Natalizumab (Nz) is a humanized monoclonal antibody to alpha‐4 integrin that deters such inflammation by suppressing CNS migration of lymphocytes, thus reducing relapse rates and disabilities in patients with RRMS.2
Progressive multifocal leukoencephalopathy (PML) is a demyelinating disease of the brain that has been reported as a serious adverse event in Nz–treated patients. PML is caused by the JC virus (JCV), an ubiquitous polyomavirus that asymptomatically infects more than 50% of healthy adults. PML usually occurs in immunodeficient individuals, including AIDS, hematologic malignancies and organ transplant recipients. As of 5 December 2018, there have been 801 confirmed Nz‐PML cases in MS patients.3, 4 There are a few reports regarding the outcome of Nz‐associated PML (Nz‐PML), but the management of Nz‐PML remains a matter of debate.5
In the absence of a consensus on management of Nz‐PML, the reported empirical attempts include Nz discontinuation to allow recovery of brain's immune surveillance, plasma exchange (PLEX) to expedite elimination of Nz, mirtazapine as a potential blocker of virus entry into cells, and mefloquine for its possible anti‐JCV proliferative properties. While the use of PLEX has been challenged as potentially detrimental,5, 6 mefloquine and mirtazapine's in vitro effectiveness remains clinically unconfirmed. When immune reconstitution inflammatory syndrome (IRIS) develops, high‐dose intravenous and tapering oral doses of corticosteroids have been utilized. Since CCR5–positive T cells have been suspected in the pathogenesis of IRIS, the CCR5 receptor antagonist maraviroc has been used both as an add‐on or a substitute for corticosteroids.7 This is based on the hypothesis that by reducing trafficking of CCR5‐T cells in the brain, maraviroc would exert an antiinflammatory effect without immune suppression.7, 8 However, its usefulness has been debated.9
Filgrastim (also known as granulocyte‐colony stimulating factor G‐CSF) is used widely to promote and restore the immune system after intense immunosuppression and chemotherapy. It promotes the production of granulocytes, lymphocytes and antigen presenting cells (APC), while also increasing the adhesive properties of T cells to extracellular matrix components independently of VLA‐4 integrin receptors, which are blocked by Nz.10, 11, 12 These immune–stimulating properties of filgrastim led us to consider it for treatment of Nz‐PML with a goal of restoring the immune competence within the CNS. We hypothesized that filgrastim would induce a rise in lymphocytes that could enter the brain despite the blocking effect of Nz, thereby achieving immune recovery faster and more effectively than through simple Nz withdrawal.13, 14
We present the management and favorable clinical outcome of Nz‐PML in a cohort of 17 MS patients treated with filgrastim for purpose of accelerated JCV elimination by immune activation.
Methods
We performed a retrospective analysis of 17 Nz‐PML patients at a single tertiary referral center from 2010 to 2017. We reviewed the clinical symptoms, diagnostic methods, therapeutic interventions, survival outcome and MS modifying therapy (MSMT) after Nz‐PML.
Fifteen of 17 patients were symptomatic at PML diagnosis and 2 were asymptomatic. The latter were discovered during routine MRI surveillance that was done every 4–12 months. PML diagnosis was established according to consensus criteria.3 One patient had histology–confirmed PML by brain biopsy. Fifteen patients had virologically–confirmed PML with positive JCV DNA PCR in CSF, and one patient had both positive biopsy results as well as JCV DNA detection in CSF.
One patient's PML evolved following initial presentation as JCV granule cell neuronopathy. The patient presented with progressive cerebellar symptoms without clear PML lesions on MR imaging. CSF analysis showed > 180,000 JCV DNA copies/mL. Convincing radiographic evidence of PML finally appeared 2 months after symptom onset.15
PML‐IRIS was defined as paradoxical, abrupt and sudden worsening of PML signs and symptoms in the setting of immune reconstitution after Nz interruption, as well as the presence of contrast enhancement in the PML lesions on MRI. The iconographic features of IRIS were enhancement at the periphery and/or inside of the PML lesion, swelling and perilesional edema.5, 16, 17 Inflammatory response of PML‐IRIS was managed with corticosteroids. Patients received seizure prophylaxis with levetiracetam 500 mg twice a day at the time of IRIS diagnosis and were treated with antiepileptic drugs if they developed seizures.
We monitored the evolution of Nz‐PML neurologic deficits over 2 years, MR imaging features and their posttreatment outcome. Clinical assessments were scored on a Karnofsky scale of 0–100 (full functionality expressed as 100). Patients with meaningful recovery were those who recovered to or near their baseline and resumed their premorbid activities. Subsequent activity of MS was assessed clinically by the number of relapses and Expanded Disability Status Scale (EDSS), as well as radiologically by the appearance of new MS lesions on MRI over the period of follow‐up.
Clinical and demographic characteristics of the 17 patients were analyzed using percentage and number for categorical variables and using mean and standard deviation (SD) for numerical variables.
Results
Study cohort
Baseline demographics, disease characteristics and outcome of 17 Nz‐PML MS patients are shown in Table 1. Clinical characteristics are summarized in Table 2. The mean age was 43.6 (9.5) years. Fourteen patients (82%) were women. Of 17 patients, two were previously treated with immunosuppressant medications. One received cladribine and another cyclophosphamide before Nz treatment. PML occurred on average after 49 (24) infusions. The mean patient weight at diagnosis was 67.94 kg (SD 18.37) and mean body mass index (BMI) 24.6 (SD 5.83), indicating values that straddle the normal‐overweight boundary. The most frequent initial PML symptoms were limb weakness (47.1%), gait impairment (41%) and speech dysfunction (29.4%). Frontal lesions were observed in 35.3% of the cases and imaging studies showed multifocal lesions in 58.8% of the patients. The mean JCV load in CSF was 60,799 copies/ml and the median 755 copies/ml (11–500,000).
Table 1.
General characteristics of MS patients with Nz‐PML
| Age at MS onset, mean (SD) | 30.1 (8.5) |
| Gender, n (%) | |
| Female | 14 (82) |
| Male | 3 (18) |
| NZ infusion (month), mean (SD) | 49.1 (24) |
| Age at PML onset, mean (SD) | 43.6 (9.5) |
| PML symptoms, n (%) | |
| Cognitive dysfunction | 2 (11.8) |
| Seizure | 1 (5.9) |
| Gait dysfunction/ataxia | 7 (41) |
| Limb weakness | 8 (47.1) |
| Paresthesia | 2 (11.8) |
| Visual defects | 2 (11.8) |
| Speech dysfunction | 5 (29.4) |
| Asymptomatic | 2 (11.8) |
| PML localization | |
| Temporal | 3 (17.6) |
| Parietal | 5 (29.4) |
| Frontal | 6 (35.3) |
| Multifocal | 10 (58.8) |
| Cerebellar | 2 (11.8) |
| Occipital | 1 (5.9) |
| PML diagnostic method | |
| Clinical | 15 (88.2) |
| JCV PCR | 16 (94.1) |
| Biopsy | 2 (11.8) |
| Imaging | 17 (100) |
| G‐CSF treatment, n (%) | 17 (100) |
| IRIS n (%) | 15 (88.2) |
| Other treatment for PML, n (%) | |
| Mefloquine | 14 (82) |
| Maraviroc | 9 (53) |
| Mirtazapine | 15 (88) |
| PLEX | 8 (47) |
| Outcome after PML | |
| Alive, n (%) | 17 (100) |
| PML relapse, n (%) | 0 |
| MS relapse post | |
| Within 1 year | 3 (18) |
| Beyond 1 year | 5 (29) |
Table 2.
Clinical characteristics of Natalizumab‐associated PML patients
| Patients | Number of Nz infusions | Age at PML diagnosis, years | PML localization in MRI | CSF JCV PCR copies/mL | PLEX | Time to IRIS from Nz withdrawal, days | EDSS at PML diagnosis | EDSS at last follow up |
|---|---|---|---|---|---|---|---|---|
| 1 | 24 | 38 | Left temporal and parietal lobe | 153 | No | 86 | 9 | 7 |
| 2 | 24 | 38 | Left temporal lobe | 845 | Yes | 37 | 1.5 | 1.5 |
| 3 | 60 | 39 | Left temporal, right frontal, parietal lobe | 300 | No | 74 | 6 | 5.5 |
| 4 | 45 | 38 | Frontal, temporal and parietal lobes right occipital lobe and right cerebellar peduncle** | 1063 | Yes | 77 | 4 | 6.5 |
| 5 | 28 | 42 | Left frontal and parietal lobe | 666 | Yes | * | 3.5 | 1.5 |
| 6 | 54 | 41 | Left frontal and temporal, thalamus, cerebral peduncle, corpus callosum and right frontal lobe | Positive | Yes | * | 3.5 | 2.5 |
| 7 | 48 | 51 | Right parietal and frontal lobes | 14,648 | Yes | 63 | 8 | 8 |
| 8 | 36 | 45 | Right cerebellar | 1236 | No | 14 | 3.5 | 2 |
| 9 | 42 | 64 | Left parietal and frontal lobe | Negative | Yes | 63 | 6 | 7 |
| 10 | 70 | 60 | Frontal lobes, right temporal and right thalamus | Positive | Yes | 49 | 6 | 7 |
| 11 | 48 | 41 | Left frontal lobe | 151,116 | No | * | 6 | 5.5 |
| 12 | 77 | 37 | Left frontal, bilateral frontal lobes, internal capsules, lentiform nuclei and cerebral peduncles | 180,000 | No | 88 | 8.5 | 7.5 |
| 13 | 17 | 33 | Bilateral frontal lobes | 126 | No | 70 | 2.5 | 2.5 |
| 14 | 70 | 36 | Left cerebellar peduncle | 371 | No | 42 | 2 | 1.5 |
| 15 | 24 | 41 | Right insula and frontal lobe | 658 | No | 35 | 5.5 | 5.5 |
| 16 | 108 | 61 | Left temporal and parietal lobe, and corpus callosum | 500,000 | Yes | * | 5 | * |
| 17 | 60 | 37 | Right occipital lobe | 11 | No | 49 | 3.5 | 3.5 |
PML, progressive multifocal leukoencephalopathy; VL, viral load; CSF, cerebrospinal fluid; IRIS, immune reconstitutio inflammatory syndrome; JCV, JC virus; Nz, Natalizumab; *, no data available, ** enhancing lessions at PML onset.
Therapies after Nz discontinuation
Nz was discontinued in all 17 patients after PML diagnosis and eight (47.1%) underwent 3 days of PLEX over 1 week. To facilitate immunoactivating elimination of the JCV and accelerate IRIS, all patients were treated with daily filgrastim 5 mcg/kg subcutaneously until approximate doubling of the baseline absolute lymphocyte counts (ALC). The mean duration of filgrastim administration was 9.94 days (SD 5.56). The amplified mean ALC was 5908/μL within an average of 7.75 days (SD 6.54), denoting a 2.95‐fold increase from mean baseline of 2000/μL (the concurrent mean maximum WBC was 52,730/μL); No patient experienced activation or worsening of MS with filgrastim. The only side effect of filgrastim was bone pain and did not lead to interruption of treatment. Anticipation of PML‐IRIS and close monitoring for its occurrence/progression was done by clinical follow‐ups and weekly contrast brain MRIs. PML‐IRIS verifiably occurred in 15 of 17 (88.2%) patients (two unverified patients were partly managed off‐site), including eight who were treated with PLEX. Mean time to PML‐IRIS was 57.4 days (SD 22) after the last Nz infusion and 13.6 days (SD 9.25) after PLEX/filgrastim treatment initiation. There was no difference in the timing of IRIS between the PLEX–treated and untreated patients. Upon developing clinical worsening as corroborated by contrast enhancement on brain MRI indicative of PML‐IRIS, patients received intravenous (IV) methylprednisolone 1000 mg daily for 3–5 days followed by 2–4 weeks of oral corticoid taper. In addition, 14 patients received mefloquine (250 mg po weekly) and 15 patients received mirtazapine (15–30 mg po daily) at the time of PML diagnosis for their potential anti‐JCV properties. Nine patients also received oral CCR5 antagonist maraviroc (150 mg BID) for its non‐immunosuppressing anti‐inflammatory properties to dampen IRIS overshoot.
Figure 1 shows two representative patients with PML‐IRIS treated with IV methylprednisolone (IVMP) 1000 mg daily for 5 days followed by 4 weeks of oral prednisone taper who had favorable outcomes. Conversely, the condition of two patients worsened with prematurely administered corticosteroids for nascent IRIS while their brain MRIs revealed enlarging PML lesions, indicating immunosuppression induced PML expansion. Corticosteroids were discontinued in both patients. One patient received a second course of filgrastim, which rekindled more robust PML‐IRIS as determined by recurrence of enhancement in PML lesions on MRI (Fig. 2).
Figure 1.
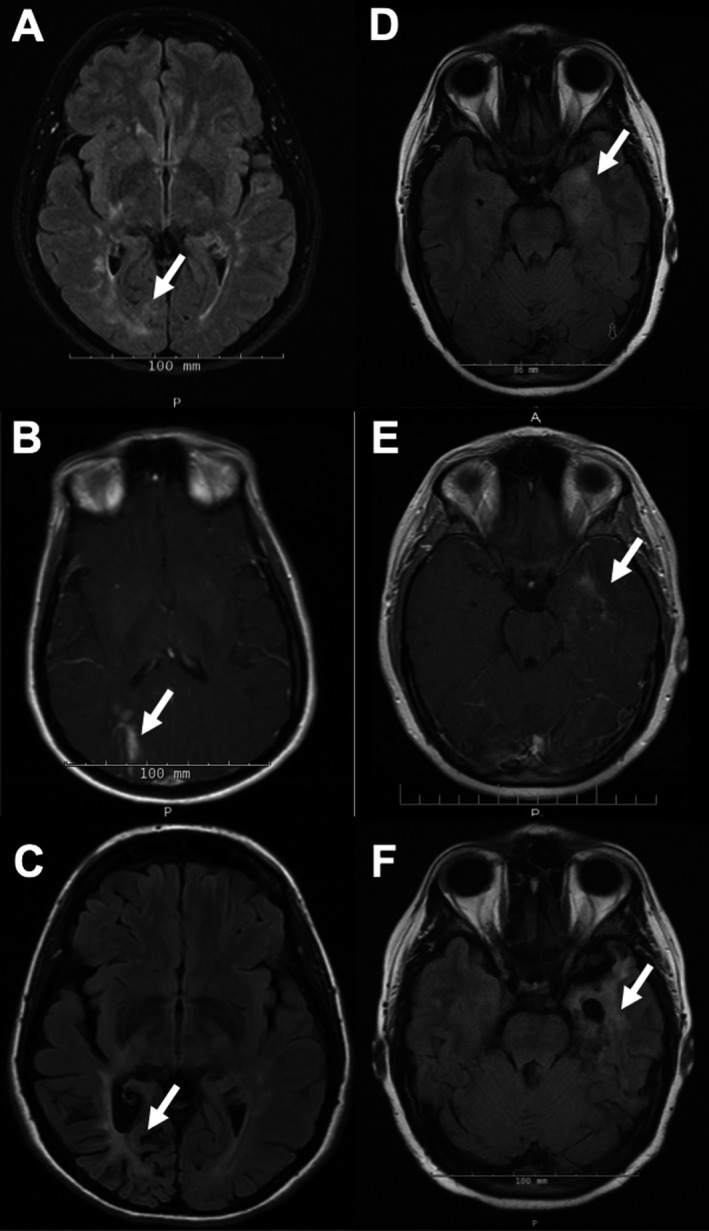
Evolution of PML lesions on MRI after filgrastim treatment in two patients. MRI findings of patient #1 (A–C) and #2 (D–F) demonstrate a PML lesion at symptom onset, during IRIS after filgrastim treatment and 2–3 years later: (A) Axial fluid‐attenuated inversion recovery (FLAIR) image shows a PML lesion in the right occipital lobe (arrow). (B) Axial postcontrast T1‐weighted image demonstrates peripheral enhancement in the lesion (arrow) 14 days after peak ALC on filgrastim treatment and 49 days after Nz withdrawal. (C) Axial FLAIR image shows atrophy at the site of the PML lesion 3 years after symptoms onset. There were no new MS lesions off MSMT (not shown here). (D) Axial FLAIR image shows a PML lesion in the left temporal lobe (arrow). (E) Axial postcontrast T1‐weighted image demonstrates peripheral enhancement in the lesion (arrow) within 1 week of peak ALC on filgrastim treatment and 37 days after Nz withdrawal. (F) Axial FLAIR image shows atrophy at the site of the PML lesion 2 years after onset. There were no new MS lesions off MSMT (not shown here).
Figure 2.
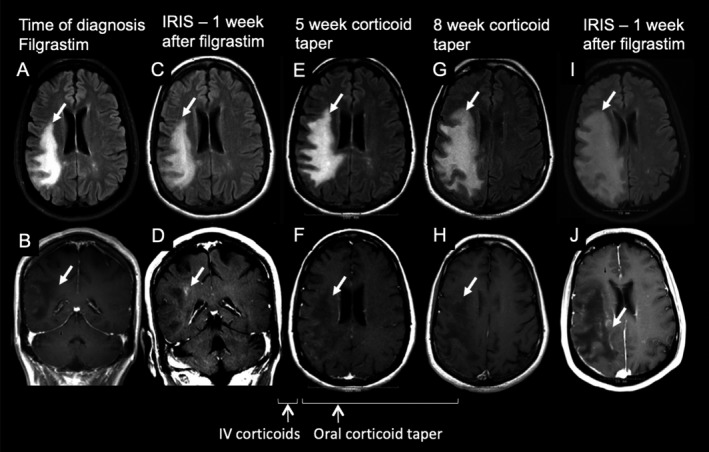
Progression of PML lesions after premature corticosteroid treatment. Axial FLAIR (A, C, E, G, I) and postcontrast T1‐weighted images (B, D, F, H, J) demonstrate PML lesion in the right frontoparietal area (arrows) of one representative MS patient. A, B) The PML lesion is devoid of contrast enhancement at the time of PML diagnosis. (C, D) Contrast enhancement on MRI and clinical worsening indicative of IRIS occurred 9 days after peak ALC on filgrastim treatment and 63 days after Nz withdrawal. The patient received intravenous methylprednisolone followed by oral prednisone taper. Three to 4 weeks later, while on prednisone taper, the patient began to worsen clinically. Repeat brain MRI exams revealed increased size PML lesions without contrast enhancement (E, F, G, and H). Prednisone was discontinued and filgrastim reinstituted. There was evidence of a more robust IRIS by contrast enhancement on MRI 1 week after the second filgrastim treatment (I, J).
Clinical evolution
There were no PML‐IRIS–related fatalities but one patient committed suicide 2.5 years later. Four patients recovered to baseline and three near baseline (41%) with Karnofsky scores 80–100, including the two representative patients shown in Figure 1. Three patients (18%) improved to midrange functionality of a Karnofsky score 60, while seven (41%) had poor outcomes requiring full care (Karnofsky = <40). Inadequate recoveries were related to extensive PML lesions in context of delayed diagnosis, ineffective IRIS and premature use of corticosteroids for underdeveloped IRIS, including the representative patient shown in Figure 2. Eleven of 17 patients had post‐IRIS CSF assays within 3–6 months, all of which revealed clearance of JCV by negative PCR.
Three of 17 (18%) patients had MS relapse within the first year and five (29%) beyond 1 year after Nz‐PML. One of three who experienced a relapse was treated with monthly IV corticosteroids. No significant changes were detected in EDSS scores over 2 years after PML‐IRIS ((EDSS −0.2(SD 1.1) P = 0.56)).
Eight of 17 patients (47%) required resumption of MS therapies on an average of 26 months after Nz discontinuation. Three patients were treated with dimethyl fumarate (DMF), another three with glatiramer acetate (GA) and two with mycophenolate mofetil (MMF). One MS relapse occurred on MMF. No MS relapses occurred with DMF and GA. No recurrences of PML occurred with these MS therapies.
Discussion
Survival and outcome on Nz‐PML
Our cohort of Nz‐PML patients had a 100% survival 2 years after PML onset. This is higher than the 71%, 75.4% and 92% survival previously reported in Nz‐PML patients.18, 19, 20 Patients’ weights and BMIs did not emerge as modulatory of the PML risk, indicating an even representation of normal‐ and excess–weight individuals. Asymptomatic PML, contrast enhancement at the time of diagnosis – a sign of immune restauration‐ and limited brain involvement at PML diagnosis are associated with better outcomes. Attentive clinical care and the use of other therapies at the time of diagnosis might stand as confounding factors. However, our cohort had a 100% survival rate despite having a lower proportion of asymptomatic PML (11.8%) and contrast enhancement at the time of diagnosis (5.9%) compared with a study that had a 92% survival rate (15.4% asymptomatic PML and 35.9% contrast enhancement at the time of diagnosis). Therefore, the higher survival in our cohort may be secondary to an earlier development of IRIS. Of note, PML‐IRIS development was not related to the administration of mefloquine, maraviroc and mirtazapine in the same study.20
Filgrastim helps recovery from leukopenia after immune ablation and chemotherapy. Filgrastim also increases the ALC and the adhesive properties of T cells to extracellular matrix components independently of the integrin α4β1 presence on their surface.11, 12 Therefore, filgrastim could counter and evade the integrin α4β1 blocking effect of Nz, which prevents endothelial adhesion and extravasation of T cells into the CNS.21 The filgrastim‐induced endothelium‐adhering lymphocytes are free of Nz exposure, possessing unencumbered functionality. Our data show that filgrastim was well tolerated, increased the ALC by nearly threefold from baseline, and could be used to induce a rapid immune reconstitution, thus limiting the spread of JCV and achieving the containment of PML by accelerating IRIS. None of the patients suffered MS relapses after filgrastim 5 mcg/kg/d, which has been shown to occur at doses higher than 15 mcg/kg/d.12, 23
Use of immunotherapy, specifically immunostimulation, as opposed to antiviral–based strategies, is highly relevant in the treatment of rapidly progressive viral infections. This work provides evidence that selective immunostimulation, without activation of a coexistent autoimmune disease, is possible and warrants further investigation. This approach may be applicable to patients with other autoimmune diseases who have developed PML as a complication of their immunotherapies.
Management of PML‐IRIS
PML‐IRIS is defined as the paradoxical, abrupt and sudden worsening of PML signs and symptoms in the setting of immune reconstitution after Nz interruption, as well as the presence of contrast enhancement in the PML lesions on MRI, but precise criteria for diagnosis of IRIS are lacking. Contrast enhancement on MRI is usually minimal or absent in PML lesions.22 Indeed, only one of our patients showed contrast enhancement at the time of PML diagnosis. Therefore, contrast enhancement in PML lesions has been used as a marker of PML‐IRIS. Prior studies demonstrated that the imaging pattern of inflammation at the time of PML diagnosis and during PML‐IRIS are not distinct entities, but they differ in their extent of inflammation. Indeed, the severity of inflammation increases at the PML‐IRIS stage. This is characterized by alteration of the blood–brain barrier including new enhancing lesions, swelling and perilesional edema.5, 16
Taking into account that initial signs of immune restoration were associated with a better outcome and higher survival rate, the induction and modulation of IRIS is a fundamental element in the management of patients with Nz‐PML. Administration of filgrastim to our patients has resulted in quicker development of IRIS (57.4 days [SD 22] vs. 82.5 ± 29.2 days from Nz withdrawal).20 PLEX–induced removal of Nz has been used widely to induce immune recovery.7 But a recent study including 42 Nz‐PML patients who receive PLEX did not improve the clinical outcome in Nz‐PML.5, 8 Similarly, our data suggests that PLEX had no effect on the outcome of PML.
Abrupt immune reconstitution may precipitate severe IRIS, a condition that is life‐threatening and that requires treatment of the inflammatory brain infiltrate to minimize the risk of brain edema and herniation. While corticosteroids help contain inflammation in PML‐IRIS, their premature or prolonged administration for IRIS that is not yet full blown promotes immunosuppression and may contribute to detrimental worsening of PML, as seen in two of our patients.24 Specifically, corticosteroids dampen interferon‐gamma driven JCV–specific T‐cell responses in MS patients.25, 26 In addition, most patients received mirtazapine and mefloquine based on in vitro data showing anti‐JCV activity.27, 28 However, those medications have not shown efficacy against PML in humans and their possible benefit in our patients could not be discerned.29, 30 Although maraviroc has been reported as possibly useful in Nz‐PML patients by inhibiting IRIS overshoot, it remains unconfirmed, including the experience in eight of our 17 patients.31 Similarly to PLEX, maraviroc had no impact on PML outcome, IRIS severity, and post PML state of patients’ MS. This may be due in part to accelerated IRIS induction in our patients and much lower dose (150 mg BID) when compared to one used by others.7 Still, our findings are supported by a large clinical study demonstrating that maraviroc does not confer meaningful protection from the occurrence of IRIS.32
In our cohort, the combination of attentive clinical care, filgrastim–induced immune activation as well as careful and well‐timed use of IRIS modulation appears to have been beneficial to patients with Nz‐PML, without enhancing MS disease activity.
MS management post Nz‐PML
None of the patients showed recurrence of PML after resumption of MS treatment, which indicates that treating MS post Nz‐PML carries no risk of PML reactivation. These results are similar to another cohort of Nz‐PML patients.33 However, in our cohort MS activity was controlled with medications that are considered of lesser efficacy than Nz. Reactivation of MS after Nz discontinuation was also lower than expected in this previously higher disease activity cohort. Three of 17 (17%) patients had MS relapse within the first year and five (29%) beyond 1 year post Nz‐PML. A previous study showed the probability of MS relapse to be 45% within the first year after Nz discontinuation whereas Nz interruption resulted in occurrence of MRI disease activity as early as 12 weeks in patients who did not develop PML.34
Filgrastim may exert immunomodulatory changes in MS that have possibly ameliorated autoimmune responses and restored immune tolerance in our patients. Those properties, though described in other medical settings, have never been investigated in MS. Further research on filgrastim can delineate the differences between protective immunity and autoimmunity, and identify practical strategies to treat opportunistic infections in the setting of autoimmune diseases and immunosuppression.35, 36
Paradoxically, JCV infection of the brain leading to PML may have played a role in the amelioration of the MS clinical course in our patients. Infections can either promote or diminish autoimmunity. A number of different viruses bear both enhancing and protective capacity. They can modulate the outcome of an autoimmune disease depending on the context.37, 38, 39, 40 During viral infections, T cells may lose function in a process called exhaustion. Exhausted T cells play an important role in determining outcome in autoimmune diseases. CD8 + T‐cells exhaustion is characterized by high expression of coinhibitory receptors such as programmed cell death–1(PD‐1). We have shown that PML patients harbor lymphocytes with significant PD‐1 expression. CD8+ T‐cell exhaustion was associated with favorable prognosis in autoimmune disease.41, 42, 43 Therefore, modulating T‐cell exhaustion may become a novel therapeutic approach for MS.
In conclusion, our data indicate that the immunostimulatory effects of filgrastim may be beneficial in Nz‐PML and that the course of MS may be ameliorated by PML. Further research is warranted as it may contribute to better understanding and treatment of autoimmune diseases and opportunistic infections. There are several limitations on our study. It is single‐center study with small number of patients, confounding variables and retrospective design. Prospective controlled studies, including larger number of patients are needed to confirm these findings.
Author Contributions
Dusan Stefoski, Roumen Balabanov, Rasha Waheed, Michael Ko, Igor J Koralnik, and Fabian Sierra Morales have contributed to the study concept and design, analysis and interpretation of the data, as well as drafting/revising of the manuscript for content.
Conflict of Interest
The authors have no additional financial relationships or conflicts of interest relevant to this article to disclose.
Acknowledgments
This study was supported in part by R01 NS 047029 and NS 074995 to IJK.
Funding Information
This study was supported in part by R01 NS 047029 and NS 074995 to IJK; National Institutes of Health (NIH), specifically National Institute of Neurological Disorders and Stroke (NINDS).
Funding Statement
This work was funded by National Institutes of Health grants NS 074995 and R01 NS 047029; National Institute of Neurological Disorders and Stroke grant .
References
- 1. Compston A, Coles A. Multiple sclerosis. Lancet 2008;372:1502–1517. [DOI] [PubMed] [Google Scholar]
- 2. McCormack P. Natalizumab: a review of its use in the management of relapsing‐remitting multiple sclerosis. Drugs 2013;73:1463–1481. [DOI] [PubMed] [Google Scholar]
- 3. Berger JR, Aksamit AJ, Clifford DB, et al. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology 2013;80:1430–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. TYSABRI® (natalizumab): PML Incidence in Patients Receiving TYSABRI. Biogen Data on File. https://medinfo.biogen.com/ 2018
- 5. Scarpazza C, Prosperini L, De Rossi N, et al. To do or not to do? Plasma exchange and timing of steroid administration in progressive multifocal leukoencephalopathy. Ann Neurol 2017;82:697–705. [DOI] [PubMed] [Google Scholar]
- 6. Landi D, De Rossi N, Zagaglia S, et al. No evidence of beneficial effects of plasmapheresis in natalizumab‐associated PML. Neurology 2017;88:1144–1152. [DOI] [PubMed] [Google Scholar]
- 7. Giacomini PS, Rozenberg A, Metz I, et al. Maraviroc and JC virus‐associated immune reconstitution inflammatory syndrome. N Engl J Med 2014;370:486–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khatri BO, Man S, Giovannoni G, et al. Effect of plasma exchange in accelerating natalizumab clearance and restoring leukocyte function. Neurology 2009;72:402–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scarpazza C, Prosperini L, Mancinelli CR, et al. Is maraviroc useful in multiple sclerosis patients with natalizumab‐related progressive multifocal leukoencephalopathy? J Neurol Sci 2017;15:233–237. [DOI] [PubMed] [Google Scholar]
- 10. Mehta H, Malandra M, Corey S. CSF and GM‐CSF in neutropenia. J Immunol 2015;195:1341–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arat M, Arslan O, Gürman G, et al. The impact of granulocyte colony stimulating factor at content of donor lymphocytes collected for cellular immunotherapy. Transfus Apher Sci 2004;30:9–15. [DOI] [PubMed] [Google Scholar]
- 12. Snir O, Lavie G, Achiron A, Bank I. G‐CSF enhances the adhesion of encephalitogenic T cells to extracellular matrix components: a possible mechanism for exacerbation of multiple sclerosis. J Neuroimmunol 2006;172:145–155. [DOI] [PubMed] [Google Scholar]
- 13. Ko M, Balabanov R, Stefoski D. Facilitation of progressive multifocal leukoencephalopathy progression in natalizumab‐treated multiple sclerosis patients by suppression of viral clearance with corticosteroids (P01.180). Neurology 2013;80(7 Supplement): P01.180. [Google Scholar]
- 14. Lehmann H, Kruger K, Fink G, Schroeter M. Progressive multifocal leukoencephalopathy after interferon beta‐1a monotherapy. J Neurol 2015;262:771–773. [DOI] [PubMed] [Google Scholar]
- 15. Agnihotri S, Dang X, Carter J, et al. JCV GCN in a natalizumab‐treated MS patient is associated with mutations of the VP1 capsid gene. Neurology 2014;83:727–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wattjes MP, Wijburg MT, van Eijk J, et al. Inflammatory natalizumab‐associated PML: baseline characteristics, lesion evolution and relation with PML‐IRIS. J Neurol Neurosurg Psychiatry 2018;89:535–541. [DOI] [PubMed] [Google Scholar]
- 17. Gheuens S, Smith DR, Wang X, et al. Simultaneous PML‐IRIS after discontinuation of natalizumab in a patient with MS. Neurology 2012;78:1390–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clifford DB, De Luca A, Simpson DM, et al. Natalizumab‐associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol 2010;9:438–446. [DOI] [PubMed] [Google Scholar]
- 19. Dong T, Richman S, Wattjes M, et al. Outcome and survival of asymptomatic PML in natalizumab‐treated MS patients. Ann Clin Transl Neurol 2014;1:755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Prosperini L, de Rossi N, Scarpazza C, et al. Natalizumab‐related progressive multifocal leukoencephalopathy in multiple sclerosis: findings from an Italian independent registry. PLoS ONE 2016;11:e0168376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Putzki N, Baranwal MK, Tettenborn B, et al. Effects of natalizumab on circulating B cells, T regulatory cells and natural killer cells. Eur Neurol 2010;63:311–317. [DOI] [PubMed] [Google Scholar]
- 22. Sahraian MA, Radue EW, Eshaghi A, et al. Review progressive multifocal leukoencephalopathy: a review of the neuroimaging features and differential diagnosis. Eur J Neurol 2012;19:1060–1069. [DOI] [PubMed] [Google Scholar]
- 23. Openshaw H, Stuve O, Antel JP, et al. Multiple sclerosis flares associated with recombinant granulocyte colony‐stimulating factor. Neurology 2000;54:2147–2150. [DOI] [PubMed] [Google Scholar]
- 24. Tan K, Roda R, Ostrow L, et al. PML‐IRIS in patients with HIV infection: clinical manifestations and treatment with steroids. Neurology 2009;72:1458–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Antoniol C, Jilek S, Schluep M, et al. Impairment of JCV‐specific T‐cell response by corticotherapy: effect on PML‐IRIS management? Neurology 2012;79:2258–2264. [DOI] [PubMed] [Google Scholar]
- 26. Koralnik IJ, Clifford DB. Comment: avoiding detrimental effects of corticosteroids on JC virus T‐cell responses–primum non nocere. Neurology 2012;79:2263. [DOI] [PubMed] [Google Scholar]
- 27. Elphick GF, Querbes W, Jordan JA, et al. The human polyomavirus, JCV, uses serotonin receptors to infect cells. Science 2004;306:1380–1383. [DOI] [PubMed] [Google Scholar]
- 28. Brickelmaier M, Lugovskoy A, Kartikeyan R, Reviriego‐Mendoza MM. Identification and characterization of mefloquine efficacy against JC virus in vitro. Antimicrob Agents Chemother 2009;53:1840–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marzocchetti A, Tompkins T, Clifford DB, et al. Determinants of survival in progressive multifocal leukoencephalopathy. Neurology 2009;73:1551–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Clifford DB, Nath A, Cinque P, et al. A study of mefloquine treatment for progressive multifocal leukoencephalopathy: results and exploration of predictors of PML outcomes. J Neurovirol 2013;19:351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hodecker SC, Stürner KH, Becker V, et al. Maraviroc as possible treatment for PML‐IRIS in natalizumab‐treated patients with MS. Neurol Neuroimmunol Neuroinflamm 2017;4:e325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sierra‐Modero JG, Ellenberg SS, Rassool MS, et al. Effect of the CCR34 antagonist maraviroc on the occurrence of immune reconstitution inflammatory syndrome on HIV (CADIRIS): a double‐blind, randomized, placebo‐controlled trial. Lancet HIV 2014;e60–e67. [DOI] [PubMed] [Google Scholar]
- 33. Maillart E, Vidal JS, Brassat D, et al. Natalizumab‐PML survivors with subsequent MS treatment: clinico‐radiologic outcome. Neurol Neuroimmunol Neuroinflamm 2017;10:1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Papeix C, Vukusic S, Casey R, et al. Risk of relapse after natalizumab withdrawal: results from the French TYSEDMUS cohort. Neurol Neuroimmunol Neuroinflamm 2016;3:e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Franzke A, Piao W, Lauber J, et al. G‐CSF as immune regulator in T cells expressing the G‐CSF receptor: implications for transplantation and autoimmune diseases. Blood 2003;102:734–739. [DOI] [PubMed] [Google Scholar]
- 36. Rutella S, Zavala F, Danese S, et al. Granulocyte colony‐stimulating factor: a novel mediator of T cell tolerance. J Immunol 2005;175:7085–7091. [DOI] [PubMed] [Google Scholar]
- 37. Steelman A. Infection as an Environmental Trigger of Multiple Sclerosis Disease Exacerbation. Front Immunol 2015;6:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Day CL, Kaufmann DE, Kiepiela P, et al. PD‐1 expression on HIV‐specific T cells is associated with T‐cell exhaustion and disease progression. Nature 2006;443:350–354. [DOI] [PubMed] [Google Scholar]
- 39. Keir ME, Liang SC, Guleria I, et al. Tissue expression of PD‐L1 mediates peripheral T cell tolerance. J Exp Med 2006;203:883–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Filippi C, Estes E, Oldham J. Immunoregulatory mechanisms triggered by viral infections protect from type 1 diabetes in mice. J Clin Invest 2009;119:1515–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Blackburn SD, Shin H, Haining WN, et al. Coregulation of CD8 + T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol 2009;10:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tan CS, Bord E, Broge T, et al. Increased Program Cell Death ‐ 1 (PD‐1) Expression on T Lymphocytes of Patients with Progressive Multifocal Leukoencephalopathy. J Acquir Immune Defic Syndr 2012;60:244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McKinney EF, Lee JC, Jayne DR, et al. T‐cell exhaustion, co‐stimulation and clinical outcome in autoimmunity and infection. Nature 2015;523:612. [DOI] [PMC free article] [PubMed] [Google Scholar]


