Abstract
Salmonella and Campylobacter are important gastroenteric pathogens. Arcobacter butzleri is an emerging enteric pathogen. Data on the frequencies of these poultry-associated pathogens on meat products sold in sub-Saharan Africa are scarce. This study aimed to analyze the frequency of Salmonella, Campylobacter, and Arcobacter antibiotic resistance and underlying mechanisms of resistance to fluoroquinolones in locally produced and imported poultry sold in urban Ghana. Chicken meat was collected and cultured on standard media. Bacterial strains were identified by biochemical methods and by mass spectrometry. Antibiotic susceptibility was tested by disk diffusion. Ciprofloxacin-resistant strains were assessed for molecular mechanisms of resistance. Among 200 samples, comprising 34% (n = 68) from the Ghanaian poultry industry and 66% (n = 132) from imports, 9% (n = 17) contained Salmonella, 11% (n = 22) Campylobacter, and 26.5% (n = 53) A. butzleri. Higher overall contamination frequencies were found in local meat. Most common Salmonella serovars identified were Kentucky (n/N = 5/16; 31%) and Poona (n/N = 4/16; 25%). Campylobacter were C. coli (n/N = 10/19; 53%) and C. jejuni (n/N = 9/19; 47%). Resistance to fluoroquinolones was high with 63% (n = 10), 75% (n = 15), and 52% (n = 25) in Salmonella, Campylobacter, and Arcobacter, respectively. A link between Salmonella Kentucky [sequence type (ST) 198] and a ciprofloxacin minimum inhibitory concentration of 16 μg/mL was found. Salmonella Poona-ST308 revealed transferable qnrB2 fluoroquinolone resistance genes. Markedly high frequencies of resistant Salmonella, Campylobacter, and Arcobacter predominant in locally produced meat represent a probable transmission reservoir for human infections. These findings highlight the need for implementation of surveillance systems that focus on food hygiene, use of antibiotics in animal husbandry, and continuous monitoring of the quality of meat products from imports.
Keywords: poultry, sub-Saharan Africa, enteric bacteria, antibiotic resistance, mechanisms of resistance
Introduction
Globally, 1 in 10 child deaths during the first 5 years of life results from diarrheal disease, causing ∼800,000 fatalities worldwide annually, most occurring in sub-Saharan Africa (SSA) and South Asia (Kotloff et al., 2017). Campylobacter and nontyphoidal Salmonella (NTS) are among the leading bacterial pathogens isolated from patients with diarrhea in both developed and developing countries (Krumkamp et al., 2015). These pathogens are predominantly transmitted through food products, with poultry meat being identified as one of the major reservoirs (Geilhausen et al., 1996). Another emerging swine-, poultry-, and waterfowl-associated pathogens are Arcobacter spp. (Corry and Atabay, 2001), especially Arcobacter butzleri, previously described as cause of gastroenteritis and invasive bloodstream infections in humans (Collado and Figueras, 2011). In particular, NTS have continuously been associated with foodborne cross-border outbreaks worldwide with a recent outbreak early in 2018 in eight U.S. states (https://www.cdc.gov/salmonella/outbreaks.html). The steadily increasing global trade of meat products between developing and industrialized countries bears the risk of spreading the animal-associated pathogens across different countries and continents. This threat is mainly a problem in countries without surveillance systems where pathogens can be imported or exported undetected. A high risk is posed to SSA regions, where data on the frequency of the bacteria in both locally produced and imported poultry are scarce and poorly monitored.
Salmonella and Campylobacter isolated from humans and other animals with reduced ciprofloxacin susceptibility and multidrug-resistance profiles have been rapidly emerging in recent years, with considerably high reported prevalences from Asia, Europe, and Africa (EFSA and ECDC, 2015; Pham et al., 2015; Abdi et al., 2017). Antibiotic use in animal rearing for growth promotion and for the prevention and treatment of infections has been identified as responsible for the increase of multidrug-resistant bacteria in meat products (Kluytmans et al., 2013). Although in industrialized countries steps have been taken toward the control of the use of antimicrobials in food-producing animals, in SSA the use of antimicrobials remains largely unregulated (Maron et al., 2013).
This study aimed to investigate and compare the frequency of Salmonella enterica, Campylobacter spp., and Arcobacter spp. in imported and locally produced poultry sold in urban Ghana. In addition, antimicrobial resistance in the mentioned bacteria is examined with special emphasis on genetic mechanisms of resistance to fluoroquinolones.
Materials and Methods
Study site and sample collection
In a cross-sectional study from May to December 2015, 200 chicken meat samples from local Ghanaian poultry (fresh meat) and from imports (frozen meat) were collected in Kumasi, the capital of the Ashanti region of Ghana. Sampling was based on availability. In total, 75 supermarkets were identified for sample collection of imported frozen meat, which was kept in freezers. Each supermarket was sampled once and the origin of the meat from imports was retrieved from packaging. If meat from different countries was available, one piece from each country was sampled per supermarket. Chickens originating from small local farms were purchased live from 19 open markets. Each merchant was visited on one occasion only. The chickens, which had been kept in cages, were slaughtered on purchase. For each sample (fresh and frozen), ∼15 g of leg, including skin, was collected and placed into a sterile homogenizing plastic bag and returned to the laboratory at 4–8°C in a cool box.
Bacterial detection and identification
The meat was homogenized in the collection bag using a pestle and mortar. The sample was evenly distributed among the different enrichment broths for Salmonella (Selenite Broth; Oxoid, Basingstoke, UK), Campylobacter (Preston No 2; Oxoid), and Arcobacter (Arcobacter enrichment broth; Oxoid). The selenite broth was incubated with loose tops at 35–37°C for 18–24 h in normal atmosphere. Preston- and Arcobacter enrichment broths were incubated at 35–37°C for 18–24 h with loose tops under microaerophilic conditions (CampyGen sachets in a candle jar; Oxoid). The selenite broth was subsequently cultured on Xylose Lysine Deoxycholate agar (Oxoid) and incubated at 35–37°C for 18–24 h in normal atmosphere. Salmonella strains were identified by a latex agglutination test (Oxoid) and by the analytical profile index test (API 20E; bioMérieux, Marcy l'Etoile, France). Preston No. 2 broth was further cultured on selective Karmali agar (Oxoid), using a filter technique as described by Atabay et al. (2003). The plates were incubated at 35–37°C for 18–24 h under microaerophilic conditions. Negative cultures were incubated for another 4 d. For Arcobacter, Müller Hinton agar (Oxoid) with 5% sheep blood was used for culture of the broth using the filter technique as described for Campylobacter. Plates were incubated for 18–24 h at 30°C in microaerophilic conditions (CampyGen sachets in a candle jar; Oxoid) and if negative, for 4 more days. Preliminary confirmation of Campylobacter and Arcobacter was done by oxidase test and Gram staining. All bacterial strains were sent to Germany on dry ice where species identification was confirmed by mass spectrometry (MALDI-TOF MS; Bruker UK Limited, England). Salmonella serotyping was performed following the White–Kauffmann–Le Minor scheme (Grimont and Weill, 2007).
Antibiotic susceptibility testing
Antibiotic susceptibility was tested using locally available antibiotics by the disk diffusion method and interpreted following the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines (http://www.eucast.org). Quality control of susceptibility testing was performed according to EUCAST (QC table v.5). Salmonella were tested for ampicillin, amoxicillin/clavulanic acid, tetracycline, chloramphenicol, cefotaxime, ceftazidime, trimethoprim/sulfamethoxazole, and ciprofloxacin. Salmonella isolates exhibiting resistance to ampicillin, trimethoprim/sulfamethoxazole, and chloramphenicol were defined as multidrug resistant (Eibach et al., 2016). Ciprofloxacin minimum inhibitory concentrations (MICs) for Salmonella were determined by Etest (Oxoid) and interpreted as ciprofloxacin resistant with an MIC >0.06 μg/mL. Salmonella isolates were screened for extended-spectrum beta-lactamase production (ESBL) using cefotaxime and ceftazidime disks.
Campylobacter were tested for tetracycline, ciprofloxacin, and erythromycin. Resistance to all three classes of antibiotics was defined as multidrug resistant (Magiorakos et al., 2012). Arcobacter isolates were tested for ciprofloxacin. To date, no specific breakpoints for defining resistance in A. butzleri are available. Thus, for interpretation, the absence of a zone of inhibition for ciprofloxacin was considered as an indicator for quinolone resistance.
Molecular characterization of Salmonella isolates
Salmonella, Campylobacter, and Arcobacter DNA were extracted using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) following the manufacturer's instructions. Salmonella exhibiting ciprofloxacin resistance were further screened for plasmid-located genes [aac(6′)Ib(-cr), qnrA, qnrB, qnrC, qnrD, and qnrS], contributing to aminoglycoside and fluoroquinolone resistance (Pfeifer et al., 2009; Pietsch et al., 2017). Furthermore, DNA gyrase and/or DNA topoisomerase IV genes (gyrA, gyrB, parC, and parE) were screened for point mutations (Chau et al., 2007). For all Salmonella isolates, sequence types (STs) were determined by multilocus sequence typing according to a previously published protocol (http://enterobase.warwick.ac.uk).
Ciprofloxacin-resistant Campylobacter were assessed for point mutations in the gyrA gene as described by Tang et al. (2017). After shipment to Germany, 48 of 53 A. butzleri isolates could be grown. All isolates were examined for point mutations in the quinolone resistance-determining region (QRDR) of the gyrA gene using the amplification and sequencing primers described by Abdelbaqi et al. (2007). Campylobacter jejuni ssp. jejuni NCTC 11168 and 81-176, C. coli RM 2228, and Arcobacter butzleri LMG 6620 served as reference strains.
Statistical analysis
Dichotomous variables were described using frequencies and their proportion. Prevalence ratios (PRs) along with their 95% confidence intervals (CIs) were calculated to show associations between dichotomous variables. Missing values were excluded from the analysis; hence, in some calculations the denominator differs. All analyses were conducted using Stata Statistical Software 14 (StataCorp LP, College Station, TX).
Results
Meat sources
In total, 200 chicken meat samples, comprising 68 (34%) samples from the local Ghanaian poultry industry and 132 (66%) from imports, were collected within Kumasi. The Ghanaian meat was exclusively sold at open markets, whereas imported meat was primarily sold in supermarkets/cold stores. Only frozen meat from the Netherlands (n = 4; 11%) and the United States (n = 1; 3%) was also collected from open markets. The majority of imported meat products originated from the Netherlands (n/N = 38/132; 29%), the United States (n/N = 31/132; 23%), and Brazil (n/N = 31/132; 23%). Other countries included Belgium (n/N = 8/132; 6%), Germany (n/N = 3/132; 2%), Poland (n/N = 3/132, 2%), Ireland (n/N = 2/132, 2%), and Turkey (n/N = 1/132, 1%). Countries of origin were not traceable for 11% (n/N = 15/132) of the samples.
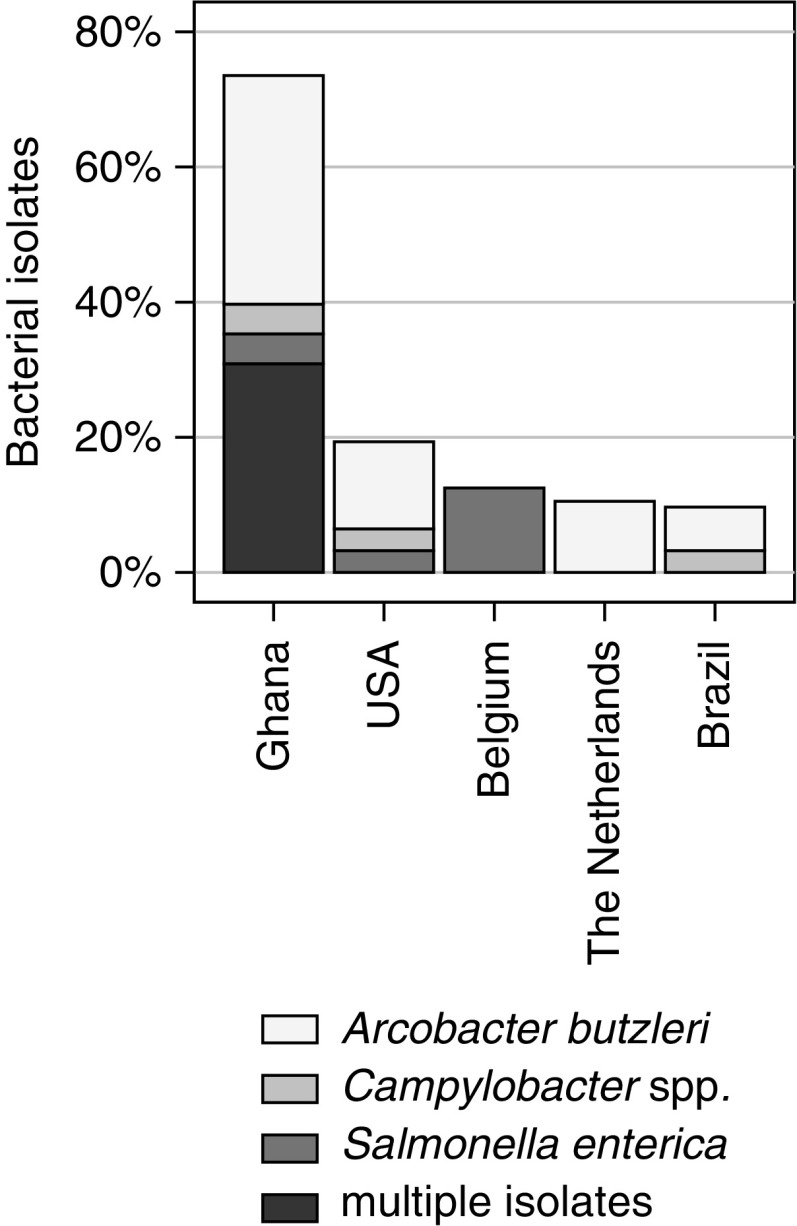
Isolates: of all collected samples, 9% (n = 17) were positive for Salmonella, 11% (n = 22) for Campylobacter, and 27% (n = 53) for Arcobacter. In local meat, Salmonella was detected in 19% (n/N = 13/68), Campylobacter in 28% (n/N = 19/68), and Arcobacter in 62% (n/N = 42/68) of the samples. In contrast, in imported meat, Salmonella, Campylobacter, and Arcobacter were found in 3% (n/N = 4/132), 2% (n/N = 3/132), and 8% (n/N = 11/132), respectively. Accordingly, there was a significant difference between locally sourced compared with imported meat products with higher frequencies in local meat for Salmonella (PR = 6.3; 95% CI: 2.1–16.6), Campylobacter (PR = 12.3; 95% CI: 3.8–40.1), and Arcobacter (PR = 7.4; 95% CI: 4.1–13.5). Multiple pathogens (pathogens under investigation found simultaneously) were primarily observed in local meat wherein 18 of 68 (26%) samples were concurrently positive with two different bacteria and 3 of 68 (4%) meat samples contained three bacterial genera. The country of origin of bacterial isolates from imported meat (considering countries with at least two samples) is summarized in Figure 1. Salmonella serovars identified included Kentucky (n/N = 5/16; 31%), Poona (n/N = 4/16; 25%), Agama (n/N = 3/16; 19%), Enteritidis (n/N = 2/16; 13%), Kaapstad (n/N = 1/16; 6%), and Ajiobo (n/N = 1/16; 6%). One serovar could not be identified due to loss of the isolate during culturing. Campylobacter comprised the species C. coli (n/N = 10/19, 53%) and C. jejuni (n/N = 9/19, 47%). Three Campylobacter spp. were lost during culturing, the species could not be identified. All Arcobacter spp. (n/N = 53/53, 100%) were A. butzleri.
FIG. 1.
Frequencies of bacterial isolates in 200 meat samples of different origin. The category multiple isolates contains at least two different pathogens of Salmonella enterica, Arcobacter butzleri, or Campylobacter spp.
Antibiotic resistance: the highest rate of resistance for Salmonella was revealed for tetracycline (n/N = 16/16; 100%) followed by ciprofloxacin (n/N = 10/16; 63%) and ampicillin (n/N = 9/16; 56%; Table 1). One imported Salmonella Agama isolate showed multidrug resistance. All Salmonella were sensitive to cefotaxime and ceftazidime with no isolates exhibiting ESBL production. The 10 ciprofloxacin-resistant isolates were all from Ghanaian poultry (except one sample whose origin could not be determined). Three ST198 Salmonella Kentucky isolates with a ciprofloxacin MIC of 16 μg/mL showed several mutations, including double mutations in gyrA and in parC (Table 2). Five isolates within the serovars Poona and Kentucky carried the plasmid-mediated quinolone resistance (PMQR) gene qnrB2 and qnrB19, respectively. The four Salmonella Poona isolates carried additionally the aminoglycoside resistance gene aacA4 gene.
Table 1.
Antibiotic Susceptibility for Salmonella and Campylobacter
| No. of resistant isolates (%) | ||
|---|---|---|
| Drug | Campylobacter (n = 20) | Salmonella (n = 16) |
| Ampicillin | — | 9 (56) |
| Amoxicillin/clavulanic acid | — | 5 (31) |
| Cefotaxime | — | 0 (0) |
| Ceftazidime | — | 0 (0) |
| Chloramphenicol | — | 1 (6) |
| Ciprofloxacin | 15 (75) | 10 (63) |
| Erythromycin | 6 (30) | — |
| Tetracycline | 14 (70) | 16 (100) |
| Trimethoprim/sulfamethoxazole | — | 11 (69) |
Campylobacter: two missing intermediate results interpreted as resistant; Salmonella: one missing; “—” indicates not tested.
Table 2.
Mechanisms of Quinolone Resistance for Salmonella
| Serovar | Mechanism | MICcip (μg/mL) | No. of isolates | MLST | Country of origin |
|---|---|---|---|---|---|
| Salmonella Enteritidis | D87G gyrA | 0.25 | 1 | ST11 | Ghana |
| Salmonella Agama | D87G gyrA | 2 | 1 | ST28 | Ghana |
| Salmonella Poona | T57S parC**, qnrB2 | 0.5 | 3 | ST308 | Ghana |
| Salmonella Poona | T57S parC**, qnrB2 | 0.5 | 1 | Unknown | Unknown* |
| Salmonella Kentucky | D87Y + S83F gyrA; T57S, S80I parC | 16 | 3 | ST198 | Ghana |
| Salmonella Kentucky | T57S parC, qnrB19 | 0.5 | 1 | ST314 | Ghana |
Isolate imported: unknown country of origin.
T57S mutation in parC is not or doubtfully responsible for resistance phenotype (Wasyl et al. 2014).
CIP, ciprofloxacin; MLST, multilocus sequence typing; ST, sequence type.
Resistance in Campylobacter was highest for ciprofloxacin (n/N = 15/20, 75%) and tetracycline (n/N = 17/20, 85%). Multidrug resistance was detected in four strains (n/N = 4/20, 20%; Table 1).
As already mentioned, no breakpoints have been defined for A. butzleri susceptibility testing. However, 25 of 53 (47%) isolates were observed with no measurable zones of inhibition (ø < 6 mm) for ciprofloxacin. Although only single Thr86IIe gyrA mutations were detected in C. coli, two C. jejuni isolates revealed double or triple point mutations (Table 3). For A. butzleri, the Thr85Ile point mutation could be associated with quinolone resistance (Abdelbaqi et al., 2007), which was detected in 15 isolates. These 15 isolates are a subset of the 25 isolates with no inhibition zone for ciprofloxacin (Table 3).
Table 3.
Point Mutations in Quinolone Resistance-Determining Region of gyrA in Ciprofloxacin-Resistant Campylobacter coli and Campylobacter jejuni and Arcobacter butzleri
| Species | Mutation in gyrA | No. of isolates |
|---|---|---|
| Campylobacter jejuni | Ser22Gly, Thr86Ile, Asn203Ser | 3 |
| Thr86Ile, Asn203Ser* | 1 | |
| Thr86IIe | 1 | |
| Campylobacter coli | Thr86IIe | 9 |
| Arcobacter butzleri | Thr86IIe | 15 |
One isolate imported from Brazil; remaining isolates from Ghana.
Discussion
Results from this study highlight the relevance of S. enterica, C. coli/C. jejuni and A. butzleri in poultry sold in Ghana. The proportion of contamination was higher for locally produced poultry than for imported meat products. In comparison, previous studies from Ghana found similar frequencies for Campylobacter and Salmonella in poultry, suggesting locally produced poultry as an important source for the spread of the studied bacteria (Sackey et al., 2001; Adu-Gyamfi et al., 2012). Numerous reports on poultry-associated NTS outbreaks due to contaminated meat products have been described worldwide, highlighting the potential of this pathogen to cause such outbreaks, which emphasizes the need for control especially within countries with weakened or absent surveillance systems (Niehaus et al., 2011). Reports for Arcobacter are limited but the frequencies found in this study were lower than that found in a study conducted in Nigeria where 32% of chicken samples contained Arcobacter (Adesiji et al., 2012). Furthermore, the demonstrated data showed that the microbiological quality with regard to the bacteria under investigation was better for frozen imported meat, which urges Ghanaian public health authorities and policy makers to enforce guidelines and regulations on food hygiene to meet the same hygienic standards. In the United States, a common practice is chlorination of meat products to reduce bacterial contamination (Paul et al., 2017). Although chlorination has been banned in Europe (EFSA, 2010), a series of measures along the production line were implemented, such as protective clothing for farm workers, hygienic slaughtering practices, and adequate transportation and storage. In comparison, the freshly slaughtered animals at local markets in Ghana did not undergo any of the mentioned procedures.
As for frozen meat products, the increasing demand of chicken in Africa including Ghana has resulted in large amounts of imports from other countries. The United States, Brazil, and Europe (mainly the Netherlands and Belgium) are the main countries for poultry exports, having contributed to 66% of poultry imports to Ghana in 2016 (https://comtrade.un.org) and encompassing 82% among the samples from imports. Although low temperatures seem to have no significant effect on the viability of certain bacterial genera such as, for example, on Escherichia as outlined in a study by Eibach et al. (2018), Campylobacter spp. and Arcobacter spp. are known to show reduced viability when exposed to low temperatures such as freezing (Chan et al., 2001). This effect could have been a further contributing factor accounting for lower frequencies in imported meat. Nevertheless, imported meat products do still represent a transmission reservoir for the studied bacteria. This observation was also highlighted by a European-wide baseline study conducted by the European Food Safety Authority (EFSA), which showed that member state prevalence in poultry varied from 4.9% to 100% for Campylobacter and 0% to 26.6% for Salmonella (EFSA, 2010). Country-specific differences were also seen in the EFSA conducted study, hence when looking at imported meat products, these should not be seen as a whole but data should be monitored country specific. As a rule, both imported and locally produced poultry could have been contaminated at all points of the animal production and food processing chain, including handling on the farm, during slaughter, and distribution. This fact should be taken into account when interpreting data. In either case, human contamination after product handling and consumption is unlikely but cannot be excluded. In contrast, cross-contamination from one animal to another during slaughtering could have occurred, which may have increased the overall number of bacterial isolates. However, regardless of the contaminating source, the studied bacteria found on sold meat products do represent without doubt a potential source of infections for the end consumer.
Antibiotic resistance to locally available drugs was considerably high. In particular fluoroquinolone resistance poses a significant public health challenge as it is among the key agents in use for the treatment of infections in Ghana. In animal husbandry, tetracyclines, penicillin/streptomycin combinations, and sulfonamides form the predominant antibiotic classes (Raufu et al., 2014). For the same antibiotic classes, the highest resistance rates were observed in this study. There is strong evidence that antibiotic use in animal husbandry can lead to selection and then transmission of multidrug-resistant bacteria to humans (Chang et al., 2015). Even though antibiotic treatment for infections that are typically self-limiting is not usually indicated, severe infections or invasive infections might require treatment. Hence, the spread of multidrug-resistant bacteria within communities, for which no alternative drugs are available, might compromise current treatment strategies in Ghana.
Quinolone resistance is typically a consequence of specific mutations in gyrase and topoisomerase IV genes (Aldred et al., 2014). The present data demonstrate mutations in the QRDR of the gyrA gene of S. enterica, C. jejuni, C. coli, and A. butzleri isolates. Furthermore, previously reported PMQR genes and the aminoglycoside acetyltransferase gene aacA4 have been detected in S. enterica isolates (Aldred et al., 2014).
There was a significant contamination of Ghanaian poultry with the Salmonella serovar Kentucky (ST198). Salmonella Kentucky ST198 first emerged in Egypt and has since been associated with high-level ciprofloxacin resistance (Raufu et al., 2014). In the past years, this ST has spread to several countries, including SSA, the Middle East, and Europe (Le Hello et al., 2011). Previous investigations in broiler farms and slaughterhouses in Ghana demonstrated one dominant Salmonella Kentucky strain but the ST was not investigated (Andoh et al., 2016). Another interesting ST found in this study was Salmonella Poona ST308 with plasmid-mediated resistance to ciprofloxacin. One isolate of this ST was recently reported from a human infection in Ghana (Kudirkiene et al., 2018). Further studies are needed that focus on the spread and epidemiology of these circulating strains within Ghana. This study had a few limitations. Although for each bacterial detection ∼5 g of sample was used, larger quantities might have increased the level of bacterial detection. The sample size is quite small, was drawn only from urban areas, and focused on chicken originating from small farms. Therefore, our results may not be representative of all chicken consumed in Ghana.
Conclusions
Salmonella, Campylobacter, and Arcobacter predominantly in locally produced meat represent a potential transmission reservoir for human infections. Also, the observed overall high level of resistance seen in locally available antibiotics, in particular, to fluoroquinolones is concerning. This level of resistance urges the implementation of strict surveillance systems that focus on food hygiene as well as on the use of antibiotics in animal farming. Furthermore, health authorities need to inform the public about emerging resistant strains in the communities such as the highly ciprofloxacin-resistant Salmonella Kentucky-ST198 and Salmonella Poona ST308; molecular investigations are the essential precondition for this. Surveillance programs also need to be coordinated with controlling the microbiological quality of imported meat and the food production end consumer chain by increasing awareness on food hygiene in the population.
Acknowledgments
We especially thank Wolfgang Rabsch for his continuous support and commitment to the study. The authors also thank Sibylle Müller-Bertling, Kirstin Ganske, Susanne Kulbe, Marita Wahnfried, and Doris Winter for excellent technical laboratory assistance. This work was supported by the German Centre for Infection Research (DZIF) (funding number 8000 201-3).
Disclosure Statement
The authors declare no conflict of interest.
References
- Abdelbaqi K, Ménard A, Prouzet-Mauleon V, Bringaud F, Lehours P, Mégraud F. Nucleotide sequence of the gyrA gene of Arcobacter species and characterization of human ciprofloxacin-resistant clinical isolates. FEMS Immunol Med Microbiol 2007;49:337–345 [DOI] [PubMed] [Google Scholar]
- Abdi RD, Mengstie F, Beyi AF, Beyene T, Waktole H, Mammo B, Ayana D, Abunna F. Determination of the sources and antimicrobial resistance patterns of Salmonella isolated from the poultry industry in Southern Ethiopia. BMC Infect Dis 2017;17:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adesiji YO, Alli OT, Adekanle MA, Jolayemi JB. Prevalence of Arcobacter, Escherichia coli, Staphylococcus aureus and Salmonella spp. in retail chicken, pork, beef, goat meat in Osogbo, Nigeria. Sierra Leon J of Biomed Res 2012;3:8–12 [Google Scholar]
- Adu-Gyamfi A, Torgby-Tetteh W, Appiah V. Microbiological quality of chicken sold in Accra and determination of D10-value of E. coli. Food Nutr Sci 2012;3:6 [Google Scholar]
- Aldred KJ, Kerns RJ, Osheroff N. Mechanism of quinolone action and resistance. Biochemistry 2014;53:1565–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andoh LA, Dalsgaard A, Obiri-Danso K, Newman MJ, Barco L, Olsen JE. Prevalence and antimicrobial resistance of Salmonella serovars isolated from poultry in Ghana. Epidemiol Infect 2016;144:3288–3299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atabay HI, Aydin F, Houf K, Sahin M, Vandamme P. The prevalence of Arcobacter spp. on chicken carcasses sold in retail markets in Turkey, and identification of the isolates using SDS-PAGE. Int J Food Microbiol 2003;81:21–28 [DOI] [PubMed] [Google Scholar]
- Chan KF, Le Tran H, Kanenaka RY, Kathariou S. Survival of clinical and poultry-derived isolates of Campylobacter jejuni at a low temperature (4 degrees C). Appl Environ Microbiol 2001;67:4186–4191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q, Wang W, Regev-Yochay G, Lipsitch M, Hanage WP. Antibiotics in agriculture and the risk to human health: How worried should we be? Evol Appl 2015;8:240–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau TT, Campbell JI, Galindo CM, Van Minh Hoang N, Diep TS, Nga TT, Van Vinh Chau N, Tuan PQ, Page AL, Ochiai RL, Schultsz C, Wain J, Bhutta ZA, Parry CM, Bhattacharya SK, Dutta S, Agtini M, Dong B, Honghui Y, Anh DD, Canh DG, Naheed A, Albert MJ, Phetsouvanh R, Newton PN, Basnyat B, Arjyal A, La TT, Rang NN, Phuong LT, Van Be Bay P, von Seidlein L, Dougan G, Clemens JD, Vinh H, Hien TT, Chinh NT, Acosta CJ, Farrar J, Dolecek C. Antimicrobial drug resistance of Salmonella enterica serovar Typhi in Asia and molecular mechanism of reduced susceptibility to the fluoroquinolones. Antimicrob Agents Chemother 2007;51:4315–4323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado L, Figueras MJ. Taxonomy, epidemiology, and clinical relevance of the genus Arcobacter. Clin Microbiol Rev 2011;24:174–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corry J, Atabay HI. Poultry as a source of Campylobacter and related organisms. Symp Ser Soc Appl Microbiol 2001;30:96–114 [DOI] [PubMed] [Google Scholar]
- EFSA 2010 scientific report. Analysis of the baseline survey on the prevalence of Campylobacter in broiler batches and of Campylobacter and Salmonella prevalence estimates. EFSA J 2010;8:1503 [Google Scholar]
- EFSA and ECDC scientific report 2015. EU Summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food 2013. EFSA J 2015;13:4036 [Google Scholar]
- Eibach D, Al-Emran HM, Dekker D, Krumkamp R, Adu-Sarkodie Y, Cruz Espinoza LM, Ehmen C, Boahen K, Heisig P, Im J, Jaeger A, von Kalckreuth V, Pak GD, Panzner U, Park SE, Reinhardt A, Sarpong N, Schütt-Gerowitt H, Wierzba TF, Marks F, May J. The emergence of reduced ciprofloxacin susceptibility in Salmonella enterica causing bloodstream infections in Rural Ghana. Clin Infect Dis 2016;62(Suppl. 1):S32–S36 [DOI] [PubMed] [Google Scholar]
- Eibach D, Dekker D, Gyau Boahen K, Wiafe C. Akenten, Sarpong N, Belmar Campos C, Berneking L, Aepfelbacher M, Krumkamp R, Owusu-Dabo E, May J. Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in local and imported poultry meat in Ghana. Vet Microbiol 2018;217:7–12 [DOI] [PubMed] [Google Scholar]
- Geilhausen B, Schütt-Gerowitt H, Aleksic S, Koenen R, Mauff G, Pulverer G. Campylobacter and Salmonella contaminating fresh chicken meat. Zentralbl Bakteriol 1996;284:241–245 [DOI] [PubMed] [Google Scholar]
- Grimont P, Weill FX. Antigenic Formulae of the Salmonella serovars, 9th ed. Paris, France: WHO Collaborating Centre for Reference and Research on Salmonella, Institut Pasteur, 2007 [Google Scholar]
- Kluytmans JA, Overdevest IT, Willemsen I, Kluytmans-van den Bergh MF, van der Zwaluw K, Heck M, Rijnsburger M, Vandenbroucke-Grauls CM, Savelkoul PH, Johnston BD, Gordon D, Johnson JR. Extended-spectrum β-lactamase-producing Escherichia coli from retail chicken meat and humans: Comparison of strains, plasmids, resistance genes, and virulence factors. Clin Infect Dis 2013;56:478–487 [DOI] [PubMed] [Google Scholar]
- Kotloff KL, Platts-Mills JA, Nasrin D, Roose A, Blackwelder WC Levine MM. Global burden of diarrheal diseases among children in developing countries: Incidence, etiology, and insights from new molecular diagnostic techniques. Vaccine 2017;35(49 Pt A):6783–6789 [DOI] [PubMed] [Google Scholar]
- Krumkamp R, Sarpong N, Schwarz NG, Adlkofer J, Loag W, Eibach D, Hagen RM, Adu-Sarkodie Y, Tannich E, May J. Correction: Gastrointestinal infections and diarrheal disease in ghanaian infants and children: An outpatient case-control study. PLoS Negl Trop Dis 2015;9:e0003728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudirkiene E, Andoh LA, Ahmed S, Herrero-Fresno A, Dalsgaard A, Obiri-Danso K, Olsen JE. The use of a combined bioinformatics approach to locate antibiotic resistance genes on plasmids from whole genome sequences of Salmonella enterica serovars from humans in Ghana. Front Microbiol 2018;9:1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Hello S, Hendriksen RS, Doublet B, Fisher I, Nielsen EM, Whichard JM, Bouchrif B, Fashae K, Granier SA, Jourdan-Da Silva N, Cloeckaert A, Threlfall EJ, Angulo FJ, Aarestrup FM, Wain J, Weill FX. International spread of an epidemic population of Salmonella enterica serotype Kentucky ST198 resistant to ciprofloxacin. J Infect Dis 2011;204:675–684 [DOI] [PubMed] [Google Scholar]
- Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, harbarth S, Hindler JF, Kahlmeter G, Olsson-Lijequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A WE.ber JT, Monnet DL. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for aquired resistance. Clin Microbiol Infect 2012;18:268–281 [DOI] [PubMed] [Google Scholar]
- Maron DF, Smith TJ, Nachman KE. Restrictions on antimicrobial use in food animal production: An international regulatory and economic survey. Global Health 2013;9:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehaus AJ, Apalata T, Coovadia YM, Smith AM, Moodley P. An outbreak of foodborne salmonellosis in rural KwaZulu-Natal, South Africa. Foodborne Pathog Dis 2011;8:693–697 [DOI] [PubMed] [Google Scholar]
- Paul NC, Sullivan TS, Shah DH. Differences in antimicrobial activity of chlorine against twelve most prevalent poultry-associated Salmonella serotypes. Food Microbiol 2017;64:202–209 [DOI] [PubMed] [Google Scholar]
- Pfeifer Y, Matten J, Rabsch W. Salmonella enterica serovar Typhi with CTX-M beta-lactamase, Germany. Emerg Infect Dis 2009;15:1533–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham NT, Ushijima H, Trinh QD, Khamrin P, Komine-Aizawa S, Okitsu S, Maneekarn N, Hayakawa S. Prevalence and antimicrobial resistance of Campylobacter jejuni and Campylobacter coli from adult hospitalized patients with diarrhea in Thailand. Clin Lab 2015;61:1809–1812 [DOI] [PubMed] [Google Scholar]
- Pietsch M, Eller C, Wendt C, Holfelder M, Falgenhauer L, Fruth A, Grössl T, Leistner R, Valenza G, Werner G, Pfeifer Y. Molecular characterisation of extended-spectrum β-lactamase (ESBL)-producing Escherichia coli isolates from hospital and ambulatory patients in Germany. Vet Microbiol 2007;200:130–137 [DOI] [PubMed] [Google Scholar]
- Raufu I, Fashae K, Ameh JA, Ambali A, Ogunsola FT, Coker AO, Hendriksen RS. Persistence of fluoroquinolone-resistant Salmonella enterica serovar Kentucky from poultry and poultry sources in Nigeria. J Infect Dev Ctries 2014;8:384–388 [DOI] [PubMed] [Google Scholar]
- Sackey BA, Mensah P, Collison E, Sakyi-Dawson E. Campylobacter, Salmonella, Shigella and Escherichia coli in live and dressed poultry from metropolitan Accra. Int J Food Microbiol 2001;71:21–28 [DOI] [PubMed] [Google Scholar]
- Tang Y, Sahin O, Pavlovic N, LeJeune J, Carlson J, Wu Z, Dai L, Zhang Q. Rising fluoroquinolone resistance in Campylobacter isolated from feedlot cattle in the United States. Sci Rep 2017;7:494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasyl D, Hoszowski A, Zając M. Prevalence and characterisation of quinolone resistance mechanisms in Salmonella spp. Vet Microbiol 2014;171:307–314 [DOI] [PubMed] [Google Scholar]