Graphical Abstract
Graphical Abstract.
Keywords: Ischaemia/reperfusion injury , Myocardial infarction , Nervous system , Cardioprotection
Abstract
Acute myocardial infarction (AMI) and the heart failure (HF) that often complicates this condition, are among the leading causes of death and disability worldwide. To reduce myocardial infarct (MI) size and prevent heart failure, novel therapies are required to protect the heart against the detrimental effects of acute ischaemia/reperfusion injury (IRI). In this regard, targeting cardiac innervation may provide a novel therapeutic strategy for cardioprotection. A number of cardiac neural pathways mediate the beneficial effects of cardioprotective strategies such as ischaemic preconditioning and remote ischaemic conditioning, and nerve stimulation may therefore provide a novel therapeutic strategy for cardioprotection. In this article, we provide an overview of cardiac innervation and its impact on acute myocardial IRI, the role of extrinsic and intrinsic cardiac neural pathways in cardioprotection, and highlight peripheral and central nerve stimulation as a cardioprotective strategy with therapeutic potential for reducing MI size and preventing HF following AMI. This article is part of a Cardiovascular Research Spotlight Issue entitled ‘Cardioprotection Beyond the Cardiomyocyte’, and emerged as part of the discussions of the European Union (EU)-CARDIOPROTECTION Cooperation in Science and Technology (COST) Action, CA16225.
This article is part of the Spotlight Issue on Cardioprotection Beyond the Cardiomyocyte.
1. Introduction
Acute myocardial infarction (AMI) and the heart failure that often complicates this condition, are among the leading causes of death and disability worldwide. To reduce myocardial infarct (MI) size and prevent heart failure, novel therapies are required to protect the heart against acute ischaemia/reperfusion injury (IRI). In this regard, the dense cardiac network of parasympathetic and sympathetic nerves and their interactions with the intrinsic cardiac nerve system (ICNS), may provide novel targets for cardioprotection. This cardiac neural network influences myocardial rhythm and contractile function, and the susceptibility to acute IRI. It also contributes to cardioprotective strategies such as ischaemic preconditioning (IPC) and remote ischaemic conditioning (RIC). In this article, we provide an overview of cardiac innervation with a focus on acute myocardial IRI, the role of extrinsic and intrinsic cardiac innervation in cardioprotection, and highlight peripheral and central nerve stimulation as a cardioprotective strategy with therapeutic potential for improving clinical outcomes in AMI patients.
2. An overview of the cardiac neural network
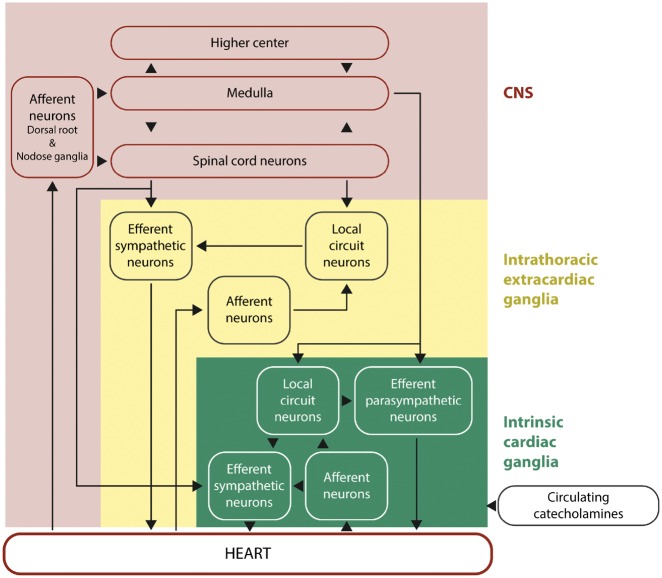
The heart is innervated by a complex interacting hierarchal network of neural pathways within the central nervous system (CNS), intrathoracic extracardiac ganglia, and intrinsic cardiac ganglia of the ICNS (see Figure1).1 The heart is supplied and controlled by sympathetic and parasympathetic nerves, which receive sensory inputs from heart, blood vessels, and other organs. The parasympathetic nerves interface with ganglionic neurons of the ICNS, whereas the sympathetic nerves traverse ganglia without synapsing on ganglionic neurons, and together they provide beat-to-beat regulation of heart rhythm and contractile function. Sympathetic stimulation increases heart rate and cardiac contractile function through activation of beta-adrenoceptors, and vagal activation reduces heart rate and in some species, cardiac contractile function, through activation of muscarinic receptors (reviewed in references 2 and 3).
Figure 1.
Hierarchy of cardiac innervation to the heart. This figure shows the complex and hierarchal interactions between the different components of the neural pathways of the CNS, intrathoracic extracardiac ganglia, and intrinsic cardiac ganglia of the intrinsic cardiac nervous system. This figure has been modified from Armour with permission.1
Pre-ganglionic fibres of the parasympathetic nervous system (SNS) arise from the medulla oblongata, and via the vagal nerves, secrete acetylcholine (Ach) which binds to the nicotinic Ach receptors on the plasma membrane of post-ganglionic fibres. These in turn secrete Ach, which binds to the type 2 muscarinic Ach receptors present on the plasma membrane of cardiac cells in the sinoatrial (SA) node, atrioventricular (AV) node, left ventricle, and to some extent also other parts of the heart, resulting in a reduction in contraction rate of cardiac muscle by shortening its action potential duration (APD) and conduction velocity, by hyperpolarizing SA nodal cells that reduce heart rate.
Within the myocardium there exists an ICNS, comprising cardiac ganglia and interconnecting neurons (known as ganglionic plexuses), which process sensory information and modulate efferent post-ganglionic parasympathetic and sympathetic activity within the heart, in the absence of any central modulation (reviewed in reference 3). The extrinsic parasympathetic and sympathetic nerves access the ICNS arterially, around the roots of the pulmonary artery and aortic root, and interface with the venous portion of the heart around the roots of the pulmonary veins and superior vena cava. The number of cardiac ganglia varies between species from 19 in mice to over 800 in humans, and they are mainly located on the dorsal atrial surface, around the base of the aorta and pulmonary artery, dorsal and ventral to the pulmonary veins, and on the anterior ventricular surface. From these cardiac ganglia, intrinsic cardiac nerves extend epicardially from ganglionic plexuses to innervate the atria, interatrial septum and the ventricles. A number of neurochemicals shave been found within the ICNS, the presence of which highlight the existence of both parasympathetic and sympathetic nervous components within the atria and ventricles. The majority of the cardiac ganglia are cholinergic (containing choline acetyltransferase, responsible for the synthesis of acetylcholine) which innervate supraventricular myocardium in and around the sinoatrial and atrioventricular nodes as well as the left ventricle, and these co-exist with both neuronal nitric oxide synthase (nNOS) [responsible for producing nitric oxide (NO)], and vasoactive intestinal peptide. The cardiac ganglia also include adrenergic nerve fibres (containing tyrosine hydroxylase, for the production of noradrenaline) within the left and right coronary subplexuses that innervate the ventricles, with which neuropeptide Y (NPY) is co-released. Within the ICNS there are also neuronal subpopulations that are non-adrenergic and non-cholinergic.4 Activation of the ICNS can result in local and/or remote cardiac changes with effects on cardiac function and rhythm that are dependent on location. The ganglionic plexuses can modulate post-ganglionic parasympathetic nerve activity and selectively modulate vagal control of heart rate, atrio-ventricular conduction and left ventricular inotropy.5–7 The ganglionic plexuses may also help mediate the differential effects of sympathetic nerves stimulation of the heart, with nerves arising from the left sympathetic chain influencing LV contractile function and electrical conduction via the AVN to a greater degree than the right, whilst the nerves arising from the right sympathetic chain have a more significant modulator effect on sinus rate via the sinoatrial node.8 The ICNS can respond to a variety of stimuli including acute IRI and influence cardiac function on a beat-to-beat basis and have been implicated in both acute IRI and cardioprotection.9
3. Cardiac innervation and acute myocardial IRA
Sensory nerve endings may detect consequences of acute ischaemia, such as hypoxia, lactate, K+ and low pH, which stimulate cardiac sensory nerves to release their neuropeptide transmitters.3,10 Local afferent function of these sensory nerves may have a strong influence on cardiac function through cardio-cardiac reflexes and initiate adaptive responses due to their NO and vasoactive neuropeptides, such as calcitonin gene-related peptide (CGRP), substance P, somatostatin.10–12 Indeed, in selective sensory desensitization by capsaicin, a ligand for TRPV channels, cardiac sensory nerves were demonstrated to strongly influence gene expression patterns in rat hearts,13 regulation of the cardiac NO-cGMP system,11 SERCA function,14 with potential effects on cardiac function. Moreover, cardiac sensory nerves play a role in acute myocardial injury and adaptation to ischaemic stress,12,15 and in the mechanism of doxorubicin-induced heart failure.16 Intact cardiac sensory nerves have been shown to protect against acute IRI-induced cell death via the local release of NO and cytoprotective neuropeptides.12 Sympathetic efferent nerve terminals release norepinephrine (NE) and exacerbate IRI-induced cardiac cell death directly and indirectly by deterioration of oxygen supply and by increasing oxygen demand.2
Cardiac sympathetic afferent denervation attenuates cardiac remodelling and improves cardiovascular function in rats with heart failure.17 Modulation of neural networks outside the heart can also impact on post-AMI remodelling with renal nerve denervation preventing adverse post-AMI LV remodelling and preserving vascular function in both spontaneously hypertensive rats and normotensive rats, effects which were mediated by reduced neprilysin activity and preservation of circulating natriuretic peptide levels.18 Interestingly, blockade of beta-adrenoceptors directly in the brain via chronic intracerebroventricular administration of metoprolol attenuated post-AMI LV remodelling in a rat model of myocardial infarction-induced heart failure, suggesting that the action of certain beta-blockers in the brain could contribute to the beneficial effect of beta-blockers in the failing heart.19
Cardiac sympathetic neurons in the stellate ganglia co-express NPY and the neurotransmitter NE. Following acute myocardial IRI, axonal damage and the inflammatory response to injury, result in suppression of NPY and NE expression, and enhanced expression of neuropeptides such as vasoactive intestinal peptide, substance P, and galanin. Habecker et al.20 observed extensive axon damage after AMI, and this was associated with a significant increase in galanin (a peptide which promotes regeneration of sensory neurons21) in cardiac sympathetic neurons in the left ventricle, suggesting the existence of an endogenous protective strategy based on neuropeptides in cardiac sympathetic neurons. The susceptibility to acute myocardial IRI differs between cardiomyocytes and neurons, and found that cardiac sympathetic neurons are more susceptible to acute myocardial IRI than cardiomyocytes.22
Most cardiac neurons of the ICNS are perivascular, making them susceptible to acute myocardial IRI, thereby setting an environment for neuronal remodelling following AMI. In response to acute myocardial IRI, pathological and degenerative changes to the cardiac ganglia occur with the appearance of cytoplasmic inclusions, a feature in common with neuronal degeneration disorders. Acute myocardial IRI induces reorganization and remodelling within ganglionic plexuses of the ICNS in the first 7 days post-AMI, resulting in increased adrenergic sensitivity and enhanced nNOS expression within parasympathetic post-ganglionic neurons within the ICNS.23 Pathological features of damaged cardiac nerves include enlargement, and degenerative changes to dendrites and axons, and the appearance of cytoplasmic inclusions.24,25 Neuronal remodelling also occurs within regions of non-infarcted myocardium, presumably enabling a compensatory response in remote myocardium and impacting on post-AMI cardiac remodelling. In this regard, it has been proposed that enhanced nNOS expression plays a protective role, attenuating the initial increase in centrally derived sympathetic activity and facilitating parasympathetic neuronal inputs.23 However, the actual interplay between the ICNS and extrinsic vagal or sympathetic nerves in the setting of AMI needs to be further elucidated.
3.1 Protection of cardiac neurons against acute myocardial IRI
The majority of studies investigating cardioprotective strategies for protecting the heart against the detrimental effects of acute IRI have focused on preventing cell death of cardiomyocytes and only few studies have explored the beneficial effects on cardiac neurons. During acute myocardial ischaemia, damage to cardiac sympathetic neurons results in the release of NE into the myocardial interstitial space. IPC can reduce myocardial NE levels following acute myocardial IRI in rat and rabbit hearts.26–28 Miura et al.28 demonstrated that the detrimental effects of acute myocardial IRI on cardiac sympathetic nerves were reduced by IPC, and the mechanism underlying this neuroprotective effect was attributed to KATP channel opening. The neurotrophin, nerve growth factor (NGF) is known to support survival and differentiation of sympathetic neurons, and is elevated following AMI in a spinal nerve-dependent manner (thoracic epidural anaesthesia prevented the increase in NGF following AMI).29 Strande et al.30 have shown that NGF administered prior to ischaemia reduced MI size in an in vivo rat AMI model, and this effect was mediated through PI3K and NOS. A recent clinical study has shown that limb RIC can reduce the muscle sympathetic nerve activity in the forearm induced by acute ischaemia, and this was associated with decreased production of an erythrocyte marker of oxidative stress and the reduction of NO availability, and ameliorated ischaemic reactive hyperaemia.31 Further studies are required to investigate whether cardioprotective strategies can protect cardiac parasympathetic neurons and the ICNS against the detrimental effects of acute myocardial IRI.
3.2 Cardiac innervation and ventricular arrhythmias following acute myocardial IRA
Ischaemia can directly provoke cardiomyocyte electrical instability, APD heterogeneity, and arrhythmias as a result of ATP depletion, lactate production, reactive oxygen species, K+ accumulation, and other substances e.g. endothelin, which enhances the response of perivascular afferent nerves to autonomic reflexes. Some myocardial ischaemic events can be triggered and enhanced by abnormal central autonomic activity such as emotional stress leading to an imbalance in cardiac sympathovagal tone, reflexly increasing cardiac sympathetic activity leading to further coronary vasoconstriction.32,33 This is often accompanied by vagal withdrawal perpetuating the clinical scenario. Since the ICNS has an integrative role and can exert considerable influence on cardiodynamics, it is possible that significant interaction occurs between the heart’s ‘little brain’3 locally and with peripheral nerves that mediate important mechanisms underlying arrhythmogenesis during acute myocardial IRI. Vagal control of heart rate and release of Ach in non-ischaemic ventricular regions are both blunted following a developed myocardial infarct,34 showing that regional ischaemia can affect non-ischaemic sites both proximal and distal to the insult site.
Preclinical studies support the notion that SNS activity is pro-arrhythmic,35,36 whilst vagal nerve stimulation is anti-arrhythmic.37 High levels of vagal activity exert powerful anti-arrhythmic effects, which can counter the effects of acute ischaemia and sympathetic activation. Mechanisms are complex and include indirect effects of accentuated antagonism against sympathetic activity and direct protective effects on electrophysiological parameters, Ca2+-handling and other important factors such as inflammation and gap junctions.38 Increased dispersion of repolarization is an important mechanism in ventricular arrhythmogenesis, and sympathetic stimulation increases dispersion of repolarization in vivo,39 especially in the ischaemic border which increases propensity to arrhythmias.40
In an innervated isolated heart preparation,41 the kinetics of the APD restitution relationship appear to be a key mechanism by which sympathetic stimulation precipitates ventricular fibrillation (VF), resulting in a steepening of APD-restitution slope—this facilitates alternans and hence wave-breaks to generate VF.42 Vagal nerve stimulation, on the other hand, protects against VF initiation by flattening the slope, an effect which is mediated via NO from neuronal NO synthase,43 a property which appears to be independent of muscarinic activation.44 This NO-mediated protection has been shown to be important with intrapericardial perfusion of L-arginine increasing NO synthase activity, and protecting against VF in open chest dogs during acute coronary artery occlusion.45 Recent proof-of-concept evidence suggests that vagal stimulation via low-level tragus stimulation can reduce arrhythmias related to acute myocardial IRI in patients with STEMI, a finding which needs to be confirmed in larger studies.46
Cardiac remodelling as a result of AMI exaggerates influences on dispersion and APD kinetics which results in increased arrhythmogeneity coupled with cardiac fibrosis, regional denervation,47 and adaptive nerve sprouting and heterogeneous hyperinnervation.48 Neural remodelling also occurs in the stellate ganglion,49 and ICNS which further promotes instability in the already arrhythmogenic environment.25 Preclinical studies have shown a beneficial effect of reducing sympathetic tone through renal artery denervation on ventricular arrhythmias associated to post-AMI LV remodelling.50,51
Clinically, left cardiac sympathetic denervation is effective in reducing arrhythmia burden in otherwise refractory ventricular arrhythmias,52 but with accompanying side effects. Recent evidence supports a cardiotopic arrangement whereby functionally distinct neurons arise from discrete regions of the sympathetic chain,53 which should be targeted for more focused therapy. On the other hand, vagal protection against VF initiation appears to be mediated through a specific population of anti-fibrillatory nitrergic neurons,54 although other indirect and non-arrhythmic mechanisms may also be at work including anti-inflammatory actions and effects on gap junctions.38 Clinical studies using implanted vagal nerve stimulators in patients with heart failure have not produced positive outcomes to date.55 Much work is needed to understand the mechanisms underlying the autonomic modulation of lethal arrhythmias especially following AMI, in order to develop effective therapeutic options.
3.3 Coronary vascular effects of cardiac sympathetic and vagal innervation in myocardial IRI
Both, cardiac sympathetic and vagal nerve activation impact on coronary blood flow through changes in heart rate with secondary effects on MI size.56 Their direct effect on the coronary circulation is more immediate and short-lasting such that it is of greater importance in acute episodes of reversible ischaemic injury than in AMI. Sympathetic activation during exercise, excitement, or pain not only increases heart rate and cardiac contractile function through activation of β-adrenoceptors but also coronary vasoconstriction of epicardial and resistive vessels through activation of α-adrenoceptors.57 In the presence of coronary stenosis, α-adrenergic coronary vasoconstriction is powerful enough to induce lactate production and ischaemic contractile dysfunction.58,59 Acute myocardial ischaemia then elicits a further positive-feedback activation of cardiac sympathetic nerves which then results in progressive α-adrenergic coronary vasoconstriction but can be eliminated by spinal anaesthesia.33 Coronary collateral vessels in dogs have no functional α-adrenoceptors. Hence, the blood flow into collateral-dependent myocardium is not reduced by sympathetic activation.60 Accordingly, chronic sympathetic denervation does not increase collateral blood flow or reduce infarct size after 3 h coronary occlusion in conscious dogs,61 and the same is true in anaesthetized rabbits.62,63 However, chronic sympathetic denervation in mice attenuates post-infarct inflammation and adverse remodelling.64 In anaesthetized pigs, carvedilol but not propranolol improved coronary blood flow after 3 h coronary occlusion/reperfusion and reduced coronary no-reflow, suggesting an action through α- rather than ß-adrenoceptor blockade.65 α-Adrenergic coronary vasoconstriction contributes to acute myocardial ischaemia also in humans.66 In particular, the cardiac sympatho-excitatory reflex elicits α-adrenergic coronary vasoconstriction during stenting in patients with stable angina and with AMI, and α-blockade may therefore improve blood flow during reperfusion following AMI.67
Activation of cardiac vagal nerves reduces MI size, not only through HR reduction, but through a number of mechanisms, including improved mitochondrial function, attenuated formation of reactive oxygen species, and inflammation.68 There is no evidence that cardiac vagal nerve activation improves collateral blood flow during coronary occlusion. However, cardiac vagal nerve activation just prior to reperfusion not only reduces MI size69,70 but also decreases areas of no-reflow after reperfusion.70 Vagal activation by electrical stimulation of the auricular tragus also reduced MI size in patients with AMI.46
3.4 Cardiac innervation and inflammation
The sympathetic control of the immune cell system has been investigated in conditions such as rheumatoid arthritis, asthma, sepsis, and colitis. Peripheral effects of SNS activation have been linked to the release of monocytes from the bone marrow,71 macrophage programming,72 cytokine expression of various immune cells,73 and B cell antibody production.74 More recently, the SNS has been suggested to play a role in the immune response to cardiovascular disease.75
Following AMI, the inflammatory response to acute myocardial IRI plays a critical role in determining MI size and subsequent LV remodelling (reviewed in references 76 and 77). Recent studies have investigated the role of the cardiac SNS in the regulation of the inflammatory response to AMI. In a murine model of AMI, Ziegler et al.64 surgically removed the right and the left superior cervical ganglia, which resulted in near complete loss of myocardial sympathetic innervation in the LV anterior wall. Although this method of cardiac sympathetic denervation did not affect acute myocardial injury and MI size, it did attenuate myocardial inflammation (with less infiltration of macrophages, neutrophils and T cells), and prevent subsequent adverse LV remodelling in terms of less cardiomyocyte hypertrophy, and preserved cardiac function. These findings confirm the importance of chronic SNS activation in post-AMI heart failure as a contributor to adverse LV remodelling. However, the mechanisms through which the cardiac SNS modulates the inflammatory response post-AMI is not known and requires further study. Interestingly, the interaction between the SNS and the immune cell system appears to be mutual, with a study showing less sympathetic hyperinnervation of remote myocardium post-AMI following chemical depleting macrophages with systemic clodronate.78
4. Cardiac innervation and cardioprotection
A number of experimental studies have investigated the role of cardiac innervation, and more recently the ICNS in endogenous cardioprotective strategies.79 Pacing-induced preconditioning requires intact cardiac capsaicin-sensitive sensory innervation, and the release of NO and CGRP from capsaicin-sensitive nerves may be involved in the mechanism of pacing-induced preconditioning.
Cardiomyocytes per se are capable of synthesizing and releasing ACh, an intrinsic cholinergic system which is known as the non-neuronal cholinergic system within the heart.80 Ach is also produced in the myocardium during acute myocardial ischaemia, and exogenous acetylcholine can be a trigger of IPC cardioprotection.81 Bilateral vagotomy did not inhibit ischaemia-induced Ach release in the myocardium.82,83 The role of Ach in the ICNS as a mediator of IPC has recently demonstrated the involvement of intrinsic cardiac ganglia. In an isolated perfused rat heart subjected to acute IRI, the ganglion blocker, hexamethonium, and the muscarinic receptor antagonist, atropine, abrogated IPC cardioprotection. Interestingly, IPC increased acetylcholine in the perfusate, and the cardioprotection induced by this perfusate in a naïve rat heart was also blocked by hexamethonium.84 However, the mechanism through which IPC stimulates the intrinsic cardiac ganglion is not clear, and whether this pathway is operative in vivo is not known. In contrast to these findings, an earlier study by Kudej et al.85 had found that intact cardiac nerves were not required for classical IPC in a porcine acute myocardial IRI model but were required for the second window of protection through the activation of α1-adrenergic receptor and increased expression of iNOS and COX-2. Atropine and bilateral vagotomy did not abolish the infarct-limiting effects of classical IPC in rats.86,87
In the field of cardioprotection, most studies have focused on the role of peripheral and cardiac innervation in RIC, the phenomenon by which brief cycles of non-lethal ischaemia and reperfusion to an organ or tissue away from the heart is able to protect the heart against AMI.88–94
4.1 Cardiac innervation and cardioprotection by RIC
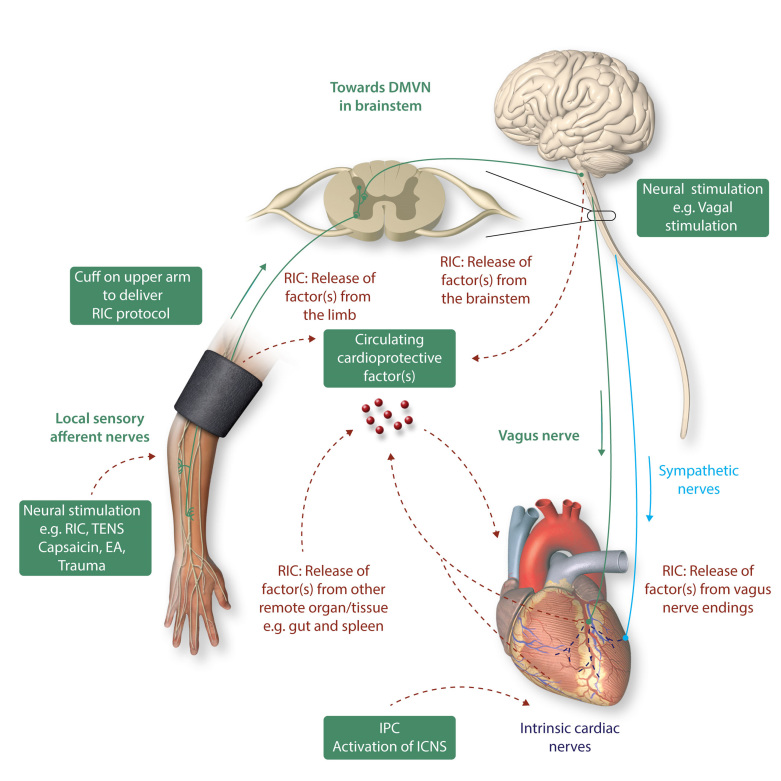
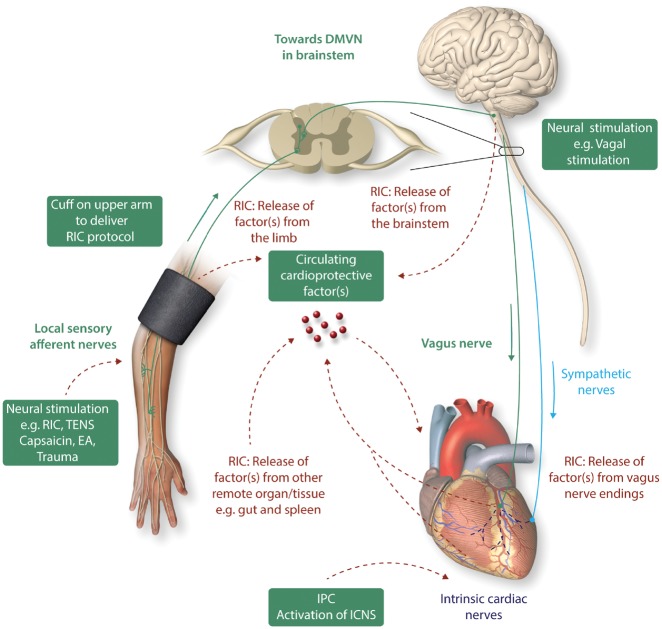
The actual mechanisms underlying cardioprotection induced by RIC remain unclear, although a neuro-hormonal pathway has been implicated (Figure 2).95 With respect to the neural component of RIC cardioprotection, an intact neural pathway is required for application of RIC to the remote organ or tissue. The neural element to the stimulus was demonstrated in some of the earliest experimental studies of RIC, which showed that the cardioprotection induced for example by transient mesenteric ischaemia was completely abrogated when animals were pre-treated with the ganglion blocker hexamethonium.96 Resection of the neural pathway to the lower limb abolished RIC-induced cardioprotection by transient limb ischaemia,87,97,98 showing the dependency of the RIC stimulus upon neural connections between the remote organ and the heart.
Figure 2.
Cardiac innervation and cardioprotection. The heart is innervated by the cardiac sympathetic and parasympathetic afferent and efferent neural pathways which interact with intrinsic cardiac nerves within the heart to modulate myocardial function, susceptibility to acute IRI, and cardiac arrhythmias. Cardioprotection induced by endogenous strategies such as IPC and RIC can modulate the intrinsic cardiac nerves and peripheral sensory afferent nerves in the limb and the vagus nerve, respectively. IPC cardioprotection in the isolated perfused has shown to be dependent on the function of intrinsic cardiac nerves within the heart. RIC which comprises brief non-lethal cycles of ischaemia and reperfusion to the limb, via cuff inflation/deflation, causes local autacoid release. This in turn activates sensory afferent neurons which relay, via the spinal cord, to the dorsal nucleus of vagal nerve (DMVN) in the CNS. Activation of nuclei within the DMVN results in increased vagal nerve firing to the heart which, via release of Ach and subsequent activation of muscarinic Ach receptors induces the cardioprotective phenotype. In addition, following activation of afferent sensory neurons in the conditioned limb, there is release of a dialysable cardioprotective factor into the systemic circulation. The source of this factor remains unknown, although possibilities include: (i) from the conditioned limb itself, (ii) from the central nervous system, (iii) from pre-/post-ganglionic parasympathetic nerve endings within the heart, and (iv) from a non-conditioned remote organ/tissue such as the gut or spleen. Neural stimulation of sensory afferent nerves [by RIC, transcutaneous nerve stimulation (TENS), trauma, EA, or topical capsaicin] or of the vagus nerve can induce cardioprotection. This figure has been modified from Sivaraman et al. 91
However, these observations predated the finding that a key component of RIC is the release of cardioprotective substances into the blood, the plasma, and plasma dialysate from animals and humans subjected to transient limb ischaemia being highly cardioprotective when used to perfuse naïve hearts subjected to prolonged ischaemia, or when used to pre-treat isolated cardiomyocytes subjected to simulated IRI.99,100 While the identification of the substance (or substances) released by RIC remain to be determined completely, there is little doubt that their release is dependent upon intact neural pathways to the triggering organ. For example, the aforementioned abrogation of RIC by femoral nerve transection prior to limb RIC was associated with failure to release humoral factor(s), the plasma dialysate from such animals having no cardioprotective activity when tested in Langendorff preparation.101 The testing of ‘cardioprotectivity’ of plasma in this way has proven to be a useful biomarker for dissecting the neuro-humoral pathways potentially involved in other conditioning stimuli.102 It is perhaps unsurprising, given the earlier discussion, that direct stimulation of the femoral nerve leads to release of humoral factor(s) and recapitulates the cardioprotectivity associated with transient limb ischaemia.103 However other, less direct, neural stimuli appear also to invoke this neuro-humoral response. For example, it has long been known that local IPC of the heart and other organs involves the stimulation of capsaicin-sensitive sensory nerves (C-sensory fibres),104 and more recently both surgical incision (presumably via stimulation of sensory fibres) and direct stimulation of sensory nerves in the skin (using topical capsaicin) was shown to induce potent ‘remote’ cardioprotection.105 In subsequent studies, topical capsaicin87,103 and stimulation of sensory nerves via transcutaneous nerve stimulation106 were both shown to release cardioprotective humoral factor(s) into the blood. Interestingly, this humoral response was abolished by pre-treatment with topical DMSO (a sensory nerve blocker) and intra-arterial injection of the NO donor SNAP, presumably via the neuro-inhibitory effects of NO on unmyelinated sensory nerves. Similarly, although conceivably working via other signalling pathways, the ‘preconditioning’ effect of targeted electro-acupuncture (EA) was associated with release of humoral factor(s) and can provide equally potent cardioprotection to that of RIC induced by transient limb ischaemia in experimental animals.107 Interestingly, EA has been shown to be cardioprotective in the clinical setting, where it reduces peri-operative myocardial injury in patients undergoing cardiac surgery.108,109
Activation of the somatosensory system, the spinal cord, and the autonomic nervous system have been shown to mediate the release of yet unidentified humoral factor(s) that elicit the response in the target organ in the setting of RIC. The sensory afferent nerve appears to be the pivotal communication from the conditioned limb or organ as release of the humoral mediator following RIC depends on an intact sensory pathway.101,110,111 The stimulus may not only originate from local IRI in the conditioned organ or limb, but may also be initiated by local surgical trauma, which appears to recruit similar signalling pathways within the heart as RIC.105,111,112
Spinal cord involvement in RIC has been supported by loss of RIC cardioprotection with spinal cord transection at T7-T10, or intrathecal spinal opioid receptor blockade with naloxone, and MI size reduction can be recapitulated via spinal cord stimulation at C8-T2.113,114 It appears that cardiac sympathetic nerves are involved in the observed MI size reduction upon spinal cord stimulation, and this cardioprotective effect is attenuated by the α1-blocker, prazosin, and the beta-blocker, timolol.114 It is well-established that systemic administration of morphine is cardioprotective, but it has recently shown that lower doses of morphine can be administered intrathecally into the cerebrospinal fluid to induce cardioprotection.115–117 This protective effect appears to be mediated by spinal µ-opioid receptors and signals through the spinal NOS-NO-cGMP pathway.116,117
The efferent cardioprotective efficacy of the humoral mediator on the myocardium is dependent on functioning intrinsic neural loops and recruitment of intrinsic cardiac ganglia, which regulate cardiac neural activity in the absence of any extracardiac neural input. Transmission via intrinsic cardiac ganglia is dependent on acetylcholine release to activate nicotinic acetylcholine receptors (nAchR) on the post-ganglionic nerve and initiate a nerve impulse. The ganglionic blocker, hexamethonium, which prevents transmission of information at the ganglia by antagonizing nAchR, abrogates protection by local bradykinin administration or RIC in most, 96,105,118 but not all studies.119 Another ganglionic blocker, trimetaphan, also abrogates the protection by RIC from ischaemia–reperfusion induced endothelial dysfunction in humans.120
Further studies are clearly required, but the potential role of direct or indirect neural stimulation as a cardioprotective strategy is compelling, and further understanding of the neural component of the neuro-humoral pathways of RIC may be important in understanding the vagaries of response when RIC is used clinically.
4.2 Vagal nerve stimulation and cardioprotection
The role of vagal stimulation, either as part of a remote stimulus or via direct stimulation is an emerging area of interest in the field of cardioprotection. Electrical stimulation of the vagal nerve is cardioprotective. Vagal stimulation reduced MI size in an in vivo rat AMI model when performed either prior to ischaemia or a the onset of reperfusion, with preconditioning vagal stimulation activating the Akt/GSK-3β muscarinic pathway, whereas post-conditioning vagal stimulation activated α7-nicotinic acetylcholine receptors and JAK2, independently of the cholinergic anti-inflammatory pathway.121 In a comprehensive study, Donato et al.122 demonstrated the involvement of these neural pathways in RIC induced cardioprotection via limb ischaemia. The need for afferent innervation to the limb was confirmed, since cardioprotection was abrogated in animals undergoing femoral and sciatic nerve transection. More importantly, prior transection of the spinal cord, or the left and right vagus nerves at the mid-cervical level, or pre-treatment with atropine, also abolished the cardioprotective effect of remote preconditioning by transient limb ischaemia. Mastitskaya et al.123,124 further dissected the role of vagal innervation in experiments using highly selective sectioning of different branches of the vagus nerve. The authors concluded that the posterior gastric branch of the vagus alone was pivotal in signal transduction of the preconditioning stimulus from limb to heart. Although they did not prepare plasma dialysate for confirmation of a coincident humoral signal, Mastitskaya concluded that their results suggest ‘that the circulating factor (or factors) of RPc are produced and released into the systemic circulation by the visceral organ(s) innervated by the posterior gastric branch of the vagus nerve’. In a different study that vagal stimulation induced the release of glucagon-like peptide 1 (GLP1),125 and GLP-1 signalling has been shown to limit MI size in isolated hearts and intact pigs,126 as well as in proof-of-concept clinical trials.127 Although the signal transduction involved is not clear, there is increasing evidence that GLP-1 signalling induces a metabolic shift towards glycolysis in cardiomyocytes which is independent of insulin.128,129
Interestingly, it has recently been shown in pigs and rats that the vago-splenic axis is required for RIC cardioprotection.130 Splenic denervation or splenectomy abolished protection and muscarinergic stimulation of an isolated perfused spleen released a substance which reduced infarct size in an isolated perfused heart, indicating that the integrity of the vago-splenic axis is essential for RIC cardioprotection. However, the nature of the spleen-derived cardioprotective substance was not identified. Also, the role of the vago-splenic axis in the more clinically relevant remote ischaemic per-conditioning or post-conditioning, was not investigated, but this limitation applies also to all other of the above studies. Although the underlying mechanisms are not known, it is proposed that the spleen acts as a source of neuroprotective,131 and cardioprotective substances.130 Most recently, acute cardioprotection via vagal nerve stimulation has been tested in the clinical setting of AMI with the demonstration that transcutaneous vagal activation by low-level electrical stimulation at the right tragus reducing MI size.46,68
Chronic neuropathic pain impacts on the susceptibility to acute myocardial IRI,132 and MI size was reduced in a murine model of chronic neuropathic pain. This cardioprotective effect could be recapitulated via activation of anterior nucleus of paraventricular thalamus (PVA)-dependent parasympathetic pathway, as evidenced by the fact that pharmacological inhibition of Erk activation in the PVA abolished neuropathic pain-induced cardioprotection, whereas activation of PVA neurons pharmacologically, or by optogenetic stimulation, induced cardioprotection.
4.3 Anaesthesia and cardioprotection by neural stimulation
Any anaesthesia impacts on the autonomic nervous system and its balance. Of particular concern with respect to cardioprotection is the use of pentobarbital anaesthesia in experimental studies since pentobarbital augments sympathetic activity and its impact on ischaemic/reperfused myocardium. Accordingly, sympathetic denervation augments ischaemic myocardial blood flow and reduces MI size in pentobarbital-anaesthetized dogs,133,134 and this effect is not seen in conscious, chronically instrumented dogs.61 Of even greater concern is the use of propofol in experimental and clinical studies on cardioprotection.135 Propofol interferes with γ-aminobutyrate-mediated central nervous control of cardiac vagal nerves.136,137 Propofol, in contrast to volatile anaesthesia, interferes with the cardioprotection by RIC in rats138 and in patients undergoing cardiovascular surgery,139–141 and this interference may have accounted for the apparent lack of cardioprotection in two large randomized clinical trials.142–144
4.4 Diabetic neuropathy as modulator of cardioprotection by RIC
The efficacy of IPC is decreased in animal and human models of diabetes mellitus,145–151 while the responses to RIC in humans with diabetes have been varying.110,152,153 Depending on the presence of peripheral neuropathy, dialysed plasma from diabetic patients subjected to RIC has revealed differential responses. Plasma from diabetic patients without neuropathy was cardioprotective in naïve recipient rabbit hearts, while plasma from patients with peripheral neuropathy failed to provide cardioprotective plasma.110 The findings confirm the interaction between the neural and humoral components of RIC and that release of the humoral mediator following RIC is dependent on an intact sensory innervation in the conditioned limb.98
As described above, the vagal nerve is an essential neural mediator for limb RIC cardioprotection and facilitates the release of the blood-borne mediator.100,122,123 However, studies exploring the impact of autonomic neuropathy upon the efficacy of RIC in a human context have not been identified. Despite deprivation of extracardiac innervation, experimental studies using isolated hearts have demonstrated consistent attenuation of the efficacy of RIC by diabetes. Acute myocardial IRI appears to be dependent on diabetes duration, but the efficacy of RIC is not.150,154,155 Moreover, the majority of studies have been conducted in young experimental animals with a low likelihood of diabetic complications. Although extracardiac autonomic neuropathy may be involved, it does not seem to be a leading mechanism behind the impaired response to RIC in diabetic individuals.
Degenerative changes and reduced numbers of nerve fibres and intracardiac ganglia have been demonstrated in patients and animal models of type 1 and 2 diabetes,156–158 and the density of cholinergic nerves may be changed in diabetic rats.159 The functional impact of disarrays in intrinsic neural cardiac loops and cardiac ganglia, which regulate cardiac neural activity and intracellular signalling pathways involved in cytoprotection, and interference with RIC is currently unknown.
5. Clinical implications and future perspectives
Limb RIC appears to the most promising strategy for limiting MI size in patients with AMI.160 There is compelling evidence that neural stimulation is a key element in triggering and coordinating RIC cardioprotection, but the contribution of the neural network is complex and depends on the type of RIC intervention (pre-, per-, and post-conditioning), the animal species and other factors, and that may be additive or redundant. Elucidating the exact role of the different neuronal pathways involved in each situation appears as an essential step to bring the maximal benefit of RIC strategies to patients. It should help optimize RIC protocols for different clinical contexts, as the type of ischaemic insult to the myocardium, age, sex, comorbidities, and co-medications, as well as to identify situations of resistance to RIC strategies and opportunities for combination therapies. It should also help to develop new treatments that could reproduce the cardioprotection afforded by remote ischaemia with pharmacological or physical methods or combinations of both. Among these new treatments, different modalities of direct nerve stimulation and neuromodulation appear as a promising, safe, and effective strategy. In this regard, transcutaneous vagal nerve stimulation has been shown to reduce MI size in AMI patients,46 and EA has been reported to reduce peri-operative myocardial injury patients undergoing cardiac surgery.108,109 Finally, limb RIC appears to be the most promising strategy for limiting MI size in patients with AMI,160 and whether it can improve clinical outcomes is being tested in the CONDI2/ERIC-PPCI trial,161 which reports its result in Summer 2019.
Conflict of interest: H.E.B. is a shareholder in CellAegis Inc. P.F. is a founder and CEO of Pharmahungary, a Group of R&D companies. All other authors have no relevant disclosures.
Funding
This work was supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre to D.H.; British Heart Foundation (FS/10/039/28270 to D.H.; FS/12/2/29300, PG/13/57/30385, and RG/17/3/32774 to G.A.N.); Duke-National University Singapore Medical School to D.H.]; Singapore Ministry of Health’s National Medical Research Council under its Clinician Scientist-Senior Investigator scheme (NMRC/CSA-SI/0011/2017 to D.H.) and Collaborative Centre Grant scheme (NMRC/CGAug16C006 to D.H.); Singapore Ministry of Education Academic Research Fund Tier 2 (MOE2016-T2-2-021 to D.H.); the Instituto de Salud Carlos III, CIBERCV-Instituto de Salud Carlos III, Spain [grant CB16/11/00479, co-funded with European Regional Development Fund-FEDER contribution to (DGD)]; and grants (PIE/2013-00047 and PI 17/1397 to D.G.D.); the German Research Foundation (SFB 1116, B08 to G.H.); The Danish Council for Strategic Research (11-108354 to H.E.B.); Novo Nordisk Foundation (Conditioning Based Intervention Strategies – ConBis to H.E.B.), Trygfonden; the National Research, Development and Innovation Office of Hungary (NVKP_16-1-2016-0017; OTKA KH 125570; OTKA 115378 to P.F.); the Higher Education Institutional Excellence Programme of the Ministry of Human Capacities in Hungary, within the framework of the Therapeutic Development thematic programme of the Semmelweis University to P.F. This article is based upon work from COST Action EU-CARDIOPROTECTION CA16225 supported by COST (European Cooperation in Science and Technology).
References
- 1. Armour JA. Potential clinical relevance of the ‘little brain’ on the mammalian heart. Exp Physiol 2008;93:165–176. [DOI] [PubMed] [Google Scholar]
- 2. Kingma JG, Simard D, Rouleau JR.. Influence of cardiac nerve status on cardiovascular regulation and cardioprotection. World J Cardiol 2017;9:508–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wake E, Brack K.. Characterization of the intrinsic cardiac nervous system. Auton Neurosci 2016;199:3–16. [DOI] [PubMed] [Google Scholar]
- 4. Allen E, Coote JH, Grubb BD, Batten TF, Pauza DH, Ng GA, Brack KE.. The electrophysiological effects of nicotinic and electrical stimulation of intrinsic cardiac ganglia in the absence of extrinsic autonomic nerves in the rabbit heart. Heart Rhythm 2018;15:1698–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gatti PJ, Johnson TA, Phan P, Jordan IK III, Coleman W, Massari VJ.. The physiological and anatomical demonstration of functionally selective parasympathetic ganglia located in discrete fat pads on the feline myocardium. J Auton Nerv Syst 1995;51:255–259. [DOI] [PubMed] [Google Scholar]
- 6. Gray AL, Johnson TA, Ardell JL, Massari VJ.. Parasympathetic control of the heart. II. A novel interganglionic intrinsic cardiac circuit mediates neural control of heart rate. J Appl Physiol (1985) 2004;96:2273–2278. [DOI] [PubMed] [Google Scholar]
- 7. Dickerson LW, Rodak DJ, Fleming TJ, Gatti PJ, Massari VJ, McKenzie JC, Gillis RA.. Parasympathetic neurons in the cranial medial ventricular fat pad on the dog heart selectively decrease ventricular contractility. J Auton Nerv Syst 1998;70:129–141. [DOI] [PubMed] [Google Scholar]
- 8. Winter J, Tanko AS, Brack KE, Coote JH, Ng GA.. Differential cardiac responses to unilateral sympathetic nerve stimulation in the isolated innervated rabbit heart. Auton Neurosci 2012;166:4–14. [DOI] [PubMed] [Google Scholar]
- 9. Beaumont E, Salavatian S, Southerland EM, Vinet A, Jacquemet V, Armour JA, Ardell JL.. Network interactions within the canine intrinsic cardiac nervous system: implications for reflex control of regional cardiac function. J Physiol 2013;591:4515–4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Franco-Cereceda A, Lundberg JM.. Actions of calcitonin gene-related peptide and tachykinins in relation to the contractile effects of capsaicin in the guinea-pig and rat heart in vitro. Naunyn Schmiedebergs Arch Pharmacol 1988;337:649–655. [DOI] [PubMed] [Google Scholar]
- 11. Csont T, Csonka C, Kovacs P, Jancso G, Ferdinandy P.. Capsaicin-sensitive sensory neurons regulate myocardial nitric oxide and cGMP signaling. Eur J Pharmacol 2003;476:107–113. [DOI] [PubMed] [Google Scholar]
- 12. Ferdinandy P, Csont T, Csonka C, Torok M, Dux M, Nemeth J, Horvath LI, Dux L, Szilvassy Z, Jancso G.. Capsaicin-sensitive local sensory innervation is involved in pacing-induced preconditioning in rat hearts: role of nitric oxide and CGRP? Naunyn Schmiedebergs Arch Pharmacol 1997;356:356–363. [DOI] [PubMed] [Google Scholar]
- 13. Zvara A, Bencsik P, Fodor G, Csont T, Hackler L Jr., Dux M, Furst S, Jancso G, Puskas LG, Ferdinandy P.. Capsaicin-sensitive sensory neurons regulate myocardial function and gene expression pattern of rat hearts: a DNA microarray study. FASEB J 2006;20:160–162. [DOI] [PubMed] [Google Scholar]
- 14. Bencsik P, Kupai K, Giricz Z, Gorbe A, Huliak I, Furst S, Dux L, Csont T, Jancso G, Ferdinandy P.. Cardiac capsaicin-sensitive sensory nerves regulate myocardial relaxation via S-nitrosylation of SERCA: role of peroxynitrite. Br J Pharmacol 2008;153:488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang L, Wang DH.. TRPV1 gene knockout impairs postischemic recovery in isolated perfused heart in mice. Circulation 2005;112:3617–3623. [DOI] [PubMed] [Google Scholar]
- 16. Katona M, Boros K, Santha P, Ferdinandy P, Dux M, Jancso G.. Selective sensory denervation by capsaicin aggravates adriamycin-induced cardiomyopathy in rats. Naunyn Schmiedebergs Arch Pharmacol 2004;370:436–443. [DOI] [PubMed] [Google Scholar]
- 17. Wang HJ, Wang W, Cornish KG, Rozanski GJ, Zucker IH.. Cardiac sympathetic afferent denervation attenuates cardiac remodeling and improves cardiovascular dysfunction in rats with heart failure. Hypertension 2014;64:745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Polhemus DJ, Trivedi RK, Gao J, Li Z, Scarborough AL, Goodchild TT, Varner KJ, Xia H, Smart FW, Kapusta DR, Lefer DJ.. Renal sympathetic denervation protects the failing heart via inhibition of neprilysin activity in the kidney. J Am Coll Cardiol 2017;70:2139–2153. [DOI] [PubMed] [Google Scholar]
- 19. Gourine A, Bondar SI, Spyer KM, Gourine AV.. Beneficial effect of the central nervous system beta-adrenoceptor blockade on the failing heart. Circ Res 2008;102:633–636. [DOI] [PubMed] [Google Scholar]
- 20. Habecker BA, Anderson ME, Birren SJ, Fukuda K, Herring N, Hoover DB, Kanazawa H, Paterson DJ, Ripplinger CM.. Molecular and cellular neurocardiology: development, and cellular and molecular adaptations to heart disease. J Physiol (Lond) 2016;594:3853–3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mahoney SA, Hosking R, Farrant S, Holmes FE, Jacoby AS, Shine J, Iismaa TP, Scott MK, Schmidt R, Wynick D.. The second galanin receptor GalR2 plays a key role in neurite outgrowth from adult sensory neurons. J Neurosci 2003;23:416–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Werner RA, Maya Y, Rischpler C, Javadi MS, Fukushima K, Lapa C, Herrmann K, Higuchi T.. Sympathetic nerve damage and restoration after ischemia-reperfusion injury as assessed by (11)C-hydroxyephedrine. Eur J Nucl Med Mol Imaging 2016;43:312–318. [DOI] [PubMed] [Google Scholar]
- 23. Hardwick JC, Ryan SE, Beaumont E, Ardell JL, Southerland EM.. Dynamic remodeling of the guinea pig intrinsic cardiac plexus induced by chronic myocardial infarction. Auton Neurosci 2014;181:4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hopkins DA, Macdonald SE, Murphy DA, Armour JA.. Pathology of intrinsic cardiac neurons from ischemic human hearts. Anat Rec 2000;259:424–436. [DOI] [PubMed] [Google Scholar]
- 25. Rajendran PS, Nakamura K, Ajijola OA, Vaseghi M, Armour JA, Ardell JL, Shivkumar K.. Myocardial infarction induces structural and functional remodelling of the intrinsic cardiac nervous system. J Physiol (Lond) 2016;594:321–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Seyfarth M, Richardt G, Mizsnyak A, Kurz T, Schomig A.. Transient ischemia reduces norepinephrine release during sustained ischemia. Neural preconditioning in isolated rat heart. Circ Res 1996;78:573–580. [DOI] [PubMed] [Google Scholar]
- 27. de Jong JW, Cargnoni A, Bradamante S, Curello S, Janssen M, Pasini E, Ceconi C, Bunger R, Ferrari R.. Intermittent v continuous ischemia decelerates adenylate breakdown and prevents norepinephrine release in reperfused rabbit heart. J Mol Cell Cardiol 1995;27:659–671. [DOI] [PubMed] [Google Scholar]
- 28. Miura T, Kawamura S, Tatsuno H, Ikeda Y, Mikami S, Iwamoto H, Okamura T, Iwatate M, Kimura M, Dairaku Y, Maekawa T, Matsuzaki M.. Ischemic preconditioning attenuates cardiac sympathetic nerve injury via ATP-sensitive potassium channels during myocardial ischemia. Circulation 2001;104:1053–1058. [DOI] [PubMed] [Google Scholar]
- 29. Yue W, Guo Z.. Blockade of spinal nerves inhibits expression of neural growth factor in the myocardium at an early stage of acute myocardial infarction in rats. Br J Anaesth 2012;109:345–351. [DOI] [PubMed] [Google Scholar]
- 30. Strande JL, Routhu KV, Lecht S, Lazarovici P.. Nerve growth factor reduces myocardial ischemia/reperfusion injury in rat hearts. J Basic Clin Physiol Pharmacol 2013;24:81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lambert EA, Thomas CJ, Hemmes R, Eikelis N, Pathak A, Schlaich MP, Lambert GW.. Sympathetic nervous response to ischemia-reperfusion injury in humans is altered with remote ischemic preconditioning. Am J Physiol Heart Circ Physiol 2016;311:H364–H370. [DOI] [PubMed] [Google Scholar]
- 32. Taggart P, Critchley H, Lambiase PD.. Heart-brain interactions in cardiac arrhythmia. Heart 2011;97:698–708. [DOI] [PubMed] [Google Scholar]
- 33. Heusch G, Deussen A, Thamer V.. Cardiac sympathetic nerve activity and progressive vasoconstriction distal to coronary stenoses: feed-back aggravation of myocardial ischemia. J Auton Nerv Syst 1985;13:311–326. [DOI] [PubMed] [Google Scholar]
- 34. Kawada T, Yamazaki T, Akiyama T, Mori H, Uemura K, Miyamoto T, Sugimachi M, Sunagawa K.. Disruption of vagal efferent axon and nerve terminal function in the postischemic myocardium. Am J Physiol Heart Circ Physiol 2002;283:H2687–H2691. [DOI] [PubMed] [Google Scholar]
- 35. Verrier RL, Thompson PL, Lown B.. Ventricular vulnerability during sympathetic stimulation: role of heart rate and blood pressure. Cardiovasc Res 1974;8:602–610. [DOI] [PubMed] [Google Scholar]
- 36. Schwartz PJ. The autonomic nervous system and sudden death. Eur Heart J 1998;19 Suppl F:F72–F80. [PubMed] [Google Scholar]
- 37. Vanoli E, De Ferrari GM, Stramba-Badiale M, Hull SS Jr, Foreman RD, Schwartz PJ.. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ Res 1991;68:1471–1481. [DOI] [PubMed] [Google Scholar]
- 38. Ng GA. Vagal modulation of cardiac ventricular arrhythmia. Exp Physiol 2014;99:295–299. [DOI] [PubMed] [Google Scholar]
- 39. Han J, Moe GK.. Nonuniform recovery of excitability in ventricular muscle. Circ Res 1964;14:44–60. [DOI] [PubMed] [Google Scholar]
- 40. Opthof T, Coronel R, Vermeulen JT, Verberne HJ, van Capelle FJ, Janse MJ.. Dispersion of refractoriness in normal and ischaemic canine ventricle: effects of sympathetic stimulation. Cardiovasc Res 1993;27:1954–1960. [DOI] [PubMed] [Google Scholar]
- 41. Ng GA, Brack KE, Coote JH.. Effects of direct sympathetic and vagus nerve stimulation on the physiology of the whole heart—a novel model of isolated Langendorff perfused rabbit heart with intact dual autonomic innervation. Exp Physiol 2001;86:319–329. [DOI] [PubMed] [Google Scholar]
- 42. Ng GA, Brack KE, Patel VH, Coote JH.. Autonomic modulation of electrical restitution, alternans and ventricular fibrillation initiation in the isolated heart. Cardiovasc Res 2007;73:750–760. [DOI] [PubMed] [Google Scholar]
- 43. Brack KE, Patel VH, Coote JH, Ng GA.. Nitric oxide mediates the vagal protective effect on ventricular fibrillation via effects on action potential duration restitution in the rabbit heart. J Physiol 2007;583:695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brack KE, Coote JH, Ng GA.. Vagus nerve stimulation protects against ventricular fibrillation independent of muscarinic receptor activation. Cardiovasc Res 2011;91:437–446. [DOI] [PubMed] [Google Scholar]
- 45. Fei L, Baron AD, Henry DP, Zipes DP.. Intrapericardial delivery of L-arginine reduces the increased severity of ventricular arrhythmias during sympathetic stimulation in dogs with acute coronary occlusion: nitric oxide modulates sympathetic effects on ventricular electrophysiological properties. Circulation 1997;96:4044–4049. [DOI] [PubMed] [Google Scholar]
- 46. Yu L, Huang B, Po SS, Tan T, Wang M, Zhou L, Meng G, Yuan S, Zhou X, Li X, Wang Z, Wang S, Jiang H.. Low-level tragus stimulation for the treatment of ischemia and reperfusion injury in patients with ST-segment elevation myocardial infarction: a proof-of-concept study. JACC Cardiovasc Interv 2017;10:1511–1520. [DOI] [PubMed] [Google Scholar]
- 47. Stanton MS, Tuli MM, Radtke NL, Heger JJ, Miles WM, Mock BH, Burt RW, Wellman HN, Zipes DP.. Regional sympathetic denervation after myocardial infarction in humans detected noninvasively using I-123-metaiodobenzylguanidine. J Am Coll Cardiol 1989;14:1519–1526. [DOI] [PubMed] [Google Scholar]
- 48. Cao JM, Fishbein MC, Han JB, Lai WW, Lai AC, Wu TJ, Czer L, Wolf PL, Denton TA, Shintaku IP, Chen PS, Chen LS.. Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation 2000;101:1960–1969. [DOI] [PubMed] [Google Scholar]
- 49. Ajijola OA, Yagishita D, Reddy NK, Yamakawa K, Vaseghi M, Downs AM, Hoover DB, Ardell JL, Shivkumar K.. Remodeling of stellate ganglion neurons after spatially targeted myocardial infarction: neuropeptide and morphologic changes. Heart Rhythm 2015;12:1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jackson N, Gizurarson S, Azam MA, King B, Ramadeen A, Zamiri N, Porta-Sanchez A, Al-Hesayen A, Graham J, Kusha M, Masse S, Lai PF, Parker J, John R, Kiehl TR, Nair GK, Dorian P, Nanthakumar K.. Effects of renal artery denervation on ventricular arrhythmias in a postinfarct model. Circ Cardiovasc Interv 2017;10:e004172.. [DOI] [PubMed] [Google Scholar]
- 51. Zhang B, Li X, Chen C, Jiang W, Lu D, Liu Q, Wang K, Yan Y, Jiang Z, Geng J, Xu H, Shan Q.. Renal denervation effects on myocardial fibrosis and ventricular arrhythmias in rats with ischemic cardiomyopathy. Cell Physiol Biochem 2018;46:2471–2479. [DOI] [PubMed] [Google Scholar]
- 52. Vaseghi M, Barwad P, Malavassi Corrales FJ, Tandri H, Mathuria N, Shah R, Sorg JM, Gima J, Mandal K, Saenz Morales LC, Lokhandwala Y, Shivkumar K.. Cardiac sympathetic denervation for refractory ventricular arrhythmias. J Am Coll Cardiol 2017;69:3070–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chauhan RA, Coote J, Allen E, Pongpaopattanakul P, Brack KE, Ng GA.. Functional selectivity of cardiac preganglionic sympathetic neurones in the rabbit heart. Int J Cardiol 2018;264:70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jones JF. Cardiac defibrillator neurones. J Physiol (Lond) 2009;587:2715.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ng GA. Neuro-cardiac interaction in malignant ventricular arrhythmia and sudden cardiac death. Auton Neurosci 2016;199:66–79. [DOI] [PubMed] [Google Scholar]
- 56. Heusch G. Heart rate in the pathophysiology of coronary blood flow and myocardial ischaemia: benefit from selective bradycardic agents. Br J Pharmacol 2008;153:1589–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Heusch G, Deussen A, Schipke J, Thamer V, Alpha 1, Alpha 2.. adrenoceptor-mediated vasoconstriction of large and small canine coronary arteries in vivo. J Cardiovasc Pharmacol 1984;6:961–968. [DOI] [PubMed] [Google Scholar]
- 58. Heusch G, Deussen A.. The effects of cardiac sympathetic nerve stimulation on perfusion of stenotic coronary arteries in the dog. Circ Res 1983;53:8–15. [DOI] [PubMed] [Google Scholar]
- 59. Seitelberger R, Guth BD, Heusch G, Lee JD, Katayama K, Ross J Jr.. Intracoronary alpha 2-adrenergic receptor blockade attenuates ischemia in conscious dogs during exercise. Circ Res 1988;62:436–442. [DOI] [PubMed] [Google Scholar]
- 60. Harrison DG, Chilian WM, Marcus ML.. Absence of functioning alpha-adrenergic receptors in mature canine coronary collaterals. Circ Res 1986;59:133–142. [DOI] [PubMed] [Google Scholar]
- 61. Shen YT, Knight DR, Vatner SF, Randall WC, Thomas JX Jr.. Responses to coronary artery occlusion in conscious dogs with selective cardiac denervation. Am J Physiol 1988;255:H525–H533. [DOI] [PubMed] [Google Scholar]
- 62. Matsuki T, Cohen MV, Holt G, Ayling J, Hearse DJ, Downey JM.. Chronic whole body sympathectomy fails to protect ischemic rabbit hearts. Am J Physiol 1989;256:H1322–H1327. [DOI] [PubMed] [Google Scholar]
- 63. Haessler R, Wolff RA, Chien GL, Davis RF, Van Winkle DM.. High spinal anesthesia does not alter experimental myocardial infarction size or ischemic preconditioning. J Cardiothorac Vasc Anesth 1997;11:72–79. [DOI] [PubMed] [Google Scholar]
- 64. Ziegler KA, Ahles A, Wille T, Kerler J, Ramanujam D, Engelhardt S.. Local sympathetic denervation attenuates myocardial inflammation and improves cardiac function after myocardial infarction in mice. Cardiovasc Res 2018;114:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhao J, Yang Y, You S, Cui C, Gao R.. Carvedilol preserves endothelial junctions and reduces myocardial no-reflow after acute myocardial infarction and reperfusion. Int J Cardiol 2007;115:334–341. [DOI] [PubMed] [Google Scholar]
- 66. Heusch G, Baumgart D, Camici P, Chilian W, Gregorini L, Hess O, Indolfi C, Rimoldi O.. alpha-adrenergic coronary vasoconstriction and myocardial ischemia in humans. Circulation 2000;101:689–694. [DOI] [PubMed] [Google Scholar]
- 67. Gregorini L, Marco J, Kozakova M, Palombo C, Anguissola GB, Marco I, Bernies M, Cassagneau B, Distante A, Bossi IM, Fajadet J, Heusch G.. Alpha-adrenergic blockade improves recovery of myocardial perfusion and function after coronary stenting in patients with acute myocardial infarction. Circulation 1999;99:482–490. [DOI] [PubMed] [Google Scholar]
- 68. Heusch G. Vagal cardioprotection in reperfused acute myocardial infarction. JACC Cardiovasc Interv 2017;10:1521–1522. [DOI] [PubMed] [Google Scholar]
- 69. Arimura T, Saku K, Kakino T, Nishikawa T, Tohyama T, Sakamoto T, Sakamoto K, Kishi T, Ide T, Sunagawa K.. Intravenous electrical vagal nerve stimulation prior to coronary reperfusion in a canine ischemia-reperfusion model markedly reduces infarct size and prevents subsequent heart failure. Int J Cardiol 2017;227:704–710. [DOI] [PubMed] [Google Scholar]
- 70. Uitterdijk A, Yetgin T, Te Lintel HM, Sneep S, Krabbendam-Peters I, van Beusekom HM, Fischer TM, Cornelussen RN, Manintveld OC, Merkus D, Duncker DJ.. Vagal nerve stimulation started just prior to reperfusion limits infarct size and no-reflow. Basic Res Cardiol 2015;110:508.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mendez-Ferrer S, Lucas D, Battista M, Frenette PS.. Haematopoietic stem cell release is regulated by circadian oscillations. Nature 2008;452:442–447. [DOI] [PubMed] [Google Scholar]
- 72. Gabanyi I, Muller PA, Feighery L, Oliveira TY, Costa-Pinto FA, Mucida D.. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell 2016;164:378–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Nance DM, Sanders VM.. Autonomic innervation and regulation of the immune system (1987-2007). Brain Behav Immun 2007;21:736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kasprowicz DJ, Kohm AP, Berton MT, Chruscinski AJ, Sharpe A, Sanders VM.. Stimulation of the B cell receptor, CD86 (B7-2), and the beta 2-adrenergic receptor intrinsically modulates the level of IgG1 and IgE produced per B cell. J Immunol 2000;165:680–690. [DOI] [PubMed] [Google Scholar]
- 75. Abboud FM, Harwani SC, Chapleau MW.. Autonomic neural regulation of the immune system: implications for hypertension and cardiovascular disease. Hypertension 2012;59:755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hernandez-Resendiz S, Chinda K, Ong SB, Cabrera-Fuentes H, Zazueta C, Hausenloy DJ.. The role of redox dysregulation in the inflammatory response to acute myocardial ischaemia-reperfusion injury—adding fuel to the fire. Curr Med Chem 2018;25:1275–1293. [DOI] [PubMed] [Google Scholar]
- 77. Ong SB, Hernandez-Resendiz S, Crespo-Avilan GE, Mukhametshina RT, Kwek XY, Cabrera-Fuentes HA, Hausenloy DJ.. Inflammation following acute myocardial infarction: multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol Ther 2018;186:73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wernli G, Hasan W, Bhattacherjee A, Rooijen N, Smith PG.. Macrophage depletion suppresses sympathetic hyperinnervation following myocardial infarction. Basic Res Cardiol 2009;104:681–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Murry CE, Jennings RB, Reimer KA.. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 1986;74:1124–1136. [DOI] [PubMed] [Google Scholar]
- 80. Saw EL, Kakinuma Y, Fronius M, Katare R.. The non-neuronal cholinergic system in the heart: a comprehensive review. J Mol Cell Cardiol 2018;125:129–139. [DOI] [PubMed] [Google Scholar]
- 81. Cohen MV, Yang XM, Liu GS, Heusch G, Downey JM.. Acetylcholine, bradykinin, opioids, and phenylephrine, but not adenosine, trigger preconditioning by generating free radicals and opening mitochondrial K(ATP) channels. Circ Res 2001;89:273–278. [DOI] [PubMed] [Google Scholar]
- 82. Kawada T, Akiyama T, Shimizu S, Kamiya A, Uemura K, Li M, Shirai M, Sugimachi M.. Detection of endogenous acetylcholine release during brief ischemia in the rabbit ventricle: a possible trigger for ischemic preconditioning. Life Sci 2009;85:597–601. [DOI] [PubMed] [Google Scholar]
- 83. Kawada T, Yamazaki T, Akiyama T, Sato T, Shishido T, Inagaki M, Takaki H, Sugimachi M, Sunagawa K.. Differential acetylcholine release mechanisms in the ischemic and non-ischemic myocardium. J Mol Cell Cardiol 2000;32:405–414. [DOI] [PubMed] [Google Scholar]
- 84. Pickard JMJ, Burke N, Davidson SM, Yellon DM.. Intrinsic cardiac ganglia and acetylcholine are important in the mechanism of ischaemic preconditioning. Basic Res Cardiol 2017;112:11.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kudej RK, Shen YT, Peppas AP, Huang CH, Chen W, Yan L, Vatner DE, Vatner SF.. Obligatory role of cardiac nerves and alpha1-adrenergic receptors for the second window of ischemic preconditioning in conscious pigs. Circ Res 2006;99:1270–1276. [DOI] [PubMed] [Google Scholar]
- 86. Richard V, Blanc T, Kaeffer N, Tron C, Thuillez C.. Myocardial and coronary endothelial protective effects of acetylcholine after myocardial ischaemia and reperfusion in rats: role of nitric oxide. Br J Pharmacol 1995;115:1532.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Basalay M, Barsukevich V, Mastitskaya S, Mrochek A, Pernow J, Sjoquist PO, Ackland GL, Gourine AV, Gourine A.. Remote ischaemic pre- and delayed postconditioning—similar degree of cardioprotection but distinct mechanisms. Exp Physiol 2012;97:908–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P.. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation 1993;87:893–899. [DOI] [PubMed] [Google Scholar]
- 89. Hausenloy DJ, Yellon DM.. Remote ischaemic preconditioning: underlying mechanisms and clinical application. Cardiovasc Res 2008;79:377–386. [DOI] [PubMed] [Google Scholar]
- 90. Pickard JM, Botker HE, Crimi G, Davidson B, Davidson SM, Dutka D, Ferdinandy P, Ganske R, Garcia-Dorado D, Giricz Z, Gourine AV, Heusch G, Kharbanda R, Kleinbongard P, MacAllister R, McIntyre C, Meybohm P, Prunier F, Redington A, Robertson NJ, Suleiman MS, Vanezis A, Walsh S, Yellon DM, Hausenloy DJ.. Remote ischemic conditioning: from experimental observation to clinical application: report from the 8th Biennial Hatter Cardiovascular Institute Workshop. Basic Res Cardiol 2015;110:453.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sivaraman V, Pickard JM, Hausenloy DJ.. Remote ischaemic conditioning: cardiac protection from afar. Anaesthesia 2015;70:732–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Heusch G, Botker HE, Przyklenk K, Redington A, Yellon D.. Remote ischemic conditioning. J Am Coll Cardiol 2015;65:177–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Heusch G. 25 years of remote ischemic conditioning: from laboratory curiosity to clinical outcome. Basic Res Cardiol 2018;113:15.. [DOI] [PubMed] [Google Scholar]
- 94. Kleinbongard P, Skyschally A, Heusch G.. Cardioprotection by remote ischemic conditioning and its signal transduction. Pflugers Arch 2017;469:159–181. [DOI] [PubMed] [Google Scholar]
- 95. Basalay MV, Davidson SM, Gourine AV, Yellon DM.. Neural mechanisms in remote ischaemic conditioning in the heart and brain: mechanistic and translational aspects. Basic Res Cardiol 2018;113:25.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Gho BC, Schoemaker RG, van den Doel MA, Duncker DJ, Verdouw PD.. Myocardial protection by brief ischemia in noncardiac tissue. Circulation 1996;94:2193–2200. [DOI] [PubMed] [Google Scholar]
- 97. Dong JH, Liu YX, Ji ES, He RR.. Limb ischemic preconditioning reduces infarct size following myocardial ischemia-reperfusion in rats. Sheng Li Xue Bao 2004;56:41–46. [PubMed] [Google Scholar]
- 98. Lim SY, Yellon DM, Hausenloy DJ.. The neural and humoral pathways in remote limb ischemic preconditioning. Basic Res Cardiol 2010;105:651–655. [DOI] [PubMed] [Google Scholar]
- 99. Shimizu M, Tropak M, Diaz RJ, Suto F, Surendra H, Kuzmin E, Li J, Gross G, Wilson GJ, Callahan J, Redington AN.. Transient limb ischaemia remotely preconditions through a humoral mechanism acting directly on the myocardium: evidence suggesting cross-species protection. Clin Sci 2009;117:191–200. [DOI] [PubMed] [Google Scholar]
- 100. Pickard JM, Davidson SM, Hausenloy DJ, Yellon DM.. Co-dependence of the neural and humoral pathways in the mechanism of remote ischemic conditioning. Basic Res Cardiol 2016;111:50.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Steensrud T, Li J, Dai X, Manlhiot C, Kharbanda RK, Tropak M, Redington A.. Pretreatment with the nitric oxide donor SNAP or nerve transection blocks humoral preconditioning by remote limb ischemia or intra-arterial adenosine. Am J Physiol Heart Circ Physiol 2010;299:H1598–H1603. [DOI] [PubMed] [Google Scholar]
- 102. Botker HE, Hausenloy D, Andreadou I, Antonucci S, Boengler K, Davidson SM, Deshwal S, Devaux Y, Di LF, Di SM, Efentakis P, Femmino S, Garcia-Dorado D, Giricz Z, Ibanez B, Iliodromitis E, Kaludercic N, Kleinbongard P, Neuhauser M, Ovize M, Pagliaro P, Rahbek-Schmidt M, Ruiz-Meana M, Schluter KD, Schulz R, Skyschally A, Wilder C, Yellon DM, Ferdinandy P, Heusch G.. Practical guidelines for rigor and reproducibility in preclinical and clinical studies on cardioprotection. Basic Res Cardiol 2018;113:39.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Redington KL, Disenhouse T, Strantzas SC, Gladstone R, Wei C, Tropak MB, Dai X, Manlhiot C, Li J, Redington AN.. Remote cardioprotection by direct peripheral nerve stimulation and topical capsaicin is mediated by circulating humoral factors. Basic Res Cardiol 2012;107:1–10. [DOI] [PubMed] [Google Scholar]
- 104. Li YJ, Xiao ZS, Peng CF, Deng HW.. Calcitonin gene-related peptide-induced preconditioning protects against ischemia-reperfusion injury in isolated rat hearts. Eur J Pharmacol 1996;311:163–167. [DOI] [PubMed] [Google Scholar]
- 105. Jones WK, Fan GC, Liao S, Zhang JM, Wang Y, Weintraub NL, Kranias EG, Schultz JE, Lorenz J, Ren X.. Peripheral nociception associated with surgical incision elicits remote nonischemic cardioprotection via neurogenic activation of protein kinase C signaling. Circulation 2009;120:S1–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Merlocco AC, Redington KL, Disenhouse T, Strantzas SC, Gladstone R, Wei C, Tropak MB, Manlhiot C, Li J, Redington AN.. Transcutaneous electrical nerve stimulation as a novel method of remote preconditioning: in vitro validation in an animal model and first human observations. Basic Res Cardiol 2014;109:406.. [DOI] [PubMed] [Google Scholar]
- 107. Redington KL, Disenhouse T, Li J, Wei C, Dai X, Gladstone R, Manlhiot C, Redington AN.. Electroacupuncture reduces myocardial infarct size and improves post-ischemic recovery by invoking release of humoral, dialyzable, cardioprotective factors. J Physiol Sci 2013;63:219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Yang L, Yang J, Wang Q, Chen M, Lu Z, Chen S, Xiong L.. Cardioprotective effects of electroacupuncture pretreatment on patients undergoing heart valve replacement surgery: a randomized controlled trial. Ann Thorac Surg 2010;89:781–786. [DOI] [PubMed] [Google Scholar]
- 109. Zhang F, Yu X, Xiao H.. Cardioprotection of electroacupuncture for enhanced recovery after surgery on patients undergoing heart valve replacement with cardiopulmonary bypass: a randomized control clinical trial. Evid Based Complement Alternat Med 2017;2017:6243630.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Jensen RV, Stottrup NB, Kristiansen SB, Botker HE.. Release of a humoral circulating cardioprotective factor by remote ischemic preconditioning is dependent on preserved neural pathways in diabetic patients. Basic Res Cardiol 2012;107:285.. [DOI] [PubMed] [Google Scholar]
- 111. Gross ER, Hsu AK, Urban TJ, Mochly-Rosen D, Gross GJ.. Nociceptive-induced myocardial remote conditioning is mediated by neuronal gamma protein kinase C. Basic Res Cardiol 2013;108:381.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Song Y, Shan JG, Xue Z, Wang SY, Xu H, Liu Y, Guo YS, Ren X.. Remote postconditioning induced by trauma protects the mouse heart against ischemia reperfusion injury. Involvement of the neural pathway and molecular mechanisms. Cardiovasc Drugs Ther 2016;30:271–280. [DOI] [PubMed] [Google Scholar]
- 113. Wong GT, Lu Y, Mei B, Xia Z, Irwin MG.. Cardioprotection from remote preconditioning involves spinal opioid receptor activation. Life Sci 2012;91:860–865. [DOI] [PubMed] [Google Scholar]
- 114. Southerland EM, Milhorn DM, Foreman RD, Linderoth B, DeJongste MJ, Armour JA, Subramanian V, Singh M, Singh K, Ardell JL.. Preemptive, but not reactive, spinal cord stimulation mitigates transient ischemia-induced myocardial infarction via cardiac adrenergic neurons. Am J Physiol Heart Circ Physiol 2007;292:H311–H317. [DOI] [PubMed] [Google Scholar]
- 115. Groban L, Vernon JC, Butterworth J.. Intrathecal morphine reduces infarct size in a rat model of ischemia-reperfusion injury. Anesth Analg 2004;98:903–909. [DOI] [PubMed] [Google Scholar]
- 116. Lu Y, Hu J, Zhang Y, Dong CS, Wong GT.. Remote intrathecal morphine preconditioning confers cardioprotection via spinal cord nitric oxide/cyclic guanosine monophosphate/protein kinase G pathway. J Surg Res 2015;193:43–51. [DOI] [PubMed] [Google Scholar]
- 117. Jiang L, Hu J, He S, Zhang L, Zhang Y.. Spinal neuronal NOS signaling contributes to morphine cardioprotection in ischemia reperfusion injury in rats. J Pharmacol Exp Ther 2016;358:450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Schoemaker RG, van Heijningen CL.. Bradykinin mediates cardiac preconditioning at a distance. Am J Physiol Heart Circ Physiol 2000;278:H1571–H1576. [DOI] [PubMed] [Google Scholar]
- 119. Weinbrenner C, Nelles M, Herzog N, Sarvary L, Strasser RH.. Remote preconditioning by infrarenal occlusion of the aorta protects the heart from infarction: a newly identified non-neuronal but PKC-dependent pathway. Cardiovasc Res 2002;55:590–601. [DOI] [PubMed] [Google Scholar]
- 120. Loukogeorgakis SP, Panagiotidou AT, Broadhead MW, Donald A, Deanfield JE, MacAllister RJ.. Remote ischemic preconditioning provides early and late protection against endothelial ischemia-reperfusion injury in humans: role of the autonomic nervous system. J Am Coll Cardiol 2005;46:450–456. [DOI] [PubMed] [Google Scholar]
- 121. Buchholz B, Kelly J, Munoz M, Bernatene EA, Mendez DN, Gonzalez Maglio DH, Dominici FP, Gelpi RJ.. Vagal stimulation mimics preconditioning and postconditioning of ischemic myocardium in mice by activating different protection mechanisms. Am J Physiol Heart Circ Physiol 2018;314:H1289–H1297. [DOI] [PubMed] [Google Scholar]
- 122. Donato M, Buchholz B, Rodriguez M, Perez V, Inserte J, Garcia-Dorado D, Gelpi RJ.. Role of the parasympathetic nervous system in cardioprotection by remote hindlimb ischaemic preconditioning. Exp Physiol 2013;98:425–434. [DOI] [PubMed] [Google Scholar]
- 123. Mastitskaya S, Marina N, Gourine A, Gilbey MP, Spyer KM, Teschemacher AG, Kasparov S, Trapp S, Ackland GL, Gourine AV.. Cardioprotection evoked by remote ischaemic preconditioning is critically dependent on the activity of vagal pre-ganglionic neurones. Cardiovasc Res 2012;95:487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Mastitskaya S, Basalay M, Hosford PS, Ramage AG, Gourine A, Gourine AV.. Identifying the source of a humoral factor of remote (pre)conditioning cardioprotection. PLoS One 2016;11:e0150108.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Basalay MV, Mastitskaya S, Mrochek A, Ackland GL, Del Arroyo AG, Sanchez J, Sjoquist PO, Pernow J, Gourine AV, Gourine A.. Glucagon-like peptide-1 (GLP-1) mediates cardioprotection by remote ischaemic conditioning. Cardiovasc Res 2016;112:669–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Timmers L, Henriques JP, de Kleijn DP, Devries JH, Kemperman H, Steendijk P, Verlaan CW, Kerver M, Piek JJ, Doevendans PA, Pasterkamp G, Hoefer IE.. Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J Am Coll Cardiol 2009;53:501–510. [DOI] [PubMed] [Google Scholar]
- 127. Lonborg J, Vejlstrup N, Kelbaek H, Botker HE, Kim WY, Mathiasen AB, Jorgensen E, Helqvist S, Saunamaki K, Clemmensen P, Holmvang L, Thuesen L, Krusell LR, Jensen JS, Kober L, Treiman M, Holst JJ, Engstrom T.. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J 2012;33:1491–1499. [DOI] [PubMed] [Google Scholar]
- 128. Bao W, Aravindhan K, Alsaid H, Chendrimada T, Szapacs M, Citerone DR, Harpel MR, Willette RN, Lepore JJ, Jucker BM.. Albiglutide, a long lasting glucagon-like peptide-1 analog, protects the rat heart against ischemia/reperfusion injury: evidence for improving cardiac metabolic efficiency. PLoS One 2011;6:e23570.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Alburquerque-Béjar JJ, Barba I, Inserte J, Miró-Casas E, Ruiz-Meana M, Poncelas M, Vilardosa Ú, Valls-Lacalle L, Rodríguez-Sinovas A, Garcia-Dorado D.. Combination therapy with remote ischaemic conditioning and insulin or exenatide enhances infarct size limitation in pigs. Cardiovasc Res 2015;107:246–254. [DOI] [PubMed] [Google Scholar]
- 130. Lieder HR, Kleinbongard P, Skyschally A, Hagelschuer H, Chilian WM, Heusch G.. Vago-splenic axis in signal transduction of remote ischemic preconditioning in pigs and rats. Circ Res 2018;123:1152–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Chen C, Jiang W, Liu Z, Li F, Yang J, Zhao Y, Ran Y, Meng Y, Ji X, Geng X, Du H, Hu X.. Splenic responses play an important role in remote ischemic preconditioning-mediated neuroprotection against stroke. J Neuroinflammation 2018;15:167.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Cheng YF, Chang YT, Chen WH, Shih HC, Chen YH, Shyu BC, Chen CC.. Cardioprotection induced in a mouse model of neuropathic pain via anterior nucleus of paraventricular thalamus. Nat Commun 2017;8:826.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Jones CE, Devous MD Sr, Thomas JX Jr, DuPont E.. The effect of chronic cardiac denervation on infarct size following acute coronary occlusion. Am Heart J 1978;95:738–746. [DOI] [PubMed] [Google Scholar]
- 134. Jones CE, Beck LY, DuPont E, Barnes GE.. Effects of coronary ligation of the chromically sympathectomized dog ventricle. Am J Physiol 1978;235:H429–H434. [DOI] [PubMed] [Google Scholar]
- 135. Heusch G. Remote ischemic conditioning in cardiovascular surgery. J Cardiovasc Pharmacol Ther 2017;22:297–301. [DOI] [PubMed] [Google Scholar]
- 136. McDougall SJ, Bailey TW, Mendelowitz D, Andresen MC.. Propofol enhances both tonic and phasic inhibitory currents in second-order neurons of the solitary tract nucleus (NTS). Neuropharmacology 2008;54:552–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Wang X, Huang ZG, Gold A, Bouairi E, Evans C, Andresen MC, Mendelowitz D.. Propofol modulates gamma-aminobutyric acid-mediated inhibitory neurotransmission to cardiac vagal neurons in the nucleus ambiguus. Anesthesiology 2004;100:1198–1205. [DOI] [PubMed] [Google Scholar]
- 138. Behmenburg F, van CP, Bunte S, Brandenburger T, Heinen A, Hollmann MW, Huhn R.. Impact of anesthetic regimen on remote ischemic preconditioning in the rat heart in vivo. Anesth Analg 2018;126:1377–1380. [DOI] [PubMed] [Google Scholar]
- 139. Kottenberg E, Thielmann M, Bergmann L, Heine T, Jakob H, Heusch G, Peters J.. Protection by remote ischemic preconditioning during coronary artery bypass graft surgery with isoflurane but not propofol—a clinical trial. Acta Anaesthesiol Scand 2012;56:30–38. [DOI] [PubMed] [Google Scholar]
- 140. Kottenberg E, Musiolik J, Thielmann M, Jakob H, Peters J, Heusch G.. Interference of propofol with signal transducer and activator of transcription 5 activation and cardioprotection by remote ischemic preconditioning during coronary artery bypass grafting. J Thorac Cardiovasc Surg 2014;147:376–382. [DOI] [PubMed] [Google Scholar]
- 141. Zangrillo A, Musu M, Greco T, Di Prima AL, Matteazzi A, Testa V, Nardelli P, Febres D, Monaco F, Calabro MG, Ma J, Finco G, Landoni G.. Additive effect on survival of anaesthetic cardiac protection and remote ischemic preconditioning in cardiac surgery: a Bayesian network meta-analysis of randomized trials. PLoS One 2015;10:e0134264.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Hausenloy DJ, Candilio L, Evans R, Ariti C, Jenkins DP, Kolvekar S, Knight R, Kunst G, Laing C, Nicholas J, Pepper J, Robertson S, Xenou M, Clayton T, Yellon DM.. Remote ischemic preconditioning and outcomes of cardiac surgery. N Engl J Med 2015;373:1408–1417. [DOI] [PubMed] [Google Scholar]
- 143. Meybohm P, Bein B, Brosteanu O, Cremer J, Gruenewald M, Stoppe C, Coburn M, Schaelte G, Boning A, Niemann B, Roesner J, Kletzin F, Strouhal U, Reyher C, Laufenberg-Feldmann R, Ferner M, Brandes IF, Bauer M, Stehr SN, Kortgen A, Wittmann M, Baumgarten G, Meyer-Treschan T, Kienbaum P, Heringlake M, Schon J, Sander M, Treskatsch S, Smul T, Wolwender E, Schilling T, Fuernau G, Hasenclever D, Zacharowski K.. A multicenter trial of remote ischemic preconditioning for heart surgery. N Engl J Med 2015;373:1397–1407. [DOI] [PubMed] [Google Scholar]
- 144. Heusch G, Gersh BJ.. ERICCA and RIPHeart: two nails in the coffin for cardioprotection by remote ischemic conditioning? Probably not! Eur Heart J 2016;37:200–202. [DOI] [PubMed] [Google Scholar]
- 145. Tosaki A, Engelman DT, Engelman RM, Das DK.. The evolution of diabetic response to ischemia/reperfusion and preconditioning in isolated working rat hearts. Cardiovasc Res 1996;31:526–536. [PubMed] [Google Scholar]
- 146. Bouchard JF, Lamontagne D.. Protection afforded by preconditioning to the diabetic heart against ischaemic injury. Cardiovasc Res 1998;37:82–90. [DOI] [PubMed] [Google Scholar]
- 147. Kersten JR, Toller WG, Gross ER, Pagel PS, Warltier DC.. Diabetes abolishes ischemic preconditioning: role of glucose, insulin, and osmolality. Am J Physiol Heart Circ Physiol 2000;278:H1218–H1224. [DOI] [PubMed] [Google Scholar]
- 148. Kristiansen SB, Lofgren B, Stottrup NB, Khatir D, Nielsen-Kudsk JE, Nielsen TT, Botker HE, Flyvbjerg A.. Ischaemic preconditioning does not protect the heart in obese and lean animal models of type 2 diabetes. Diabetologia 2004;47:1716–1721. [DOI] [PubMed] [Google Scholar]
- 149. Tsang A, Hausenloy DJ, Mocanu MM, Carr RD, Yellon DM.. Preconditioning the diabetic heart: the importance of AKT phosphorylation. Diabetes 2005;54:2360–2364. [DOI] [PubMed] [Google Scholar]
- 150. Whittington HJ, Babu GG, Mocanu MM, Yellon DM, Hausenloy DJ.. The diabetic heart: too sweet for its own good? Cardiol Res Pract 2012;2012:845698.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Sivaraman V, Hausenloy DJ, Wynne AM, Yellon DM.. Preconditioning the diabetic human myocardium. J Cell Mol Med 2010;14:1740–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Sloth AD, Schmidt MR, Munk K, Schmidt M, Pedersen L, Toft Sorensen H, Botker HE, Bottcher M, Kaltoft A, Terkelsen C, Andersen N, Hansen T, Trautner S, Lassen J, Christiansen E, Krusell L, Kristensen S, Thuesen L, Nielsen S, Rehling M, Nielsen T.. Impact of cardiovascular risk factors and medication use on the efficacy of remote ischaemic conditioning: post hoc subgroup analysis of a randomised controlled trial. BMJ Open 2015;5:e006923.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Kottenberg E, Thielmann M, Kleinbongard P, Frey UH, Heine T, Jakob H, Heusch G, Peters J.. Myocardial protection by remote ischaemic pre-conditioning is abolished in sulphonylurea-treated diabetics undergoing coronary revascularisation. Acta Anaesthesiol Scand 2014;58:453–462. [DOI] [PubMed] [Google Scholar]
- 154. Hjortbak MV, Hjort J, Povlsen JA, Jensen RV, Stottrup NB, Laursen MR, Jespersen NR, Lofgren B, Botker HE.. Influence of diabetes mellitus duration on the efficacy of ischemic preconditioning in a Zucker diabetic fatty rat model. PLoS One 2018;13:e0192981.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Povlsen JA, Lofgren B, Dalgas C, Birkler RI, Johannsen M, Stottrup NB, Botker HE.. Protection against myocardial ischemia-reperfusion injury at onset of type 2 diabetes in Zucker diabetic fatty rats is associated with altered glucose oxidation. PLoS One 2013;8:e64093.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Schramm E, Wagner M, Nellessen U, Inselmann G.. Ultrastructural changes of human cardiac atrial nerve endings in diabetes mellitus. Eur J Clin Invest 2000;30:311–316. [DOI] [PubMed] [Google Scholar]
- 157. Kamal AA, Tay SS, Wong WC.. The cardiac ganglia in streptozotocin-induced diabetic rats. Arch Histol Cytol 1991;54:41–49. [DOI] [PubMed] [Google Scholar]
- 158. Batulevicius D, Frese T, Peschke E, Pauza DH, Batuleviciene V.. Remodelling of the intracardiac ganglia in diabetic Goto-Kakizaki rats: an anatomical study. Cardiovasc Diabetol 2013;12:85.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Mabe AM, Hoover DB.. Remodeling of cardiac cholinergic innervation and control of heart rate in mice with streptozotocin-induced diabetes. Auton Neurosci 2011;162:24–31. [DOI] [PubMed] [Google Scholar]
- 160. Hausenloy DJ, Botker HE, Engstrom T, Erlinge D, Heusch G, Ibanez B, Kloner RA, Ovize M, Yellon DM, Garcia-Dorado D.. Targeting reperfusion injury in patients with ST-segment elevation myocardial infarction: trials and tribulations. Eur Heart J 2017;38:935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Hausenloy DJ, Kharbanda R, Rahbek SM, Moller UK, Ravkilde J, Okkels JL, Engstrom T, Garcia Ruiz JM, Radovanovic N, Christensen EF, Sorensen HT, Ramlall M, Bulluck H, Evans R, Nicholas J, Knight R, Clayton T, Yellon DM, Botker HE.. Effect of remote ischaemic conditioning on clinical outcomes in patients presenting with an ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Eur Heart J 2015;36:1846–1848. [PubMed] [Google Scholar]