This editorial refers to ‘Association between resting amygdalar activity and abnormal cardiac function in women and men: a retrospective cohort study’, by M. Fiechter et al., pp. 625–632.
Women have adverse cardiovascular morbidity and mortality despite having less obstructive coronary artery disease (CAD).1 In addition to a higher burden of cardiac risk factors and inflammation, psychological risk factors are highly prevalent in women and these factors such as depression, anxiety, acute and chronic stress, are associated with adverse outcomes, particularly in women. Prior work has shown that women are more susceptible than men to mental stress-induced myocardial ischaemia, emotional stress-related angina, and takotsubo/stress-induced cardiomyopathy.1–3 Given these observations, it is clear that the brain–heart axis plays an important role in women, but specific mechanisms remain unclear.
In this issue, Fiechter et al.4 report on novel findings of sex differences in the association of resting amygdalar activation and cardiac function in a retrospective cohort study. Subjects in this study underwent myocardial single-photon emission computerized tomography (SPECT) for suspected or known CAD. They also had a 18-fluorodeoxyglucose positron emission tomography (18F-FDG PET) for detection or staging of malignancies or inflammatory disorders, including the brain where amygdalar activation is known to be related to mental stress. Three-hundred and two patients, out of which 29.1% were women, were retrospectively evaluated to determine if there was an association between enhanced amygdalar metabolic activity and cardiac measures of perfusion and function. The major novel findings include: (i) in women, a decrease in left ventricular ejection fraction and fixed perfusion defects correlated with higher resting amgydalar activation, while there was no correlation in men; (ii) reversible myocardial ischaemia was not associated with amygdalar activation in women or men; and (iii) coronary artery calcification was not associated with amygdalar activation.
The main strength of the study is analysing the results using sex as a biological variable (SABV) and combining the clinically ordered SPECT and 18F-FDG PET datasets, establishing links between the amygdala stress-based neural circuitry and cardiac function possible. The findings add to the emerging literature on stress-related brain activation in cardiovascular disease.5,6 While the retrospective nature of this study precluding actual stress testing, and relatively smaller sample size of women are limitations, the results intriguingly suggest a potentially relevant sex difference regarding neural amygdala activation and cardiovascular disease. Study findings of amydalar activation associated with left ventricular ejection fraction should be extended to heart failure with preserved ejection fraction, a problem that predominates in women. Moreover, it would be interesting to see if amygdalar activation was associated with diastolic dysfunction and associated markers (B-type natriuretic peptide, troponin, 6 min walk distance, functional status), which was not evaluated in this study. Nearly one-half of the patients in this study were on beta-blockers, and there was a significant difference in beta-blocker use between men and women (52.3% vs. 38.6%, P = 0.015). It is interesting to speculate whether blocking the vasodilating beta adrenergic receptors in the presence of increased vasoconstrictor nerve activity from amygdalar activation is contributing to abnormal microvascular reactivity and myocardial injury.7
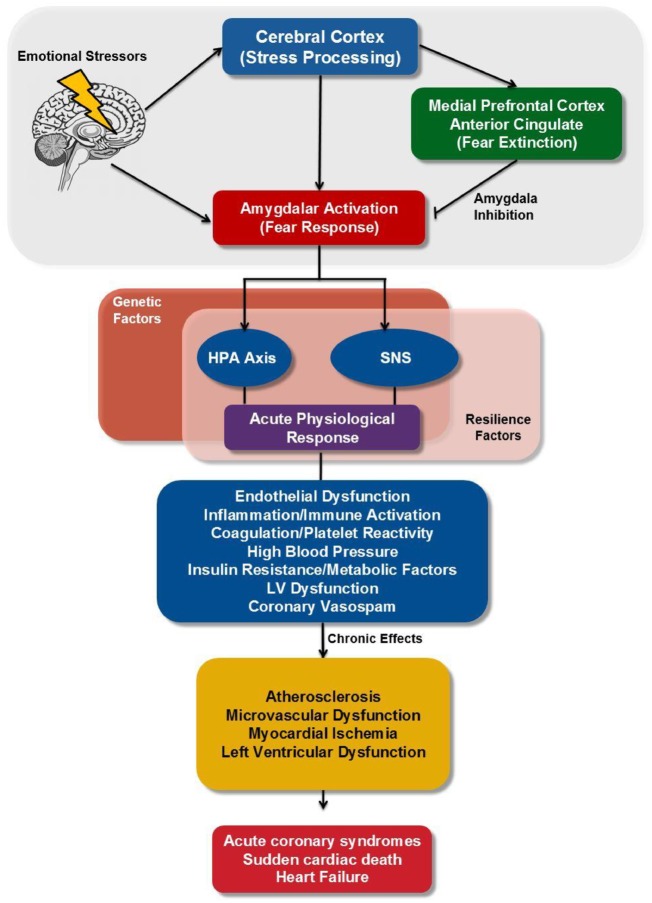
Physiological and psychological stress affects many brain areas that are intimately interconnected, including the highly evolved prefrontal cortex (PFC) and more primitive structures like the amygdala, the hippocampus, and the locus coeruleus (LC) (Figure 1). Upon exposure to a stressful stimulus, the amygdala activates the hypothalamus–pituitary–adrenal axis via projections to the hypothalamus, and the sympathetic nervous system through projections to autonomic control sites, including the parabrachial nucleus, nucleus tractus solitarius, rostroventral lateral medulla, and dorsal motor nucleus of the vagus.2,8–11 This results in an acute physiological response with the release of cortisol and norepinephrine, and an increase in systolic blood pressure and heart rate.2 Furthermore, the amygdala mediates fear conditioning, whereby a previously neutral stimulus (e.g. a cold day), can trigger a fear response after it is paired with a traumatic event. The amygdala is able to sustain a stress response long after a trauma is over. Alternatively, circuits within the PFC are needed to extinguish a conditioned response to a traumatic event and return to normative behaviour by inhibiting the amygdala.12 During chronic stress exposure, there is a remarkable weakening in the executive functions of the medial PFC, while concomitantly strengthening the primitive emotional responses of the amygdala and the tonic firing of the noradrenergic LC.2,8,9 As a consequence, that tonic amygdalar activation results in chronic sympathetic activation leading to immune activation with increased inflammation13 and endothelial dysfunction.14 Amygdala is also implicated in modulation of autonomic outflow in response to physiological stress.15 As found in the present investigation, sex differences in amygdala (de)activation, and its role in autonomic cardiovascular control, have indeed previously been reported16; however, how these sex differences change with age/hormone status, or may be potentiated with disease, remains poorly understood and warrants future research.
Figure 1.
Neurobiology of stress and cardiovascular function. Brain regions involved in stress (amygdala, medial prefrontal cortex, and anterior cingulate) have outputs directly or indirectly through the hypothalamus and the medial prefrontal cortex to neurohormonal systems (cortisol and norepinephrine) affected by stress. These pathways mediate increased heart rate and blood pressure, and chronic activation leads to increased inflammation and endothelial dysfunction, conferring risk of coronary heart disease. HPA, hypothalamic-pituitary-adrenal; SNS, sympathetic nervous system.
Taken together, and combined with these innovative SABV analyses, these results suggest that neurally mediated factors contribute differentially in women and men. Specifically, stress-related amygdala activation appears to contribute to myocardial injury and decrease in left ventricular ejection fraction. This malignant cascade of events can lead to acute coronary syndromes and sudden cardiac death,2,3,6 suggesting that investigation regarding modulation of amygdalar activation by pharmacologic means or bio-behavioural approaches to improved cardiac outcomes should be pursued.
Funding
The National Heart, Lung and Blood Institutes (Nos. N01-HV-68161, N01HV-68162, N01-HV-68163, N01-HV-68164, U0164829, U01 HL649141, U01 HL649241, K23HL105787, T32HL69751, R01 HL090957), and National Institute on Aging grant 1R03AG032631; the National Center for Research Resources (GCRC grant MO1-RR00425); the National Center for Advancing Translational Sciences (UL1TR000124); and the Gustavus and Louis Pfeiffer Research Foundation, Danville, NJ, the Gilead Sciences, The Women’s Guild of Cedars-Sinai Medical Center, Los Angeles, CA, The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, PA, and QMED, Inc., Laurence Harbor, NJ, the Edythe L. Broad and the Constance Austin Women’s Heart Research Fellowships, Cedars-Sinai Medical Center, Los Angeles, California, the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles, The Society for Women’s Health Research (SWHR), Washington, D.C., the Linda Joy Pollin Women’s Heart Health Program, the Erika Glazer Women’s Heart Health Project, and the Adelson Family Foundation, Cedars-Sinai Medical Center, Los Angeles, California.
Conflict of interest: P.K.M., B.B.L. and M.D.N.: None; C.N.B.M. has been a speaker for Abbott Diagnostics and served on the board of directors for iRhythm.
References
- 1. Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL, Camici PG, Chilian WM. et al. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation 2017;135:1075–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vaccarino V, Bremner JD.. Behavioral, emotional and neurobiological determinants of coronary heart disease risk in women. Neurosci Biobehav Rev 2017;74:297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vaccarino V, Sullivan S, Hammadah M, Wilmot K, Al Mheid I, Ramadan R. et al. Mental stress-induced-myocardial ischemia in young patients with recent myocardial infarction: sex differences and mechanisms. Circulation 2018;137:794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fiechter MR, Burger I, Bengs S, Treyer V, Becker A. et al. Association between resting amygdalar activity and abnormal cardiac function in women and men: a retrospective cohort study. Eur Heart J Cardiovasc Imaging 2019;20:625–32. [DOI] [PubMed] [Google Scholar]
- 5. Templin C, Hanggi J, Klein C, Topka MS, Hiestand T, Levinson RA. et al. Altered limbic and autonomic processing supports brain-heart axis in takotsubo syndrome. Eur Heart J 2019;40:1183–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tawakol A, Ishai A, Takx RA, Figueroa AL, Ali A, Kaiser Y. et al. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet 2017;389:834–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Robertson RM, Wood AJ, Vaughn WK, Robertson D.. Exacerbation of vasotonic angina pectoris by propranolol. Circulation 1982;65:281–5. [DOI] [PubMed] [Google Scholar]
- 8. Arnsten AF, Raskind MA, Taylor FB, Connor DF.. The effects of stress exposure on prefrontal cortex: translating basic research into successful treatments for post-traumatic stress disorder. Neurobiol Stress 2015;1:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bremner JD, Campanella C, Khan Z, Shah M, Hammadah M, Wilmot K. et al. Brain correlates of mental stress-induced myocardial ischemia. Psychosom Med 2018;80:515–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cechetto DF, Calaresu FR.. Central pathways relaying cardiovascular afferent information to amygdala. Am J Physiol 1985;248:R38–45. [DOI] [PubMed] [Google Scholar]
- 11. Danielsen EH, Magnuson DJ, Gray TS.. The central amygdaloid nucleus innervation of the dorsal vagal complex in rat: a phaseolus vulgaris leucoagglutinin lectin anterograde tracing study. Brain Res Bull 1989;22:705–15. [DOI] [PubMed] [Google Scholar]
- 12. Maren S, Phan KL, Liberzon I.. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci 2013;14:417–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lima BB, Hammadah M, Wilmot K, Pearce BD, Shah A, Levantsevych O. et al. Posttraumatic stress disorder is associated with enhanced interleukin-6 response to mental stress in subjects with a recent myocardial infarction. Brain Behav Immun 2019;75:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shah A, Chen C, Campanella C, Kasher N, Evans S, Reiff C. et al. Brain correlates of stress-induced peripheral vasoconstriction in patients with cardiovascular disease. Psychophysiology 2019;56:e13291.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ulrich-Lai YM, Herman JP.. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 2009;10:397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kimmerly DS, Wong S, Menon R, Shoemaker JK.. Forebrain neural patterns associated with sex differences in autonomic and cardiovascular function during baroreceptor unloading. Am J Physiol Regul Integr Comp Physiol 2007;292:R715–22. [DOI] [PubMed] [Google Scholar]