Abstract
Myocardial tissue tracking imaging techniques have been developed for a more accurate evaluation of myocardial deformation (i.e. strain), with the potential to overcome the limitations of ejection fraction (EF) and to contribute, incremental to EF, to the diagnosis and prognosis in cardiac diseases. While most of the deformation imaging techniques are based on the similar principles of detecting and tracking specific patterns within an image, there are intra- and inter-imaging modality inconsistencies limiting the wide clinical applicability of strain. In this review, we aimed to describe the particularities of the echocardiographic and cardiac magnetic resonance deformation techniques, in order to understand the discrepancies in strain measurement, focusing on the potential sources of variation: related to the software used to analyse the data, to the different physics of image acquisition and the different principles of 2D vs. 3D approaches. As strain measurements are not interchangeable, it is highly desirable to work with validated strain assessment tools, in order to derive information from evidence-based data. There is, however, a lack of solid validation of the current tissue tracking techniques, as only a few of the commercial deformation imaging softwares have been properly investigated. We have, therefore, addressed in this review the neglected issue of suboptimal validation of tissue tracking techniques, in order to advocate for this matter.
Keywords: strain, speckle tracking imaging, feature tracking, tagging, echocardiography, cMR, review
Introduction
Assessment of cardiac contractile function remains a challenge in current cardiology. Indeed, ejection fraction (EF), the traditional parameter used to describe left ventricular (LV) function, presents significant limitations,1 related to its volumetric nature, suboptimal reproducibility, and inability to reflect regional LV function. This has prompted for a more in-depth characterization of LV mechanics through non-invasive evaluation of myocardial deformation, i.e. strain. Strain2 is the deformation produced by the application of a force; myocardial strain represents percent change in myocardial length from relaxed to contractile state. Unlike EF, strain allows studying the different spatial components of contractile function in either longitudinal strain (LS), circumferential strain (CS), or radial strain (RS) directions, both globally and regionally. However, similar to EF, strain represents a load-dependent estimation of cardiac function and neither is able to depict the true myocardial contractility.
Assessment of LV deformation through quantification of strain has witnessed considerable development, from echocardiographic determined velocity of circumferential fibre shortening,3 cardiac magnetic resonance (cMR) tissue tagging,4 tissue Doppler echocardiography5–8 to current speckle tracking echocardiography (STE), and feature tracking (FT) approaches.9–12 Alterations of strain were found to occur in the setting of maintained EF13,14 and were reported to provide additional prognostic value over EF alone in a multitude of clinical scenarios, ranging from asymptomatic adults without a previous history of cardiac pathology (as participants of MESA and Framingham studies)15,16 to valvular heart disease (in particular aortic stenosis13) and heart failure with preserved and reduced EF.17,18 Therefore, deformation imaging techniques have become extremely popular and, being applied to numerous research questions, have resulted in an extensive number of published papers, with ‘myocardial strain’ keyword search hitting nearly 8000 results in PubMed alone. Notwithstanding the enthusiastic scientific interest, myocardial deformation assessment has only partly breached the clinical setting, as several concerns have been raised regarding its robustness in the real-life scenario.
In this review, we attempt to summarize the general principles and technical particularities of current deformation imaging modalities, with particular emphasis on factors explaining differences in measurement values among methods. Further, we aim to provide an overview of current state of validation and intra- and inter-modality comparison.
General principles of deformation imaging techniques
Myocardial deformation can be assessed both from echocardiographic and cMR images, following a similar general workflow, with specific analysis algorithms implemented for each imaging modality. Most deformation imaging techniques share the common principle that specific patterns or features are identified within an image and followed over time in the subsequent images of the sequence by searching the most probable correspondence in successive image frames.19 Then, local tissue deformation can be estimated by repeating the process for the entire time sequence.
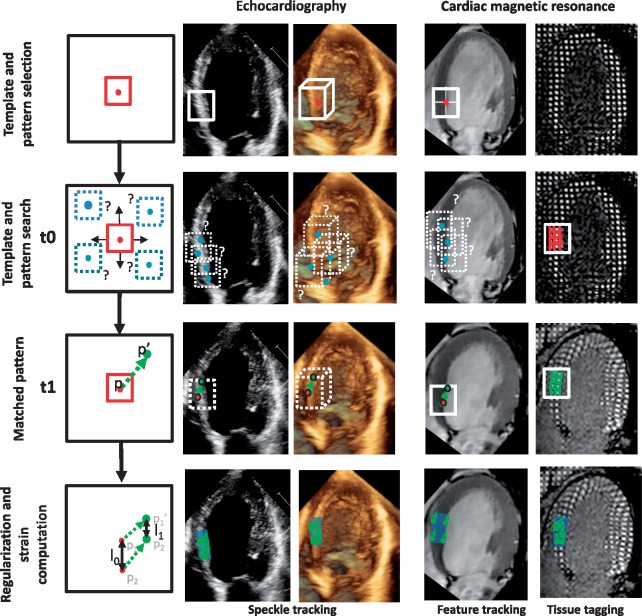
Tissue tracking—general workflow
Typical workflow of tissue tracking is shown in Figure 1. Generally, the initial step is to recognize the key cardiac events: end-diastole (ED) and end-systole (ES). The second step is the definition of a region of interest encompassing the myocardial wall, by semi-automatic contouring of the endocardial and epicardial borders either in ED or in ES or both. Segmentation is a critical step as it defines the set of points that will be tracked, introducing variability depending on the user and the segmentation algorithm.10 Finally, the region of interest is tracked throughout the cardiac cycle, and strain curves are computed, possibly post-processed. Either the end-systolic or peak systolic strain can be reported.
Figure 1.
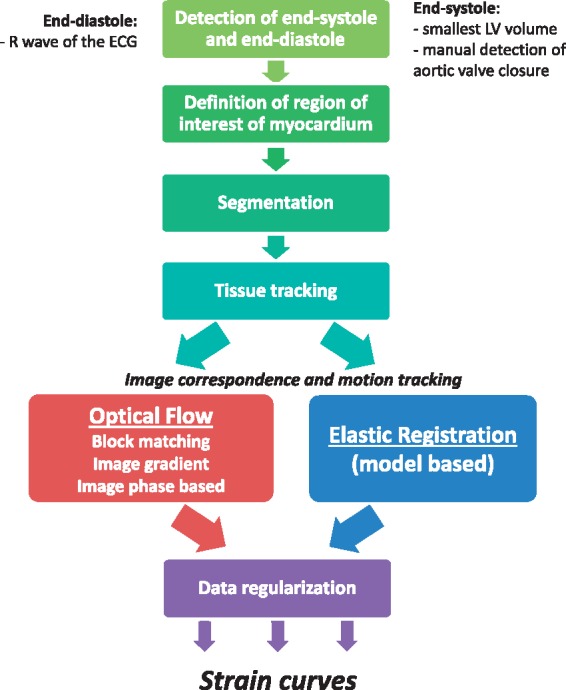
General workflow of strain computation.
Technology of tissue tracking—analysis algorithms
Echocardiographic and cMR deformation imaging softwares employ different algorithms to process the image in order to estimate the local myocardial motion. Some techniques exploit specificities of the imaging modality [e.g. cMR tagging], while others are generic and can be applied to any modality (e.g. block-matching techniques for STE and FT). A more detailed technical discussion is included in the Supplementary data online.
Specific modalities of tissue tracking and strain imaging
The tissue tracking strategies can be applied to echocardiographic (2D or 3D), cMR (cine or tagged images), sometimes extending these strategies to account for specificities of the imaging modality (Figure 2).The particularities of these techniques to estimate myocardial deformation are discussed below, while advantages and shortcomings of each method are summarized in the following sections.
Figure 2.
The principles of tissue tracking techniques illustrated on different imaging modalities. The myocardial speckled pattern (on 2D and 3D echocardiography), or anatomical features (on cine-cMR images), or tagging information (on cMR tagging) are identified within an image and followed over time in the subsequent images of the sequence by searching the most probable pattern correspondence.
cMR tagging
cMR tagging4 magnetically labels different regions in the myocardium, by creating, prior to image acquisition, locally induced perturbations of the magnetization with selective radiofrequency saturation planes20 resulting in dark lines. When the saturation pulses are applied in two orthogonal planes, the resulting tagging pattern forms a grid of intrinsic tissue markers known as tags. Because the magnetization is a property of the tissue, the tag lines move along with the tissue in which they are created, deforming during contraction. Tag using harmonic phase imaging (HARP) technique will find the best optical flow for matching the multiple ‘channels’ of the tagged acquisitions, each tag direction corresponding to one channel. Thus, tracking the tag deformation allows direct evaluation of the myocardial deformation or strain. Variations of tagging for strain computation are Strain Encoding magnetic resonance imaging (SENC)21 and Displacement Encoding with Stimulated Echos (DENSE).22 In these techniques, encoding is applied through plane and pixel intensities directly relate to the amount of tissue deformation.
cMR tagging has been widely accepted as the reference standard imaging modality for strain quantification after extensive validation in vitro23 and in vivo24–31 and has allowed the development of the first models of normal and abnormal myocardial motion in humans.24,29,32–35 The main advantage of tagging is that deformation is directly measured by physical properties of the tissue. Yet, cMR tagging also has certain limitations (Table 1). Tagged images have low temporal resolution reaching at the best 20–30 frames/heart-beat. Furthermore, tag deposition in the beginning of systole starts after detection of R wave and introduces a delay of approximately 30 ms. Thus tag deposition may not be exactly at the beginning of cardiac contraction, potentially leading to underestimation of strain, especially at high heart rates. The spatial resolution of tags, as well as the ratio of tag spacing to slice thickness, are also important factors for reliable strain measurements. For this reason, the accuracy of strain estimates from cMR tagging is lower at the endocardial border and in thin-walled regions of the LV, and cMR tagging estimates essentially mid-wall rather than endocardial strain. Finally, tagging requires dedicated acquisition sequence and time-consuming post-processing using specific software solutions such as HARP. Therefore, cMR tagging has mainly remained a research tool and has not undergone as widespread use as more recent methods to measure strain.
Table 1.
Spatial and temporal resolution and strength and weaknesses of different imaging modalities
| 2DSTE | 3DSTE | cMR-FT | cMR Tagging | cMR SENC | cMR DENSE | |
|---|---|---|---|---|---|---|
| Spatial | 0.2–0.3 mm | 0.4–0.5 mm | 1–2 mm in plane | >1 mm in plane | 1.5–2 mm | 1.5–2 mm |
| 6–10 mm through plane | 5–7 mm through plane | |||||
| Temporal | 40–60 frames/s | 20–50 frames/s | 25–35 phases/heart-beat | 20–30 phases/heart-beat | 20–30 phases/heart-beat | 20–30 phases/heart-beat |
| Strengths |
|
|
|
|
|
|
| Weaknesses |
|
|
|
|
|
|
Speckle tracking echocardiography
STE is currently the widest available technique to quantify myocardial deformation,36 mainly because it can be performed on conventional B-Mode images, assuming that image quality is sufficient.
STE analyses LV deformation by tracking cardiac motion from image intensities. Features being tracked can include image contours and image texture, more specifically, the naturally occurring speckled pattern of the myocardium when imaged by ultrasound.37 For tracking the speckle texture, block-matching method is a commonly used technique. It automatically identifies a pattern within a region or block of interest, compares it to all possible matching regions within the search region and finds the position of the best matching block compared with the original one. STE can be applied to 2D, and more recently to 3D echocardiographic images. Optical flow methods have also been applied to echocardiographic images, as well as elastic registration, all of them being able to capture motion and to a certain extent deformation as demonstrated on synthetic images.38 STE has high spatial and temporal resolution, but depending on the algorithm, typically evaluates speckle motion mainly at endocardial border of the LV, and relatively less in the myocardium.
cMR-FT
cMR-FT is a relatively new 2D imaging technique that can be applied to standard cMR cine SSFP sequences, gaining popularity by allowing measurement of myocardial deformation without the need for dedicated acquisition and complex post-processing.12,19
cMR-FT is mainly based on a block-matching approach. It first identifies anatomic features in the cMR image along the myocardial boundaries, defines region of interests around these locations and track them along the cardiac cycle by looking for the most similar region in the next image. Advantages of FT is that strain can be computed on conventional SSFP cine images using several commercial softwares. In contrast to STE and cMR tagging, FT does not seem to distinguish intramyocardial features, as the grey level distribution in cine SSFP images in relatively homogenous. Furthermore, similar to tagging, cine-cMR images have substantially lower spatial and temporal resolution than STE (Table 1).
Sources of variations and intra- and inter-modality inconsistencies
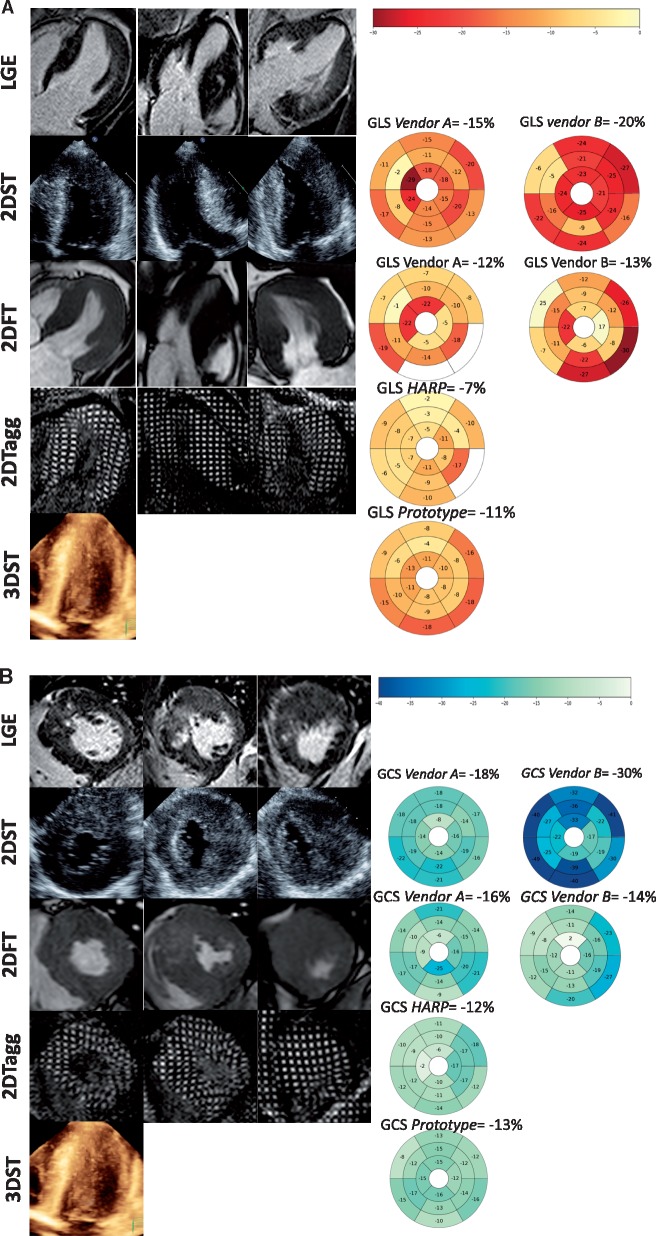
The differences in imaging modalities and competitive methodologies result in intra- and inter-modality inconsistencies in deformation estimation as illustrated by Figure 3A and B. These are explained by several factors, as listed below and summed up in Table 2.
Figure 3.
Example of differences in global and regional strain estimates (A longitudinal and B circumferential) by different modalities and softwares in a patient with hypertrophic cardiomyopathy. Regional strain values are represented in 17 and 18 segment colour-coded bullseyes plots. Excluded segments due to poor image quality or tracking are not colour-coded.
Table 2.
Summary of sources of variations and intra- and inter-modality inconsistencies
| Imaging modality related factors | Quality of the acquisition process |
| Spatial and temporal resolution | |
| Segmentation misalignment between imaging modalities | |
| Software-related factors | Spatial and temporal smoothing |
| Size of the search region | |
| Favouring tracking in a certain myocardial layer | |
| Computation of Lagrangian or Eulerian strain | |
| Calculation of global strain values | |
| Definition of end-diastole and end-systole | |
| Operator-related factors | Definition of regions of interest |
| Experience and training |
Imaging modality related factors
A first set of factors potentially influencing deformation quantification is the quality of the acquisition process, varying between operators and modalities. Ensuring reproducible and accurate breathing control is therefore a key requirement for all modalities.
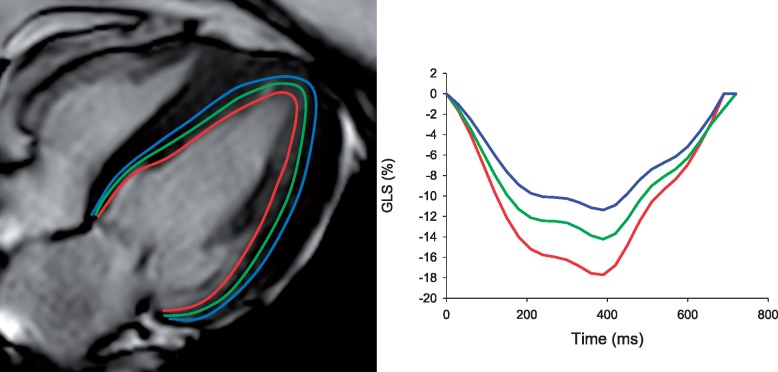
A second set of modality-dependent factors relate to the spatial and temporal resolution of the images. Both resolutions are crucial to ensure the complete characterization of myocardial deformation over successive time frames. If the temporal and spatial resolution is too low (Figure 4), the local patterns may become less comparable, an effect known as image de-correlation and displacements may become harder to detect.19 Precisely, the temporal resolution of cMR and 3DSTE is lower than that of 2DSTE, and inferior to Tissue Doppler Imaging for example, meaning that cMR and 3DSTE are more prone to miss the short-lived events during the isovolumic period. Another parameter that may influence strain values is the reference method. For instance in tagged images, tag deposition is delayed relative to electrocardiogram signal detection, which leads to underestimation of strain (Figure 5). On the other hand, STE has a higher spatial resolution than cMR, which is however blunted by a low signal-to-noise ratio. The particularity of the spatial resolution of the ultrasound images is a lower lateral than axial resolution and lower in-depth resolution.39 This means that most reliable results are obtained closer to the centre line of the image, at smaller depths.
Figure 4.
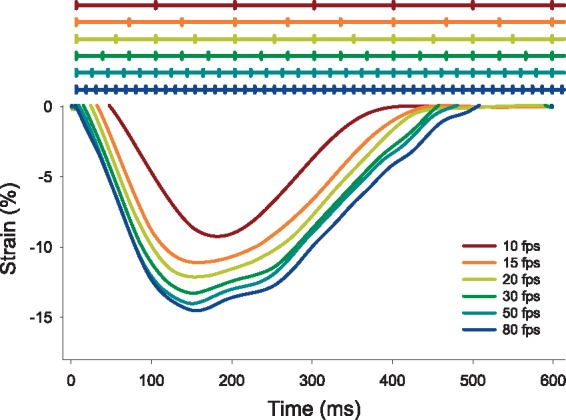
Influence of temporal resolution on strain measurement (data extrapolated from a high temporal resolution STE image undersampled at lower frame rates).
Figure 5.
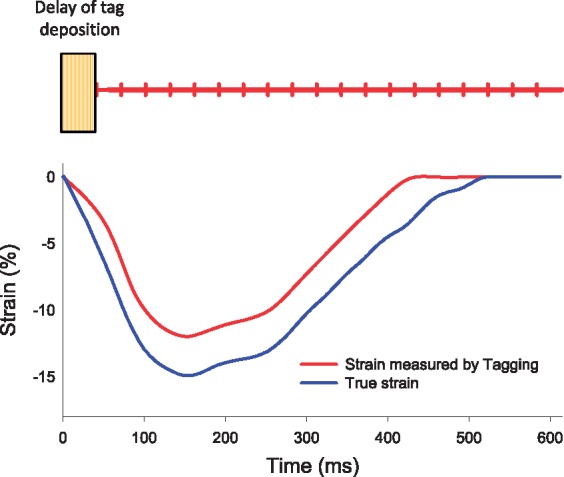
Influence of tag deposition delay of strain computation in cMR tagging [data extrapolated from a high resolution STE image acquired at high heart rate (120 bpm)].
Finally, a last source of potential discrepancies in 2D regional deformation values between modalities is the difficulty to match myocardial segments. This segmentation misalignment between imaging modalities is explained by the fact that 2D imaging planes are not necessarily the same when acquired by echocardiography and cMR due to different scanning angles, therefore, complete correspondence between segments is not achievable. For any 2D technique, the pattern within a region of interest is detected and tracked along the image plane. However, as LV deformation is a 3D phenomenon, involving a combination of apex-to-base shortening and simultaneous twisting, the myocardial patterns have a complex 3D motion. Therefore, the pattern within a region of interest defined in a 2D image plane, might move out of the scanning plane during the cardiac cycle. Moreover, unlike cMR, the imaging planes of 2D echo might not depict the true apex in long- and short-axis views, effect known as foreshortening. The through-plane motion and foreshortening represent a limitation of 2D analysis, which are overcome by 3D techniques.
Software-related factors
Several factors related to the specificities of implementing image tracking algorithms can also heavily influence deformation values.
ST algorithms apply spatial and temporal smoothing to regularize the results in order to reduce noise, which can affect the measurement robustness by missing significant localized abnormalities in the case of spatial smoothing or by masking rapid events in case of temporal smoothing.19,40 Also, as strain is computed from the spatial derivatives of the displacement, different regularization strategies (thus affecting motion smoothness) can dramatically affect the range of deformation values computed from the displacement field, at least when considering single material points or small regions. Therefore, parameters based on local estimates are more prone to variability than those based on an integrative combination, i.e. global strains are more stable and reliable than segmental strains.
For block-matching algorithms, the size of the search region must be carefully tuned.19 In general, solving for displacements between short distance regions is challenging and may explain why usually RS (computed on the small distance between endo- and epicardium) is less reliable than LS and CS41 that are computed over larger regions.
Favouring the tracking in a certain myocardial layer, i.e. endocardial rather than transmural could alter the strain values, as given the fibre orientation, the deformation of the endocardial layer is more important than in the mid and epicardial layers. The level of endocardial strain detection by the software is probably the most important factor (Figure 6) inducing intra- and inter- modality variability in strain measurement. Importantly, the level of layer detection may also vary among imaging methods. In particular, as mentioned before, tags are mainly detected in mid-wall, whereas STE mainly follows endocardial markers.
Figure 6.
Influence of endocardial layer position on strain measurements. (Example of different layer positions on endocardial strain in a cMR-FT image).
Other software-related factors that introduce variability are:
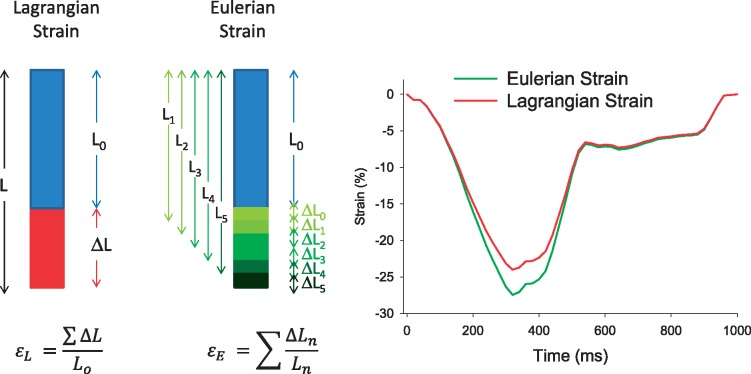
Computation of Lagrangian or Eulerian strain, as the two formulas (represented in Figure 4) will result in slightly different values, with Eulerian strain having higher absolute values (Figure 7).
Calculation of global strain values, either by using the entire myocardial length or by averaging values computed at segmental level, will give different results.42
Definition of ED and ES, which has been shown to have a major influence on the accuracy of strain measurements, up to the point that changing ED or ES by only four frames can significantly impact strain values to as much as 20–40% relative changes in ES GLS.43
Figure 7.
Difference in Eulerian and Lagrangian strain computation.
Operator-related factors
Most strain analysis softwares require manual drawing of the myocardial contours. As these contours define the regions/points being tracked, different operators contouring differently will obtain different deformation values. For all techniques, operator experience and training is an important factor in accuracy of measurements. Indeed most validation studies were performed in highly experienced centres and core-labs and may not translate to overall clinical practice. With the advent of machine learning and fully automated analysis this factor may become less important in the future.
Practical aspects
Tissue tracking software platforms may use different algorithms for measuring deformation and presentation of results, therefore, two aspects become critically important: validation of each specific analysis software and consensus reporting among software package vendors. Validating non-invasive tools used for clinical practice is, however, a challenging task, and has not been done for routine parameters as EF for example. Additionally, EF is subjected to inter-modality (echocardiography vs. cMR) and inter-vendor (different 3DE analysis softwares) variability on top of the suboptimal inter and intra-observer reproducibility.
Validation
Before implementation in the clinical setting, any new strain imaging method requires a complex process of validation: on synthetic data sets, in vitro and in vivo experiments, and validation in humans. In opposition to the enthusiastic number of published papers assessing the clinical usefulness of strain, there is a notable lack of validation studies. Indeed, while numerous studies have performed inter-technique comparisons, which can only demonstrate their relative performance, true validation studies for STE, and cMR-FT studies have only been performed for few commercial softwares of the numerous available alternatives. Additionally, most of the clinical validation studies have included a very small number of subjects in the healthy control group and even smaller number in the diseased group, which can only hardly represent the diverse pathological phenotypes encountered in clinical practice.
Validation on synthetic data sets
Synthetic data sets are computer generated images and constitute the first step when testing a new software. In such a controlled environment, the deformation values to be measured are known (i.e. ground truth), while parameters as motion rate, wall thickness and EF can be synthetically altered to simulate different cardiac conditions.
Currently, open-access libraries of 2D and 3D simulated ultrasound datasets, as well as simulated cine cMR, are being built to facilitate performance analysis of different software packages in order to promote quality assurance.48–50 The tested strain imaging methods have shown promising results, and efforts have been made to reach the level of realism of the real ultrasound and cMR images.
In vitro validation
The next step used for validation of strain techniques is by using physical cardiac phantoms in which motion is mechanically controlled. In this case, the motion of the phantom is compared to the ground truth obtained by sonomicrometry recordings. Sonomicrometry is a technique of measuring distances between piezoelectric crystals based on the speed of acoustic signals through the medium they are embedded in. Both 2DST44 and 3DST46,47 methods have been validated in vitro and have shown good accuracy (see Table 3), while for FT there is currently no validation on phantoms.
Table 3.
In vitro validation of 2DSTE and 3DSTE
| Study | Model | Method | Reference | Software | Strain | Conclusion |
|||
|---|---|---|---|---|---|---|---|---|---|
| r/ICC | Bias ± 2 SD (%) | 95% CI | |||||||
| 2DSTE | |||||||||
| Korinek et al.44 | Phantom | Different motion rates (n = 23) | Sono | GE EchoPAC PC_2D strain, | Long | r = 0.99 | 0.7 ± 2.2 | −3.6 to 5 | Promising |
| Amzulescu et al.45 | Phantom | Different motion rates and stroke volumes (n = 35) | Sono | Qlab 10.3 Philips | Long | ICC = 0.89 | 3 ± 2.8 | −8.2 to 2.5 | Good for Long |
| 3DSTE | |||||||||
| Heyde et al.46 | Phantom | Different motion rates (n = 7) | Sono | In-house software |
|
|
Adequate | ||
| Hjertaas et al.47 | Phantom | Different motion rates and stroke volumes (n = 15) | Sono | GE EchoPAC BT11 |
|
|
|
|
Accurate for Long and Circ, not for Rad |
However, the models used to mimic motion are generally simple and do not represent the true complex cardiac deformation, while cardiac anatomic structures as trabeculations/valves are not represented.
In vivo validation
In order to approximate the real-life conditions, an in vivo design to validate strain measurements is required. Different open-chest animal models have been used and myocardial deformation values have been compared to sonomicrometry. Studies investigating 2DSTE and 3DSTE (Table 4) have reported overall good agreement of strain by STE with sonomicrometry measurements. Generally, while LS 2DSTE seems to perform well across studies, recent reports describe suboptimal correlation and larger bias for CS and RS by 2DSTE.56 For 3DSTE, LS and CS are accurate compared to sonomicrometry, while RS has been shown to be less reliable.59 To date, FT has not been tested in vivo.
Table 4.
In vivo validation of 2DSTE and 3DSTE
| Study | Model | Method | Reference | Software | Strain | Conclusion |
|||
|---|---|---|---|---|---|---|---|---|---|
| r/ICC | Bias ± 2 SD (%) | 95% CI | |||||||
| 2DSTE | |||||||||
| Korinek et al.44 | 16 pigs | Baseline, LAD ligation | Sono | GE EchoPAC PC_2D strain |
|
r = 0.94 | −1.1 ± 7.5 | −15.8 to 3.9 | Promising |
| Toyoda et al.51 | 6 dogs | Dobutamine | Sono | US customized software | Rad | r = 0.92 | Promising | ||
| Langeland et al.52 | 5 sheep | Baseline, CX ligation, esmolol, dobutamine | Sono | In-house software (SPEQLE 2D) |
|
|
|
|
Promising |
| Amundsen et al.53 | 9 dogs | Baseline, saline loading, LAD occlusion | Sono | MathLab-based custom made programme |
|
|
|
Accurate | |
| Reant et al.54 | 10 pigs | Baseline, LAD occlusion, dobutamine | Sono | GE EchoPAC |
|
|
Real potential | ||
| Pirat et al.55 | 7 dogs | Baseline, LAD occlusion, esmolol, dobutamine | Sono | Siemens VVI |
|
|
Accurate | ||
| Heyde et al.56 | 5 sheep | Baseline, CX ligation, esmolol, dobutamine | Sono | GE EchoPAC v110.0.0, |
|
|
|
|
Circ and Radial overestimate |
| 3DSTE | |||||||||
| Seo et al.57 | 10 sheep | Baseline, LAD ligation, dobutamine, propranolol | Sono | Toshiba 3D wall motion tracking |
|
|
Reliable | ||
| Heyde et al.58 | 14 sheep | Baseline, CX ligation, dobutamine, esmolol | Sono | In-house STE software |
|
|
Acceptable accuracy | ||
| Bouchez et al.59 | 13 sheep | Baseline, dobutamine, CX occlusion | Sono | SIemens eSie volume mechanics |
|
|
|
Good for Long and Circ, less accurate for Rad | |
Similar to the in vitro validation, this approach allows measurement of deformation in only a few LV regions, where the sonomicrometry crystals are located, and image quality is better than in standard clinical settings, therefore, not representative.
Clinical validation
Unlike in vitro and in vivo settings, image quality is different and motion can be calculated in more than one region for all strain components. Clinical validation in humans is achieved by using another previously validated imaging modality, such as cMR tagging, as reference framework. Studies investigating the reliability of speckle and tissue tracking techniques compared to cMR tagging in humans have shown lower accuracy than for the pre-clinical validation, but generally demonstrated satisfactory results (Table 5). Four studies compared commercial45,60,61 or custom developed 2DSTE62 vs. cMR-tagging with modest to good correlations and acceptable bias. However, it was not always performed for all strain directions45,60,61 but most often only for LS.62 Correlation was acceptable for GLS, but less for GCS and importantly agreement was poor at regional level.45 In addition, there was overestimation of LS and CS and spatial inhomogeneity in particular at apical level. 3DSTE has been compared to cMR tagging by three studies evaluating the CS direction in healthy controls63 and small number of diseased,64 using software from two different manufacturers or prototype software.65 While the correlation was good, one study found that CS was overestimated by 3DSTE.63 Another study found better agreement of 3DSTE than 2DSTE with cMR tagging.65 Part of the inter-modality differences have been largely attributed to the technical specifications of each software,45,65 namely the tracking algorithms, which, despite contouring transmural regions of interest, may lead to higher deformation values when endocardial layer is predominantly tracked than when a transmural approach is favoured. Additionally, most clinical studies have assessed global strains, and the few attempting to validate segmental strain in patients45,53,65 show conflicting results, the most recent ones questioning the reliability of regional deformation assessment.45,65
Table 5.
Clinical validation of 2DSTE, 3DSTE, and cMR-FT
| Conclusion |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Patients | Method | Reference | Software | Strain | r/ICC | Bias ± 2SD (%) | 95% CI | |
| 2DSTE | |||||||||
| Amundsen et al.53 | 7 MI, 4 NL | cMR tagging | MathLab-based custom made programme | Long | r = 0.87 | −9.1 to 8 | Accurate | ||
| Cho et al.60 | 30 CAD | cMR tagging | GE EchoPAC BT04 |
|
|
|
|
Modest performance | |
| Bansal et al.61 | 30 CAD | cMR tagging | GE EchoPAC-PC v6.0 |
|
|
Feasible | |||
| Amundsen et al.62 | 10 MI, 11 NL | cMR tagging | GE EchoPAC-PC v6.0 In-house STE software | Long |
|
|
Suitable | ||
| Amzulescu et al.45 | 75 DYS , 30 HCM, 31 NL | cMR tagging | Philips QLAB 10.3 |
|
|
|
|
Best for GLS, suitable for GCS, suboptimal for segmental strain | |
| 3DSTE | |||||||||
| Kleijn et al.63 | 45 NL | Mid-ventricular | cMR tagging | Toshiba 3D wall motion tracking software | Circ | 0.8 | 10 ± 1.7 | 6.7–13.2 | Circ overestimates strain |
| Zhou et al.64 | 12 NL, 12 DCM, 11 HTA | Apical and mid-ventricular | cMR tagging | SIemens eSie Volume Mechanics | Circ |
|
|
|
Feasible |
| Amzulescu et al.65 | 63 DYS, 27 HCM 91 NL | cMR tagging | Philips Prototype software |
|
|
|
|
GLS, GCS accurate, suboptimal for segmental strain | |
| cMR-FT | |||||||||
| Hor et al.66 | 191 Duchenne muscular dystrophy, 42 NL | Mid-ventricular | cMR tagging | TomTec Diogenes | Circ | 0.89 | −4 to 3.5 | No under or overestimation. | |
| Harrild et al.67 | 13 NL, 11 HCM | Mid-ventricular | cMR tagging | Customized software programme (Cardiotool) | Circ | 1 ± 9 | −16.6 to 18.6 | No under or overestimation. | |
| Augustine et al.68 | 145 NL | 20 NL had cMR tagging | cMR tagging | Tomtec 2D Cardiac Performance analysis |
|
|
|
Long and Rad overestimate | |
| Wu et al.69 | 10 NL + 10 left bundle branch, 10 HCM | Endocardial and mid-wall layer | cMR tagging mid-wall | TomTec Diogenes | Circ | Segmental Mid FT ICC: 0.58 (0.14–0.80) | Circ overestimates, segmental FT unreliable. | ||
| Moody et al.70 | 35 NL + 10 DCM | Endocardial layer | cMR tagging endo-, mid-, epi-, transmural | TomTec Diogenes |
|
|
|
Sufficient agreement. | |
| Singh et al.71 | 18 aortic stenosis | Endo, endo/epi average | cMR tagging | TomTec Diogenes |
|
ICC = 0.54 | 3.6 ± 3.3 | −2.9 to 10.2 | Long and Circ overestimate |
CAD, coronary artery disease; DCM, dilated cardiomyopathy; DYS, dysfunction; HCM, hypertrophic cardiomyopathy; MI, myocardial infarct, NL, normal, healthy volunteers.
For FT, reports of the clinical validation data vs. cMR tagging are conflicting, with some studies showing good agreement,66,70 while others describe strain overestimation of certain strain deformation directions.68,69,71 Similar to STE, it has been generally concluded that segmental deformation assessment with FT is less reliable than global strain estimation.69
Intervendor agreement
An increasing number of studies evaluating differences between STE software manufacturers have consistently reported significant intervendor variability for 2D GLS measurement.72–76 Therefore, the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE) have set up a task force to assess sources of STE measurement variability in partnership with the industry,42 aiming to standardize STE in order to potentially extend its clinical application. Subsequent to the task force initiative, different manufacturers have released improved software versions and the inter-vendor agreement for 2DSTE GLS has improved.77,78 However, 2D regional STE measurements are still subject to important variability among vendors.78,79
Intervendor agreement has been investigated for 3DSTE as well and, similar to 2DSTE, strain measurements were discordant, depending on the tested imaging equipment and analysis software. While GLS seemed less affected, GCS had acceptable intervendor agreement and GRS had the highest variability.80
A similar issue is anticipated for FT, as inconsistencies between the commercially available softwares have been demonstrated, with acceptable differences for GLS and GCS, but considerable disagreement for GRS.81,82
Therefore, variations in proprietary software are responsible of suboptimal intervendor agreement of strain measurements and constitute a significant limitation to the implementation of STE and FT techniques. Cross-platform standardization is needed in order to expand deformation imaging methods beyond the current research oriented environment.
Normal strain values
The application of myocardial strain to quantify deformation in pathological states requires the definition of a normal range. As shown by Figure 8, reported normal ranges vary largely between the different deformation imaging modalities. In particular, heterogeneity was larger for GRS than GLS and GCS. Besides the technical factors described above, patient-related factors (age, gender, and ethnicity) and haemodynamic factors (heart rate and blood pressure) constitute other potential influences.83,84,92
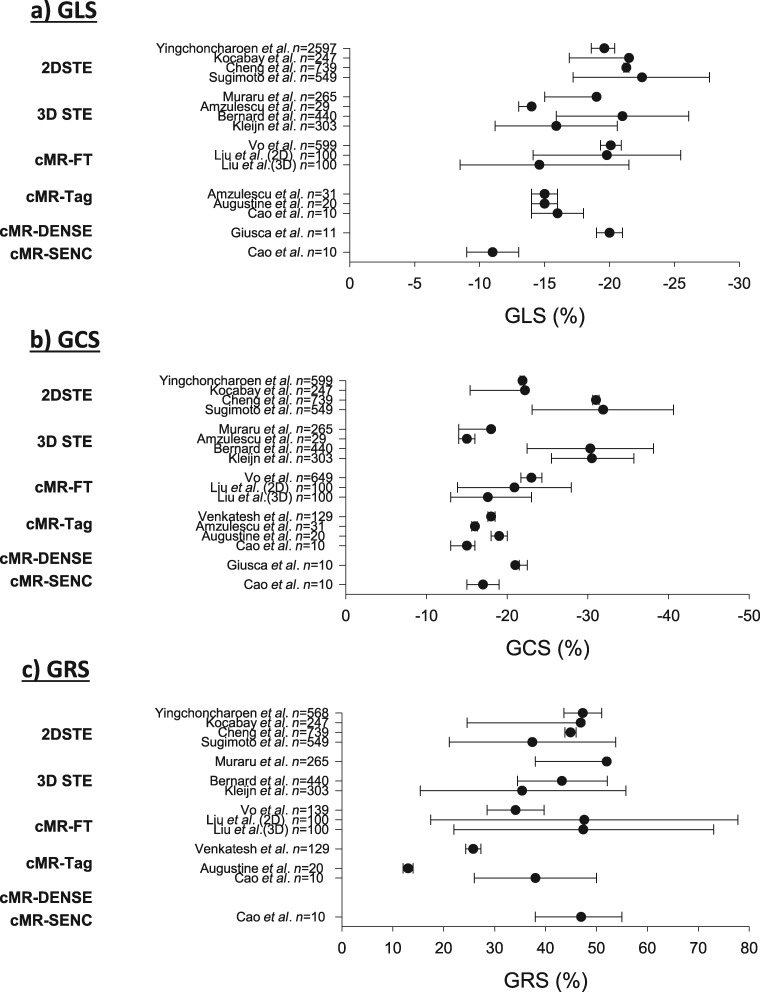
Figure 8.
Reported normal (mean and 95% confidence interval) global strain values in healthy subjects for different imaging modalities. Data from refs.45,65,68,82–91 Normal GLS, GCS, and GRS values were compared using random effects models weighted by inverse variance and heterogeneity between methods was compared using the Cochran Q test and the inconsistency factor. For all strain measurements, I2 and Q indicated significant heterogeneity among studies and methods.
Clinical implications
For the aforementioned reasons, in clinical practice, a global strain parameter rather than a segmental strain value should be favoured when estimating LV function. And, as the reported technical limitations, validation issues and intervendor agreement pertain particularly to GCS and GRS, and less to GLS, the preferred global strain parameter should be GLS. Additionally, baseline and follow-up strain measurements need to be obtained using the same modality, analysis system, and software version. As deformation estimation techniques are less dependent on segmentation variability than EF calculation, strain measurements have proven to be more reproducible than EF.93–95
Therefore, when facing two imperfect parameters of systolic function estimation, i.e. EF and strain, the clinician should take into account the potential benefits and disadvantages of each.
Conclusion
While multiple studies have shown the usefulness of strain quantification for risk stratification in various cardiac disease,9,39 the main limitation remains that strain values vary among methods, modalities and software version.96 Therefore, method and software specific cut-off values need currently to be used. Another major caveat, which remains largely neglected, is the lack of proper validation of most methods vs. absolute and objective reference standard.
To allow accurate deformation estimates and avoid unnecessary variability between products and methods, it should be mandatory that each strain quantification method undergoes rigorous validation using a multi-step process before wide-use for research purposes, and even more, for clinical implementation. Despite the difficulties, such an approach of widespread validation and cross-modality and vendor standardization needs to be applied to allow further development of this technology and successful clinical utilization of these methods.
Funding
Grant support by the Fondation Nationale de la Recherche Scientifique of the Belgian Government (FRSM PDR 19488731).
Conflict of interest: H.L. and M.D.C. are employed by Philips Medical Systems. The Cliniques Universitaires St. Luc have a master research agreement with Philips Medical Systems. All remaining authors have declared no conflicts of interest.
Supplementary Material
References
- 1. Konstam MA, Abboud FM.. Ejection fraction: misunderstood and overrated (changing the paradigm in categorizing heart failure). Circulation 2017;135:717–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mirsky I, Parmley WW.. Assessment of passive elastic stiffness for isolated heart muscle and the intact heart. Circ Res 1973;33:233–43. [DOI] [PubMed] [Google Scholar]
- 3. Domanski MJ, Follmann D, Mirsky II.. A new approach to assessing regional and global myocardial contractility. Echocardiography 1997;14:1–8. [DOI] [PubMed] [Google Scholar]
- 4. Zerhouni EA, Parish DM, Rogers WJ, Yang A, Shapiro EP.. Human heart: tagging with MR imaging–a method for noninvasive assessment of myocardial motion. Radiology 1988;169:59–63. [DOI] [PubMed] [Google Scholar]
- 5. Sutherland GR, Stewart MJ, Groundstroem KW, Moran CM, Fleming A, Guell-Peris FJ.. Color Doppler myocardial imaging: a new technique for the assessment of myocardial function. J Am Soc Echocardiogr 1994;7:441–58. [DOI] [PubMed] [Google Scholar]
- 6. Heimdal A, Støylen A, Torp H, Skjærpe T.. Real-time strain rate imaging of the left ventricle by ultrasound. J Am Soc Echocardiogr 1998;11:1013–9. [DOI] [PubMed] [Google Scholar]
- 7. Edvardsen T, Gerber BL, Garot J, Bluemke DA, Lima JA, Smiseth OA.. Quantitative assessment of intrinsic regional myocardial deformation by Doppler strain rate echocardiography in humans: validation against three-dimensional tagged magnetic resonance imaging. Circulation 2002;106:50–6. [DOI] [PubMed] [Google Scholar]
- 8. Urheim S, Edvardsen T, Torp H, Angelsen B, Smiseth OA.. Myocardial strain by Doppler echocardiography. Validation of a new method to quantify regional myocardial function. Circulation 2000;102:1158–64. [DOI] [PubMed] [Google Scholar]
- 9. Collier P, Phelan D, Klein A.. A test in context: myocardial strain measured by speckle-tracking echocardiography. J Am Coll Cardiol 2017;69:1043–56. [DOI] [PubMed] [Google Scholar]
- 10. Jasaityte R, Heyde B, D’Hooge J.. Current state of three-dimensional myocardial strain estimation using echocardiography. J Am Soc Echocardiogr 2013;26:15–28. [DOI] [PubMed] [Google Scholar]
- 11. Muraru D, Niero A, Rodriguez-Zanella H, Cherata D, Badano L.. Three-dimensional speckle-tracking echocardiography: benefits and limitations of integrating myocardial mechanics with three-dimensional imaging. Cardiovasc Diagn Ther 2018;8:101–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Claus P, Omar AMS, Pedrizzetti G, Sengupta PP, Nagel E.. Tissue tracking technology for assessing cardiac mechanics: principles, normal values, and clinical applications. JACC Cardiovasc Imaging 2015;8:1444–60. [DOI] [PubMed] [Google Scholar]
- 13. Delgado V, Tops LF, van Bommel RJ, van der Kley F, Marsan NA, Klautz RJ. et al. Strain analysis in patients with severe aortic stenosis and preserved left ventricular ejection fraction undergoing surgical valve replacement. Eur Heart J 2009;30:3037–47. [DOI] [PubMed] [Google Scholar]
- 14. Kraigher-Krainer E, Shah AM, Gupta DK, Santos A, Claggett B, Pieske B. et al. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol 2014;63:447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Choi E-Y, Rosen BD, Fernandes VRS, Yan RT, Yoneyama K, Donekal S. et al. Prognostic value of myocardial circumferential strain for incident heart failure and cardiovascular events in asymptomatic individuals: the Multi-Ethnic Study of Atherosclerosis. Eur Heart J 2013;34:2354–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cheng S, McCabe EL, Larson MG, Merz AA, Osypiuk E, Lehman BT. et al. Distinct aspects of left ventricular mechanical function are differentially associated with cardiovascular outcomes and all-cause mortality in the community. J Am Heart Assoc 2015;4:e002071.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Park JJ, Park JB, Park JH, Cho GY.. Global longitudinal strain to predict mortality in patients with acute heart failure. J Am Coll Cardiol 2018;71:1947–57. [DOI] [PubMed] [Google Scholar]
- 18. Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Liu L. et al. Prognostic importance of impaired systolic function in heart failure with preserved ejection fraction and the impact of spironolactone. Circulation 2015;132:402–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pedrizzetti G, Claus P, Kilner PJ, Nagel E.. Principles of cardiovascular magnetic resonance feature tracking and echocardiographic speckle tracking for informed clinical use. J Cardiovasc Magn Reson 2016;18:51.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Axel L, Dougherty L.. MR imaging of motion with spatial modulation of magnetization. Radiology 1989;171:841–5. [DOI] [PubMed] [Google Scholar]
- 21. Osman NF, Sampath S, Atalar E, Prince JL.. Imaging longitudinal cardiac strain on short-axis images using strain-encoded MRI. Magn Reson Med 2001;46:324–34. [DOI] [PubMed] [Google Scholar]
- 22. Kim D, Gilson WD, Kramer CM, Epstein FH.. Myocardial tissue tracking with two-dimensional cine displacement-encoded MR imaging: development and initial evaluation. Radiology 2004;230:862–71. [DOI] [PubMed] [Google Scholar]
- 23. Young AA, Axel L, Dougherty L, Bogen DK, Parenteau CS.. Validation of tagging with MR imaging to estimate material deformation. Radiology 1993;188:101–8. [DOI] [PubMed] [Google Scholar]
- 24. Garot J, Bluemke DA, Osman NF, Rochitte CE, McVeigh ER, Zerhouni EA. et al. Fast determination of regional myocardial strain fields from tagged cardiac images using harmonic phase MRI. Circulation 2000;101:981–8. [DOI] [PubMed] [Google Scholar]
- 25. Yeon SB, Reichek N, Tallant BA, Lima JA, Calhoun LP, Clark NR. et al. Validation of in vivo myocardial strain measurement by magnetic resonance tagging with sonomicrometry. J Am Coll Cardiol 2001;38:555–61. [DOI] [PubMed] [Google Scholar]
- 26. Lima JA, Jeremy R, Guier W, Bouton S, Zerhouni EA, McVeigh E. et al. Accurate systolic wall thickening by nuclear magnetic resonance imaging with tissue tagging: correlation with sonomicrometers in normal and ischemic myocardium. J Am Coll Cardiol 1993;21:1741–51. [DOI] [PubMed] [Google Scholar]
- 27. Thomas D, Ferrari VA, Janik M, Kim DH, Pickup S, Glickson JD. et al. Quantitative assessment of regional myocardial function in a rat model of myocardial infarction using tagged MRI. MAGMA 2004;17:179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu W, Chen J, Ji S, Allen JS, Bayly PV, Wickline SA. et al. Harmonic phase MR tagging for direct quantification of Lagrangian strain in rat hearts after myocardial infarction. Magn Reson Med 2004;52:1282–90. [DOI] [PubMed] [Google Scholar]
- 29. McVeigh ER, Zerhouni EA.. Noninvasive measurement of transmural gradients in myocardial strain with MR imaging. Radiology 1991;180:677–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhou R, Pickup S, Glickson JD, Scott CH, Ferrari VA.. Assessment of global and regional myocardial function in the mouse using cine and tagged MRI. Magn Reson Med 2003;49:760–4. [DOI] [PubMed] [Google Scholar]
- 31. Azhari H, Weiss JL, Rogers WJ, Siu CO, Shapiro EP.. A noninvasive comparative study of myocardial strains in ischemic canine hearts using tagged MRI in 3-D. Am J Physiol 1995;268:H1918–26. [DOI] [PubMed] [Google Scholar]
- 32. Clark NR, Reichek N, Bergey P, Hoffman EA, Brownson D, Palmon L. et al. Circumferential myocardial shortening in the normal human left ventricle. Assessment by magnetic resonance imaging using spatial modulation of magnetization. Circulation 1991;84:67–74. [DOI] [PubMed] [Google Scholar]
- 33. Moore CC, McVeigh ER, Zerhouni EA.. Quantitative tagged magnetic resonance imaging of the normal human left ventricle. Top Magn Reson Imaging 2000;11:359–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McVeigh ER. MRI of myocardial function: motion tracking techniques. Magn Reson Imaging 1996;14:137–50. [DOI] [PubMed] [Google Scholar]
- 35. Young AA, Axel L.. Three-dimensional motion and deformation of the heart wall: estimation with spatial modulation of magnetization–a model-based approach. Radiology 1992;185:241–7. [DOI] [PubMed] [Google Scholar]
- 36. Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F. et al. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr 2010;23:351–69; quiz 453–5. [DOI] [PubMed] [Google Scholar]
- 37. Perk G, Tunick PA, Kronzon I.. Non-Doppler two-dimensional strain imaging by echocardiography–from technical considerations to clinical applications. J Am Soc Echocardiogr 2007;20:234–43. [DOI] [PubMed] [Google Scholar]
- 38. De Craene M, Marchesseau S, Heyde B, Gao H, Alessandrini M, Bernard O. et al. 3D strain assessment in ultrasound (Straus): a synthetic comparison of five tracking methodologies. IEEE Trans Med Imaging 2013;32:1632–46. [DOI] [PubMed] [Google Scholar]
- 39. Smiseth OA, Torp H, Opdahl A, Haugaa KH, Urheim S.. Myocardial strain imaging: how useful is it in clinical decision making? Eur Heart J 2016;37:1196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bijnens BH, Cikes M, Claus P, Sutherland GR.. Velocity and deformation imaging for the assessment of myocardial dysfunction. Eur J Echocardiogr 2009;10:216–26. [DOI] [PubMed] [Google Scholar]
- 41. Langeland S, Wouters PF, Claus P, Leather HA, Bijnens B, Sutherland GR. et al. Experimental assessment of a new research tool for the estimation of two-dimensional myocardial strain. Ultrasound Med Biol 2006;32:1509–13. [DOI] [PubMed] [Google Scholar]
- 42. Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R. et al. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J Am Soc Echocardiogr 2015;28:183–93. [DOI] [PubMed] [Google Scholar]
- 43. Mada RO, Lysyansky P, Daraban AM, Duchenne J, Voigt JU.. How to define end-diastole and end-systole?: impact of timing on strain measurements. JACC Cardiovasc Imaging 2015;8:148–57. [DOI] [PubMed] [Google Scholar]
- 44. Korinek J, Wang J, Sengupta PP, Miyazaki C, Kjaergaard J, McMahon E. et al. Two-dimensional strain—a Doppler-independent ultrasound method for quantitation of regional deformation: validation in vitro and in vivo. J Am Soc Echocardiogr 2005;18:1247–53. [DOI] [PubMed] [Google Scholar]
- 45. Amzulescu MS, Langet H, Saloux E, Manrique A, Boileau L, Slimani A. et al. Head-to-head comparison of global and regional two-dimensional speckle tracking strain versus cardiac magnetic resonance tagging in a multicenter validation study. Circ Cardiovasc Imaging 2017;10:e006530. [DOI] [PubMed] [Google Scholar]
- 46. Heyde B, Cygan S, Choi HF, Lesniak-Plewinska B, Barbosa D, Elen A. et al. Regional cardiac motion and strain estimation in three-dimensional echocardiography: a validation study in thick-walled univentricular phantoms. IEEE Trans Ultrason Ferroelectr Freq Control 2012;59:668–82. [DOI] [PubMed] [Google Scholar]
- 47. Hjertaas JJ, Fossa H, Dybdahl GL, Gruner R, Lunde P, Matre K.. Accuracy of real-time single- and multi-beat 3-D speckle tracking echocardiography in vitro. Ultrasound Med Biol 2013;39:1006–14. [DOI] [PubMed] [Google Scholar]
- 48. Alessandrini M, Chakraborty B, Heyde B, Bernard O, De Craene M, Sermesant M. et al. Realistic vendor-specific synthetic ultrasound data for quality assurance of 2-D speckle tracking echocardiography: simulation pipeline and open access database. IEEE Trans Ultrason Ferroelectr Freq Control 2018;65:411–22. [DOI] [PubMed] [Google Scholar]
- 49. Zhou Y, Giffard-Roisin S, De Craene M, Camarasu-Pop S, D'Hooge J, Alessandrini M. et al. A framework for the generation of realistic synthetic cardiac ultrasound and magnetic resonance imaging sequences from the same virtual patients. IEEE Trans Med Imaging 2018;37:741–54. [DOI] [PubMed] [Google Scholar]
- 50. Alessandrini M, De Craene M, Bernard O, Giffard-Roisin S, Allain P, Weese J. et al. A pipeline for the generation of realistic 3D synthetic echocardiographic sequences: methodology and open-access database. IEEE Trans Med Imaging 2015;34:1436–51. [DOI] [PubMed] [Google Scholar]
- 51. Toyoda T, Baba H, Akasaka T, Akiyama M, Neishi Y, Tomita J. et al. Assessment of regional myocardial strain by a novel automated tracking system from digital image files. J Am Soc Echocardiogr 2004;17:1234–8. [DOI] [PubMed] [Google Scholar]
- 52. Langeland S, D’hooge J, Wouters PF, Leather HA, Claus P, Bijnens B. et al. Experimental validation of a new ultrasound method for the simultaneous assessment of radial and longitudinal myocardial deformation independent of insonation angle. Circulation 2005;112:2157–62. [DOI] [PubMed] [Google Scholar]
- 53. Amundsen BH, Helle-Valle T, Edvardsen T, Torp H, Crosby J, Lyseggen E. et al. Noninvasive myocardial strain measurement by speckle tracking echocardiography: validation against sonomicrometry and tagged magnetic resonance imaging. J Am Coll Cardiol 2006;47:789–93. [DOI] [PubMed] [Google Scholar]
- 54. Reant P, Labrousse L, Lafitte S, Bordachar P, Pillois X, Tariosse L. et al. Experimental validation of circumferential, longitudinal, and radial 2-dimensional strain during dobutamine stress echocardiography in ischemic conditions. J Am Coll Cardiol 2008;51:149–57. [DOI] [PubMed] [Google Scholar]
- 55. Pirat B, Khoury DS, Hartley CJ, Tiller L, Rao L, Schulz DG. et al. A novel feature-tracking echocardiographic method for the quantitation of regional myocardial function: validation in an animal model of ischemia-reperfusion. J Am Coll Cardiol 2008;51:651–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Heyde B, Jasaityte R, Barbosa D, Robesyn V, Bouchez S, Wouters P. et al. Elastic image registration versus speckle tracking for 2-D myocardial motion estimation: a direct comparison in vivo. IEEE Trans Med Imaging 2013;32:449–59. [DOI] [PubMed] [Google Scholar]
- 57. Seo Y, Ishizu T, Enomoto Y, Sugimori H, Yamamoto M, Machino T. et al. Validation of 3-dimensional speckle tracking imaging to quantify regional myocardial deformation. Circ Cardiovasc Imaging 2009;2:451–9. [DOI] [PubMed] [Google Scholar]
- 58. Heyde B, Bouchez S, Thieren S, Vandenheuvel M, Jasaityte R, Barbosa D. et al. Elastic image registration to quantify 3-D regional myocardial deformation from volumetric ultrasound: experimental validation in an animal model. Ultrasound Med Biol 2013;39:1688–97. [DOI] [PubMed] [Google Scholar]
- 59. Bouchez S, Heyde B, Barbosa D, Vandenheuvel M, Houle H, Wang Y. et al. In-vivo validation of a new clinical tool to quantify three-dimensional myocardial strain using ultrasound. Int J Cardiovasc Imaging 2016;32:1707–14. [DOI] [PubMed] [Google Scholar]
- 60. Cho GY, Chan J, Leano R, Strudwick M, Marwick TH.. Comparison of two-dimensional speckle and tissue velocity based strain and validation with harmonic phase magnetic resonance imaging. Am J Cardiol 2006;97:1661–6. [DOI] [PubMed] [Google Scholar]
- 61. Bansal M, Cho GY, Chan J, Leano R, Haluska BA, Marwick TH.. Feasibility and accuracy of different techniques of two-dimensional speckle based strain and validation with harmonic phase magnetic resonance imaging. J Am Soc Echocardiogr 2008;21:1318–25. [DOI] [PubMed] [Google Scholar]
- 62. Amundsen BH, Crosby J, Steen PA, Torp H, Slordahl SA, Stoylen A.. Regional myocardial long-axis strain and strain rate measured by different tissue Doppler and speckle tracking echocardiography methods: a comparison with tagged magnetic resonance imaging. Eur J Echocardiogr 2009;10:229–37. [DOI] [PubMed] [Google Scholar]
- 63. Kleijn SA, Brouwer WP, Aly MF, Russel IK, de Roest GJ, Beek AM. et al. Comparison between three-dimensional speckle-tracking echocardiography and cardiac magnetic resonance imaging for quantification of left ventricular volumes and function. Eur Heart J Cardiovasc Imaging 2012;13:834–9. [DOI] [PubMed] [Google Scholar]
- 64. Zhou X, Thavendiranathan P, Chen Y, Cheng L, Qian Z, Liu S. et al. Feasibility of automated three-dimensional rotational mechanics by real-time volume transthoracic echocardiography: preliminary accuracy and reproducibility data compared with cardiovascular magnetic resonance. J Am Soc Echocardiogr 2016;29:62–73. [DOI] [PubMed] [Google Scholar]
- 65. Amzulescu MS, Langet H, Saloux E, Manrique A, Slimani A, Allain P. et al. Improvements of myocardial deformation assessment by three-dimensional speckle-tracking versus two-dimensional speckle-tracking revealed by cardiac magnetic resonance tagging. J Am Soc Echocardiogr 2018;31:1021–33. [DOI] [PubMed] [Google Scholar]
- 66. Hor KN, Gottliebson WM, Carson C, Wash E, Cnota J, Fleck R. et al. Comparison of magnetic resonance feature tracking for strain calculation with harmonic phase imaging analysis. JACC Cardiovasc Imaging 2010;3:144–51. [DOI] [PubMed] [Google Scholar]
- 67. Harrild DM, Han Y, Geva T, Zhou J, Marcus E, Powell AJ.. Comparison of cardiac MRI tissue tracking and myocardial tagging for assessment of regional ventricular strain. Int J Cardiovasc Imaging 2012;28:2009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Augustine D, Lewandowski AJ, Lazdam M, Rai A, Francis J, Myerson S. et al. Global and regional left ventricular myocardial deformation measures by magnetic resonance feature tracking in healthy volunteers: comparison with tagging and relevance of gender. J Cardiovasc Magn Reson 2013;15:8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wu L, Germans T, Guclu A, Heymans MW, Allaart CP, van Rossum AC.. Feature tracking compared with tissue tagging measurements of segmental strain by cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2014;16:10.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Moody WE, Taylor RJ, Edwards NC, Chue CD, Umar F, Taylor TJ. et al. Comparison of magnetic resonance feature tracking for systolic and diastolic strain and strain rate calculation with spatial modulation of magnetization imaging analysis. J Magn Reson Imaging 2015;41:1000–12. [DOI] [PubMed] [Google Scholar]
- 71. Singh A, Steadman CD, Khan JN, Horsfield MA, Bekele S, Nazir SA. et al. Intertechnique agreement and interstudy reproducibility of strain and diastolic strain rate at 1.5 and 3 Tesla: a comparison of feature-tracking and tagging in patients with aortic stenosis. J Magn Reson Imaging 2015;41:1129–37. [DOI] [PubMed] [Google Scholar]
- 72. Farsalinos KE, Daraban AM, Unlu S, Thomas JD, Badano LP, Voigt JU.. Head-to-Head comparison of global longitudinal strain measurements among nine different vendors: the EACVI/ASE inter-vendor comparison study. J Am Soc Echocardiogr 2015;28:1171–81.e2. [DOI] [PubMed] [Google Scholar]
- 73. Nelson MR, Hurst RT, Raslan SF, Cha S, Wilansky S, Lester SJ.. Echocardiographic measures of myocardial deformation by speckle-tracking technologies: the need for standardization? J Am Soc Echocardiogr 2012;25:1189–94. [DOI] [PubMed] [Google Scholar]
- 74. Costa SP, Beaver TA, Rollor JL, Vanichakarn P, Magnus PC, Palac RT.. Quantification of the variability associated with repeat measurements of left ventricular two-dimensional global longitudinal strain in a real-world setting. J Am Soc Echocardiogr 2014;27:50–4. [DOI] [PubMed] [Google Scholar]
- 75. Nagata Y, Takeuchi M, Mizukoshi K, Wu VC, Lin FC, Negishi K. et al. Intervendor variability of two-dimensional strain using vendor-specific and vendor-independent software. J Am Soc Echocardiogr 2015;28:630–41. [DOI] [PubMed] [Google Scholar]
- 76. D'Hooge J, Barbosa D, Gao H, Claus P, Prater D, Hamilton J. et al. Two-dimensional speckle tracking echocardiography: standardization efforts based on synthetic ultrasound data. Eur Heart J Cardiovasc Imaging 2016;17:693–701. [DOI] [PubMed] [Google Scholar]
- 77. Yang H, Marwick TH, Fukuda N, Oe H, Saito M, Thomas JD. et al. Improvement in strain concordance between two major vendors after the strain standardization initiative. J Am Soc Echocardiogr 2015;28:642–8.e7. [DOI] [PubMed] [Google Scholar]
- 78. Shiino K, Yamada A, Ischenko M, Khandheria BK, Hudaverdi M, Speranza V. et al. Intervendor consistency and reproducibility of left ventricular 2D global and regional strain with two different high-end ultrasound systems. Eur Heart J Cardiovasc Imaging 2017;18:707–16. [DOI] [PubMed] [Google Scholar]
- 79. Mirea O, Pagourelias ED, Duchenne J, Bogaert J, Thomas JD, Badano LP. et al. Intervendor differences in the accuracy of detecting regional functional abnormalities: a report from the EACVI-ASE strain standardization task force. JACC Cardiovasc Imaging 2018;11:25–34. [DOI] [PubMed] [Google Scholar]
- 80. Badano LP, Cucchini U, Muraru D, Al Nono O, Sarais C, Iliceto S.. Use of three-dimensional speckle tracking to assess left ventricular myocardial mechanics: inter-vendor consistency and reproducibility of strain measurements. Eur Heart J Cardiovasc Imaging 2013;14:285–93. [DOI] [PubMed] [Google Scholar]
- 81. Schuster A, Stahnke VC, Unterberg-Buchwald C, Kowallick JT, Lamata P, Steinmetz M. et al. Cardiovascular magnetic resonance feature-tracking assessment of myocardial mechanics: intervendor agreement and considerations regarding reproducibility. Clin Radiol 2015;70:989–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Cao JJ, Ngai N, Duncanson L, Cheng J, Gliganic K, Chen Q.. A comparison of both DENSE and feature tracking techniques with tagging for the cardiovascular magnetic resonance assessment of myocardial strain. J Cardiovasc Magn Reson 2018;20:26.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Muraru D, Cucchini U, Mihăilă S, Miglioranza MH, Aruta P, Cavalli G. et al. Left ventricular myocardial strain by three-dimensional speckle-tracking echocardiography in healthy subjects: reference values and analysis of their physiologic and technical determinants. J Am Soc Echocardiogr 2014;27:858–71.e1. [DOI] [PubMed] [Google Scholar]
- 84. Cheng S, Larson MG, McCabe EL, Osypiuk E, Lehman BT, Stanchev P. et al. Age- and sex-based reference limits and clinical correlates of myocardial strain and synchrony: the Framingham Heart Study. Circ Cardiovasc Imaging 2013;6:692–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Yingchoncharoen T, Agarwal S, Popovic ZB, Marwick TH.. Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr 2013;26:185–91. [DOI] [PubMed] [Google Scholar]
- 86. Kocabay G, Muraru D, Peluso D, Cucchini U, Mihaila S, Padayattil-Jose S. et al. Normal left ventricular mechanics by two-dimensional speckle-tracking echocardiography. Reference values in healthy adults. Rev Esp Cardiol (Engl Ed) 2014;67:651–8. [DOI] [PubMed] [Google Scholar]
- 87. Bernard A, Addetia K, Dulgheru R, Caballero L, Sugimoto T, Akhaladze N. et al. 3D echocardiographic reference ranges for normal left ventricular volumes and strain: results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging 2017;18:475–83. [DOI] [PubMed] [Google Scholar]
- 88. Kleijn SA, Pandian NG, Thomas JD, Perez de Isla L, Kamp O, Zuber M. et al. Normal reference values of left ventricular strain using three-dimensional speckle tracking echocardiography: results from a multicentre study. Eur Heart J Cardiovasc Imaging 2015;16:410–6. [DOI] [PubMed] [Google Scholar]
- 89. Vo HQ, Marwick TH, Negishi K.. MRI-derived myocardial strain measures in normal subjects. JACC Cardiovasc Imaging 2018;11:196–205. [DOI] [PubMed] [Google Scholar]
- 90. Venkatesh BA, Donekal S, Yoneyama K, Wu C, Fernandes VR, Rosen BD. et al. Regional myocardial functional patterns: quantitative tagged magnetic resonance imaging in an adult population free of cardiovascular risk factors: the multi-ethnic study of atherosclerosis (MESA). J Magn Reson Imaging 2015;42:153–9. [DOI] [PubMed] [Google Scholar]
- 91. Giusca S, Korosoglou G, Zieschang V, Stoiber L, Schnackenburg B, Stehning C. et al. Reproducibility study on myocardial strain assessment using fast-SENC cardiac magnetic resonance imaging. Sci Rep 2018;8:14100.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Andre F, Steen H, Matheis P, Westkott M, Breuninger K, Sander Y. et al. Age- and gender-related normal left ventricular deformation assessed by cardiovascular magnetic resonance feature tracking. J Cardiovasc Magn Reson 2015;17:25.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Knackstedt C, Bekkers SC, Schummers G, Schreckenberg M, Muraru D, Badano LP. et al. Fully automated versus standard tracking of left ventricular ejection fraction and longitudinal strain: the FAST-EFs Multicenter Study. J Am Coll Cardiol 2015;66:1456–66. [DOI] [PubMed] [Google Scholar]
- 94. Medvedofsky D, Kebed K, Laffin L, Stone J, Addetia K, Lang RM. et al. Reproducibility and experience dependence of echocardiographic indices of left ventricular function: side-by-side comparison of global longitudinal strain and ejection fraction. Echocardiography 2017;34:365–70. [DOI] [PubMed] [Google Scholar]
- 95. Barbier P, Mirea O, Cefalu C, Maltagliati A, Savioli G, Guglielmo M.. Reliability and feasibility of longitudinal AFI global and segmental strain compared with 2D left ventricular volumes and ejection fraction: intra- and inter-operator, test-retest, and inter-cycle reproducibility. Eur Heart J Cardiovasc Imaging 2015;16:642–52. [DOI] [PubMed] [Google Scholar]
- 96. Reichek N. Myocardial strain: still a long way to go. Circ Cardiovasc Imaging 2017;10:e007145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.