Graphical Abstract
Graphical Abstract.
Keywords: Ischaemia, Reperfusion, Cardioprotection, Haematopoietic cells, Exosomes
Abstract
During an ST-elevation myocardial infarction (STEMI), the myocardium undergoes a prolonged period of ischaemia. Reperfusion therapy is essential to minimize cardiac injury but can paradoxically cause further damage. Experimental procedures to limit ischaemia and reperfusion (IR) injury have tended to focus on the cardiomyocytes since they are crucial for cardiac function. However, there is increasing evidence that non-cardiomyocyte resident cells in the heart (as discussed in a separate review in this Spotlight series) as well as circulating cells and factors play important roles in this pathology. For example, erythrocytes, in addition to their main oxygen-ferrying role, can protect the heart from IR injury via the export of nitric oxide bioactivity. Platelets are well-known to be involved in haemostasis and thrombosis, but beyond these roles, they secrete numerous factors including sphingosine-1 phosphate (S1P), platelet activating factor, and cytokines that can all strongly influence the development of IR injury. This is particularly relevant given that most STEMI patients receive at least one type of platelet inhibitor. Moreover, there are large numbers of circulating vesicles in the blood, including microvesicles and exosomes, which can exert both beneficial and detrimental effects on IR injury. Some of these effects are mediated by the transfer of microRNA (miRNA) to the heart. Synthetic miRNA molecules may offer an alternative approach to limiting the response to IR injury. We discuss these and other circulating factors, focussing on potential therapeutic targets relevant to IR injury. Given the prevalence of comorbidities such as diabetes in the target patient population, their influence will also be discussed. This article is part of a Cardiovascular Research Spotlight Issue entitled ‘Cardioprotection Beyond the Cardiomyocyte’, and emerged as part of the discussions of the European Union (EU)-CARDIOPROTECTION Cooperation in Science and Technology (COST) Action, CA16225.
This article is part of the Spotlight Issue on Cardioprotection Beyond the Cardiomyocyte.
1. Introduction
During an ST-elevation myocardial infarction (STEMI) the myocardium undergoes a prolonged period of ischaemia. Reperfusion therapy is essential to minimize cardiac injury but can paradoxically cause further damage.1 Experimental procedures to limit ischaemia and reperfusion (IR) injury have been developed.1–3 These strategies include ischaemic conditioning applied before ischaemia (preconditioning or IPC), after ischaemia (postconditioning or IPost), or to a distal organ or limb (remote conditioning, RIC). In addition, numerous pharmacological strategies activate either the PI3K/AKT (Reperfusion Injury Salvage Kinase, RISK), JAK/STAT (survivor activating factor enhancement or SAFE), or cGMP/PKG signalling pathways. These pathways have various effects on cardiomyocytes, but inhibition of the mitochondrial permeability transition pore (MPTP) has been described as a common end effector.1 Furthermore, the mechanism of cardioprotection may also involve global changes in cardiac gene expression.4,5
Unfortunately, despite success in limiting IR injury in experimental animal models, the above approaches have not translated well to the clinical setting.1,6 Possible reasons for this have been extensively discussed.1,2,6,7 One reason is likely to be the prevalence of comorbidities such as dyslipidaemia, diabetes, and age in the STEMI patient population, which can impede cardioprotective strategies.8 Another reason is that many patients are already taking drugs (e.g. statins) or are administered drugs (e.g. platelet P2Y12 inhibitors) that are known to influence cardioprotection.8 It may also be relevant that, because of their crucial role in cardiac function, most cardioprotection studies have focussed on protecting the cardiomyocytes. However, increasing evidence suggests that solely targeting cardiomyocytes may be insufficient to protect the heart in the complex scenario of STEMI, and a multi-target approach may be necessary.9 In this regard, it may also be important to consider the roles played by innate immunity and inflammation, and the nervous system in addition to non-cardiomyocyte cells resident in the heart—topics which are discussed in separate reviews in this Spotlight series.10–12 Here, we address the importance of circulating blood cells and factors in IR injury and cardioprotection. We examine the role played by platelets, erythrocytes, and the extracellular vesicles (EVs) they release into the blood. While thrombosis is clearly a fundamental cause of coronary occlusion and myocardial ischaemia, factors targeting the thrombus and clotting factors may exert cardioprotective effects independent of occlusion. Furthermore, non-vesicular ribonucleic acid (RNA) may be an important cardioprotective approach. Lymphocytes play a complex role in IR injury. Circulating B- and T-lymphocytes are recruited to the injured myocardium in the days following infarction, and contribute to healing after acute myocardial infarction (AMI), but there is also some evidence that T cells contribute to acute myocardial IR injury. The role of lymphocytes and other immune cells is discussed in detail in an accompanying review in this series.11
While there are certainly roles for circulating cells and factors in the longer time-scale of response to IR including inflammation and ventricular remodelling, we focus on their role in initial myocardial injury following acute IR injury.
2. Platelets
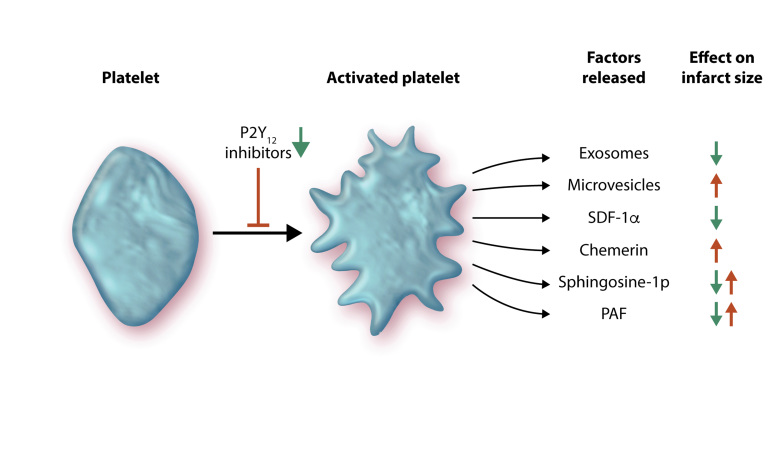
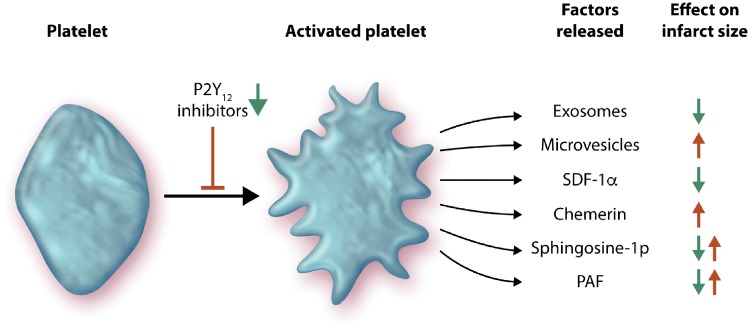
Platelets are small, anucleate cell fragments whose primary function is to initiate haemostasis in response to small vessel injury. However, they are emerging as important factors in the regulation of vascular homeostasis in many organs, including the heart. When activated, platelets can initiate haemostasis to prevent bleeding and eventually propagate a thrombus. STEMI is the consequence of coronary occlusion by thrombosis following plaque rupture. The latter promotes platelet adhesion and aggregation, thereby contributes to the blood clotting process. Platelets release various factors that may influence the heart during IR including cytokines, microRNAs (miRNAs), chemerin, sphingosine-1-phosphate (S1P), and platelet-activating factor (Figure 1), thereby affecting the non-thrombogenic properties of the vascular endothelium and disturbing cardiomyocyte functions.
Figure 1.
Ischaemia and reperfusion causes the activation of platelets, which subsequently release a multitude of factors with divergent effects on infarct size. These include exosomes and microvesicles (both types of extracellular vesicles), SDF-1α, chemerin, sphingosine-1 phosphate, and PAF. P2Y12 inhibitors can prevent platelet activation and can also reduce infarct size (see text for details).
Activated platelets release a variety of factors that can affect IR injury. Some of these are vaso-active, as discussed further in an accompanying review.13 Other factors may act directly on cardiomyocytes and worsen their response to IR. For example, activated platelets release chemerin, an adipokine involved in inflammation, obesity, insulin resistance, and metabolic syndrome.14 Acting through chemokine-like receptor1 (CMKLR1 or ChemR23), it reduces AKT phosphorylation, activates caspase-9, and induces apoptosis in murine cardiomyocytes, which suggests it could also increase cardiac IR injury.15
Platelets also release numerous factors which may activate cardioprotective RISK and SAFE pathways. For example, they are a major source of CXCL12 (stromal cell derived factor-1α, SDF-1α), which is released upon activation and can reduce IR injury in rodent and human myocardium, in addition to promoting longer time-scale repair mechanisms.16,17
Platelet-derived S1P appears to make an important contribution to protection from IR injury. Platelets contain sphingosine kinase, which can transform membrane sphingosine into S1P for storage and release.18 S1P can have both pro- and anti-aggregatory effects via G-protein coupled receptors (GPCRs) on platelets.18 Importantly, S1P can also directly induce myocardial protection, apparently via S1P1, S1P2, and S1P3 receptors in cardiomyocytes, leading to activation of the RISK and SAFE pathways.19–22 It has also been reported that PAK1/AKT/NOS3 signalling may mediate cardioprotection by S1P.22,23 Mice lacking both S1P2 and S1P3 receptors have 50% smaller infarcts after IR,22 but it is not clear which cell type mediates this effect. Nevertheless, studies demonstrate that S1P is a pivotal mediator of cardioprotection and can trigger IPC and IPost. Indeed, S1P mediates powerful cardioprotection in isolated mouse hearts.24,25
Diabetes can increase platelet hyperactivity and oxidative stress leading to cardiovascular complications.26 These alterations may, at least in part, be responsible for the reduced ability to induce cardioprotection in models of uncontrolled diabetes.8 Pre-treatment of isolated rat hearts with platelets from healthy subjects was protective against IR injury, whereas platelets from diabetic subjects were not, possibly due to altered release of S1P.27
Cardioprotective strategies such as IPC and IPost can induce the release of S1P.25,26 Whether these manoeuvres affect S1P release from platelets is not clear. Nevertheless, P2Y12 inhibitors induce a conditioning-like cardioprotection that requires both platelets and S1P.29–31 Platelets collected from patients with acute coronary syndrome (ACS) increased injury when perfused through isolated rat hearts, and this cytotoxicity was blocked by P2Y12 inhibitors.32 However, prevention of platelet aggregation alone is not protective in vivo, since infarct size is unaltered in thrombocytopenic rats that remain untreated.31 All P2Y12 antagonists tested to date have been found to be cardioprotective in animals, and neither IPC nor IPost can add protection to that induced by the anti-platelet drugs.29,33 Since virtually all percutaneous coronary intervention (PCI) patients are treated with P2Y12 inhibitors, it is important that future cardioprotective interventions are tested in an animal model receiving a P2Y12 antagonist. The failure to clinically translate IPost-mimetics, which had appeared so protective in animal studies29,33 has led to the mistaken assumption that animal hearts are not appropriate models of human hearts. But even in animal studies, IPost was unable to confer further infarct size reduction in an animal concomitantly treated with a P2Y12 antagonist.29 It would therefore appear that in the presence of a P2Y12 antagonist, further cardioprotection can be achieved only if the intervention has a different mechanism of protection from the platelet inhibitor.19,29,33,34 This approach should pave the way to translation of cardioprotective protocols into successful clinical treatments.
The phosphoglyceride platelet activating factor (PAF) is produced and released by platelets, endothelial cells and leucocytes.35 PAF acts as an autocrine/paracrine mediator on various cell types including cardiomyocytes, endothelial cells, smooth muscle cells, and platelets.35 PAF has a dual role in IR.35 IR causes the release of high quantities of PAF (1–10 nmol/L) with direct and indirect negative effects on coronary and cardiac functions, including a strong arrhythmogenic effect.35 At very low concentrations (pM), PAF has a cardioprotective effect similar that that elicited by IPC.35–37 Cardioprotection by PAF involves activation of the RISK kinase pathway, including protein kinase C (PKC), AKT, and NOS.36 Interestingly, a PAF-receptor antagonist impairs the infarct-sparing effect of both IPC and PAF.36
Although PAF or other endogenous factors within platelets may participate in triggering IPC-induced cardioprotection, they appear not to be required for cardioprotection by IPC, since thrombocytopenia did not abolish cardioprotection by IPC.19 However, platelets might still affect infarction in patients with coronary artery disease or comorbidities such as diabetes where platelets may be activated. Thus, platelets not only affect haemostasis and thrombosis, but platelet-derived products including EVs can have a profound effect on infarct size and cardioprotection.
3. Erythrocytes
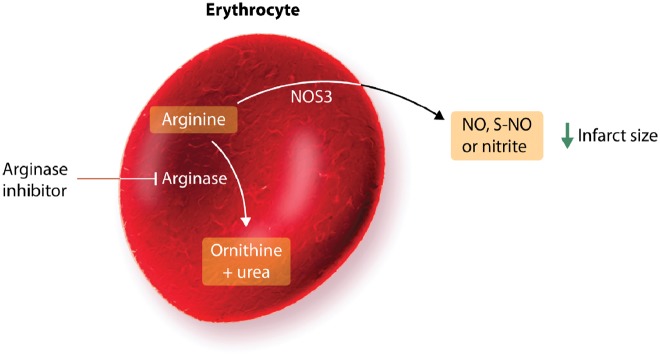
It is well-known that erythrocytes are involved in the regulation of the cardiovascular system via mechanisms that include their interaction with the endothelium.38–40 These mechanisms include the export of NO-like bioactivity and ATP that exert important cardiovascular effects. Additionally, erythropoietin, a kidney-derived cytokine that has the ability to increase red blood cell mass, can protect cardiomyocytes from apoptotic cell death through NOS3-derived NO production.41 In the setting of IR, erythrocytes were originally suggested to protect the isolated rat heart from IR injury via a NOS-dependent mechanism.42 This was supported by the observation that mice with blood cells lacking NOS3 had lower circulating nitrite and developed larger infarcts following IR than control mice, supporting a role of erythrocyte NOS3 under in vivo conditions.41,43 It was subsequently shown that export of NOS3-derived NO bioactivity from erythrocytes induced cardioprotection in the isolated heart.44 This effect was tightly controlled by the enzyme arginase which is known to reciprocally regulate NO formation by competing with NOS3 for the substrate L-arginine. Consequently, inhibition of erythrocyte arginase induces cardioprotection via a mechanism that is entirely dependent on erythrocyte NOS3 (Figure 2).44
Figure 2.
Erythrocytes contain endothelial nitric oxide synthase (NOS3), which protects the heart via the production of nitric oxide (NO), S-nitrosothiols (S-NO), or nitrite. Since NOS3 competes with arginase for the common substrate arginine, inhibition of arginase can be cardioprotective.
Interestingly, arginase is up-regulated in erythrocytes in type 2 diabetes—an important comorbidity in patients with STEMI.45,46 Accordingly, it was recently demonstrated that erythrocytes from both mice and patients with type 2 diabetes markedly impair recovery of cardiac systolic function, increase left ventricular end-diastolic pressure and increase infarct size following IR in comparison with erythrocytes from control mice or healthy humans.46 The underlying mechanism behind this effect was increased arginase activity in erythrocytes which led to a decrease in NO production. It further resulted in increased reactive oxygen species (ROS) production due to uncoupling of NOS3 and increased expression of NADPH oxidase (NOX2) in erythrocytes.47 The ROS species hydrogen peroxide produced by erythrocytes activates endothelial cell arginase and NOX1, which results in endothelial oxidative stress and impaired endothelium-dependent relaxation.47 Thus, available data suggest that erythrocytes are prominently involved in events occurring during IR by export of NO bioactivity under strict control of erythrocyte arginase. Furthermore, erythrocytes are important targets for cardioprotective therapies including arginase and ROS inhibition. In addition, erythrocyte NOS/NO bioactivity has been suggested to be associated with RIC via increased erythrocyte deformability.48 Finally, erythrocyte dysfunction characterized by increased arginase activity and ROS production leading to endothelial dysfunction aggravates IR injury and increases infarct size in type 2 diabetes.46 Therefore, erythrocytes represent an important potential target for cardioprotection.
4. Extracellular vesicles and circulating miRNA
In addition to circulating cells, blood contains large numbers of EVs.49–52 Most of these EVs originate from platelets and erythrocytes, but they are also produced by other circulating cells such as leucocytes, and by vascular cells particularly the endothelium. Historically, the cardiovascular field has focussed on the population of larger EVs, called microvesicles (MVs), in part because they are relatively easy to study using methods such as flow cytometry. Over the past few years, there has been increasing interest in the smaller type of EVs called exosomes, particularly because of their apparent signalling role.49–52 As we discuss, these different types of EVs below, it is important to be aware that results can be highly dependent on the isolation and purification methods used, and that the purity and specific fractions of exosomes achieved using commonly used isolation methods can be quite variable.50,52,53 Furthermore, while EVs certainly contain miRNA, miRNA is also found in the blood complexed to lipoproteins and Argonaute proteins, and the relative importance of these different vehicles for the transfer of miRNA is highly debated.
4.1 Exosomes
Exosomes are nano-sized (50–150 nm diameter) lipid bilayer vesicles released from cells when multivesicular bodies fuse with the plasma membrane.50,52 Exosomes are secreted by all cell types and act as universal propagators of intercellular communication. Since high concentrations of exosomes are found in the blood (∼1010 per mL54), they have been hypothesized to mediate the transmission of the cardioprotective signal of RIC.55 Indeed, RIC was shown to increase the concentration of exosomes in the blood,54 and in 2014, the first evidence that cardioprotection by RIC might be transmitted by EVs, most likely exosomes, was obtained.56 In this study, pre-treatment with exosomes from conditioned donor hearts attenuated infarct size in non-preconditioned recipient hearts undergoing IR.56 Recently, it has been shown that exosomes derived from the plasma of rats subjected to RIC play a role in reducing oxidative stress-mediated injury.57
Platelets are a major source of circulating EVs, releasing both exosomes and MVs.52,58 Platelet EVs are also present in atherosclerotic plaques.59 Several stimuli can augment the release of EVs, including physical-chemical stresses and pro-apoptotic stimuli. Platelet exosomes appear to have an anti-thrombotic effect.49 Platelet-derived EVs can transfer RNAs to recipient cells and influence their activity.49,60 While studies suggest an important role for platelet-derived miRNAs in haemostasis, thrombosis, and unstable coronary syndromes,60 it is less clear whether miRNA, either from platelets or EVs, could act rapidly enough to influence acute infarct formation after IR.
Exosomes are increasingly being exploited for their therapeutic cardioprotective role in progenitor/stem cell-based therapy.50 Molecules and EVs secreted by progenitor cells appear to create a reparative and regenerative milieu in the tissue microenvironment, which may be more important than the differentiation potential of the cells themselves. Exosomes purified from culture medium conditioned by resident cardiac progenitor cells (Exo-CPC), but not exosomes released from normal dermal fibroblasts, are cardioprotective and proangiogenic in vivo.61,62 Exo-CPC injected into the infarct border zone reduced scar size, increased viable mass and vessel density, and improved global heart function after myocardial infarction (MI) in mice.61,62 Not only CPC-derived exosomes can induce a cardioprotective signal, since differences are observed when comparing CPC with exosomes from patient-matched, bone-marrow derived, mesenchymal stem cell (BMC). Although Exo-BMC provide some cardioprotection after AMI, they are not as effective as Exo-CPC.63
The exact mechanism by which exosomes protect cardiomyocytes from IR injury has yet to be elucidated but it may involve the exosome’s cargo of mRNA, short non-coding RNA (miRNAs, Y-RNA) and/or proteins.61,62,64 Given their abundance and specific expression within tissue-specific exosomes, miRNAs appear to be an important component, although there are some aspects that are not yet clear, including how transferred miRNAs are incorporated into an endogenous RISC complex and mediate their effect in competition with large amounts of host miRNA.65 The most highly enriched miRNAs in Exo-CPC include miR-146a-3p, miR-132, and miR-210.61,62 Gain and loss-of-function studies revealed antiapoptotic and proangiogenic properties of these miRNAs. Plasma exosomes induced by RIC transfer miR-24 and decrease oxidative stress-mediated apoptosis into cardiomyocytes by downregulating expression of the pro-apoptotic protein Bim.57
CPC cultured as 3-D cardiospheres (CDC) release exosomes that contain many short RNAs that are unique to CDCs compared with fibroblast-derived exosomes.64 The most abundant RNA species found in CDC-exosomes is a Y RNA fragment (EV-YF1).64 Its relative abundance in CDC-exosomes correlates with an indirect in vivo reduction of cardiomyocyte apoptosis, by increasing expression of the known cardioprotective cytokine interleukin 10 into macrophages within the ischaemic area.64 CDCs-exosomes reduced infarct size 48 h after reperfusion when injected in rats subjected to 45 min coronary artery occlusion.66 In this case, cardioprotection was mediated by miR-181b, as demonstrated by the loss of cardioprotection caused by miR-181b antagomir and by the fact that inert exosomes from fibroblasts, after enrichment with miR-181b, were able to reduce infarct size.66 Cardioprotection was found to be related to the expression of the pro-inflammatory genes NOS2 and TNF, protein kinase C δ (PKCδ), and increased macrophage polarization.66
The protein cargo of plasma-derived exosomes includes heat-shock protein 70 (HSP70), which plays a crucial role in pro-survival effects of circulating exosomes when used in ex vivo, in vivo, and in vitro settings of IR.54 Extracellular exosome-mediated signal activates ERK1/2 in cardiomyocytes, which trigger toll-like receptor (TLR4) leading to phosphorylation of the cardioprotective protein HSP27.54 When any of these proteins are selectively blocked, or HSP70 is absent from the surface of exosomes, the cardioprotective signal is not propagated.54 Diabetes impairs the cardioprotective activity of exosomes.67 However, exosomes from non-diabetic rats retained the ability to protect cardiomyocytes from diabetic rats, indicating that exosome therapy can still be effective despite the hyperglycaemic environment found in diabetic patients.67 Among the most highly expressed protein on Exo-CPC is pregnancy-associated plasma protein-A (PAPP-A), a protease that releases active insulin growth factor 1, a key cardioprotective agent. PAPP-A appears to be required for Exo-CPC to improve functional recovery after permanent coronary artery occlusion.63
Small animal studies suggest that exosomes could revolutionize medicine due to their potent effects on cell behaviour including cardioprotection. However, there is a long route to the final goal of clinical benefits in patients using exosome-based therapeutics.52 Little is known about what is the most active fraction of collected samples for exosome studies. Improved techniques for the isolation of defined size-ranges of exosome populations are needed.52 It will also be important to develop methods to target exosomes to the heart to limit their potential side effects on other tissues. Finally, although some research groups have recently begun to approach the technical challenge of isolating GMP (good laboratory procedures)-grade exosomes,68 several technical and regulatory aspects will need to be overcome to enable the large-scale production of exosomes.52 Furthermore, pre-clinical large animals studies will be necessary before exosomes can be considered as a realistic therapeutic approach for cardioprotection.
4.2 Microvesicles
MVs, also known as microparticles or ectosomes, are a heterogeneous population of EVs formed by outward budding and/or shedding of the plasma membrane. This process can occur in several cell types, including endothelial cells, erythrocytes, leucocytes, platelets, and cardiomyocytes.49,52 MVs are also heterogeneous in their size and molecular composition.49,52 Although initially considered plasma membrane fragments emanating from platelets as part of the coagulation process,69 it is now established that MVs are important players in intercellular communication, since they can convey proteins, lipids, nucleic acids, and other molecules with biological activity such as cytokines, hormones, and coagulation factors between distant cells.70 Circulating MVs have been implicated in several physiological functions such as the coagulation process, reticulocyte maturation, angiogenesis, tissue repair and inflammation.50,52,70 The concentration of MVs in the plasma is estimated to be approximately 2–4 × 108 per mL.70 In healthy subjects, the majority are of platelet origin as indicated by the presence of CD41, while the remaining MVs derive from granulocytes, ECs, erythrocytes, and monocytes.70 In contrast to the beneficial effects of platelet exosomes, noted above, platelet MVs can promote interactions between platelets, endothelial cells, and monocytes favouring atherogenesis.49,51,70,71 Since MVs contain procoagulant platelet membrane components, they can potentiate the coagulation response.49
The number of circulating MVs increases in patients with heart failure and vascular inflammation, most likely due to platelet activation.49,52 Moreover, the number of circulating procoagulant MVs is elevated in patients with ACS and chronic ischaemic heart disease.52 Endothelial-derived MVs can increase in heart failure, hypertension, coronary artery disease, and carotid artery disease, possibly due to endothelial injury and dysfunction.72
In addition to their importance as biomarkers, MVs elicit biological responses in recipient cells which may depend on the cell-type and of origin as well as its physiological status.52 For example, platelet-derived MVs injected into the myocardium induced angiogenesis and stimulated post-ischaemic revascularization in a rat model of MI.73 IPC increased the number of circulating MVs derived from platelets, endothelial cells and erythrocytes, and administration of these MVs significantly alleviated damage to the myocardium and restored cardiac function after IR injury by inhibiting endoplasmic reticulum stress.74 MVs from mesenchymal stem cells overexpressing GATA-4 were found to be cardioprotective, and this was attributed to an increase in miR-221 levels in the MVs, which were taken up by cardiomyocytes and silenced the pro-apoptotic protein PUMA.75
On the other hand, IR may cause the release of MVs that are more damaging. MVs released from endothelial cells after IR were pro-apoptotic and pro-oxidative to cardiomyocytes.76 Furthermore, MVs originating from cardiomyocytes and endothelial cells following AMI can also be internalized by infiltrating monocytes and regulate local inflammatory responses.77
4.3 Non-vesicular non-coding RNAs
The majority (98%) of RNA molecules in the body are non-coding RNA molecules.78,79 These include ribosomal RNA, transfer RNA, microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs).78 miRNAs are single-stranded RNA molecules, 21–23 nucleotides in length that affect gene expression by binding to particular mRNAs and promote their degradation or inhibiting their translation into proteins. LncRNAs and circRNAs regulate the expression of genes via a complex array of epigenetic, post-transcriptional, and translational modes. The effects of miRNAs are sequence-specific, but each miRNA can affect numerous mRNA molecules and each mRNA can be affected by numerous miRNAs. Since non-coding RNA molecules are involved in ‘fine tuning’ of the expression of proteins in numerous signalling processes in the body, there is great interest in developing approaches to administer them systemically as therapeutic agents.
The miR-15 family was reported to be detrimental in IR.80 In this study, a locked nucleic acid-modified (LNA)-anti-miR complementary to the seed region of the miR-15 family (LNA-miR-15), administered intravenously at the onset of reperfusion, limited infarct size in mice subjected to 75 min ischaemia followed by 24 h reperfusion.80 Targeting miR-15 also prevented the decrease in Pdk4 (a key regulator of mitochondrial function) and Sgk1 (an inhibitor of cardiomyocyte apoptosis).80
In pigs subject to 60 min ischaemia followed by reperfusion, administration of LNA-miR-92a 5 min prior to reperfusion reduced infarct size and left ventricular function improved.81 However, a benefit was only seen after catheter-based delivery, and not by intravenous infusion. LNA-92a also increased capillary density and decreased leucocyte infiltration and cardiomyocyte cell death.81
In rabbits, intravenous administration of liposomal-encapsulated miR-145 immediately after reperfusion (following 30 min coronary artery occlusion) reduced myocardial infarct size and improved left ventricular function 2 weeks after infarction.82 The target of miR-145 was found to be fibroblast growth factor receptor substrate 2, and its effect was at least partially mediated by the activation of autophagy.82
Following physical or pharmacological interventions, changes in the expression of certain non-coding RNAs might be expected to reveal those that represent the most promising targets. Several studies have explored which non-coding RNAs are affected by IR or interventions such as IPC and IPost and have tested whether these non-coding RNAs have protective effects in experimental models.4
Using unbiased miRNA omics approach, several miRNAs were identified that affected by IPC and IPost and termed these miRNAs protectomiRs. Transfection of protectomiRs (specific miRNA mimics or antagomirs as appropriate) into cardiac myocytes validated their cardiocytoprotective efficacy. In particular, a miR-125b* mimic was shown to be of high relevance for cardioprotection.83,84 As expected, the concentration of numerous non-coding RNA molecules is altered by ischaemia, IR, conditioning stimuli and medications. Several group have shown that by offsetting these changes with specific agonists or antagonist, the protective effects of various interventions are lost. For example, inhibiting miR-499 abolishes the protective effect of post-conditioning85; the protective effect of pioglitazone in vitro against simulated IR is dependent on downregulating miR-29 levels. Hence, 3-day pre-treatment with antagomirs against miR-29a or 29c attenuated apoptosis and limited infarct size in an in vivo rat model of 30 min ischaemia/24 h reperfusion.86
Another example of a cardioprotective miRNA is miR-21. In an isolated heart model, infarct size was smaller when mice had been pre-treated 24 h previously with synthetic miR-21.87 However, miR-21 also appears to contribute to remodelling and fibrosis in the failing heart.88
Although this review focusses primarily on studies in which non-coding RNAs or their antagonists was administered around the time of reperfusion because of their relevance to patients with STEMI, it should be noted that benefit for RNA-based therapies has also been seen in models of permanent coronary artery ligation and/or when treatment is administered prior to the onset of ischaemia.79
In general, the use of non-coding RNA based-therapy as a short-term therapy to mitigate IR injury and reduce infarct size in patients presenting with STEMI requires rapid and specific delivery of the RNA molecules to the heart, and a rapid onset of action.4 In the clinical setting such therapeutic agents should either be given intravenously during the ischaemic phase or intravenously or intra-coronary during primary PCI, and must be able to enter the cells rapidly and have a rapid effect gene expression. Approaches are being developed to aid intracellular RNA delivery or chemically modify RNA to permit its direct cellular uptake.89,90 However, clinical translation of these pharmaceutical agents is still at an early stage.
5. Thrombosis and blood clotting factors
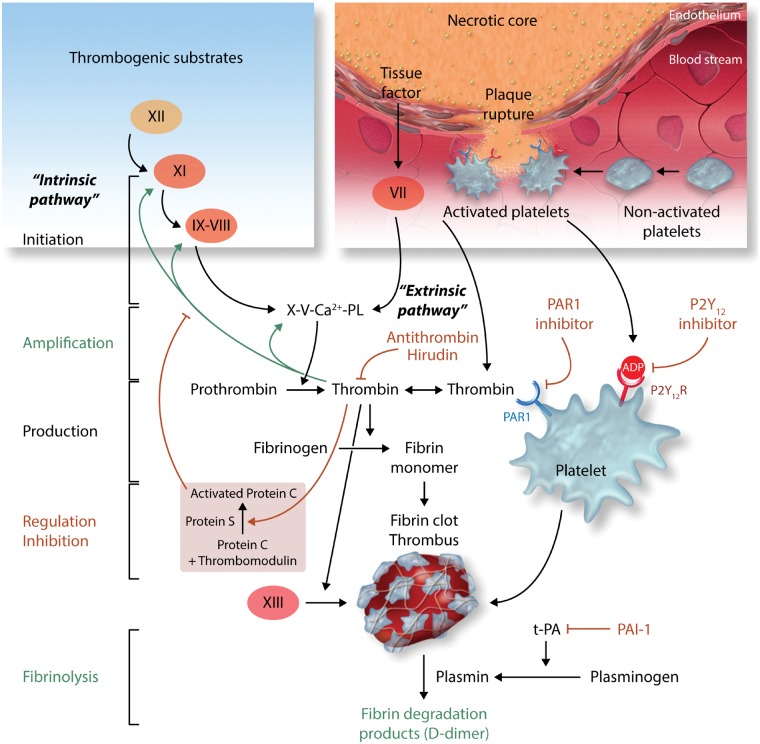
Several interventions targeting blood clotting factors have been found to limit infarct size independently of the haemostatic function of these proteins. Blood coagulation is initiated when plasma factor VII (FVII) binds to its cellular receptor tissue factor (TF), (expressed on deeper cell layers of the vessel wall), and is converted to the active protease FVIIa (extrinsic initiation pathway) (Figure 3).91 The TF:FVIIa complex activates the factors IX and X resulting in the generation of only minute amounts of thrombin that immediately start to amplify its own production by activating factors XI, VIII, and V. These reactions of the intrinsic pathway result in the mass production of thrombin, now available to induce fibrin formation and blood clotting. Additionally, thrombin activates platelets, endothelial cells, and cardiomyocytes, by cleavage of protease activated receptors (PARs) and the subsequent activation of intracellular signalling pathways.92 The intrinsic regulation of blood coagulation is achieved by the thrombin-induced generation of the thrombomodulin-protein C pathway, resulting in an effective control of thrombin generation.
Figure 3.
Initiation, amplification and feedback anticoagulant mechanisms in the coagulation cascade. The different phases, from initiation of coagulation due to exposure of tissue factor and binding of its ligand factor VII/VIIa either at a wound/extravascular site or in the intravascular compartment (microvesicles), designated as ‘extrinsic pathway’, to amplification and production of thrombin by the positive feedback reactions of the ‘intrinsic pathway’ are indicated. In parallel to fibrin clot formation, the majority of thrombin will distantly bind to its endothelial cell receptor thrombomodulin to induce the activation of protein C (PC) into APC, which limits further thrombin production by degrading the procoagulant cofactors VIIIa and Va. While these reactions are sufficient to achieve wound healing upon physiological haemostasis, when an atherosclerotic plaque ruptures, thrombogenic substrates are exposed that can initiate (auto-) activation of the factor XII-dependent reactions of the contact phase, resulting in enhanced thrombin generation and hence, fibrin clot formation and eventually thrombosis. The inhibitors mentioned in the text are indicated in red.
TF initiates the clotting cascade and is a major prothrombotic factor.93 Increased plasma TF levels, associated with EVs, are observed in patients with AMI, reflecting enhanced intravascular procoagulant activity.93 Experimental studies indicate that cardiac IR increases TF activity. In vivo studies in rabbit models have shown that anti-TF therapy prevented the transient decrease in regional myocardial blood flow, reduced platelet and fibrin(ogen) accumulation, and reduced infarct size.94 Thrombin may also contribute to the pathology of IR injury, since in a rabbit model of IR, selective inhibition of thrombin by recombinant hirudin (lepirudin) decreased infarct size.94 The TF-thrombin pathway may also contribute to myocardial injury by an additional mechanism that is not dependent on fibrin deposition but involves activation of PARs on vascular endothelial cells and cardiac myocytes.94 Since myocardial IR injury is partly mediated by thrombin and several cellular responses to thrombin are mediated by PARs, PARs have been extensively investigated as potential targets for cardioprotection.
The four known PARs are GPCRs that are activated by several serine proteases, including coagulation and mast cell-derived proteases. For example, thrombin cleaves and activates PAR-1, -3, and -4 on a variety of cells and thereby activates each of these receptors, whose new amino-terminal portion serves as leached ligand.95 PAR-1 is the high-affinity receptor for thrombin and is expressed by several cell types in the heart, including cardiomyocytes and cardiac fibroblasts. Since PAR-1 is expressed as a ‘cell-bound substrate’ of thrombin on both platelets and immune cells, hormonal doses of the enzyme are sufficient to provoke platelet aggregation or a variety of immune responses in the context of inflammation and cardiovascular disease.96 Treatment of rats or isolated hearts with a selective PAR-1 antagonist, reduced infarct size in a dose-dependent manner and increased ventricular recovery following IR.97 When PAR-1 is cleaved by thrombin it releases a 41-amino-acid peptide called parstatin. Both parstatin and its putative signal peptide (N-terminal fragment 1-26), reduced infarct size when administered to rats prior to IR.98,99 The underlying mechanisms may involve the known cardioprotective pathways including the RISK and the MPTP pathway.99
Activated protein C (APC) is a serine protease that serves as natural anticoagulant with an important role in regulating thrombin formation and the extent of fibrin formation. It is recognized by the endothelial protein C receptor and alters signalling of the thrombin-PAR-1 complex. In a mouse model of acute IRI, administration of APC significantly reduced myocardial infarct size,100–102 with PAR-1 required for this process.101 Interestingly, a variant APC, lacking catalytic activity, remained protective, implying that the protection from IRI is independent of its proteolytic activity.100 Furthermore, infarct size reduction depended on its PAR-1 signalling, but not its anticoagulant properties.102
Although APC serves as a major ligand/activator of PAR-2 on immune cells, its role in IRI is controversially discussed and the protease(s) that activate PAR-2 during cardiac IRI are not known.94 Infarct size was significantly reduced in PAR-2−/− mice subjected to 30 min ischaemia and 2 h reperfusion, as well as exhibiting decreasing oxidative/nitrative stress.103 In contrast, infusion of a PAR-2-activating peptide reduced infarct size in isolated perfused rat hearts.104 Interestingly, this peptide showed additive protection with an IPC protocol of 2 min ischaemia followed by 10 min reperfusion.105 Furthermore, the PAR-2 agonist peptide SLIGRL reduced infarct size when administered to rats at the time of reperfusion, via a pathway involving ERK1/2 and PKC.106 SLIGRL also reduced infarct size in isolated rats hearts, via pathways involving PKCε or PKA, and transient receptor potential vanilloid type 1 -dependent release of calcitonin gene-related peptide and substance P.107
PAR-4 knockout mice exhibited reduced infarct size after acute IR. This may be due to protection from a Src- and epidermal growth factor receptor-dependent pathway of JNK-induced apoptosis.108 Two structurally unrelated PAR-4 antagonists reduced infarct size in rats when administered prior to ischaemia either in vitro or in vivo, via a mechanism that appears to involve adenosine.109
Plasmin is the main enzyme that dissolves fibrin blood clots. The main function of plasminogen activator inhibitor type-1 (PAI-1 or SERPIN E1) is to oppose the plasmin activation cascade, thereby maintaining the clot. Increased expression of PAI-1 is profibrotic in hearts subjected to MI. A markedly greater extent of infarction was observed in PAI-1 knockout mice compared with controls and this was associated with haemorrhage and inflammation.110
Overall, pharmaceutical agents targeting thrombosis and blood clotting factors appear have multiple benefits in the setting of IR, including benefits independent of haemostasis, although these are not always easy to completely separate mechanistically.
6. Conclusion
As can be seen from this review, circulating cells and factors can strongly impact IR injury via various mechanisms. Erythrocytes, for example, can export NO bioactivity and be cardioprotective. Platelets, in addition to their role in haemostasis and thrombosis, secrete a large number of factors that can influence the development of IR injury both positively and negatively. Erythrocytes, platelets, and other cell types can release both MVs and exosomes which may have both detrimental or protective characteristics in the setting of IR. These effects may be mediated by the transfer of miRNA to cardiomyocytes, or through ligand-receptor signalling or other mediators (e.g. NO).
In many of the experiments described, the end target is likely to be the cardiomyocyte, since ultimately, it is these cells that must be preserved in order to limit infarct size and retain contractile function. However, there are other important aspects to IR injury such as endothelial damage and microvascular obstruction which may be targets. Furthermore, the interactions between thrombus, clotting factors and circulating haematopoietic cells have not yet been clarified in terms of IR injury. With greater understanding of PAR-1 and PAR-4 signalling pathways and their role in IR injury may come opportunities for better tailored therapies to prevent tissue injury.
One firm conclusion that can be drawn is that it is important to consider the interaction of potential cardioprotective agents with comedications such as platelet inhibitors, since these appear to have cardioprotective actions independent of their role in haemostasis. Furthermore, comorbidities such as diabetes can impact not only the induction of cardioprotection in the target cardiomyocytes, but can also influence the function of platelets, erythrocytes and EVs, and consequently impair their ability to mediate cardioprotection. Finally, future studies of potential cardioprotective agents should consider not just their direct effect on cardiomyocytes, but on indirect effects that may be mediated via circulating blood cells and factors.
Acknowledgements
This article is based upon work from COST Action EU-CARDIOPROTECTION CA16225 supported by COST (European Cooperation in Science and Technology). The images used in the Graphical Abstract for this article were downloaded from Servier Medical Art (https://smart.servier.com) on the 22/11/18 under a Creative Commons Attribution 3.0 Unported License.
Conflict of interest: P.F. is the founder and CEO of Pharmahungary Group, a group of R&D companies. I.A., L.B., Y.B., H.A.C.-F., M.V.C., S.D., J.M.D., H.G., C.P., J.P., P.P. and K.T.P. declare no conflicts of interest.
Funding
This work was supported by the British Heart Foundation [PG/16/85/32471 and PG/18/44/33790 to S.M.D.]; National Institute for Health Research University College London Hospitals Biomedical Research Centre [to S.M.D.]; Università degli Studi di Torino [PAGP_RIC_LOC_16_01; PAGP_RILO_17_01; PENC_RILO_17_01 to P.P. and C.P.]; Astra Zeneca, Boehringer Ingelheim Pharmaceuticals [to Y.B.]; The Russian Government Program for competitive growth of Kazan Federal University, Kazan (Russian Federation) [to H.A.C.F. and K.T.P.]; SHF-Foundation [SHF/FG657P/2017 to H.A.C.F.]; the von Behring-Röntgen-Foundation (Marburg, Germany) [to H.A.C.F.]; the National Research, Development and Innovation Office of Hungary [NVKP_16-1-2016-0017; OTKA KH 125570; OTKA 115378 to P.F.]; the Higher Education Institutional Excellence Programme of the Ministry of Human Capacities in Hungary, within the framework of the Therapeutic Development thematic programme of the Semmelweis University [to P.F.]; the European Regional Development Fund (ERDF) through the Operational Program for Competitiveness Factors (COMPETE) [PAC ‘NETDIAMOND’ POCI-01-0145-FEDER-016385; HealthyAging2020 CENTRO-01-0145-FEDER-000012-N2323; POCI-01-0145-FEDER-007440 and FCT-UID/NEU/04539/2013 to CNC.IBILI to H.G.]; the Excellence Cluster Cardio-pulmonary System (ECCPS) of the German Research Foundation (Bonn, Germany) [to K.T.P.]. This article is based upon work from COST Action EU-CARDIOPROTECTION CA16225 supported by COST (European Cooperation in Science and Technology).
References
- 1. Hausenloy DJ, Botker HE, Engstrom T, Erlinge D, Heusch G, Ibanez B, Kloner RA, Ovize M, Yellon DM, Garcia-Dorado D.. Targeting reperfusion injury in patients with ST-segment elevation myocardial infarction: trials and tribulations. Eur Heart J 2017;38:935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hausenloy DJ, Garcia-Dorado D, Botker HE, Davidson SM, Downey J, Engel FB, Jennings R, Lecour S, Leor J, Madonna R, Ovize M, Perrino C, Prunier F, Schulz R, Sluijter JPG, Van Laake LW, Vinten-Johansen J, Yellon DM, Ytrehus K, Heusch G, Ferdinandy P.. Novel targets and future strategies for acute cardioprotection: position paper of the European Society of Cardiology Working Group on Cellular Biology of the Heart. Cardiovasc Res 2017;113:564–585. [DOI] [PubMed] [Google Scholar]
- 3. Hausenloy DJ, Barrabes JA, Botker HE, Davidson SM, Di Lisa F, Downey J, Engstrom T, Ferdinandy P, Carbrera-Fuentes HA, Heusch G, Ibanez B, Iliodromitis EK, Inserte J, Jennings R, Kalia N, Kharbanda R, Lecour S, Marber M, Miura T, Ovize M, Perez-Pinzon MA, Piper HM, Przyklenk K, Schmidt MR, Redington A, Ruiz-Meana M, Vilahur G, Vinten-Johansen J, Yellon DM, Garcia-Dorado D.. Ischaemic conditioning and targeting reperfusion injury: a 30 year voyage of discovery. Basic Res Cardiol 2016;111:70.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Perrino C, Barabasi AL, Condorelli G, Davidson SM, De Windt L, Dimmeler S, Engel FB, Hausenloy DJ, Hill JA, Van Laake LW, Lecour S, Leor J, Madonna R, Mayr M, Prunier F, Sluijter JPG, Schulz R, Thum T, Ytrehus K, Ferdinandy P.. Epigenomic and transcriptomic approaches in the post-genomic era: path to novel targets for diagnosis and therapy of the ischaemic heart? Position Paper of the European Society of Cardiology Working Group on Cellular Biology of the Heart. Cardiovasc Res 2017;113:725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Varga ZV, Giricz Z, Bencsik P, Madonna R, Gyongyosi M, Schulz R, Mayr M, Thum T, Puskas LG, Ferdinandy P.. Functional genomics of cardioprotection by ischemic conditioning and the influence of comorbid conditions: implications in target identification. Curr Drug Targets 2015;16:904–911. [DOI] [PubMed] [Google Scholar]
- 6. Heusch G. Critical issues for the translation of cardioprotection. Circ Res 2017;120:1477–1486. [DOI] [PubMed] [Google Scholar]
- 7. Lecour S, Bøtker HE, Condorelli G, Davidson SM, Garcia-Dorado D, Engel FB, Ferdinandy P, Heusch G, Madonna R, Ovize M, Ruiz-Meana M, Schulz R, Sluijter JP, Van Laake LW, Yellon DM, Hausenloy DJ.. ESC Working Group Cellular Biology of the Heart: position paper: improving the preclinical assessment of novel cardioprotective therapies. Cardiovasc Res 2014;104:399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ferdinandy P, Hausenloy DJ, Heusch G, Baxter GF, Schulz R.. Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev 2014;66:1142–1174. [DOI] [PubMed] [Google Scholar]
- 9. Davidson SM, Ferdinandy P, Andreadou I, Bøtker HE, Heusch G, Ibáñez B, Ovize M, Schulz R, Yellon DM, Hausenloy DJ, Garcia-Dorado D.. Multi-target strategies to reduce myocardial ischemia/reperfusion injury. J Am Coll Cardiol 2019;73:86–96. [DOI] [PubMed] [Google Scholar]
- 10. Zuurbier CJ, Abbate A, Cabrera-Fuentes HA, Cohen MV, Collino M, De Kleijn DPV, Downey JM, Pagliaro P, Preissner KT, Takahashi M, Davidson SM.. Innate immunity as a target for acute cardioprotection. Cardiovasc Res 2019;115:1131–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Andreadou I, Cabrera-Fuentes HA, Devaux Y, Frangogiannis NG, Frantz S, Guzik T, Liehn EA, Gomes CPC, Schulz R, Hausenloy DJ.. Immune cells as targets for cardioprotection: new players and novel therapeutic opportunities. Cardiovasc Res 2019;115:1117–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hausenloy DJ, Bøtker HE, Ferdinandy P, Heusch G, André Ng G, Redington A, Garcia-Dorado D.. Cardiac innervation in acute myocardial ischaemia/reperfusion injury and cardioprotection. Cardiovasc Res 2019;115:1167–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hausenloy DJ, Chilian W, Crea F, Davidson SM, Ferdinandy P, Garcia-Dorado D, van Royen N, Schulz R, Heusch G.. The coronary circulation in acute myocardial ischaemia/reperfusion injury: a target for cardioprotection. Cardiovasc Res 2019;115:1143–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Du XY, Zabel BA, Myles T, Allen SJ, Handel TM, Lee PP, Butcher EC, Leung LL.. Regulation of chemerin bioactivity by plasma carboxypeptidase N, carboxypeptidase B (activated thrombin-activable fibrinolysis inhibitor), and platelets. J Biol Chem 2009;284:751–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rodriguez-Penas D, Feijoo-Bandin S, Garcia-Rua V, Mosquera-Leal A, Duran D, Varela A, Portoles M, Rosello-Lleti E, Rivera M, Dieguez C, Gualillo O, Gonzalez-Juanatey JR, Lago F.. The adipokine chemerin induces apoptosis in cardiomyocytes. Cell Physiol Biochem 2015;37:176–192. [DOI] [PubMed] [Google Scholar]
- 16. Malik A, Bromage DI, He Z, Candilio L, Hamarneh A, Taferner S, Davidson SM, Yellon DM.. Exogenous SDF-1alpha protects human myocardium from hypoxia-reoxygenation injury via CXCR4. Cardiovasc Drugs Ther 2015;29:589–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ziff OJ, Bromage DI, Yellon DM, Davidson SM.. Therapeutic strategies utilizing SDF-1alpha in ischaemic cardiomyopathy. Cardiovasc Res 2018;114:358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vito CD, Hadi LA, Navone SE, Marfia G, Campanella R, Mancuso ME, Riboni L.. Platelet-derived sphingosine-1-phosphate and inflammation: from basic mechanisms to clinical implications. Platelets 2016;27:393–401. [DOI] [PubMed] [Google Scholar]
- 19. Cohen MV, Yang XM, White J, Yellon DM, Bell RM, Downey JM.. Cangrelor-mediated cardioprotection requires platelets and sphingosine phosphorylation. Cardiovasc Drugs Ther 2016;30:229–232. [DOI] [PubMed] [Google Scholar]
- 20. Knapp M. Cardioprotective role of sphingosine-1-phosphate. J Physiol Pharmacol 2011;62:601–607. [PubMed] [Google Scholar]
- 21. Vessey DA, Li L, Honbo N, Karliner JS.. Sphingosine 1-phosphate is an important endogenous cardioprotectant released by ischemic pre- and postconditioning. Am J Physiol Heart Circ Physiol 2009;297:H1429–H1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Means CK, Xiao CY, Li Z, Zhang T, Omens JH, Ishii I, Chun J, Brown JH.. Sphingosine 1-phosphate S1P2 and S1P3 receptor-mediated AKT activation protects against in vivo myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 2007;292:H2944–H2951. [DOI] [PubMed] [Google Scholar]
- 23. Egom EE, Mohamed TM, Mamas MA, Shi Y, Liu W, Chirico D, Stringer SE, Ke Y, Shaheen M, Wang T, Chacko S, Wang X, Solaro RJ, Fath-Ordoubadi F, Cartwright EJ, Lei M.. Activation of Pak1/AKT/eNOS signaling following sphingosine-1-phosphate release as part of a mechanism protecting cardiomyocytes against ischemic cell injury. Am J Physiol Heart Circ Physiol 2011;301:H1487–H1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jin ZQ, Goetzl EJ, Karliner JS.. Sphingosine kinase activation mediates ischemic preconditioning in murine heart. Circulation 2004;110:1980–1989. [DOI] [PubMed] [Google Scholar]
- 25. Lecour S, Smith RM, Woodward B, Opie LH, Rochette L, Sack MN.. Identification of a novel role for sphingolipid signaling in TNF alpha and ischemic preconditioning mediated cardioprotection. J Mol Cell Cardiol 2002;34:509–518. [DOI] [PubMed] [Google Scholar]
- 26. Jung JH, Tantry US, Gurbel PA, Jeong YH.. Current antiplatelet treatment strategy in patients with diabetes mellitus. Diabetes Metab J 2015;39:95–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Russo I, Femmino S, Barale C, Tullio F, Geuna S, Cavalot F, Pagliaro P, Penna C.. Cardioprotective properties of human platelets are lost in uncontrolled diabetes mellitus: a study in isolated rat hearts. Front Physiol 2018;9:875.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Karliner JS. Sphingosine kinase and sphingosine 1-phosphate in the heart: a decade of progress. Biochim Biophys Acta 2013;1831:203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang XM, Liu Y, Cui L, Yang X, Liu Y, Tandon N, Kambayashi J, Downey JM, Cohen MV.. Platelet P2Y(1)(2) blockers confer direct postconditioning-like protection in reperfused rabbit hearts. J Cardiovasc Pharmacol Ther 2013;18:251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nylander S, Schulz R.. Effects of P2Y12 receptor antagonists beyond platelet inhibition—comparison of ticagrelor with thienopyridines. Br J Pharmacol 2016;173:1163–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tani M, Sano T, Ito M, Igarashi Y.. Mechanisms of sphingosine and sphingosine 1-phosphate generation in human platelets. J Lipid Res 2005;46:2458–2467. [DOI] [PubMed] [Google Scholar]
- 32. Barrabes JA, Inserte J, Mirabet M, Quiroga A, Hernando V, Figueras J, Garcia-Dorado D.. Antagonism of P2Y12 or GPIIb/IIIa receptors reduces platelet-mediated myocardial injury after ischaemia and reperfusion in isolated rat hearts. Thromb Haemost 2010;104:128–135. [DOI] [PubMed] [Google Scholar]
- 33. Yang XM, Cui L, Alhammouri A, Downey JM, Cohen MV.. Triple therapy greatly increases myocardial salvage during ischemia/reperfusion in the in situ rat heart. Cardiovasc Drugs Ther 2013;27:403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cohen MV, Downey JM.. The impact of irreproducibility and competing protection from P2Y12 antagonists on the discovery of cardioprotective interventions. Basic Res Cardiol 2017;112:64.. [DOI] [PubMed] [Google Scholar]
- 35. Penna C, Bassino E, Alloatti G.. Platelet activating factor: the good and the bad in the ischemic/reperfused heart. Exp Biol Med (Maywood) 2011;236:390–401. [DOI] [PubMed] [Google Scholar]
- 36. Penna C, Alloatti G, Cappello S, Gattullo D, Berta G, Mognetti B, Losano G, Pagliaro P.. Platelet-activating factor induces cardioprotection in isolated rat heart akin to ischemic preconditioning: role of phosphoinositide 3-kinase and protein kinase C activation. Am J Physiol Heart Circ Physiol 2005;288:H2512–H2520. [DOI] [PubMed] [Google Scholar]
- 37. Montrucchio G, Alloatti G, Mariano F, de Paulis R, Comino A, Emanuelli G, Camussi G.. Role of platelet-activating factor in the reperfusion injury of rabbit ischemic heart. Am J Pathol 1990;137:71–83. [PMC free article] [PubMed] [Google Scholar]
- 38. Cortese-Krott MM, Kelm M.. Endothelial nitric oxide synthase in red blood cells: key to a new erythrocrine function? Redox Biol 2014;2:251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yedgar S, Koshkaryev A, Barshtein G.. The red blood cell in vascular occlusion. Pathophysiol Haemost Thromb 2002;32:263–268. [DOI] [PubMed] [Google Scholar]
- 40. Poz D, De Falco E, Pisano C, Madonna R, Ferdinandy P, Balistreri CR.. Diagnostic and prognostic relevance of Red blood cell distribution width for vascular aging and cardiovascular diseases. Rejuvenation Res 2018;doi:10.1089/rej.2018.2094. [DOI] [PubMed] [Google Scholar]
- 41. Burger D, Lei M, Geoghegan-Morphet N, Lu X, Xenocostas A, Feng Q.. Erythropoietin protects cardiomyocytes from apoptosis via up-regulation of endothelial nitric oxide synthase. Cardiovasc Res 2006;72:51–59. [DOI] [PubMed] [Google Scholar]
- 42. Yang BC, Nichols WW, Mehta JL.. Cardioprotective effects of red blood cells on ischemia and reperfusion injury in isolated rat heart: release of nitric oxide as a potential mechanism. J Cardiovasc Pharmacol Ther 1996;1:297–306. [DOI] [PubMed] [Google Scholar]
- 43. Merx MW, Gorressen S, van de Sandt AM, Cortese-Krott MM, Ohlig J, Stern M, Rassaf T, Godecke A, Gladwin MT, Kelm M.. Depletion of circulating blood NOS3 increases severity of myocardial infarction and left ventricular dysfunction. Basic Res Cardiol 2014;109:398.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yang J, Gonon AT, Sjoquist PO, Lundberg JO, Pernow J.. Arginase regulates red blood cell nitric oxide synthase and export of cardioprotective nitric oxide bioactivity. Proc Natl Acad Sci USA 2013;110:15049–15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhou Z, Mahdi A, Tratsiakovich Y, Zahorán S, Kövamees O, Nordin F, Uribe Gonzalez AE, Alvarsson M, Östenson CG, Andersson DC, Hedin U, Hermesz E, Lundberg JO, Yang J, Pernow J.. Erythrocytes from patients with type 2 diabetes induce endothelial dysfunction via arginase I. J Am Coll Cardiol 2018;72:769–780. [DOI] [PubMed] [Google Scholar]
- 46. Yang J, Zheng X, Mahdi A, Zhou Z, Tratsiakovich Y, Jiao T, Kiss A, Kovamees O, Alvarsson M, Catrina SB, Lundberg JO, Brismar K, Pernow J.. Red blood cells in type 2 diabetes impair cardiac post-ischemic recovery through an arginase-dependent modulation of nitric oxide synthase and reactive oxygen species. JACC Basic Transl Sci 2018;3:450–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhou ZC, Mahdi A, Tratsiakovich Y, Zahorán S, Kövamees O, Nordin F, Uribe Gonzalez AE, Alvarsson M, Östenson C-G, Andersson DC, Hedin U, Hermesz E, Lundberg JO, Yang JN, Pernow J.. Erythrocytes from patients with type 2 diabetes induce endothelial dysfunction via arginase I. J Am Coll Cardiol 2018;72:769–780. [DOI] [PubMed] [Google Scholar]
- 48. Grau M, Kollikowski A, Bloch W.. Remote ischemia preconditioning increases red blood cell deformability through red blood cell-nitric oxide synthase activation. Clin Hemorheol Microcirc 2016;63:185–197. [DOI] [PubMed] [Google Scholar]
- 49. Boulanger CM, Loyer X, Rautou PE, Amabile N.. Extracellular vesicles in coronary artery disease. Nat Rev Cardiol 2017;14:259–272. [DOI] [PubMed] [Google Scholar]
- 50. Davidson SM, Yellon DM.. Exosomes and cardioprotection—a critical analysis. Mol Aspects Med 2018;60:104–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lawson C, Vicencio JM, Yellon DM, Davidson SM.. Microvesicles and exosomes: new players in metabolic and cardiovascular disease. J Endocrinol 2016;228:R57–R71. [DOI] [PubMed] [Google Scholar]
- 52. Sluijter JPG, Davidson SM, Boulanger CM, Buzas EI, de Kleijn DPV, Engel FB, Giricz Z, Hausenloy DJ, Kishore R, Lecour S, Leor J, Madonna R, Perrino C, Prunier F, Sahoo S, Schiffelers RM, Schulz R, Van Laake LW, Ytrehus K, Ferdinandy P.. Extracellular vesicles in diagnostics and therapy of the ischaemic heart: position paper from the Working Group on Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res 2018;114:19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Baranyai T, Herczeg K, Onodi Z, Voszka I, Modos K, Marton N, Nagy G, Mager I, Wood MJ, El Andaloussi S, Palinkas Z, Kumar V, Nagy P, Kittel A, Buzas EI, Ferdinandy P, Giricz Z.. Isolation of exosomes from blood plasma: qualitative and quantitative comparison of ultracentrifugation and size exclusion chromatography methods. PLoS One 2015;10:e0145686.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vicencio JM, Yellon DM, Sivaraman V, Das D, Boi-Doku C, Arjun S, Zheng Y, Riquelme JA, Kearney J, Sharma V, Multhoff G, Hall AR, Davidson SM.. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J Am Coll Cardiol 2015;65:1525–1536. [DOI] [PubMed] [Google Scholar]
- 55. Yellon DM, Davidson SM.. Exosomes: nanoparticles involved in cardioprotection? Circ Res 2014;114:325–332. [DOI] [PubMed] [Google Scholar]
- 56. Giricz Z, Varga ZV, Baranyai T, Sipos P, Paloczi K, Kittel A, Buzas EI, Ferdinandy P.. Cardioprotection by remote ischemic preconditioning of the rat heart is mediated by extracellular vesicles. J Mol Cell Cardiol 2014;68:75–78. [DOI] [PubMed] [Google Scholar]
- 57. Minghua W, Zhijian G, Chahua H, Qiang L, Minxuan X, Luqiao W, Weifang Z, Peng L, Biming Z, Lingling Y, Zhenzhen W, Jianqing X, Huihui B, Xiaozhong W, Xiaoshu C.. Plasma exosomes induced by remote ischaemic preconditioning attenuate myocardial ischaemia/reperfusion injury by transferring miR-24. Cell Death Dis 2018;9:320.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ.. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood 1999;94:3791–3799. [PubMed] [Google Scholar]
- 59. Leroyer AS, Isobe H, Leseche G, Castier Y, Wassef M, Mallat Z, Binder BR, Tedgui A, Boulanger CM.. Cellular origins and thrombogenic activity of microparticles isolated from human atherosclerotic plaques. J Am Coll Cardiol 2007;49:772–777. [DOI] [PubMed] [Google Scholar]
- 60. McManus DD, Freedman JE.. MicroRNAs in platelet function and cardiovascular disease. Nat Rev Cardiol 2015;12:711–717. [DOI] [PubMed] [Google Scholar]
- 61. Barile L, Lionetti V, Cervio E, Matteucci M, Gherghiceanu M, Popescu LM, Torre T, Siclari F, Moccetti T, Vassalli G.. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc Res 2014;103:530–541. [DOI] [PubMed] [Google Scholar]
- 62. Ibrahim AG, Cheng K, Marban E.. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports 2014;2:606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Barile L, Cervio E, Lionetti V, Milano G, Ciullo A, Biemmi V, Bolis S, Altomare C, Matteucci M, Disilvestre D, Fertig TE, Torre T, Demertzis S, Mauri P, Moccetti T, Vassalli G.. Cardioprotection by cardiac progenitor cell-secreted exosomes: role of pregnancy-associated plasma protein-A. Cardiovasc Res 2018;114:992–1005. [DOI] [PubMed] [Google Scholar]
- 64. Cambier L, de Couto G, Ibrahim A, Echavez AK, Valle J, Liu W, Kreke M, Smith RR, Marbán L, Marbán E.. Y RNA fragment in extracellular vesicles confers cardioprotection via modulation of IL-10 expression and secretion. EMBO Mol Med 2017;9:337–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tkach M, Thery C.. Communication by extracellular vesicles: where we are and where we need to go. Cell 2016;164:1226–1232. [DOI] [PubMed] [Google Scholar]
- 66. de Couto G, Gallet R, Cambier L, Jaghatspanyan E, Makkar N, Dawkins JF, Berman BP, Marbán E.. Exosomal MicroRNA transfer into macrophages mediates cellular postconditioning. Circulation 2017;136:200–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Davidson SM, Riquelme JA, Takov K, Vicencio JM, Boi-Doku C, Khoo V, Doreth C, Radenkovic D, Lavandero S, Yellon DM.. Cardioprotection mediated by exosomes is impaired in the setting of type II diabetes but can be rescued by the use of non-diabetic exosomes in vitro. J Cell Mol Med 2018;22:141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Andriolo G, Provasi E, Lo Cicero V, Brambilla A, Soncin S, Torre T, Milano G, Biemmi V, Vassalli G, Turchetto L, Barile L, Radrizzani M.. Exosomes from human cardiac progenitor cells for therapeutic applications: development of a GMP-grade manufacturing method. Front Physiol 2018;9:1169.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol 1967;13:269–288. [DOI] [PubMed] [Google Scholar]
- 70. Freyssinet JM, Toti F.. Formation of procoagulant microparticles and properties. Thromb Res 2010;125(Suppl. 1):S46–S48. [DOI] [PubMed] [Google Scholar]
- 71. Badimon L, Suades R, Fuentes E, Palomo I, Padro T.. Role of platelet-derived microvesicles as crosstalk mediators in atherothrombosis and future pharmacology targets: a link between inflammation, atherosclerosis, and thrombosis. Front Pharmacol 2016;7:293.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Badimon L, Suades R, Arderiu G, Peña E, Chiva-Blanch G, Padró T.. Microvesicles in atherosclerosis and angiogenesis: from bench to bedside and reverse. Front Cardiovasc Med 2017;4:77.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Brill A, Dashevsky O, Rivo J, Gozal Y, Varon D.. Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc Res 2005;67:30–38. [DOI] [PubMed] [Google Scholar]
- 74. Liu M, Wang Y, Zhu Q, Zhao J, Wang Y, Shang M, Liu M, Wu Y, Song J, Liu Y.. Protective effects of circulating microvesicles derived from ischemic preconditioning on myocardial ischemia/reperfusion injury in rats by inhibiting endoplasmic reticulum stress. Apoptosis 2018;23:436–448. [DOI] [PubMed] [Google Scholar]
- 75. Yu B, Gong M, Wang Y, Millard RW, Pasha Z, Yang Y, Ashraf M, Xu M.. Cardiomyocyte protection by GATA-4 gene engineered mesenchymal stem cells is partially mediated by translocation of miR-221 in microvesicles. PLoS One 2013;8:e73304.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhang Q, Shang M, Zhang M, Wang Y, Chen Y, Wu Y, Liu M, Song J, Liu Y.. Microvesicles derived from hypoxia/reoxygenation-treated human umbilical vein endothelial cells promote apoptosis and oxidative stress in H9c2 cardiomyocytes. BMC Cell Biol 2016;17:25.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Loyer X, Zlatanova I, Devue C, Yin M, Howangyin KY, Klaihmon P, Guerin CL, Kheloufi M, Vilar J, Zannis K, Fleischmann BK, Hwang DW, Park J, Lee H, Menasche P, Silvestre JS, Boulanger CM.. Intra-cardiac release of extracellular vesicles shapes inflammation following myocardial infarction. Circ Res 2018;123:100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bar C, Chatterjee S, Thum T.. Long noncoding RNAs in cardiovascular pathology, diagnosis, and therapy. Circulation 2016;134:1484–1499. [DOI] [PubMed] [Google Scholar]
- 79. Ong SB, Katwadi K, Kwek XY, Ismail NI, Chinda K, Ong SG, Hausenloy DJ.. Non-coding RNAs as therapeutic targets for preventing myocardial ischemia-reperfusion injury. Expert Opin Ther Targets 2018;22:247–261. [DOI] [PubMed] [Google Scholar]
- 80. Hullinger TG, Montgomery RL, Seto AG, Dickinson BA, Semus HM, Lynch JM, Dalby CM, Robinson K, Stack C, Latimer PA, Hare JM, Olson EN, van Rooij E.. Inhibition of miR-15 protects against cardiac ischemic injury. Circ Res 2012;110:71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hinkel R, Penzkofer D, Zuhlke S, Fischer A, Husada W, Xu QF, Baloch E, van Rooij E, Zeiher AM, Kupatt C, Dimmeler S.. Inhibition of microRNA-92a protects against ischemia/reperfusion injury in a large-animal model. Circulation 2013;128:1066–1075. [DOI] [PubMed] [Google Scholar]
- 82. Higashi K, Yamada Y, Minatoguchi S, Baba S, Iwasa M, Kanamori H, Kawasaki M, Nishigaki K, Takemura G, Kumazaki M, Akao Y, Minatoguchi S.. MicroRNA-145 repairs infarcted myocardium by accelerating cardiomyocyte autophagy. Am J Physiol Heart Circ Physiol 2015;309:H1813–H1826. [DOI] [PubMed] [Google Scholar]
- 83. Varga ZV, Zvara A, Farago N, Kocsis GF, Pipicz M, Gaspar R, Bencsik P, Gorbe A, Csonka C, Puskas LG, Thum T, Csont T, Ferdinandy P.. MicroRNAs associated with ischemia-reperfusion injury and cardioprotection by ischemic pre- and postconditioning: protectomiRs. Am J Physiol Heart Circ Physiol 2014;307:H216–H227. [DOI] [PubMed] [Google Scholar]
- 84. Varga ZV, Agg B, Ferdinandy P.. miR-125b is a protectomiR: a rising star for acute cardioprotection. J Mol Cell Cardiol 2018;115:51–53. [DOI] [PubMed] [Google Scholar]
- 85. Zhu J, Yao K, Wang Q, Guo J, Shi H, Ma L, Liu H, Gao W, Zou Y, Ge J.. Ischemic postconditioning-regulated miR-499 protects the rat heart against ischemia/reperfusion injury by inhibiting apoptosis through PDCD4. Cell Physiol Biochem 2016;39:2364–2380. [DOI] [PubMed] [Google Scholar]
- 86. Ye Y, Hu Z, Lin Y, Zhang C, Perez-Polo JR.. Downregulation of microRNA-29 by antisense inhibitors and a PPAR-gamma agonist protects against myocardial ischaemia-reperfusion injury. Cardiovasc Res 2010;87:535–544. [DOI] [PubMed] [Google Scholar]
- 87. Yin C, Wang X, Kukreja RC.. Endogenous microRNAs induced by heat-shock reduce myocardial infarction following ischemia-reperfusion in mice. FEBS Lett 2008;582:4137–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S.. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 2008;456:980–984. [DOI] [PubMed] [Google Scholar]
- 89. Sultana N, Magadum A, Hadas Y, Kondrat J, Singh N, Youssef E, Calderon D, Chepurko E, Dubois N, Hajjar RJ, Zangi L.. Optimizing cardiac delivery of modified mRNA. Mol Ther 2017;25:1306–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Mellis D, Caporali A.. MicroRNA-based therapeutics in cardiovascular disease: screening and delivery to the target. Biochem Soc Trans 2018;46:11–21. [DOI] [PubMed] [Google Scholar]
- 91. Franchi F, Rollini F, Angiolillo DJ.. Antithrombotic therapy for patients with STEMI undergoing primary PCI. Nat Rev Cardiol 2017;14:361–379. [DOI] [PubMed] [Google Scholar]
- 92. Antoniak S, Pawlinski R, Mackman N.. Protease-activated receptors and myocardial infarction. IUBMB Life 2011;63:383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Suefuji H, Ogawa H, Yasue H, Kaikita K, Soejima H, Motoyama T, Mizuno Y, Oshima S, Saito T, Tsuji I, Kumeda K, Kamikubo Y, Nakamura S.. Increased plasma tissue factor levels in acute myocardial infarction. Am Heart J 1997;134:253–259. [DOI] [PubMed] [Google Scholar]
- 94. Mackman N. The role of the tissue factor-thrombin pathway in cardiac ischemia-reperfusion injury. Semin Vasc Med 2003;3:193–198. [DOI] [PubMed] [Google Scholar]
- 95. Vu TK, Hung DT, Wheaton VI, Coughlin SR.. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell 1991;64:1057–1068. [DOI] [PubMed] [Google Scholar]
- 96. Samad F, Ruf W.. Inflammation, obesity, and thrombosis. Blood 2013;122:3415–3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Strande JL, Hsu A, Su J, Fu X, Gross GJ, Baker JE.. SCH 79797, a selective PAR1 antagonist, limits myocardial ischemia/reperfusion injury in rat hearts. Basic Res Cardiol 2007;102:350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Routhu KV, Tsopanoglou NE, Strande JL.. Parstatin(1-26): the putative signal peptide of protease-activated receptor 1 confers potent protection from myocardial ischemia-reperfusion injury. J Pharmacol Exp Ther 2010;332:898–905. [DOI] [PubMed] [Google Scholar]
- 99. Strande JL, Widlansky ME, Tsopanoglou NE, Su J, Wang J, Hsu A, Routhu KV, Baker JE.. Parstatin: a cryptic peptide involved in cardioprotection after ischaemia and reperfusion injury. Cardiovasc Res 2009;83:325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wildhagen KC, Schrijver R, Beckers L, ten Cate H, Reutelingsperger CP, Lutgens E, Nicolaes GA.. Effects of exogenous recombinant APC in mouse models of ischemia reperfusion injury and of atherosclerosis. PLoS One 2014;9:e101446.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Loubele ST, Spek CA, Leenders P, van Oerle R, Aberson HL, Hamulyák K, Ferrell G, Esmon CT, Spronk HM, ten Cate H.. Activated protein C protects against myocardial ischemia/reperfusion injury via inhibition of apoptosis and inflammation. Arterioscler Thromb Vasc Biol 2009;29:1087–1092. [DOI] [PubMed] [Google Scholar]
- 102. Nazir S, Gadi I, Al-Dabet MM, Elwakiel A, Kohli S, Ghosh S, Manoharan J, Ranjan S, Bock F, Braun-Dullaeus RC, Esmon CT, Huber TB, Camerer E, Dockendorff C, Griffin JH, Isermann B, Shahzad K.. Cytoprotective activated protein C averts Nlrp3 inflammasome-induced ischemia-reperfusion injury via mTORC1 inhibition. Blood 2017;130:2664–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Antoniak S, Rojas M, Spring D, Bullard TA, Verrier ED, Blaxall BC, Mackman N, Pawlinski R.. Protease-activated receptor 2 deficiency reduces cardiac ischemia/reperfusion injury. Arterioscler Thromb Vasc Biol 2010;30:2136–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Napoli C, Cicala C, Wallace JL, de Nigris F, Santagada V, Caliendo G, Franconi F, Ignarro LJ, Cirino G.. Protease-activated receptor-2 modulates myocardial ischemia-reperfusion injury in the rat heart. Proc Natl Acad Sci USA 2000;97:3678–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Napoli C, De Nigris F, Cicala C, Wallace JL, Caliendo G, Condorelli M, Santagada V, Cirino G.. Protease-activated receptor-2 activation improves efficiency of experimental ischemic preconditioning. Am J Physiol Heart Circ Physiol 2002;282:H2004–H2010. [DOI] [PubMed] [Google Scholar]
- 106. Jiang R, Zatta A, Kin H, Wang N, Reeves JG, Mykytenko J, Deneve J, Zhao ZQ, Guyton RA, Vinten-Johansen J.. PAR-2 activation at the time of reperfusion salvages myocardium via an ERK1/2 pathway in in vivo rat hearts. Am J Physiol Heart Circ Physiol 2007;293:H2845–H2852. [DOI] [PubMed] [Google Scholar]
- 107. Zhong B, Wang DH.. Protease-activated receptor 2-mediated protection of myocardial ischemia-reperfusion injury: role of transient receptor potential vanilloid receptors. Am J Physiol Regul Integr Comp Physiol 2009;297:R1681–R1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kolpakov MA, Rafiq K, Guo X, Hooshdaran B, Wang T, Vlasenko L, Bashkirova YV, Zhang X, Chen X, Iftikhar S, Libonati JR, Kunapuli SP, Sabri A.. Protease-activated receptor 4 deficiency offers cardioprotection after acute ischemia reperfusion injury. J Mol Cell Cardiol 2016;90:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Strande JL, Hsu A, Su J, Fu X, Gross GJ, Baker JE.. Inhibiting protease-activated receptor 4 limits myocardial ischemia/reperfusion injury in rat hearts by unmasking adenosine signaling. J Pharmacol Exp Ther 2008;324:1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zaman AK, Fujii S, Schneider DJ, Taatjes DJ, Lijnen HR, Sobel BE.. Deleterious effects of lack of cardiac PAI-1 after coronary occlusion in mice and their pathophysiologic determinants. Histochem Cell Biol 2007;128:135–145. [DOI] [PubMed] [Google Scholar]