Graphical Abstract
Graphical Abstract.
Keywords: Coronary circulation, Microvascular obstruction, Cardioprotection, Ischaemia, Reperfusion
Abstract
The coronary circulation is both culprit and victim of acute myocardial infarction. The rupture of an epicardial atherosclerotic plaque with superimposed thrombosis causes coronary occlusion, and this occlusion must be removed to induce reperfusion. However, ischaemia and reperfusion cause damage not only in cardiomyocytes but also in the coronary circulation, including microembolization of debris and release of soluble factors from the culprit lesion, impairment of endothelial integrity with subsequently increased permeability and oedema formation, platelet activation and leucocyte adherence, erythrocyte stasis, a shift from vasodilation to vasoconstriction, and ultimately structural damage to the capillaries with eventual no-reflow, microvascular obstruction (MVO), and intramyocardial haemorrhage (IMH). Therefore, the coronary circulation is a valid target for cardioprotection, beyond protection of the cardiomyocyte. Virtually all of the above deleterious endpoints have been demonstrated to be favourably influenced by one or the other mechanical or pharmacological cardioprotective intervention. However, no-reflow is still a serious complication of reperfused myocardial infarction and carries, independently from infarct size, an unfavourable prognosis. MVO and IMH can be diagnosed by modern imaging technologies, but still await an effective therapy. The current review provides an overview of strategies to protect the coronary circulation from acute myocardial ischaemia/reperfusion injury. This article is part of a Cardiovascular Research Spotlight Issue entitled ‘Cardioprotection Beyond the Cardiomyocyte’, and emerged as part of the discussions of the European Union (EU)-CARDIOPROTECTION Cooperation in Science and Technology (COST) Action, CA16225.
This article is part of the Spotlight Issue on Cardioprotection Beyond the Cardiomyocyte.
1. Introduction
Reperfusion is the only way to salvage ischaemic myocardium from infarction, but reperfusion per se also inflicts additional injury, such that the resulting myocardial infarct (MI) size is determined by both ischaemia- and reperfusion-induced injury.1–3 There is still an unmet medical need for adjunct cardioprotection on top of timely reperfusion.4,5 In type II myocardial infarction and in the absence of epicardial coronary artery occlusion, the distinction of ischaemia and reperfusion is less obvious, but there is still infarction and cardioprotection is needed.6 Numerous animal experiments have provided robust evidence that MI size can be reduced by mechanical or pharmacological interventions before (preconditioning), during (perconditioning), or after (postconditioning) myocardial ischaemia. However, the translation of cardioprotection to clinical practice has been largely disappointing so far, for many reasons, including lack of rigor and reproducibility in experimental studies, as well as conceptual and technical faults in clinical trial design.7–10 One important conceptual reason for failure of translation may relate to the focus of cardioprotection studies on the cardiomyocyte, and the neglect of other tissues in the heart, notably the coronary circulation.11
2. The coronary circulation in acute myocardial ischaemia/reperfusion injury
The coronary circulation is both culprit and victim of acute myocardial ischaemia/reperfusion injury (IRI), and as such a prime target for cardioprotection. Acute ST-segment elevation myocardial infarction (STEMI) is induced by rupture of an epicardial coronary atherosclerotic plaque with superimposed thrombosis, which occludes the epicardial coronary artery completely and renders the dependent perfusion territory ischaemic; residual blood flow to the perfusion territory then depends entirely on the coronary collateral circulation which varies interindividually and largely depends on its prior adaptation to pre-existing epicardial coronary atherosclerotic narrowing. More recent studies have emphasized the increasing importance of atherosclerotic plaque erosion rather than rupture, particularly in statin-treated patients and particularly for the induction of non-STEMI.12 The epicardial coronary artery with its culprit lesion is also the target of interventional therapy by dilatation/stenting with or without thrombectomy. Such percutaneous coronary intervention (PCI) may not only restore epicardial coronary blood flow but at the same tissue dislodge atherothrombotic debris from the culprit lesion and embolize it into the coronary microcirculation.13
The coronary circulation distal to the epicardial atherosclerotic culprit lesion is not virgin, but characterized by endothelial dysfunction through the typical risk factors (aging, hypertension, hyperlipidaemia, diabetes etc.) which characterize atherosclerosis in general.11 More specifically, the coronary circulation distal to epicardial stenoses remodels, with atrophy of the vascular wall in larger coronary arteries and hypertrophy of the vascular wall in smaller arteries and arterioles,14,15 and its autoregulatory vasomotor responses are attenuated.15 The coronary microcirculation as such is not only exposed to atherothrombotic debris, which is dislodged from the epicardial culprit lesion and causes microembolization, microinfarcts, and a subsequent inflammatory response,16–18 but also the release of vasoconstrictor, pro-thrombotic and pro-inflammatory soluble substances from the culprit lesion, notably serotonin, thromboxane A2, and TNFα.19,20 In consequence of coronary microembolization and in response to these soluble substances, coronary vasodilator reserve is severely impaired.18,21
3. Effects of acute myocardial ischaemia/reperfusion injury on the coronary vasculature
3.1 Endothelium, pericytes, and glycocalyx
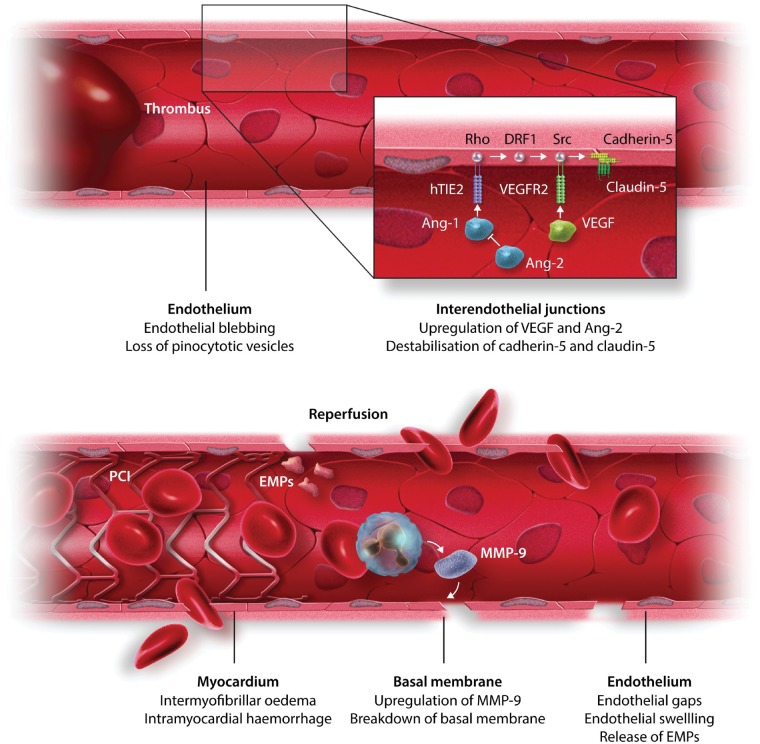
Coronary endothelial cells are relatively resistant to ischaemia and survive hypoxia in vitro for several days.22 However, in vivo, the interruption of antegrade pulsatile flow and shear stress induces swelling and blebbing of endothelial cells.23 The actual disruption of the endothelium and subsequent extravasation of cells after reperfusion are probably facilitated by destabilization of the cellular junctions. Reperfused endothelium experiences altered Ca2+ homeostasis, increased cytosolic calcium activates the endothelial contractile elements and their contraction promotes the formation of intercellular gaps which increase permeability to large molecules.24 Activated endothelial cells and platelets result in the expression of adhesion molecules and subsequent adhesion of platelets and platelet-leucocyte aggregates to the coronary microvasculature.25 Also, the release of cytokines impairs the stability of cell junctions and increases vascular permeability via activation of Src26 and dissociation of the VEGFR2/vascular endothelial (VE)-cadherin complex (Figure 1).27 NLRP3 inflammasome activation in endothelial cells may initiate caspase 1-mediated cell death.29 Endothelium-initiated inflammation together with pro-inflammatory effects of debris from cardiomyocyte necrosis result in recruitment of inflammatory cells and release of pro-inflammatory factors, including vascular endothelial growth factor (VEGF),30 matrix metalloproteases, thrombin, myeloperoxidase,31 and platelet activating factor.32 These factors, in turn, increase vascular permeability and result in myocardial oedema by different mechanisms, including activation of eNOS in caveolae by VEGF.33,34 Angiopoietin-1 and angiopoietin-like peptide 4 have protective effects via stablization of endothelial cell junctions.30,35
Figure 1.
Potential mechanisms underlying capillary damage following AMI. During thrombotic coronary occlusion and interruption of flow, the endothelium shows morphological and functional changes, including swelling and blebbing and loss of endothelial junctions via release of angiopoietins and VEGF. Instantaneous opening of the coronary vessel by placement of a coronary stent induces additional damage leading to endothelial gaps, extravasation of erythrocytes, and intramyocardial haemorrhage. Figure modified with permission from Betgem et al.28
Pericytes induce vasoconstriction of the cerebral microvasculature, thereby contributing to entrapment of red and white blood cells in areas of no-reflow in the post-ischaemic brain.36 Although pericytes are present in high numbers in the coronary microvasculature,37 their role in the heart remains unclear. In the acutely reperfused rat heart, capillary obstruction was associated with the presence of pericytes, with reduced capillary diameter, suggesting that cardiac pericytes may also constrict coronary capillaries and reduce microvascular blood flow after acute myocardial infarction (AMI). The pericyte relaxant adenosine increased capillary diameter, decreased capillary obstruction, and increased perfusion volume.38 Cardiac pericytes may therefore represent a novel therapeutic target for protecting the coronary microvasculature following AMI.
The glycocalyx is a matrix structure which covers endothelial cells and pericytes. The coronary glycocalyx is sensitive to acute myocardial IRI,39 and its shedding contributes to the development of oedema,40 and leucocyte,41 and platelet42 adherence. TNFα is involved in glycocalyx degradation,43 and nitric oxide (NO) is protective.44 Thus, the glycocalyx may be a novel target for coronary vascular cardioprotection.
3.2 Oedema
Intracellular water accounts for more than 75% of myocardial water content, and reperfusion induces cardiomyocyte swelling immediately upon coronary reflow.45 Osmotic swelling contributes to sarcolemmal rupture and cell death, and hyperosmotic reperfusion can reduce myocardial oedema and MI size.46,47 In surviving cardiomyocytes, intracellular oedema is reversed by restoration of activation of ion pumps, notably sarcolemmal Na+/K+-ATPase.48 During ischaemia, the accumulation of metabolites increases interstitial osmolality, and the exposure to normo-osmotic blood at reperfusion induces immediate interstitial oedema. Interstitial oedema then diminishes as catabolite washout eliminates the osmotic gradient between the intravascular and the interstitial compartments,45 but there is a second wave of oedema caused by increased vascular permeability. Serial cardiovascular magnetic resonance (CMR) imaging studies have revealed such bimodal pattern of myocardial oedema after reperfusion in pigs and humans.49,50
3.3 Platelets
Platelets contribute to many processes relevant to acute IRI, including vascular integrity, lymphangiogenesis and tissue regeneration.51 After AMI, platelets play a biphasic role, initially recruiting neutrophils and amplifying the inflammatory response, and later releasing factors that actively support the resolution of inflammation.51 Upon activation, platelets release a variety of nucleotides, neurotransmitters, and over 300 proteins from secretory α-granules, dense granules, and lysosomal granules.52 Activated platelets also release microvesicles and exosomes which contain miRNA and lipids. The released substances are involved in platelet aggregation and coagulation. Some, such as sphingosine-1-phosphate (S1P),53–56 and platelet-activating factor,57,58 can exert direct cardioprotective effects on cardiomyocytes, but their protective effect depends on the actual concentrations and circumstances. Other factors can affect the coronary microvasculature, including serotonin, growth factors, cytokines and chemokines. Intriguingly, both anti- and pro-angiogenic factors (e.g. VEGF and SDF1α) can be released from platelet α-granules under different circumstances.59
Endothelial cells produce prostacyclins, NO and adenosine that inhibit platelet aggregation and adhesion. When activated, however, they express adhesion molecules and release von Willebrand factor, which activates platelets, causing them to form a plug. Conversely, activated platelets release vasoconstrictive compounds such as ADP, serotonin and thromboxane A2.60
Studies in isolated, perfused hearts have shown that platelets can be cardioprotective. The barrier function of coronary microvessels in the isolated perfused rat heart is improved after perfusion of platelet-rich plasma.61 Myocardial injury measured by cardiac enzymes and function in rat hearts subject to IRI was decreased by perfusion with either washed rat platelets or with the supernatant of activated rat platelets.62 The precise mechanism is unclear but may involve the release of S1P, adenosine, serotonin, or thromboxane A2.62 Perfusion of guinea pig hearts with constituents released by platelets helped to maintain the integrity of the coronary endothelium after IRI.63 The specific action of platelets in a given situation appears to depend on their state of activation.57,58,64 In rat hearts subjected to acute myocardial IRI, perfusion with platelets from AMI patients increased coronary resistance and myocardial injury when compared with perfusion with platelets from healthy volunteers.65 Such injury was prevented by the P2Y12 receptor antagonist cangrelor and the glycoprotein IIb/IIIa receptor blocker abciximab, suggesting that early inhibition of platelet activation may be cardioprotective.65
Given the complex, multi-factorial role of platelets, in vivo studies provide more clinically relevant information than in vitro studies, which are more reductionist and mechanistic in nature.66 Pigs were administered the platelet integrin αIIbβ3 receptor antagonist lamifiban prior to reperfusion after 55 min myocardial ischaemia. Lamifiban inhibited platelet aggregation and had a potent antithrombotic effect at the culprit lesion as expected, but did not reduce microvascular platelet accumulation or MI size.67 Similarly, in a mouse in vivo model of 30 min left coronary artery ligation followed by 24 h reperfusion, MI size was not affected by inhibition of platelet adhesion or aggregation, but reduced by inhibition of platelet activation along with improved perfusion, suggesting a possible effect on the microvasculature.68 Ultimately, even if activated platelets do release substances with protective effects on the endothelium, treatment of AMI patients will always include platelet inhibition, given the importance of their primary pro-thrombotic activity.65 To complicate matters even further, experimental data suggest that P2Y12 receptor inhibition using ticagrelor or cangrelor at the onset of reperfusion can itself reduce MI size,69 but whether this cardioprotective effect is mediated on the coronary vasculature or the cardiomyocyte is not clear.
4. Microvascular obstruction as a target for cardioprotection
Microvascular obstruction (MVO) following AMI is primarily a reperfusion phenomenon, which manifests clinically as coronary no-reflow in the infarct-related artery following primary PCI, and has been defined as the ‘inability to reperfuse a previously ischaemic region’.70 The pathophysiology underlying MVO is complex and multifactorial and has been attributed to: endothelial swelling and blebbing obstructing capillary blood flow, cardiomyocyte swelling compressing capillaries, platelet activation and aggregation, capillary obstruction due to red and white blood cell stasis, and coronary microembolization (reviewed in Ref.11). Severe MVO can result in capillary destruction and extravasation of red blood cells into the myocardium—termed intramyocardial haemorrhage (IMH), a condition which portends to worse prognosis following AMI. MVO following reperfusion of sustained myocardial ischaemia is always associated with infarction.71 The MVO and no-reflow areas are always contained within the infarcted tissue and not seen in the risk area which has remained viable.72 Also, there is infarction without MVO/no-reflow. These observations would put MVO as a consequence of myocardial infarction rather than its cause. However, MI size is robustly identified and quantified no earlier than after several hours of reperfusion, for technical reasons.71 Therefore, any early and transient MVO which may have contributed to infarct extension may have gone unnoticed. In response to cardioprotective interventions, effects on MI size and on MVO can be dissociated. In pigs, local and remote ischaemic conditioning procedures reduce MI size but not areas of no-reflow.73 Conversely, delayed hypothermia during reperfusion only reduces no-reflow but not MI size.74 Mechanistically, the same factors which cause cardiomyocyte death (necrosis, apoptosis, etc.) can also cause death of endothelial and vascular smooth muscle cells, i.e. hypoxia per se with re-oxygenation and consequent enhanced formation of reactive oxygen species (ROS). Intracellular and interstitial oedema, intravascular platelet and erythrocyte aggregates and early inflammatory responses contribute to MVO and cardiomyocyte death, but their contribution to MVO and cardiomyocyte death may differ. At this point, the causality between MVO and cardiomyocyte cell death remains unresolved, and the two phenomena must be considered as separate but intimately related, possibly because of their identical underlying mechanisms. MVO and coronary no-reflow occur frequently even after prompt epicardial recanalization of the infarct-related artery,75 and strongly impact on patient prognosis.76 Several therapies for preventing MVO, which have been successfully tested in experimental models of AMI, have failed in the translation to AMI patients.10,11
4.1 Invasive and non-invasive methods for assessment of coronary no-reflow and MVO
The thrombolysis in myocardial infarction (TIMI) score grades blood flow in epicardial vessels.77 However, MVO may occur in nearly 50% of patients with TIMI flow 3. Angiographic methods characterizing dye penetration within the myocardium, the myocardial blush grade (MBG) and TIMI myocardial perfusion grade, have been developed to shift attention to coronary microcirculatory flow.78,79 The gold standard for assessing coronary microvascular function is coronary blood flow by thermodilution or flow velocity by Doppler which in combination with quantitative coronary angiography of epicardial coronary arteries also provides volumetric coronary blood flow.80 MVO is characterized by systolic retrograde and diminished anterograde flow, and by rapid deceleration of diastolic flow. Such impaired coronary flow velocity pattern following primary PCI is associated with future cardiovascular events.81 The index of microvascular resistance assessed by thermodilution provides a more reproducible assessment of the coronary microcirculation and predicts acute microvascular injury, left ventricular functional recovery, and clinical outcomes after STEMI.82,83
Incomplete ST-segment resolution (STR) has been related to MVO and worse clinical outcome after primary PCI.84 A consensus is still lacking over which electrocardiogram (ECG) leads should be analysed, the optimal timing of ECG analysis, and whether standard ECG or continuous ECG monitoring is preferable.85 Myocardial contrast echocardiography (MCE) utilizes ultrasound to visualize contrast microbubbles with a rheology similar to that of erythrocytes, and lack of contrast opacification due to MVO predicts poor functional recovery after STEMI.86 MCE, however, is limited by moderate spatial resolution and operator dependency. CMR allows multi-slice imaging with high-tissue contrast and high spatial resolution, enabling accurate quantification, and localization of MVO and MI size. CMR-defined MVO correlates with angiographic and invasive indices of MVO87 and is associated with worse outcome.88 MVO is diagnosed as: (i) lack of gadolinium uptake on first pass perfusion (<1 min of contrast administration), (ii) lack of early gadolinium enhancement (<2–3 min of contrast administration), and (iii) lack of late gadolinium enhancement (LGE) (10–15 min after contrast administration).89 Although first pass perfusion and early contrast gadolinium enhancement detect the presence of MVO with greater sensitivity than LGE, the presence of MVO on LGE is a stronger predictor of clinical outcomes following STEMI.89
5. Intramyocardial haemorrhage as a target for cardioprotection
IMH can develop after reperfusion of an infarct-related coronary artery. In dog hearts with 50 to 60 min coronary occlusion and reperfusion IMH develops in the central core of the infarct; ultrastructurally, the endothelium is interrupted at several locations.90,91 In patients, IMH was first observed at autopsy after lytic therapy of AMI.92 IMH is not germane to thrombolysis but frequently observed also after mechanical reperfusion and associated with unfavourable clinical outcome.93 This relation with adverse clinical outcome is even stronger than that of MI size or MVO.94 IMH is associated with larger MI size, longer treatment delay and the use of glycoprotein IIb/IIIa inhibitors.95 IMH is not only a bystander phenomenon; extravasation of erythrocytes, leucocytes and finally iron deposition further increase myocardial damage via a sustained inflammatory reaction.96,97 Without reperfusion, IMH will not occur as shown both in experimental models,98 and at autopsy of patients with non-reperfused AMI.99 In an ex vivo reperfusion rat model, the endothelial barrier function for microspheres of 0.1 µm diameter was lost in hearts exposed to initial 30 min ischaemia followed by 60 min reperfusion, whereas the barrier function remained intact after 30 min ischaemia without reperfusion, along with better preservation of endothelial cellular junctions and less endothelial cell damage.100 Given this sequence of events, a therapeutic window apparently exists to prevent microvascular damage and subsequent IMH upon reperfusion.
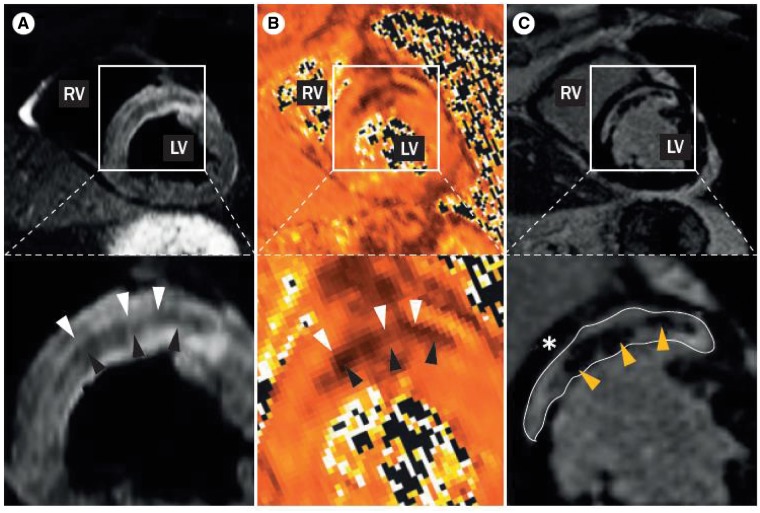
The first large series of CMR-scanning acutely after STEMI demonstrated specific changes in the infarct core in up to 50% of patients treated with primary PCI.88 Using LGE, many patients displayed infarct areas completely devoid of contrast.88 Subsequently, contrast-free sequences were introduced to specifically detect IMH.101,102 The degradation of erythrocytes and release of oxyhaemoglobin, de-oxyhaemoglobin, and methaemoglobin change the CMR tissue characteristics, as reflected by a relative decrease in relaxation time and thus relative signal attenuation within the infarct zone. Iron deposition in the form of ferritin and hemosiderin also induces signal attenuation (Figure 2). T2* shows the lowest increase upon oedema and the highest relative decrease upon haemorrhage and thus theoretically is the most accurate sequence to detect IMH.96 Whether or not CMR-defined MVO and IMH are separate entities is still debated. In a combined patient and pig study, there was a very large overlap between LGE detected MVO and T2-detected IMH. These areas were confined to the infarct core and displayed massive haemorrhage and complete microvascular destruction. Actual MVO was only observed in the infarct border zone.103
Figure 2.
Intramyocardial haemorrhage following AMI on cardiac MRI. (A) On T2-weighted images relaxation times and thus signal strength increase due to myocardial oedema formation after AMI (white arrow heads). In case of IMH, haemoglobin degradation products lead to a relative decrease in relaxation time, and thus a relative signal attenuation within the MI zone (black arrow heads). (B) On T2* images a relatively lower increase is observed with myocardial oedema (white arrow heads), and a relative higher decrease is observed upon IMH (black arrow heads), providing a stronger signal separation when compared with T2. (C) On LGE images the hypointense core indicates that no gadolinium entered the infarct core (yellow arrow heads). Overall infarct area is indicated by the hyperintense signal of the gadolinium that is retained within the tissue (white line). Note the large overlap between MVO as assessed by LGE and IMH as assessed by T2 and T2*. Figure modified with permission from Betgem et al.28
6. Coronary collateral angiogenesis
Brief episodes of ischaemia and reperfusion induced by ischaemic preconditioning (IPC) enable the preservation of endothelial function of coronary arterioles following acute myocardial IRI.104 Coronary endothelial function is sensitive to acute myocardial IRI, in that the vasodilatory action of thrombin under normal conditions is reversed to a vasoconstrictive effect following IRI,105 and this original observation by Ku has been confirmed by many groups.106,107 A well-developed coronary collateral circulation protects against lethal acute myocardial IRI by maintaining perfusion to the area at risk. Apparently, similar underlying mechanisms are shared by both IPC of cardiomyocytes and coronary collateral growth. Activation of hypoxia-inducible factor (HIF) ap dissecting whether the cardioprotective effects of ischaemic ears critical for IPC,108 and HIF-dependent genes are required for coronary collateral growth in a model of episodic myocardial ischaemia.109,110 Mitochondrial function also appears to be critical for both IPC,111 and for coronary collateral growth.112 Collateral angiogenesis cannot be recruited acutely for cardioprotection but is important for the healing and remodelling following acute myocardial infarction.113,114
7. Targeting the coronary vasculature for cardioprotection
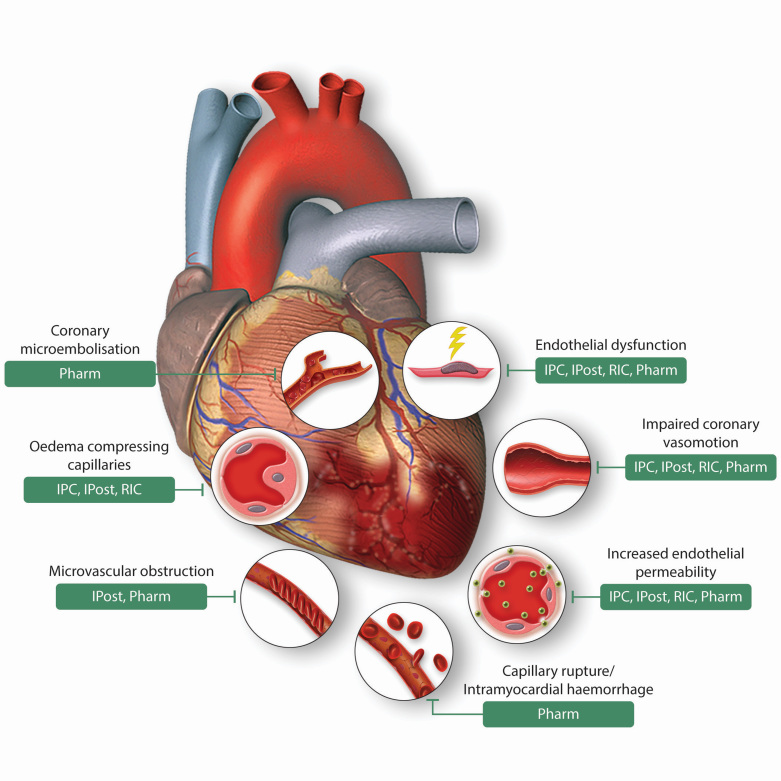
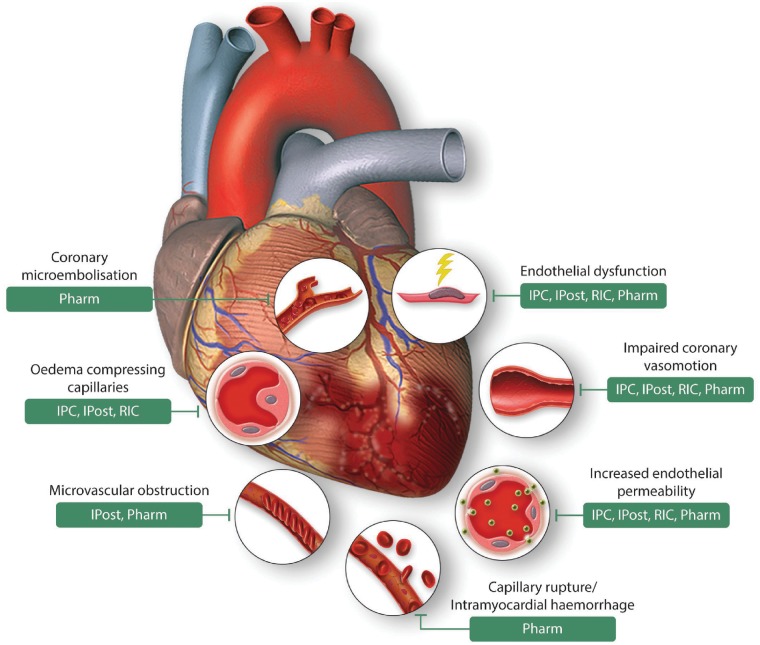
Interventions to protect the coronary vasculature following acute IRI sustained during AMI have been targeted to endothelial dysfunction, loss of endothelial integrity, microembolization, impaired vasomotor function, cardiomyocyte and endothelial swelling compressing capillaries, and capillary rupture with IMH (Figure 3).
Figure 3.
Effects of acute myocardial ischaemia/reperfusion injury on the coronary vasculature, and therapeutic vascular targets for cardioprotection. This scheme depicts the diverse consequences of acute myocardial ischaemia/reperfusion injury on the coronary vasculature following acute myocardial infarction, and highlights the vascular targets of endogenous cardioprotective strategies (IPC, ischaemic preconditioning, IPost, ischaemic postconditioning, and RIC, remote ischaemic conditioning) and Pharmacological agents (Pharm). Figure modified with permission from Heusch et al.11
The heart can be protected from cell death by different endogenous cardioprotective strategies, collectively termed ‘ischaemic conditioning’ [reviewed in Ref.115] and comprising the application of one or more brief cycles of non-lethal ischaemia and reperfusion to the heart itself, either prior to the lethal ischaemic episode (IPC),116 or at the onset of reperfusion (ischaemic postconditioning (IPost).117 Such cardioprotective stimulus can also be applied to an organ or tissue away from the heart [remote ischaemic conditioning (RIC)],118–122 either prior to [remote ischaemic preconditioning (RIPC)],123 or during the lethal ischaemic episode [remote ischaemic perconditioning (RIPerC)],124 or at the onset of reperfusion [remote ischaemic postconditioning (RIPost)].125 The majority of experimental and clinical studies have focused on the cardioprotective effects of ischaemic conditioning on cardiomyocytes and neglected the coronary vasculature. However, dissecting whether the cardioprotective effects of ischaemic conditioning protects the coronary vasculature independently of cardiomyocytes is challenging, given the intimate and potentially causal relationship between damage to the coronary vasculature and cardiomyocyte death following AMI.71
7.1 Protecting the coronary vasculature with IPC
IPC, in addition to reducing MI size, can protect the coronary vasculature, as evidenced by less endothelial damage,126 increased flow-mediated dilator response to vasodilators such as adenosine and nitric oxide or a reactive hyperaemia stimulus,104,127–130 less neutrophil adherence,127 and improved endothelial integrity.131 Mechanisms implicated in IPC include adenosine,132,133 KATP channel opening,132,134 signalling ROS,135 bradykinin B1 receptor activation,136 prostaglandin E2,137 NO,138 attenuated formation of detrimental ROS,139 reduced endothelin-1,140 enhanced eNOS function,141 and preservation of endothelial tight junctions.131 However, some studies failed to show beneficial effects with IPC on coronary no-reflow73,142 or coronary vasomotor response.143 The interaction of coronary microembolization with ischaemic conditioning is complex.13 Prior coronary microembolization does not induce IPC,13 and conversely IPC does not protect from coronary microembolization.144 Coronary microembolization induces however delayed protection from infarction through upregulation of TNFα.145
In patients with pre-infarction angina (a clinical example of IPC)146,147 reperfusion,148 coronary microvascular reflow and flow reserve were improved following AMI, suggesting coronary vascular protection with endogenous IPC by pre-infarct angina.149 Whether or not pre-infarction angina is a form of IPC is still under debate, and whether or not pre-infarction angina is protective under all circumstances is questionable, given the phenomenon of hyperconditioning.150 In any event, the need to apply the protective stimulus prior to the lethal ischaemic insult has prevented the clinical application of IPC in AMI patients in whom the onset of acute myocardial ischaemia cannot be anticipated.
7.2 Protecting the coronary vasculature with IPost
IPost can be applied at the onset of reperfusion, making its use in STEMI patients at the time of primary PCI possible. In the first description of MI-limitation by IPost,117 less myocardial oedema, reduced neutrophil adherence and decreased endothelial P-selectin expression, and improved vasodilator response to acetylcholine were observed. In pigs, smaller MI size, less MVO, improved endothelial function, and preserved coronary blood flow were observed after 2 h of reperfusion with IPost.151 A more recent study reported less oedema and MVO, but no reduction in MI size with IPost and RIC in a closed-chest pig infarction model.152 Other studies failed to show any beneficial effects of IPost on MVO73,153,154; one of these studies also found no reduction in MI size with IPost,153 but the others did demonstrate a smaller MI size with IPost.73,154 The dissociation between the beneficial effects of IPost on MVO and MI size are difficult to interpret at this time. Concomitant IPost and coronary microembolization, as probably occurs during further manipulation of the culprit lesion just after established reperfusion, has been shown to not impair protection by IPost.155
In the clinical setting, the beneficial effects of IPost on MVO appeared to mirror its MI-limiting effect.156 Reduction of MI size went along with limitation of MVO by 50% with IPost (both by CMR).156 In primary PCI-treated STEMI patients less coronary no-reflow with IPost was reflected by improved TIMI grade, STR, MBG, and corrected TIMI frame count.157 Also, IPost reduced MI size, and improved coronary blood flow and endothelium-dependent vasodilator function following STEMI.158 However, other clinical studies have failed to demonstrate an effect of IPost on MVO, but these studies also showed no effect of IPost on MI size.153,159 Some studies have even reported detrimental effects of IPost with larger MI size, but in these studies there was no detrimental effect on coronary microvascular function.160,161
7.3 Protecting the coronary vasculature with limb RIC
IPost requires further manipulation of the culprit coronary lesion, thereby limiting its clinical application. In contrast, RIC can be induced non-invasively by one or more cycles of brief non-lethal ischaemia and reperfusion to the limb.162 In human volunteers, serial inflations and deflations of a pneumatic cuff on the upper arm improved post-ischaemic endothelial function (as measured by increased blood flow response to acetylcholine) in the contralateral arm.162 Using the same model, limb RIC induced an early and a delayed vasculoprotective effect 24–48 h following the stimulus in healthy volunteers and in patients with atherosclerosis, which was blocked by the KATP channel blocker glibenclamide,163 required a neural pathway, which was blocked by pharmacological ganglionic blockade164 and was effective even when limb RIC was performed during the acute forearm IRI. An endothelial-protective effect from limb RIC was also present with daily limb RIC for 7 days,165 and still present 8 days following the protective stimulus,166 suggesting that a chronic daily limb RIC stimulus may be able to extend the window of vascular protection. Long-term nitroglycerine and limb RIC each separately reduced MI size in rats and attenuated the endothelial dysfunction from forearm ischaemia/reperfusion in healthy volunteers, but in combination abrogated any protection both in the heart and in the peripheral vasculature.167
Coronary vascular resistance was reduced and coronary blood flow improved with limb RIC in pigs at baseline and following acute myocardial IRI, and this effect was blocked by KATP channel blockade with glibenclamide but not by femoral nerve transection.168 In healthy human volunteers, limb RIC increased coronary flow velocity (by Doppler), suggesting a hyperaemic response with RIC.169 In patients undergoing PCI for stable coronary artery disease (CAD), limb RIC reduced periprocedural myocardial injury and rapidly increased distal coronary occlusive pressure, reflecting improved coronary collateral blood flow.170 Also in patients undergoing PCI for stable CAD, RIC improved coronary vasomotor responses to acetylcholine, reflecting better endothelial function.171,172 However, several clinical studies have reported reductions in MI size with limb RIC in STEMI patients treated by primary PCI, but have not found any beneficial effects on coronary no-reflow or MVO,159,173 suggesting that the cardioprotective effects of limb RIC in STEMI patients may be targeted towards ischaemic cardiomyocytes rather than the coronary vasculature.
7.4 Pharmacological strategies for protecting the coronary vasculature
Many pharmacological agents have been tested for their protective effects on the coronary vasculature, and only an overview is provided here. A number of drugs are currently given in the cardiac characterization laboratory to treat coronary no-reflow in STEMI patients following PCI, and these include nitrates, calcium channel blockers, and adenosine. Although these drugs can induce coronary vasodilation and in some case reduce MVO, these interventions do not appear to improve clinical outcomes following primary PCI.174–176 Most pharmacological agents used to induce coronary vascular protection also have protective effects on the cardiomyocyte, i.e. adenosine, NO donors, calcium antagonists, and P2Y12 inhibitors, making it difficult to separate vascular from cardiomyocyte protection. Some novel approaches have been tried to reduce coronary no-reflow and prevent MVO in experimental studies.9
Administration of angiopoietin-like peptide 4 at reperfusion to target the endothelial gap-junction VE-cadherin complex and preserve coronary endothelial integrity following acute myocardial IRI reduced MI size, decreased myocardial oedema, and prevented MVO and IMH.29 Opening of the mitochondrial permeability transition pore (MPTP) during reperfusion is a critical determinant of cell death from acute IRI, and its inhibition at reperfusion using cyclosporine-A (CSA) reduced MI size in small animal AMI models,177,178 although in large animals the effect of CSA has been mixed.179–181 CSA reduced MI size in an initial clinical study of primary PCI-treated STEMI patients,182 but failed to improve clinical outcomes in two subsequent large clinical studies.183,184 In one pig study, CSA reduced both MI size and MVO154; however, whether this was due to a direct vasculoprotective effect of CSA or occurred secondary to myocardial salvage is not clear. Nitroglycerine can induce a preconditioning-like protection of the coronary vasculature, the peripheral vasculature and the myocardium,147,167 and its mechanisms are still not fully elucidated, may depend on dose and duration of administration and may include hitherto unrecognized effects on the MPTP.185
Therapeutic hypothermia limits MI size in experimental IRI studies when initiated during ischaemia, whereas clinical studies using invasive interventions to achieve hypothermia have had limited success primarily due to logistical issues. Hypothermia in rabbit hearts reduced coronary no-reflow following acute IRI, when delayed into reperfusion, even when there was no MI limiting effect,74 raising the possibility for an extended window for vascular protection following AMI. Mild hypothermia using a non-invasive ThermoSuit System initiated during ischaemia reduced MI size and prevented coronary no-reflow in rabbit and rat models of acute myocardial IRI186; whether or not such protection would be effective if applied at the onset of reperfusion needs to be tested.
8. Effect of comorbidities and co-medications on coronary vascular protection
Comorbidities and co-medications can confound cardioprotection elicited by ischaemic conditioning strategies.187 In pigs with acute IRI, IPost improved endothelial function and reduced MVO in healthy animals, but failed to do so in the presence of hypercholesterolaemia.151 The abrogation of IPost-induced cardioprotection was attributed to detrimental effects of hypercholesterolaemia on NOS levels. In another study, IPC provided significant microvascular protection in the skeletal muscle from prolonged IRI in normal, but not in diabetic rats.188 In young men, flow-mediated dilation (FMD) decreased significantly after IRI without but not with prior IPC; such protection by IPC was attenuated in elderly patients.189 In smokers, the IPC-induced increase in forearm blood flow response to acetylcholine seen in healthy volunteers was blunted, while the responses to sodium nitroprusside before and after the IPC stimulus were similar.190 In contrast to age and smoking, neither hypertension,191 nor reduced left ventricular ejection fraction192 affected the protective response of RIC on FMD,191 or coronary flow reserve (by transthoracic Doppler).192
Of note, in most studies on comorbidities animals are untreated. Acute rosuvastatin prevented the development of IRI-induced conduit artery endothelial dysfunction.193 In contrast, chronic rosuvastatin did not prevent the development of IRI-induced endothelial dysfunction.194 The anti-diabetic sulfonylurea glibenclamide abolished RIC- and IPost- induced protection on forearm endothelial function in humans during acute IRI.163,195 On the other hand, re-establishment of normoglycaemia by islet cell transplantation restored the cardioprotection, as reflected by reduced infarct size, from IPost which had been lost in diabetes.196 The RIC-induced prevention of FMD impairment following IRI was abrogated by cyclooxygenase (COX) 2 inhibition.197 Non-selective COX inhibition with aspirin 325 mg and ibuprofen or specific COX-2 inhibition with celecoxib inhibited the protective effects of rosuvastatin in the setting of IRI. In contrast, low dose aspirin (81 mg daily)—as given for the prevention on coronary artery disease—did not have such inhibitory effects.198 Often, low dose aspirin is combined with P2Y12-inhibition: clopidogrel given 24 h prior to an episode of IRI limited the adverse effects of ischaemia on endothelial function.199 While acute treatment with NO donors might protect endothelial function, such protection might be lost with the development of nitrate tolerance, and nitrate tolerance may also interfere with the vascular protection by RIC.167 In contrast, inhibition of phosphodiesterase 5 with sildenafil provided sustained protection of the endothelium from adverse IRI effects on vascular function.200
In summary, while there appears to be an effect of comorbidities and co-treatments in peripheral vascular beds, almost nothing is known on their interactions on cardioprotective interventions in the coronary circulation.
9. Future perspectives
MVO and no-reflow are serious consequences of reperfused AMI which carry an adverse prognosis. As such these phenomena require attention. Currently, the causal relationship between cardiomyocyte and coronary microvascular injury is not clear. Likewise, it is not clear to what extent protective interventions target the cardiomyocyte, the coronary circulation, or both. Clearly, however, there is a need for protection of the coronary circulation beyond infarct size reduction. At this point, there is no intervention or substance which would specifically protect the coronary circulation from IRI. However, the development of specific or additive protective strategies for the coronary circulation is an unmet medical need. Protection is needed from enhanced permeability, enhanced platelet and leucocyte adherence and transmigration, impaired vasomotion, capillary obstruction by erythrocytes, platelets and leucocytes, and ultimately capillary destruction and haemorrhage. Thus, all structural elements of the coronary vascular wall from glycocalyx to endothelium to smooth muscle and adventitia need protection. At this point, the most promising protective substance/molecule to achieve such multi-faceted protection appears to be angiopoietin-like peptide 4.29
Conflict of interest: P.F. is the founder and CEO of Pharmahungary, a Group of R&D companies. All other authors have no relevant conflict of interest.
Funding
This work was supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre [to S.M.D. and D.J.H.]; British Heart Foundation (FS/10/039/28270 to D.J.H.); Duke-National University Singapore Medical School [to D.J.H.]; Singapore Ministry of Health’s National Medical Research Council under its Clinician Scientist-Senior Investigator scheme [NMRC/CSA-SI/0011/2017 to D.J.H.] and Collaborative Centre Grant scheme [NMRC/CGAug16C006 to D.J.H.); Singapore Ministry of Education Academic Research Fund Tier 2 [MOE2016-T2-2-021 to D.J.H.]; German Research Foundation [He 1320/18-3 and SFB 1116 B8 to G.H.] and [SFB/CRC 1213 B05 to R.S.]; National Research, Development and Innovation Office of Hungary [NVKP_16-1-2016-0017; OTKA KH 125570; OTKA 115378]; the Higher Education Institutional Excellence Programme of the Ministry of Human Capacities in Hungary, within the framework of the Therapeutic Development thematic programme of the Semmelweis University [to P.F.]; the Instituto de Salud Carlos III, CIBERCV-Instituto de Salud Carlos III, Spain [CB16/11/00479, co-funded with European Regional Development Fund-FEDER contribution to D.G.D.]; and PIE/2013-00047 and PI 17/1397 [to D.G.D.]. This article is based upon work from COST Action EU-CARDIOPROTECTION CA16225 supported by COST (European Cooperation in Science and Technology).
References
- 1. Yellon DM, Hausenloy DJ.. Myocardial reperfusion injury. N Engl J Med 2007;357:1121–1135. [DOI] [PubMed] [Google Scholar]
- 2. Ibanez B, Heusch G, Ovize M, Van de Werf F.. Evolving therapies for myocardial ischemia/reperfusion injury. J Am Coll Cardiol 2015;65:1454–1471. [DOI] [PubMed] [Google Scholar]
- 3. Kleinbongard P, Amanakis G, Skyschally A, Heusch G.. Reflection of cardioprotection by remote ischemic perconditioning in attenuated ST-segment elevation during ongoing coronary occlusion in pigs: evidence for cardioprotection from ischemic injury. Circ Res 2018;122:1102–1108. [DOI] [PubMed] [Google Scholar]
- 4. Hausenloy DJ, Botker HE, Engstrom T, Erlinge D, Heusch G, Ibanez B, Kloner RA, Ovize M, Yellon DM, Garcia-Dorado D.. Targeting reperfusion injury in patients with ST-segment elevation myocardial infarction: trials and tribulations. Eur Heart J 2017;38:935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heusch G, Gersh BJ.. The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: a continual challenge. Eur Heart J 2017;38:774–784. [DOI] [PubMed] [Google Scholar]
- 6. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD.. Fourth universal definition of myocardial infarction (2018). Eur Heart J 2018; doi: 10.1093/eurheartj/ehy462 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 7. Hausenloy DJ, Erik BH, Condorelli G, Ferdinandy P, Garcia-Dorado D, Heusch G, Lecour S, van Laake LW, Madonna R, Ruiz-Meana M, Schulz R, Sluijter JP, Yellon DM, Ovize M.. Translating cardioprotection for patient benefit: position paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res 2013;98:7–27. [DOI] [PubMed] [Google Scholar]
- 8. Bulluck H, Yellon DM, Hausenloy DJ.. Reducing myocardial infarct size: challenges and future opportunities. Heart 2016;102:341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hausenloy DJ, Garcia-Dorado D, Botker HE, Davidson SM, Downey J, Engel FB, Jennings R, Lecour S, Leor J, Madonna R, Ovize M, Perrino C, Prunier F, Schulz R, Sluijter JPG, van Laake LW, Vinten-Johansen J, Yellon DM, Ytrehus K, Heusch G, Ferdinandy P.. Novel targets and future strategies for acute cardioprotection: position paper of the European Society of Cardiology Working Group on Cellular Biology of the Heart. Cardiovasc Res 2017;113:564–585. [DOI] [PubMed] [Google Scholar]
- 10. Heusch G. Critical issues for the translation of cardioprotection. Circ Res 2017;120:1477–1486. [DOI] [PubMed] [Google Scholar]
- 11. Heusch G. The coronary circulation as a target of cardioprotection. Circ Res 2016;118:1643–1658. [DOI] [PubMed] [Google Scholar]
- 12. Partida RA, Libby P, Crea F, Jang IK.. Plaque erosion: a new in vivo diagnosis and a potential major shift in the management of patients with acute coronary syndromes. Eur Heart J 2018;39:2070–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heusch G, Skyschally A, Kleinbongard P.. Coronary microembolization and microvascular dysfunction. Int J Cardiol 2018;258:17–23. [DOI] [PubMed] [Google Scholar]
- 14. Mills I, Fallon JT, Wrenn D, Sasken H, Gray W, Bier J, Levine D, Berman S, Gilson M, Gewirtz H.. Adaptive responses of coronary circulation and myocardium to chronic reduction in perfusion pressure and flow. Am J Physiol 1994;266:H447–H457. [DOI] [PubMed] [Google Scholar]
- 15. Sorop O, Merkus D, de Beer VJ, Houweling B, Pistea A, McFalls EO, Boomsma F, van Beusekom HM, van der Giessen WJ, VanBavel E, Duncker DJ.. Functional and structural adaptations of coronary microvessels distal to a chronic coronary artery stenosis. Circ Res 2008;102:795–803. [DOI] [PubMed] [Google Scholar]
- 16. Dorge H, Neumann T, Behrends M, Skyschally A, Schulz R, Kasper C, Erbel R, Heusch G.. Perfusion-contraction mismatch with coronary microvascular obstruction: role of inflammation. Am J Physiol Heart Circ Physiol 2000;279:H2587–H2592. [DOI] [PubMed] [Google Scholar]
- 17. Thielmann M, DöRge H, Martin C, Belosjorow S, Schwanke U, van de Sand A, Konietzka I, BüChert A, KrüGer A, Schulz R, Heusch G, Myocardial dysfunction with coronary microembolization: signal transduction through a sequence of nitric oxide, tumor necrosis factor-alpha, and sphingosine. Circ Res 2002;90:807–813. [DOI] [PubMed] [Google Scholar]
- 18. Herrmann J, Haude M, Lerman A, Schulz R, Volbracht L, Ge J, Schmermund A, Wieneke H, von BC, Eggebrecht H, Baumgart D, Heusch G, Erbel R.. Abnormal coronary flow velocity reserve after coronary intervention is associated with cardiac marker elevation. Circulation 2001;103:2339–2345. [DOI] [PubMed] [Google Scholar]
- 19. Leineweber K, Bose D, Vogelsang M, Haude M, Erbel R, Heusch G.. Intense vasoconstriction in response to aspirate from stented saphenous vein aortocoronary bypass grafts. J Am Coll Cardiol 2006;47:981–986. [DOI] [PubMed] [Google Scholar]
- 20. Kleinbongard P, Bose D, Baars T, Mohlenkamp S, Konorza T, Schoner S, Elter-Schulz M, Eggebrecht H, Degen H, Haude M, Levkau B, Schulz R, Erbel R, Heusch G.. Vasoconstrictor potential of coronary aspirate from patients undergoing stenting of saphenous vein aortocoronary bypass grafts and its pharmacological attenuation. Circ Res 2011;108:344–352. [DOI] [PubMed] [Google Scholar]
- 21. Skyschally A, Schulz R, Erbel R, Heusch G.. Reduced coronary and inotropic reserves with coronary microembolization. Am J Physiol Heart Circ Physiol 2002;282:H611–H614. [DOI] [PubMed] [Google Scholar]
- 22. Stempien-Otero A, Karsan A, Cornejo CJ, Xiang H, Eunson T, Morrison RS, Kay M, Winn R, Harlan J.. Mechanisms of hypoxia-induced endothelial cell death. Role of p53 in apoptosis. J Biol Chem 1999;274:8039–8045. [DOI] [PubMed] [Google Scholar]
- 23. Maxwell L, Gavin JB.. The role of post-ischaemic reperfusion in the development of microvascular incompetence and ultrastructural damage in the myocardium. Basic Res Cardiol 1991;86:544–553. [DOI] [PubMed] [Google Scholar]
- 24. Kasseckert SA, Schafer C, Kluger A, Gligorievski D, Tillmann J, Schluter KD, Noll T, Sauer H, Piper HM, Abdallah Y.. Stimulation of cGMP signalling protects coronary endothelium against reperfusion-induced intercellular gap formation. Cardiovasc Res 2009;83:381–387. [DOI] [PubMed] [Google Scholar]
- 25. Scotland RS, Cohen M, Foster P, Lovell M, Mathur A, Ahluwalia A, Hobbs AJ.. C-type natriuretic peptide inhibits leukocyte recruitment and platelet-leukocyte interactions via suppression of P-selectin expression. Proc Natl Acad Sci USA 2005;102:14452–14457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weis S, Shintani S, Weber A, Kirchmair R, Wood M, Cravens A, McSharry H, Iwakura A, Yoon YS, Himes N, Burstein D, Doukas J, Soll R, Losordo D, Cheresh D.. Src blockade stabilizes a Flk/cadherin complex, reducing edema and tissue injury following myocardial infarction. J Clin Invest 2004;113:885–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weis S, Cui J, Barnes L, Cheresh D.. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J Cell Biol 2004;167:223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Betgem RP, de Waard GA, Nijveldt R, Beek AM, Escaned J, van RN.. Intramyocardial haemorrhage after acute myocardial infarction. Nat Rev Cardiol 2015;12:156–167. [DOI] [PubMed] [Google Scholar]
- 29. Liu Y, Lian K, Zhang L, Wang R, Yi F, Gao C, Xin C, Zhu D, Li Y, Yan W, Xiong L, Gao E, Wang H, Tao L.. TXNIP mediates NLRP3 inflammasome activation in cardiac microvascular endothelial cells as a novel mechanism in myocardial ischemia/reperfusion injury. Basic Res Cardiol 2014;109:415. [DOI] [PubMed] [Google Scholar]
- 30. Galaup A, Gomez E, Souktani R, Durand M, Cazes A, Monnot C, Teillon J, Le JS, Bouleti C, Briois G, Philippe J, Pons S, Martin V, Assaly R, Bonnin P, Ratajczak P, Janin A, Thurston G, Valenzuela DM, Murphy AJ, Yancopoulos GD, Tissier R, Berdeaux A, Ghaleh B, Germain S.. Protection against myocardial infarction and no-reflow through preservation of vascular integrity by angiopoietin-like 4. Circulation 2012;125:140–149. [DOI] [PubMed] [Google Scholar]
- 31. Mollenhauer M, Friedrichs K, Lange M, Gesenberg J, Remane L, Kerkenpaß C, Krause J, Schneider J, Ravekes T, Maass M, Halbach M, Peinkofer G, Saric T, Mehrkens D, Adam M, Deuschl FG, Lau D, Geertz B, Manchanda K, Eschenhagen T, Kubala L, Rudolph TK, Wu Y, Tang WHW, Hazen SL, Baldus S, Klinke A, Rudolph V.. Myeloperoxidase mediates postischemic arrhythmogenic ventricular remodeling. Circ Res 2017;121:56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bitencourt CS, Bessi VL, Huynh DN, Menard L, Lefebvre JS, Levesque T, Hamdan L, Sohouhenou F, Faccioli LH, Borgeat P, Marleau S.. Cooperative role of endogenous leucotrienes and platelet-activating factor in ischaemia-reperfusion-mediated tissue injury. J Cell Mol Med 2013;17:1554–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weis SM, Cheresh DA.. Pathophysiological consequences of VEGF-induced vascular permeability. Nature 2005;437:497–504. [DOI] [PubMed] [Google Scholar]
- 34. Feng Y, Venema VJ, Venema RC, Tsai N, Behzadian MA, Caldwell RB.. VEGF-induced permeability increase is mediated by caveolae. Invest Ophthalmol Vis Sci 1999;40:157–167. [PubMed] [Google Scholar]
- 35. Lee SW, Won JY, Lee HY, Lee HJ, Youn SW, Lee JY, Cho CH, Cho HJ, Oh S, Chae IH, Kim HS.. Angiopoietin-1 protects heart against ischemia/reperfusion injury through VE-cadherin dephosphorylation and myocardiac integrin-β1/ERK/caspase-9 phosphorylation cascade. Mol Med 2011;17:1095–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O’Farrell FM, Buchan AM, Lauritzen M, Attwell D.. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014;508:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nees S, Weiss DR, Senftl A, Knott M, Forch S, Schnurr M, Weyrich P, Juchem G.. Isolation, bulk cultivation, and characterization of coronary microvascular pericytes: the second most frequent myocardial cell type in vitro. Am J Physiol Heart Circ Physiol 2012;302:H69–H84. [DOI] [PubMed] [Google Scholar]
- 38. O'Farrell FM, Mastitskaya S, Hammond-Haley M, Freitas F, Wah WR, Attwell D.. Capillary pericytes mediate coronary no-reflow after myocardial ischaemia. Elife 2017;6. pii:e29280. doi:10.7554/eLife.29280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Becker BF, Chappell D, Jacob M.. Endothelial glycocalyx and coronary vascular permeability: the fringe benefit. Basic Res Cardiol 2010;105:687–701. [DOI] [PubMed] [Google Scholar]
- 40. van den Berg BM, Vink H, Spaan JA.. The endothelial glycocalyx protects against myocardial edema. Circ Res 2003;92:592–594. [DOI] [PubMed] [Google Scholar]
- 41. Chappell D, Dorfler N, Jacob M, Rehm M, Welsch U, Conzen P, Becker BF.. Glycocalyx protection reduces leukocyte adhesion after ischemia/reperfusion. Shock 2010;34:133–139. [DOI] [PubMed] [Google Scholar]
- 42. Chappell D, Brettner F, Doerfler N, Jacob M, Rehm M, Bruegger D, Conzen P, Jacob B, Becker BF.. Protection of glycocalyx decreases platelet adhesion after ischaemia/reperfusion: an animal study. Eur J Anaesthesiol 2014;31:474–481. [DOI] [PubMed] [Google Scholar]
- 43. Chappell D, Hofmann-Kiefer K, Jacob M, Rehm M, Briegel J, Welsch U, Conzen P, Becker BF.. TNF-alpha induced shedding of the endothelial glycocalyx is prevented by hydrocortisone and antithrombin. Basic Res Cardiol 2009;104:78–89. [DOI] [PubMed] [Google Scholar]
- 44. Bruegger D, Rehm M, Jacob M, Chappell D, Stoeckelhuber M, Welsch U, Conzen P, Becker BF.. Exogenous nitric oxide requires an endothelial glycocalyx to prevent postischemic coronary vascular leak in guinea pig hearts. Crit Care 2008;12:R73.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Garcia-Dorado D, Andres-Villarreal M, Ruiz-Meana M, Inserte J, Barba I.. Myocardial edema: a translational view. J Mol Cell Cardiol 2012;52:931–939. [DOI] [PubMed] [Google Scholar]
- 46. Garcia-Dorado D, Theroux P, Munoz R, Alonso J, Elizaga J, Fernandez-Aviles F, Botas J, Solares J, Soriano J, Duran JM.. Favorable effects of hyperosmotic reperfusion on myocardial edema and infarct size. Am J Physiol 1992;262:H17–H22. [DOI] [PubMed] [Google Scholar]
- 47. Andres-Villarreal M, Barba I, Poncelas M, Inserte J, Rodriguez-Palomares J, Pineda V, Garcia-Dorado D.. Measuring water distribution in the heart: preventing edema reduces ischemia-reperfusion injury. J Am Heart Assoc 2016;5. pii:e003843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Inserte J, Garcia-Dorado D, Hernando V, Soler-Soler J.. Calpain-mediated impairment of Na+/K+-ATPase activity during early reperfusion contributes to cell death after myocardial ischemia. Circ Res 2005;97:465–473. [DOI] [PubMed] [Google Scholar]
- 49. Fernandez-Jimenez R, Sanchez-Gonzalez J, Aguero J, Garcia-Prieto J, Lopez-Martin GJ, Garcia-Ruiz JM, Molina-Iracheta A, Rossello X, Fernandez-Friera L, Pizarro G, Garcia-Alvarez A, Dall'armellina E, Macaya C, Choudhury RP, Fuster V., Ibanez B.. Myocardial edema after ischemia/reperfusion is not stable and follows a bimodal pattern: imaging and histological tissue characterization. J Am Coll Cardiol 2015;65:315–323. [DOI] [PubMed] [Google Scholar]
- 50. Fernandez-Jimenez R, Barreiro-Perez M, Martin-Garcia A, Sanchez-Gonzalez J, Aguero J, Galan-Arriola C, Garcia-Prieto J, Diaz-Pelaez E, Vara P, Martinez I, Zamarro I, Garde B, Sanz J, Fuster V, Sanchez PL, Ibanez B.. Dynamic edematous response of the human heart to myocardial infarction: implications for assessing myocardial area at risk and salvage. Circulation 2017;136:1288–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Walsh TG, Poole AW.. Do platelets promote cardiac recovery after myocardial infarction: roles beyond occlusive ischemic damage. Am J Physiol Heart Circ Physiol 2018;314:H1043–H1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pagel O, Walter E, Jurk K, Zahedi RP.. Taking the stock of granule cargo: platelet releasate proteomics. Platelets 2017;28:119–128. [DOI] [PubMed] [Google Scholar]
- 53. Theilmeier G, Schmidt C, Herrmann J, Keul P, SchäFers M, Herrgott I, Mersmann J, Larmann J, Hermann S, Stypmann J, Schober O, Hildebrand R, Schulz R, Heusch G, Haude M, von Wnuck LK, Herzog C, Schmitz M, Erbel R, Chun J, Levkau B.. High-density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation 2006;114:1403–1409. [DOI] [PubMed] [Google Scholar]
- 54. Keul P, van Borren MM, Ghanem A, Muller FU, Baartscheer A, Verkerk AO, Stumpel F, Schulte JS, Hamdani N, Linke WA, van LP, Matus M, Schmitz W, Stypmann J, Tiemann K, Ravesloot JH, Alewijnse AE, Hermann S, Spijkers LJ, Hiller KH, Herr D, Heusch G, Schafers M, Peters SL, Chun J, Levkau B.. Sphingosine-1-phosphate receptor 1 regulates cardiac function by modulating Ca2+ sensitivity and Na+/H+ exchange and mediates protection by ischemic preconditioning. J Am Heart Assoc 2016;5. pii:e003393. doi:10.1161/JAHA.116.003393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Karliner JS, Honbo N, Summers K, Gray MO, Goetzl EJ.. The lysophospholipids sphingosine-1-phosphate and lysophosphatidic acid enhance survival during hypoxia in neonatal rat cardiac myocytes. J Mol Cell Cardiol 2001;33:1713–1717. [DOI] [PubMed] [Google Scholar]
- 56. Zhang J, Honbo N, Goetzl EJ, Chatterjee K, Karliner JS, Gray MO.. Signals from type 1 sphingosine 1-phosphate receptors enhance adult mouse cardiac myocyte survival during hypoxia. Am J Physiol Heart Circ Physiol 2007;293:H3150–H3158. [DOI] [PubMed] [Google Scholar]
- 57. Penna C, Bassino E, Alloatti G.. Platelet activating factor: the good and the bad in the ischemic/reperfused heart. Exp Biol Med (Maywood) 2011;236:390–401. [DOI] [PubMed] [Google Scholar]
- 58. Russo I, Femmino S, Barale C, Tullio F, Geuna S, Cavalot F, Pagliaro P, Penna C.. Cardioprotective properties of human platelets are lost in uncontrolled diabetes mellitus: a study in isolated rat hearts. Front Physiol 2018;9:875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Battinelli EM, Markens BA, Italiano JE Jr.. Release of angiogenesis regulatory proteins from platelet alpha granules: modulation of physiologic and pathologic angiogenesis. Blood 2011;118:1359–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pearson PJ, Schaff HV, Vanhoutte PM.. Acute impairment of endothelium-dependent relaxations to aggregating platelets following reperfusion injury in canine coronary arteries. Circ Res 1990;67:385–393. [DOI] [PubMed] [Google Scholar]
- 61. McDonagh PF. Platelets reduce coronary microvascular permeability to macromolecules. Am J Physiol 1986;251:H581–H587. [DOI] [PubMed] [Google Scholar]
- 62. Yang BC, Virmani R, Nichols WW, Mehta JL.. Platelets protect against myocardial dysfunction and injury induced by ischemia and reperfusion in isolated rat hearts. Circ Res 1993;72:1181–1190. [DOI] [PubMed] [Google Scholar]
- 63. Heindl B, Zahler S, Welsch U, Becker BF.. Disparate effects of adhesion and degranulation of platelets on myocardial and coronary function in postischaemic hearts. Cardiovasc Res 1998;38:383–394. [DOI] [PubMed] [Google Scholar]
- 64. Mirabet M, Garcia-Dorado D, Inserte J, Barrabes JA, Lidon RM, Soriano B, Azevedo M, Padilla F, Agullo L, Ruiz-Meana M, Massaguer A, Pizcueta P, Soler-Soler J.. Platelets activated by transient coronary occlusion exacerbate ischemia-reperfusion injury in rat hearts. Am J Physiol Heart Circ Physiol 2002;283:H1134–H1141. [DOI] [PubMed] [Google Scholar]
- 65. Barrabes JA, Inserte J, Mirabet M, Quiroga A, Hernando V, Figueras J, Garcia-Dorado D.. Antagonism of P2Y12 or GPIIb/IIIa receptors reduces platelet-mediated myocardial injury after ischaemia and reperfusion in isolated rat hearts. Thromb Haemost 2010;104:128–135. [DOI] [PubMed] [Google Scholar]
- 66. Botker HE, Hausenloy D, Andreadou I, Antonucci S, Boengler K, Davidson SM, Deshwal S, Devaux Y, Di LF, Di SM, Efentakis P, Femmino S, Garcia-Dorado D, Giricz Z, Ibanez B, Iliodromitis E, Kaludercic N, Kleinbongard P, Neuhauser M, Ovize M, Pagliaro P, Rahbek-Schmidt M, Ruiz-Meana M, Schluter KD, Schulz R, Skyschally A, Wilder C, Yellon DM, Ferdinandy P, Heusch G.. Practical guidelines for rigor and reproducibility in preclinical and clinical studies on cardioprotection. Basic Res Cardiol 2018;113:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Barrabes JA, Garcia-Dorado D, Mirabet M, Lidon RM, Soriano B, Ruiz-Meana M, Pizcueta P, Blanco J, Puigfel Y, Soler-Soler J.. Lack of effect of glycoprotein IIb/IIIa blockade on myocardial platelet or polymorphonuclear leukocyte accumulation and on infarct size after transient coronary occlusion in pigs. J Am Coll Cardiol 2002;39:157–165. [DOI] [PubMed] [Google Scholar]
- 68. Pachel C, Mathes D, Arias-Loza AP, Heitzmann W, Nordbeck P, Deppermann C, Lorenz V, Hofmann U, Nieswandt B, Frantz S.. Inhibition of platelet GPVI protects against myocardial ischemia-reperfusion injury. Arterioscler Thromb Vasc Biol 2016;36:629–635. [DOI] [PubMed] [Google Scholar]
- 69. Yang XM, Liu Y, Cui L, Yang X, Liu Y, Tandon N, Kambayashi J, Downey JM, Cohen MV.. Platelet P2Y(1)(2) blockers confer direct postconditioning-like protection in reperfused rabbit hearts. J Cardiovasc Pharmacol Ther 2013;18:251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Krug A, de Rochemont Du Mesnil, Korb G.. Blood supply of the myocardium after temporary coronary occlusion. Circ Res 1966;19:57–62. [DOI] [PubMed] [Google Scholar]
- 71. Heusch G, Kleinbongard P, Skyschally A.. Myocardial infarction and coronary microvascular obstruction: an intimate, but complicated relationship. Basic Res Cardiol 2013;108:380.. [DOI] [PubMed] [Google Scholar]
- 72. Kloner RA, King KS, Harrington M.. No-reflow phenomenon in heart and brain. Am J Physiol Heart Circ Physiol 2018;315:H550–H562. [DOI] [PubMed] [Google Scholar]
- 73. Skyschally A, Amanakis G, Neuhauser M, Kleinbongard P, Heusch G.. Impact of electrical defibrillation on infarct size and no-reflow in pigs subjected to myocardial ischemia-reperfusion without and with ischemic conditioning. Am J Physiol Heart Circ Physiol 2017;313:H871–H878. [DOI] [PubMed] [Google Scholar]
- 74. Hale SL, Herring MJ, Kloner RA.. Delayed treatment with hypothermia protects against the no-reflow phenomenon despite failure to reduce infarct size. J Am Heart Assoc 2013;2:e004234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Niccoli G, Scalone G, Lerman A, Crea F.. Coronary microvascular obstruction in acute myocardial infarction. Eur Heart J 2016;37:1024–1033. [DOI] [PubMed] [Google Scholar]
- 76. Eitel I, de Waha S, Wohrle J, Fuernau G, Lurz P, Pauschinger M, Desch S, Schuler G, Thiele H.. Comprehensive prognosis assessment by CMR imaging after ST-segment elevation myocardial infarction. J Am Coll Cardiol 2014;64:1217–1226. [DOI] [PubMed] [Google Scholar]
- 77. Morishima I, Sone T, Okumura K, Tsuboi H, Kondo J, Mukawa H, Matsui H, Toki Y, Ito T, Hayakawa T.. Angiographic no-reflow phenomenon as a predictor of adverse long-term outcome in patients treated with percutaneous transluminal coronary angioplasty for first acute myocardial infarction. J Am Coll Cardiol 2000;36:1202–1209. [DOI] [PubMed] [Google Scholar]
- 78. van 't Hof AW, Liem A, Suryapranata H, Hoorntje JC, de Boer MJ, Zijlstra F.. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: myocardial blush grade. Zwolle Myocardial Infarction Study Group. Circulation 1998;97:2302–2306. [DOI] [PubMed] [Google Scholar]
- 79. Niccoli G, Cosentino N, Spaziani C, Fracassi F, Tarantini G, Crea F.. No-reflow: incidence and detection in the cath-lab. Curr Pharm Des 2013;19:4564–4575. [DOI] [PubMed] [Google Scholar]
- 80. Bulluck H, Foin N, Tan JW, Low AF, Sezer M, Hausenloy DJ.. Invasive assessment of the coronary microcirculation in reperfused ST-segment-elevation myocardial infarction patients: where do we stand? Circ Cardiovasc Interv 2017;10. pii:e004373. doi:10.1161/CIRCINTERVENTIONS.116.004373. [DOI] [PubMed] [Google Scholar]
- 81. Yamamuro A, Akasaka T, Tamita K, Yamabe K, Katayama M, Takagi T, Morioka S.. Coronary flow velocity pattern immediately after percutaneous coronary intervention as a predictor of complications and in-hospital survival after acute myocardial infarction. Circulation 2002;106:3051–3056. [DOI] [PubMed] [Google Scholar]
- 82. van de Hoef TP, Bax M, Meuwissen M, Damman P, Delewi R, de Winter RJ, Koch KT, Schotborgh C, Henriques JP, Tijssen JG, Piek JJ.. Impact of coronary microvascular function on long-term cardiac mortality in patients with acute ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv 2013;6:207–215. [DOI] [PubMed] [Google Scholar]
- 83. Fahrni G, Wolfrum M, De Maria GL, Cuculi F, Dawkins S, Alkhalil M, Patel N, Forfar JC, Prendergast BD, Choudhury RP, Channon KM, Banning AP, Kharbanda RK.. Index of microcirculatory resistance at the time of primary percutaneous coronary intervention predicts early cardiac complications: insights from the OxAMI (Oxford Study in Acute Myocardial Infarction) cohort. J Am Heart Assoc 2017;6. pii:e005409. doi:10.1161/JAHA.116.005409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Schroder R. Prognostic impact of early ST-segment resolution in acute ST-elevation myocardial infarction. Circulation 2004;110:e506–e510. [DOI] [PubMed] [Google Scholar]
- 85. Infusino F, Niccoli G, Fracassi F, Roberto M, Falcioni E, Lanza GA, Crea F.. The central role of conventional 12-lead ECG for the assessment of microvascular obstruction after percutaneous myocardial revascularization. J Electrocardiol 2014;47:45–51. [DOI] [PubMed] [Google Scholar]
- 86. Galiuto L, Garramone B, Scara A, Rebuzzi AG, Crea F, La TG, Funaro S, Madonna M, Fedele F, Agati L.. The extent of microvascular damage during myocardial contrast echocardiography is superior to other known indexes of post-infarct reperfusion in predicting left ventricular remodeling: results of the multicenter AMICI study. J Am Coll Cardiol 2008;51:552–559. [DOI] [PubMed] [Google Scholar]
- 87. Nijveldt R, Beek AM, Hirsch A, Stoel MG, Hofman MB, Umans VA, Algra PR, Twisk JW, van Rossum AC.. Functional recovery after acute myocardial infarction: comparison between angiography, electrocardiography, and cardiovascular magnetic resonance measures of microvascular injury. J Am Coll Cardiol 2008;52:181–189. [DOI] [PubMed] [Google Scholar]
- 88. Wu KC, Zerhouni EA, Judd RM, Lugo-Olivieri CH, Barouch LA, Schulman SP, Blumenthal RS, Lima JA.. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation 1998;97:765–772. [DOI] [PubMed] [Google Scholar]
- 89. Bulluck H, Dharmakumar R, Arai AE, Berry C, Hausenloy DJ.. Cardiovascular magnetic resonance in acute ST-segment-elevation myocardial infarction: recent advances, controversies, and future directions. Circulation 2018;137:1949–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Jennings R, Sommers H, Smyth G, Lack H, Linn H.. Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. Arch Pathol 1960;70:68–78. [PubMed] [Google Scholar]
- 91. Kloner RA, Ganote CE, Jennings RB.. The “no-reflow” phenomenon after temporary coronary occlusion in the dog. J Clin Invest 1974;54:1496–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Fujiwara H, Onodera T, Tanaka M, Fujiwara T, Wu DJ, Kawai C, Hamashima Y.. A clinicopathologic study of patients with hemorrhagic myocardial infarction treated with selective coronary thrombolysis with urokinase. Circulation 1986;73:749–757. [DOI] [PubMed] [Google Scholar]
- 93. Ganame J, Messalli G, Dymarkowski S, Rademakers FE, Desmet W, Van De WF, Bogaert J.. Impact of myocardial haemorrhage on left ventricular function and remodelling in patients with reperfused acute myocardial infarction. Eur Heart J 2009;30:1440–1449. [DOI] [PubMed] [Google Scholar]
- 94. Carrick D, Haig C, Ahmed N, McEntegart M, Petrie MC, Eteiba H, Hood S, Watkins S, Lindsay MM, Davie A, Mahrous A, Mordi I, Rauhalammi S, Sattar N, Welsh P, Radjenovic A, Ford I, Oldroyd KG, Berry C.. Myocardial hemorrhage after acute reperfused ST-segment-elevation myocardial infarction: relation to microvascular obstruction and prognostic significance. Circ Cardiovasc Imaging 2016;9:e004148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Amier RP, Tijssen RYG, Teunissen PFA, Fernandez-Jimenez R, Pizarro G, Garcia-Lunar I, Bastante T, van de Ven PM, Beek AM, Smulders MW, Bekkers SCAM, van RN, Ibanez B, Nijveldt R.. Predictors of intramyocardial hemorrhage after reperfused ST-segment elevation myocardial infarction. J Am Heart Assoc 2017;6. pii:e005651. doi:10.1161/JAHA.117.005651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wang G, Yang HJ, Kali A, Cokic I, Tang R, Xie G, Yang Q, Francis J, Li S, Dharmakumar R.. Influence of myocardial hemorrhage on staging of reperfused myocardial infarctions with T2 cardiac magnetic resonance imaging: insights into the dependence on infarction type with ex vivo validation. JACC Cardiovasc Imaging 2018;S1936-878X(18)30128-1. doi:10.1016/j.jcmg.2018.01.018 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bulluck H, Rosmini S, Abdel-Gadir A, White SK, Bhuva AN, Treibel TA, Fontana M, Ramlall M, Hamarneh A, Sirker A, Herrey AS, Manisty C, Yellon DM, Kellman P, Moon JC, Hausenloy DJ.. Residual myocardial iron following intramyocardial hemorrhage during the convalescent phase of reperfused ST-segment-elevation myocardial infarction and adverse left ventricular remodeling. Circ Cardiovasc Imaging 2016;9. pii:e004940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Higginson LA, White F, Heggtveit HA, Sanders TM, Bloor CM, Covell JW.. Determinants of myocardial hemorrhage after coronary reperfusion in the anesthetized dog. Circulation 1982;65:62–69. [DOI] [PubMed] [Google Scholar]
- 99. Mathey DG, Schofer J, Kuck KH, Beil U, Kloppel G.. Transmural, haemorrhagic myocardial infarction after intracoronary streptokinase. Clinical, angiographic, and necropsy findings. Br Heart J 1982;48:546–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hollander MR, de Waard GA, Konijnenberg LS, Meijer-van Putten RM, van den Brom CE, Paauw N, de Vries HE, van de Ven PM, Aman J, Van Nieuw-Amerongen GP, Hordijk PL, Niessen HW, Horrevoets AJ, van RN.. Dissecting the effects of ischemia and reperfusion on the coronary microcirculation in a rat model of acute myocardial infarction. PLoS One 2016;11:e0157233.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Pedersen SF, Thrysoe SA, Robich MP, Paaske WP, Ringgaard S, Botker HE, Hansen ES, Kim WY.. Assessment of intramyocardial hemorrhage by T1-weighted cardiovascular magnetic resonance in reperfused acute myocardial infarction. J Cardiovasc Magn Reson 2012;14:59.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bulluck H, Rosmini S, Abdel-Gadir A, Bhuva AN, Treibel TA, Fontana M, Gonzalez-Lopez E, Ramlall M, Hamarneh A, Sirker A, Herrey AS, Manisty C, Yellon DM, Moon JC, Hausenloy DJ.. Diagnostic performance of T1 and T2 mapping to detect intramyocardial hemorrhage in reperfused ST-segment elevation myocardial infarction (STEMI) patients. J Magn Reson Imaging 2017;46:877–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Robbers LF, Eerenberg ES, Teunissen PF, Jansen MF, Hollander MR, Horrevoets AJ, Knaapen P, Nijveldt R, Heymans MW, Levi MM, van Rossum AC, Niessen HW, Marcu CB, Beek AM, van RN.. Magnetic resonance imaging-defined areas of microvascular obstruction after acute myocardial infarction represent microvascular destruction and haemorrhage. Eur Heart J 2013;34:2346–2353. [DOI] [PubMed] [Google Scholar]
- 104. DeFily DV, Chilian WM.. Preconditioning protects coronary arteriolar endothelium from ischemia-reperfusion injury. Am J Physiol 1993;265:H700–H706. [DOI] [PubMed] [Google Scholar]
- 105. Ku DD. Coronary vascular reactivity after acute myocardial ischemia. Science 1982;218:576–578. [DOI] [PubMed] [Google Scholar]
- 106. Lefer AM, Tsao PS, Lefer DJ, Ma XL.. Role of endothelial dysfunction in the pathogenesis of reperfusion injury after myocardial ischemia. FASEB J 1991;5:2029–2034. [DOI] [PubMed] [Google Scholar]
- 107. Piana RN, Shafique T, Dai HB, Sellke FW.. Epicardial and endocardial coronary microvascular responses: effects of ischemia-reperfusion. J Cardiovasc Pharmacol 1994;23:539–546. [DOI] [PubMed] [Google Scholar]
- 108. Cai Z, Zhong H, Bosch-Marce M, Fox-Talbot K, Wang L, Wei C, Trush MA, Semenza GL.. Complete loss of ischaemic preconditioning-induced cardioprotection in mice with partial deficiency of HIF-1 alpha. Cardiovasc Res 2008;77:463–470. [DOI] [PubMed] [Google Scholar]
- 109. Matsunaga T, Warltier DC, Tessmer J, Weihrauch D, Simons M, Chilian WM.. Expression of VEGF and angiopoietins-1 and -2 during ischemia-induced coronary angiogenesis. Am J Physiol Heart Circ Physiol 2003;285:H352–H358. [DOI] [PubMed] [Google Scholar]
- 110. Toyota E, Warltier DC, Brock T, Ritman E, Kolz C, O’Malley P, Rocic P, Focardi M, Chilian WM.. Vascular endothelial growth factor is required for coronary collateral growth in the rat. Circulation 2005;112:2108–2113. [DOI] [PubMed] [Google Scholar]
- 111. Heusch G. Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res 2015;116:674–699. [DOI] [PubMed] [Google Scholar]
- 112. Pung YF, Rocic P, Murphy MP, Smith RA, Hafemeister J, Ohanyan V, Guarini G, Yin L, Chilian WM.. Resolution of mitochondrial oxidative stress rescues coronary collateral growth in Zucker obese fatty rats. Arterioscler Thromb Vasc Biol 2012;32:325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Heil M, Schaper W.. Influence of mechanical, cellular, and molecular factors on collateral artery growth (arteriogenesis). Circ Res 2004;95:449–458. [DOI] [PubMed] [Google Scholar]
- 114. Heusch G, Libby P, Gersh B, Yellon D, Bohm M, Lopaschuk G, Opie L.. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet 2014;383:1933–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hausenloy DJ, Barrabes JA, Botker HE, Davidson SM, Di LF, Downey J, Engstrom T, Ferdinandy P, Carbrera-Fuentes HA, Heusch G, Ibanez B, Iliodromitis EK, Inserte J, Jennings R, Kalia N, Kharbanda R, Lecour S, Marber M, Miura T, Ovize M, Perez-Pinzon MA, Piper HM, Przyklenk K, Schmidt MR, Redington A, Ruiz-Meana M, Vilahur G, Vinten-Johansen J, Yellon DM, Garcia-Dorado D.. Ischaemic conditioning and targeting reperfusion injury: a 30 year voyage of discovery. Basic Res Cardiol 2016;111:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Murry CE, Jennings RB, Reimer KA.. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 1986;74:1124–1136. [DOI] [PubMed] [Google Scholar]
- 117. Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J.. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol 2003;285:H579–H588. [DOI] [PubMed] [Google Scholar]
- 118. Hausenloy DJ, Yellon DM.. Remote ischaemic preconditioning: underlying mechanisms and clinical application. Cardiovasc Res 2008;79:377–386. [DOI] [PubMed] [Google Scholar]
- 119. Sivaraman V, Pickard JM, Hausenloy DJ.. Remote ischaemic conditioning: cardiac protection from afar. Anaesthesia 2015;70:732–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Pickard JM, Botker HE, Crimi G, Davidson B, Davidson SM, Dutka D, Ferdinandy P, Ganske R, Garcia-Dorado D, Giricz Z, Gourine AV, Heusch G, Kharbanda R, Kleinbongard P, MacAllister R, McIntyre C, Meybohm P, Prunier F, Redington A, Robertson NJ, Suleiman MS, Vanezis A, Walsh S, Yellon DM, Hausenloy DJ.. Remote ischemic conditioning: from experimental observation to clinical application: report from the 8th Biennial Hatter Cardiovascular Institute Workshop. Basic Res Cardiol 2015;110:453.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Heusch G, Botker HE, Przyklenk K, Redington A, Yellon D.. Remote ischemic conditioning. J Am Coll Cardiol 2015;65:177–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Heusch G. 25 years of remote ischemic conditioning: from laboratory curiosity to clinical outcome. Basic Res Cardiol 2018;113:15.. [DOI] [PubMed] [Google Scholar]
- 123. Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P.. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation 1993;87:893–899. [DOI] [PubMed] [Google Scholar]
- 124. Schmidt MR, Smerup M, Konstantinov IE, Shimizu M, Li J, Cheung M, White PA, Kristiansen SB, Sorensen K, Dzavik V, Redington AN, Kharbanda RK.. Intermittent peripheral tissue ischemia during coronary ischemia reduces myocardial infarction through a KATP-dependent mechanism: first demonstration of remote ischemic perconditioning. Am J Physiol Heart Circ Physiol 2007;292:H1883–H1890. [DOI] [PubMed] [Google Scholar]
- 125. Kerendi F, Kin H, Halkos ME, Jiang R, Zatta AJ, Zhao ZQ, Guyton RA, Vinten-Johansen J.. Remote postconditioning. Brief renal ischemia and reperfusion applied before coronary artery reperfusion reduces myocardial infarct size via endogenous activation of adenosine receptors. Basic Res Cardiol 2005;100:404–412. [DOI] [PubMed] [Google Scholar]
- 126. Kaeffer N, Richard V, Francois A, Lallemand F, Henry JP, Thuillez C.. Preconditioning prevents chronic reperfusion-induced coronary endothelial dysfunction in rats. Am J Physiol 1996;271:H842–H849. [DOI] [PubMed] [Google Scholar]
- 127. Kurzelewski M, Czarnowska E, Maczewski M, Beresewicz A.. Effect of ischemic preconditioning on endothelial dysfunction and granulocyte adhesion in isolated guinea-pig hearts subjected to ischemia/reperfusion. J Physiol Pharmacol 1999;50:617–628. [PubMed] [Google Scholar]
- 128. Richard V, Kaeffer N, Tron C, Thuillez C.. Ischemic preconditioning protects against coronary endothelial dysfunction induced by ischemia and reperfusion. Circulation 1994;89:1254–1261. [DOI] [PubMed] [Google Scholar]
- 129. Thourani VH, Nakamura M, Duarte IG, Bufkin BL, Zhao ZQ, Jordan JE, Shearer ST, Guyton RA, Vinten-Johansen J.. Ischemic preconditioning attenuates postischemic coronary artery endothelial dysfunction in a model of minimally invasive direct coronary artery bypass grafting. J Thorac Cardiovasc Surg 1999;117:383–389. [DOI] [PubMed] [Google Scholar]
- 130. Gattullo D, Linden RJ, Losano G, Pagliaro P, Westerhof N.. Ischaemic preconditioning changes the pattern of coronary reactive hyperaemia in the goat: role of adenosine and nitric oxide. Cardiovasc Res 1999;42:57–64. [DOI] [PubMed] [Google Scholar]
- 131. Li Z, Jin ZQ.. Ischemic preconditioning enhances integrity of coronary endothelial tight junctions. Biochem Biophys Res Commun 2012;425:630–635. [DOI] [PubMed] [Google Scholar]
- 132. Bouchard JF, Lamontagne D.. Mechanisms of protection afforded by preconditioning to endothelial function against ischemic injury. Am J Physiol 1996;271:H1801–H1806. [DOI] [PubMed] [Google Scholar]
- 133. Merkus D, Stepp DW, Jones DW, Nishikawa Y, Chilian WM.. Adenosine preconditions against endothelin-induced constriction of coronary arterioles. Am J Physiol Heart Circ Physiol 2000;279:H2593–H2597. [DOI] [PubMed] [Google Scholar]
- 134. Beresewicz A, Maczewski M, Duda M.. Effect of classic preconditioning and diazoxide on endothelial function and O2- and NO generation in the post-ischemic guinea-pig heart. Cardiovasc Res 2004;63:118–129. [DOI] [PubMed] [Google Scholar]
- 135. Kaeffer N, Richard V, Thuillez C.. Delayed coronary endothelial protection 24 hours after preconditioning: role of free radicals. Circulation 1997;96:2311–2316. [DOI] [PubMed] [Google Scholar]
- 136. Bouchard JF, Chouinard J, Lamontagne D.. Role of kinins in the endothelial protective effect of ischaemic preconditioning. Br J Pharmacol 1998;123:413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Bouchard JF, Chouinard J, Lamontagne D.. Participation of prostaglandin E2 in the endothelial protective effect of ischaemic preconditioning in isolated rat heart. Cardiovasc Res 2000;45:418–427. [DOI] [PubMed] [Google Scholar]
- 138. Laude K, Favre J, Thuillez C, Richard V.. NO produced by endothelial NO synthase is a mediator of delayed preconditioning-induced endothelial protection. Am J Physiol Heart Circ Physiol 2003;284:H2053–H2060. [DOI] [PubMed] [Google Scholar]
- 139. Maczewski M, Duda M, Pawlak W, Beresewicz A.. Endothelial protection from reperfusion injury by ischemic preconditioning and diazoxide involves a SOD-like anti-O2-mechanism. J Physiol Pharmacol 2004;55:537–550. [PubMed] [Google Scholar]
- 140. Duda M, Czarnowska E, Kurzelewski M, Konior A, Beresewicz A.. Ischemic preconditioning prevents endothelial dysfunction, P-selectin expression, and neutrophil adhesion by preventing endothelin and O2-generation in the post-ischemic guinea-pig heart. J Physiol Pharmacol 2006;57:553–569. [PubMed] [Google Scholar]
- 141. Kim SJ, Zhang X, Xu X, Chen A, Gonzalez JB, Koul S, Vijayan K, Crystal GJ, Vatner SF, Hintze TH.. Evidence for enhanced eNOS function in coronary microvessels during the second window of protection. Am J Physiol Heart Circ Physiol 2007;292:H2152–H2158. [DOI] [PubMed] [Google Scholar]
- 142. Bauer B, Simkhovich BZ, Kloner RA, Przyklenk K.. Does preconditioning protect the coronary vasculature from subsequent ischemia/reperfusion injury? Circulation 1993;88:659–672. [DOI] [PubMed] [Google Scholar]
- 143. Loke KE, Woodman OL.. Preconditioning improves myocardial function and reflow, but not vasodilator reactivity, after ischaemia and reperfusion in anaesthetized dogs. Clin Exp Pharmacol Physiol 1998;25:552–558. [DOI] [PubMed] [Google Scholar]
- 144. Skyschally A, Gres P, Heusch P, Martin C, Haude M, Erbel R, Schulz R, Heusch G.. Preinfarction angina: no interference of coronary microembolization with acute ischemic preconditioning. J Mol Cell Cardiol 2005;39:355–361. [DOI] [PubMed] [Google Scholar]
- 145. Skyschally A, Gres P, Hoffmann S, Haude M, Erbel R, Schulz R, Heusch G.. Bidirectional role of tumor necrosis factor-alpha in coronary microembolization: progressive contractile dysfunction versus delayed protection against infarction. Circ Res 2007;100:140–146. [DOI] [PubMed] [Google Scholar]
- 146. Tomai F, Crea F, Chiariello L, Gioffre PA.. Preinfarction angina and myocardial preconditioning. Cardiologia 1999;44:963–967. [PubMed] [Google Scholar]
- 147. Heusch G. Nitroglycerin and delayed preconditioning in humans: yet another new mechanism for an old drug? Circulation 2001;103:2876–2878. [DOI] [PubMed] [Google Scholar]
- 148. Andreotti F, Sciahbasi A, De ME, Maseri A.. Preinfarction angina and improved reperfusion of the infarct-related artery. Thromb Haemost 1999;82 Suppl 1:68–72. [PubMed] [Google Scholar]
- 149. Colonna P, Cadeddu C, Montisci R, Ruscazio M, Selem AH, Chen L, Onnis E, Meloni L, Iliceto S.. Reduced microvascular and myocardial damage in patients with acute myocardial infarction and preinfarction angina. Am Heart J 2002;144:796–803. [DOI] [PubMed] [Google Scholar]
- 150. Whittaker P, Przyklenk K.. From ischemic conditioning to ‘hyperconditioning’: clinical phenomenon and basic science opportunity. Dose Response 2014;12:650–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Zhao JL, Yang YJ, You SJ, Cui CJ, Gao RL.. Different effects of postconditioning on myocardial no-reflow in the normal and hypercholesterolemic mini-swines. Microvasc Res 2007;73:137–142. [DOI] [PubMed] [Google Scholar]
- 152. Baranyai T, Giricz Z, Varga ZV, Koncsos G, Lukovic D, Makkos A, Sarkozy M, Pavo N, Jakab A, Czimbalmos C, Vago H, Ruzsa Z, Toth L, Garamvolgyi R, Merkely B, Schulz R, Gyongyosi M, Ferdinandy P.. In vivo MRI and ex vivo histological assessment of the cardioprotection induced by ischemic preconditioning, postconditioning and remote conditioning in a closed-chest porcine model of reperfused acute myocardial infarction: importance of microvasculature. J Transl Med 2017;15:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Bodi V, Ruiz-Nodar JM, Feliu E, Minana G, Nunez J, Husser O, Martinez-Elvira J, Ruiz A, Bonanad C, Monmeneu JV, Lopez-Lereu MP, Forteza MJ, de Dios E, Hervas A, Moratal D, Gomez C, Mainar L, Sanchis J, Mainar V, Valencia J, Diaz A, Noguera I, Chaustre F, Chorro FJ.. Effect of ischemic postconditioning on microvascular obstruction in reperfused myocardial infarction. Results of a randomized study in patients and of an experimental model in swine. Int J Cardiol 2014;175:138–146. [DOI] [PubMed] [Google Scholar]
- 154. Zalewski J, Claus P, Bogaert J, Driessche NV, Driesen RB, Galan DT, Sipido KR, Buszman P, Milewski K, Van de Werf F.. Cyclosporine A reduces microvascular obstruction and preserves left ventricular function deterioration following myocardial ischemia and reperfusion. Basic Res Cardiol 2015;110:18.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Skyschally A, Walter B, Heusch G.. Coronary microembolization during early reperfusion: infarct extension, but protection by ischaemic postconditioning. Eur Heart J 2013;34:3314–3321. [DOI] [PubMed] [Google Scholar]
- 156. Mewton N, Thibault H, Roubille F, Lairez O, Rioufol G, Sportouch C, Sanchez I, Bergerot C, Cung TT, Finet G, Angoulvant D, Revel D, Bonnefoy-Cudraz E, Elbaz M, Piot C, Sahraoui I, Croisille P, Ovize M.. Postconditioning attenuates no-reflow in STEMI patients. Basic Res Cardiol 2013;108:383.. [DOI] [PubMed] [Google Scholar]
- 157. Dong M, Mu N, Guo F, Zhang C, Ren F, Li J, Tao Z, Yang J, Li G.. The beneficial effects of postconditioning on no-reflow phenomenon after percutaneous coronary intervention in patients with ST-elevation acute myocardial infarction. J Thromb Thrombolysis 2014;38:208–214. [DOI] [PubMed] [Google Scholar]
- 158. Ma XJ, Zhang XH, Li CM, Luo M.. Effect of postconditioning on coronary blood flow velocity and endothelial function in patients with acute myocardial infarction. Scand Cardiovasc J 2006;40:327–333. [DOI] [PubMed] [Google Scholar]
- 159. Eitel I, Stiermaier T, Rommel KP, Fuernau G, Sandri M, Mangner N, Linke A, Erbs S, Lurz P, Boudriot E, Mende M, Desch S, Schuler G, Thiele H.. Cardioprotection by combined intrahospital remote ischaemic perconditioning and postconditioning in ST-elevation myocardial infarction: the randomized LIPSIA CONDITIONING trial. Eur Heart J 2015;36:3049–3057. [DOI] [PubMed] [Google Scholar]
- 160. Freixa X, Bellera N, Ortiz-Perez JT, Jimenez M, Pare C, Bosch X, De Caralt TM, Betriu A, Masotti M.. Ischaemic postconditioning revisited: lack of effects on infarct size following primary percutaneous coronary intervention. Eur Heart J 2012;33:103–112. [DOI] [PubMed] [Google Scholar]
- 161. Tarantini G, Favaretto E, Marra MP, Frigo AC, Napodano M, Cacciavillani L, Giovagnoni A, Renda P, De Biasio V, Plebani M, Mion M, Zaninotto M, Isabella G, Bilato C, Iliceto S.. Postconditioning during coronary angioplasty in acute myocardial infarction: the POST-AMI trial. Int J Cardiol 2012;162:33–38. [DOI] [PubMed] [Google Scholar]
- 162. Kharbanda RK, Mortensen UM, White PA, Kristiansen SB, Schmidt MR, Hoschtitzky JA, Vogel M, Sorensen K, Redington AN, MacAllister R.. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation 2002;106:2881–2883. [DOI] [PubMed] [Google Scholar]
- 163. Loukogeorgakis SP, Williams R, Panagiotidou AT, Kolvekar SK, Donald A, Cole TJ, Yellon DM, Deanfield JE, MacAllister RJ.. Transient limb ischemia induces remote preconditioning and remote postconditioning in humans by a K(ATP)-channel dependent mechanism. Circulation 2007;116:1386–1395. [DOI] [PubMed] [Google Scholar]
- 164. Loukogeorgakis SP, Panagiotidou AT, Broadhead MW, Donald A, Deanfield JE, MacAllister RJ.. Remote ischemic preconditioning provides early and late protection against endothelial ischemia-reperfusion injury in humans: role of the autonomic nervous system. J Am Coll Cardiol 2005;46:450–456. [DOI] [PubMed] [Google Scholar]
- 165. Luca MC, Liuni A, McLaughlin K, Gori T, Parker JD.. Daily ischemic preconditioning provides sustained protection from ischemia-reperfusion induced endothelial dysfunction: a human study. J Am Heart Assoc 2013;2:e000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Jones H, Hopkins N, Bailey TG, Green DJ, Cable NT, Thijssen DH.. Seven-day remote ischemic preconditioning improves local and systemic endothelial function and microcirculation in healthy humans. Am J Hypertens 2014;27:918–925. [DOI] [PubMed] [Google Scholar]
- 167. Hauerslev M, Mork SR, Pryds K, Contractor H, Hansen J, Jespersen NR, Johnsen J, Heusch G, Kleinbongard P, Kharbanda R, Botker HE, Schmidt MR.. Influence of long-term treatment with glyceryl trinitrate on remote ischemic conditioning. Am J Physiol Heart Circ Physiol 2018;315:H150–H158. [DOI] [PubMed] [Google Scholar]
- 168. Shimizu M, Konstantinov IE, Kharbanda RK, Cheung MH, Redington AN.. Effects of intermittent lower limb ischaemia on coronary blood flow and coronary resistance in pigs. Acta Physiol (Oxf) 2007;190:103–109. [DOI] [PubMed] [Google Scholar]
- 169. Zhou K, Yang B, Zhou XM, Tan CM, Zhao Y, Huang C, Liao XB, Xiao HB.. Effects of remote ischemic preconditioning on the flow pattern of the left anterior descending coronary artery in normal subjects. Int J Cardiol 2007;122:250–251. [DOI] [PubMed] [Google Scholar]
- 170. Xu Y, Yu Q, Yang J, Yuan F, Zhong Y, Zhou Z, Wang N.. Acute hemodynamic effects of remote ischemic preconditioning on coronary perfusion pressure and coronary collateral blood flow in coronary heart disease. Acta Cardiol Sin 2018;34:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Corcoran D, Young R, Cialdella P, McCartney P, Bajrangee A, Hennigan B, Collison D, Carrick D, Shaukat A, Good R, Watkins S, McEntegart M, Watt J, Welsh P, Sattar N, McConnachie A, Oldroyd KG, Berry C.. The effects of remote ischaemic preconditioning on coronary artery function in patients with stable coronary artery disease. Int J Cardiol 2018;252:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Heusch G. Protection of the human coronary circulation by remote ischemic conditioning. Int J Cardiol 2018;252:35–36. [DOI] [PubMed] [Google Scholar]
- 173. White SK, Frohlich GM, Sado DM, Maestrini V, Fontana M, Treibel TA, Tehrani S, Flett AS, Meier P, Ariti C, Davies JR, Moon JC, Yellon DM, Hausenloy DJ.. Remote ischemic conditioning reduces myocardial infarct size and edema in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv 2015;8:178–188. [DOI] [PubMed] [Google Scholar]
- 174. White SK, Hausenloy DJ, Moon JC.. Imaging the myocardial microcirculation post-myocardial infarction. Curr Heart Fail Rep 2012;9:282–292. [DOI] [PubMed] [Google Scholar]
- 175. Bulluck H, Hausenloy DJ.. Microvascular obstruction: the bane of myocardial reperfusion. Rev Esp Cardiol (Engl Ed) 2015;68:919–920. [DOI] [PubMed] [Google Scholar]
- 176. Heusch G, Kleinbongard P, Bose D, Levkau B, Haude M, Schulz R, Erbel R.. Coronary microembolization: from bedside to bench and back to bedside. Circulation 2009;120:1822–1836. [DOI] [PubMed] [Google Scholar]
- 177. Hausenloy DJ, Maddock HL, Baxter GF, Yellon DM.. Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning? Cardiovasc Res 2002;55:534–543. [DOI] [PubMed] [Google Scholar]
- 178. Hausenloy DJ, Duchen MR, Yellon DM.. Inhibiting mitochondrial permeability transition pore opening at reperfusion protects against ischaemia-reperfusion injury. Cardiovasc Res 2003;60:617–625. [DOI] [PubMed] [Google Scholar]
- 179. Skyschally A, Schulz R, Heusch G.. Cyclosporine A at reperfusion reduces infarct size in pigs. Cardiovasc Drugs Ther 2010;24:85–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Hausenloy DJ, Boston-Griffiths EA, Yellon DM.. Cyclosporin A and cardioprotection: from investigative tool to therapeutic agent. Br J Pharmacol 2012;165:1235–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Lim WY, Messow CM, Berry C.. Cyclosporin variably and inconsistently reduces infarct size in experimental models of reperfused myocardial infarction: a systematic review and meta-analysis. Br J Pharmacol 2012;165:2034–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, Elbelghiti R, Cung TT, Bonnefoy E, Angoulvant D, Macia C, Raczka F, Sportouch C, Gahide G, Finet G, Andre-Fouet X, Revel D, Kirkorian G, Monassier JP, Derumeaux G, Ovize M.. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med 2008;359:473–481. [DOI] [PubMed] [Google Scholar]
- 183. Cung TT, Morel O, Cayla G, Rioufol G, Garcia-Dorado D, Angoulvant D, Bonnefoy-Cudraz E, Guerin P, Elbaz M, Delarche N, Coste P, Vanzetto G, Metge M, Aupetit JF, Jouve B, Motreff P, Tron C, Labeque JN, Steg PG, Cottin Y, Range G, Clerc J, Claeys MJ, Coussement P, Prunier F, Moulin F, Roth O, Belle L, Dubois P, Barragan P, Gilard M, Piot C, Colin P, De PF, Morice MC, Ider O, Dubois-Rande JL, Unterseeh T, Le BH, Beard T, Blanchard D, Grollier G, Malquarti V, Staat P, Sudre A, Elmer E, Hansson MJ, Bergerot C, Boussaha I, Jossan C, Derumeaux G, Mewton N, Ovize M.. Cyclosporine before PCI in patients with acute myocardial infarction. N Engl J Med 2015;373:1021–1031. [DOI] [PubMed] [Google Scholar]
- 184. Ottani F, Latini R, Staszewsky L, La VL, Locuratolo N, Sicuro M, Masson S, Barlera S, Milani V, Lombardi M, Costalunga A, Mollichelli N, Santarelli A, De CN, Sganzerla P, Boi A, Maggioni AP, Limbruno U.. Cyclosporine A in reperfused myocardial infarction: the multicenter, controlled, open-label CYCLE trial. J Am Coll Cardiol 2016;67:365–374. [DOI] [PubMed] [Google Scholar]
- 185. Bibli SI, Papapetropoulos A, Iliodromitis EK, Daiber A, Randriamboavonjy V, Steven S, Brouckaert P, Chatzianastasiou A, Kypreos KE, Hausenloy DJ, Fleming I, Andreadou I.. Nitroglycerin limits infarct size through S-nitrosation of Cyclophilin D: a novel mechanism for an old drug. Cardiovasc Res 2019;115:625–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Herring MJ, Dai W, Hale SL, Kloner RA.. Rapid induction of hypothermia by the ThermoSuit system profoundly reduces infarct size and anatomic zone of no reflow following ischemia-reperfusion in rabbit and rat hearts. J Cardiovasc Pharmacol Ther 2015;20:193–202. [DOI] [PubMed] [Google Scholar]
- 187. Ferdinandy P, Hausenloy DJ, Heusch G, Baxter GF, Schulz R.. Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev 2014;66:1142–1174. [DOI] [PubMed] [Google Scholar]
- 188. Wang WZ, Jones S, Stepheson LL, Khiabani KT, Zamboni WA.. Microvascular protection induced by late preconditioning was abolished in STZ-induced acute diabetic rats. J Reconstr Microsurg 2002;18:689–696. [DOI] [PubMed] [Google Scholar]
- 189. van dM I, Riksen N, Seeger JP, Schreuder TH, Borm GF, Eijsvogels TM, Hopman MT, Rongen GA, Thijssen DH.. Aging attenuates the protective effect of ischemic preconditioning against endothelial ischemia-reperfusion injury in humans. Am J Physiol Heart Circ Physiol 2013;304:H1727–H1732. [DOI] [PubMed] [Google Scholar]
- 190. Nakamura S, Kimura M, Goto C, Noma K, Yoshizumi M, Chayama K, Kihara Y, Higashi Y.. Cigarette smoking abolishes ischemic preconditioning-induced augmentation of endothelium-dependent vasodilation. Hypertension 2009;53:674–681. [DOI] [PubMed] [Google Scholar]
- 191. Moro L, Pedone C, Mondì A, Nunziata E, Antonelli Incalzi R.. Effect of local and remote ischemic preconditioning on endothelial function in young people and healthy or hypertensive elderly people. Atherosclerosis 2011;219:750–752. [DOI] [PubMed] [Google Scholar]
- 192. Kono Y, Fukuda S, Hanatani A, Nakanishi K, Otsuka K, Taguchi H, Shimada K.. Remote ischemic conditioning improves coronary microcirculation in healthy subjects and patients with heart failure. Drug Des Devel Ther 2014;8:1175–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Liuni A, Luca MC, Gori T, Parker JD.. Rosuvastatin prevents conduit artery endothelial dysfunction induced by ischemia and reperfusion by a cyclooxygenase-2-dependent mechanism. J Am Coll Cardiol 2010;55:1002–1006. [DOI] [PubMed] [Google Scholar]
- 194. Liuni A, Luca MC, Gori T, Parker JD.. Loss of the preconditioning effect of rosuvastatin during sustained therapy: a human in vivo study. Am J Physiol Heart Circ Physiol 2012;302:H153–H158. [DOI] [PubMed] [Google Scholar]
- 195. Okorie MI, Bhavsar DD, Ridout D, Charakida M, Deanfield JE, Loukogeorgakis SP, MacAllister RJ.. Postconditioning protects against human endothelial ischaemia-reperfusion injury via subtype-specific KATP channel activation and is mimicked by inhibition of the mitochondrial permeability transition pore. Eur Heart J 2011;32:1266–1274. [DOI] [PubMed] [Google Scholar]
- 196. Przyklenk K, Maynard M, Greiner DL, Whittaker P.. Cardioprotection with postconditioning: loss of efficacy in murine models of type-2 and type-1 diabetes. Antioxid Redox Signal 2011;14:781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Liu X, Sha O, Cho EY.. Remote ischemic postconditioning promotes the survival of retinal ganglion cells after optic nerve injury. J Mol Neurosci 2013;51:639–646. [DOI] [PubMed] [Google Scholar]
- 198. Kwong W, Liuni A, Zhou K, Parker JD.. Cyclooxygenase inhibition and rosuvastatin-induced vascular protection in the setting of ischemia-reperfusion: a human in vivo study. Vascul Pharmacol 2015;71:159–165. [DOI] [PubMed] [Google Scholar]
- 199. Kwong W, Parker JD.. The effect of clopidogrel on the response to ischemia reperfusion. J Cardiovasc Pharmacol Ther 2017;22:368–373. [DOI] [PubMed] [Google Scholar]
- 200. McLaughlin K, Lytvyn Y, Luca MC, Liuni A, Gori T, Parker JD.. Repeated daily dosing with sildenafil provides sustained protection from endothelial dysfunction caused by ischemia and reperfusion: a human in vivo study. Am J Physiol Heart Circ Physiol 2014;307:H888–H894. [DOI] [PubMed] [Google Scholar]