Abstract
Objective
Respiratory abnormalities such as upper airway obstruction are common in Parkinson's disease (PD) and are an important cause of mortality and morbidity. We tested the effect of pedunculopontine region (PPNr) stimulation on respiratory maneuvers in human participants with PD, and separately recorded PPNr neural activity reflected in the local field potential (LFP) during these maneuvers.
Methods
Nine patients with deep brain stimulation electrodes in PPNr, and seven in globus pallidus interna (GPi) were studied during trials of maximal inspiration followed by forced expiration with stimulation OFF and ON. Local field potentials (LFPs) were recorded in the unstimulated condition.
Results
PEFR increased from 6.41 ± 0.63 L/sec in the OFF stimulation state to 7.5 L ± 0.65 L/sec in the ON stimulation state (z = −2.666, df = 8, P = 0.024). Percentage improvement in PEFR was strongly correlated with proximity of the stimulated electrode contact to the mesencephalic locomotor region in the rostral PPN (r = 0.814, n = 9, P = 0.008). Mean PPNr LFP power increased within the alpha band (7–11 Hz) during forced respiratory maneuvers (1.63 ± 0.16 μV2/Hz) compared to resting breathing (0.77 ± 0.16 μV2/Hz; z = −2.197, df = 6, P = 0.028). No changes in alpha activity or spirometric indices were seen with GPi recording or stimulation. Percentage improvement in PEFR was strongly positively correlated with increase in alpha power (r = 0.653, n = 14 (7 PPNr patients recorded bilaterally), P = 0.0096).
Interpretation
PPNr stimulation in PD improves indices of upper airway function. Increased alpha‐band activity is seen within the PPNr during forced respiratory maneuvers. Our findings suggest a link between the PPNr and respiratory performance in PD.
Introduction
Respiratory abnormalities are common in Parkinson's Disease (PD) and are a major cause of death.1 Such abnormalities include abnormal respiratory patterns, aspiration pneumonia and chronic airflow limitation.2, 3 The latter is believed to be caused by upper airway obstruction (UAO) due to weakness, hypokinesia or aberrant contraction of the striated musculature around the upper airway2, 4 and has been suggested to be an important factor in secretion retention, atelectasis, and aspiration pneumonia.1, 2, 5
Irregular and jerky movements of both the glottis and supraglottic structures with sudden and intermittent closure of the airway are seen on fiberoptic endoscopic examination of the upper airways in extrapyramidal disorders including PD. In addition, there is often a fixed reduction in laryngeal diameter believed to be due to tonic activation of vocal fold adductors.2 These abnormalities have been reflected in respiratory function tests signifying UAO, manifesting as diminished peak expiratory flow rate (PEFR) and maximal flow at 50% of forced vital capacity (FEF50), with an increased ratio of forced expiratory volume in one‐second (FEV1) to PEFR.2, 4, 6
The nonmotor benefits of deep brain stimulation (DBS) have attracted recent interest.7, 8 We have previously shown that stimulation of selected subcortical areas can improve PEFR by up to 30% in humans treated for movement and chronic pain disorders, an effect which is presumed to be mediated by changes in autonomic outflow to airway smooth muscle rather than changes in inspiratory/expiratory muscle tone.9 The sites stimulated in this previous study were the periaqueductal grey matter of the midbrain, implicated in integrated behavioral responses10, 11 and in the central autonomic network,12 and the subthalamic nucleus. Other sites used as controls in that study were the globus pallidus interna (GPi) and the sensory thalamus. Although stimulation of the GPi and sensory thalamus are therapeutic in movement and chronic pain disorders, respectively, they are not implicated in the brain's cardiorespiratory neurocircuitry and indeed they conferred no change in lung function.
The mesencephalic locomotor region (MLR), which includes the rostral PPN,13, 14, 15 has been shown to modulate autonomic variables in decerebrate or anesthetized animals.16, 17 In particular, animal studies show glutamatergic neurones from the MLR project directly to the medullary respiratory generator and play a key role in changes in respiration linked to motion.18 PPN region (PPNr) stimulation is a novel therapy for freezing of gait and postural instability in PD19, 20, 21, 22 and is suggested to improve control of axial musculature.21 Accordingly, we hypothesize that PPNr may improve UAO in patients with PD. In addition, we define whether changes in local patterns of oscillatory synchrony implicated in axial motor control may also be evident in voluntary respiration. PPNr oscillations tend to synchronize within the 7–11 Hz band,23 and this activity increases during, and correlates with, gait performance.24 Hence our second hypothesis is that voluntary respiration will be accompanied by increases in alpha activity in the PPNr.
Methods
Patients
Nine patients with PD (meeting UK Brain Bank Criteria) manifesting with freezing of gait or postural instability, receiving chronic bilateral PPN region (PPNr) stimulation were recruited from centers in Oxford, UK, and Brisbane, Australia. Ethical approval was obtained from both centers in addition to written informed consent. Indications for this treatment and implantation technique have been reviewed elsewhere.19 PPNr stimulation was of a mean amplitude 2.8 volts (V) (range 2.2–4.3 V), mean frequency 32 Hz (range 20–40 Hz) and a 60 microsecond pulse width. Patient and stimulation characteristics are summarized in Table 1. Seven cases with implanted GPi electrodes for dystonia in Oxford, UK, were used as the control group. Mean GPi age was 51 years (range 22–66 years), two males, five females, mean amplitude 2.4 volts (range 2–3 volts), mean frequency 114 Hz (range 60–130 Hz) and a 90 microsecond pulse width.
Table 1.
Demographic and stimulation parameters in patients receiving pedunculopontine nucleus region stimulation
| Age (yrs)/Sex | Stimulation parameters (Voltage, Pulse width, Frequency) |
|---|---|
| 47/M | 2.2v, 60 μsec, 35 Hz |
| 77/M | Left 2.5v, Right 2.8v, 60 μsec, 35 Hz |
| 62/F | 4v, 60 μsec, 35 Hz |
| 73/M | 4.3v, 60 μsec, 35 Hz |
| 73/F | 3v, 60 μsec, 35 Hz |
| 57/M | 2.2v, 60 μsec, 20 Hz |
| 56/M | 2.5v, 60 μsec, 20 Hz |
| 68/M | 3.0v, 60 μsec, 40 Hz |
| 54/M | 2v, 60 μsec, 30 Hz |
Respiratory maneuvers
Testing was undertaken by a single clinician trained in the supervision of spirometry by the lung function laboratory within the Department of Respiratory Medicine, Churchill Hospital, UK, and at the center in Brisbane, and patients were asked to attend for testing having taken their normal PD medications. Patients were trained to perform spirometry according to the European Respiratory Society guidelines.25 All patients were able to perform spirometry competently. Patients were tested whilst sitting comfortably upright in a chair with the neck in a neutral position. No nose clip was applied. A thoracic girdle was applied to continuously transduce changes in thoracic circumference related to breathing. After a period of resting breathing, a forced respiratory maneuver, consisting of a maximal inspiration followed by a forced expiration was performed. A clinical Spirolab 2 spirometer (Medical International Research USA, Inc) recorded lung function indices. Patients performed three trials of maximal inspiration followed by forced expiration each for the conditions of stimulation OFF and ON. Stimulation parameters were the same as at the patient's regular therapeutic settings. The ON/OFF order was randomized and patients were blinded to whether stimulation was ON or OFF, however, the experimenter was not blinded to patient condition. There was a 10‐minute wash‐out period between DBS ON and DBS OFF conditions based on previous findings that cardiorespiratory parameter changes occur within seconds to minutes of deep brain stimulation adjustments.9, 26, 27
Electrophysiological recordings
In patients with externalized electrodes, it was possible to simultaneously record local field potentials (LFPs) during resting breathing and forced respiratory maneuvers in the OFF stimulation state. As a control, LFPs were recorded during the same conditions from patients with GPi electrodes, as this nucleus has previously not produced changes in lung function when stimulated.9 Recordings were made from adjacent contacts of each deep brain macroelectrode (Medtronic, model 3389) in a bipolar configuration. Signals were filtered at 0.5–500 Hz and amplified (10,000x) using isolated CED 1902 amplifiers and digitized using CED 1401 Mark II at a rate of 2.5 kHz (Cambridge Electronic Design, Cambridge, UK). LFPs were displayed online and saved onto hard disk using Spike II (CED) and subsequently analyzed offline. Fast Fourier Transformations were applied to decompose the LFP signal into its constituent frequencies, specifically the 7–11 Hz band, although the full power spectrum was reviewed for the presence of artifact prior to filtering. Mean power within the 7–11 Hz band during forced respiratory maneuvers across all trials was compared to mean 7–11 Hz band power during randomly selected periods of resting breathing.
Upper airway & other spirometry measurements
We recorded PEFR, defined as the highest flow achieved from a maximum forced expiration started without hesitation from a position of maximal lung inflation,28 maximal flow at 50% of forced vital capacity (FEF50), forced expiratory volume in one‐second (FEV1) defined as the maximal volume of air exhaled in the first second of a forced expiration from a position of full inspiration,25 and forced vital capacity (FVC), defined as the maximal volume of air exhaled with maximally forced effort from a maximal inspiration.25 FEV1/PEFR and FEV1/FVC ratios were derived. Primary outcome variables were those indicating upper airway flow, namely PEFR, FEF50, and FEV1/PEFR ratio2, 4, 6.
Electrode contact mapping
Preoperative MRI was fused to post‐operative head computerized tomograms (CT) using image fusion software (Radionics Inc., Burlington, MA, USA). Postoperative CT images were acquired on the day of surgery. Rostro‐caudal distances of the active electrode contacts from the pontomesencephalic (PM) line were recorded whereby increasing positivity depicted an increasingly rostral location. To illustrate the locations of the PPN electrodes, anatomical landmarks were used to transform the electrode positions onto the Montreal Neurological Institute (MNI) standard and structural brain template of 152 averaged brains using the FMRIB Software Library (FSL).29, 30
Statistical analysis
Data were analyzed using the Statistical Package for Social Sciences (SPSS version 11, SPSS Inc., Chicago, IL). The Kolmogorov–Smirnov Test demonstrated that PEFR percentage improvement, FVC, and mean electrode contact depth data were normally distributed. To compare the difference between condition means, paired samples t‐tests were applied to FVC data, whereas the Wilcoxon signed‐rank test was applied to the non‐Gaussian distributed variables. All tests were two tailed. P < 0.05 after correction for multiple comparisons were considered significant.31 Percentage PEFR improvement with stimulation was correlated with the mean of the active contact depths of the two electrodes in each patient using Pearson's correlation coefficient. Means and standard error of the means are given in the text.
Results
Indices of upper airway function
PEFR improved in patients receiving PPNr stimulation, and the increase was significant from 6.41 ± 0.63 L/sec in the OFF stimulation state to 7.5 ± 0.65 L/sec in the ON stimulation state (z = −2.666, df = 8, P = 0.024; Fig. 1). Within the control group, PEFR was not changed by GPi stimulation. Mean PEFR was 8.31 ± 1.11 L/sec in the OFF stimulation state compared to 8.33 ± 1.06 L/sec in the ON stimulation state (z = −0.340, df = 6, P = 0.734).
Figure 1.
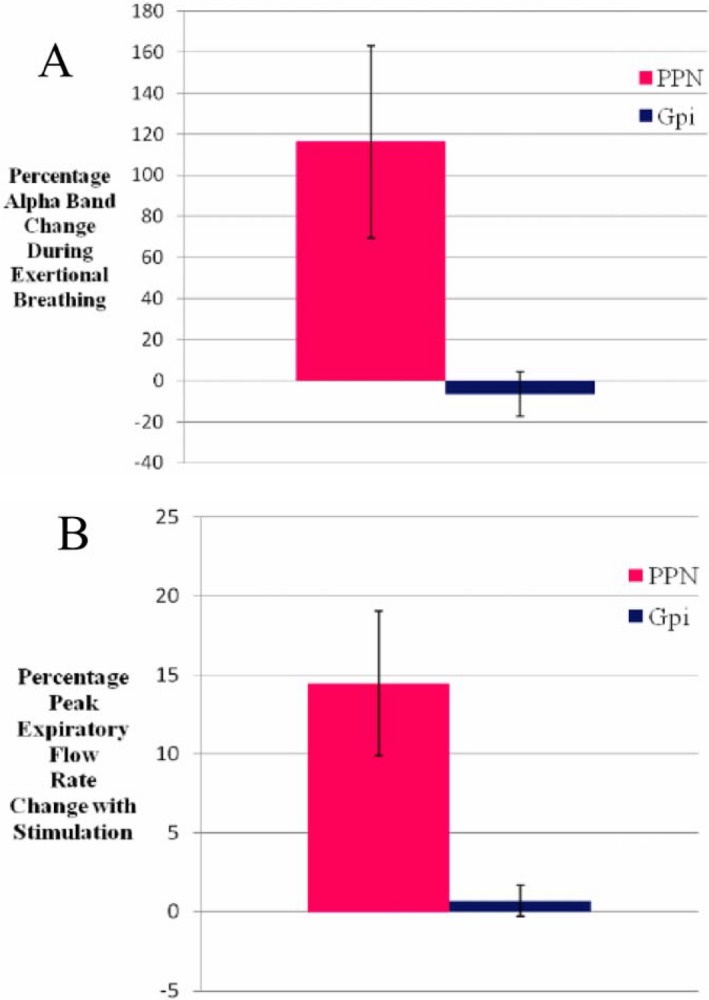
Percentage changes in (A) Mean alpha band synchronization during forced respiratory maneuvers compared to resting breathing in the PPN region (red) versus the GPi (blue); and (B) Mean peak expiratory flow rate across all patients with stimulation of the PPN region (red) versus the GPi (blue). Error bars represent standard errors.
In the PPNr group, FEF50 was lower than predicted in the OFF DBS state with a mean predicted percentage of 90% compared to 100% ON DBS. Mean FEF50 increased from 3.45 ± 0.36 L/sec to 3.83 ± 0.5 L/sec with PPN stimulation, although this did not reach significance (P = 0.063). Mean FEV1 change was only marginal, increasing with PPNr stimulation, and this was not significant (2.68 ± 0.18 L Off vs. 3.00 ± 0.31 L On DBS, P = 0.139; See Table 2).
Table 2.
Change in indices of upper airway function ON versus OFF PPN region stimulation
| Stimulation | PEFR Mean ± SE (L/sec) | FEV1/PEFR Mean ± SE (mL/L per min) | FEF50 Mean ± SE (L/sec) |
|---|---|---|---|
| ON |
7.45 (± 0.65) |
6.75 (± 0.42) |
3.83 (± 0.54) |
| OFF |
6.41 (±0.63) |
7.21 (± 0.45) |
3.45 (± 0.36) |
| z, df | −2.666, 8 | −2.666, 8 | −1.863, 6 |
| P | 0.024* | 0.016* | 0.063 |
SE, Standard error. * indicates significant (p < 0.05)
The ratio of FEV1/PEFR improved with PPNr stimulation in all patients. The criteria for UAO (FEV1/PEFR ratio > 8.5 mL/L per min3) were satisfied in one patient with a mean FEV1/PEFR ratio of 8.83 mL/L per min without stimulation. Mean FEV1/PEFR ratio was reduced to 8.19 mL/L per min with stimulation and therefore no longer within the UAO critical range. The same patient's mean PEFR improved from 5.7 to 7.4L/sec with stimulation (29.9% improvement). Across all patients, mean FEV1/PEFR ratio improved with stimulation from 7.21 ± 0.45 mL/L per min to 6.75 ± 0.42 mL/L per min (z = −2.666, df = 8, P = 0.016). Given that the FEV1 change was only marginal, the change in FEV1/PEFR ratio with stimulation appears to reflect increases in PEFR only, suggesting an upper rather than lower airway function improvement.
General indices of lung function
There was an increase in mean FVC from 3.22 ± 0.21L to 3.50 ± 0.30L, which was not significant (t = 1.298, df = 8, P = 0.231). No patients showed an FEV1/FVC ratio of less than 70% with stimulation ON or OFF stimulation, which would have suggested overall airway obstruction.32 FEV1/FVC ratio showed no significant change between conditions (OFF 83.5 ± 2.8% vs. ON stimulation 85.3 ± 3.4%; z = −1.540, df = 8, P = 0.123).
Stimulation depth versus upper airway function
Active PPNr electrode contact depth amongst patients was distributed along the rostro‐caudal length of the PPNr (see Fig. 2). Percentage improvement in PEFR was strongly correlated with the rostral location of active contacts (see Fig. 3). Mean active contact depth from the PM line explained 66% of the variance of percentage PEFR improvement with stimulation (r = 0.814, n = 9 and P = 0.008). Thus, the more rostral the stimulation, the greater the percentage PEFR improvement.
Figure 2.
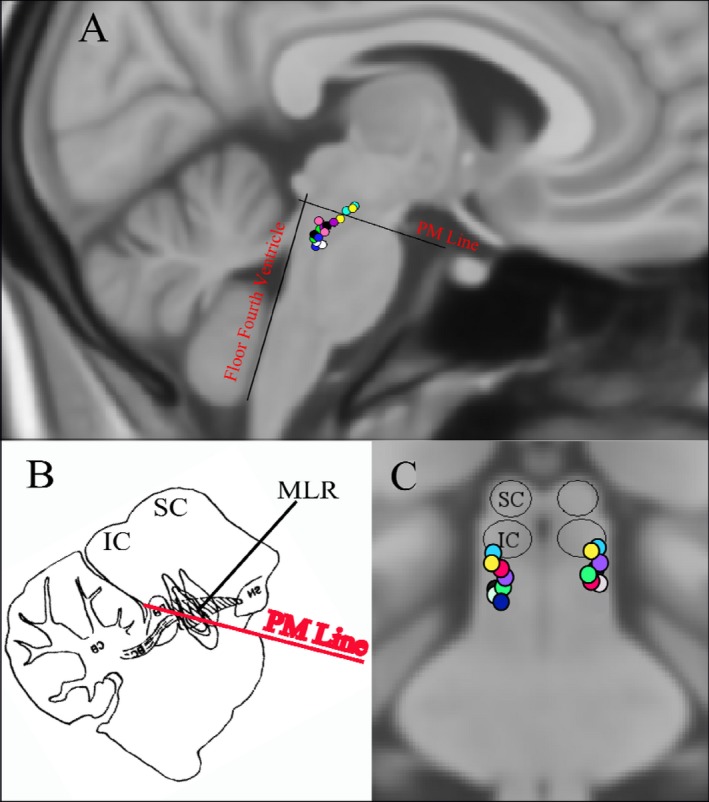
Location of active pedunculopontine region electrode contacts in Montreal Neurological Institute standard space. (A) Sagittal section; (B) Equivalent rat sagittal section demonstrating the mesencephalic locomotor region (MLR) above the pontomesencephalic (PM) line (adapted from Chong & Bedford); (C) Coronal section.
Figure 3.
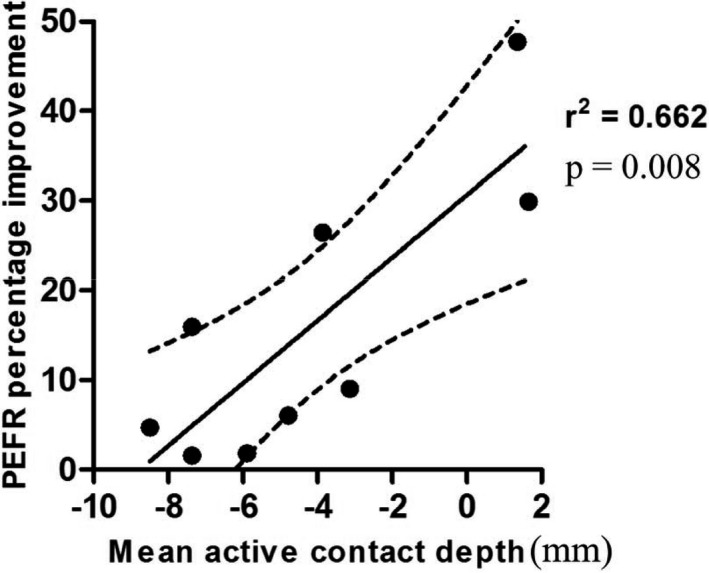
Scatterplot demonstrating the correlation between mean active contact depth from the pontomesencephalic line versus improvement in peak expiratory flow rate.
Electrophysiology: alpha band activity
PPNr LFPs were recorded in seven cases and GPi LFPs were recorded in seven cases. Mean PPNr LFP power was significantly higher within the alpha band during forced respiratory maneuvers (1.63 ± 0.16 μV2/Hz) compared to resting breathing (0.77 ± 0.16 μV2/Hz; z = −2.197, df = 6, P = 0.028) (see Fig. 4). Alpha band power increased during maximal inspiration and peaked during forced expiration, before returning to baseline levels (see Fig. 5).
Figure 4.
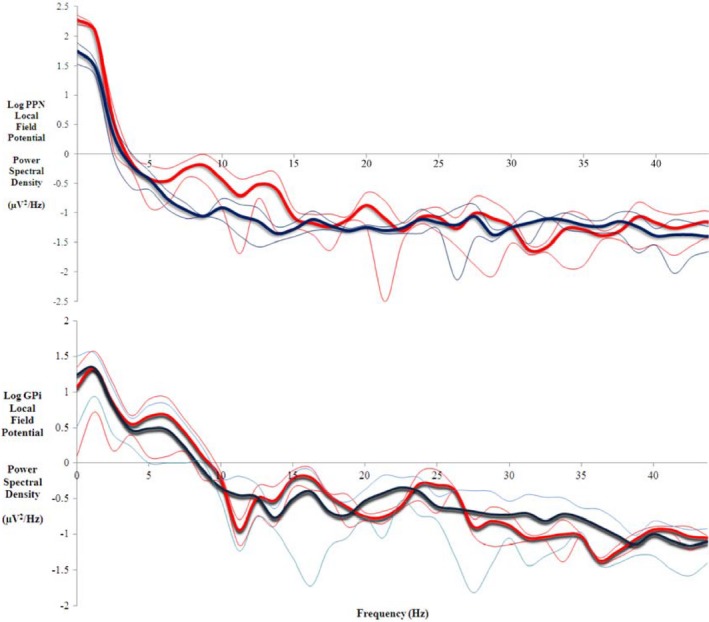
Local field potential power spectra showing increased alpha band (7‐11 Hz) synchronisation during forced respiratory manoeuvres (red) compared to resting breathing (blue) across all trials in two representative patients with 95% confidence limits as thin lines. Upper: Pedunculopontine Nucleus (PPN) region in one patient; Lower: Globus Pallidus interna (GPi) in one patient.
Figure 5.
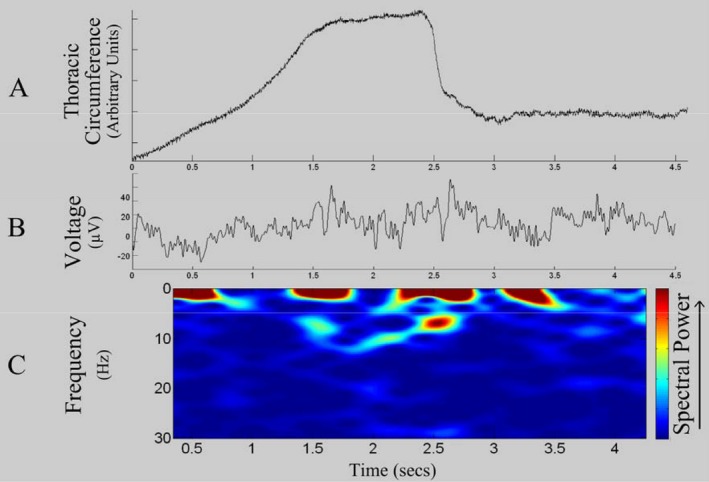
Simultaneously recorded physiological signals in a representative patient. (A) Respiratory trace showing increase in thoracic circumference during maximal inspiration followed by a rapid decrease in circumference during forced expiration; (B) Raw LFP during exertional respiratory maneuver; (C) Time frequency spectrogram demonstrating an increase in alpha 7–11 Hz power during maximal inspiration and forced expiration.
In contrast, no change in mean alpha power within the control GPi nuclei was seen during forced respiratory maneuvers (1.25 ± 1.49 μV2/Hz) compared to resting breathing trials (1.38 ± 1.60 μV2/Hz; z = −1.693, df = 6, P = 0.090) (see Fig. 4).
Across all PPNr and GPi patients studied, percentage increase in PEFR on stimulation was strongly positively correlated with percentage increase in alpha power during spirometry (Spearman's r = 0.653, n = 14, P = 0.0096).
Discussion
The main finding of this study is that low‐frequency electrical stimulation of the PPNr produced a significant increase in spirometric indices of upper airway function, whereas recordings from the same stimulation target revealed an increase in alpha activity during the spirometric maneuvers of maximal inspiration and forced expiration compared to resting breathing in PD patients. No such changes were seen when stimulating and recording from the GPi.
Upper airway dysfunction in PD is an important cause of morbidity and can lead to death. UAO manifests as a reduced PEFR and FEF50, and an increased FEV1/PEFR ratio.2, 4, 6 In our study, improvements were seen in all three of these indices with two of the three reaching statistical significance. PEFR captures the initial 15 milliseconds of forced expiration. This likely reflects upper airway flow as firstly, this early expiratory period receives a major contribution from the large proximal airways, and secondly because it is predominantly limited by turbulence of flow in the large upper airways.2 In contrast, FEV1 captures a longer time window and reflects lower airway performance as well. There was no significant change in FEV1 in our study and the changes in FEV1/PEFR ratio were due to increases in PEFR. Therefore, PPNr stimulation predominantly improved upper, rather than lower, airway performance. This may be a property of PPNr connectivity influencing only one end of the respiratory tree or alternatively may reflect the fact that it is the upper airways which are affected by PD and there is thus more scope to produce a detectable change in their performance. Although changes in clinical outcomes were not formally recorded in this patient group, we would anticipate that clinical consequences of improved upper airway performance would include improvements in symptoms of dyspnea and reduction in problems such as sleep disordered breathing, which has been associated with worse functional outcomes following stroke.33 However, further clinical studies are required to demonstrate this. There was a strong correlation between percentage PEFR improvement and a more rostral location of stimulation within the PPNr. The rostral PPN is within the MLR. Chong & Bedford described arterial blood pressure and heart rate increases in addition to locomotion in rats after electrical stimulation of the MLR,17 and a subset of neurons within the MLR have also been identified as being responsible for increasing ventilation with locomotion.34 In the present study, stimulation was in the rostral PPN above the PM line, at the level of the inferior colliculus and rostral to the parabrachial nucleus. Stimulation was lateral and separate from the periaqueductal gray area. The two patients (yellow and cyan active contacts in Fig. 2) who experienced the greatest PEFR improvement in our study received stimulation to the equivalent area in the human.
The link between the PPNr and respiratory function was strengthened by our finding of an increase in local alpha activity upon maximal inspiration and forced expiration. This change was not observed in GPi, making it unlikely to be a cardiovascular or mechanical epiphenomenon of the respiratory maneuver. Could this activity reflect the activation of the PPNr outflow that innervates the respiratory musculature, given that driving this area at lower frequencies than used at other motor targets for DBS improves spirometric function? It may be that DBS modulates the activity of PPNr cholinergic neurons, which normally have an inhibitory effect on muscle tone,35, and thus improves function of respiratory muscles, which have diminished function in PD.36 PPNr alpha power has also been shown to increase in PD patients during walking,24 which is again improved by low‐frequency PPNr stimulation, and thus the signal may be related to changes in activity in the MLR as discussed above.
An alternative hypothesis is that alpha band synchronization may reflect attentional changes associated with the spirometric maneuver. Indeed, the PPN forms part of the ascending arousal system, a region implicated in alertness. It is postulated that alpha activity may be an index of active suppression to block out distracters to facilitate focusing on a desired subject.37,38 The improved upper airway function in this study might then thus been the result of an improved focus of internal attention during the task, as has been argued for voluntary limb movements.23
It is also possible that current spread to other brain regions such as the Kolliker‐Fuse nucleus and the lateral parabrachial nucleus may have been responsible for the respiratory effects of stimulation, rather than direct stimulation of the PPN proper. The parabrachial/Kolliker‐Fuse complex in the dorsal brainstem is known to have various modulatory effects on respiratory responses depending on the precise site of stimulation, including promotion of hyperpnea, inspiratory response, and apnea, (e.g., Chamberlin and Saper, 199439) and also to be important for locomotor‐respiratory entrainment,40 which may be significant in the context of neuromodulation that is known to improve gait.
There are a number of potential study limitations that should be borne in mind. First, we acknowledge that electrode positions were based on post‐op CT scans carried out on the day of surgery. Repeat CT scans were not acquired at the time of testing, and there was therefore a theoretical potential for small degrees of electrode migration between the post‐op CT and the time of testing. All patients had PD and therefore the electrophysiological activity seen in the PPNr may be a feature specific to the PD phenotype and thus it may not be possible to generalize these results to non‐PD subjects. The GPi patients suffered from dystonia rather than PD. The lack of electrophysiological change is consistent with studies that have shown no change in oscillatory activity during anticipation or performance of exercise despite increases in heart rate, mean arterial blood pressure, and respiratory rate.41 Second, the lack of spirometric improvement with GPi stimulation, is consistent with previous evidence showing no lung function change9 nor change in cardiovascular performance37 after GPi stimulation. None of the patients studied had known respiratory deficits and so the improvements we detected may have been near ceiling level; future studies are necessary to evaluate whether upper airway function can be improved still further in symptomatic patients, and whether the improvements seen in the present patients translate to longer term improvements in respiratory outcomes such as diminished risk of aspiration.
Conclusion
This is the first interventional study in humans to demonstrate an improvement in lung function by electrical stimulation of the PPN region. Improvement was in upper airway function in PD patients. The sites stimulated also synchronized their activity in the 7–11 Hz alpha band during forced spirometric maneuvers. The mesencephalic locomotor region has previously been shown to modulate cardiovascular performance in animals and in this study low‐frequency stimulation was associated with a greater lung function improvement when delivered to rostral part of the PPN region. Thus this study suggests a functional and electrophysiological link between the PPN region, particularly its rostral portion, and upper airway performance. It remains to be seen if this is true outside of PD, and whether the scale of the effect could, under circumstances of impaired lung function, afford symptomatic improvement.
Author Contribution
JH, AG: Conception and design of study. JH, JSB, SW, PS, TC: Identification of subjects, collection and analysis of data. JH, HR, TA, SM, SCM, ALG: Drafting manuscript and editing manuscript
Conflicts of Interest
Prof Green is on an Executive Advisory Board (Movement Disorders) for Abbott and holds a consultancy agreement with Abbott. He also has a Consultancy agreement with Renishaw plc. He has given Expert testimony (unrelated) and receives Royalties from Oxford University Press (unrelated). He holds an MRC grant (unrelated to this project).
Acknowledgments
This study was supported by a research grant from Medtronic Europe S.A. and grants from the Oxford Biomedical Research Centre of the UK NIHR, the Norman Collisson Foundation, the Wolfson Charitable Trust and the National Health and Medical Research Council (Australia).
Funding Information
This study was supported by a research grant from Medtronic Europe S.A. and grants from the Oxford Biomedical Research Centre of the UK NIHR, the Norman Collisson Foundation, the Wolfson Charitable Trust and the National Health and Medical Research Council (Australia).
Funding Statement
This work was funded by Medtronic grant ; Oxford Biomedical Research Centre grant ; Norman Collisson Foundation grant ; Wolfson Charitable Trust grant ; National Health and Medical Research Council (Australia) grant .
References
- 1. Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17:427–442. [DOI] [PubMed] [Google Scholar]
- 2. Vincken WG, Gauthier SG, Dolfuss RE, et al. Involvement of upper airway muscles in extrapyramidal disorders: a cause of airflow limitation. N Engl J Med 1984;311:438–442. [DOI] [PubMed] [Google Scholar]
- 3. Vincken WG, Darauay CM, Cosio MG. Reversibility of upper airway obstruction after levodopa therapy in Parkinson's disease. Chest 1989;96:210–212. [DOI] [PubMed] [Google Scholar]
- 4. Hovestadt A, Bogaard JM, Meerwaldt JD, van der Meche FGA. Pulmonary function in Parkinson's disease. J Neurol Neurosurg Psychiatry 1989;52:329–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lilker ES, Woolf CR. Pulmonary function in Parkinson's syndrome: the effect of thalamotomy. Can Med Assoc J 1968;99:752–757. [PMC free article] [PubMed] [Google Scholar]
- 6. Empey DW. Assessment of upper airways obstruction. BMJ 1972;3:503–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Green AL, Stone E, Sitsapesan H, et al. Switching off micturition using deep brain stimulation at midbrain sites. Ann Neurol 2012;72:144–147. [DOI] [PubMed] [Google Scholar]
- 8. Hyam JA, Kringelbach ML, Silburn PA, et al. The autonomic effects of deep brain stimulation ‐ a therapeutic opportunity. Nat Rev Neurol 2012b;8:391–400. [DOI] [PubMed] [Google Scholar]
- 9. Hyam JA, Brittain JS, Paterson DJ, et al. Controlling the lungs via the brain: a novel neurosurgical method to improve lung function in humans. Neurosurgery 2012a;70:469–477. [DOI] [PubMed] [Google Scholar]
- 10. Carrive P, Bandler R. Viscerotopic organization of neurones subserving hypotensive reactions within the midbrain periaqueductal grey: a correlative functional and anatomical study. Brain Res 1991;541:206–215. [DOI] [PubMed] [Google Scholar]
- 11. Bittencourt AS, Carobrez AP, Zamprogno LP, et al. Organization of single components of defensive behaviors within distinct columns of periaqueductal gray matter of the rat: role of N‐methyl‐D‐aspartic acid glutamate receptors. Neuroscience 2004;125:71–89. [DOI] [PubMed] [Google Scholar]
- 12. Benarroch E. Central autonomic network: functional organization and clinical correlations. Chapters 1&2, New York: Wiley‐Blackwell, 1998. [Google Scholar]
- 13. Inglis WL, Winn P. The pedunculopontine segmental nucleus: where the striatum meets the reticular formation. Prog Neurobiol 1995;47:1–29. [DOI] [PubMed] [Google Scholar]
- 14. Skinner RD, Kinjo N, Henderson V, Garcia‐Rill E. Locomotor projections from the pedunculopontine nucleus to the spinal cord. NeuroReport 1990a;1:183–186. [DOI] [PubMed] [Google Scholar]
- 15. Skinner RD, Kinjo N, Ishikawa Y, et al. Locomotor projections form the pedunculopontine nulceus to the medioventral medulla. NeuroReport 1990b;1:207–210. [DOI] [PubMed] [Google Scholar]
- 16. Bedford TG, Loi PK, Crandall CC. A model of dynamic exercise: the decerebrate rat locomotor preparation. J Appl Physiol 1992;72:121–127. [DOI] [PubMed] [Google Scholar]
- 17. Chong RKY, Bedford TG. Heart rate, blood pressure, and running speed responses to mesencephalic locomotor region stimulation in anaesthetised rats. Eur J Neurosci 1997;434:280–284. [DOI] [PubMed] [Google Scholar]
- 18. Gariepy J‐F, Missaghi K, Chevallier S, et al. Specific neural substrate linking respiration to locomotion. PNAS 2012;109:E84–E92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Plaha P, Gill SS. Bilateral deep brain stimulation of the pedunculopontine nucleus for Parkinson's disease. NeuroReport 2005;16:1883–1887. [DOI] [PubMed] [Google Scholar]
- 20. Thevathasan W, Coyne TJ, Hyam JA, et al. Pedunculopontine nucleus stimulation improves gait freezing in Parkinson disease. Neurosurgery 2011a;69:1248–1253; discussion 1254. [DOI] [PubMed] [Google Scholar]
- 21. Thevathasan W, Pogosayan A, Hyam JA, et al. A block to pre‐prepared movement in gait freezing, relieved by pedunculopontine nucleus stimulation. Brain 2011b;134:2085–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thevathasan W, Cole MH, Graepel CL, et al. A spatiotemporal analysis of gait freezing and the impact of pedunculopontine nucleus stimulation. Brain 2012a;135:1446–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Androulidakis AG, Mazzone P, Litvak V, et al. Oscillatory activity in the pedunculopontine area of patients with Parkinson's disease. Exp Neurol 2008;211:59–66. [DOI] [PubMed] [Google Scholar]
- 24. Thevathasan W, Pogosyan A, Hyam JA, et al. Alpha oscillations in the pedunculopontine nucleus correlate with gait performance in parkinsonism. Brain 2012b;135:148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of Spirometry. Eur Respir J 2005;26:319–338. [DOI] [PubMed] [Google Scholar]
- 26. Green AL, Wang S, Owen SLF, et al. Deep brain stimulation can regulate arterial blood pressure in awake humans. NeuroReport 2005;16:1741–1745. [DOI] [PubMed] [Google Scholar]
- 27. Green AL, Hyam JA, Williams C, et al. Intra‐operative deep brain stimulation of the periaqueductal grey matter modulates blood pressure and heart rate variability in humans. Neuromodulation 2010;3:174–181. [DOI] [PubMed] [Google Scholar]
- 28. Quanjer PH, Lebowitz MD, Gregg I, et al. Peak expiratory flow: conclusions and recommendations of the European Respiratory Society. Eur Respir J 1997;10 (Suppl):24,2s–8s. [PubMed] [Google Scholar]
- 29. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 2004;23(S1):208–219. [DOI] [PubMed] [Google Scholar]
- 30. Woolrich MW, Jbabdi S, Patenaude B, et al. Bayesian analysis of neuroimaging data in FSL. NeuroImage 2009;45:S173–S186. [DOI] [PubMed] [Google Scholar]
- 31. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B 1995;57:289–300. [Google Scholar]
- 32. Pauwels RA, Buist AS, Calverley PMA, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) workshop summary. Respir Crit Care Med 2001;163:1256–1276. [DOI] [PubMed] [Google Scholar]
- 33. Turkington PM, Allgar V, Bamford J, et al. Effect of upper airway obstruction in acute stroke on functional outcome at 6 months. Thorax 2004;59:367–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gariepy JF, Missaghi K, Chevallier S, et al. Specific neural substrate linking respiration to locomotion. Proc Natl Acad Sci USA 2012;109:5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Takakusaki K, Obara K, Nozu T, Okumura T. Modulatory effects of the GABAergic basal ganglia neurons on the PPN and the muscle tone inhibitory system in cats. Arch Ital Biol 2011;149:385–405. [DOI] [PubMed] [Google Scholar]
- 36. Vercueil L, Linard JP, Wuyam B, et al. Breathing pattern in patients with Parkinson's disease. Respir Physiol 1999;118:163–172. [DOI] [PubMed] [Google Scholar]
- 37. Ward LM. Synchronous neural oscillations and cognitive processes. Trends Cogn Sci 2003;7:553–559. [DOI] [PubMed] [Google Scholar]
- 38. Thornton JM, Aziz TZ, Schlugman D, Paterson DJ. Electrical stimulation of the midbrain increases heart rate and arterial blood pressure in awake humans. J Physiol 2002;539(Pt 2):615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chamberlin NL, Saper CB. Topographic organization of respiratory responses to glutamate microstimulation of the parabrachial nucleus in the rat. J Neurosci 1994;14:6500–6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Giraudin A, Le Bon‐Jego M, Cabirol M‐J, et al. J Neurosci 2012;32:11841–11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Green AL, Wang S, Purvis S, et al. Identifying cardiorespiratory neurocircuitry involved in central command during exercise in humans. J Physiol 2007;578:605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]


