Abstract
Essential tremor (ET) is one of the most common adult movement disorders, characterized by clinical tremor and other nonmotor symptoms. It is a progressive disease that shares features with other neurodegenerative diseases. ET is a complex disease with both genetic and environmental underpinnings. While genetic forms of ET are well recognized, the role of environmental and lifestyle factors in ET has been debated. Studies suggest that exposure to neurotoxic compounds such as β‐carboline alkaloids and ethanol are potential risk factors for ET, while antioxidant intake may be protective. In addition, smoking acts as a protective factor in ET, parallel to its effects in other neurological diseases. New evidence points to pesticide and lead exposure as potential risk factors. There is growing evidence to suggest that environmental and lifestyle factors play a role in ET but additional research is needed in order to completely understand their cause and effect association. There is also a need for larger case‐control and prospective cohort studies across different populations to further evaluate the etiological importance of these factors in ET.
Introduction to Essential Tremor
Essential tremor (ET) is a chronic neurological disease more prevalent among the elderly. It is one of the most common adult movement disorders, with reported pooled prevalence of 0.9% across all ages, 4.6–6.3% above age 60–65, and up to 21.7% above age 95.1 ET is characterized by action or postural tremors at frequencies 4–12 Hz,2 most commonly involving upper limbs, but also the head, face or jaw, trunk, and lower limbs.2, 3 Besides tremor, ET patients may also exhibit signs of cerebellar involvement, including gait ataxia and vestibulocerebellar involvement.3, 4
While well recognized as a movement disorder, increasing evidence suggests associations with nonmotor symptoms including cognitive,5, 6 psychiatric,7, 8 and sensory symptoms including hearing and olfactory impairment,9, 10 dementia,11, 12 anxiety, depression, and personality changes.13, 14 The heterogeneity of clinical presentations suggests that ET is a more complex clinical syndrome with a defining feature of action tremor as opposed to a monosymptomatic disorder, although kinetic tremors remain a core feature. While genetic forms of ET are well recognized, the role of environmental and lifestyle factors in ET has been debated. Several emerging studies have examined associations between environmental, lifestyle factors, and ET. We provide a concise and focused review of the current knowledge of lifestyle and environmental factors in ET, highlight potential new trends and areas of focus for future studies.
Pathophysiology of ET
The pathophysiology of ET has been debated. Some studies establish evidence of a degenerative process, while others suggest functional dysregulation of neuronal transmission. Postmortem studies report two main subgroups of pathology, one with the presence of Lewy bodies in the brainstem, and the other without Lewy bodies but with degenerative changes of the cerebellum, marked by Purkinje cells loss and axonal swelling.15, 16 Some hypothesized that Purkinje cell pathology may be part of the cellular cascade leading to ET,17 causing remodeling of the cerebellar cortex leading to cerebellar degeneration.17
The presence of Lewy bodies and protein aggregation in brain tissue18 supports an anatomical basis for clinical similarities between ET and Parkinson's disease (PD) (such as common feature of tremor), although Lewy bodies are reported only in a small proportion of ET patients.19 ET patients are more likely to develop PD compared to healthy individuals,20 suggesting that early cellular degenerative changes may be common to both diseases. Neuroimaging has demonstrated iron accumulation in basal ganglia of ET patients,21 a finding that has been similarly observed in other neurodegenerative diseases including PD.
Genetic Contribution in ET
The genetics basis of ET has been supported by evidence of heredity in familial ET. Linkage studies have mapped susceptibility loci at gene regions on chromosome 3q13 (ETM1) in Icelandic families,22 chromosome 2p22‐25 (ETM2) in American families,23 and chromosome 6p23 in North American families (ETM3).24 Genome‐wide association studies identified mutations in the Leucine‐rich repeat and Ig domain‐containing Nogo receptor interacting protein‐1 gene (LINGO1)25 and solute carrier family 1‐glial affinity glutamate transporter‐member 2 (SLC1A2) gene.26 Exome sequencing studies identified mutations in the fused in sarcoma/translated in liposarcoma (FUS/TLS) gene in a Canadian family,27 the HTRA2 gene in a Turkish family,28 and variants of TENM4 gene in the Spanish population.29 Current studies suggest an autosomal dominant inheritance pattern with low penetrance in familial ET, and a non‐Mendelian pattern, for example, multifactorial inheritance in sporadic ET.30 Inherited neurological channelopathies may also contribute to disease pathogenesis, supported with recent functional data.31 Despite the strong hereditary component in monogenic ET, few disease genetic variants have been identified. The presence of complex non‐Mendelian inheritance patterns or environmental confounding may be contributing factors.
Environmental and Lifestyle Effects on ET
Nongenetic factors may have significant effects on ET, suggested by the high preponderance of ET cases without family history or affected relatives, together with reported 60–63% concordance in monozygotic twins in twin studies.32, 33
Lifestyle and environmental factors are known to play an important role in other neurological disorders. Earlier studies in ET have investigated effects of dietary factors including β‐carboline alkaloids, meat, caffeine, and alcohol intake, as well as smoking habits and occupational toxin exposures of pesticides and solvents (Fig. 1).
Figure 1.
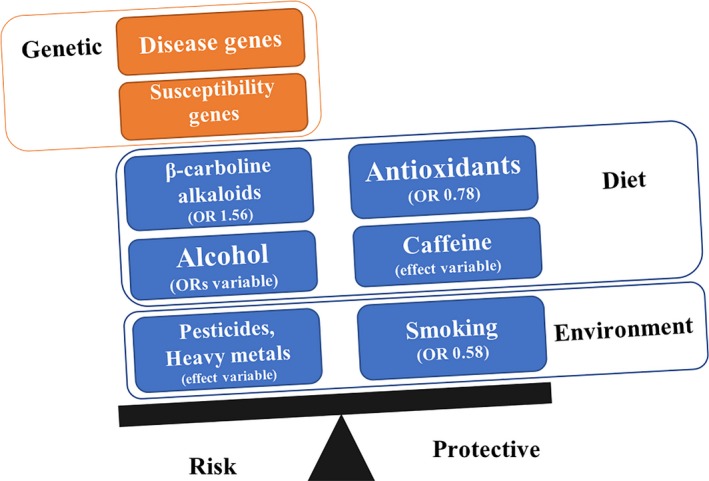
Complex genetic and environmental factors affecting essential tremor.
Lifestyle factors
Diet
Diet is a key component of life. The effects of β‐carboline alkaloids found in meat and dietary antioxidants have been studied with no clear conclusion. New evidence suggests that ethanol, previously thought to have tremor‐relieving effects, may be a risk factor as a cerebellar toxin. Evidence on the protective effects of caffeine consumption remains inconclusive. The studies on dietary components in ET have been summarized (Tables 1 and 2).
Table 1.
Summary of case‐control studies on dietary exposure in essential tremor
| Categories | Authors | Year | Sample size | Country | Exposure | Results |
|---|---|---|---|---|---|---|
| Antioxidants | Scarmeas & Louis 52 | 2007 | 148 ET, 250 controls | USA | Mediterranean diet |
Higher adherence to diet was associated with lower odds for ET [0.78 (0.61–0.99); P = 0.042]. Compared to lowest adherence tertile, middle tertile [0.41 (0.16–1.05)], and highest tertile [0.29 (0.10–0.82)] had a lower ET odds [P for trend 0.021] |
| Louis et al. 51 | 2005 | 156 cases, 220 controls | USA | Vitamin E, vitamin C | No significant difference in vitamin E and C consumption between ET cases and controls | |
| Meat (harmane) | Louis et al. 46 | 2008 | 125 cases, 125 controls | USA | Meat consumptions, meat doneness level |
Higher total current meat consumption was associated with ET (OR = 1.006, P = 0.04) only in male ET cases. Male cases had higher odds of being in the highest than lowest quartile of total current meat consumption (adjusted OR = 21.36, P = 0.001). No significant difference between meat doneness level in cases and controls. |
| Louis et al. 45 | 2005 | 106 cases, 161 controls | USA | Blood harmane concentration, animal protein intake |
Higher log (blood harmane concentration) in ET cases than controls. Similar amounts of animal protein consumption and caloric intakes in ET cases and controls. |
|
| Harmane | Louis et al. 38 | 2013 | 130 cases, 138 controls | Spain | Blood harmane concentration | Blood harmane concentrations higher in ET compared to controls, but did not reach statistical significance |
| Louis et al. 40 | 2013 | 70 cases, 27 controls | USA | Brain harmane concentration |
Mean brain harmane concentration was higher in ET than controls. Mean concentration was 35% higher in ET cases compared to controls after adjustment for postmortem interval and freezer time. |
|
| Louis et al. 37 | 2008 | 150 cases, 135 controls | USA | Blood harmane concentration |
Higher Log (blood harmane concentration) in ET compared to controls (ORadjusted 1.56, 95% CI 1.01–2.42, P = 0.04) Higher odds of ET OR 1.90 (95% CI 1.07–3.39, P = 0.029) in highest vs. lowest log (blood harmane concentration) tertile. |
|
| Louis et al. 36 | 2002 | 100 cases, 100 controls | USA | Blood harmane concentration |
Higher mean log (blood harmane concentration) in cases than controls. Median harmane = 5.21 g(−10)/mL in cases and 2.28 g(−10)/mL in controls) (P = 0.005). |
|
| Caffeine | Prakash et al. 66 | 2006 |
79 cases, 100 controls |
Singapore | Caffeine consumption |
Caffeine consumption in ET patients higher than control group (nonsignificant in multivariate analysis). No significant correlation with disease duration or total tremor score in ET patients |
| Louis et al. 57 | 2004 | 130 cases, 175 controls | USA | Caffeine consumption | No correlation between daily caffeine intake in milligrams and disease duration, total tremor score, or performance‐based test score in ET cases. |
Table 2.
Summary of studies on alcohol in essential tremor
| Authors | Year | Study type | Sample size | Country | Exposure | Results |
|---|---|---|---|---|---|---|
| Nicoletti et al. 63 | 2011 | Case‐control | 83 cases, 245 controls | Italy | Wine |
Negative association between essential tremor and wine consumption preceding the onset of disease OR = 0.23 (0.08–0.64) with a significant dose effect 1–2 glass of wine per day: OR 0.32 >3 glass of wine per day: OR 0.14 |
| Louis & Michalec 58 | 2014 | Case‐control | 354 cases, 370 controls | USA | Ethanol |
No significant difference in average daily ethanol intake between cases and controls. Among cases, there was no correlation between average daily ethanol intake and tremor severity. |
| Louis et al. 62 | 2009 | Prospective cohort study | 3285 (76 ET cases) | Spain | Ethanol |
Baseline number of drink‐years was marginally associated with a higher risk of incident ET (RR = 1.003) Highest baseline drink‐year quartile doubled the risk of incident ET (RR = 2.29), with decreasing RR down the quartiles With each higher drink‐year quartile, the risk of incident ET increased an average of 23%. |
| Jiménez‐Jiménez et al. 56 | 2007 | Case‐control | 142 cases, 284 controls | Spain | Ethanol |
Increased exposure to alcohol was significantly associated with higher age of ET onset. Time of exposure to alcohol was correlated with age at onset of ET Total consumption of alcohol was not correlated with age at onset of ET |
| Louis et al. 57 | 2004 | Case‐control | 130 cases, 175 controls | USA | Ethanol | No significant difference in ethanol intake between ET cases and controls |
β‐carboline alkaloids and meat consumption
β‐carboline alkaloids are a class of highly neurotoxic compounds. Harmaline is primarily used to generate tremors in animal models for ET.34 Harmane, which has tremor‐producing effects,35 is the most abundant dietary β‐alkaloid found in meat, and also the most potent and well studied of these compounds.
Studies showed a clear association between blood harmane concentration and ET, with higher concentrations found in cases compared to controls.36, 37, 38 Harmane levels were associated with disease severity, measured by tremor severity and olfactory deficit.39 Further studies supported the neurotoxic action of harmane in brain tissue by correlating brain harmane concentrations with ET.40 Blood harmane concentration was a significant predictor of cerebellar N‐acetylaspartate/total creatine (NAA/tCr), an MRI neuroimaging marker indicating cerebellar neuronal degeneration.41
However, associations with dietary β‐carboline alkaloid consumption were less established. The main source of β‐carboline alkaloids is in one's diet, with dietary intake being 50 times greater than endogenous production,42 and cooking at high temperatures leads to increased concentrations in foods.43, 44 However, dietary studies showed no difference in animal protein intake in ET.45 A later study by the same group reported higher total meat consumption in male ET cases compared to controls but not in women.46 These findings may be influenced by higher meat intake in the male population, or suggest that relative dietary contributions of meat to disease pathogenesis may differ between men and women. The authors reported no significant difference in meat doneness level, suggesting that the effect of heat on cooking may not be significant.46 These studies failed to identify any clear correlation between animal protein consumption and blood harmane concentrations,45 suggesting that dietary protein consumption may not be the ideal surrogate measure for biological levels of harmane in the body. The lack of association with dietary harmane suggests that it may be useful to focus on the effects of its metabolism.
The cross‐sectional study design of existing studies do not account for prediagnosis diet in ET cases. Additionally, dietary data were captured with the Food Frequency Questionnaire, which is reflective of current diet but not past diet. This may be addressed with future cohort studies collecting more comprehensive dietary data before incidence of ET.
Antioxidants
The diet represents the main source of antioxidants in the human body. Prominent antioxidants include Vitamins C and E, β‐carotene, and coenzyme Q. Dietary antioxidants may have a protective effect on some neurodegenerative diseases.47, 48, 49, 50 However, its role in ET has not been studied in detail.
Direct antioxidant consumption of vitamins C and E was not significantly different between ET and healthy controls.51 In contrast to isolated dietary antioxidant components, the evaluation of composite diets may prove to be more promising. Higher adherence to the Mediterranean composite diet, known for its high antioxidant content, was associated with lower odds of ET (OR [0.78 (0.61–0.99)].52 Additionally, subjects in the highest tertile of diet adherence had lower ET risk.51 Assessment of whole diets may be more effective in capturing overall antioxidant intake as opposed to isolated components. Future studies should evaluate whole diets to quantify antioxidant intake more comprehensively.
Ethanol
Alcohol consumption has been studied in ET, but mostly for its tremor‐relieving effects. Alcohol was historically found to reduce tremor severity transiently. While the mechanism of action remains unclear, suppressive effects on the inferior olivary nuclei53, 54 and GABA transmission pathways55 have been suggested.
Contrary to this knowledge, weak or nonsignificant associations with alcohol intake were reported with ET.56, 57 A quantitative study found no correlation between average ethanol intake and tremor severity evaluated using a validated clinical rating scale.58 The recent development of more objective and stringent methods of quantifying tremor severity may have proven these tremor‐relieving effects to be less significant than expected.
Ethanol is a well‐established cerebellar toxin, demonstrating degenerative effects leading to Purkinje cell loss and cerebellar atrophy.59, 60, 61 This suggests that ethanol is more likely a risk factor. However, this has been difficult to study as the tremor‐relieving effects of alcohol have led to confounding links with chronic alcoholism in ET, complicating any causal effects of alcohol. Nonetheless, some studies addressed this limitation by investigating baseline alcohol consumption with incident ET, reporting a direct association between alcohol and ET onset (Table 2). In a Spanish population‐based case‐control study, baseline alcohol intake was marginally associated with increased risk of incident ET after adjusting for smoking and depressive symptoms.62 A quantitative analysis reported that the highest baseline alcohol intake tertile was associated with twice the risk of developing ET, with an increase of incident ET risk by 23% per tertile increase in alcohol intake.62 These studies support the hypothesis that ethanol consumption is a contributory risk factor of ET.
In contrast, studies on wine consumption have reported conflicting results. In a small Italian case‐control study, prediagnosis wine consumption had an inverse negative correlation with ET [OR 0.35(0.18, 0.69)]63 (Table 2), suggesting that wine may be a protective factor. This apparent effect may be attributed to the rich antioxidant content of wine acting in opposition to the neurotoxic effects of alcohol. There may be value in investigating different types of alcohol for their differing effects on ET.
The contradicting effects of various dietary components reflect the difficulties in assessing the role of diet in ET. The balance of antioxidant and oxidative components in individual foods appears to play a role in disease pathogenesis. Current studies are limited by small sample size and cross‐sectional design. There is a need for larger studies with more comprehensive dietary evaluation to assess the effects holistically, specifically focusing on antioxidant components. Further, longitudinal studies will also be necessary to demonstrate any causal effects of alcohol on ET.
Caffeine
Caffeine has been studied as a potential risk factor in ET. Endogenous adenosine activation was found to depress excitatory transmission in mouse models, reducing tremor.64 Caffeine acts as a nonselective adenosine antagonist, and may trigger or exacerbate tremor clinically. Additionally, brewed coffee contains β‐carboline alkaloids formed during the brewing process.65
However, studies failed to find any correlation with caffeine consumption (Table 1). A New York study reported no correlation between daily caffeine intake with ET disease duration or tremor severity.57 A separate study in Singapore similarly showed no significant correlation between caffeine intake and disease duration or tremor scores.66
Sleep
Sleep disturbances are part of the nonmotor symptoms of ET. However, the association between sleep and ET has not been well studied. One prospective population‐based study reported shorter sleep duration to be associated with increased risk of incident ET.67 This remains a potential area for future studies.
Environmental factors
Smoking
The protective effect of smoking has been reported by a series of Spanish case‐control studies (Table 3). A Spanish population‐based study reported that ever‐smokers had half the risk of ET compared to never‐smokers. A dose‐dependent response was reported in the same study, with smokers in the highest pack‐year tertile having only one‐third the risk of ET compared to never‐smokers.68 In the same cohort, smoking was associated with slightly lower risk of incident ET, and adjusted Cox proportional hazards model analysis supported a similar dose‐dependent association.69 Another Spanish study reported a correlation between increased period of smoking exposure and earlier age of ET onset, although there were no significant associations with the amount of smoking exposure.56
Table 3.
Summary of studies on environmental exposures in essential tremor
| Authors | Year | Study type | Sample size | Country | Exposure | Results |
|---|---|---|---|---|---|---|
| Louis et al. 69 | 2008 | Prospective cohort study | 3348 (77 ET cases) | Spain | Smoking |
The highest baseline pack‐year tertile was associated with lower risk of incident ET. Cases in the highest pack‐year tertile were only one‐third as likely to develop ET compared with nonsmokers (RR 0.29, 95% CI 0.09–0.90, P = 0.03) |
| Benito‐Leon et al. 68 | 2008 | Case‐control | 221 cases, 663 controls | Spain | Smoking |
Ever‐smokers are less likely to have ET than the never‐smokers (OR 0.58, 95% CI 0.40–0.84, P = 0.004). Inverse association between number of pack‐years and ET (adjusted OR 0.991, 95% CI 0.984–0.997, P = 0.005). Ever‐smokers in the highest pack‐year tertile were one‐third as likely to have ET than the never‐smokers (adjusted OR 0.39, 95% CI 0.22–0.69, P = 0.001). |
| Jiménez‐Jiménez et al. 56 | 2007 | Case‐control | 142 cases, 284 controls | Spain | Smoking |
Time of exposure to smoking was correlated with age at onset of ET. No associations found with total amount of smoking. |
| Azevedo & Meyer 75 | 2017 | Case‐control | 51 cases, 204 controls | Portugal | Pesticide exposure |
Exposure to 16 to 16.9 years of pesticide use had highest odds of essential tremor (ORadj 4.60; 95% CI: 1.29–16.41) No significant correlation of ET with other durations of pesticide exposure, no clear dose‐response trend. |
| Yao et al. 74 | 2015 | Cross‐sectional | 5932 (216 ET cases) | China | Pesticide exposure | Past pesticide exposure was found to be greater in ET compared to controls. |
| Dogu et al. 77 | 2007 | Case‐control | 105 cases, 105 controls | Turkey | Lead exposure | Higher median blood lead concentration in ET cases (2.7 microg/dL) compared to controls (1.5 microg/dL) |
| Louis et al. 73 | 2006 | Case‐control | 136 cases, 144 controls | USA | Pesticide exposure | No significant difference in serum concentrations of organochlorine pesticides between ET cases and controls. |
The basis of this protective effect remains unclear, but may draw some similarities to the effects of smoking in other neurological diseases. In cellular in vitro experimental systems, nicotine activity at nicotinic acetylcholine receptors has been shown to provide protection from neurotoxic insults, with nicotine pretreatment reducing neurotoxicity in cell systems.70, 71, 72 Studies in animal models have demonstrated that tobacco constituents can protect against nigrostriatal damage. This formed the basis for the protective effect of smoking in PD, where nicotine‐stimulated dopamine release from nigrostriatal dopaminergic neurons via stimulation of the nicotinic acetylcholine receptors provides neuroprotection.
To date, the protective effect of smoking on ET has been shown in studies limited to Spain and within a similar cohort. Studies from other populations are lacking. We cannot exclude the possibility that the protective effect of smoking may be specific to genetic or other environmental factors limited to this selected Spanish population. Future prospective studies in other cohorts and countries would be useful in confirming the generalizability of these findings.
Pesticides and farming
Pesticide exposure, particularly organochlorine pesticides, has been linked to tremors. However, current evidence has been inconclusive. There was no difference in serum concentrations of six organochlorine pesticides in ET cases compared to controls.73 A similar lack of association with self‐reported pesticide exposure was reported by two case‐control studies in Spain and Singapore56, 66 (Table 3).
In contrast to these earlier studies, a large study cohort in China reported a positive association between self‐reported pesticide exposure and ET.74 Another study in Portugal reported significantly higher pesticide exposure in ET.75 Although this study reported that having 16–16.9 years of exposure led to highest ET risk, there was no significant dose‐dependent trend noted across the range of exposure durations reported,75 making it difficult to interpret these results in the context of other studies. A major limitation of these two studies is that they were not published in English, hence details of the study could not be assessed. Larger studies are needed to reassess the effect of pesticide exposure in ET, and more quantitative methods of assessing pesticide exposure are required. Self‐reported values may not be sufficiently sensitive to detect differences between ET and controls. Future studies may consider the use of surrogate measures such as occupational exposures or serum biomarkers together with self‐reported exposures.
Other exposures – lead, heavy metals
Exposure to heavy metals, specifically lead and manganese, has been studied in a small number of studies. Lead exposure has been shown to cause neurological disorders with tremors. ET patients had higher blood lead concentrations compared to controls, reported in studies conducted in New York76 and Turkey77 (Table 3). The effect of lead toxicity may be explained by its destructive effects on cerebellar tissue and Purkinje neurons. Subsequently, no new studies have investigated the effects of lead exposure in ET.
Besides lead, the role of exposures to other metals and solvents in ET is inconclusive. Case‐control studies have found no significant difference in occupational exposure to manganese and organic solvents78 or iron–manganese alloys56 in ET cases compared to controls, although iron–manganese alloy exposure was associated with increased age of ET onset.56
There is a lack of studies evaluating these toxic exposures, likely due to the difficulties in quantifying these exposures accurately. The use of serum biomarker levels may be more reliable markers of such exposures as opposed to questionnaires.
Limitations of studies
There are several limitations of current literature on environmental factors in ET. First, the diagnostic criteria and definition of ET remain variable across several studies. Several studies employ clinical criteria in accordance with the Consensus statement of the Movement Disorder Society on Tremor. Studies based on the cohort recruited from Columbia University Medical Center (CUMC) employ additional diagnostic criteria, evaluating for ET using the validated Washington Heights‐Inwood Genetic Study of Essential Tremor (WHIGET) criteria. Other studies employed clinical criteria based on tremor features and family history. The lack of uniformity in the definition and diagnosis of ET makes it difficult to draw reliable comparisons across the various studies.
Current studies recognize and define ET as a single disease, but do not account for the heterogeneity of the disease. Heterogeneity in clinical features, such as tremor type, location, severity, and progression, as well as in age of onset and pharmacological reactions suggests that ET may be further subdivided in multiple disease subtypes.79 Pathogenesis pathways may differ between these clinical subtypes, confounding current findings. Future studies may consider classifying ET subtypes based on predominant features instead of assessing ET cases as a monosymptomatic group.
Existing studies have variable exclusion criteria for comorbid conditions. Most studies excluded patients exhibiting signs of dystonia and PD. Other movement disorders with predominant features of tremor, such as spinocerebellar ataxia, were not excluded in a portion of the studies, though these are likely to be rare.80 Additionally, some studies did not account for or exclude tremor attributable to other conditions such as hyperthyroidism, Wilson's disease, or anxiety disorders, which can be misleading in the clinical setting. Tremors due to concomitant drug use were also not excluded in multiple studies.
Methods of data collection on the environmental exposure studied should be validated with good reliability. Most studies conducted in‐person interviews using questionnaires, administered by trained testers or the investigators themselves. Dietary exposures were generally captured using the Willett Semi‐Quantitative Food Frequency Questionnaire, a validated questionnaire to assess current diet. Other environmental exposures such as smoking and alcohol intake were assessed with structured questionnaires. However, these questionnaires have generally not been validated in the study population. The use of follow‐up or repeated administrations of the questionnaire at separate time points would be useful in validating the sensitivity and repeatability of these questionnaires within each study cohort.
A major limitation is that a majority of these studies were conducted by the same research groups using the same cohort of subjects, hence capturing data within limited geographical locations. Dietary studies were largely limited to the USA, with subjects recruited from CUMC. Studies on smoking and ethanol consumption generally recruited subjects from the Neurologic Disorders in Central Spain (NEDICES) study, which only included cases from a select number of communities in Central Spain. There is limited data from other countries, making it difficult to extrapolate the validity of these findings in other countries or in different ethnic populations. There is a need for wider coverage to allow us to draw conclusions on the generalizability of these findings.
Publication bias is an inherent limitation that may lead to biased sampling when evaluating current literature. Studies reporting higher effect sizes and significant findings are more likely to be published, leading to bias in the published literature. The current findings may not be representative of actual effects of environmental factors, which may be less promising than reported.
Current studies are limited by small sample sizes and cross‐sectional design, making it difficult to establish any clear temporal association of these possible risk and protective factors. Prospective cohort studies or longitudinal studies will allow temporal associations to be evaluated. Additionally, serum biomarker data were often single time‐point measurements, which fluctuate with dietary variation. Multiple time‐point data should be measured with standardized methodology to enhance study validity. Larger nested case‐control studies or multi‐center studies may be more effective in deriving clearer conclusions on the effects of environmental exposures in ET, and to confirm or disprove the findings from earlier studies.
Future directions
Current evidence suggests that environmental and lifestyle factors play an important role in ET, although their underlying pathogenetic pathways are not well understood. There is a need to extend the study of these environmental factors to larger populations, using nested case‐control studies in different populations to assess the generalizability of the results. Prospective cohort studies or other longitudinal study designs will be necessary in future to establish the etiological role of these lifestyle factors in ET more clearly.
The study of dietary factors will also benefit from more comprehensive evaluation of the diet instead of various dietary components in isolation. There is added potential of incorporating culture‐specific dietary habits into the study of dietary factors. There may be a need for better surrogate measures of dietary components and toxin exposures in the environment to serve as more reliable markers of exposure. Questionnaires used to quantify exposure should be validated and reliable, with proven repeatability to ensure that exposure information is captured accurately.
Conclusions
ET is a complex disease contributed by both genetic and environmental factors. While reviewing the past and current evidence for lifestyle and environmental factors as risk or protective factors in ET, we have identified certain factors including exposure to neurotoxic compounds such as β‐carboline alkaloids and ethanol as potential risk factors for ET, while antioxidant intake may be protective. Revisiting previously studied environmental factors in ET have also renewed our understanding of these factors. Smoking acts as a protective factor in ET, parallel to its effects in other neurological diseases. New evidence suggests that pesticide and lead exposure may also be potential risk factors. However, there are many limitations of current studies that have to be addressed and findings have to be replicated in independent cohorts and validated. There is a need for larger, nested case‐control studies or prospective cohort studies across different populations, before more conclusive statements can be made on the etiological importance of various lifestyle and environmental factors in ET.
Conflict of Interest
None declared.
Acknowledgments
The authors thank the support of National Medical Research Council for funding ET research (EK‐T, STaR investigator award).
Funding Information
The authors thank the support of National Medical Research Council for funding ET research (EK‐T, STaR investigator award).
Funding Statement
This work was funded by National Medical Research Council grant .
References
- 1. Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord 2010;25:534–541. [DOI] [PubMed] [Google Scholar]
- 2. Deuschl G, Elble R. Essential tremor–neurodegenerative or nondegenerative disease towards a working definition of ET. Mov Disord 2009;24:2033–2041. [DOI] [PubMed] [Google Scholar]
- 3. Poston KL, Rios E, Louis ED. Action tremor of the legs in essential tremor: prevalence, clinical correlates, and comparison with age‐matched controls. Parkinsonism Relat Disord 2009;15:602–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Koster B, Deuschl G, Lauk M, et al. Essential tremor and cerebellar dysfunction: abnormal ballistic movements. J Neurol Neurosurg Psychiatry 2002;73:400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Louis ED, Benito‐Leon J, Vega‐Quiroga S, Bermejo‐Pareja F; Neurological Disorders in Central Spain Study G . Faster rate of cognitive decline in essential tremor cases than controls: a prospective study. Eur J Neurol 2010;17:1291–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benito‐Leon J, Louis ED, Sanchez‐Ferro A, Bermejo‐Pareja F. Rate of cognitive decline during the premotor phase of essential tremor: a prospective study. Neurology 2013;81:60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tan EK, Fook‐Chong S, Lum SY, et al. Non‐motor manifestations in essential tremor: use of a validated instrument to evaluate a wide spectrum of symptoms. Parkinsonism Relat Disord 2005;11:375–380. [DOI] [PubMed] [Google Scholar]
- 8. Miller KM, Okun MS, Fernandez HF, et al. Depression symptoms in movement disorders: comparing Parkinson's disease, dystonia, and essential tremor. Mov Disord 2007;22:666–672. [DOI] [PubMed] [Google Scholar]
- 9. Ondo WG, Sutton L, Dat Vuong K, et al. Hearing impairment in essential tremor. Neurology 2003;61:1093–1097. [DOI] [PubMed] [Google Scholar]
- 10. Louis ED, Bromley SM, Jurewicz EC, Watner D. Olfactory dysfunction in essential tremor: a deficit unrelated to disease duration or severity. Neurology 2002;59:1631–1633. [DOI] [PubMed] [Google Scholar]
- 11. Bermejo‐Pareja F, Louis ED, Benito‐Leon J; Neurological Disorders in Central Spain Study G . Risk of incident dementia in essential tremor: a population‐based study. Mov Disord 2007;22:1573–1580. [DOI] [PubMed] [Google Scholar]
- 12. Thawani SP, Schupf N, Louis ED. Essential tremor is associated with dementia: prospective population‐based study in New York. Neurology 2009;73:621–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Louis ED, Benito‐Leon J, Bermejo‐Pareja F; Neurological Disorders in Central Spain Study G . Self‐reported depression and anti‐depressant medication use in essential tremor: cross‐sectional and prospective analyses in a population‐based study. Eur J Neurol 2007;14:1138–1146. [DOI] [PubMed] [Google Scholar]
- 14. Chatterjee A, Jurewicz EC, Applegate LM, Louis ED. Personality in essential tremor: further evidence of non‐motor manifestations of the disease. J Neurol Neurosurg Psychiatry 2004;75:958–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Louis ED, Faust PL, Vonsattel JP. Purkinje cell loss is a characteristic of essential tremor. Parkinsonism Relat Disord 2011;17:406–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Louis ED, Faust PL, Ma KJ, et al. Torpedoes in the cerebellar vermis in essential tremor cases vs. controls. Cerebellum 2011;10:812–819. [DOI] [PubMed] [Google Scholar]
- 17. Louis ED. From neurons to neuron neighborhoods: the rewiring of the cerebellar cortex in essential tremor. Cerebellum 2014;13:501–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ghika A, Kyrozis A, Potagas C, Louis ED. Motor and non‐motor features: differences between patients with isolated essential tremor and patients with both essential tremor and Parkinson's disease. Tremor Other Hyperkinet Mov (NY) 2015;5:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Louis ED, Faust PL, Vonsattel JP, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain 2007;130(Pt 12):3297–3307. [DOI] [PubMed] [Google Scholar]
- 20. Benito‐Leon J, Louis ED, Bermejo‐Pareja F; Neurological Disorders in Central Spain Study G . Risk of incident Parkinson's disease and parkinsonism in essential tremor: a population based study. J Neurol Neurosurg Psychiatry 2009;80:423–425. [DOI] [PubMed] [Google Scholar]
- 21. Sharifi S, Nederveen AJ, Booij J, van Rootselaar AF. Neuroimaging essentials in essential tremor: a systematic review. Neuroimage Clin 2014;5:217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gulcher JR, Jonsson P, Kong A, et al. Mapping of a familial essential tremor gene, FET1, to chromosome 3q13. Nat Genet 1997;17:84–87. [DOI] [PubMed] [Google Scholar]
- 23. Higgins JJ, Pho LT, Nee LE. A gene (ETM) for essential tremor maps to chromosome 2p22‐p25. Mov Disord 1997;12:859–864. [DOI] [PubMed] [Google Scholar]
- 24. Shatunov A, Sambuughin N, Jankovic J, et al. Genomewide scans in North American families reveal genetic linkage of essential tremor to a region on chromosome 6p23. Brain 2006;129(Pt 9):2318–2331. [DOI] [PubMed] [Google Scholar]
- 25. Stefansson H, Steinberg S, Petursson H, et al. Variant in the sequence of the LINGO1 gene confers risk of essential tremor. Nat Genet 2009;41:277–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thier S, Lorenz D, Nothnagel M, et al. Polymorphisms in the glial glutamate transporter SLC1A2 are associated with essential tremor. Neurology 2012;79:243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Merner ND, Girard SL, Catoire H, et al. Exome sequencing identifies FUS mutations as a cause of essential tremor. Am J Hum Genet 2012;91:313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Unal Gulsuner H, Gulsuner S, Mercan FN, et al. Mitochondrial serine protease HTRA2 p.G399S in a kindred with essential tremor and Parkinson disease. Proc Natl Acad Sci USA 2014;111:18285–18290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hor H, Francescatto L, Bartesaghi L, et al. Missense mutations in TENM4, a regulator of axon guidance and central myelination, cause essential tremor. Hum Mol Genet 2015;24:5677–5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ma S, Davis TL, Blair MA, et al. Familial essential tremor with apparent autosomal dominant inheritance: should we also consider other inheritance modes? Mov Disord 2006;21:1368–1374. [DOI] [PubMed] [Google Scholar]
- 31. Smith P, Arias R, Sonti S, et al. A drosophila model of essential tremor. Sci Rep 2018;8:7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tanner CM, Goldman SM, Lyons KE, et al. Essential tremor in twins: an assessment of genetic vs environmental determinants of etiology. Neurology 2001;57:1389–1391. [DOI] [PubMed] [Google Scholar]
- 33. Lorenz D, Frederiksen H, Moises H, et al. High concordance for essential tremor in monozygotic twins of old age. Neurology 2004;62:208–211. [DOI] [PubMed] [Google Scholar]
- 34. Du W, Aloyo VJ, Harvey JA. Harmaline competitively inhibits [3H]MK‐801 binding to the NMDA receptor in rabbit brain. Brain Res 1997;770:26–29. [DOI] [PubMed] [Google Scholar]
- 35. Louis ED, Zheng W. Beta‐carboline alkaloids and essential tremor: exploring the environmental determinants of one of the most prevalent neurological diseases. Sci World J 2010;10:1783–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Louis ED, Zheng W, Jurewicz EC, et al. Elevation of blood beta‐carboline alkaloids in essential tremor. Neurology 2002;59:1940–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Louis ED, Jiang W, Pellegrino KM, et al. Elevated blood harmane (1‐methyl‐9H‐pyrido[3,4‐b]indole) concentrations in essential tremor. Neurotoxicology 2008;29:294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Louis ED, Benito‐Leon J, Moreno‐Garcia S, et al. Blood harmane (1‐methyl‐9H‐pyrido[3,4‐b]indole) concentration in essential tremor cases in Spain. Neurotoxicology 2013;34:264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Louis ED, Rios E, Pellegrino KM, et al. Higher blood harmane (1‐methyl‐9H‐pyrido[3,4‐b]indole) concentrations correlate with lower olfactory scores in essential tremor. Neurotoxicology 2008;29:460–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Louis ED, Factor‐Litvak P, Liu X, et al. Elevated brain harmane (1‐methyl‐9H‐pyrido[3,4‐b]indole) in essential tremor cases vs. controls. Neurotoxicology 2013;38:131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Louis ED, Zheng W, Mao X, Shungu DC. Blood harmane is correlated with cerebellar metabolism in essential tremor: a pilot study. Neurology 2007;69:515–520.17679670 [Google Scholar]
- 42. Pfau W, Skog K. Exposure to beta‐carbolines norharman and harman. J Chromatogr B Analyt Technol Biomed Life Sci 2004;802:115–126. [DOI] [PubMed] [Google Scholar]
- 43. Skog K. Cooking procedures and food mutagens: a literature review. Food Chem Toxicol 1993;31:655–675. [DOI] [PubMed] [Google Scholar]
- 44. Layton DW, Bogen KT, Knize MG, et al. Cancer risk of heterocyclic amines in cooked foods: an analysis and implications for research. Carcinogenesis 1995;16:39–52. [DOI] [PubMed] [Google Scholar]
- 45. Louis ED, Zheng W, Applegate L, et al. Blood harmane concentrations and dietary protein consumption in essential tremor. Neurology 2005;65:391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Louis ED, Keating GA, Bogen KT, et al. Dietary epidemiology of essential tremor: meat consumption and meat cooking practices. Neuroepidemiology 2008;30:161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liu Z, Zhou T, Ziegler AC, et al. Oxidative stress in neurodegenerative diseases: from molecular mechanisms to clinical applications. Oxid Med Cell Longev 2017;2017:2525967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Golbe LI, Farrell TM, Davis PH. Case‐control study of early life dietary factors in Parkinson's disease. Arch Neurol 1988;45:1350–1353. [DOI] [PubMed] [Google Scholar]
- 49. Sano M, Ernesto C, Thomas RG, et al. A controlled trial of selegiline, alpha‐tocopherol, or both as treatment for Alzheimer's disease. The Alzheimer's Disease Cooperative Study. N Engl J Med 1997;336:1216–1222. [DOI] [PubMed] [Google Scholar]
- 50. Li H, Ding F, Xiao L, et al. Food‐derived antioxidant polysaccharides and their pharmacological potential in neurodegenerative diseases. Nutrients 2017;9:778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Louis ED, Jurewicz EC, Parides MK. Case‐control study of nutritional antioxidant intake in essential tremor. Neuroepidemiology 2005;24:203–208. [DOI] [PubMed] [Google Scholar]
- 52. Scarmeas N, Louis ED. Mediterranean diet and essential tremor. A case‐control study. Neuroepidemiology 2007;29:170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Loewenstein Y. A possible role of olivary gap‐junctions in the generation of physiological and pathological tremors. Mol Psychiatry 2002;7:129–131. [DOI] [PubMed] [Google Scholar]
- 54. Boecker H, Wills AJ, Ceballos‐Baumann A, et al. The effect of ethanol on alcohol‐responsive essential tremor: a positron emission tomography study. Ann Neurol 1996;39:650–658. [DOI] [PubMed] [Google Scholar]
- 55. Kralic JE, Criswell HE, Osterman JL, et al. Genetic essential tremor in gamma‐aminobutyric acidA receptor alpha1 subunit knockout mice. J Clin Invest 2005;115:774–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jimenez‐Jimenez FJ, de Toledo‐Heras M, Alonso‐Navarro H, et al. Environmental risk factors for essential tremor. Eur Neurol 2007;58:106–113. [DOI] [PubMed] [Google Scholar]
- 57. Louis ED, Jurewicz EC, Applegate L, et al. Semiquantitative study of current coffee, caffeine, and ethanol intake in essential tremor cases and controls. Mov Disord 2004;19:499–504. [DOI] [PubMed] [Google Scholar]
- 58. Louis ED, Michalec M. Semi‐quantitative data on ethanol consumption in 354 ET cases and 370 controls. J Neurol Sci 2014;347:174–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dlugos CA. Ethanol‐related increases in degenerating bodies in the Purkinje neuron dendrites of aging rats. Brain Res 2008;1221:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Andersen BB. Reduction of Purkinje cell volume in cerebellum of alcoholics. Brain Res 2004;1007:10–18. [DOI] [PubMed] [Google Scholar]
- 61. Yokota O, Tsuchiya K, Terada S, et al. Alcoholic cerebellar degeneration: a clinicopathological study of six Japanese autopsy cases and proposed potential progression pattern in the cerebellar lesion. Neuropathology 2007;27:99–113. [DOI] [PubMed] [Google Scholar]
- 62. Louis ED, Benito‐Leon J, Bermejo‐Pareja F. Population‐based study of baseline ethanol consumption and risk of incident essential tremor. J Neurol Neurosurg Psychiatry 2009;80:494–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nicoletti A, Mostile G, Cappellani R, et al. Wine drinking and essential tremor: a possible protective role. Mov Disord 2011;26:1310–1315. [DOI] [PubMed] [Google Scholar]
- 64. Bekar L, Libionka W, Tian GF, et al. Adenosine is crucial for deep brain stimulation‐mediated attenuation of tremor. Nat Med 2008;14:75–80. [DOI] [PubMed] [Google Scholar]
- 65. Herraiz T. Identification and occurrence of the bioactive beta‐carbolines norharman and harman in coffee brews. Food Addit Contam 2002;19:748–754. [DOI] [PubMed] [Google Scholar]
- 66. Prakash KM, Fook‐Choong S, Yuen Y, Tan EK. Exploring the relationship between caffeine intake and essential tremor. J Neurol Sci 2006;251:98–101. [DOI] [PubMed] [Google Scholar]
- 67. Benito‐Leon J, Louis ED, Bermejo‐Pareja F. Short sleep duration heralds essential tremor: a prospective, population‐based study. Mov Disord 2013;28:1700–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Benito‐Leon J, Louis ED, Bermejo‐Pareja F; Neurological Disorders in Central Spain Study G . Population‐based case‐control study of cigarette smoking and essential tremor. Mov Disord 2008;23:246–252. [DOI] [PubMed] [Google Scholar]
- 69. Louis ED, Benito‐Leon J, Bermejo‐Pareja F; Neurological Disorders in Central Spain Study G . Population‐based prospective study of cigarette smoking and risk of incident essential tremor. Neurology 2008;70:1682–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Quik M, Perez XA, Bordia T. Nicotine as a potential neuroprotective agent for Parkinson's disease. Mov Disord 2012;27:947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Picciotto MR, Zoli M. Neuroprotection via nAChRs: the role of nAChRs in neurodegenerative disorders such as Alzheimer's and Parkinson's disease. Front Biosci 2008;13:492–504. [DOI] [PubMed] [Google Scholar]
- 72. Shimohama S. Nicotinic receptor‐mediated neuroprotection in neurodegenerative disease models. Biol Pharm Bull 2009;32:332–336. [DOI] [PubMed] [Google Scholar]
- 73. Louis ED, Factor‐Litvak P, Parides M, et al. Organochlorine pesticide exposure in essential tremor: a case‐control study using biological and occupational exposure assessments. Neurotoxicology 2006;27:579–586. [DOI] [PubMed] [Google Scholar]
- 74. Yao Y, Wang Y, Yang X. Related factors and prevalence for the essential tremor disease of Uygur residents in Hetian, Xinjiang UygurAutonomous Region. Zhonghua Yi Xue Za Zhi 2015;95:69–72. [PubMed] [Google Scholar]
- 75. Azevedo MFA, Meyer A. Essential tremor in endemic disease control agents exposed to pesticides: a case‐control study. Cad Saude Publ 2017;33:e00194915. [DOI] [PubMed] [Google Scholar]
- 76. Louis ED, Jurewicz EC, Applegate L, et al. Association between essential tremor and blood lead concentration. Environ Health Perspect 2003;111:1707–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dogu O, Louis ED, Tamer L, et al. Elevated blood lead concentrations in essential tremor: a case‐control study in Mersin, Turkey. Environ Health Perspect 2007;115:1564–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Louis ED, Applegate LM, Factor‐Litvak P, et al. Essential tremor: occupational exposures to manganese and organic solvents. Neurology 2004;63:2162–2164. [DOI] [PubMed] [Google Scholar]
- 79. Louis ED. ‘Essential tremor’ or ‘the essential tremors’: is this one disease or a family of diseases? Neuroepidemiology 2014;42:81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tan EK, Tong J, Pavanni R, et al. Genetic analysis of SCA 2 and 3 repeat expansions in essential tremor and atypical Parkinsonism. Mov Disord 2007;22:1971–1974. [DOI] [PubMed] [Google Scholar]


