Abstract
Increasing evidence indicates that immune system dysfunction affects anti‐N‐methyl‐D‐aspartate receptor (NMDAR) encephalitis. This study aims to investigate the relationship between adhesion molecules and the pathophysiology in anti‐NMDAR encephalitis. Soluble forms of Intercellular adhesion molecule‐1 (sICAM‐1), vascular adhesion molecule‐1 (sVCAM‐1), and L‐selectin (sL‐selectin), were measured in the CSF and serum of 26 participants with anti‐NMDAR encephalitis, 11 patients with schizophrenia and 22 patients with noninflammatory disorders. CSF levels of sICAM‐1, sVCAM‐1 and sL‐selectin were significantly elevated in the anti‐NMDAR encephalitis group. sVCAM‐1 levels were positively associated with modified Rankin scale score in anti‐NMDAR encephalitis patients at the onset and 3‐month follow‐up.
Introduction
Anti‐N‐Methyl‐D‐aspartate receptor (NMDAR) encephalitis is an autoimmune disorder with diverse psychiatric and neurological features, most commonly psychosis, disorientation, amnesia, seizures, and a complex movement disorder.1 This condition is most common in young women with or without tumors (ovarian teratoma usually).2 Most patients with anti‐NMDAR encephalitis develop a multistage illness that progresses from psychosis, memory deficits, seizures, speech disorder, movement disorder, autonomic symptoms, and central hypoventilation.3
The compromise of blood‐brain barrier (BBB) integrity is closely related to the progression of autoimmune diseases.4, 5 Adhesion molecules facilitate the process of leukocyte migration and further modulate the permeability of the BBB to immune cells.6, 7, 8 Intercellular adhesion molecule‐1 (ICAM‐1), vascular adhesion molecule‐1 (VCAM‐1), and L‐selectin engaged with leukocyte integrins activates diverse signaling pathways in endothelial cells, result in the reorganization of junction complexes further mediate leukocyte adhesion migration.7, 9
ICAM‐1 (CD54) is a transmembrane protein that is upregulated on endothelial and epithelial cells at sites of inflammation. It mediates the vascular adhesion and paracellular migration of leukocytes expressing activated LFA‐1 (CD11a) and Mac‐1 (CD11b). Soluble ICAM‐1 promotes angiogenesis and serves an indicator of vascular endothelial cell activation or damage. VCAM‐1 (CD106) is induced in endothelial cells by inflammatory cytokines including TNF‐α and IL‐1β. This induces clustering of VCAM‐1 and activation of intracellular signaling pathways that induce phosphorylation of tight junction proteins, resulting in their disassembly and redistribution from the cell border.10 L‐selectin (Leukocyte Selectin, CD62L) is a cell surface glycoprotein expressed constitutively on a wide variety of leukocytes.11 Acting in cooperation with P‐selectin and E‐selectin, L‐Selectin mediates the initial interaction of circulating leukocytes on the endothelium.9 This initial interaction, also involving ICAM‐1 and VCAM‐1, leads eventually to extravasation of the white blood cell through the blood vessel wall into the extracellular matrix tissue. The majority of myeloid cells, B cells and virgin T cells express L‐selectin, cleavage of L‐selectin from the cell surface results in a high circulating level of functionally active soluble L‐selectin.6 Multiple studies indicated that L‐selectin, P‐selectin E‐selectin collaborate to mediate the initial binding of leukocytes to endothelium at sites of tissue injury and inflammation.12, 13, 14
We have reported several inflammatory indicators or immune‐modulate factors highly expressed in cerebrospinal fluid (CSF) or serum of patients with Anti‐NMDAR encephalitis,15, 16 but the profiles of molecules facilitate the cells migration remain unclear. In this study, we measured the expression of soluble forms of ICAM‐1 (sICAM‐1), VCAM‐1 (sVCAM‐1), and L‐selectin (sL‐selectin) in patients with anti‐NMDAR encephalitis. We also examined whether the soluble forms of these cell adhesion molecules can be measured as a diagnose or prognosis biomarker for anti‐NMDAR encephalitis.
Methods and Materials
Subjects and behavioral assessments
Fifty‐nine study participants aged between 9 and 60 years were recruited in this study. Participants consisted of 26 patients with anti‐NMDAR encephalitis, 11 Schizophrenia (SP) and 22 noninflammation diseases (NID) control patients. The diagnosis of anti‐NMDAR encephalitis was confirmed using revised anti‐NMDAR encephalitis diagnosis criteria of 2016,17 including the presence of clinical manifestations and detection of anti‐NMDAR antibodies in the CSF. Patients met DSM‐IV criteria for SP were recruited. NID controls including 16 cases of cerebrovascular disease and six cases of movement disorders. Both the SP and NID control groups were negative for specific CSF and serum antibodies.
CSF and serum samples in this study were obtained from patients within 3 days of their admission, nine pair of CSF and serum samples from patients with NMDAR encephalitis were collected at 3‐month follow‐up. Patients with anti‐NMDAR encephalitis received evaluation of neurological condition using modified Rankin scale (mRS) scores both at admission and 3‐month follow‐up after discharge.
The study protocol was approved by the Ethics Committee of Nanfang Hospital, Southern Medical University, each participant provided written informed consent to participate. A detailed medical history was obtained from all patients.
Measurement of sICAM‐1, sVCAM‐1, and sL‐selectin
CSF samples were collected using polypropylene tubes and centrifuged at 4000g for 10 min. Blood samples were collected using BD Vacutainer Serum Separation tubes and let clot for 20 min at room temperature. Sera were centrifuged at 2000g for 10 min and stored at −80°C until the assay. Levels of sICAM‐1 were determined with sICAM‐1 Quantikine ELISA kits (R&D Systems) with sensitivities of 0.254 ng/mL. Levels of VCAM‐1 were determined with sVCAM‐1 Quantikine ELISA kits (R&D Systems) with sensitivities of 1.26 ng/mL. Levels of sL‐selectin were determined with sL‐selectin ELISA Kit (Abnova) with sensitivities of 0.195 ng/mL. The assays were performed according to the protocols recommended by the manufacturers. All standards and samples were assayed in duplicate. Optical densities were determined on a CLARIOstar Microplate Reader (BMG LabTech).
Statistical analysis
Analyses were conducted with GraphPad Prism 7.0 software. Data were tested for normality and found to be nonnormally distributed. Accordingly, data are presented as median (interquartile range), Kruskal–Wallis analysis was used to evaluate differences between multiple groups and Dunn's multiple comparison test was used for post‐hoc analysis. The diagnose potential of soluble adhesion molecules in anti‐NMDAR encephalitis were assessed with the receiving operating characteristic (ROC) curve analysis. Correlation analysis was performed with Spearman analysis. All analyses were two‐tailed, and values of P < 0.05 were considered statistically significant.
Results
Clinical characteristics
Demographic and clinical characteristics of patient were presented in Table 1. All patients with anti‐NMDAR encephalitis were positive for anti‐NMDAR antibody tests in CSF and 21 patients were positive for serum tests, four patients were detected with ovarian teratoma. There were no statistically significant differences on sex or age in each group.
Table 1.
Clinical characteristics of patients
| Anti‐NMDAR encephalitis | SP | NID | |
|---|---|---|---|
| Number of patients (n) | 26 | 11 | 22 |
| Gender (female/male) | 14/12 | 7/4 | 11/11 |
| Age (years) | 33 (17.5–53.25)* | 40 (27–53) | 38 (23.75–51.75) |
| Serum albumin (g/L) | 38.6 (33.55–41.4) | – | 40.55 (37.53–44.33) |
| CSF albumin (g/L) | 0.35 (0.22–0.77) | – | 0.34 (0.23–0.45) |
| Number of symptoms (n) | |||
| Prodrome (headache, fever) | 13 | 0 | 0 |
| Behavior and cognition disorder | 22 | 0 | 0 |
| Memory deficit | 14 | 0 | 0 |
| Speech disorder | 15 | 0 | 0 |
| Seizures | 18 | 0 | 0 |
| Movement disorder | 11 | 0 | 0 |
| Loss of consciousness | 15 | 0 | 0 |
| Autonomic symptoms | 11 | 0 | 0 |
| Central hypoventilation | 9 | 0 | 0 |
| Cerebellar ataxia | 0 | 0 | 0 |
| Hemiparesis | 2 | 0 | 0 |
| EEG change | 21 | 0 | 0 |
| Ovarian teratoma | 4 | 0 | 0 |
| Onset mRS | 4 (3.75–5) | – | – |
| 3 months’ mRS | 3 (2–3) | – | – |
| CSF anti‐NMDAR antibody | 26 | 0 | 0 |
| Serum anti‐NMDAR antibody | 21 | 0 | 0 |
SP, schizophrenia; NID, non‐inflammatory disorders.
Data were presented as the median (interquartile range).
CSF and serum levels of adhesion molecules in patients with anti‐NMDAR encephalitis
Median CSF levels of sICAM‐1, sVCAM‐1, and sL‐selectin were all elevated in the anti‐NMDAR encephalitis group compared with the SP group (sICAM‐1: P = 0.006, sVCAM‐1: P = 0.001, sL‐selectin: P = 0.001) or NID control group (sICAM‐1: P = 0.001, sVCAM‐1: P < 0.001, sL‐selectin: P = 0.003) (Fig. 1A–C, respectively). Serum levels of sVCAM‐1 were also significantly reduced, by approximately a 50%, in the anti‐NMDAR encephalitis group (335.69 ng/mL; 225.05–462.78 ng/mL) in comparison with the SP group (218.94 ng/mL; 169.37–313.73 ng/mL; P = 0.002) and NID control group (216.43 ng/mL; 208.66–231.47 ng/mL; P < 0.001) (Fig. 1E). There were no significant differences in serum levels of sICAM‐1 or sL‐selectin between the anti‐NMDAR encephalitis participants and controls (Fig. 1D and F). Correlation between CSF and serum levels of sICAM‐1, sVCAM‐1, and sL‐selectin levels in anti‐NMDAR encephalitis patients were also tested, only sL‐selectin showed a positive correlation between CSF and serum levels (P = 0.001, r = 0595) (Fig. 2C). In the comparison of patients with anti‐NMDAR encephalitis and non‐anti‐NMDAR encephalitis, the area under the ROC curve (AUC) was 0.981 for CSF sVCAM‐1, which was superior to others (CSF sICAM‐1: 0.787, CSF sL‐selectin: 0.740, serum sICAM‐1: 0.516, serum sVCAM‐1: 0.801, serum sL‐selectin: 0.556), the optimal cut‐off values for CSF sVCAM‐1 were 5.25 ng/mL. Besides, combination of sICAM, sVCAM, and sL‐selectin in CSF and serum did not show an improved diagnose potential for anti‐NMDAR encephalitis (Table 2).
Figure 1.
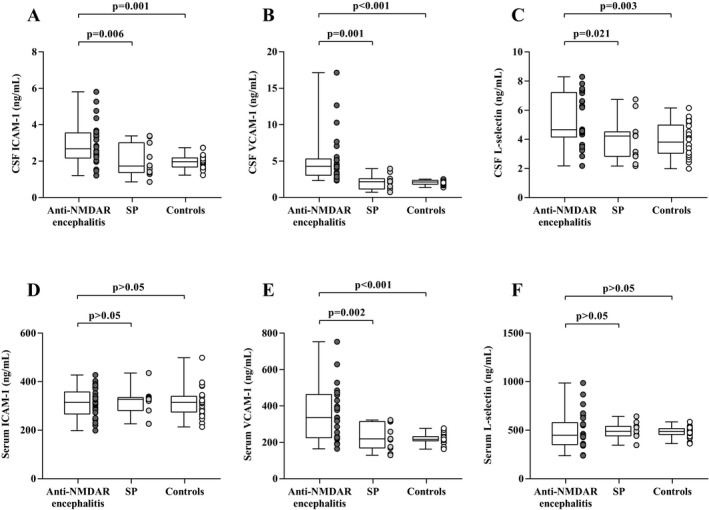
CSF and Serum concentration of sICAM‐1, sVCAM‐1 and sL‐selectin in anti‐NMDAR encephalitis, SP and NID patients (A–F).
Figure 2.
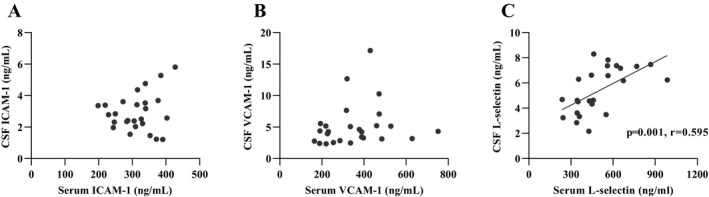
Correlation between CSF and serum levels of sICAM‐1, sVCAM‐1 and sL‐selectin levels in anti‐NMDAR encephalitis patients (A–C) (Spearman test).
Table 2.
ROC analysis of CSF and serum adhesion molecules
| AUC | 95% CI | |
|---|---|---|
| Anti‐NMDAR encephalitis versus non‐Anti‐NMDAR encephalitis | ||
| CSF ICAM‐1 | 0.787 | 0.647–0.926 |
| CSF VCAM‐1 | 0.981 | 0.951–1.010 |
| CSF L‐Selectin | 0.740 | 0.600–0.880 |
| Serum ICAM‐1 | 0.516 | 0.350–0.682 |
| Serum VCAM‐1 | 0.801 | 0.669–0.930 |
| Serum L‐Selectin | 0.556 | 0.380–0.732 |
| CSF ICAM/serum ICAM | 0.774 | 0.636–0.913 |
| CSF VCAM/serum VCAM | 0.719 | 0.568–0.869 |
| CSF L‐Selectin/serum L‐Selectin | 0.771 | 0.639–0.903 |
| CSF ICAM‐1 + CSF VCAM‐1 + CSF L‐Selectin | 0.960 | 0.919–1 |
| serum ICAM‐1 + serum VCAM‐1 + serum L‐Selectin | 0.902 | 0.823–0.981 |
| CSF ICAM‐1 + serum ICAM‐1 | 0.780 | 0.649–0.91 |
| CSF VCAM‐1 + serum VCAM‐1 | 0.951 | 0.9–1 |
| CSF L‐Selectin + serum L‐Selectin | 0.767 | 0.644–0.89 |
CSF, cerebrospinal fluid; AUC, area under the curve; CI, confidence interval.
Altered CSF levels of adhesion molecules in the follow‐up for anti‐NMDAR encephalitis patients
We obtained 3‐month follow‐up CSF and serum samples from nine patients with anti‐NMDAR encephalitis and found that the levels of all three adhesion molecules in CSF and serum were significantly decreased (Fig. 3). The change in CSF sVCAM‐1 levels was most pronounced, from (5.08 ng/mL; 3.30–8.96 ng/mL) (onset) to (4.15 ng/mL; 3.09–6.03 ng/mL) (6‐month follow‐up) (P = 0.008), although the latter was still higher than controls (P < 0.001).
Figure 3.
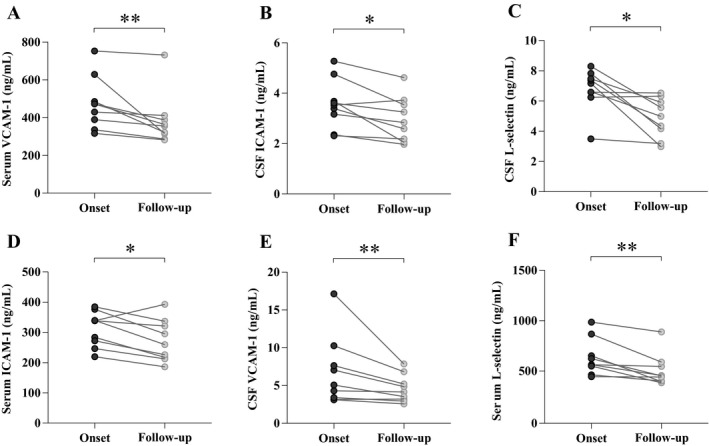
Levels of CSF sICAM‐1, sVCAM‐1 and sL‐selectin in the nine patients with anti‐NMDAR encephalitis who received a follow‐up test during remission (P values: * <0.05; ** <0.01).
Associations of adhesion molecules levels with mRS scores and clinical behaviorals
We then examined whether there was correlation between the levels of adhesion molecules and neurological condition among patients with anti‐NMDAR encephalitis. A significant positive correlation was observed between onset mRS score and the CSF levels of adhesion molecules (sICAM‐1: r = 0.612; P = 0.001, sVCAM‐1: r = 0.498; P = 0.01, sL‐selectin: r = 0.582; P = 0.002) (Fig. 4A–C), serum levels of sVCAM‐1 and sL‐selectin were also associated with mRS score at disease onset (sVCAM‐1: r = 0.614; P = 0.001, sL‐selectin: r = 0.417; P = 0.034) (Fig. 4D and E). However, only CSF sVCAM‐1 levels were found to be correlated with mRS score at 3‐month follow‐up (r = 0.439; P = 0.025) (Fig. 4B). Besides, no significant association was found between adhesion molecules and the appearance of specific clinical features, such as prodromal symptoms, cognitive deficits, dyskinesia, and tumor (Table S1).
Figure 4.
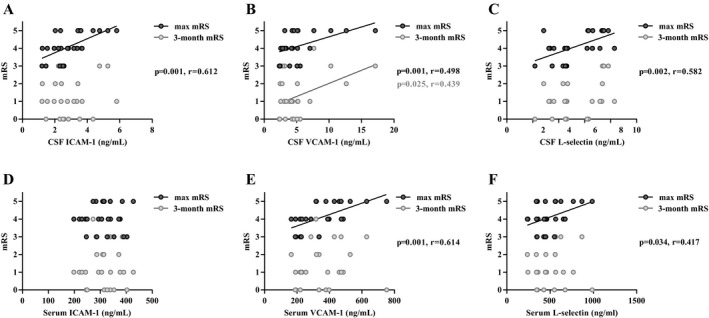
Correlation of CSF and serum levels of sICAM‐1, sVCAM‐1 and sL‐selectin with onset mRS and 3 months’ mRS in anti‐NMDAR encephalitis patients (A–F) (Spearman test).
Discussion
Anti‐NMDAR encephalitis is characterized by antibody‐mediated autoimmune responses to the CNS, the main symptoms being cognitive impairment and mental psychiatric symptoms, 80% of patients were found with early cerebrospinal fluid abnormalities, mainly characterized by lymphocytosis.18, 19 Literatures also demonstrate central nervous system inflammatory infiltration during anti‐NMDAR encephalitis, mainly characterized by perivascular lymphocytic infiltration.20
Both circulatory and central systemic inflammation could affect the production and migration of anti‐NMDAR antibodies and plasma cells.21, 22 Studies have found that breakdown of BBB integrity is relevant to the present and severity of neuropsychiatric symptoms caused by anti‐NMDAR antibodies.23, 24 Considering the high seroprevalence of anti‐NMDAR antibodies in healthy individuals,24 changes in BBB permeability may be essential for the pathogenesis of anti‐NMDAR receptor encephalitis.
The recruitment of circulating leukocytes to target tissues is a critical stage in the inflammatory diseases, it is controlled by multiple steps that mediate initial leukocyte binding, rolling along the surface of the blood vessels, and subsequent adhesion to the target. ICAM‐1, VCAM‐1, and L‐selectin facilitate leukocyte migration across the BBB, which may take part in the pathophysiology of anti‐NMDAR encephalitis (Fig. S1).
In this study, anti‐NMDAR encephalitis patients showed significantly higher CSF sICAM‐1, sVCAM‐1, and sL‐selectin levels when compared with SP or NID groups, whereas there was no difference regarding serum sICAM‐1 and sL‐selectin levels. There was a significant association between the CSF levels of all three adhesion molecules and disease severity, but only serum sVCAM‐1 levels are associated with disease prognosis.
ICAM‐1 is expressed on leukocytes, endothelial cells and upregulated in response to inflammatory signals.25 Although the primary source of sICAM‐1 under normal human conditions has not been fully elucidated, the literature indicates that a large source of sICAM‐1 is derived from endothelial cells and is elevated in patients with various inflammatory syndromes.26, 27 Studies have shown that ICAM‐1 is involved in the pathogenesis of a variety of autoimmune diseases.28, 29 In mice model of multiple sclerosis, ICAM‐1‐/‐ or mutant mice showed reduced T‐cell infiltration in the CNS and lower clinical grading.30 VCAM‐1 is expressed by activated endothelial cells and macrophages,7 and the circulating form has been shown critical role to promote monocyte chemotaxis in responding to inflammatory diseases.31, 32, 33 In our study, VCAM‐1 was found significant increased in CSF and serum, and associated with mRS both at baseline and 3‐month follow‐up, initial prodromal symptoms in patients with anti‐NMDAR encephalitis may be related to the role of VCAM‐1 in inducing leukocyte aggregation and migration. If the patient responds to treatment, the level of VCAM‐1 will decrease rapidly, with decreased leukocyte infiltration, and the apoptosis of plasmablast, resulting in a slow decline in antibody titer and recovery. Long‐term high expression of VCAM‐1 may result persisted CNS inflammation with severe neurological conditions.
The soluble forms of both VCAM‐1 and L‐selectin have been reported with chemotactic effects on B cells.34, 35 The role of B cells in anti‐NMDAR encephalitis has been well established, after crossing BBB, the memory B cells would undergo restimulation and maturation into antibody producing plasma cells,36 VCAM‐1 and L‐selectin may be involved in maintaining the long‐term presence of B cells in the CNS and sustained intrathecal antibody synthesis, which affects the outcome of anti‐NMDAR encephalitis.
Evidence suggested a link between adhesion molecules and cognitive impairment,37, 38 however, it was not found in patients with anti‐NMDAR encephalitis, probably due to differences in the mechanisms that lead to cognitive function changes in anti‐NMDAR encephalitis, mainly by antibody‐mediated changes in neuronal function. The expression of adhesion factors in SP patients differed from varied reports,39, 40 our results found no significant difference when comparing with other noninflammatory CNS diseases, but due to the small number of samples, caution should be exercised when interpreting these results.
Since early treatment is closely linked to the prognosis of anti‐NMDAR encephalitis, sensitive biomarkers would be particularly useful.41 In our study, sVCAM‐1 levels in CSF correlate with mRS scores both at onset and 3‐month follow‐up, suggesting a high level of sVCAM‐1 may indicate severe clinical outcomes and poor prognosis for NMDAR encephalitis. Thus, changes in CSF sVCAM‐1 combined with anti‐NMDAR antibody titers may be promising indicators for predicting anti‐NMDAR encephalitis.
Conclusion
Although the etiopathology of anti‐NMDAR encephalitis remain unclear, there are increasing evidences that dysfunction in the immune system involved in this disease. Data for this study showed that adhesion molecules, sICAM‐1, sVCAM‐1, and sL‐selectin are elevated in patients with anti‐NMDAR encephalitis. These elevated levels were associated with high mRS score, and could act as an indicator for the severity and prognosis of anti‐NMDAR encephalitis, further investigations aimed at determining the interaction between immune cells and behavior in patients with anti‐NMDAR encephalitis are needed.
Ethics Statement
The study protocol was approved by the Ethics Committee of Nanfang Hospital, Southern Medical University, each participant provided written informed consent to participate.
Author Contributions
H. W., W. X. and S. P. conceived this study and designed the experiments. Y. D., C. Y., Z. Z., Y. P., J. C., H. X., Y. C. and K. O. collected the samples and clinical data. YD and CY performed the experiments and analyzed the data. Y. D., H. W. and W. X. wrote the manuscript. All authors read and approved the final manuscript and agreed to submit it for publication.
Conflict of Interest
All authors read and approved the final manuscript. They declare no conflicts of interest.
Supporting information
Figure S1. ICAM‐1, VCAM‐1, and L‐selectin and their ligands at leucocytes and CNS endothelial cells.
Table S1. Correlation between clinical manifestations and the level of adhesion factors in anti‐NMDAR encephalitis patients.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (81673950, 81760413, 81760902). Guangdong Provincial Science and Technology plan projects (2017A020215182, 2016A020215101). Tackling Key Problems in Science and Technology and Trial Production of New Products in Liuzhou (No. 2016G020213, NO2014J030407) and Guangxi Natural Fund Project (No. 2017GXNSFBA198114).
Funding Information
This work was funded by the National Natural Science Foundation of China (81673950, 81760413, 81760902). Guangdong Provincial Science and Technology plan projects (2017A020215182, 2016A020215101). Tackling Key Problems in Science and Technology and Trial Production of New Products in Liuzhou (No. 2016G020213, NO2014J030407) and Guangxi Natural Fund Project (No. 2017GXNSFBA198114).
Funding Statement
This work was funded by National Natural Science Foundation of China grants 81673950, 81760413, and 81760902; Guangdong Provincial Science and Technology grants 2017A020215182 and 2016A020215101.
Contributor Information
Wei Xie, Email: xieweizn@fimmu.com.
Honghao Wang, Email: wang_whh@163.com.
Reference
- 1. Dalmau J, Gleichman AJ, Hughes EG, et al. Anti‐NMDA‐receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008;7:1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Granerod J, Ambrose HE, Davies NWS, et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population‐based prospective study. Lancet Infect Dis 2010;10:835–844. [DOI] [PubMed] [Google Scholar]
- 3. Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long‐term outcome in patients with anti‐NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 2013;12:157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ortiz GG, Pacheco‐Moises FP, Macias‐Islas MA, et al. Role of the blood‐brain barrier in multiple sclerosis. Arch Med Res 2014;45:687–697. [DOI] [PubMed] [Google Scholar]
- 5. Kebir H, Kreymborg K, Ifergan I, et al. Human TH17 lymphocytes promote blood‐brain barrier disruption and central nervous system inflammation. Nat Med 2007;13:1173–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patel KD, Cuvelier SL, Wiehler S. Selectins: critical mediators of leukocyte recruitment. Semin Immunol 2002;14:73–81. [DOI] [PubMed] [Google Scholar]
- 7. Wu TC. The role of vascular cell adhesion molecule‐1 in tumor immune evasion. Cancer Res 2007;67:6003–6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abadier M, Haghayegh Jahromi N, Cardoso Alves L, et al. Cell surface levels of endothelial ICAM‐1 influence the transcellular or paracellular T‐cell diapedesis across the blood‐brain barrier. Eur J Immunol 2015;45:1043–1058. [DOI] [PubMed] [Google Scholar]
- 9. Angiari S. Selectin‐mediated leukocyte trafficking during the development of autoimmune disease. Autoimmun Rev 2015;14:984–995. [DOI] [PubMed] [Google Scholar]
- 10. Schlesinger M, Bendas G. Vascular cell adhesion molecule‐1 (VCAM‐1)–an increasing insight into its role in tumorigenicity and metastasis. Int J Cancer 2015;136:2504–2514. [DOI] [PubMed] [Google Scholar]
- 11. Bouillet P, O'Reilly LA. CD95, BIM and T cell homeostasis. Nat Rev Immunol 2009;9:514–519. [DOI] [PubMed] [Google Scholar]
- 12. Berardi C, Decker PA, Kirsch PS, et al. Plasma and serum L‐selectin and clinical and subclinical cardiovascular disease: the Multi‐Ethnic Study of Atherosclerosis (MESA). Transl Res 2014;163:585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yoshikawa N, Noda K, Shinoda H, et al. Serum vascular adhesion protein‐1 correlates with vascular endothelial growth factor in patients with type II diabetes. J Diabetes Complications 2013;27:162–166. [DOI] [PubMed] [Google Scholar]
- 14. Doring A, Wild M, Vestweber D, et al. E‐ and P‐Selectin are not required for the development of experimental autoimmune encephalomyelitis in C57BL/6 and SJL mice. J Immunol 2007;179:8470–8479. [DOI] [PubMed] [Google Scholar]
- 15. Ai P, Zhang X, Xie Z, et al. The HMGB1 is increased in CSF of patients with an Anti‐NMDAR encephalitis. Acta Neurol Scand 2018;137:277–282. [DOI] [PubMed] [Google Scholar]
- 16. Liu B, Ai P, Zheng D, et al. Cerebrospinal fluid pentraxin 3 and CD40 ligand in anti‐N‐menthyl‐d‐aspartate receptor encephalitis. J Neuroimmunol 2018;315:40–44. [DOI] [PubMed] [Google Scholar]
- 17. Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 2016;15:391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dalmau J. NMDA receptor encephalitis and other antibody‐mediated disorders of the synapse: the 2016 Cotzias Lecture. Neurology 2016;87:2471–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martinez‐Hernandez E, Horvath J, Shiloh‐Malawsky Y, et al. Analysis of complement and plasma cells in the brain of patients with anti‐NMDAR encephalitis. Neurology 2011;77:589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tuzun E, Zhou L, Baehring JM, et al. Evidence for antibody‐mediated pathogenesis in anti‐NMDAR encephalitis associated with ovarian teratoma. Acta Neuropathol 2009;118:737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Castillo‐Gomez E, Kastner A, Steiner J, et al. The brain as immunoprecipitator of serum autoantibodies against N‐Methyl‐D‐aspartate receptor subunit NR1. Ann Neurol 2016;79:144–151. [DOI] [PubMed] [Google Scholar]
- 22. Ehrenreich H. Autoantibodies against the N‐methyl‐d‐aspartate receptor subunit NR1: untangling apparent inconsistencies for clinical practice. Front Immunol 2017;8:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Castillo‐Gomez E, Oliveira B, Tapken D, et al. All naturally occurring autoantibodies against the NMDA receptor subunit NR1 have pathogenic potential irrespective of epitope and immunoglobulin class. Mol Psychiatry 2017;22:1776–1784. [DOI] [PubMed] [Google Scholar]
- 24. Hammer C, Stepniak B, Schneider A, et al. Neuropsychiatric disease relevance of circulating anti‐NMDA receptor autoantibodies depends on blood‐brain barrier integrity. Mol Psychiatry 2014;19:1143–1149. [DOI] [PubMed] [Google Scholar]
- 25. Siu G, Hedrick SM, Brian AA. Isolation of the murine intercellular adhesion molecule 1 (ICAM‐1) gene. ICAM‐1 enhances antigen‐specific T cell activation. J Immunol 1989;143:3813–3820. [PubMed] [Google Scholar]
- 26. Erturk K, Tastekin D, Bilgin E, et al. Serum activated leukocyte cell adhesion molecule and intercellular adhesion molecule‐1 in patients with gastric cancer: can they be used as biomarkers? Biomed Pharmacother 2016;77:86–91. [DOI] [PubMed] [Google Scholar]
- 27. Dorr O, Liebetrau C, Mollmann H, et al. Soluble fms‐Like tyrosine kinase‐1 and endothelial adhesion molecules (intercellular cell adhesion molecule‐1 and vascular cell adhesion molecule‐1) as predictive markers for blood pressure reduction after renal sympathetic denervation. Hypertension 2014;63:984–990. [DOI] [PubMed] [Google Scholar]
- 28. Uzawa A, Mori M, Masuda S, et al. Markedly elevated soluble intercellular adhesion molecule 1, soluble vascular cell adhesion molecule 1 levels, and blood‐brain barrier breakdown in neuromyelitis optica. Arch Neurol 2011;68:913–917. [DOI] [PubMed] [Google Scholar]
- 29. Hu X, Barnum SR, Wohler JE, et al. Differential ICAM‐1 isoform expression regulates the development and progression of experimental autoimmune encephalomyelitis. Mol Immunol 2010;47:1692–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bullard DC, Hu X, Crawford D, et al. Expression of a single ICAM‐1 isoform on T cells is sufficient for development of experimental autoimmune encephalomyelitis. Eur J Immunol 2014;44:1194–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liao B, Tang Y, Hu F, et al. Serum levels of soluble vascular cell adhesion molecules may correlate with the severity of dengue virus‐1 infection in adults. Emerg Microbes Infect 2015;4:e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rios DRA, Alpoim PN, Godoi LC, et al. Increased levels of sENG and sVCAM‐1 and decreased levels of VEGF in severe preeclampsia. Am J Hypertens 2016;29:1307–1310. [DOI] [PubMed] [Google Scholar]
- 33. Kuessel L, Wenzl R, Proestling K, et al. Soluble VCAM‐1/soluble ICAM‐1 ratio is a promising biomarker for diagnosing endometriosis. Hum Reprod 2017;32:770–779. [DOI] [PubMed] [Google Scholar]
- 34. Leuker CE, Labow M, Muller W, et al. Neonatally induced inactivation of the vascular cell adhesion molecule 1 gene impairs B cell localization and T cell‐dependent humoral immune response. J Exp Med 2001;193:755–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tang ML, Steeber DA, Zhang XQ, et al. Intrinsic differences in L‐selectin expression levels affect T and B lymphocyte subset‐specific recirculation pathways. J Immunol 1998;160:5113–5121. [PubMed] [Google Scholar]
- 36. Makuch M, Wilson R, Al‐Diwani A, et al. N‐methyl‐D‐aspartate receptor antibody production from germinal center reactions: therapeutic implications. Ann Neurol 2018;83:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tchalla AE, Wellenius GA, Sorond FA, et al. Elevated soluble vascular cell adhesion molecule‐1 is associated with cerebrovascular resistance and cognitive function. J Gerontol A Biol Sci Med Sci 2017;72:560–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li G, Xiong K, Korff A, et al. Increased CSF E‐Selectin in clinical Alzheimer's disease without altered CSF Abeta42 and Tau. J Alzheimers Dis 2015;47:883–887. [DOI] [PubMed] [Google Scholar]
- 39. Schwartz M, Kipnis J. A conceptual revolution in the relationships between the brain and immunity. Brain Behav Immun 2011;25:817–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kipnis J, Cohen H, Cardon M, et al. T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc Natl Acad Sci USA 2004;101:8180–8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leypoldt F, Hoftberger R, Titulaer MJ, et al. Investigations on CXCL13 in anti‐N‐methyl‐D‐aspartate receptor encephalitis: a potential biomarker of treatment response. JAMA Neurol 2015;72:180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. ICAM‐1, VCAM‐1, and L‐selectin and their ligands at leucocytes and CNS endothelial cells.
Table S1. Correlation between clinical manifestations and the level of adhesion factors in anti‐NMDAR encephalitis patients.


