Abstract
Cigarette craving is a cardinal feature of smoking, which is the leading preventable cause of death. Despite its clinical relevance, there remains a pressing need to develop new approaches for controlling craving. Although olfactory cues (OCs) are especially well suited to reduce-affectively charged cravings, there has been surprisingly little research on the topic. We investigated the strategic use of OCs to reduce cigarette craving. Abstinent smokers (N=232) initially sampled and rated a series of OCs. Participants then were exposed to in vivo smoking cues, which produced robust cigarette cravings. During peak craving, they were randomly assigned to sniff one of three types of OCs (all of which they had previously sampled) while their craving, and a set of responses thought to be associated with craving, were assessed. OCs that a participant had rated as pleasant reduced craving more than did exposure to odor blank (i.e., neutral) or tobacco-related OCs. This effect persisted over the course of 5-min. In addition, smokers with the most specific autobiographical memory systems were most responsive to the craving-reducing effects of pleasant OCs. About 90% of participants reported they could imagine using a pleasant OC to curb their craving in the natural environment. The present data suggest that OCs show promise for controlling cravings and highlight the need to conduct further research to test whether OCs may prove useful alone or in combination with existing approaches as a smoking cessation intervention.
Keywords: cigarette, smoking, tobacco, craving, olfaction
General Scientific Summary
Given observed relations between craving and smoking relapse, novel approaches to craving relief are sorely needed. This laboratory study revealed that, following exposure to a lit cigarette to generate a peak craving state in abstinent smokers, the strategic use of olfactory cues reduced craving throughout the course of a five-minute assessment. These findings support continued investigation of olfactory cues as a potential component of a smoking cessation intervention.
Smoking rates have fallen over the past 50 years; nevertheless, nearly 40-million Americans still smoke (Centers for Disease Control and Prevention, 2016), a fraction of the billion smokers worldwide (World Health Organization, 2008). Smoking is the chief preventable cause of death in the US (Centers for Disease Control and Prevention, 2014). Most adult smokers want to quit, and about half report trying in the past year (Centers for Disease Control and Prevention, 2011). Yet nearly half who try to quit relapse within two weeks. Even with nicotine replacement, relapse is common, leading to calls for new treatments (Baker et al., 2011). Novel interventions are urgently needed to help the millions who wish to quit, and psychology’s emergence as a hub science supports its unique role in this effort (Dimoff, Sayette, & Norcross, 2017; Green McDonald, O’Connell, & Suls, 2015).
Craving (used interchangeably with “urge”) is central to addiction, can predict relapse, and is a criterion for substance use disorders in DSM-5 (American Psychiatric Association, 2013; Sayette & Tiffany, 2013). Both laboratory (see Sayette, 2016) and field (Gwaltney, Shiffman & Sayette, 2005) research indicate that smokers’ ability, and confidence in their ability, to cope with urges weaken during the very moments that they are most needed. Fully 71% of relapsing smokers who had mastered a set of coping skills reported using none of these skills during the lapse (Brandon, Tiffany, Obremski, & Baker, 1990; see also McCarthy et al., 2010). Urges that are not satisfied can be tiring and unpleasant (Tiffany, 1992), and concerns about potential craving can prevent smokers from even trying to quit (Orleans, Rimer, Cristinzio, Keintz, & Fleisher, 1991).
Craving is defined many ways but generally is thought to be a strong desire to smoke (Sayette et al., 2000). As articulated by Baker, Morse, and Sherman (1987), we view craving to be affective in nature, reflecting the processing of motivationally significant stimuli (Sayette, Martin, Hull, Wertz, & Perrott, 2003). Like other emotions (Salovey, 1992), cravings influence cognitive processes. Urge-induced shifts in cognition hamper self-regulation, leading smoking and relapse to become more probable (see Sayette & Creswell, 2016). An often neglected research domain that offers clues for understanding why craving may precipitate relapse is temporal cognition. Time appears to pass more slowly when individuals are struggling with a craving than when they are not craving (Klein et al., 2003, Sayette et al. 2005), perhaps leading to what Vohs and Schmeichel (2003) refer to as an “extended now” period. Pertinent to the present study, when smokers are in a peak-craving state and asked to predict the trajectory of their craving over a specified period of time (e.g., the next 30 minutes) during which they are not permitted to smoke, they overestimate its duration and intensity (Sayette, Loewenstein, Kirchner, & Travis, 2005). In other words, they feel that without being able to smoke, their already high craving will unremittingly worsen. In actuality, however, even unresolved cravings tend to dissipate naturally (Marlatt 1985, Niaura et al. 1999; Sayette et al., 2005), suggesting that during peak craving, smokers' beliefs that cravings are intractable are typically false. These inaccurate predictions made while in a peak craving state suggest that urges alter the perceived consequences of resisting temptation (e.g., “my craving will get increasingly unbearable if I don’t smoke”), which may lead to failures in self-regulation (Baumeister, 2017). The present study evaluated not only craving reduction but anticipated craving trajectories if smoking were not permitted.
Craving Intervention
There have been widespread efforts to curb cigarette cravings (Ferguson & Shiffman, 2009; Levy et al., 2010). Pharmacologic interventions include nicotine replacement and psychotropic medications such as Bupropion and Varenicline (Brandon et al., 2011; Fiore et al., 2008). These approaches have had some success but also have limitations, such as aversive side effects, continued nicotine dependence, and are slow to take effect. Even nicotine gums can take as long as 15-min to work (Shiffman et al., 2003). Faster acting methods are indicated, as ½ of smokers who lapse after craving do so within 11-min of urge onset (Ferguson & Shiffman, 2009).
Behavioral approaches to craving reduction include exercise, meditation, coping skills and acupuncture. Although there remains good reason to study these methods, to date they have had mixed success (Marcus et al., 2005) and have some limitations such as being inconvenient and hard to master. Rose and colleagues have tested a conceptually distinct alternative to these approaches, known as airway sensory replacement. Pleasurable sensations accompanying smoking (e.g., taste, respiratory tract sensations) provide a rich set of smoking cues, and these sensations may be crucial in relieving craving and facilitating smoking abstinence (Rose, Salley, Behm, Bates, & Westman, 2010). These investigators tested the impact of inhaling various substances designed to simulate the sensory experience of smoking on craving relief (e.g., citric acid, capsaicin), but acknowledge that its long-term success is still unknown and “no satisfactory approach [to airway sensory replacement] has yet been developed” (Rose, 2006, p. 281).
Clearly, there is a pressing need for new interventions addressing the impact of craving on smoking (Baker et al., 2011). Conceptually, analysis of the link between craving and tobacco use requires understanding of the interaction of emotional and cognitive processes that promote smoking. The strategic administration of olfactory cues (OCs), either alone or in tandem with existing methods, may offer a novel approach to reducing craving and related shifts in the processing of smoking-related information.
Olfactory methods of craving relief.
In contrast to airway sensory replacement, in which the products simulate and remind one of smoking, the olfactory approach tested here aims to distract a smoker away from thoughts of smoking. The premise that OCs are better suited than other sensory cues to reduce affectively-charged cravings receives support from multiple sources. Cravings are emotional “visceral” experiences (Baker et al, 1987; Loewenstein, 1996; Nordgren & Chou, 2011). Olfaction is directly related to the amygdala and orbitofrontal cortex (a.k.a. the “secondary olfactory cortex” Shepherd, 2007), two structures linked to drug craving (Wilson, Sayette, & Fiez, 2004). Emotions are effectively manipulated through olfaction (Engen, 1982; Gottfried, 2010; Herz, 1998; 2012), a finding that extends to cigarette craving (Perkins et al., 2001; Towner, Ybasco, Rezai, Rose, & Contrada, 1991). This is unsurprising, as humans enjoy an outstanding sense of smell (McGann, 2017). Although OCs are known to provoke craving, surprisingly there has been almost no research testing the ability of OCs to attenuate cravings.
Recently, the study of olfaction – and its potential to alter cognition, emotion, behavior and physiology – has emerged as a worthy area of scientific inquiry (Herz, 2009). Scientific advances have occurred across diverse areas (Haviland-Jones, Wilson, & Freyberg, 2016). For instance, orthonasal OCs (i.e., sniffing odors) affect memory (Herz, 1998; Herz & Engen, 1996; Moss, Cook, Wesnes, & Duckett, 2003), interpersonal preferences (Li, Moallem, Paller, & Gottfried, 2007), behavior (Herz, Schankler, & Beland, 2004; Holland, Hendriks, & Aarts, 2005), and improve pain tolerance (Prescott & Wilkie, 2007), anxiety (Kadohisa, 2013), and cardiovascular and immunological markers of health (Herz, 2016). Further, OCs are known to affect time perception (Millot, Laurent, & Casini, 2016; Zhou, Feng, Chen, & Zhou, 2018). Finally, OCs even may be used during sleep to reduce smoking behavior due to aversive conditioning (Arzi et al., 2014). These observations suggest that olfactory stimuli may play an important role in the attenuation of urges.
Preliminary study.
In an initial proof-of-concept study, we evaluated the immediate impact of individually selected OCs on cigarette craving relief (Sayette & Parrott, 1999). Because individuals can experience different reactions to the same OCs (Engen, 1982), we offered participants a wide sample of OCs to identify particular ones that each person found to be pleasant or unpleasant. Accordingly, abstinent smokers first sniffed eight OCs, rating each on several dimensions (e.g., pleasantness). Participants next were exposed to smoking cues to create a peak urge. They then sniffed one of three OCs (their most pleasant, least pleasant, or odor blank1) and immediately rated their urge. Results indicated that exposure to pleasant or unpleasant OCs (similarly) reduced urge to smoke relative to exposure to the control OC. The drop in urge among those assigned to sniff a pleasant or unpleasant odor was about double that found for the odor blank stimulus (Sayette & Parrott, 1999). Two subsequent studies from different laboratories report similar data for food urges (Firmin, Gillette, Hobbs, & Wu, 2016; Kemps & Tiggemann, 2013). The latter group also included an auditory comparison group and found OCs to be uniquely potent in curbing craving. They noted that OCs offer a “less cognitively demanding alternative to imagery-based craving reduction techniques” (p. 1552).
Study rationale.
The findings from our initial study of primarily college-age smokers (mean age = 22.7) raise several questions related to the impact of this method. First, data are in line with the idea that provocative OCs distract a person away from their craving experience. If this explanation is accurate, then exposing craving smokers to a tobacco-related OC presumably would not reduce craving. If tobacco OCs also reduced craving, however, it would support an alternative perspective to distraction, in which merely simulating a smoking experience (Rose, 2006), or imagining consumption (Morewedge et al., 2010, though see Kavanagh et al., 2005) might attenuate a craving. The present study therefore included a tobacco OC condition.
Second, the drop in urge observed in Sayette and Parrott (1999) might have dissipated in mere seconds. Because that study assessed urge just once, within seconds of sniffing the OC, the duration of the OC effect remains unclear. Thus, an aim of the present study was to determine if the craving-reducing effects of a pleasant OC would extend for several minutes. If an OC temporarily attenuated craving and controlled expectations about the trajectory of the craving experience, it would buy a smoker precious time to regain focus and possibly manage the high-risk situation well enough that the threat is eliminated (e.g., the moment of “clarity” allows the smoker to leave a party). The premise is that not all time is created equal, and the moments close to peak craving are critical for determining how one will cope with the craving. From a relapse prevention perspective (Marlatt & Gordon, 1985), demonstrating that the impact of a craving-reducing OC lasts at least a few minutes may prove valuable if it targets high-risk moments.
Third, from a cognitive perspective, OCs are known to be especially effective in eliciting autobiographical memories (Herz, 1998; Saive, Royet, & Plailly, 2014). For instance, OCs evoke more specifically detailed, emotional and evocative autobiographical memories than do visual cues (Chu & Downes, 2002; Herz & Schooler, 2002; Herz, Eliassen, Beland & Souza, 2004). The most popular theory of autobiographical memory organization is drawn from the Self-Memory System (Conway, 2005; Conway & Pleydell-Pearce, 2000). This model proposes that autobiographical memories are reconstructed during retrieval by accessing a collection of self-relevant knowledge organized from broad to specific. The information that is retrieved when recalling an autobiographical memory falls into varying levels of specificity. Research indicates that individual differences in degree of autobiographical memory specificity predict a range of clinical, developmental, and social psychological phenomena (Goddard, Dritschel, & Burton, 1996; Wang, 2008). For instance, depressive symptomatology is associated with less detailed autobiographical memory recall (Williams et al., 2007). Assuming strategic use of OCs reduces peak craving partly by eliciting autobiographical memories that interfere with urge processing, assuming these memories are unrelated to smoking, this effect should be especially pronounced among those with the most specific autobiographical memory systems. The current study tested this proposition using the Sentence Completion for Events of the Past Test (SCEPT: Raes et al., 2007) to measure generative autobiographical retrieval. The SCEPT instructs participants to complete sentence stems in any way they would like as long as the response makes sense with the stem and that each sentence is about a different topic (sentence stem examples: Last year… I will never forget…). AM specificity scores from the SCEPT were obtained by summing the number of specific memories provided by the participant in the sentence completions. A memory was considered specific if it referred to an event that occurred at a particular time and that lasted less than one day (Raes et al., 2007; Williams et al., 2007), otherwise the memory was coded as non-specific. As is the case in the present study, the SCEPT is typically used as a trait measure of autobiographical memory. Accordingly, we administered it while participants were neither craving nor being exposed to OCs.
Fourth, in contrast to other senses, olfactory associations once formed are highly affected by proactive interference but resistant to retroactive interference (Lawless & Engen, 1977). That is, the first association made to an OC is hard to unlearn, while subsequent associations to the same scent are hard to form. This replicated and widely cited finding (see Herz, 2012) bodes well for its use for craving relief, as repeatedly pairing a positive odor with peak craving unlikely will cause the OC to lose its initial pleasant associations and instead become linked to the unpleasantness of an unrequited craving (i.e., OCs that reduce craving during an initial trial should maintain their therapeutic effects into the future). The present study tested this assumption by evaluating the impact of OCs on urge relief on successive days.
In addition to addressing these four questions raised by the findings of Sayette and Parrott (1999), the present study had a practical aim, namely to evaluate the extent to which participants thought use of OCs might serve as an effective approach to craving relief outside the laboratory. A challenge for behavioral interventions concerns motivation to use them (Kober & Mell, 2015). Regardless of how OCs work in the lab, skepticism associated with using olfactory stimuli could undermine their potential utility. Because our initial research indicated that pleasant and unpleasant OCs were similarly effective in reducing urge (Sayette & Parrott, 1999), the present study focused on pleasant OCs, assuming they would be more appealing to smokers than unpleasant or potentially harmful OCs (e.g., smelling salts) as a potential real-world intervention.
In this study, nicotine-deprived smokers initially rated the pleasantness of 12 OCs. They next were exposed to smoking cues, a manipulation that together with nicotine deprivation, has effectively increased self-reported urge (see Sayette & Tiffany, 2013). One of the previously administered OCs (either the one they previously rated as most pleasant, their own brand of tobacco, or an odor blank control) was then administered to smokers during this high craving state and reported urge and other craving-related processes were assessed over a 5-min interval. We predicted that (a) pleasant OCs would reduce reported urge more than a tobacco-related or odor blank OC, (b) this effect would persist across 5-min, and (c) the effect would not be subject to retroactive interference assessed at a subsequent experimental session. We also hypothesized that a pleasant OC would reduce the anticipated intensity and duration of cravings if participants were unable to smoke, and that this effect would extend to our measures of the value and expectancies attributed to smoking a cigarette. Moreover, it was hypothesized that pleasant OCs would be particularly effective in relieving craving among smokers with more specific and detailed autobiographical memory systems.
Method
This study received approval from the University of Pittsburgh Institutional Review Board.
OC Pilot Testing
Pilot testing to select the set of pleasant OCs for the study was adapted from our prior research (Herz, 2004; Herz & Cupchik, 1992; 1995; Sayette & Parrott, 1999). Participants sampled multiple odors to determine which ones were perceived to be most pleasant. To reduce confounding effects of nonolfactory sensory input, odors were soaked into dry interflo pellets, covered in cotton, and presented in opaque white 2-in high containers, an approach that has been used extensively (Herz & Cuphcik, 1995; Herz, Beland & Hellerstein, 2004). We pilot tested 30 odors, including those used in our initial study (Sayette & Parrott, 1999) and others provided by the International Flavors and Fragrance (IFF) corporation, while asking participants to complete an aroma questionnaire (AQ: Herz & Cupchik, 1995). The aroma questionnaire assessed responses from 1 (e.g., extremely unpleasant) to 9 (e.g., extremely pleasant), with 5 labeled “neutral” on pleasantness, intensity, and familiarity, and recorded emotional associations or memories participants had to the odor (Herz & Cupchik, 1992; Sugiyama et al., 2010). The aim was to generate a set of odors for which each participant could rate at least two as very pleasant. Piloting, along with use of the participant’s brand of tobacco and odor blank (the same odor container was presented but there was no odor inside), yielded a final set of 12 OCs for use in this study.
Experimental Design
Following a phone screen and follow-up lab screening visit, there were two experimental sessions. In EXP session 1, abstinent smokers were randomly assigned to one of three OCs during peak craving: 1) pleasant; 2) smoking-related; or 3) odor blank, allocating 75% as many participants to conditions 2 and 3, relative to condition 1. The increased sampling for condition 1 reflected the need for increased power to evaluate potential shifts in the effectiveness of pleasant OCs across days. In EXP session 2 (occurring at the same time of day as EXP session 1), half the participants in condition 1 were randomly assigned to sniff their original (pleasant) OC from session 1, while half sniffed a new pleasant OC (e.g., if a participant had rated lemon and lily of the valley as similarly pleasant and lemon was selected for session #1, then they would either receive the lemon again or the lily of the valley at session #2). [Our initial study revealed that all but two participants in the pleasant group identified at least two OCs tied for their maximal pleasantness rating (Sayette & Parrott, 1999).] When there was a tie, the top two OCs were randomly assigned to the two sessions. In addition, 1/3 of the participants in the remaining two conditions were randomly selected to switch and receive a pleasant OC, while the rest received the same tobacco or odor blank OC as in EXP session 1. This created five groups of about 45 participants for EXP session 2: 1) original pleasant OC; 2) different pleasant OC; 3) switch from tobacco or odor blank to a pleasant OC; 4) tobacco OC; and 5) odor blank. This design aimed to control for potentially confounding variables such as prior exposure to the lab or to the same OC.
Participants
Two hundred thirty-two smokers2 (107 female, 125 male) aged 18–55 years completed the study. All participants were required to smoke 10-30 cigarettes per day for at least 12 continuous months prior to the study. To assess exclusionary criteria, participants completed an initial telephone interview and attended a screening session. Participants were excluded if they reported a medical condition that contraindicated nicotine, were illiterate, unable to understand the basic math needed to execute the smoking choice task (described below), were using nicotine products (e.g., e-cigarettes, nicotine patch) other than cigarettes, were interested in quitting or cutting down on their smoking in the next 30 days, recorded carbon monoxide (CO) levels outside the range of 10 to 55 ppm, or were unable to detect a sample odor on a forced recognition task (see screening session below). On the basis of self-reported assessment, the final sample was 49.6% Caucasian, 42.6% African American, 6.5% multiracial, 0.9% Asian, and 0.4% American Indian. Participants’ mean age was 40.5 years (SD = 10.8). They smoked an average of 15.5 cigarettes per day (SD = 4.9).
Procedure
Telephone screening.
Ads for the study indicated that we were looking for people to participate in a three-part research project. The ad continued, “To participate, you must currently smoke cigarettes, be 18-55 years old, in good health, speak fluent English, be willing to fill out questionnaires, and to not smoke before two sessions.” Ads concluded with the phrase, “Earn $150 for completing this study” and the lab phone number. Potential participants who responded to our ads underwent a phone interview to exclude those not meeting selection criteria. Those who met selection criteria were asked to attend a 2-hr screening session at the Alcohol and Smoking Research Laboratory (ASRL) to determine whether they would qualify for a subsequent pair of experimental sessions lasting up to two hours each. Everyone was paid $20.00 at the screening session whether or not they were deemed eligible for the main study.
Screening session.
At the ASRL screening session, 342 participants provided informed consent and were required to show photo identification. The consent form indicated that the purpose of the study was to gain a better understanding of the effects of smoking opportunity in smokers and did not call attention to olfactory cues and craving. Participants next recorded a baseline CO reading and completed a brief interview regarding their current medical status and medication usage. We then administered a brief odor screening test, in which participants smelled six identical jars and identified the one containing an odor (an odor of comparable intensity to the study odors). [Only three individuals (1% of our sample) were excluded based on this test.] Participants next completed a battery of forms assessing age, gender, ethnicity, education, and income with standard forms used in the ASRL. Fifty participants were deemed ineligible and were paid and permitted to leave. Another 59 either failed to show, or recorded CO readings that were too high to be used, for one of the two subsequent experimental sessions. Eligible participants completed additional questionnaires assessing their smoking history, level of nicotine dependence, and other smoking-related constructs, as well as a standardized odor threshold test (Sniffin Sticks: Hummel et al., 2007; Kobal et al., 2000), to determine whether the various experimental conditions were comprised of participants (all of whom were above threshold for participation based on the initial odor perception test) with similar levels of odor perception. (See supplemental material for list of questionnaires administered to participants, including those not addressed in the present report.) Next, eligible participants were invited to attend two 2-hr lab sessions spaced one day apart for which they would be paid $150.00 in total: $20 for this screening/assessment session, $30 for the first EXP session, and $100 for the second EXP session. Participants were told to bring a pack of their preferred brand of cigarettes and to abstain from smoking for 8-hr before the experimental session. They also were told that breath samples would be collected to ensure that they had abstained from smoking.
Experimental Sessions (Sessions 1 and 2)
Sessions began between 1:00 p.m. and 4:00 p.m. Participants brought their preferred brand of cigarettes and lighter to the sessions. Procedures were modeled after our prior work (Sayette & Parrott, 1999). Figure 1 presents a timeline of the procedures. To check compliance with smoking abstinence instructions, participants reported the last time they smoked and provided a CO sample (Bedfont Scientific piCO+ Smokerlyzer). The requirement was that CO not exceed half their initial (nonabstinent) CO level or was < 10 ppm. Those who either reported smoking within 8-hr of arrival, or who produced a CO inconsistent with abstinence, were not permitted to participate in the session and were asked to reschedule. Participants presented their pack of cigarettes and lighter to the experimenter.
Figure 1.
Time Line (in minutes) for Experimental Sessions
Sampling.
After CO recording, participants sampled the 12 OCs drawn from our piloting. One OC typically perceived as unpleasant (amyl vinyl carbinol, often described as a “mushroom odor”) was included so that participants would be motivated to use a wider range on the scale and to mitigate against a cumulative positive mood induction. The other OCs included cumin, chocolate, apple, peppermint, vanilla, lemon, lily of the valley and an odor blank (no odor container). We also included two tobacco OCs, Amsterdam Shag and Danish Export (Scandinavian Tobacco Group Lane Ltd.) and the tobacco odor derived from the participant’s preferred brand. These three tobacco odors were generated by placing loose tobacco covered in cotton in the same white containers used with all the odors. Order of OC presentation was randomized, with the caveats that participants always received a pleasant OC first and last, and that they never received two non-pleasant OCs consecutively. The experimenter instructed participants through a two-way intercom to unscrew the lid on the container and sniff inside (approximately 6-sec). Following initial exposure to each of the OCs, participants completed the aroma questionnaire while continuing to sniff the container as often as they wished.
Craving Induction
Following a 15-min rest, during which participants completed a distractor task rating the attractiveness of a set of slides, a tray with an opaque plastic cover was placed on the table in front of them. Participants were asked not to touch the tray until instructed. When told, they lifted the cover, revealing their cigarettes, a lighter and ashtray. Participants removed a cigarette from the pack and lit it without putting it in their mouths (by holding it in the flame for several seconds until the tobacco began to burn). Participants were told that they could not smoke the cigarette. They next were asked to put down the lighter and to hold the cigarette comfortably in their dominant hand while staring at it but not placing it in their mouths. After 10-sec, participants verbally rated their urge to smoke (see measures below). Participants then extinguished the cigarette and placed it back in the ashtray.
Peak Craving OC Exposure
Participants opened a drawer in the table and removed a container corresponding to the OC for their condition. They were told to unscrew the lid and sniff for about 2-sec (one inhalation, Laing, 1983), and provided a verbal report of their urge. Next participants completed two craving related response measures (about 5-min), during which they sniffed the OC and rated their urge to smoke about every 60-sec. (See craving response measures below for description.) Participants then were asked if they wished to smoke. For those who wanted to smoke (all but eight participants), the smoking choice task was administered. Participants then were paid, reminded about their final session the next day (EXP session 2), and permitted to leave.
EXP session 2 was similar to EXP session 1, with the following changes. After smoking cue exposure, participants received either the same OC as in EXP session 1 or a different OC (see experimental design above). Participants next completed one urge rating assessment immediately after extinguishing their cigarette. If they wished to smoke, they were escorted outside the building to a smoking area, after which they completed a final packet of questionnaires. This packet included the SCEPT to assess autobiographical memory specificity and the contemplation ladder, a 0-10 scale with 0 = “No thought of quitting” and 10 = “Taking action to quit (e.g., cutting down, enrolling in a program)” (Biener, & Abrams, 1991). Participants then completed a post-experimental form, were debriefed, and paid.
Craving Response Measures.
Reported urge to smoke.
Urge to smoke was rated using a scale ranging from 0 (no urge to smoke at all) to 100 (strongest urge to smoke that I have ever felt). This scale has been highly sensitive to craving manipulations (see Heckman et al., 2013). During both sessions, participants completed this measure prior to smoking cue exposure and during smoking cue exposure, and then six times during OC exposure in EXP session # 1 and once during EXP Session #2.
Smoking Consequences Questionnaire-Brief (SCQ-B) (Sayette et al., 2005).
Beliefs about negative and positive consequences of smoking were rated to indicate the probability that a series of 24 desirable and undesirable consequences of smoking would occur (no timeframe was provided). The SCQ-B is an abbreviated version of the original SCQ (Copeland et al., 1995).
Anticipated Duration and Intensity Scale (ADIS).
Participants reported on a 0-100 scale their anticipated urge if not permitted to smoke over the next 5, 10, 15, 25, 35, and 45 min (Sayette et al., 2005).
Smoking Choice Task.
To assess the valuation of smoking, participants reported the least amount of money they would accept to postpone smoking for an additional 5-min (Sayette et al., 2001). Participants first were asked if they wished to smoke. For those who wanted to smoke, the smoking choice task was administered. The eight participants who declined were scored as requiring $0 to wait 5-min (Sayette, Martin, Wertz, Shiffman, & Perrott, 2001). The rest were told that we already had written down the maximum amount we would pay. They were further instructed that if their value exceeded ours, they could smoke immediately but would not receive additional money. They were told that if their value was less than ours, we would pay them what they asked in return for their 5-min smoking delay. The critical data were the minimal amounts participants reported they would accept to delay smoking for 5-min. (Participants did not actually postpone their smoking and received $5 extra, regardless of their responses.)
Due to concerns with anchoring effects associated with repeated assessment, the SCQ-B, ADIS, and smoking choice tasks only were administered at EXP Session #1. Further, in the case of the smoking choice task, the “choice” is no longer meaningful during EXP session 2, as participants now understand due to their experience at EXP session #1 that they will not actually need to wait five minutes before being able to smoke.
Results
Baseline
The primary aims of the study focused on EXP Session #1, which included three OC conditions. To ensure equivalence between groups, a series of analyses was conducted to determine if the three groups were similar across key measures. Random assignment led to groups that did not differ on age, gender, nicotine dependence [as assessed by the Nicotine Dependence Syndrome Scale (NDSS: Shiffman, Waters, & Hickox, 2004) and the revised Fagerstrom Test for Nicotine Dependence (FTND: Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991)], odor perception, race, ethnicity, or level of education (p’s >. 15). Across groups, participants’ baseline and post-smoking cue exposure urges did not differ. There also were no group differences in urge increases from baseline to smoking cue exposure (p’s > 0.39).
Manipulation Checks
In order to evaluate the urge-reducing effects of the OCs, it was crucial that the smoking cue exposure procedure elicit a strong urge to smoke. Mean urge during smoking cue exposure was high (M = 82.13, SD = 19.81), comparing favorably to peak urges in past studies (see Wertz & Sayette, 2001). A repeated measures ANOVA revealed a significant increase in reported urge from baseline (M = 69.40, SD = 22.15) to smoking cue exposure, F (1, 228) = 97.34, p < .001, providing support for the efficacy of our urge induction to generate a peak urge state.
By design, during OC sampling the three OC groups were expected to differ on pleasantness ratings for the specific OC they eventually would be administered following smoking cue exposure. The mean pleasantness (SD) rating from the aroma questionnaire for participants exposed to a (a) pleasant OC was 8.57 (.65), (b) tobacco OC was 4.57 (1.97), and (c) odor blank OC was 4.84 (1.38). As expected, groups differed on this dimension, F (2, 228) = 224.05, p < .001. Participants in the pleasant condition reported their selected OC to be significantly more pleasant than did those in the other two conditions (p’s < .001), whose values did not differ from each other (p’s >.25). Also, as expected, the mood rating from the aroma questionnaireshowed the same pattern, F (2, 221) = 27.59, p <.001. Participants in the pleasant condition [M = 7.17 (.17)] reported higher positive mood than did those in the tobacco [M = 5.43 (.20)] or odor blank [M = 5.66 (.20)] conditions, respectively (p’s < .001), with the latter two conditions being similar to each other (p > .43). In sum, our manipulation succeeded in creating a pleasant condition that led to greater pleasantness ratings and a more pleasant mood than did the tobacco and odor blank conditions.
As in our prior work, the groups also differed on the remaining aroma questionnaire items. Specifically, significant group differences for the particular OC that was presented following smoking cue-exposure emerged for intensity, F (2, 228) = 112.23, p < .001, memory3, F (2, 210) = 80.05, p < .001, and familiarity, F (2, 227) = 138.41, p < .001. Pleasant OC values were significantly higher than tobacco, which in turn were significantly higher than odor blank (p’s < .002).
Effect of OC on Reported Urge
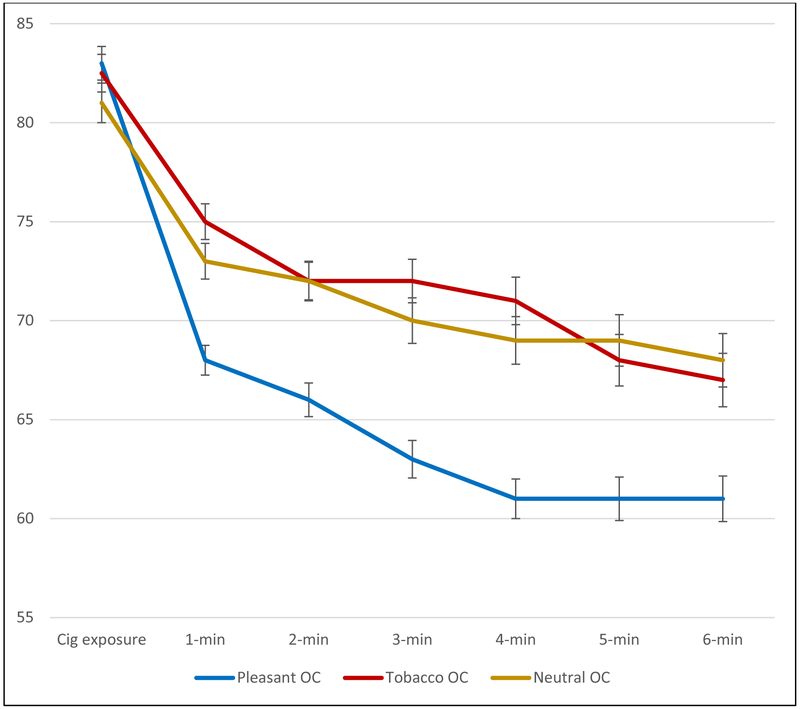
To examine the effects of OCs on urge report, a 3 (OC condition) X 6 (Time) repeated measures ANCOVA was computed, with the six OC exposure assessments as the within-subject variable and pre- and post-smoking cue exposure urges as covariates. As expected, both covariates (pre- and post-smoking cue urges) were significant, F’s > 22, p’s < .001. There also was an effect of time, F (5, 1130) = 5.07, p < .001, partial η2= .022). Most relevant to the study hypotheses, there was a main effect for OC condition, F (2, 226) = 5.33, p = .006 (partial η2 = .045; 90% confidence intervals ranging from .0079 to .0907). Collapsing across the six OC urge assessments, participants who were presented with a pleasant OC reported a 19.3 point drop in urge, which was significantly greater than those receiving the Tobacco OC (M =11.7 points), p < .006 (partial η2= .045; 90% confidence intervals ranging from .0073 to .1067), and significantly greater than those receiving the odor blank (M = 11.2 points), p < .011 (partial η2 = .040; 90% confidence intervals ranging from .0050 to .0994). Follow-up analyses were conducted at each of the six time points. For the first five time periods there was a significant effect of OC condition (all F’s > 3.23, p’s <.042; partial η2s ranged from .028 to .049; 90% confidence intervals ranging from .0006 to .0961). (As seen in Figure 2, the smallest difference among the first five assessments appeared following the first OC exposure assessment.) At the final (6th) assessment, the effect of OC was no longer significant, F (2, 226) = 2.74, p < .067) (see Figure 2). The absence of a significant effect at this final period is due to urge ratings in the odor blank and tobacco groups starting to return to lower, pre-smoking cue-exposure, levels. That is, the urge-reducing effect of the pleasant OC during this final time period appears to be maintained. Pleasant OCs were equally effective across gender and level of nicotine dependence (p’s > .88).
Figure 2.
Mean Urge Ratings (0-100 scale)
Effects of OC on Craving-Related Responses
ADIS:
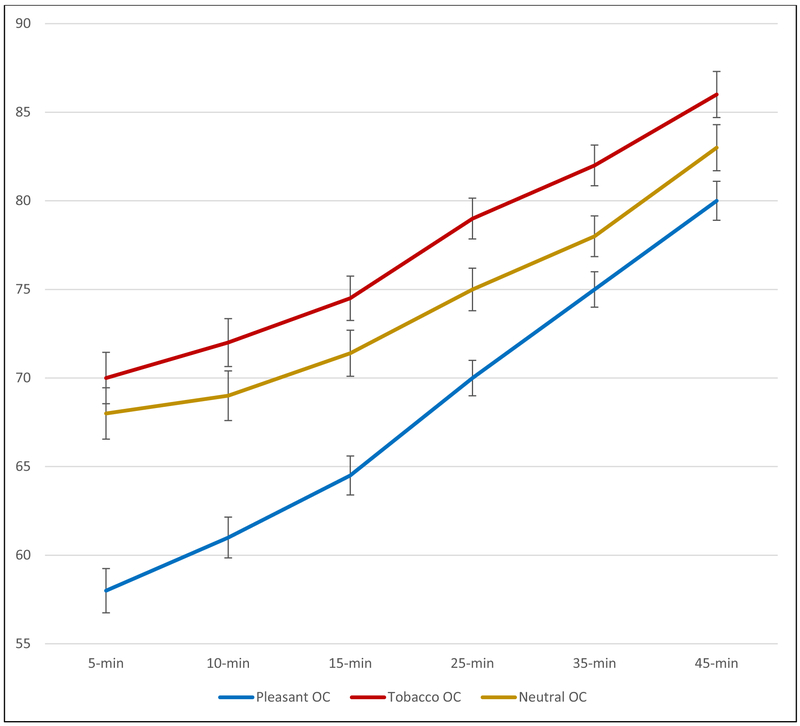
To examine the effects of sniffing an OC on anticipated urge over a 45-min interval, a 3 (OC condition) X 6 (Time) repeated measures ANCOVA, with the six ADIS assessments (anticipating urge at 5-, 10-, 15-, 25-, 35-, and 45-min) as the within-subject variables and pre- and post-smoking cue exposure urges as covariates, was computed. Both covariates (pre- and post-smoking cue urges) were significant, F’s > 8.2, p’s < .005. There also was an effect of time F (5, 1090) = 15.93, p < .001, partial η2=.068). Pertinent to the hypotheses, there was a main effect of OC condition across time, F (2, 218) = 5.43, p = .006. Collapsing across the six OC anticipated urge assessments, participants who were presented with a pleasant OC anticipated their urge would be 68.1, which was significantly lower than those receiving the Tobacco OC (M = 76.1), p < .001 (partial η2= .077; 90% confidence intervals ranging from .0223 to .1478), and significantly lower than those receiving the odor blank (M = 74.7), p < 032 (partial η2 = .030; 90% confidence intervals ranging from .0013 to .0846. Follow-up analyses were conducted at each of the six anticipated time points. For the first four time periods there was a significant effect of OC condition (F’s > 4.17, p’s < .018, partial η2s ranged from .037 to .055; 90% confidence intervals ranged from .0037 to .1116). At the final two assessments (anticipated urge at time 35-min and 45-min), the effect of OC was no longer significant, F (2, 218) = 2.93 and 1.47 (p’s = .056 and .232, respectively). As depicted in Figure 3, exposure to a pleasant OC led to significantly lower anticipated urges, with the effect starting to dissipate toward the end of the 45-min anticipation interval. We next examined whether the drop in urge following initial OC exposure accounted for the impact of OC on the ADIS. Results showed that once the urge rating following initial OC exposure (just prior to completing the ADIS) was included in the analyses, the overall effect of OC on ADIS scores was no longer significant, F (2, 217) = 2.71, p = .068.
Figure 3.
Anticipated Urge Ratings (0-100 scale) Completed Just After Olfactory Cue Exposure
Smoking choice:
As in our prior studies, the smoking choice measure was not normally distributed (skew = 2.7, kurtosis = 10.8). Although median scores were in the expected direction, with those receiving a pleasant OC requiring $3.25 to postpone smoking for five minutes, compared to $4.38 and $4.75 for those receiving the tobacco and the odor blank, respectively, a nonparametric Van der Waerden test failed to reach significance (p = .390).
SCQ-brief:
A one-way ANOVA failed to reveal an effect of OC condition (p = .378).
Role of Autobiographical Memory Systems on OC-Related Urge Reduction.
We next tested the extent to which individual differences in autobiographical memory specificity (assessed with the SCEPT) moderated the impact of OC condition on urge (using the mean value of the six OC urge assessments). Scores on the SCEPT were equivalent across the three OC conditions, which is expected, as it was administered following ad lib smoking when cravings were diminished. Controlling for pre- and post-smoking cue exposure urges and OC condition, specificity did not predict OC urge (p > .66). Addressing our hypothesis, however, the SCEPT specificity × OC condition interaction was significant, F (2, 221) = 5.32, p = .006 (partial η2 = .046; 90% confidence intervals ranges from .0364 to .0079). To evaluate this interaction, we examined the correlation between autobiographical memory specificity and urge drop (i.e., post-smoking cue exposure urge minus the mean value of the six OC urge assessments) in each of the three conditions. Those in the pleasant OC condition with the most specific autobiographical memory systems displayed the greatest drop in urge, r(93)=.244, p = .019. In contrast, there was no relation between specificity and urge change in either of the other two OC conditions, r’s= −.068 and −.217 for odor blank and tobacco, respectively (p’s >.15).
Tests for habituation across two days.
We also tested the impact of OCs on urge during EXP session 2. We initially analyzed urge following OC exposure for those who were exposed to the same OC that they had received at EXP session 1. Similar to EXP session 1, results of an ANCOVA (with OC condition as the independent variable and baseline urge and post smoking cue exposure urge serving as covariates) revealed a main effect of OC, F (2, 132) = 3.85, p =.024 (partial η2 = .055; 90% confidence intervals ranged from .0400 to .1186). Smokers in the pleasant condition showed a significantly greater drop in urge (M = 9.2) than did either those in the tobacco (M = 4.4) or odor blank (M = 3.8) conditions (p’s < .038), with the latter two conditions not differing from each other (p > .57). We also examined whether the same pleasant OC, due to possible habituation, would be less effective than a different but similarly rated pleasant OC on this second day. Pleasantness ratings did not differ among participants who were randomly assigned to receive the same (n = 48, M = 8.08, SD = 1.13) vs. a different (n = 46, M = 7.74, SD = 1.41) pleasant OC on session 2 (p > .19) (partial η2 = .018; 90% confidence intervals ranged from .0000 to .0854). We then contrasted urge relief among these two groups during EXP session 2, again including baseline urge and post smoking cue exposure urge as covariates. There was no evidence of decay in the effectiveness of the original pleasant OC, F (1, 90) = 0.48, p > .49 (partial η2 = .005; 90% confidence intervals ranged from .0000 to .0544). (In fact, the urge ratings were in the opposite direction one would expect if there were habituation). Finally, there were no differences in urge relief between those receiving the same pleasant OC on session 2 and either of the two groups that had received a pleasant OC for the first time during session 2 (having received either a tobacco (p > .12) (partial η2 = .037; 90% confidence intervals ranged from .0000 to .1305) or odor blank (p > .99) (partial η2 = .000; 90% confidence intervals ranged from .0000 to .0000) OC during session 1.
Interest in Using Pleasant OCs.
Although the current study did not test the effects of OCs on actually quitting, we did collect data following EXP session 2 on participants (n = 174)4 who had received a detectable OC (i.e., either a pleasant or a tobacco OC) regarding whether or not they could imagine using an OC (a “nicely scented product”) to lower their urge if they were trying to quit smoking and were experiencing an intense urge. Eighty-nine percent reported affirmatively, supporting the potential viability of OCs as part of a smoking cessation treatment. Further, 93% indicated they could imagine using OCs if they were not trying to quit but were forced to abstain from smoking for a certain period of time.
Discussion
Choosing to smoke the “next” cigarette ranks among the most harmful decisions one can make. While most smokers generally recognize this danger, during “hot” moments of temptation the appeal of smoking increases, learned coping skills (or quit-smoking messages) can be ignored, and in many cases the smoking habit persists (Sayette, Lowenstein, Griffin, & Black, 2008). This dire public health challenge also poses a conceptual puzzle, namely to understand the interplay between cognitive and emotional processes driving the smoking habit. Progress has been slow to develop strategies to reverse or limit these hot decisions to smoke. It is vital that innovative approaches be tested to help the millions of smokers wishing to quit, especially during high-risk periods when cravings are most pronounced (i.e., during withdrawal and when exposed to smoking cues) (Baker et al., 2011). Strategic use of olfactory stimuli may offer a novel approach to curbing cravings and controlling the accompanying shifts in processing of smoking-related information that may enhance the likelihood of smoking.
Our major finding was that pleasant OCs attenuated fairly intense cigarette craving across a 5-min period, relative to tobacco or no odor OCs. These data replicate our initial test of this concept, which reported an immediate effect of OC on urge, comparing well to the onset of urge relief of even the fastest acting NRT products (Du, Borders, Selmani, & Waverczak, 2015), and provide further evidence that the impact of pleasant OCs can persist for as long as 5-min. Across the 5-min interval, the drop in urge from peak in the pleasant group approached 20 points on the 0-100 scale, about double that found for the control OCs, virtually the same magnitude of urge reduction found in our preliminary study (Sayette & Parrott, 1999). These findings also are in accord with research demonstrating an OC-induced drop in food cravings (Firmin et al., 2016; Kemps & Tiggemann, 2013). Together, these findings suggest that sniffing an OC identified a priori by the participant to be pleasant reduces peak urges. Figure 2 also suggests that unlike a pleasant OC, sniffing a tobacco OC is no better at urge reduction than sniffing an odor blank. Indeed, many participants noted during debriefing that the tobacco OC reminded them of their urge, distinct from simulating a smoking experience, which might have reduced an urge (Rose, 2006). Admittedly, we cannot rule out the possibility that the tobacco OC, which was not a perfect representation of a smoker’s burning cigarette, also might have been distracting, and thus we stop short of claiming that the evidence for distraction as a mechanism of OC urge relief is conclusive. Our data also reveal that OC’s had stronger effects for participants who had higher urges prior to sniffing. Whether this suggests that OCs are especially effective for those with strong urges or simply a methodological issue (i.e., those with higher urge scores have more room to drop) remains an open question.
There also was some indication that exposure to a pleasant OC affected cognitive processes putatively linked to smoking. Specifically, pleasant OC exposure tempered the anticipated intensity and duration of the expectation of an unrequited urge for 30-min, likely offering a more realistic, less alarming appraisal of the craving experience (Marlatt, 1985; Sayette et al., 2005). Presumably, if smokers believe their urge will reach intolerable levels if they do not smoke, then they may acquiesce to the urge rather than try to resist it (Sayette, 2016). This finding suggests that not only does a pleasant OC reduce urge, but it may also alter processes believed to relate to smoking-related decision making. If, as Baumeister (2017) argues, relapse is linked to a faulty ability to predict future states, these data suggest that OCs may play a key role in promoting healthier decision-making during cravings. This intriguing finding should be tempered by the nonsignificant effects of OC on smoking choice and perceived smoking consequences, though it also should be noted that this latter measure was developed to detect stable traits rather than subtle momentary shifts (Copeland et al., 1995). We did not counterbalance the various craving-related response measures, as our aim was not to test the relative sensitivity of each. Instead, we prioritized the urge rating scale as it is by far the most widely used of our measures and would best permit this study to connect to the broader literature. We believe that the current findings justify additional research with a variety of smokers and measures of smoking-related decision making to more comprehensively assess the impact of OC on urge responding.
Data also suggested that individuals with the most specific autobiographical memory systems showed the greatest effects when using a pleasant OC to control their urge. To our knowledge, this is the first evidence to implicate autobiographical memory in the relation between pleasant OCs and craving relief. Because we designed the study such that olfactory cues would lead to direct retrieval of autobiographical memories (i.e., a bottom-up, stimulus driven approach), the underlying mechanisms are thought to reflect automatic processes unrelated to executive functioning (Conway & Pleydell-Pearce, 2000).
We had hoped to obtain additional evidence for this prospect by assessing actual autobiographical memories during the initial OC sampling. During OC sampling, we asked participants to write down whether each OC triggered a memory and if so to describe it. In an ongoing study, we have switched this method and have begun instead to use a structured interview to assess memories and are finding participants to be more open to describing such memories than they were when writing them down. Nevertheless, the link between autobiographical specificity and urge reduction following administration of a pleasant OC is intriguing, and we hope it will foster additional testing outside our laboratory. More broadly, research is needed to further unpack the ways in which olfactory stimuli influence cognitive and affective processes associated with craving.
We offered a subset of our participants either the same or a different pleasant OC on a second session, and, consistent with prior research indicating that OCs can be resistant to proactive interference (Lawless & Engen, 1977), we found the same (repeated) pleasant OC to be as effective as a new pleasant OC in reducing urge. While the differences on the second day between pleasant compared to tobacco or blank OCs were equally significant in EXP session 2 as in EXP session 1, power was less robust for some of these analyses. It is also the case that across the board the levels of urge reduction after placing the cigarette in the ashtray were lower in EXP session 2. It also remains unclear how effective OCs would be if repeated across many days (perhaps a menu of individually-tailored OCs would be beneficial)? Consequently, it would be useful to continue to evaluate the potential for habituation across sessions in future research.
Only 1% of the more than 300 smokers screened were excluded due to inadequate odor detection. That is, the direct odor administration used in the study can be used with even heavy daily smokers. Moreover, neither gender nor nicotine dependence moderated the impact of pleasant OCs on urge reduction, suggesting that the presentation of OCs can be effective for a broad range of smokers.
In line with our emphasis on pleasant OCs, 89% of participants reported that they could imagine using an OC in the future if they were trying to quit and were experiencing an urge to smoke, supporting the potential viability of OCs as part of a smoking cessation treatment. Further, 93% indicated they would consider using OCs if they were not trying to quit but were forced to abstain from smoking for a certain period of time.
A key remaining question that was not addressed here concerns the impact of OCs on actual smoking behavior among individuals wishing to quit. We believe there is a solid foundation for proposing that OCs may prove to be a useful component of a smoking cessation program and note that a small field study recently found that OCs not only curbed cigarette craving but increased the lag between urge and use (Cordell & Buckle, 2013). Whether or not strategic use of OCs (in conjunction with extant treatment approaches or even by itself) might help a subset of smokers quit is worthy of investigation. To conduct a rigorous test of these questions, however, requires research using a variety of delivery systems and integration with other pharmacological and behavioral elements of successful smoking cessation. We believe that the data provided in the present laboratory study is pivotal in motivating such additional efforts, including ecological momentary assessment research examining smokers in their natural environment.
The present study also did not contrast olfactory cues with other sensory stimuli, precluding our ability to demonstrate that olfactory cues are uniquely potent in relieving craving. We reiterate, however, that a prior study with food craving did aim to address this issue by contrasting olfactory and auditory cues and found superior effects for olfaction (Kemps & Tiggemann, 2013). Moreover, compared to any other senses, olfaction is directly tied to emotional processing in the brain (Aggleton & Mishkin, 1986; Cahill et al., 1995). Therefore, we believe that OCs represent an especially good class of sensory cue for affecting emotions, including craving. It also remains to be seen whether OCs can help relieve cigarette craving that is not specifically the result of nicotine deprivation and exposure to a lit cigarette. Results suggest that OCs can help control cravings for a 5-min span. This time frame may be sufficient to enable a smoker to take action to avoid a relapse, or may hold down the fort long enough for a nicotine replacement product to exert its effects. As noted by Shiffman et al. (2003), the pharmacokinetic profile for acute nicotine replacement products such as gum and nasal spray may take several minutes to exert effects. By way of comparison, the nicotine (Nicorette) gum condition in their study led to about a 25-point drop in urge (on a 0-100 scale) after 15-min. (which was the first time period they assessed), a drop that is larger than that found in the pleasant OC condition. A subsequent study found use of Nicorette gum to drop cravings about 25 points after just 6-min (Niaura et al., 2005). It is also possible that 5-min may be long enough for the urge to begin to subside naturally (Sayette et al., 2005). As observed in Figure 2, after 5-min the drop in urge found in the pleasant condition still was holding steady, yet it remains unclear just how long the urge relief effect would last beyond 5-min.
The urge reduction found here and in our prior study (Sayette & Parrott, 1999), along with studies showing similar effects for food craving, and more generally the emerging body of scientific research suggesting OCs can affect processes as diverse as memory, pain tolerance, and anxiety, combine to support continued investigation of this often overlooked sensory system. Yet it is not entirely clear how to interpret even a 20-point drop on a rating scale. While urge is traditionally considered to be a linear construct, with each point on the scale reflecting a similar unit of craving, such assumptions remain untested (Sayette & Tiffany, 2013). There may be key thresholds of craving above which there is an increased probability of experiencing an “all systems go” response (Sayette et al., 2003) and the likelihood of acting on the desire increases. Such thresholds are hard to capture, and likely differ across person and situation, but research on this topic is important in order to better contextualize the impact of a drop from about 80 to 60 on a 0-100 urge scale.
Finally, though the data are consistent with the proposition that pleasant OCs relieve urge by distracting smokers from their craving (and even suggest a role for autobiographical memory), future research is needed to offer a more mechanistic understanding of the potential routes through which OCs attenuate craving. It remains unclear whether pleasant OCs simply offer distraction, or whether this distraction is a function of the impact of OCs on a variety of craving-related processes, including: working memory (OCs may compete with urges for the same working memory resources); delay discounting (OCs may help to reestablish cognitive control or, conversely, may compete for the same reward processes); habitual responding (OCs may help to override overlearned drug use behaviors); affective interference (OCs may induce emotional states that disrupt the internal stimulus setting events for craving 5 or that are incompatible with a withdrawal-based urge, see Baker et al., 1987); and response inhibition (OCs may bolster inhibitory control processes). These possibilities are compatible with recent neurobehavioral models that aim to identify subtle cognitive impairment that supports addiction, with craving-related processes linked to a number of coordinated brain networks (Bickel, Jarmolowicz, Mueller, & Gatchalian, 2011; Zilverstand, Huang, Alia-Klein & Goldstein, 2018). We currently are conducting fMRI research aimed at better understanding why smokers may experience processing shifts while craving, and in particular, how OCs may attenuate these responses.
In summary, despite disappointing relapse rates, there have been few new approaches to smoking cessation in general, and to craving relief in particular. The ability of olfactory stimuli to uniquely alter craving states and cognitive processes associated with smoking, and disrupt well-learned smoking routines, would offer a distinct and novel method for reducing cravings, and our results to this end are promising. Accordingly, continued investigation of the link between OCs and craving relief provides opportunities to enhance smoking cessation efforts, while simultaneously offering a conceptually rich platform for examining the relations among emotion, cognition, and olfaction.
Supplementary Material
Acknowledgments
This research was supported by the National Cancer Institute (R01 CA184779 and R01CA184779-03S). We thank Stephen Warrenburg, Aleksey Dumer, Krystyna Rankin and The International Flavors and Fragrance (IFF) corporation for providing us with many of the olfactory cues used in the study. We also thank Catharine Fairbairn, John Dimoff, Calvin Salazar, Chloe Reshetar-Jost, Stephen Susa, Brandon Jenkins, and Sarah Bobrzynski for assistance. Mary Marchetti is now at the University of Oregon. The self-reported urge data reported here were presented in a symposium at the annual meeting of the Society for the Study of Motivation, San Francisco, CA, May 2018. This study received approval from the University of Pittsburgh Institutional Review Board study # PRO13110547.
Footnotes
An odor blank OC condition was included to evaluate a potential confound in which drops in reported urge during post-cue exposure odor administration could be attributed to the act of sniffing, per se. This condition also provided a control for the effect of time on urges to smoke. That is, once the smoking cue exposure manipulation was completed (and the smoker extinguished the cigarette and placed it back in the ashtray), there might be a tendency for urges to drop from maximal levels.
One participant was excluded from the study due to procedural abnormalities—a decision that was made at the time—and then later replaced.
For those who did report a memory we examined memory intensity, specificity, and pleasantness. However, because < 15% of participants in tobacco and odor blank OC conditions reported a memory, we did not conduct further analyses with this item. We address this low response rate in the discussion.
This question was not asked of participants in the odor blank condition as they never received a scented OC during smoking cue exposure. One odor blank participant was mistakenly administered this question, and their response is not included.
We thank an anonymous reviewer for this suggestion.
Contributor Information
Michael A. Sayette, University of Pittsburgh
Mary A. Marchetti, University of Pittsburgh
Rachel S. Herz, Brown University
Lea M. Martin, University of Pittsburgh
Molly A. Bowdring, University of Pittsburgh
References
- Aggleton JP & Mishkin M (1986). The amygdala: Sensory gateway to the emotions In Plutchik R, Kellerman H (Eds.), Emotion: Theory, research and experience. Vol 3: Biological foundations of emotion, Orlando: Academic Press. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Arzi A, Holtzman Y, Samnon P, Eshel N, Harel E, & Sobel N (2014). Olfactory aversive conditioning during sleep reduces cigarette-smoking behavior. Journal of Neuroscience, 34, 15382–15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Mermelstein R, Collins LM, Piper ME, Jorenby DE, Smith SS, … Fiore MC (2011). New methods for tobacco dependence treatment research. Annals of Behavioral Medicine, 41, 192–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Morse E, & Sherman JE (1987). The motivation to use drugs: A psychobiological analysis of urges In Rivers C (Ed.), The Nebraska Symposium on Motivation: Alcohol Use and Abuse (pp. 257–323). Lincoln: University of Nebraska Press. [PubMed] [Google Scholar]
- Baumeister RF (2017). Addiction, cigarette smoking, and voluntary control of action: Do cigarette smokers lose their free will? Addictive Behaviors Reports, 5, 67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biener L & Abrams DB (1991). The contemplation ladder: Validation of a measure of readiness to consider smoking cessation. Health Psychology, 10, 360–365. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mueller ET & Gatchalian KM (2011). The behavioral economics and neuroeconomics of reinforcer pathologies: implications for etiology and treatment of addiction. Current Psychiatry Reports, 13, 406–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon TH, Drobes DJ, Unrod M, Heckman* BW, Oliver* JA, Roetzheim RC, Karver SB, & Small BJ (2011). Varenicline effects on craving, cue reactivity, and smoking reward. Psychopharmacology, 218, 391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon TH, Tiffany ST, Obremski KM, & Baker TB (1990). Postcessation cigarette use: The process of relapse. Addictive Behaviors, 15, 105–114. [DOI] [PubMed] [Google Scholar]
- Brown KW & Ryan RW (2003). The Benefits of being present: Mindfulness ad its role in psychological well-being. Journal of Personality and Social Psychology, 84, 822–848. [DOI] [PubMed] [Google Scholar]
- Cahill L, Babinsky R, Markowilsch HJ & McGaugh J (1995). Amygdala and emotional memory. Nature, 377, 295–296. [DOI] [PubMed] [Google Scholar]
- Carver CS, Scheier MF, & Weintraub JK (1989). Assessing coping strategies: A theoretically based approach. Journal of Personality and Social Psychology, 56, 267–283. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2016). Current cigarette smoking among adults—United States, 2000-2004. Morbidity and Mortality Weekly Report, 65, 1205–1211. Accessed 2017 Mar 28. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2014). The health consequences of smoking—50 years of progress: A report of the surgeon general. Accessed 2015 Aug 17. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2011). Quitting smoking among adults—United States, 2001–2010. Morbidity and Mortality Weekly Report, 60(44), 1513–1519. Accessed 2012 Jun 7. [PubMed] [Google Scholar]
- Chu S, & Downes JJ (2002). Proust nose best: Odors are better cues of autobiographical memory. Memory & Cognition, 30(4), 511–518. [DOI] [PubMed] [Google Scholar]
- Conway MA (2005). Memory and the self. Journal of Memory and Language, 53(4), 594–628. [Google Scholar]
- Conway MA, & Pleydell-Pearce CW (2000). The construction of autobiographical memories in the self-memory system. Psychological Review, 107(2), 261–288. [DOI] [PubMed] [Google Scholar]
- Copeland AL, Brandon TH, & Quinn EP (1995). The Smoking Consequences Questionnaire-Adult: Measurement of smoking outcome expectancies of experienced smokers. Psychological Assessment, 7, 484–494. [Google Scholar]
- Cordell B & Buckle J (2013). The effects of aromatherapy on nicotine craving on a U.S. campus: A small comparison study. The Journal of Alternative and Complementary Medicine 19, 709–713. [DOI] [PubMed] [Google Scholar]
- Costa PT Jr., & McCrae RR (1992). Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI): Professional manual. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Dimoff JD, Sayette MA, & Norcross JC (2017). Addiction training in clinical psychology: Are we keeping up with the rising epidemic? American Psychologist, 72, 689–695. [DOI] [PubMed] [Google Scholar]
- Du D, Borders J, Selmani A & Waverczak W (2015). A pilot study to investigate the efficacy of nicotine oral soluble film, lozenge and gum in relief of acute smoking cue-provoked craving for cigarette in low dependence smokers. Journal of Smoking Cessation 10, 87–95. [Google Scholar]
- Engen T (1982). The perception of odors. New York, NY: Academic Press. [Google Scholar]
- Feeney BC, & Thrush RL (2010). Relationship influences on exploration in adulthood: The characteristics and function of a secure base. Journal of Personality and Social Psychology, 98, 57–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SG, & Shiffman S (2009). The relevance and treatment of cue-induced cravings in tobacco dependence. Journal of Substance Abuse Treatment, 36, 235–243. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, … Wewers ME (2008). Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: US Public Health Service. [Google Scholar]
- Firmin MW, Gillette AL, Hobbs TE, & Wu D (2016). Effects of olfactory sense on chocolate craving. Appetite, 105, 700–704. [DOI] [PubMed] [Google Scholar]
- Goddard L, Dritschel B, & Burton A (1996). Role of autobiographical memory in social problem solving and depression. Journal of Abnormal Psychology, 105(4), 609–616. [DOI] [PubMed] [Google Scholar]
- Gottfried JA (2010). Olfaction and its pleasures: Human neuroimaging perspectives In Gottfried JA (Ed.), Pleasures of the brain (pp. 125–145). New York: Oxford University Press. [Google Scholar]
- Green McDonald P, O'Connell M, & Suls J (2015). Cancer control falls squarely within the province of the psychological sciences. American Psychologist, 70(2), 61–74. [DOI] [PubMed] [Google Scholar]
- Gwaltney CJ, Shiffman S, & Sayette MA (2005). Situational correlates of abstinence self-efficacy. Journal of Abnormal Psychology, 114, 649–660. [DOI] [PubMed] [Google Scholar]
- Haviland-Jones JM, Wilson P, & Freyberg R (2016). Olfaction: Explicit and implicit emotional processing In Barrett LF, Lewis M & Haviland-Jones JM (Eds.), Handbook of emotions (pp. 196–218). Guilford Press. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, & Fagerstrom KO (1991). The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction, 86, 1119–1127. [DOI] [PubMed] [Google Scholar]
- Heatherton TF & Polivy J (1991). Development and validation of a scale for measuring state self-esteem. Journal of Personality and Social Psychology, 60, 895–910. [Google Scholar]
- Heckman BW, Kovacs MA, Marquinez NS, Meltzer LR, Tsambarlis ME, et al. (2013). Effects of mood manipulations on cigarette craving: a meta-analysis. Addiction, 108, 2068–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks PS, Wood SB, Baker MR, Delucchi KL, & Hall SM (2011). The smoking abstinence Questionnaire: Measurement of smokers’ abstinence-related expectancies. Addiction, 106, 716–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz RS (1998). Are odors the best cues to memory? A cross-modal comparison of associative memory stimuli. Annals of the New York Academy of Sciences, 855, 670–674. [DOI] [PubMed] [Google Scholar]
- Herz RS (2004). A naturalistic analysis of autobiographical memories triggered by olfactory visual and auditory stimuli. Chemical Senses, 29, 217–224. [DOI] [PubMed] [Google Scholar]
- Herz RS (2009). Aromatherapy facts and fictions: A scientific analysis of olfactory effects on mood, physiology and behavior. International Journal of Neuroscience, 119, 263–290. [DOI] [PubMed] [Google Scholar]
- Herz RS (2012). Odor memory and the special role of associative learning In Zucco GM, Herz RS & Schaal B (Eds.), Olfactory cognition: From perception and memory to environmental odours and neuroscience (pp. 95–114). Amsterdam, Netherlands: John Benjamins Publishing Company. [Google Scholar]
- Herz RS (2016). The role of odor-evoked memory in psychological and physiological health. Brain Science, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz RS, Beland SL & Hellerstein M (2004). Changing odor hedonic perception through emotional associations in humans. International Journal of Comparative Psychology, 17, 315–339. [Google Scholar]
- Herz RS, & Cupchik GC (1992). An experimental characterization of odor-evoked memories in humans. Chemical Senses, 17, 519–528. [DOI] [PubMed] [Google Scholar]
- Herz RS, & Cupchik GC (1995). The emotional distinctiveness of odor-evoked memories. Chemical Senses, 20, 517–528. [DOI] [PubMed] [Google Scholar]
- Herz RS, & Engen T (1996). Odor memory: Review and analysis. Psychonomic Bulletin & Review, 3, 300–313. [DOI] [PubMed] [Google Scholar]
- Herz RS, Eliassen JC, Beland SL, & Souza T. (2004). Neuroimaging evidence for the emotional potency of odor-evoked memory. Neuropsychologia, 42, 371–378. [DOI] [PubMed] [Google Scholar]
- Herz RS, Schankler C, & Beland S (2004). Olfaction, emotion and associative learning: Effects on motivated behavior. Motivation and Emotion, 28, 363–383. [Google Scholar]
- Herz RS, & Schooler JW (2002). A naturalistic study of autobiographical memories evoked by olfactory and visual cues: Testing the Proustian hypothesis. The American Journal of Psychology, 115, 21–32. [PubMed] [Google Scholar]
- Holland RW, Hendriks M, & Aarts H (2005). Smells like clean spirit. Nonconscious effects of scent on cognition and behavior. Psychological Science, 16, 689–693. [DOI] [PubMed] [Google Scholar]
- Hummel T, Kobal G, Gudziol H, & Mackay-Sim A (2007). Normative data for the “Sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. European Archives of Otorhinolaryngology, 264, 237–243. [DOI] [PubMed] [Google Scholar]
- Kadohisa M (2013). Effects of odor on emotion, with implications. Frontiers in Systems Neuroscience, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh DJ, May J, & Andrade J (2005). Imaginary relish and exquisite torture: The elaborated intrusion theory of desire. Psychological Review, 112, 446–467. [DOI] [PubMed] [Google Scholar]
- Kemps E, & Tiggemann M (2013). Olfactory stimulation curbs food cravings. Addictive Behaviors, 38, 1550–1554. [DOI] [PubMed] [Google Scholar]
- Klein LC, Corwin EJ, & Stine MM (2003). Smoking abstinence impairs time estimation accuracy in cigarette smokers. Psychopharmacology Bulletin, 37, 90–95. [PubMed] [Google Scholar]
- Kobal G, Klimek L, Wolfensberger M, Gudziol H, Temmel A, Owen CM, … Hummel T (2000). Multicenter investigation of 1,036 subjects using a standardized method for the assessment of olfactory function combining tests of odor identification, odor discrimination, and olfactory thresholds. European Archives of Otorhinolaryngology, 257, 205–211. [DOI] [PubMed] [Google Scholar]
- Kober H, & Mell MM (2015). Neural mechanisms underlying craving and the regulation of craving In Wilson SJ (Ed.), The wiley handbook on the cognitive neuroscience of addiction (pp. 195–218). Wiley-Blackwell. [Google Scholar]
- Laing DG (1983). Natural sniffing gives optimum odour perception for humans. Perception, 12, 99–117. [DOI] [PubMed] [Google Scholar]
- Lawless H, & Engle T (1977). Associations to odors: Interference, mnemonics, and verbal labeling. Journal of Experimental Psychology: Human Learning and Memory, 3, 52–59. [PubMed] [Google Scholar]
- Levy D, Mabry P, Graham A, Orleans CT, & Abrams D (2010). Exploring scenarios to dramatically reduce smoking prevalence: A simulation model of the three-part cessation process. American Journal of Public Health, 100, 1253–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Moallem I, Paller KA, & Gottfried JA (2007). Subliminal smells can guide social preferences. Psychological Science, 18, 1044–1049. [DOI] [PubMed] [Google Scholar]
- Lipkus IM, Green JD, Feaganes JR, Sedikides C, (2001). The relationship between attitudinal ambivalence and desire to quit smoking among college smokers. Journal of Applied Social Psychology, 31, 113–133. [Google Scholar]
- Loewenstein G (1996). Out of control visceral influences on behavior. Organizational Behavior and Human Decision Processes, 65, 272–292. [Google Scholar]
- Marcus BH, Lewis BA, Hogan J, King TK, Albrecht AE, Bock B, … Abrams DB (2005). The efficacy of moderate-intensity exercise as an aid for smoking cessation in women: A randomized controlled trial. Nicotine & Tobacco Research, 7, 871–880. [DOI] [PubMed] [Google Scholar]
- Marlatt GA (1985). Cognitive factors in the relapse process In Marlatt GA & Gordon JR (Eds.), Relapse prevention: Maintenance strategies in the treatment of addictive behaviors (pp. 128–200). New York: Guilford Press. [Google Scholar]
- Marlatt GA, & Gordon JR (Eds.). (1985). Relapse prevention: Maintenance strategies in the treatment of addictive behaviors. New York: Guilford Press. [Google Scholar]
- McCarthy DE, Piasecki TM, Jorenby DE, Lawrence DL, Shiffman S, Fiore MC, Baker TB. (2010). A multilevel analysis of nonsignificant counseling effects in a randomized smoking cessation trial Addiction, 105, 2195–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGann JP (2017). Poor human olfaction is a 19th-century myth. Science, 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morewedge CK, Huh Y, & Vosgerau J (2010). Thought for food: Imagined consumption reduces actual consumption. Science, 303, 1530–1533. [DOI] [PubMed] [Google Scholar]
- Moss M, Cook J, Wesnes K, & Duckett P (2003). Aromas of rosemary and lavender essential oils differentially affect cognition and mood in healthy adults. International Journal of Neuroscience, 113, 15–38. [DOI] [PubMed] [Google Scholar]
- Niaura RS, Abrams DB, Shadel WG, Rohsenow DJ, Monti PM, & Sirota AD (1999). Cue exposure treatment for smoking relapse prevention: a controlled clinical trial. Addiction, 94, 685–95. [DOI] [PubMed] [Google Scholar]
- Niaura RS, Sayette MA, Shiffman S, Glover ED, Nides M, Shelanski M, Shadel W, Koslo R, Robbins B, & Sorrentino J (2005). Comparative efficacy of rapid-release nicotine gum versus Nicotine Polacrilex gum in relieving smoking cue-provoked craving. Addiction, 100, 1720–1730. [DOI] [PubMed] [Google Scholar]
- Nordgren LF & Chou EY (2011). The push and pull of temptation: The bidirectional influence of temptation on self-control. Psychological Science, 22, 1386–1390. [DOI] [PubMed] [Google Scholar]
- Orleans CT, Rimer BK, Cristinzio S, Keintz MK, & Fleisher L (1991). A national survey of older smokers: Treatment needs of a growing population. Health Psychology, 10, 343–351. [DOI] [PubMed] [Google Scholar]
- Paulhus DL (1991). Measurement and control of response bias In Robinson J, Shaver P, & Wrightsman L (Eds.), Measures of personality and social psychological attitudes, Vol. 1 (pp. 17–59). San Diego, CA: Academic Press. [Google Scholar]
- Perkins KA, Gerlach D, Vender J, Grobe J, Meeker J, & Hutchison S (2001). Sex differences in the subjective and reinforcing effects of visual and olfactory cigarette smoke stimuli. Nicotine & Tobacco Research, 3, 141–150. [DOI] [PubMed] [Google Scholar]
- Prescott J, & Wilkie J (2007). Pain tolerance selectively increased by a sweet-smelling odor. Psychological Science, 18, 308–311. [DOI] [PubMed] [Google Scholar]
- Raes F, Hermans D, Williams JM, & Eelen P (2007). A sentence completion procedure as an alternative to the Autobiographical Memory Test for assessing overgeneral memory in non-clinical populations. Memory, 15, 495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE (2006). Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology, 184, 274–285. [DOI] [PubMed] [Google Scholar]
- Rose JE Salley A, Behm FM, Bates JE, & Westman EC (2010). Reinforcing effects of nicotine and nonnicotine components of cigarette smoke. Psychopharmacology, 210, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saive AL, Royet JP, & Plailly J (2014). A review on the neural bases of episodic odor memory: From laboratory-based to autobiographical approaches. Frontiers in Behavioral Neuroscience, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salovey P (1992). Mood-induced self-focused attention. Journal of Personality and Social Psychology, 62, 699–707. [DOI] [PubMed] [Google Scholar]
- Sayette MA (2016). The role of craving in substance use disorders: Theoretical and methodological issues. Annual Review of Clinical Psychology, 12, 407–433. [DOI] [PubMed] [Google Scholar]
- Sayette MA, & Creswell KG (2016). Self-regulatory failure and addiction In Vohs KD & Baumeister RF (Eds), Handbook of self-regulation: Research, theory, and applications, (pp. 571–590). New York: Guilford Press. [Google Scholar]
- Sayette MA, Loewenstein G, Griffin KM, & Black JJ (2008). Exploring the cold-to-hot empathy gap in smokers. Psychological Science, 19, 926–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Loewenstein G, Kirchner TR, & Travis T (2005). Effects of smoking urge on temporal cognition. Psychology of Addictive Behaviors, 19, 88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Martin CS, Hull JG, Wertz JM & Perrott MA (2003). Effects of nicotine deprivation on craving response covariation in smokers. Journal of Abnormal Psychology, 112, 110–118. [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Martin CS, Wertz JM, Shiffman S, & Perrott MA (2001). A Multidimensional analysis of cue-elicited craving in heavy smokers and tobacco chippers. Addiction, 96, 1419–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, & Parrott DJ (1999). Effects of olfactory stimuli on urge reduction in smokers. Experimental and Clinical Psychopharmacology, 7, 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Shiffman S, Tiffany ST, Niaura RS, Martin CS & Shadel WG (2000). The measurement of drug craving. Addiction, 95, Suppl 2, S189–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, & Tiffany ST (2013). Peak provoked craving: An alternative to smoking cue-reactivity. Addiction, 108, 1019–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriever VA, Reither N, Gerber J, Iannilli E & Hummel T (2013). Olfactory bulb volume in smokers. Experimental Brain Research 225, 153–157. [DOI] [PubMed] [Google Scholar]
- Schutte NS, Malouff JM, Hall LE, Haggerty DJ, Cooper JT Golden CJ, et al. , (1998). Development and validation of a measure of emotional intelligence. Personality and Individual Differences, 25, 167–177. [Google Scholar]
- Shepherd GM (2007). Perspectives on olfactory processing, conscious perception, and orbitofrontal cortex. Annals of the New York Academy of Sciences, 1121, 87–101. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Shadel WG, Niaura R, Khayrallah MA, Jorenby DE, Ryan CF, & Ferguson CL (2003). Efficacy of acute administration of nicotine gum in relief of cue-provoked cigarette craving. Psychopharmacology, 166, 343–350. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Waters AJ, & Hickox M (2004). The Nicotine Dependence Syndrome Scale: a multidimensional measure of nicotine dependence. Nicotine & Tobacco Research, 6, 327–348. [DOI] [PubMed] [Google Scholar]
- Smith SS, Piper ME, Bolt DM, Fiore MC, Wetter DW, Cinciripini PM, et al. (2010). Development of the Brief Wisconsin Inventory of Smoking Dependence Motives. Nicotine and Tobacco Research, 12, 489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama H, Oshida A, Thueneman P, Littell S, Katayama A, Kashiwagi M, Hikichi S & Herz RS (2010). The relationship between positive odor-evoked memories and product evaluation. 32nd Annual Meeting of the Association of Chemoreception Sciences, (pp. 67). April 2010, St Petersburg, Florida. [Google Scholar]
- Tiffany SA (1992). Critique of contemporary urge and craving research: Methodological, psychometric, and theoretical issues. Advances in Behaviour Research and Therapy, 14, 123–139. [Google Scholar]
- Towner EA, Ybasco FA, Rezai N, Rose KM, & Contrada RJ (1991). Cue reactivity in smokers: The role of cue characteristics. Health Psychology, 10, 228. [Google Scholar]
- Turner ML & Engle RW (1989). Is working memory capacity task dependent? Journal of Memory and Language, 28, 127–154. [Google Scholar]
- Vohs KD & Schmeichel BJ (2003). Self-regulation and extended now: Controlling the self alters the subjective experience of time. Journal of Personality and Social Psychology, 85, 217–230. [DOI] [PubMed] [Google Scholar]
- Wang Q (2008). Emotion knowledge and autobiographical memory across the preschool years: A cross-cultural longitudinal investigation. Cognition, 108(1), 117–135. [DOI] [PubMed] [Google Scholar]
- Wertz JM & Sayette MA (2001). A review of the effects of perceived drug use opportunity on self-reported urge. Experimental and Clinical Psychopharmacology, 9, 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Barnhofer T, Crane C, Herman D, Raes F, Watkins E, & Dalgleish T (2007). Autobiographical memory specificity and emotional disorder. Psychological Bulletin, 133, 122–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, & Fiez JA (2004). Prefrontal responses to drug cues: A neurocognitive analysis. Nature Neuroscience, 7, 211–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization: WHO. (2008). WHO Report on the Global Tobacco Epidemic. Geneva, Switzerland: Accessed 2012 Jun 7. [Google Scholar]
- Zhou B, Geng G, Chen W, & Zhou W (2018). Olfaction warps visual time perception. Cerebral Cortex, 28, 1718–1728. [DOI] [PubMed] [Google Scholar]
- Zilverstand A, Huang AS, Alia-Klein N & Goldstein RZ (2018). Neuroimaging Impaired Response Inhibition and Salience Attribution in Human Drug Addiction: A Systematic Review. Neuron, 98, 886–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.