Abstract
Purpose of Review
Research over the past few decades points to the importance of frailty, or the lack of physiologic reserve, in the natural history of chronic diseases and in modifying the impact of potential interventions. End-stage kidney disease (ESKD) and the intervention of kidney transplantation are no exception. We review the recent epidemiologic and cohort-based evidence on the association between frailty and kidney transplant outcomes and provide a framework of questions with which to approach future research endeavors and clinical practice.
Recent Findings
Frailty in kidney transplant candidates can be measured in numerous ways, including descriptive phenotype, description scores, functional testing, and surrogate measures. Regardless of the metric, the presence of frailty is strongly associated with inferior pre- and posttransplant outcomes compared to the absence of frailty. However, some frail patients with ESKD can benefit from transplant over chronic dialysis. Evidence-based approaches for identifying frail ESKD patients who can benefit from transplant over dialysis, with acceptable posttransplant outcomes, are lacking. Interventional trials to improve frailty and physical function before transplant (prehabilitation) and after transplant (rehabilitation) are also lacking.
Conclusion
Frailty is increasingly recognized as highly relevant to peritransplant outcomes, but more work is needed to: 1) tailor management to the unique needs of frail patients, both pre- and posttransplant; 2) define phenotypes of frail patients who are expected to benefit from transplant over dialysis; and 3) develop interventions to reverse frailty, both pre- and post-transplant.
Keywords: Assessment, Frailty, Kidney transplant, Outcomes, Prehabilitation, Survival
Introduction
Frailty is a state of decreased physiologic reserve and diminished ability to recover from physiologic stressors1. Functional status may decline and fail to recover after a medical illness or intervention, placing the individual at higher risk for complications. Literature on frailty and kidney transplant outcomes use a wide range of definitions and myriad of metrics including:
descriptive phenotype (Fried criteria2 );
descriptive scores (Frailty Index3, health-related quality of life [HRQOL] scores4);
physical function testing (SPPB5, measures of lower extremity strength and grip strength6);
cardiopulmonary exercise testing (peak VO2)7, other functional tests (6-minute walk test, sit-to-stand, or timed walking tests8;
other surrogate measures (days of hospitalization9, falls10 ).
Frailty exists on a spectrum, ranging from mild decrements in reserve (sometimes termed “prefrailty”) to severe functional impairment1. Frailty has been associated with poor health outcomes in almost all conditions, from community-dwelling older adults 1 to solid organ transplant recipients11.
Frailty is common in kidney transplant candidates. Studies in dialysis-dependent patients suggest a frailty prevalence of up to 70%12–14. Even among kidney transplant candidates, generally the healthiest of dialysis-dependent patients, approximately 20% meet criteria for frailty11. Advanced chronic kidney disease (CKD)/uremia and commonly-associated comorbid conditions, including anemia, diabetes mellitus and heart disease, all contribute to the frailty phenotype15. Understanding frailty and its effect on transplant outcomes therefore has significant implications for patient education and clinical management, including the pursuit of and acceptance for kidney transplantation as a treatment option.
To better assess and utilize frailty in the peri-transplant clinical setting, the following questions need answers:
Is there a frailty threshold at which the risk of transplantation exceeds that of continuing maintenance dialysis?
Should the type of transplant considered be tailored based on frailty status? For example, should some frail candidates only consider living donor kidney transplantation to minimize the risks associated with delayed graft function and further deterioration in the post-transplant course?
To what extent is frailty reversible after transplant, and how long does this process take?
What interventions may be effective to mitigate frailty, both pre- and post-transplant?
This review seeks to illuminate some of the above issues. Herein we will review the associations between frailty and transplant outcomes, an active research area over the last 5–10 years. We then seek to apply that knowledge to the questions of pre- and posttransplant management of frail patients and highlight knowledge deficits to be addressed by future research efforts.
Frailty and Outcomes Before - Transplant
Emerging literature on the association between frailty and outcomes in kidney transplant candidates and recipients are predominantly observational. These studies demonstrate a higher risk of mortality and morbidity among patients who are frail compared to non-frail patients, both before and after transplantation. It is important to note, however, that under the current candidate selection practices, survival benefit with transplantation may be seen even among some candidates who are frail.
Multiple studies have established an association between patient-reported or surrogate measures of frailty and adverse outcomes in transplant candidates on the waiting list. In a large multicenter cohort study of 1,975 patients, the Fried frailty phenotype is associated with higher waitlist mortality (HR 1.262.193.79)16. In a large retrospective study using registry data, Reese et al.17 noted that kidney transplant candidates in the lowest baseline physical function score quartile (based on the Medical Outcomes Study 36-Item Short Form Health Survey - SF-36) were less likely to undergo transplantation, more likely to be inactivated and have a lower survival at 3 years compared to the highest quartile (84% vs 92%). Survival benefit conferred by transplantation persisted in every physical function quartile. Limitations of the study include selection bias of patients both for waitlisting and for transplantation, and the use of indirect measures of physical functioning, albeit ones that have been validated in the dialysis-dependent population. In another registry data-based study, Lynch et al.9 studied whether hospital days in the first year of waitlist can be used as a measure of fitness for transplant. Based on registry data for 51,111 patients, those with higher hospitalization burden were noted to have higher waitlist mortality (1–7 hospital days: HR 1.201.241.28; 8–14 days: HR 1.421.491.56; ≥15 days: HR 1.992.072.15; versus 0 days). Those with a high hospitalization burden had lower post-transplant survival, but survival was significantly better than remaining on the waitlist. Furthermore, in a single-center study of 96 transplant candidates, Locke et al.18 observed that lean muscle mass (measured via morphometric assessments of psoas muscle attenuation and paraspinous lean volume) was associated with a small but significant decreased risk of death (HR 0.910.930.96 for higher psoas muscle attenuation and HR 0.960.98 0 99 for increase in lean paraspinous volume) over a 5–6 year follow-up period. Together, these studies demonstrate that the association between frailty and pre-transplant mortality is robust across different frailty measures. Frailty assessments may be particularly important where the patient comorbidity burden is lower, in identifying high-risk patients who may not be noticed otherwise19. These data also show that some frail ESKD patients can benefit from transplant over chronic dialysis, although how to identify those who will benefit and have acceptance posttransplant outcomes is not yet known.
One study applied an objective measure of cardiovascular reserve and arrived at a similar conclusion. Ting et al.20 studied 240 waitlisted patients followed for 5 years. They quantified cardiovascular reserve using cardiopulmonary exercise testing and found that patients with an impaired peak VO2 (based on percentage of age-predicted peak VO2) had a significantly lower survival compared to those with a better reserve. However, among patients with low cardiovascular reserve, transplanted patients had significantly greater survival compared to non-transplanted patients (HR=0.090.220.56).
Frailty and Outcomes After Transplant
Studies of post-transplant outcomes in transplant recipients have utilized both the Fried frailty phenotype and other measures of frailty. Outcomes examined include short-term (delayed graft function and hospitalization) and long-term (death) measures (Table 1). Frailty, as assessed by varying metrics in these studies, is associated with worse short-term outcomes after transplant. Frailty defined by Fried index, a combination of self-reported and objective measures, was associated with an almost 2-fold increase in risk of delayed graft function, 1.2-fold increase in risk of protracted initial hospital stay, and 1.6-fold increase in risk of hospital readmission within 1 month22. The effect of frailty on length of stay is especially pronounced in patients with depressive symptoms (1.9 fold increase in risk).23 Pre-transplant hospitalization, a surrogate measure of frailty, is also associated with increase post-transplant hospitalization (0 hospital days: 73%; 1–7 days: 70%; 8–14 days: 75%; ≥15 days: 80% hospitalization by 12 months of transplant)24.
Table 1:
Summary of recent studies of frailty and outcomes after kidney transplantation, adopted from 42.
| Reference, year | Study design and Participants | Frailty measure, timing and prevalence | Outcome/s associated with frailty | Other key findings | Study limitations |
|---|---|---|---|---|---|
| Garonzik-Wang, Arch Surg 201248 | Prospective cohort Single center 12/2008 –4/2010 N=183 (35% LDKTx) |
Fried Frailty Score 3–5 at the time of
admission for transplant Frailty at KTx: 25.1% |
DGF: 30% vs 15% in frail vs non-frail, aHR 1.131.963.36 | Deceased donor serum creatinine level and cold ischemia time was also associated with DGF. | Single center Observational Small sample size |
| McAdams-Demarco, AJT 201311 | Retrospective cohort Single center 12/2008 –12/2012 N=383 (39% LDKTx) |
Fried Frailty Score 3–5 at the time of
admission for transplant Frailty at KTx: 18.8% |
Early (within 30 days) hospital readmission (EHR): 46% vs 28% in frail vs non-frail, aRR 1.181.61 2.19. | Frailty improved EHR risk prediction by improving the area under the ROC curve and the net reclassification index. | Single center Observational Frail sample included more men (71% vs 58%, P=0.046) |
| McAdams-Demarco, JAGS 201533 | Prospective cohort Single center 12/2008 –3/2014 N=349 (37% LDKTx) Cohort overlaps with other McAdams studies |
Fried Frailty Score 3–5 at the time of
admission for transplant Frailty at KTx: 19.8% |
Change in frailty score after KTx: The only risk factor associated with improvement in frailty score was pre-KT frailty (aHR 1.712.553.82). | Pre-KT frailty status, diabetes mellitus, and DGF were independently associated with long-term changes in frailty score. | Single center Observational Short follow up |
| McAdams-Demarco Transplantation 201725 | Prospective cohort Single center N=663 12/2008–8/2015 Cohort overlaps with other McAdams studies |
Fried Frailty Score 3–5 at the time of
admission for transplant Frailty at KTx: 19.5% |
3 yr death: Associated with combinations of exhaustion and slowed walking speed (HR 1.172.435.03) and poor grip strength, exhaustion, and slowed walking speed (HR 1.142.615.97). | Frailty associated with older age (adjusted prevalence ratio aPR 1.212.224.07) IADL disability (1.723.226.06), depression (3.0211.3131.82), less than high school education (1.303.107.36), and low HRQOL (1.483.719.31). | Single center Observational |
| McAdams-Demarco, Transplantation 201834 | Prospective cohort 2 US centers N=443 (34.8% LDKTx) 5/2014–5/2017 Updated cohort from prior McAdams studies. |
Fried Frailty Score 3–5 at the time of admission for transplant | HRQOL: physical and kidney-disease specific
elements are worse in frail recipients, but mental elements are
similar. HRQOL change at 3 months post-KTx: • change in physical element 1.35 vs 0.34 points per month in frail vs non-frail • kidney disease-specific: 3.75 vs 2.41 points per month in frail vs non-frail |
Observational Single instrument Short follow-up Unclear testing protocol | |
| Nastasi, Transplantation 201827 | Prospective cohort 2 US centers N=719 Cohort overlaps with prior McAdams study |
Fried Frailty Score 3–5 at the time of
admission for transplant Lower extremity functional impairment using Short Physical Performance Battery (SPPB) at the time of admission for transplant |
Death: associated with lower extremity functional impairment (aHR 1.122.304.74), after adjustment of other risk factors including Fried Frailty Score. | Observational | |
| Alhamad ATC abstract 201626 | Retrospective cohort Single center N=383 2000–2014 |
6 minute walk test (6MWT) <1000ft | Graft failure & death: 6MWT <1000ft not associated with graft failure or patient death at 1, 3, and 5 years. At 10 years, short 6MWT <1000ft associated with graft failure (aHR 1.84.29.6) but not death. | Single center Observational | |
| Lynch, Ann Surgery 201622 | Registry based: linkage of USRDS &
Medicare claims. N=37,623 (Medicare-insured) 1/2000 –12/2010 |
Hospitalization days within 1 year before
KTx: 0: 51% 1–7: 25% 8–14: 11% 5+: 13% |
Admission in 1 yr after KTx: grade association
with pre-KTx hospitalization (aHR 1.171.281.70).
3yr death: grade association with pre-KTx hospitalization (aHR 1.201.421.70). 3yr graft loss: grade association with pre-KTx hospitalization (aHR 1.151.301.44). |
Hospitalization associated with female, prior
KTx recipients, diabetic, CHF, atherosclerotic vascular disease, and
COPD. Pre-KTx hospitalization associated with greater length of stay during transplant admission and greater service needs at discharge. |
Observational Registry data Medicare only |
| Lynch, AJT 20179 | Registry based: linkage of USRDS &
Medicare. N=51,111 (wait-listed KTx candidates) 1/2000–12/2010 |
Hospitalization days in the 1st year of
wait-list: 0: 47% 1–7: 23% 8–14: 12% 15+: 19% |
Death on waitlist: grade association with
pre-KTx hospitalization (aHR
1.241.492.07). Survival benefit of KTx vs staying on waitlist: Significant across all grades of pre-KTx hospitalization. Predictive model using admissions while on waitlist alone had higher accuracy for post-listing mortality than EPTS. |
Hospitalization associated w same risk factors as the prior Lynch study. | Observational Registry data Medicare only |
| Terjimanian, Clin Transplant 201726 | Retrospective cohort Single center 2005 – 2014 N=158 |
Morphometric age: calculated through analytic morphomics on CT scan within 1 yr pre-KTx | Death: associated with morphometric age (HR 1.031.061.08 per year) but not chronologic age. | In chronologically oldest patients, those with younger morphometric age had greater survival rates. | Observational Single center Small sample size single center Surrogate measure not established in ESRD |
CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; EPTS, expected post-transplant survival; KTx, kidney transplant; LDKTx, living donor kidney transplant; SR, self-report; PF, physical function; USRDS, United States Renal Data System; aRR, adjusted risk ratio; aHR, adjusted hazard ratio
Frailty is also associated with long-term transplant outcomes, including mortality and graft survival. The association is strong, whether frailty is measured by Fried index11 or other metrics. Among the 5 components of the Fried index, the combination of poor grip strength, low physical activity and slow walking speed were especially strongly associated with increased mortality (HR 1 142.6 15 97)25. Other studies employed alternative measures of frailty, including 6-minute walk test26, Short Physical Performance Battery27, morphometric age28, and hospitalization in the first-year of waitlist24. All these studies confirmed the strong association with frailty measures and posttransplant mortality (compared to the absence of frailty). Importantly, the studies available to date are observational, and how frailty should inform transplant candidacy is currently controversial.
Frailty and Transplant Candidacy
The kidney implantation procedure and peri-transplant immunosuppression represent significant physiologic stressors, from which the frail recipient may, by definition, have a protracted recovery (Figure 1). During the recovery period, complications may arise which further reduce physical performance. McAdams-DeMarco et al. outlined mechanisms by which frail transplant recipients may be prone to transplant complications, including increased hospitalization21, immunosuppression intolerance29, and delirium30. A threshold, or thresholds, for frailty may exist for which certain frail candidates are better off remaining on dialysis and others should only accept living donor kidneys to minimize the extent of peritransplant physiologic stress. Indeed, approximately 5% of transplant candidates were removed from the waitlist in 2016 because they were too sick for transplant31; many of them may had an unacceptably high level of irreversible frailty. The only existing surrogate measure for frailty/physical function that is mandated reporting nationally is the Karnofsky scale, which is a rough surrogate32. Lack of a systematic approach and standardized instruments to assess frailty in transplant candidates makes it difficult to determine and evaluate frailty thresholds for informing transplant candidacy.
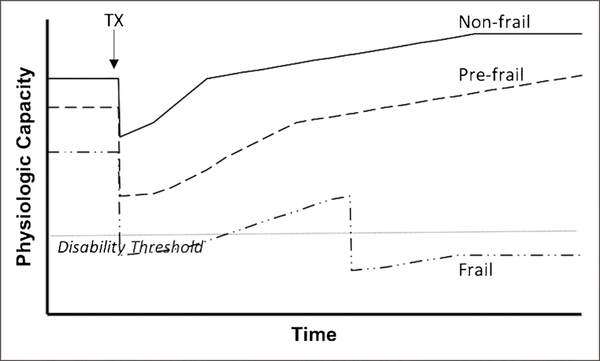
Figure 1.
A schematic of frailty as the loss of functional reserve. A non-frail patient (solid line) receives a kidney transplant (arrow): physiologic capacity decreases post-operatively and recovers to a better baseline than pretransplant. A prefrail patient (hashed line) experiences a greater decline and slower recovery of physiologic capacity, but ultimately achieves better physiologic capacity than pretransplant. A frail patient (dotted and hashed line) experiences a great decline in physiologic capacity to the point of losing dependence (horizontal dotted line), at which point recovery is prolonged and also plagued by further setbacks, which eventually results in a permanent loss of functional independence and poor outcome.
Preliminary data suggest that, under current practices at certain centers, measures of frailty may improve posttransplant. In a cohort of 349 transplant recipients at Johns Hopkins Hospital33, 20% had the frailty phenotype (meeting at least 3 of the Fried criteria), and a higher percentage became frail within 1–2 months of transplant. At 3-months post-transplant, 74% of the initially frail recipients became less frail. The choice of the Fried phenotype, a non-quantitative instrument, to quantity frailty improvement is a limitation of this study, as is the high likelihood of selection bias in the study protocol. Longitudinal fluctuations in measuring the frailty phenotype may also account for part of the observed improvement. In a follow-up study of 443 transplant recipients from Johns Hopkins and University of Michigan Hospital34, the physical and kidney-disease specific health-related quality of life (HRQOL) scores posttransplant improved in both initially frail and non-frail recipients. The improvement was more marked in frail recipients, especially in the domains of general health, effects of kidney disease, cognitive function, and social interaction. At these study centers, most frail transplant candidates appear to recover post-transplant and experience improvements in functional status and quality of life. Relatedly, in a large registry study, even patients with low physical function scores experience a survival advantage conferred by transplant over dialysis17, but this finding may be affected by unmeasured selection factors. Replication of these studies using quantitative frailty instruments, pre-specified assessment intervals, and longer follow-up at different transplant centers (with different thresholds for recipient and organ acceptance and different transplant protocols) will confirm (or disprove) these initial observations and lend empirical credence to our proposed paradigm in Figure 1.
If most frail transplant recipients improve posttransplant, then the higher mortality and adverse outcome rates in frail recipients may be attributed to either stochastic post-transplant events or the presence of patient subsets whose post-transplant trajectories diverge from non-frail patients. Predicting these “high-risk” frail candidates may enable us to refine transplant candidacy criteria and avoid the unfortunate outcome of making a patient worse with a failed transplant. All existing studies on this topic confront the inescapable limitations that: 1) stochastic post-transplant events cannot be predicted; 2) a strong selection bias exists, as perceived frailty is already contributing to decisions of transplantation; and 3) a model to be used in shared decision making regarding whether to proceed with a transplant or remain on maintenance dialysis will need an extremely high degree of accuracy (i.e. ability to provide the correct estimates for the probability of an adverse outcome). Existing models include the Estimated Post-Transplant Survival (EPTS) score35 which accounts for only age and limited comorbidities (diabetes, length of time on dialysis, and prior transplant), and various models including measures of frailty9,11,32. In the most discriminating of these models9 (based on days of hospitalizations in the first year of waitlist), recipients in the highest category of risk have 3-year death or graft loss rates of 30%: whether such a failure rate warrants proceeding with kidney transplant is a decision to be made at the individual level by shared decision making.
Timing of Frailty Assessments
The timing of pre-transplant frailty assessment warrants brief discussion. Literature to date has reported frailty measures at one pretransplant time point, mostly immediately prior to transplant11 or at study enrollment20. Such timing is practical and useful for research studies but requires modification for useful clinical practice. Measuring frailty at the time of transplant evaluation can help inform transplant candidacy and type of transplant offered but may miss deceased donor transplant candidates who become frail while awaiting transplant36. Measuring frailty immediately prior to transplant can help guide post-transplant management but will have little bearing on decisions and counseling regarding transplant candidacy. Ideally, frailty will be assessed longitudinally while awaiting a transplant and more frequently as candidates move toward the top of the wait-list (Figure 2). Such reassessment should be framed in the context of other patient factors, such as chronologic age, social support and other comorbidity. However, for the vast majority of transplant candidates whose primary or nephrology care is not delivered by transplant center affiliates, repeat frailty testing will necessitate either repeat visits to transplant centers or close coordination between transplant centers and local nephrology practices and dialysis units. Implementation of full, longitudinal pre-transplant frailty monitoring will require a more integrated care coordination model than what is currently available.
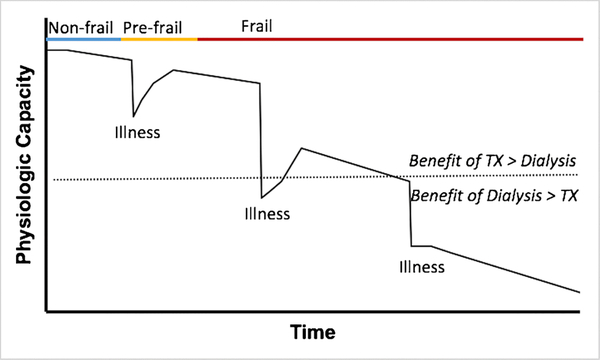
Figure 2.
A schematic of the functional trajectory of a patient on the kidney transplant waitlist. Re-assessments, especially after major illnesses, are crucial in properly phenotyping patients on the frailty spectrum and making the appropriate transplant-related decisions. TX: transplant. Benefit: Projected benefit of transplant or dialysis.
Frailty and Pretransplant Management
In addition to better assessment of frail patients with ESKD who are expected to benefit from transplant, effective interventions to modify frailty may allow more frail patients to become suitable transplant candidates. However, the optimal strategy to address frailty before transplantation is not known. Physical activity interventions, with or without nutritional interventions, have demonstrated reduction of frailty measures in community-dwelling elderly adults37. Whether this can be consistently achieved in the advanced CKD population is debatable. Physical or exercise therapy delivery in the advanced CKD population is challenging. Implementation of intradialytic or supervised interdialytic exercise is limited by staffing challenges, competing priorities, and reimbursement38,39. Home-based exercise therapy may result in a modest improvement in 6-minute walk test performance and lower extremity strength40 How the positive effects persist beyond the study period is unknown. The myriad of barriers to exercise reported by patients, including fatigue, comorbidities, and limitations related to dialysis access41, may explain the high attrition rate (20–50%) reported in most exercise intervention studies in the CKD population (cf Table 1 in review by Cheng et al.42. Prehabilitation, or physical rehabilitation completed prior a major procedural intervention, has shown some success in intra-abdominal surgeries (cf Table 2 in review by Cheng et al.42). In the kidney transplant setting, prehabilitation may theoretically leverage the higher motivation of patients at the top of the waitlist, but this conjecture has yet to be empirically evaluated. Response to prehabilitation, or lack thereof, may also provide an additional data point for assessing a frail patient’s ongoing kidney candidacy (Figure 3). For all these reasons, prehabilitation in kidney transplantation warrants more study.
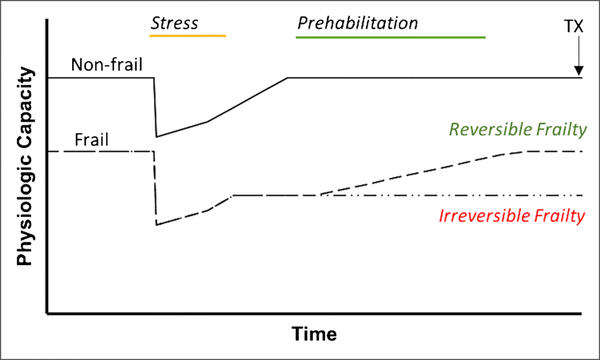
Figure 3.
A schematic of the functional trajectories of non-frail, reversibly frail, and irreversibly frail patients on the kidney transplant waitlist. A trial of prehabilitation may be useful in distinguishing the reversibly frail from irreversibly frail patient before transplant occurs.
Frailty and Posttransplant Management
There are limited data to inform modification of posttransplant care based on frailty status. Potential modifications include:
Aggressive and planned physical rehabilitation after transplantation in high-risk candidates
Immunosuppression modification
Accommodation of patients with cognitive deficits
Compared to prehabilitation, posttransplant rehabilitation is potentially easier to arrange logistically. Two randomized controlled exercise trials exist. In a US trial43 (N=54 in exercise arm), an individualized home exercise regimen, tele-monitored via phone, improved objective and self-reported physical functioning over usual care. The average age of transplant patients was quite low (40±13 in the exercise arm), and no frailty screening was done at recruitment. A small UK pilot trial44 (N=13 in exercise arm) recruited older patients within 1 year of transplant and tested the effects of 12 weeks of supervised structured exercise classes twice per week. They reported a statistically non-significant trend toward an improvement in peak VO2 attributable to aerobic training that persisted for 6 months beyond the intervention end date. Replication of these studies with higher numbers stratified by pretransplant frailty will help to delineate the benefits of rehabilitation and are necessary for obtaining insurer approval for covering the service.
The optimal approach to immunosuppression may differ between elderly frail and non-frail, non-elderly kidney transplant recipients45. The altered pharmacokinetics of medications in elderly individuals may alter their exposure to immunosuppressants. Rejection and death-censored graft failure rates decrease with increasing recipient age46,47, implying age-related immune senescence or heightened immunosuppression exposure in elderly patients under current protocols. Side-effects and immunosuppressant intolerance are also more common in frail individuals29. Overall, the balance between suppressing alloimmunity and minimizing side effects may call for lower immunosuppression in frail transplant recipients, but this hypothesis warrants focused studies.
Transplant centers under-recognize cognitive deficits, such as delirium, to which frail transplant recipients are particularly prone30. Targeted efforts to address cognitive deficits in frail transplant recipients include better delirium prevention and treatment along with targeted measures for medication safety and adherence. These are therefore reasonable steps to mitigate the downstream effects of frailty, although the optimal approach remains unknown.
Conclusions
As has been recognized in other populations, frailty is an important determinant of outcomes in kidney transplant candidates and recipients and exerts a significant impact on a patient’s course, both pre- and posttransplant. In the past 5–10 years, a proliferation of studies has demonstrated a robust link between frailty, measured by varying metrics, and transplant outcomes. These studies also provide insight into the mechanistic basis for the link and suggest possible intervention venues, including prehabilitation, rehabilitation, immunosuppression modification and closer attention to cognitive impairment. The observation that frailty may not preclude benefit from transplant over dialysis argues for liberal referral to transplant centers, allowing programs to make candidacy determinations, rather than denying referral based on perception that a patient may be too unfit. At this time, minimization of pretransplant dialysis exposure through early referral, effective education on the potential benefits of living donor transplantation, and consideration of non-standard deceased donor organs to increase transplant options are particularly important to elderly and frail patients. Defining characteristics of frail patients who can benefit from transplant over dialysis with acceptable posttransplant outcomes, is a vital research priority. Prospective study of interventions and management strategies to improve frailty and mitigate adverse outcomes are also needed. All stakeholders—including patients, referring physicians, transplant programs, policy makers, insurers, and researchers—should recognize the importance of frailty as a determinant of kidney transplant success and convene on interventions to improve transplant outcomes (Figure 4).
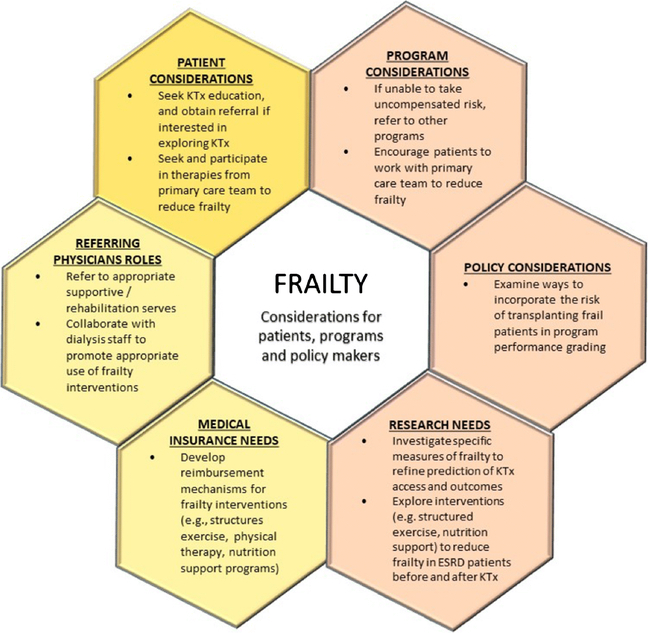
Figure 4.
Frailty considerations for key stakeholders.
Acknowledgement
JCT received funding from the John M. Sobrato Foundation. KLL received support from a grant from the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R01DK102981.
ABBREVIATIONS and ACRONYMS
- CKD
chronic kidney disease
- EPTS
Estimated Post-Transplant Survival
- ESKD
end-stage kidney disease
- HRQOL
health-related quality of life
Footnotes
Compliance with Ethical Standards
Conflict of Interest
Xingxing S. Cheng, Krista L. Lentine, Farrukh M. Koraishy, Jonathan Myers, and Jane C. Tan report no conflicts of interest or funding for this work. XSC, KLL and JCT are members of the Frailty Consensus workgroup formed by the American Society of Transplantation (AST) Kidney-Pancreas Community of Practice; KLL is the American Society of Nephrology (ASN) Quality Committee representative to the workgroup.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
* Of importance
- 1.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–762. doi: 10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol. 2001;56(3):M146–156. [DOI] [PubMed] [Google Scholar]
- 3.Mitnitski AB, Graham JE, Mogilner AJ, Rockwood K. Frailty, fitness and late-life mortality in relation to chronological and biological age. BMC Geriatr. 2002;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johansen KL, Dalrymple LS, Glidden D, et al. Association of Performance-Based and Self-Reported Function-Based Definitions of Frailty with Mortality among Patients Receiving Hemodialysis. Clin J Am Soc Nephrol. 2016;11(4):626–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. [DOI] [PubMed] [Google Scholar]
- 6.Stevens PJ, Syddall HE, Patel HP, Martin HJ, Cooper C, Aihie Sayer A. Is grip strength a good marker of physical performance among community-dwelling older people? J Nutr Health Aging. 2012;16(9):769–774. [DOI] [PubMed] [Google Scholar]
- 7.Ross R, Blair SN, Arena R, et al. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement From the American Heart Association. Circulation. 2016;134(24):e653–e699. [DOI] [PubMed] [Google Scholar]
- 8.Guyatt GH, Sullivan MJ, Thompson PJ, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132(8):919–923. [PMC free article] [PubMed] [Google Scholar]
- 9.Lynch RJ, Zhang R, Patzer RE, Larsen CP, Adams AB. First-Year Waitlist Hospitalization and Subsequent Waitlist and Transplant Outcome. Am J Transplant. 2017;17(4):1031–1041.* A powerful and comprehensive registry analysis illustrating how first-year waitlist hospitalization may be used as a surrogate for adverse waitlist and transplant outcomes.
- 10.Plantinga LC, Lynch RJ, Patzer RE, Pastan SO, Bowling CB. Association of Serious Fall Injuries among United States End Stage Kidney Disease Patients with Access to Kidney Transplantation. Clin J Am Soc Nephrol. 2018;13(4):628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McAdams-DeMarco MA, Law A, King E, et al. Frailty and mortality in kidney transplant recipients. Am J Transplant. 2015;15(1):149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bao Y, Dalrymple L, Chertow GM, Kaysen GA, Johansen KL. Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med. 2012;172(14):1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johansen KL, Chertow GM, Jin C, Kutner NG. Significance of frailty among dialysis patients. J Am Soc Nephrol JASN. 2007;18(11):2960–2967. [DOI] [PubMed] [Google Scholar]
- 14.McAdams-DeMarco MA, Law A, Salter ML, et al. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc. 2013;61(6):896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JC, Kalantar-Zadeh K, Kopple JD. Frailty and protein-energy wasting in elderly patients with end stage kidney disease. J Am Soc Nephrol. 2013;24(3):337–351. [DOI] [PubMed] [Google Scholar]
- 16.McAdams-DeMarco MA, Ying H, Thomas AG, et al. Frailty, Inflammatory Markers, and Waitlist Mortality Among Patients With End-stage Renal Disease in a Prospective Cohort Study. Transplantation. 2018;102(10):1740–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reese PP, Shults J, Bloom RD, et al. Functional status, time to transplantation, and survival benefit of kidney transplantation among wait-listed candidates. Am J Kidney Dis. 2015;66(5):837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Locke JE, Carr JJ, Nair S, et al. Abdominal lean muscle is associated with lower mortality among kidney waitlist candidates. Clin Transplant. 2017;31(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parez Fernandez M, Martinez Miguel P, Ying H, et al. Comorbidity, Frailty, and Waitlist Mortality Among Kidney Transplant Candidates of All Ages. Am J Nephrol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ting SMS, Iqbal H, Kanji H, et al. Functional cardiovascular reserve predicts survival pre-kidney and post-kidney transplantation. J Am Soc Nephrol. 2014;25(1):187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McAdams-DeMarco MA, King EA, Luo X, et al. Frailty, Length of Stay, and Mortality in Kidney Transplant Recipients: A National Registry and Prospective Cohort Study. Ann Surg. 2017;266(6):1084–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McAdams-DeMarco MA, Law A, Salter ML, et al. Frailty and early hospital readmission after kidney transplantation. Am J Transplant. 2013;13(8):2091–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konel JM, Warsame F, Ying H, et al. Depressive symptoms, frailty, and adverse outcomes among kidney transplant recipients. Clin Transplant. 2018;32(10):e13391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lynch RJ, Zhang R, Patzer RE, Larsen CP, Adams AB. Waitlist Hospital Admissions Predict Resource Utilization and Survival After Renal Transplantation. Ann Surg. 2016;264(6):1168–1173. [DOI] [PubMed] [Google Scholar]
- 25.McAdams-DeMarco MA, Ying H, Olorundare I, et al. Individual Frailty Components and Mortality in Kidney Transplant Recipients. Transplantation. 2017;101(9):2126–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alhamad T, Lentine K, Anwar S, et al. Functional capacity pre-transplantation measured by 6 minute walk test and clinical outcomes. ATC Abstracts. https://atcmeetingabstracts.com/abstract/functional-capacity-pre-transplantation-measured-by-6-minute-walk-test-and-clinical-outcomes/. Accessed August 1, 2018. [Google Scholar]
- 27.Nastasi AJ, McAdams-DeMarco MA, Schrack J, et al. Pre-Kidney Transplant Lower Extremity Impairment and Post-Kidney Transplant Mortality. Am J Transplant. 2018;18(1):189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terjimanian MN, Underwood PW, Cron DC, et al. Morphometric age and survival following kidney transplantation. Clin Transplant. 2017;31(10). [DOI] [PubMed] [Google Scholar]
- 29.McAdams-DeMarco MA, Law A, Tan J, et al. Frailty, mycophenolate reduction, and graft loss in kidney transplant recipients. Transplantation. 2015;99(4):805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haugen CE, Mountford A, Warsame F, et al. Incidence, Risk Factors, and Sequelae of Post-kidney Transplant Delirium. J Am Soc Nephrol. 2018;29(6):1752–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2016. Annual Data Report: Kidney. Am J Transplant. 18(S1):18–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bui K, Kilambi V, Rodrigue JR, Mehrotra S. Patient Functional Status at Transplant and Its Impact on Posttransplant Survival of Adult Deceased-Donor Kidney Recipients. Transplantation. August 2018. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McAdams-DeMarco MA, Isaacs K, Darko L, et al. Changes in Frailty After Kidney Transplantation. J Am Geriatr Soc. 2015;63(10):2152–2157.* The first study to report longitudinal changes in frailty markers after kidney transplant.
- 34.McAdams-DeMarco MA, Olorundare IO, Ying H, et al. Frailty and Postkidney Transplant Health-Related Quality of Life. Transplantation. 2018;102(2):291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clayton PA, McDonald SP, Snyder JJ, Salkowski N, Chadban SJ. External validation of the estimated posttransplant survival score for allocation of deceased donor kidneys in the United States. Am J Transplant. 2014;14(8):1922–1926. [DOI] [PubMed] [Google Scholar]
- 36.Chu NM, Deng A, Haugen CE, Segev DL, McAdams-DeMarco MA. Dynamic Frailty before Kidney Transplantation—Time of Measurement Matters. Transplantation. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Puts MTE, Toubasi S, Andrew MK, et al. Interventions to prevent or reduce the level of frailty in community-dwelling older adults: a scoping review of the literature and international policies. Age Ageing. 2017;46(3):383–392. doi: 10.1093/ageing/afw247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kontos PC, Miller K-L, Brooks D, et al. Factors influencing exercise participation by older adults requiring chronic hemodialysis: a qualitative study. Int Urol Nephrol. 2007;39(4):1303–1311. [DOI] [PubMed] [Google Scholar]
- 39.Thompson S, Tonelli M, Klarenbach S, Molzahn A. A Qualitative Study to Explore Patient and Staff Perceptions of Intradialytic Exercise. Clin J Am Soc Nephrol. 2016;11(6):1024–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manfredini F, Mallamaci F, D’Arrigo G, et al. Exercise in Patients on Dialysis: A Multicenter, Randomized Clinical Trial. J Am Soc Nephrol. 2017;28(4):1259–1268.* The largest randomized clinical trial on home-based exercise in patients on dialysis, with an easily implementable unsupervised home exercise regimen.
- 41.Jhamb M, McNulty ML, Ingalsbe G, et al. Knowledge, barriers and facilitators of exercise in dialysis patients: a qualitative study of patients, staff and nephrologists. BMC Nephrol. 2016;17(1):192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng XS, Myers JN, Chertow GM, et al. Prehabilitation for kidney transplant candidates: Is it time? Clin Transplant. 2017;31(8).* Review article with summary tables for evidence around physical therapy in patients on dialysis and around prehabilitation in abdominal surgery.
- 43.Painter PL, Hector L, Ray K, et al. A randomized trial of exercise training after renal transplantation. Transplantation. 2002;74(1):42–48. [DOI] [PubMed] [Google Scholar]
- 44.Greenwood SA, Koufaki P, Mercer TH, et al. Aerobic or Resistance Training and Pulse Wave Velocity in Kidney Transplant Recipients: A 12-Week Pilot Randomized Controlled Trial (the Exercise in Renal Transplant [ExeRT] Trial). Am J Kidney Dis. 2015;66(4):689–698. [DOI] [PubMed] [Google Scholar]
- 45.AST KPCOP. Frailty in kidney and kidney-pancreas transplantation: A consensus document from the American Society of Transplantation’s Kidney-Pancreas Community of Practice. 2018.* The upcoming consensus document from the American Society of Transplantation’s Kidney-Pancreas Community of Practice, with summary of the current state of frailty and directions for clinical practice and research.
- 46.Tullius SG, Tran H, Guleria I, Malek SK, Tilney NL, Milford E. The combination of donor and recipient age is critical in determining host immunoresponsiveness and renal transplant outcome. Ann Surg. 2010;252(4):662–674. [DOI] [PubMed] [Google Scholar]
- 47.Lepeytre F, Dahhou M, Zhang X, et al. Association of Sex with Risk of Kidney Graft Failure Differs by Age. J Am Soc Nephrol. 2017;28(10):3014–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garonzik-Wang JM, Govindan P, Grinnan JW, et al. Frailty and delayed graft function in kidney transplant recipients. Arch Surg. 2012;147(2):190–193. [DOI] [PubMed] [Google Scholar]