Abstract
Probiotics are live non‐pathogenic commensal organisms that exert therapeutic effects in travellers’ diarrhea, irritable bowel syndrome and inflammatory bowel disease. Little is known about mechanisms of action of commensal bacteria on intestinal motility and motility‐induced pain. It has been proposed that probiotics affect intestinal nerve function, but direct evidence for this has thus far been lacking. We hypothesized that probiotic effects might be mediated by actions on colonic intrinsic sensory neurons. We first determined whether sensory neurons were present in rat colon by their responses to chemical mucosal stimulation and identified them in terms of physiological phenotype and soma morphotype. Enteric neuron excitability and ion channel activity were measured using patch clamp recordings. We fed 109 Lactobacillus reuteri (LR) or vehicle control to rats for 9 days. LR ingestion increased excitability (threshold for evoking action potentials) and number of action potentials per depolarizing pulse, decreased calcium‐dependent potassium channel (IKCa) opening and decreased the slow afterhyperpolarization (sAHP) in sensory AH neurons, similar to the IKCa antagonists Tram‐34 and clotrimazole. LR did not affect threshold for action potential generation in S neurons. Our results demonstrate that LR targets an ion channel in enteric sensory nerves through which LR may affect gut motility and pain perception.
Keywords: ion channel, enteric nervous system, calcium‐dependent potassium channel, rat
Introduction
Probiotics are live non‐pathogenic commensal organisms that promote beneficial health effects when ingested [1]. Organisms used as probiotics are most frequently of the Lactobacillus or Bifidobacterium species, and clinically beneficial effects of probiotics have been described in travellers’ diarrhea, irritable bowel syndrome and inflammatory bowel disease [2, 3, 4, 5, 6]. Although experimental modulation of intestinal motility [7] and visceral pain [7, 8, 9] by Lactobacillus species have been convincingly demonstrated for murine colon, little is known about the mechanisms of action whereby this occurs.
The enteric nervous system (ENS) provides sensory innervation of the mucosa, with nerve endings adjacent to the mucosal side of absorptive epithelial cells, hence ideally placed to respond to luminal bacteria. Because the ENS plays a critical role in maintaining normal gut function, it represents a highly plausible major site of action through which commensal organisms can regulate many physiological functions, including intestinal motility. Giant migrating contractions and enteric neuronal signalling have recently been described as essential for visceral pain–related pseudo‐affective responses to colorectal distension in rat colon [10]. Hence, an action on the ENS by commensals could potentially reduce pain perception through relief of dysmotility.
Here, we report a mechanism whereby an orally ingested Lactobacillus species (L. reuteri, LR) affects the intrinsic sensory neurons within the musculature via ion channel–specific effects. This mechanism of action may underlie some of the therapeutic benefits of certain commensal bacteria in healthy conditions as well as in intestinal disorders. To investigate the potential action of this organism on enteric neurons, we adapted an in situ method for patch clamping developed for mouse tissue [11] that allowed recording from the ENS in intact, un‐dissociated longitudinal muscle myenteric plexus preparations from rats.
Materials and methods
All experiments used male Sprague–Dawley rats (Charles River Breeding Laboratories, Pointe‐Claire, QC, Canada) weighing 350–500 g. Rats were gavaged daily for 9 days with LR in Man–Rogosa–Sharpe liquid medium (MRS broth vehicle; Difco Laboratories, Sparks, MD, USA), or with MRS broth vehicle control or given just water. Daily feeding was with 109 LR in 0.2 ml MRS bacteria grown from frozen stocks (−80°C) and prepared for ingestion as described previously [8]. Bacterial counts were determined using a colorimeter as described previously [8]. The Animal Care Committee of McMaster University approved all experiments. After the feeding period, animals were anaesthetized and a colon segment located 2 cm from the rectum was removed and placed in oxygenated Krebs saline. Segments were opened, pinned flat, mucosa up, in Krebs saline–containing petri dishes. Longitudinal muscle myenteric plexus (LMMP) preparations with exposed myenteric plexus [12] were made by dissection in oxygenated Krebs saline. In other experiments, responses to chemical stimulation of the mucosa were recorded from neurons in juxta‐mucosa myenteric plexuses in hemi‐dissected mucosa‐LMMP preparations, where the mucosa was removed for only half of the area as described previously [13].
To prepare ganglia for patch clamping, the myenteric plexus was exposed to 0.01% protease type XI for 10 min. and the surface cleaned by sweeping with a hair, after which patch pipette seals ≥4 GΩ could be obtained [11, 14]. During this process, the outlines of individual ganglia and the neuron cell bodies they contain are readily visualized under bright‐field illumination; thus, individual neurons were patched by contacting them with the pipette under visual control [11, 14, 15, 16, 17, 18]. Current and voltage clamp recordings were made using an Axon Instruments multiclamp amplifier (Molecular Devices, Sunnyvale, CA, USA). The K+‐rich patch pipette solution and extracellular Krebs saline were the same as in Mao et al. [11]. Whole‐cell, cell‐attached and inside‐out single‐channel voltage clamp recordings were made as described in Mao et al.[11].
AH neurons were identified by the presence of a slow AHP and a hump on the relaxation phase of the action potential (AP) [11, 12, 15, 19, 20, 21]. sAHPs were recorded in current clamp mode after a single AP or after a standard triple‐spike stimulus where each spike was evoked by three supra‐threshold depolarizing pulses delivered at 50 Hz and each of 10 msec. duration [22]. Those that lacked the hump and sAHP were identified as S neurons.
Neuron excitability, rheobase (threshold) and firing discharge accommodation were measured by injecting 2‐sec.‐duration depolarizing current pulses in voltage‐recording mode. Rheobase was determined by injecting currents of increasing intensity until a single AP was evoked for the first time. The ability of the neuron to fire repetitively, a measure of accommodation, was tested by injecting a current pulse of exactly 2× rheobase intensity and counting the number of action potentials (APno) fired.
The whole‐cell voltage clamp, current recording protocol consisted of two voltage commands, the first was a 2‐sec. hyperpolarizing step voltage command and this was immediately followed by a depolarizing, quasi‐steady state, voltage ramp (speed = 25 mV/sec.). This protocol was always executed twice, the second one started immediately after the first one finished. The hyperpolarizing step command was used to test for a hyperpolarizing activated cationic current (I h) [11]. I h is a voltage‐dependent current whose conductance was calculated from the steady‐state magnitude of the current evoked by the fixed 2‐sec. hyperpolarizing voltage step according to g h=V−E h/I h, where E h=−30 mV is the I h reversal potential. The two ramp commands were used to determine the magnitude of the sAHP current. The sAHP current in guinea pig [15, 23] and mouse [11] small intestine AH neurons is essentially voltage independent [11, 15, 23], and thus its steady‐state I–V curve can be well fit using the GHK current equation. Deviations from the GHK fit are taken as evidence that the current is not voltage independent [24]. In previous in situ whole‐cell recordings using guinea pig or mouse, the difference current between successive double quasi‐steady‐state ramp voltage commands has been used as the I–V curve for the sAHP current [11, 15], and thus the current’s presence has been measured as K+ ion permeability (P K), as is frequently the case for voltage‐independent currents [24]. The GHK equation provides the relationship between conductance (g=I/V) and permeability, and is given by
| (1) |
where [K+] is in mM, and F, R, T have their usual meaning and P K is expressed in m3/s.
IKCa channel opening in cell‐attached mode was evoked by passing a 50‐msec. inward current pulse via the patch pipette [11]. P o was calculated from areas under multiple Gaussians fitted to all‐points current histograms shown as insets in Fig. 6B–E. For some neurons, inside‐out patches were pulled after cell‐attached recording of the slow AHP ion channel; thereafter, clotrimazole was applied to the cytoplasmic surface by adding 3 μM to the Krebs superfusate. These cells were always re‐patched to confirm that they were AH neurons. Cytoplasmic [Ca2+] was calculated using Maxchelator (http://www.stanford.edu/~cpatton/maxc.html).
Figure 6.
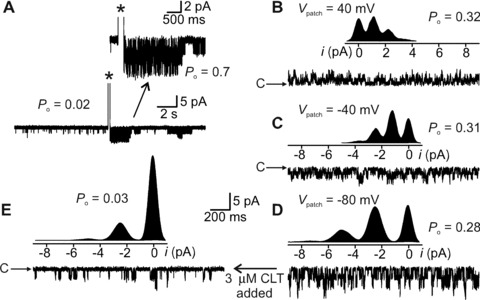
Functional identification of K+ channel whose opening generates the sAHP current. (A) Cell‐attached recording from AH cell. IKCa channel opening is infrequent before current pulse evoked AP (*), after which, P o increased from 0.02 to 0.7 (inset). P o decreased to background levels in approximately 10 sec. (B–D) Same patch as in (A) but inside‐out configuration and at trans‐patch voltages (V patch) indicated. Cytoplasmic [Ca2+]= 0.5 μM for which P o approximately 0.3 irrespective of the voltage. Inserts are all‐points current histograms of channel currents. Each distribution was fit by a sum of 4 Gaussians. Distance between equally spaced histogram peaks equals magnitude of unitary channel current. (E) Same ion channel and V patch as for (D), but the cytoplasmic face of the patch was exposed to 3 μM clotrimazole. The all‐points histogram shown above the raw channel trace indicates that the time spent in the close state was greatly increased compared with the histogram shown in (D). Binomial analysis revealed that clotrimazole reduced P o from 0.28 to 0.03. c indicates closed state in raw channel current traces.
Intrinsic sensory neurons were identified by their responses to chemical mucosal stimulation as was done previously in other species [11, 13, 25, 26].The mucosa was stimulated in hemi‐dissected preparations [13] (see Fig. 2A) with butyrate, which at physiological luminal concentrations of 1–10 mM can alter colon motility [27] or 5‐hydroxytryptamine (5‐HT) [28].
Figure 2.
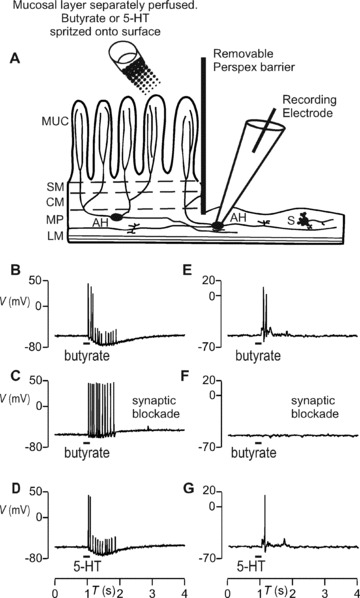
Direct (sensory) and synaptic responses of enteric neurons to 5 mM butyrate or 5 μM 5‐HT applied to the mucosa. (A) Diagram illustrating the hemi‐dissected preparation used to identify sensory responses to chemical mucosal stimulation. A transverse view of the dissected colon segment is shown; the left‐to‐right axis represents the circumference of the opened segment and the oral–anal axis runs orthogonal to the page’s plane. Chemicals were spritzed onto the mucosal layer, which was superfused with Krebs saline. Electrophysiological signals were recorded from AH cells whose processes run from MP into the mucosal layer, and from S cells that mainly innervate other inter‐ or motorneurons, or smooth muscle. The layers depicted are LM, longitudinal muscle; MP, myenteric plexus; CM, circular muscle; SM, submucosa; MUC, mucosal layer. AH cell responded with non‐synaptic burst of APs to butyrate (B) or 5‐HT (D). (E and G) S cell responded with fast EPSPs and APs to the same stimulation. (C and F) AH but not S cell responses were sensory because synaptic blockade with low Ca2+ and high Mg2+ superfusing saline did extinguish the latter but not the former.
Neural morphology was revealed post hoc as described in Mao et al.[11]. Briefly, after recording, neurons were filled with neurobiotin, which had previously been added to the intracellular patch pipette saline [11]. After overnight fixation, labelled neurons were visualized by a reaction of neurobiotin with streptavidin–Texas Red (Vector, http://www.vectorlabs.com). Neurons were visualized under fluorescence epi‐illumination [11].
Data analysis
Data were analyzed using Clampfit (Axon Instruments) and statistics calculated with Minitab (http://www.minitab.com). Descriptive data were given as mean ± S.E.M. All statistical tests were two‐tailed and performed using Student’s t‐test. Differences were considered statistically significant at P < 0.05.
Recordings were obtained from 22 AH cells from rats treated with LR, 15 from MRS broth vehicle control and 16 AH cells from water‐fed rats.
There were no statistically discernable differences in measured membrane parameters or in AP rheobase and APno between cells recorded from water‐fed animals or those given vehicle; thus, these two groups were pooled to form a combined control group. In addition, recordings were obtained from 10 S cells, treated with LR, 10 from vehicle treated and 7 water treated, for which vehicle and water groups were also pooled to make a control group.
Results
Identification of myenteric neuron phenotypes in rat colon
There are no published recordings of direct sensory responses in identified enteric neurons from the colon of any species. Thus, we first determined whether sensory neurons were present in rat colon and identified them in terms of physiological phenotype and soma morphotype. APs were evoked via the patch pipette and thresholds were tested for with 2‐sec. depolarizing current pulses. We injected neurobiotin and recorded from 24 neurons from 21 untreated rats. Of these, 14 were AH cells because they had APs with repolarization humps and an sAHP (Fig. 1A). All AH cells also had a persisting Na+ current (I Na,P), which was evident as a region of negative slope conductance that gives the I–V curve a non‐monotonic shape (Fig. 1C). Of the AH cells, 9/9 had the classical Dogiel type II morphotype, consisting of an oval soma and multiple long neurites that emerged directly from the soma (Fig. 1C). Seven cells lacked the AP hump, sAHP (Fig. 1B) and I Na,P and of these 5/5 were uniaxonal with sparse short somatic dendrites (Fig. 1D); they were thus S cells. Addition of the triarylmethane I K,Ca blockers clotrimazole (5 μM) and TRAM‐34 (1 μM) abolished the sAHP in 5/5 or 4/4 AH cells, respectively.
Figure 1.
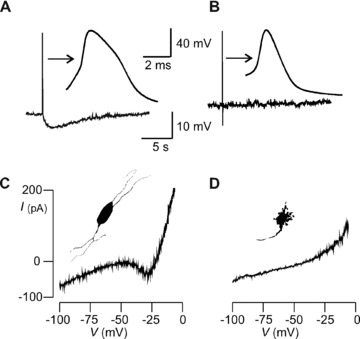
Identification of myenteric neurons in rat colon. (A & C) AH cell and (B & D) S cell. (A) AH and (B) S cell membrane voltage recording. Arrows point to action potentials on expanded time base. Hump during action potential repolarization and sAHP > 2 sec. duration after single AP distinguishes AH from S cell. (C) Non‐monotonic quasi‐steady‐state I–V curve from AH cell. Inset portrays neuron silhouette showing multipolar Dogiel type II morphotype. (D) I–V trace from S cell is monotonic and lacks region of negative conductance. Inset shows uniaxonal cell silhouette with multiple short dendrites indicating the Dogiel type I morphotype.
We identified intrinsic sensory AH neurons by their responses to chemical mucosal stimulation and the responses also served to demonstrate an intact conduction pathway between the rat colon mucosa and myenteric AH neurons. The mucosa in hemi‐dissected preparations (see Methods) from 15 untreated rats was exposed to brief 200‐msec.‐duration, 10‐μL spritzes of either 5 mM Na‐butyrate buffered to neutrality in HEPES‐Krebs saline or normal Krebs containing 5 μM 5‐hydroxytryptamine (5‐HT). Butyrate activated 5/7 AH cells via axonal APs (Fig. 2) running orthodromically from mucosa where they originate, to soma where they were recorded (Fig. 2A). Only the first two to three APs fully invaded the cell body because the sAHP that they evoke prevents soma excitation by subsequent axonal APs as they arrive. For the APs that fail to invade, electrotonic conduction from the axon led to the recording of smaller proximal process potentials as seen in Fig. 2B and D. S cells (8/11) responded trans‐synaptically with suprathreshold fEPSPs to butyrate (Fig. 2E). Blocking synaptic transmission by reducing [Ca2+] to 0.2 mM and increasing [Mg2+] to 10 mM in the superfusate [29] abolished the responses in S (Fig. 2F) but not AH cells (Fig. 2C). Because the stimuli were applied to the mucosa and not onto the neurons, the AH cells’ responses must have been sensory ones. Similarly, 5‐HT activated 5/7 AH directly and 10/11 S cells trans‐synaptically (Fig. 2D and G).
Effect of Lactobacillus ingestion on myenteric neuron properties
We next examined the excitability of identified neurons in LMMP preparations taken from animals that were fed LR or vehicle control. Figure 3A shows a typical trace taken from an AH cell in a vehicle‐fed animal for which APno= 1. Recordings from neurons with prior LR feeding revealed APno > 1 (Fig. 3B) during 2× threshold current stimulation. Overall, LR feeding decreased firing threshold (rheobase) from 8.09 ± 1.7 (24) for controls to 3.3 ± 0.8 μA/cm2 (21) (P= 0.02) (Fig. 3C). In addition, LR increased APno from 1.6 ± 0.2 (24) to 3.2 ± 0.7 (17) (P= 0.04) (Fig. 3D).
Figure 3.
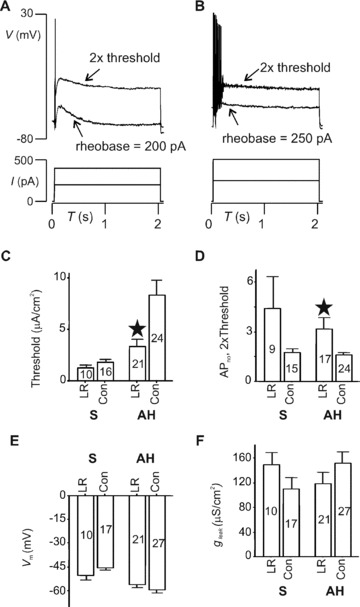
LR ingestion augments AH cell excitability. (A and B) Threshold and 2× threshold voltage responses (upper traces) to current injected (lower). AH cell from control rat (A) fired APs only at the very beginning of depolarization; one from LR‐fed rat (B) discharged seven APs. (C–E) Summary of current clamp data on passive membrane properties and excitability. (C) AP firing thresholds for either AH or S cells. Compared with control vehicle‐fed animals (Con), LR increased the number of APs discharged for AH but not S cells (D) (*P < 0.05). Test stimuli were 2 sec. depolarizations applied at 2× threshold current intensity. Membrane potential (V m) (E) and leak conductance (g leak) (F) were not altered by LR in either AH or S cells. Numbers of neurons for each category are indicated within body of bar graphs.
Summaries of soma excitability data for all neurons tested are given in Fig. 3C and D, which shows that in contrast to AH cells the excitability of S cells was not affected by ingestion of LR. Rheobases were 1.3 ± 0.3 (10) and 1.8 ± 0.3 (16) μA/cm2 for LR and control, respectively (P= 0.2); APno was 4.4 ± 1.9 (9) for the LR group compared with 1.7 ± 0.3 (15) (P= 0.2). For control AH neurons, the resting membrane potential (V m) and leak conductance (g leak) were −59 ± 2 mV (27) and 150 ± 18 (27) μS/cm2, respectively, and this was not altered by LR feeding. Control V m and g leak were −46 ± 2 mV (10) and 110 ± 19 μS/cm2 (10), respectively, for S neurons; these parameters also were not significantly altered by LR feeding (Fig. 3E and F).
The principal determinant of AH cell firing accommodation is the sAHP. We thus examined whether LR feeding altered the sAHP and found that LR reduced its duration and amplitude as illustrated in representative traces of Fig. 4A. Amplitudes and durations for the sAHP after a single AP for LR versus controls were −1.3 ± 0.8 (18) mV versus−3.8 ± 0.4 (20) mV (P= 0.02) and 3.9 ± 1.0 (18) sec. versus 13.6 ± 1.6 (20) sec., respectively (P < 0.001) (Fig. 4B and C). AH cells can fire APs in short bursts of two or more spikes in response to chemical stimulation of their receptive fields (Fig. 2). Accordingly, we tested whether the summed sAHP evoked by a select triple‐spike stimulus protocol (see Methods) was reduced by LR feeding. As shown in Fig. 4D and E, LR also reduced the magnitude of the summed sAHP. These data suggest that LR acted to increase the excitability of AH cells by alteration of the sAHP.
Figure 4.
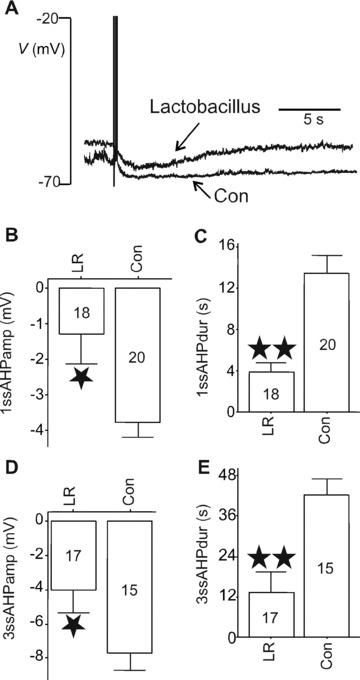
Effects of LR on sAHP. (A) Representative traces of post‐AP AHPs in AH cells from LR‐ or broth vehicle (Con)‐fed animals illustrating that LR shortened and reduced the amplitude of the sAHP. The amplitude and duration of single‐spike (1ssAHP) (B, C) or triple‐spike (3ssAHP) (D, E) sAHPs were reduced by LR ingestion. *P < 0.05, **P < 0.01. Numbers of neurons for each category are indicated within body of bar graphs.
There are essentially two conductances that underlie the sAHP in AH cells [30]: a clotrimazole/TRAM‐34–sensitive calcium‐dependent outward potassium current (I K,Ca) that drives the membrane towards E K approximately −90 mV and a hyperpolarization‐activated inward cationic current (I h) that drives it towards E h approximately −30 mV. Figure 5 illustrates how a simple combined hyperpolarizing step slow voltage ramp protocol (Fig. 5A) was used to measure both types of currents and to determine which was affected by LR. I h (Fig. 5C) was measured as the conductance increase (g h) elicited by the fixed hyperpolarizing voltage step [31] as described in Methods (see also Ref. [11] for a detailed description). The calculated conductance was divided by the whole‐cell membrane capacitance to give conductance per unit membrane area, assuming 1 μF = 1 cm2, g h= 23 ± 5 μS/cm2 (n= 11) for LR‐fed compared with 23 ± 4 (n= 9) for vehicle‐fed animals (P= 0.8).
Figure 5.
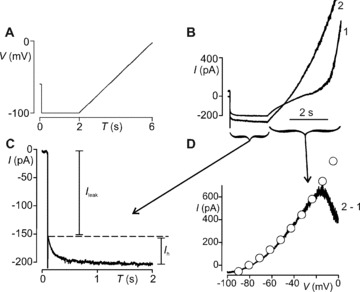
Whole‐cell currents underlying sAHP. (A) Voltage protocol for isolating I h and I K,Ca; 2‐sec. hyperpolarization step followed by 4‐sec. depolarizing ramp. The protocol was delivered twice: The first ramp (1) evoked the I K,Ca current in response to Ca2+ entry during the depolarization. The second ramp following immediately after the protocol was made while I K,Ca was active. (B) Current response to the initial 2‐sec. hyperpolarizing voltage step command. I leak is the instantaneous tail current generated by the total background currents active at the initial holding potential of –60 mV. This was followed by a slowly developing, non‐inactivating, inward current (I h). I h did not differ between protocols 1 and 2, indicating that it was not Ca2+ dependent. For protocol 2, the AHP current contributed to I leak (see onset of current trace in B). (D) The difference current (2–1) reveals voltage‐insensitive I K,Ca current. I K,Ca was well fitted (o) by the GHK current equation demonstrating that it was not voltage gated. I K,Ca permeability (P k) = 0.103 fm3/sec. for this cell. I h and I K,Ca were measured in this manner for neurons taken from LR‐fed or control animals. LR reduced P k but did not change I h (Results).
I K,Ca was measured from the I–V curves (Fig. 5D) constructed using difference currents induced by two successive slowly depolarizing ramp voltage commands [15] (Fig. 5). These ramp‐induced difference currents were Ca2+ dependent because they were reduced to zero in the absence of extracellular Ca2+ in AH cells taken from either control (n= 6) or probiotic fed (n= 3) animals. All of the I–V curves were well fit by the GHK equation demonstrating that I K,Ca is not voltage gated. The constant (P k) in the GHK equation gives a direct measure of the current magnitude in terms of ionic permeability [24]. In contrast to I h, LR reduced I K,Ca in AH cells from 0.113 ± 0.006 (n= 11) to 0.070 ± 0.017 (n= 7) fm3/sec. (P= 0.013). This current was eliminated by adding to the superfusing Krebs saline 5 μM clotrimazole (n= 5) or 1 μM TRAM34 (n= 3) but not 0.1 μM apamin (n= 3). These results show that LR feeding reduced sAHP by decreasing an outward intermediate conductance KCa type (IKCa or KCa3.1) current [32, 33] rather than augmenting I h.
To functionally identify the K+ ion channel that underlies the sAHP and was thus the one affected by probiotic ingestion, we exploited the ability of clotrimazole to block IKCa channels from their cytoplasmic side [34]. Using patches from 7 AH cells, each patch first in cell‐attached then inside‐out configuration, we determined that the K+ channel whose prolonged opening was evoked by an AP had an ‘intermediate’ 32 ± 8 pS single‐channel conductance. The channel’s prolonged opening after the AP mimicked the time course of the sAHP with an early post‐spike cell‐attached open probability (P o) approximately 0.7 (Fig. 6A). On pulling an inside‐out patch, a nominal cytoplasmic [Ca2+]= 0.5 μM gave P o approximately 0.3 with little variation due to trans‐patch voltage (Fig. 6B–D). When [Ca2+] was 5 nM, P o was less than 0.01 (not shown). Finally, clotrimazole (1 μM, n= 5) (Fig. 6D and E) or TRAM‐34 (3 μM, n= 3), also applied cytoplasmically, substantially reduced P o. Consequently, we have shown for the first time that the ion channel that opened during the sAHP as identified in cell‐attached recording mode was blocked in the inside‐out mode by specific IKCa blockers, and the same ion channel blockers also abolished the sAHP recorded in whole‐cell mode.
Discussion
Lactobacillus reuteri (LR) selectively increased the excitability of myenteric AH/Dogiel type II neurons. This was demonstrated by a decreased threshold for activation as well as an increased number of action potentials (APs) generated upon depolarization. This was due to a decreased slow afterhyperpolarization caused by a reduction in an intermediate calcium‐dependent potassium channel (IKCa). The effects of LR were mimicked by TRAM‐34 and clotrimazole, ion channel blockers that affect the same potassium channel. It is therefore likely that the effects of LR on enteric sensory nerves will translate into reduction of colonic motility since in an in vivo rat small intestine preparation, TRAM‐34, a specific IKCa channel blocker, decreased the amplitude of distention‐induced contractions by about 50%, leading to a complete cessation of fluid propulsion [35]. This interpretation is also consistent with a report that TRAM‐34 decreases rat colon peristalsis in vitro[36]. Further evidence for this interpretation comes from the observation that inhibition of AH cell excitability, by inhibition of I h, increases motility [36]. An enteric commensal organism–mediated increase in AH cell excitability might enhance the net tonic inhibition that the ENS exerts on the musculature [37, 38, 39, 40, 41, 42]. A reduction in colonic motility may also alleviate pain because active increase in muscle tension at the site of distension appears to be an essential factor for visceral pain responses [10].
An influence of commensal bacteria on immune cells and pro‐ and anti‐inflammatory cytokine production has long been cited to explain the health‐promoting actions of probiotics [43], but no clear mechanisms of action have been established. Lactobacillus commensal organisms can normalize inflammation‐related hypercontractility [44], and in germ‐free animals Lactobacillus acidophilus increases the frequency of the small intestine migrating myoelectric complex and accelerate transit; other commensal species had a converse effect [45]. In contrast, the actions of Lactobacillus species, especially reuteri, on normal gut motor behaviour are not well described. Yet there is evidence that certain commensal bacteria can alter rat gut motility [2, 46] and nociception [8] in the absence of overt inflammation. Application of several Lactobacillus species (L. acidophilus, L. casei, L. delbrueckii or L. plantarum) (VSL#3) decreases the contraction amplitude of ex vivo guinea pig colon segments [47]. Also, in vitro exposure of the mucosa of human dissected colon segments to L. rhamnosus reduces the contractile response of intestinal smooth‐muscle cells to acetylcholine [48].
Oral ingestion of Saccharomyces boulardii in the pig resulted in changes in the calbindin content in unidentified enteric neurons; however, no functional changes were reported [49]. Our data point to a mechanism that can explain how some commensal organisms might affect neuron function and is the first to provide experimental evidence of this at a cellular level and defines a molecular basis for it. It may be also significant that the putative therapeutic actions [50] of triarylmethane IKCa blockers, as immunosuppressants and in controlling secretory diarrhea, parallel some of the actions that have been ascribed to certain probiotics in inflammatory and functional bowel disorders [2, 3, 4].
We deduce that the reduction in sAHP reported here resulted from a decrease in IKCa channel current (Fig. 5), although the nature of the enteric sAHP channel has been controversial. Recordings from guinea pig or mouse AH cells have pointed towards maxi‐ [21], intermediate [11, 18] or small [51] conductance KCa channels. In addition, membrane protein expression in AH cells has been demonstrated immunohistochemically for small conductance KCa[52] and IKCa[53] channels. To provide evidence for the identity of the K channel involved, single K+ ion channels have been observed to open after the AP and the time course of their open state correlated with that of the sAHP [11, 18]. These recordings were made in cell‐attached configurations, and specific ion channel blockers were applied to the external surface of outside‐patches [11, 18]. Hence, it could not be determined with certainty that the channel that opened to produce the sAHP current was the same type as that whose opening was inhibited by the pharmacological blocker. In the present study, we applied clotrimazole, an IKCa channel blocker, to the cytoplasmic face of inside‐out patches that contained the same channel whose post‐AP opening was previously observed in cell‐attached patches, eliminating any doubt that opening of the IKCa channel underlines the sAHP.
The mechanism(s) whereby LR signals to AH cells to alter their excitability could include many plausible possible routes. Commensal bacteria are potent metabolic factories that can produce a wide variety of neurotransmitters and neuromodulator substances [54] such as GABA, 5‐hydroxytryptamine (5‐HT) or melatonin [55, 56, 57], NO and short‐chain fatty acids such as propionate and butyrate, all of which can act on enteric neurons. These substances might diffuse to reach AH cell processes in the mucosa. The action of butyrate on AH neuron somas in the absence of mucosa has previously been described by Neunlist et al.[58]. However, it is not certain that luminal butyrate crosses the epithelial layer and diffuses through the mucosal and submucosal layers to reach the myenteric plexus within the external muscular layers, to then act on the somas. In contrast, we applied butyrate to the mucosa (Fig. 2A), which is densely innervated by AH cell processes, and we recorded activity from AH cell somas in the myenteric plexus. Consequently, we could demonstrate a sensory response because application of butyrate to the mucosa directly activated the processes of AH cells (Fig. 2C). Activation of any group of AH cells by any combination of paths results in a synchronized excitation of the AH cell network [59, 60, 61]. This network consists of reciprocally connected AH cells that transmit to each other via excitatory metabotropic post‐synaptic neuropeptide or muscarinic receptors [59, 60, 61]. Activation of the receptors inhibits IKCa opening through several intracellular second messenger pathways [61, 62]. These considerations provide a framework for how diverse bacterial products might activate the metabotropic synaptic mechanisms that excite AH cells by reducing the sAHP ion channel current.
LR feeding did not affect excitability of S neurons. No effect was observed on the threshold for evoking APs, and the number of APs on depolarizing pulses was not statistically different from controls. However, as can be deduced from the average values, in some S neurons the number of APs on a depolarizing pulse was markedly >1. This is likely due to one of two factors. (1) S neuron excitability is variable; some S neurons fire phasically and others fire tonically without interruption during a prolonged depolarizing stimulus [19]. (2) When AH neurons fire, they strongly excite neighbouring S neurons by metabotropic neurotransmission [29]. Hence, in preparations in which AH neurons have increased excitability, S neuron excitability would also be expected to be increased.
Since AH neurons were taken from tissue that had been removed from the animal on the day of experimentation, they were no longer directly exposed to the probiotic metabolites. Hence, the AH neurons displayed a form of memory; earlier exposure to LR was expressed as a change in soma excitability. It has been well established that AH neurons exhibit a form of long‐term activity‐dependent post‐synaptic excitation. This long‐term excitation appears to be present in all myenteric AH neurons but is lacking in S neurons [63, 64], which can be excited by pre‐synaptic input, but this excitation does not persist more than minutes beyond the stimulation period, nor is it augmented with repetition [64]. The existence, identification and nomenclature of sensory neurons with somas in the gut wall are still under discussion [65, 66]. Hirst et al.[12, 67] proposed that AH cells might be intrinsic sensory neurons [68]. Intrinsic primary afferent neurons were identified and named by Gershon et al.[69] as those that responded to mechanical mucosal stimulation with c‐Fos expression, even under hexamethonium nicotinic blockade. Conventional AH neurons, which do not express c‐Fos when excited [70], have been shown to directly respond to mucosal chemical stimulation in the presence of total (low calcium–high magnesium) synaptic blockade [13]. The terms intrinsic primary afferent and intrinsic sensory are sometimes used to describe the same neuron [65]. Because we used mucosal stimulation to identify AH neurons as responding to chemical stimuli we have used the term intrinsic sensory to describe rat colon AH neurons in the present paper.
The in situ patch clamp recording technique we have used in the present paper has previously been applied in guinea pig [14] and mouse [11] small intestine, but the present work is the first case of its use in rat colon. Visualization of intact ganglia under bright field, the ready ability to record electrical signals from neurons in these ganglia and the successful recovery of neurobiotin‐filled cells suggest that in rat colon the myenteric ganglia also adhere to the longitudinal muscle when the circular muscle is removed. This conclusion is supported by a previous report [20] that showed that in the rat LMMP preparation enteric neurons can be recorded with sharp intracellular electrodes, and for which pan‐neuronal staining clearly showed that intact ganglia containing neurons readily adhere to the longitudinal muscle.
The present study shows, for the first time, that sensory neurons are present in rat colon and identifies them electrophysiologically and morphologically as AH neurons. As has been described for mouse [11, 71], addition of the triarylmethane IKCa blockers clotrimazole (5 μM) and TRAM‐34 (1 μM) abolished the sAHP. The correlation between AH neuron physiology and morphology as described here may not be entirely applicable to the mouse colon, for which it has been reported that >10% of Dogiel type I neurons with prominent fast synaptic input have AH neuron characteristics [72]. It is not known if mouse colon AH neurons express I Na,P.
We are currently exploring the different pathways that may be involved in the effects of LR feeding. The specificity of the effects both in terms of neuronal type and membrane mechanism points towards targeting IKCa channels in selecting therapeutically efficacious bacteria to modulate ENS function.
Acknowledgements
The Brain‐Body Institute, the Ontario Research Development Challenge Fund (ORDCF), and the Canadian Institutes of Health Research supported this work. Dr. Bingxian Wang received salary support through a Canadian Association of Gastroenterology – Canadian Institute of Health Research – Janssen fellowship.
#The authors declare that there is no conflict of interest to disclose.
References
- 1. Fioramonti J, Theodorou V, Bueno L. Probiotics: what are they? What are their effects on gut physiology Best Pract Res Clin Gastroenterol . 2003; 17: 711–24. [DOI] [PubMed] [Google Scholar]
- 2. Walker R, Buckley M. Probiotic microbes: the scientific basis. Washington , D.C. : American Society for Microbiology; 2006. [PubMed] [Google Scholar]
- 3. O’Mahony L, McCarthy J, Kelly P, et al . Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology . 2005; 128: 541–51. [DOI] [PubMed] [Google Scholar]
- 4. Gionchetti P, Lammers KM, Rizzello F, Campieri M. VSL#3: an analysis of basic and clinical contributions in probiotic therapeutics. Gastroenterol Clin North Am . 2005; 34: 499–513, ix‐x. [DOI] [PubMed] [Google Scholar]
- 5. Verdu EF, Collins SM. Irritable bowel syndrome and probiotics: from rationale to clinical use. Curr Opin Gastroenterol . 2005; 21: 697–701. [DOI] [PubMed] [Google Scholar]
- 6. Shanahan F. Irritable bowel syndrome: shifting the focus toward the gut microbiota. Gastroenterology . 2007; 133: 340–2. [DOI] [PubMed] [Google Scholar]
- 7. Rousseaux C, Thuru X, Gelot A, et al . Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med . 2007; 13: 35–7. [DOI] [PubMed] [Google Scholar]
- 8. Kamiya T, Wang L, Forsythe P, et al . Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague‐Dawley rats. Gut . 2006; 55: 191–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Verdu EF, Bercik P, Verma‐Gandhu M, et al . Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut . 2006; 55: 182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sarna SK. Enteric descending and afferent neural signaling stimulated by giant migrating contractions: essential contributing factors to visceral pain. Am J Physiol Gastrointest Liver Physiol . 2007; 292: G572–81. [DOI] [PubMed] [Google Scholar]
- 11. Mao Y, Wang B, Kunze W. Characterization of myenteric sensory neurons in the mouse small intestine. J Neurophysiol . 2006; 96: 998–1010. [DOI] [PubMed] [Google Scholar]
- 12. Hirst GD, Holman ME, Spence I. Two types of neurones in the myenteric plexus of duodenum in the guinea pig. J Physiol . 1974; 236: 303–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kunze WA, Bornstein JC, Furness JB. Identification of sensory nerve cells in a peripheral organ (the intestine) of a mammal. Neuroscience . 1995; 66: 1–4. [DOI] [PubMed] [Google Scholar]
- 14. Kunze WA, Clerc N, Furness JB, Gola M. The soma and neurites of primary afferent neurons in the guinea‐pig intestine respond differentially to deformation. J Physiol . 2000; 526: 375–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rugiero F, Gola M, Kunze WA, Reynaud JC, Furness JB, Clerc N. Analysis of whole‐cell currents by patch clamp of guinea‐pig myenteric neurones in intact ganglia. J Physiol . 2002; 538: 447–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rugiero F, Mistry M, Sage D, et al . Selective expression of a persistent tetrodotoxin‐resistant Na+ current and NaV1.9 subunit in myenteric sensory neurons. J Neurosci . 2003; 23: 2715–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vogalis F, Harvey JR, Neylon CB, Furness JB. Regulation of K+ channels underlying the slow afterhyperpolarization in enteric afterhyperpolarization‐generating myenteric neurons: role of calcium and phosphorylation. Clin Exp Pharmacol Physiol . 2002; 29: 935–43. [DOI] [PubMed] [Google Scholar]
- 18. Vogalis F, Harvey JR, Furness JB. TEA‐ and apamin‐resistant K(Ca) channels in guinea‐pig myenteric neurons: slow AHP channels. J Physiol . 2002; 538: 421–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bornstein JC, Furness JB, Kunze WA. Electrophysiological characterization of myenteric neurons: how do classification schemes relate J Auton Nerv Syst . 1994; 48: 1–15. [DOI] [PubMed] [Google Scholar]
- 20. Browning KN, Lees GM. Myenteric neurons of the rat descending colon: electrophysiological and correlated morphological properties. Neuroscience . 1996; 73: 1029–47. [DOI] [PubMed] [Google Scholar]
- 21. Kunze WA, Bornstein JC, Furness JB, Hendriks R, Stephenson DS. Charybdotoxin and iberiotoxin but not apamin abolish the slow after‐ hyperpolarization in myenteric plexus neurons. Pflugers Arch . 1994; 428: 300–6. [DOI] [PubMed] [Google Scholar]
- 22. Vogalis F, Harvey JR, Furness JB. PKA‐mediated inhibition of a novel K+ channel underlies the slow after‐hyperpolarization in enteric AH neurons. J Physiol . 2003; 548: 801–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. North RA, Tokimasa T. Persistent calcium‐sensitive potassium current and the resting properties of guinea‐pig myenteric neurones. J Physiol . 1987; 386: 333–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hille B. Ionic channels of excitable membranes. 2nd ed. Massachusetts : Sunderland ; 1984. [Google Scholar]
- 25. Kunze WA, Clerc N, Bertrand PP, Furness JB. Contractile activity in intestinal muscle evokes action potential discharge in guinea‐pig myenteric neurons. J Physiol . 1999; 517: 547–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kunze WA, Furness JB, Bertrand PP, Bornstein JC. Intracellular recording from myenteric neurons of the guinea‐pig ileum that respond to stretch. J Physiol . 1998; 506: 827–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dass NB, John AK, Bassil AK, et al . The relationship between the effects of short‐chain fatty acids on intestinal motility in vitro and GPR43 receptor activation. Neurogastroenterol Motil . 2007; 19: 66–74. [DOI] [PubMed] [Google Scholar]
- 28. Bertrand PP, Kunze WA, Bornstein JC, Furness JB, Smith ML. Analysis of the responses of myenteric neurons in the small intestine to chemical stimulation of the mucosa. Am J Physiol . 1997; 273: G422–35. [DOI] [PubMed] [Google Scholar]
- 29. Kunze WA, Furness JB, Bornstein JC. Simultaneous intracellular recordings from enteric neurons reveal that myenteric AH neurons transmit via slow excitatory postsynaptic potentials. Neuroscience . 1993; 55: 685–94. [DOI] [PubMed] [Google Scholar]
- 30. Furness JB, Kunze WA, Bertrand PP, Clerc N, Bornstein JC. Intrinsic primary afferent neurons of the intestine. Prog Neurobiol . 1998; 54: 1–18. [DOI] [PubMed] [Google Scholar]
- 31. Galligan JJ, Tatsumi H, Shen KZ, Surprenant A, North RA. Cation current activated by hyperpolarization (IH) in guinea pig enteric neurons. Am J Physiol . 1990; 259: G966–72. [DOI] [PubMed] [Google Scholar]
- 32. Wei AD, Gutman GA, Aldrich R, Chandy KG, Grissmer S, Wulff H. International Union of Pharmacology. LII. Nomenclature and molecular relationships of calcium‐activated potassium channels. Pharmacol Rev . 2005; 57: 463–72. [DOI] [PubMed] [Google Scholar]
- 33. Jensen B, Hertz M, Christophersen P, Madsen L. The Ca(2+)‐activated K(+) channel of intermediate conductance: a possible target for immune suppression. Expert Opin Ther Targets . 2002; 6: 623–36. [DOI] [PubMed] [Google Scholar]
- 34. Dunn PM. The action of blocking agents applied to the inner face of Ca(2+)‐activated K+ channels from human erythrocytes. J Membr Biol . 1998; 165: 133–43. [DOI] [PubMed] [Google Scholar]
- 35. Ferens D, Baell J, Lessene G, Smith JE, Furness JB. Effects of modulators of Ca(2+)‐activated, intermediate‐conductance potassium channels on motility of the rat small intestine, in vivo . Neurogastroenterol Motil . 2007; 19: 383–9. [DOI] [PubMed] [Google Scholar]
- 36. Strong DS, Sharkey KA, Mawe GM. Relationship between AH neuron excitability and peristalsis in normal versus inflamed guinea pig distal colon. Neurogastroenterol Motil . 2006; 18: 695: A95. [Google Scholar]
- 37. Delbro DS. Neuronal inhibition determines the gastrointestinal motor state. News Physiol Sci . 1996; 11: 67–71. [Google Scholar]
- 38. Spencer NJ, Bywater RA, Taylor GS. Disinhibition during myoelectric complexes in the mouse colon. J Auton Nerv Syst . 1998; 71: 37–47. [DOI] [PubMed] [Google Scholar]
- 39. Wood JD. Intrinsic neural control of intestinal motility. Annu Rev Physiol . 1981; 43: 33–51. [DOI] [PubMed] [Google Scholar]
- 40. Wood JD. Effects of elevated magnesium on discharge of myenteric neurons of cat small bowel. Am J Physiol . 1975; 229: 657–62. [DOI] [PubMed] [Google Scholar]
- 41. Wood JD. Excitation of intestinal muscle by atropine, tetrodotoxin, and xylocaine. Am J Physiol . 1972; 222: 118–25. [DOI] [PubMed] [Google Scholar]
- 42. Wood JD. Neuropathophysiology of functional gastrointestinal disorders. World J Gastroenterol . 2007; 13: 1313–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Marco ML, Pavan S, Kleerebezem M. Towards understanding molecular modes of probiotic action. Curr Opin Biotechnol . 2006; 17: 204–10. [DOI] [PubMed] [Google Scholar]
- 44. Verdú EF, BercÌk P, Bergonzelli GE, et al . Lactobacillus paracasei normalizes muscle hypercontractility in a murine model of postinfective gut dysfunction. Gastroenterology . 2004; 127: 826–37. [DOI] [PubMed] [Google Scholar]
- 45. Husebye E, Hellstrom PM, Sundler F, Chen J, Midtvedt T. Influence of microbial species on small intestinal myoelectric activity and transit in germ‐free rats. Am J Physiol Gastrointest Liver Physiol . 2001; 280: G368–80. [DOI] [PubMed] [Google Scholar]
- 46. Lesniewska V, Rowland I, Laerke HN, Grant G, Naughton PJ. Relationship between dietary‐induced changes in intestinal commensal microflora and duodenojejunal myoelectric activity monitored by radiotelemetry in the rat in vivo . Exp Physiol . 2006; 91: 229–37. [DOI] [PubMed] [Google Scholar]
- 47. Massi M, Ioan P, Budriesi R, et al . Effects of probiotic bacteria on gastrointestinal motility in guinea‐pig isolated tissue. World J Gastroenterol . 2006; 12: 5987–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Guarino MP, Altomare A, Stasi E, et al . Effect of acute mucosal exposure to Lactobacillus rhamnosus GG on human colonic smooth muscle cells. J Clin Gastroenterol . 2008. [DOI] [PubMed] [Google Scholar]
- 49. Kamm K, Hoppe S, Breves G, Schroder B, Schemann M. Effects of the probiotic yeast Saccharomyces boulardii on the neurochemistry of myenteric neurones in pig jejunum. Neurogastroenterol Motil . 2004; 16: 53–60. [DOI] [PubMed] [Google Scholar]
- 50. Chandy KG, Wulff H, Beeton C, Pennington M, Gutman GA, Cahalan MD. K+ channels as targets for specific immunomodulation. Trends Pharmacol Sci . 2004; 25: 280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vogalis F, Furness JB, Kunze WA. Afterhyperpolarization current in myenteric neurons of the guinea pig duodenum. J Neurophysiol . 2001; 85: 1941–51. [DOI] [PubMed] [Google Scholar]
- 52. Nakajima H, Goto H, Azuma YT, Fujita A, Takeuchi T. Functional interactions between the SK2 channel and the nicotinic acetylcholine receptor in enteric neurons of the guinea pig ileum. J Neurochem . 2007; 103: 2428–38. [DOI] [PubMed] [Google Scholar]
- 53. Furness JB, Robbins HL, Selmer IS, et al . Expression of intermediate conductance potassium channel immunoreactivity in neurons and epithelial cells of the rat gastrointestinal tract. Cell Tissue Res . 2003; 314: 179–89. [DOI] [PubMed] [Google Scholar]
- 54. Iyer LM, Aravind L, Coon SL, Klein DC, Koonin EV. Evolution of cell‐cell signaling in animals: did late horizontal gene transfer from bacteria have a role Trends Genet . 2004; 20: 292–9. [DOI] [PubMed] [Google Scholar]
- 55. Bourlioux P, Koletzko B, Guarner F, Braesco V. The intestine and its microflora are partners for the protection of the host: report on the Danone Symposium ‘The Intelligent Intestine’, held in Paris, June 14, 2002. Am J Clin Nutr . 2003; 78: 675–83. [DOI] [PubMed] [Google Scholar]
- 56. Hardeland R, Poeggeler B. Non‐vertebrate melatonin. J Pineal Res . 2003; 34: 233–41. [DOI] [PubMed] [Google Scholar]
- 57. Siragusa S, De Angelis M, Di Cagno R, Rizzello CG, Coda R, Gobbetti M. Synthesis of {gamma}‐aminobutyric acid (GABA) by lactic acid bacteria isolated from Italian cheese varieties. Appl Environ Microbiol . 2007; 73: 7283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Neunlist M, Dobreva G, Schemann M. Characteristics of mucosally projecting myenteric neurones in the guinea‐pig proximal colon. J Physiol . 1999; 517: 533–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Furness JB. The enteric nervous system. Massachusetts, Oxford, Carlton : Blackwell Publishing; 2006. [Google Scholar]
- 60. Kunze WA, Furness JB. The enteric nervous system and regulation of intestinal motility. Annu Rev Physiol . 1999; 61: 117–42. [DOI] [PubMed] [Google Scholar]
- 61. Wood JD, Kirchgessner A. Slow excitatory metabotropic signal transmission in the enteric nervous system. Neurogastroenterol Motil . 2004; 16: 71–80. [DOI] [PubMed] [Google Scholar]
- 62. Nguyen TV, Stebbing MJ, Clerc N, Kawai M, Harvey JR, Furness JB. Evidence for protein kinase involvement in long‐term postsynaptic excitation of intrinsic primary afferent neurons in the intestine. Auton Neurosci . 2004; 115: 1–6. [DOI] [PubMed] [Google Scholar]
- 63. Clerc N, Furness JB, Kunze WA, Thomas EA, Bertrand PP. Long‐term effects of synaptic activation at low frequency on excitability of myenteric AH neurons. Neuroscience . 1999; 90: 279–89. [DOI] [PubMed] [Google Scholar]
- 64. Alex G, Kunze WA, Furness JB, Clerc N. Comparison of the effects of neurokinin‐3 receptor blockade on two forms of slow synaptic transmission in myenteric AH neurons. Neuroscience . 2001; 104: 263–9. [DOI] [PubMed] [Google Scholar]
- 65. Blackshaw LA, Brookes SJ, Grundy D, Schemann M. Sensory transmission in the gastrointestinal tract. Neurogastroenterol Motil . 2007; 19: 1–19. [DOI] [PubMed] [Google Scholar]
- 66. Wood JD. Enteric nervous system: reflexes, pattern generators and motility. Curr Opin Gastroenterol . 2008; 24: 149–58. [DOI] [PubMed] [Google Scholar]
- 67. Hirst GD, Holman ME, Prosser CL, Spence I. Some properties of the neurones of Auerbach’s plexus. J Physiol . 1972; 225: 60P–1P. [PubMed] [Google Scholar]
- 68. Bornstein JC. Intrinsic sensory neurons of mouse gut: towards a detailed knowledge of enteric neural circuitry across species. J Neurophysiol . 2006; 2006: 511. [DOI] [PubMed] [Google Scholar]
- 69. Gershon MD, Kirchgessner AL. Identification, characterization and projections of intrinsic primary afferent neurones of the submucosal plexus: activity‐induced expression of c‐fos immunoreactivity. J Auton Nerv Syst . 1991; 33: 185–7. [Google Scholar]
- 70. Ritter RC, Costa M, Brookes SH. Nuclear Fos immunoreactivity in guinea pig myenteric neurons following activation of motor activity. Am J Physiol . 1997; 273: G498–507. [DOI] [PubMed] [Google Scholar]
- 71. Neylon CB, Nurgali K, Hunne B, et al . Intermediate‐conductance calcium‐activated potassium channels in enteric neurones of the mouse: pharmacological, molecular and immunochemical evidence for their role in mediating the slow afterhyperpolarization. J Neurochem . 2004; 90: 1414–22. [DOI] [PubMed] [Google Scholar]
- 72. Nurgali K, Furness JB, Stebbing MJ. Correlation of electrophysiology, shape and synaptic properties of myenteric AH neurons of the guinea pig distal colon. Auton Neurosci . 2003; 103: 50–64. [DOI] [PubMed] [Google Scholar]


