Abstract
Boron neutron capture therapy (BNCT) provides highly targeted delivery of radiation through the limited spatial distribution of its effects. This translational research/phase I clinical trial investigates whether BNCT might be developed as a treatment option for squamous cell carcinoma of head and neck (SCCHN) relying upon preferential uptake of the two compounds, sodium mercaptoundecahydro‐closo‐dodecaborate (BSH) or L‐para‐boronophenylalanine (BPA) in the tumour. Before planned tumour resection, three patients received BSH and three patients received BPA. The 10B‐concentration in tissues and blood was measured with prompt gamma ray spectroscopy. Adverse effects from compounds did not occur. After BPA infusion the 10B‐concentration ratio of tumour/blood was 4.0 ± 1.7. 10B‐concentration ratios of tumour/normal tissue were 1.3 ± 0.5 for skin, 2.1 ± 1.2 for muscle and 1.4 ± 0.01 for mucosa. After BSH infusion the 10B‐concentration ratio of tumour/blood was 1.2 ± 0.4. 10B‐concentration ratios of tumour/normal tissue were 3.6 ± 0.6 for muscle, 2.5 ± 1.0 for lymph nodes, 1.4 ± 0.5 for skin and 1.0 ± 0.3 for mucosa. BPA and BSH deliver 10B to SCCHN to an extent that might allow effective BNCT treatment. Mucosa and skin are the most relevant organs at risk.
Keywords: boron neutron capture therapy, squamous cell carcinoma of head and neck, BSH, BPA, 10B‐biodistribution
Introduction
Advanced squamous cell carcinoma of head and neck (SCCHN) remains among the most resistant tumours to treatment [1]. Current treatment involves surgery when the disease is operable, and radio(‐chemo)therapy. Research efforts focus on advanced chemotherapy and on irradiation techniques that aim to increase the precision of beam delivery to a defined volume (e.g. intensity modulated radiotherapy and particle irradiation).
A further step to optimize radiotherapy might be achieved with cellular targeting of radiation that in principle can selectively kill tumour cells whilst sparing surrounding normal tissues. One such treatment option could be boron neutron capture therapy (BNCT), which, by the limited spatial distribution of its effects, produces highly selective delivery of the irradiation. BNCT exploits the ability of the isotope 10B to capture thermal neutrons leading to the nuclear reaction 10B(n,α,γ)7Li. This reaction produces alpha particles and 7Li‐ions, which both have a high linear energy transfer (LET) and a high biological effectiveness compared with photon irradiation. The range of these particles in tissue is approximately 10 μm, restricting their effects to one cell diameter. Therefore, if 10B can be selectively delivered to tumour cells, the short range of the high‐LET particles offers the potential for a targeted irradiation of individual tumour cells.
The success of BNCT depends on the selective delivery of 10B‐atoms to the tumour cells. At present, two compounds are explored clinically:
Sodium mercaptoundecahydro‐closo‐dodecaborate (BSH, Na2 10B12H11SH) was investigated in malignant glioma [2] and in a phase I trial for glioblastoma multiforme (EORTC 11961) [3].
L‐para‐boronophenylalanine (BPA, C9H12 10BNO4) was studied in glioblastoma and melanoma [4].
The treatment of SCCHN with BNCT might be especially attractive because tumours are located near critical organs, complicating the delivery of sufficient radiation dose to eradicate the disease. Improved dose distribution might allow dose escalation within the target and an optimal sparing of normal tissue. High‐LET particles are moreover particularly advantageous in hypoxic tumours with poor radiosensitivity, which is often characteristic of SCCHN.
Assuming that BNCT offers more effective loco‐regional tumour control through such targeted high‐LET irradiation, preferential delivery of a 10B‐containing compound to the tumour (compared to surrounding normal tissue) should be demonstrated before the concept moves into the clinic. In contrast to other anticancer drugs, compounds used for BNCT do not have any therapeutic effect by themselves but are aimed exclusively at transporting 10B‐atoms to tumour cells. As a consequence, conventional methods to test the efficacy of candidate drugs are not applicable. Therefore, the EORTC trial 11001 was initiated as a translational research/phase I trial to investigate whether the compounds BSH and BPA preferentially accumulate in specific tumour entities. This article reports the results of the biodistribution study in patients suffering from SCCHN.
Materials and methods
Study design
Prior to the planned removal of the tumour, groups of three patients were infused with either BPA or BSH. Tissue and blood samples were collected and analysed as described below. The co‐administration of both compounds might lead to improved 10B‐concentration ratios of tumour to normal tissues because of different mechanisms of targeting. Therefore, the infusion of both compounds was allowed in a third group of patients if a ‘favourable’ uptake of both compounds was demonstrated. ‘Favourable’10B‐uptake was defined in the trial protocol as follows:
-
1
BSH: tumour‐normal surrounding tissue ratio more than 2, tumour to blood ratio more than 0.6;
-
2
BPA: tumour‐normal surrounding tissue ratio more than 2, tumour to blood ratio more than 1.5.
The primary end‐point was the 10B‐concentration measured by prompt gamma ray spectroscopy (PGRS). The secondary end‐point was the toxicity of the 10B‐compounds (assessed according to NCI‐CTC version 2.1). As no benefit from participation in the trial was expected for the individual patient, the number of patients included was kept to a minimum and the protocol procedures were defined not to interfere with planned surgical treatment. Descriptive statistics were applied. To avoid toxicity, the doses infused to patients were lower than the doses needed for a BNCT‐treatment but high enough to lead to measurable 10B‐concentration in the investigated tissues. The Ethics Committee of the Medical Faculty, University Duisburg‐Essen, approved the trial. The clinical trial was conducted under the requirements of the Declaration of Helsinki. All patients gave written informed consent prior to inclusion.
Patient selection
Patients with histologically proven SCCHN were eligible if surgery was planned. Other eligibility criteria were age 18 years or more, WHO performance status 2 or less, adequate haematological values, no severe concomitant disease and absence of toxic effects of previous anticancer therapies. Patients with a history of phenylketonuria, radiation to head and neck or chemotherapy within 3 months prior to the planned surgery, were excluded.
Study procedures
The quality control of compounds (produced by Katchem, Prague) and the preparation of injection solutions followed the standard operating procedures established for the EORTC‐BNCT trials [5]. BPA was complexed with fructose prior to infusion.
The infusion of compounds in the respective study groups was as follows:
-
1
Within 1 hr, 50 mg/kg BSH infused. Infusion started 12 hrs prior to tissue sampling.
-
2
Within 1 hr, 100 mg/kg BPA infused. Infusion started 2 hrs prior to tissue sampling.
Dose reduction rules were defined, in case any patient experienced severe toxicity (grade 3 according to NCI‐CTC). On days 1, 5 and 28 after surgery, toxicity was prospectively assessed.
The 10B‐concentration in samples was analysed at the High Flux Reactor Petten by PGRS, which is an established tool for 10B‐analysis in biological samples [6]. All tumours were examined histopathologically applying standard procedures. In all patients the diagnosis of squamous cell carcinoma was confirmed. Special attention was paid to the regions where samples were taken for 10B‐analysis, to confirm histologically the macroscopical appearance of samples (tumour versus tumour free).
Results
Between February 2004 and December 2007, six patients who were all eligible and had completed protocol treatment and follow‐up investigations as foreseen were included. The extent and the duration of surgery differed considerably among patients. As surgery was given priority over the trial, the sample collection period following the end of infusion was 11.3–15.3 hrs for patients infused with BSH and 0.3–7.5 hrs for patients infused with BPA. The types of normal tissues that were sampled also depended on the type of surgery and thus differed for the individual patient. Tumour samples were taken in all patients at time points close to those planned for tissue extraction according to protocol. In one patient in the BSH group but in three patients in the BPA group, repeated tumour samples were collected. This allowed the time course of 10B‐accumulation in the tumour to be observed. The 10B‐concentration ratios of tumour to normal tissues were only calculated if both samples were collected at similar time points.
10B‐uptake after BPA‐infusion
The pharmacokinetic of 10B in blood as delivered by BPA was modelled with the measured data according to a two‐compartment model [7] (Fig. 1A). The 10B‐concentration reached a maximum at the end of the BPA infusion and dropped thereafter. The mean 10B‐concentration in the tumours 1.7–3 hrs after BPA infusion was 19.0 ± 6.8 μg/g. The mean 10B‐concentration ratio of tumour to blood was 4.0 ± 1.7. The 10B‐concentration ratio of tumour/blood as a function of time followed the time course of the 10B‐concentration in blood with an initial build‐up to a maximum at about 2 hrs after the start of infusion (Fig. 1B).
Figure 1.
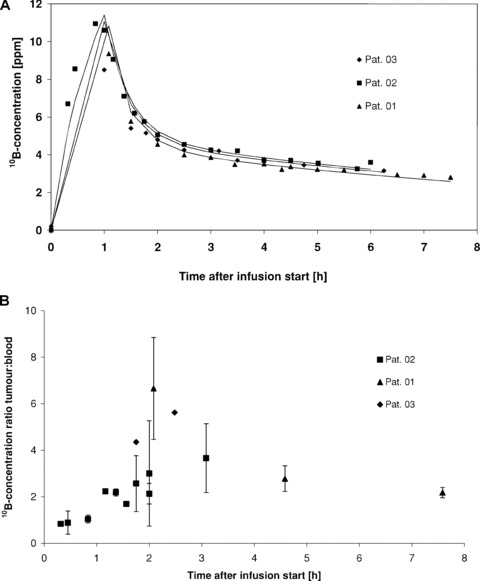
10B‐concentration in blood (A) and 10B‐concentration ratios of tumour/blood (B) in three patients as a function of time after intravenous (i.v.) infusion of 100 mg BPA/kg bw in 1 hr. The 10B‐concentrations were measured with prompt gamma ray spectroscopy (PGRS).
The absolute 10B‐concentrations and 10B‐concentration ratios of tissue/blood for individual patients are summarized in Table 1. Of the normal organs evaluated, the highest mean10B‐concentrations 1.7–3 hrs after the BPA infusion were found in skin (15.3 ± 1 μg/g), tongue (11.9 μg/g), muscle (10.3 ± 2.8 μg/g) and mucosa (10.7 ± 3.5 μg/g). Mean 10B‐concentration ratios of tumour/normal tissues were 1.3 ± 0.5 for skin, 2.1 for tongue (one sample), 2.1 ± 1.2 for muscle and 1.4 ± 0.01 for mucosa, respectively (Fig. 2). The 10B‐concentration ratio of tumour/blood clearly fulfilled the protocol definition of a ‘favourable’ ratio as did the 10B‐concentration ratios of tumour/muscle and tumour/tongue; however, the ratios of tumour/skin and tumour/mucosa did not.
Table 1.
Mean absolute 10B‐concentrations (±·S.D.) and 10B‐concentration ratios (±·S.D.) of tissue/blood following an infusion of BPA (50 mg BPA/kg bw)
| Patients | |||
|---|---|---|---|
| Patient ident. no. | 01 | 02 | 03 |
| Sex, age | Male, 44 years | Male, 69 years | Male, 50 years |
| Weight, height | 84 kg, 180 cm | 73 kg, 172 cm | 60 kg, 168 cm |
| Body surface | 2.0 m2 | 1.9 m2 | 1.7 m2 |
| Tumour stage | T2 N1 M0 | T4 N0 M0 | T3 N2b M0 |
| Histology | SCC of floor of mouth, G2–3 | SCC of maxillary sinus, orbit nasal cavity, G2 | SCC of tonsil, G2–3 |
| 10B‐concentration | n | Time after infusion start (hr) | 10B‐concentration | n | Time after infusion start (hr) | 10B‐concentration | n | Time after infusion start (hr) | |
|---|---|---|---|---|---|---|---|---|---|
| 10B‐concentration in tumour (ppm) | (a) 30.3 ± 2.0 | 3 | 2.0 | (a) 5.6 | 1 | 0.3 | (a) 22.4 | 1 | 1.8 |
| (b) 9.4 ± 1.9 | 4 | 4.5 | (b) 7.6 ± 4.3 | 3 | 0.5 | (b) 23.9 | 1 | 2.5 | |
| (c) 6.1 ± 0.6 | 5 | 7.5 | (c) 11.4 ± 1.8 | 6 | 0.8 | ||||
| (d) 20.2 ± 0 | 2 | 1.2 | |||||||
| (e) 15.5 ± 1.1 | 3 | 1.4 | |||||||
| (f) 10.5 | 1 | 1.7 | |||||||
| (g) 14.8 ± 6.9 | 3 | 1.8 | |||||||
| (h) 10.8 ± 2.2 | 4 | 2.0 | |||||||
| (i) 15.2 ± 11.4 | 4 | 2.0 | |||||||
| (j) 15.6 ± 6.3 | 2 | 3.1 | |||||||
| 10B‐concentration ratio of tumour/blood | (a) 6.7 ± 2.2 | 2.0 | (a) 0.9 | 0.3 | (a) 4.4 | 1.8 | |||
| (b) 2.8 ± 0.6 | 4.5 | (b) 0.9 ± 0.5 | 0.5 | (b) 5.6 | 2.5 | ||||
| (c) 2.2 ± 0.2 | 7.5 | (c) 1.0 ± 0.2 | 0.8 | ||||||
| (d) 2.2 ± 0 | 1.2 | ||||||||
| (e) 2.2 ± 0.2 | 1.4 | ||||||||
| (f) 1.7 | 1.7 | ||||||||
| (g) 2.6 ± 1.2 | 1.8 | ||||||||
| (h) 2.1 ± 0.4 | 2.0 | ||||||||
| (i) 3.0 ± 2.2 | 2.0 | ||||||||
| (j) 3.7 ± 1.5 | 3.1 | ||||||||
| 10B‐concentration in muscle (ppm) | (a) 6.4 ± 1.2 | 4 | 5.0 | (a) 12.2 | 1 | 2.0 | 8.3 | 1 | 3.2 |
| (b) 4.7 ± 0.3 | 5 | 7.5 | (b) 5.5 ± 2.0 | 2 | 5.8 | ||||
| 10B‐concentration ratio of muscle/blood | (a) 2.0 ± 0.4 | 5.0 | (a) 2.4 | 2.0 | 2 | 3.2 | |||
| (b) 1.7 ± 0.1 | 7.5 | (b) 1.7 ± 0.6 | 5.8 | ||||||
| 10B‐concentration in skin (ppm) | 6.3 ± 0.03 | 2 | 5.0 | (a) 16.0 ± 0.6 | 2 | 2.7 | 14.6 | 1 | 3.0 |
| (b) 5.8 ± 0.4 | 2 | 5.8 | |||||||
| 10B‐concentration ratio of skin/blood | 2.0 ± 0.01 | 5.0 | (a) 3.5 ± 0.1 | 2.7 | 3.4 | 3.0 | |||
| (b) 1.8 ± 0.1 | 5.8 | ||||||||
| 10B‐concentration in fat (ppm) | (a) 1.9 ± 0.5 | 3 | 5.0 | (a) 4.5 ± 2.3 | 6 | 2.0 | 7.7 | 1 | 3.0 |
| (b) 2.4 ± 0.7 | 4 | 7.5 | (b) 2.0 | 1 | 5.4 | ||||
| (c) 1.7 ± 0.3 | 4 | 5.8 | |||||||
| 10B‐concentration ratio of fat/blood | (a) 0.6 ± 0.2 | 5.0 | (a) 0.9 ± 0.5 | 2.0 | 1.8 | 3.0 | |||
| (b) 0.9 ± 0.2 | 7.5 | (b) 0.6 | 5.4 | ||||||
| (c) 0.5 ± 0.1 | 5.8 | ||||||||
| 10B‐concentration in LN (ppm) | nd | (a) 6.3 | 1 | 5.4 | nd | ||||
| (b) 4.6 | 1 | 5.8 | |||||||
| 10B‐concentration ratio of LN/blood | nd | (a) 1.9 | 5.4 | nd | |||||
| (b) 1.4 | 5.8 | ||||||||
| 10B‐concentration in mucosa (ppm) | nd | 10.7 ± 0.9 | 2 | 2.0 | 15.6 ± 0.9 | 2 | 1.8 | ||
| 10B‐concentration ratio of mucosa/blood | nd | 2.1 ± 0.2 | 2.0 | 3.0 ± 0.2 | 1.8 | ||||
| 10B‐concentration in tongue (ppm) | 13.8 ± 7.0 | 4 | 4.3 | nd | 11.9 | 1 | 2.5 | ||
| 10B‐concentration ratio of tongue/blood | 4.3 ± 2.2 | 4.3 | nd | 2.8 | 2.5 |
Patient ident. no., patient registration number; nd, not done; LN, lymph node (not metastatic); SCC, squamous cell carcinoma.
Figure 2.
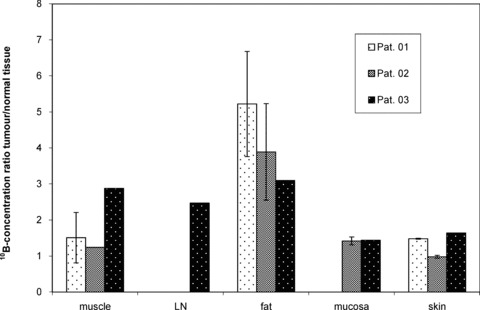
10B‐concentration ratio of tumour/normal organs in three patients after i.v. infusion of 100 mg BPA/kg bw in 1 hr. LN, lymph node.
10B‐uptake after BSH infusion
After BSH infusion, the 10B‐concentration in blood reached a maximum at the end of the infusion and dropped thereafter considerably more slowly compared with BPA (Fig. 3A). During the tissue sampling, the mean 10B‐concentration in the blood was 16.5 ± 8.3 μg/g.
Figure 3.
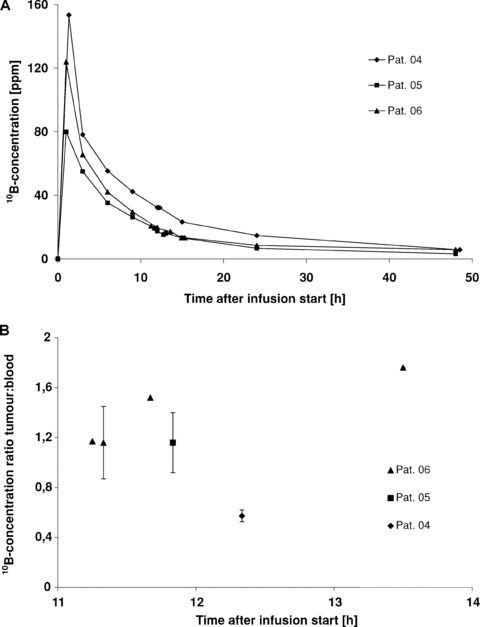
10B‐concentration in blood (A) and 10B‐ concentration ratios of tumour/blood (B) in three patients as a function of time after i.v. infusion of 50 mg BSH/kg bw in 1 hr. The 10B‐concentration was measured with PGRA.
The mean 10B‐concentration in the tumours after BSH‐infusion was 24.9 ± 4.6 μg/g; the mean 10B‐concentration ratio of tumour/blood was 1.2 ± 0.4. Figure 3B illustrates the 10B‐concentration ratio of tumour/blood as a function of time. In patient 06, tumour samples were obtained at four time points 11.3–13.6 hrs after the start of BSH infusion. In this patient, the 10B‐concentration ratio of tumour/blood increased within this observation period. However, because of the low number of tissue samples this trend could not be verified in other patients.
After BSH infusion, the highest mean 10B‐concentrations in normal tissues were measured in mucosa (20.4 ± 2.9 μg/g) and skin (19.5 ± 0.9 μg/g). Lower 10B‐concentrations were found in fat (mean 10.7 ± 0.5 μg/g), lymph nodes (9.7 ± 0.7 μg/g) and muscle (7.4 ± 0.8 μg/g). Data for individual patients are summarized in Table 2. These absolute 10B‐concentrations translate into a favourable mean 10B‐concentration ratio of tumour/muscle (3.6 ± 0.6) and quite favourable ratios of tumour/normal lymph nodes (2.5 ± 1.0) and tumour/fat (2.3 ± 0.8), whereas the 10B‐concentration ratio of tumour/skin was 1.4 ± 0.5 and the ratio of tumour/oral mucosa was 1.0 ± 0.3 only (Fig. 4). The 10B‐concentration ratios of tumour/blood, tumour/muscle, tumour/lymph nodes and tumour/fat fulfilled the definition in the study protocol for ‘favourable’10B‐concentration ratios. The ratios of tumour/skin and tumour/mucosa failed to reach ‘favourable’ ratios.
Table 2.
Mean absolute 10B‐concentrations (±·S.D.) in tissues and 10B‐concentration ratios (±·S.D.) of tissue/blood following an infusion of 50 mg BSH/kg bw
| Patients | |||
|---|---|---|---|
| Patient ident. no. | 04 | 05 | 06 |
| Sex, age | Male, 57 years | Male, 49 years | Male, 53 years |
| Weight, height | 100 kg, 180 cm | 75 kg, 182 cm | 61 kg, 168 cm |
| Body surface | 2.2 m2 | 1.9 m2 | 1.7 m2 |
| Tumour stage | T4 N2b M0 | T1 N0 M0 | T4 N2c M0 |
| Histology | SCC of oropharynx, G1 | SCC of floor of mouth, G3 | SCC of base of tongue, G2 |
| n | Time after infusion start (hr) | n | Time after infusion start (hr) | n | Time after infusion start (hr) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 10B‐concentration in tumour (ppm) | 18.5 ± 1.5 | 5 | 12.3 | 22.2 ± 4.6 | 2 | 11.8 | (a) 24.4 | 1 | 11.3 |
| (b) 24.4 ± 6.1 | 2 | 11.3 | |||||||
| (c) 29.9 | 1 | 11.7 | |||||||
| (d) 30.4 | 1 | 13.5 | |||||||
| 10B‐concentration ratio of tumour/blood | 0.6 ± 0.05 | 12.3 | 1.2 ± 0.2 | 11.8 | (a) 1.2 | 11.3 | |||
| (b) 1.2 ± 0.3 | 11.3 | ||||||||
| (c) 1.5 | 11.7 | ||||||||
| (d) 1.8 | 13.5 | ||||||||
| 10B‐concentration in muscle (ppm) | nd | 6.9 ± 0.7 | 3 | 12.8 | (a) 8.0 ± 2.7 | 2 | 14.8 | ||
| (b) 5.15 ± 0.3 | 2 | 15.8 | |||||||
| 10B‐concentration ratio of muscle/blood | nd | 0.5 ± 0.05 | 12.8 | (a) 0.4 0.2 | 14.8 | ||||
| (b) 0.4 ± 0.02 | 15.8 | ||||||||
| 10B‐concentration in muscle (ppm) | nd | 6.9 ± 0.7 | 3 | 12.8 | (a) 8.0 ± 2.7 | 2 | 14.8 | ||
| (b) 5.15 ± 0.3 | 2 | 15.8 | |||||||
| 10B‐concentration ratio of muscle/blood | nd | 0.5 ± 0.05 | 12.8 | (a) 0.4 0.2 | 14.8 | ||||
| (b) 0.4 ± 0.02 | 15.8 | ||||||||
| 10B‐concentration in skin (ppm) | nd | 20.1 ± 1.6 | 2 | 12.7 | 18.8 ± 5.6 | 3 | 14.8 | ||
| 10B‐concentration ratio of skin/blood | nd | 1.3 ± 0.1 | 12.7 | 1.4 ± 0.4 | 14.8 | ||||
| 10B‐concentration in fat [ppm] | nd | 14.1 ± 2.3 | 2 | 12.7 | 10.7 ± 0.5 | 2 | 14.8 | ||
| 10B‐concentration ratio of fat/blood | nd | 0.9 ± 0.2 | 12.7 | 0.8 ± 0.04 | 14.8 | ||||
| 10B‐concentration in LN (ppm) | nd | 12.2 ± 0.7 | 2 | 13.0 | 9.7 ± 0.04 | 2 | 15.3 | ||
| 10B‐concentration ratio of LN/blood | nd | 0.8 ± 0.04 | 13.0 | 0.73 ± 0 | 15.3 | ||||
| 10B‐concentration in mucosa (ppm) | 22.4 ± 4.4 | 2 | 12.4 | 18.4 | 1 | 11.9 | nd | ||
| 10B‐concentration ratio of mucosa/blood | 0.7 ± 0.1 | 1.0 | 11.9 | nd |
Patient ident. no., patient registration number; nd, not done; LN, lymph node (not metastatic); SCC, squamous cell carcinoma.
Figure 4.
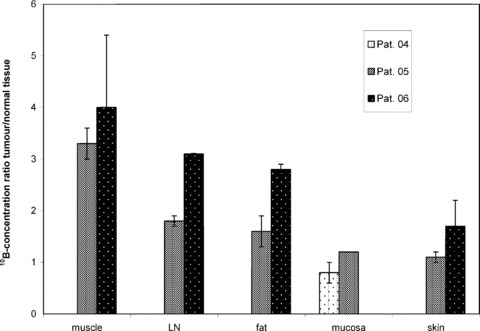
10B‐concentration ratio of tumour/normal organs in three patients after i.v. infusion of 50 mg BSH/kg bw in 1 hr. LN, lymph node.
For both compounds ‘favourable’10B‐concentration ratios of tumour/normal tissues were not reached for all normal tissues; hence, the combination of compounds could not be evaluated.
Analysis of toxicity
All adverse events were clearly related to surgery, tumour or pre‐existing diagnoses. In patients who received BPA, the following adverse events were reported: pain (grade 1, n= 1), restricted movement of the tongue (grade 1, n= 1), headache (grade 1, n= 1), depression (grade 1, n= 1), fever (grade 1, n= 1), coughing (grade 1, n= 1) and fatigue (grade 1, n= 1) (Table S1). One serious adverse event (SAE) occurred: one patient experienced alcohol withdrawal symptoms with a transitory psychotic syndrome. He developed aspiration pneumonia with pulmonary insufficiency but recovered within 6 weeks. Although explicitly asked, this patient neither admitted alcohol abuse nor was his alcohol dependence clinically evident. The complications were clearly unrelated to the BPA infusion. In patients who received BSH, the following adverse events occurred: dysphagia (grade 1, n= 1), swelling of the tongue (grade 1, n= 1), obstruction of the airway (grade 1, n= 1), thrush of the tongue (grade 1, n= 1), infection (grade 1, n= 1) and pain (grade 1, n= 2) (Table S2). One patient experienced an SAE. He was hospitalized 3 weeks after surgery because of an occlusion of a bypass of the left A. femoralis superficialis. The patient was successfully treated by thrombectomy. Because this pre‐existing arterial occlusive disease was known, the SAE was judged to be unrelated to BSH.
Discussion
Trial design
Unlike other radiotherapeutic techniques, the selective damage to tumour cells by BNCT is not achieved by the direct action of the beam but mainly by the neutron capture reactions releasing high‐LET particles only where 10B‐atoms are present. Consequently, a crucial requirement in the advancement of BNCT is the development and testing of boronated compounds. Such developments have been challenged because of the highly complex and interdisciplinary nature of BNCT, which requires expertise in many fields such as neutron physics, (boron‐)chemistry, radiobiology, radio‐oncology, specialized analytical methods [6] and pharmacology, which is usually only available at selected academic institutions. To test the suitability of a compound, its toxicity as well as the 10B‐concentration and 10B‐distribution delivered by the compound under investigation must be assessed. Knowledge of the latter characteristics is also necessary for radiation dose calculation. The presented trial design was based on animal experiments [8, 9, 10] and streamlined procedures to test such compound characteristics early in the development cycle, thus preventing therapeutic failures during costly BNCT trials involving irradiation of patients. The limited drug exposure reduces the risk for patients whilst critical proof‐of‐principle and limited data on pharmacokinetics and distribution can be gained. These data help to guide more elaborated trials based on early clinical information rather than animal models. Similar trial designs were recently allowed by the Exploratory Investigational New Drug guidance [11] by the U.S. Food and Drug Administration for evaluation of targeted anticancer agents and discussed in a focus in Clinical Cancer Research[12, 13].
Such early clinical trials without any therapeutic intent can include only a few patients. Considering this constraint and the high inter‐patient variation, the results of the actual investigation should be confirmed by a trial involving a large number of patients. In spite of the low patient number, the recruiting period was quite long. This was partly caused by the absence of any benefit for the participating patients, which made the enrolment challenging. Moreover, many patients were ineligible because of concomitant diseases caused by alcohol and nicotine, which are the most important risk factors for SCCHN.
Despite strict inclusion criteria, we observed a number of adverse events that were related to concomitant illnesses and/or surgery. Neither BSH nor BPA was toxic at the low doses infused in this trial. However, for BNCT treatment considerably higher compound doses are needed. Although serious compound‐related adverse events were not reported in the clinical trials conducted so far [2, 4, 5, 14, 15], strict monitoring of compound toxicity is necessary in any BNCT trial.
This is the first time that human data on the 10B‐concentration after infusion of BPA and BSH were collected in SCCHN and adjacent normal tissues. Furthermore, for the first time the variance of the 10B‐concentration in human tumours over time was documented in the same subject. Despite the challenge of collecting samples without disturbing the normal course of surgery, surprisingly congruent and conclusive results, as follows, were obtained.
BPA
Cellular uptake of BPA is mediated by the l‐amino acid transport system for neutral amino acids (LAT1) [16]. LAT1 is highly expressed in proliferating tissues and in many human neoplasms, presumably to support their continuous growth and proliferation [17]. This overexpression makes the LAT1 a promising target for tumour diagnosis and treatment [18, 19] and also explains the observed high 10B‐uptake not only in the tumours but also in normal proliferating tissues such as skin and mucosa. A high uptake of BPA in these two tissues was suspected by Busse et al. [4] who observed radiation dermatitis, dysphagia, xerostomia and taste disturbance following BNCT of brain tumours. Kato et al. observed similar side effects after BNCT of SCCHN [20]. Our findings also confirm the hypothesis of Coderre et al. [21] who identified oral mucosa being an organ at risk for BPA‐mediated BNCT in a rat model.
The time course of 10B‐accumulation in the tumour was documented in our study. This followed the 10B‐concentration in blood with a time lag of approximately 1 hr (Fig. 1B). The peak of the tumour/blood 10B‐concentration ratio occurred 2 hrs after the start of a 1 hr infusion, confirming this time point to be optimal for a BNCT treatment when using BPA [4, 15].
In all three patients, the tumour/blood 10B‐concentration ratio (mean 4.0 ± 1.7) appears high in comparison to the published data on glioblastomas (1.8–3.4) [22] and melanomas (1.5–4.5) [15]. Our study supplements the results of BNCT treatment studies [20, 23, 24], which were simultaneously conducted by other groups, with the scientific basis for dose calculation. The observed high tumour/blood 10B‐concentration ratios offer a good explanation of clinical observations, which showed good tumour response after BNCT of SCCHN [23, 24].
Ideally, the 10B‐concentration, which determines the irradiation dose, should be known in tissues and blood during BNCT in order to calculate the applied dose. However, because of the lack of an appropriate method, it is common practice to measure the 10B‐concentration in blood only and to assume a fixed ratio between the 10B‐concentration in blood and tissues. Data on the time course of the tumour/blood 10B‐ratio, which was varied over time in our study (Fig. 1), call into question the assumption that the 10B‐concentration in the tumour can be accurately predicted. Particularly for BPA this observation is of high relevance as the tissue/blood ratio changes considerably during the irradiation. Irradiation typically lasts 0.5–1.5 hrs at a time when the 10B‐concentration in blood drops steeply. In the Finnish SCCHN trial [23], dose calculations were based on data from studies in brain tumours. Although these assumptions were apparently sufficient to perform safe treatments, the authors emphasize the need for biodistribution studies as basis for dose calculation. Our data suggest that in the Finnish studies the dose in the tumour as well as in mucosa was underestimated.
Trials measuring the 10B‐concentration in tissues and blood cannot be replaced by assessments using positron emission tomography (PET). Labelling of BPA with 18F (18F‐fluoro‐L‐BPA) enables the estimation of BPA uptake in vivo[25] and the investigation in individual patients to see if the 18F‐fluoro‐L‐BPA‐concentration in the tumour is higher than in surrounding tissues. This approach to selection of patients for a BNCT treatment however does not allow measurement or prediction of the 10B‐concentration during treatment. The described 1.8‐ to 4.4‐fold [20, 23] accumulation of 18F‐fluoro‐L‐BPA in SCCHN compared to normal tissue could not be reproduced with our measurements of the 10B‐concentrations. Moreover, the voxel sizes for PET are quite big [20, 25], resulting in a limited spatial resolution, which makes discrimination between mucosa, skin or muscle in the head and neck region impossible.
BSH
BSH is assumed to target brain tumours by crossing the pathologically permeable blood–brain barrier (BBB) in the tumour but not the intact BBB. BSH is taken up in brain tumours to a concentration that is equal to or below the concentration in blood, but is not deposited in normal brain. This results in favourable tumour/brain ratios [14]. Indeed, in this trial we did not find a substantial accumulation of 10B delivered by BSH in the tumours in relation to blood. Nevertheless, the 10B‐concentration ratio of tumour/blood was higher than expected (1.2 ± 0.4) and twice as high as reported for glioblastoma (0.6 ± 0.2) [14]. Interestingly, we observed large differences in the 10B‐concentration between various normal tissues and high 10B‐concentration ratios (>2) between tumour/muscle, tumour/fat and tumour/normal lymph nodes. Combined with the observed high 10B‐concentration within the tumour, such ratios are sufficient for effective BNCT. However, as is the case for BPA, the 10B‐concentration ratios of tumour/skin and tumour/oral mucosa were less than 1.5, indicating that these organs are at risk during treatment.
These are the first clinical data showing a promising ratio between 10B‐concentrations in an extracerebral tumour and surrounding normal tissues after BSH infusion. The ratios of skin/blood and muscle/blood in our study were similar to those reported in other clinical [5, 14] and experimental investigations [26].
The uptake mechanism of BSH is not fully understood. The high 10B‐concentration ratios of tumour and some normal tissues cannot be explained by the uptake mechanisms proposed to date [27, 28]. Our data clearly exclude BSH uptake by diffusion [29]. Oxidation of the BSH molecule to B12H11S‐SB12H11 4− (BSSB) and B12H11S‐SOB12H11 4− (BSSOB) might play a role caused by micro milieu changes [30]. Because the 10B‐concentration in blood proved to be as high as in tumours, blood vessels of normal tissues are organs at risk. Trivillin et al.[31] suggest that the antitumour effect of BSH‐mediated BNCT might not be caused exclusively by direct irradiation of tumour cells but in part by irradiation of the tumour vascular endothelium. However, our study design does not allow the addition of new information to this topic.
In contrast to BPA, irradiation in BSH‐mediated BNCT takes place approximately 12 hrs after the infusion [3, 32] when a more stable relationship between the 10B‐concentration in blood and tissues can be assumed. Thus, the backreference from the 10B‐concentration in blood to the 10B‐concentration in the tumour for dose calculations seems to be less problematic than for BPA. Non‐invasive techniques to follow the in vivo fate of BSH are essential. The use of PET is not possible because a radioactively labelled analogue of the BSH molecule could not be synthesized. MRI might offer a solution, which unfortunately is not yet available for clinical use [33].
In summary, this trial is the first to substantiate the potential of BNCT for the treatment of SCCHN with human data on tissue uptake of the compounds BSH and BPA. BPA accumulates 10B in SCCHN to 4‐fold higher concentrations compared with blood. This results in favourable 10B‐concentration ratios of tumour/muscle, tumour/fat and tumour/normal lymph nodes. BSH delivers10B to SCCHN to similar concentrations compared with blood but demonstrated high 10B‐concentration ratios of tumour/muscle, tumour/fat and tumour/lymph nodes. Skin and mucosa are relevant organs at risk for both compounds. However, side effects to these tissues are well known in radiation oncology and not expected to be dose limiting. BNCT might show an advantage in recurrent SCCHN where few treatment options remain and in tumours located in the nasopharynx, because the known low 10B‐concentrations in normal brain promises good protection of this organ. Undoubtedly, our results justify further investigations aimed to develop BNCT as a treatment modality.
Further elucidation of biological mechanisms and the clinical significance of BNCT for SCCHN will come from future research to
-
•
to confirm the observed 10B‐ratios of tumour/blood and tumour/normal tissues at higher compound doses needed for a BNCT‐treatment and to assess the absolute 10B‐concentration that can be reached in SCCHN.
-
•
to substantiate the findings of this trial with statistically significant evidence. Therefore, clinical trials in which patients are treated with BNCT should be supplemented with a biodistribution substudy. Larger trials exclusively aimed at investigating the 10B‐distribution are difficult to justify because patients do not benefit from their participation.
-
•
to investigate whether the co‐administration of BSH and BPA results in optimized tumour/normal tissue 10B‐ratios and in a more homogenous distribution of 10B in the tumour. The definitions in the trial protocol did not permit the infusion of both compounds. Given the differing distribution of both compounds, the hypothesis that both compounds target differing tumour cell subpopulations should be investigated.
Supporting information
Table S1 Adverse events graded according to CTC criteria after BPA‐infusion. Adverse events were not related to the compound BPA but to surgery, tumor or preexisting diagnoses.
Table S2 Adverse events graded according to CTC criteria after BSH‐infusion. Adverse events were not related to the compound BSH but to surgery, tumor or preexisting diagnoses.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Acknowledgements
The work received financial supported from the European Commission contract QLK3‐CT‐1999‐01067. We gratefully acknowledge the support of Martin Stuschke (Department of Radiation Oncology, University Hospital Duisburg‐Essen). We are grateful to Anja Marr (Institute for Medical Informatics, Biometry and Epidemiology, University Duisburg‐Essen) and Alessandra Busato (Data Center, EORTC) who gave excellent support in the data management. We are indebted to Kerstin Heise (Institute for Cell Biology, University Duisburg‐Essen) for storing the tissue samples as well as to Don Grocotte for his help with linguistics.
References
- 1. Winquist E, Oliver T, Gilbert R. Postoperative chemoradiotherapy for advanced squamous cell carcinoma of the head and neck: a systematic review with meta‐analysis. Head Neck. 2007; 1: 38–46. [DOI] [PubMed] [Google Scholar]
- 2. Kageji T, Nagahiro S, Mizobuchi Y, et al . Boron neutron capture therapy using mixed epithermal and thermal neutron beams in patients with malignant glioma‐correlation between radiation dose and radiation injury and clinical outcome. Int J Radiat Oncol Biol Phys. 2006; 65: 1446–55. [DOI] [PubMed] [Google Scholar]
- 3. Sauerwein W, Zurlo A. The EORTC Boron Neutron Capture Therapy (BNCT) Group: Achievements and future projects. Eur J Cancer . 2002; 38: S31–S34. [DOI] [PubMed] [Google Scholar]
- 4. Busse PM, Harling OK, Palmer MR, et al . A critical examination of the results from the Harvard‐MIT NCT program phase I clinical trial of neutron capture therapy for intracranial disease. J Neurooncol. 2003; 62: 111–21. [DOI] [PubMed] [Google Scholar]
- 5. Wittig A, Malago M, Collette L, et al . Uptake of two 10B‐compounds in liver metastases of colorectal adenocarcinoma for extracorporeal irradiation with boron neutron capture therapy (EORTC Trial 11001). Int J Cancer. 2008; 122: 1164–71. [DOI] [PubMed] [Google Scholar]
- 6. Wittig A, Michel J, Moss RL, et al . Boron analysis and boron imaging in biological materials for boron neutron capture therapy (BNCT). Crit Rev Oncol Hematol. 2008; 68: 66–90. [DOI] [PubMed] [Google Scholar]
- 7. Kiger WS III, Palmer MR, Riley KJ, et al . Pharmacokinetic modeling for boronophenylalanine‐fructose mediated neutron capture therapy: 10B concentration predictions and dosimetric consequences. J Neurooncol. 2003; 62: 171–86. [DOI] [PubMed] [Google Scholar]
- 8. Wittig A, Arlinghaus HF, Kriegeskotte C, et al . Laser‐SNMS in tissue: a powerful tool for elemental and molecular imaging in the development of targeted drugs. Mol Cancer Ther. 2008; 7: 1763–71. [DOI] [PubMed] [Google Scholar]
- 9. Obayashi S, Kato I, Ono K, et al . Delivery of (10)boron to oral squamous cell carcinoma using boronophenylalanine and borocaptate sodium for boron neutron capture therapy. Oral Oncol. 2004; 40: 474–82. [DOI] [PubMed] [Google Scholar]
- 10. Kamida A, Obayashi S, Kato I, et al . Effects of boron neutron capture therapy on human oral squamous cell carcinoma in a nude mouse model. Int J Radiat Biol. 2006; 82: 21–29. [DOI] [PubMed] [Google Scholar]
- 11. Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates Nat Rev Drug Discov. 2004; 3: 711–5. [DOI] [PubMed] [Google Scholar]
- 12. Doroshow JH, Parchment RE. Oncologic phase 0 trials incorporating clinical pharmacodynamics: From concept to patient. Clin Cancer Res. 2008; 14: 3658–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Murgo AJ, Kummar S, Rubinstein L, et al . Designing phase 0 cancer clinical trials. Clin Cancer Res. 2008; 14: 3675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hideghéty K, Sauerwein W, Wittig A, et al . Tissue uptake of BSH in patients with glioblastoma in the EORTC 11961 phase I BNCT trial. J Neurooncol. 2003; 62: 145–56. [DOI] [PubMed] [Google Scholar]
- 15. Liberman SJ, Dagrosa A, Jimenez Rebagliati RA, et al . Biodistribution studies of boronophenylalanine‐fructose in melanoma and brain tumor patients in Argentina. Appl Radiat Isot. 2004; 61: 1095–100. [DOI] [PubMed] [Google Scholar]
- 16. Wittig A, Sauerwein WA, Coderre JA. Mechanisms of transport of p‐borono‐phenylalanine through the cell membrane in vitro . Radiat Res. 2000; 153: 173–80. [DOI] [PubMed] [Google Scholar]
- 17. Yanagida O, Kanai Y, Chairoungdua A, et al . Human L‐type amino acid transporter 1 (LAT1): characterization of function and expression in tumor cell lines. Biochim Biophys Acta. 2001; 1514: 291–302. [DOI] [PubMed] [Google Scholar]
- 18. Haase C, Bergmann R, Fuechtner F, et al . L‐type amino acid transporters LAT1 and LAT4 in cancer: uptake of 3‐O‐methyl‐6–18F‐fluoro‐L‐DOPA in human adenocarcinoma and squamous cell carcinoma in vitro and in vivo. J Nucl Med. 2007; 48: 2063–71. [DOI] [PubMed] [Google Scholar]
- 19. Kaira K, Oriuchi N, Imai H, et al . L‐type amino acid transporter 1 and CD98 expression in primary and metastatic sites of human neoplasms. Cancer Sci. 2008; 99: 2380–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kato I, Ono K, Sakurai Y, et al . Effectiveness of BNCT for recurrent head and neck malignancies. Appl Radiat Isot. 2004; 61: 1069–73. [DOI] [PubMed] [Google Scholar]
- 21. Coderre JA, Morris GM, Kalef Ezra J, et al . The effects of boron neutron capture irradiation on oral mucosa: evaluation using a rat tongue model. Radiat Res. 1999; 152: 113–8. [PubMed] [Google Scholar]
- 22. Elowitz EH, Bergland RM, Coderre JA, et al . Biodistribution of p‐boronophenylalanine in patients with glioblastoma multiforme for use in boron neutron capture therapy. Neurosurgery. 1998; 42: 463–9. [DOI] [PubMed] [Google Scholar]
- 23. Kankaanranta L, Seppala T, Koivunoro H, et al . Boron neutron capture therapy in the treatment of locally recurred head and neck cancer. Int J Radiat Oncol Biol Phys. 2007; 69: 475–82. [DOI] [PubMed] [Google Scholar]
- 24. Ariyoshi Y, Miyatake S, Kimura Y, et al . Boron neuron capture therapy using epithermal neutrons for recurrent cancer in the oral cavity and cervical lymph node metastasis. Oncol Rep. 2007; 18: 861–6. [PubMed] [Google Scholar]
- 25. Imahori Y, Ueda S, Ohmori Y, et al . Positron emission tomography‐based boron neutron capture therapy using boronophenylalanine for high‐grade gliomas: Part II. Clin Cancer Res. 1998; 4: 1833–41. [PubMed] [Google Scholar]
- 26. Bendel P, Frantz A, Zilberstein J, et al . Boron‐11 NMR of borocaptate: relaxation and in vivo detection in melanoma‐bearing mice. Magn Reson Med. 1998; 39: 439–47. [DOI] [PubMed] [Google Scholar]
- 27. Kageji T, Otersen B, Gabel D, et al . Interaction of mercaptoundecahydrododecaborate (BSH) with phosphatidylcholine: Relevance to boron neutron capture therapy. Biochim Biophys Acta. 1998; 1391: 377–83. [DOI] [PubMed] [Google Scholar]
- 28. Yoshida F, Matsumura A, Yamamoto T, et al . Enhancement of sodium borocaptate (BSH) uptake by tumor cells induced by glutathione depletion and its radiobiological effect. Cancer Lett. 2004; 215: 61–67. [DOI] [PubMed] [Google Scholar]
- 29. Heber EM, Trivillin VA, Nigg DW, et al . Homogeneous boron targeting of heterogeneous tumors for boron neutron capture therapy (BNCT): chemical analyses in the hamster cheek pouch oral cancer model. Arch Oral Biol. 2006; 51: 922–9. [DOI] [PubMed] [Google Scholar]
- 30. Pettersson OA, Carlsson J, Grusell E. Accumulation of 10B in the central degenerative areas of human glioma and colon carcinoma spheroids after sulfhydryl boron hydride administration. Cancer Res. 1992; 52: 1587–91. [PubMed] [Google Scholar]
- 31. Trivillin VA, Heber EM, Nigg DW, et al . Therapeutic success of boron neutron capture therapy (BNCT) mediated by a chemically non‐selective boron agent in an experimental model of oral cancer: A new paradigm in BNCT radiobiology. Radiat Res. 2006; 166: 387–96. [DOI] [PubMed] [Google Scholar]
- 32. Kageji T, Nagahiro S, Kitamura K, et al . Optimal timing of neutron irradiation for boron neutron capture therapy after intravenous infusion of sodium borocaptate in patients with glioblastoma. Int J Radiat Oncol Biol Phys. 2001; 51: 120–30. [DOI] [PubMed] [Google Scholar]
- 33. Bendel P, Sauerwein W. Optimal detection of the neutron capture therapy agent borocaptate sodium (BSH): a comparison between 1H and 10B NMR. Med Phys. 2001; 28: 178–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Adverse events graded according to CTC criteria after BPA‐infusion. Adverse events were not related to the compound BPA but to surgery, tumor or preexisting diagnoses.
Table S2 Adverse events graded according to CTC criteria after BSH‐infusion. Adverse events were not related to the compound BSH but to surgery, tumor or preexisting diagnoses.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item


