Abstract
To reveal the functional intrinsic niche of human embryonic stem cells (hESC) we examined the production of basement membrane (BM) proteins and the presence of their receptors in feeder‐free cell culture conditions. In addition, we investigated binding of hESCs to purified human BM proteins and identified the receptors mediating these contacts. Also, we tested whether purified human laminin (Lm) isoforms have a role in hESC self‐renewal and growth in short‐term cultures. The results show that hESCs synthesize Lm α1 and Lm α5 chains together with Lm β1 and γ1 chains suggesting the production of Lms‐111 and ‐511 into the culture medium and deposits on cells. hESCs contain functionally important integrin (Int) subunits, Int β1, α3, α6, α5, β5 and αV, as well as the Lm α5 receptor, Lutheran (Lu) glycoprotein and its truncated form, basal cell adhesion molecule (B‐CAM). In cell adhesion experiments, Int β1 was crucial for adhesion to most of the purified human BM proteins. Lu/B‐CAM mediated adhesion to Lm‐511 together with Int α3β1, and was essential for the adhesion of hESCs to embryonic feeder cells. Adhesion to Lm‐411 was mediated by Int α6β1. Lm‐511 supported hESC growth in defined medium equally well as Matrigel. These results provide consequential information of the biological role of BM in hESCs, warranting further investigation of BM biology of human pluripotent stem cells.
Keywords: human embryonic stem cell, extracellular matrix, basement membrane, laminin, integrin, B‐CAM/Lutheran, adhesion, defined cultures
Introduction
Basement membranes (BMs) are thin sheets of specialized extracellular matrix (ECM), underlying all epithelia, and surrounding muscle, endothelial, fat and Schwann cells as well as the whole central nervous system. The function of BMs is to provide structural support, divide tissues into compartments and also to regulate cellular functions. The major structural components of BMs are sheets of laminins (Lm) and type IV collagens, which are bridged by nidogen into a functional BM structure. Out of the various BM components, Lms have been shown to be the most important regulators of cellular functions [1, 2, 3]. Lms are trimeric glycoproteins composed of α, β and γ chain [3]. Among 15 recognized Lms, embryonic Lm‐111 (α1β1γ1) and Lm‐511 (α5β1γ1) are crucial during early mouse embryogenesis for the survival and development of embryonic epithelial cells [4]. Null mutations in Lm γ1 or Lm β1 chains are lethal at E5.5 due to non‐functional embryonic BM and Reichert’s membrane [5, 6]. Also, mouse embryos lacking Lm α1 die at E7.5 due to the absence of functional Reichert’s membrane [7].
Integrins are heterodimeric cell membrane receptors, consisting of non‐covalently linked α and β subunits. They mediate cell–cell and cell–ECM interactions by adhering cells to BM proteins. Mouse embryos constitutively express Int αVβ3, α6β1 and α5β1 at two‐cell stage, and integrins α3β1, α7β1, α6β1, α2β1 and α1β1 in a regulated manner during further development [4, 8]. Human embryos express Int αV subunit throughout the early embryonic development, at least until compaction [9]. Embryonic epithelial cells have been shown to express the dystroglycan glycoprotein complex, which can act as a receptor for Lm‐111 [4, 10, 11]. Besides integrins and dystroglycan complex, Lutheran glycoprotein (Lu/B‐CAM) acts as a receptor for α5‐chain containing Lms [12, 13].
Feeder cell‐free culturing of human embryonic stem cells (hESC) typically requires an ECM coating, such as Matrigel, and supplementation with basic fibroblast growth factor (bFGF) [14, 15]. A recent study indicated that bFGF might have an indirect effect on hESC cultures via hESC‐derived fibroblast‐like cells [16]. Beside other functions, FGFs modify the ECM production of cells [17]. Stem cells are located in a specific niche composed of soluble factors and the physical interactions between stem cells and the surrounding BM proteins [18, 19]. Because of the origin of hESCs, their in vivo niche is difficult to explore and there is little information available on the synthesis of BM proteins by hESCs. In order to better understand the natural ECM of hESCs, we have studied the Lm synthesis by hESCs and the interactions between hESCs and purified human BM proteins.
Materials and methods
hESC culture
The studies presented here were performed on Finnish hESC lines FES 29 and FES 30 [20]. The cells were regularly analysed for the expression of pluripotency markers (tumour recognition antigen [Tra] 1–60 and stage‐ specific embryonic antigen [SSEA]3) by flow cytometry and found to be positive (85% to 97%). The hESCs were cultured on mouse embryonic fibroblasts (mEF, isolated from day 12.5 ICR foetuses) in KnockOut Dulbecco’s Modified Eagle’s Medium (DMEM) (Invitrogen, Carlsbad, CA, USA), supplemented with 20% KnockOut serum replacement, 0.1 mM 2‐mercaptoethanol, 1× penicillin‐streptomycin‐L‐glutamine, 1× nonessential amino acids (all from Invitrogen), 1× insulin transferrin selenium (ITS) liquid media supplement (Sigma, St. Louis, MO, USA) and 6 ng/ml bFGF (Sigma). hESCs were passaged by trypsin‐like enzyme (TrypLE Select, Invitrogen). Before carrying out the experiments presented here the hESCs were adapted to feeder‐free conditions using mEF‐conditioned medium supplemented with 12 ng/ml bFGF and Matrigel (BD Biosciences, Bedford, UK) coating as described previously [14]. The feeder‐free cultures were passaged every 5 days by collagenase IV (4–5 min., 20 U/ml [Invitrogen]). Lms‐111 [21] and ‐511 [22] were purified by immunoaffinity chromatography from the culture supernatant of Jar cells. Vitronectin was purified as described [23]. Defined cultures were performed using purified human Lm‐111 (8 μg/ml), Lm‐511 (3.5 μg/ml) or vitronectin (4 μg/ml) as matrix and StemPro (Invitrogen) as culture medium using mechanical passaging.
Metabolic labelling and immunoprecipitation of culture medium, matrix and cells
hESCs were cultured in Roswell Park Memorial Institute medium (RPMI) (Sigma) without methionine, serum or serum replacement and supplemented with ITS, non‐essential amino acids, L‐Glutamine and bFGF (see above) for 30 min. After methionine starvation, 100 μCi of 35S‐labelled methionine (Perkin‐Elmer, Waltkam, MA, USA) was added to the medium and incubated overnight. To identify Lms secreted to the culture medium the medium was first collected and centrifuged. Then the medium was supplemented with normal mouse serum and with 0.5% Triton X‐100. The medium was pre‐absorbed with GammaBindPlus Sepharose (GBPS) beads (GE Healthcare‐Biosciences, Uppsala, Sweden), followed by centrifugation and absorbtion with GBPS beads pre‐coupled with monoclonal antibodies (MAb) against human Lm α1, α2, α3, α4 and α5 (Table 1).
Table 1.
Antibodies used in the study
| MAb clone | Specificity | Reference | Methods |
|---|---|---|---|
| 161EB7 | Lm α1 chain | Virtanen et al. [21] | IP |
| 5H2 | Lm α2 chain | Leivo and Engvall [48] | ICC |
| BM‐2 | Lm α3 chain | Rousselle et al. [49] | IP |
| 3H2 | Lm α4 chain | Wondimu et al. [50] | IP |
| 4C7 | Lm α5 chain | Engvall et al. [51] | IP |
| 114DG10 | Lm β1 chain | Virtanen et al. [52] | IP |
| S5F11 | Lm β2 chain | Wewer et al. [53] | ICC |
| 113BC7 | Lm γ1 chain | Määttäet al. [54] | IP |
| TS2/7 | Int α1 subunit | Hemler et al. [55] | ICC, FC |
| CLB‐10G11 | Int α2 subunit | Giltay et al. [56] | ICC, FC |
| J143 | Int α3 subunit | Fradet et al. [57] | ICC, IP, FC |
| PIB5 | Int α3 subunit | Wayner and Carter [58] | FB |
| PUJI4 | Int α4 subunit | Hemler et al. [59] | ICC, FC |
| B1E5 | Int α5 subunit | Werb et al. [60] | ICC, IP, FC |
| GoH3 | Int α6 subunit | Chemicon, Temecula, CA | ICC, IP, FC, FB |
| LM 142.69 | Int αv subunit | Cheresh and Spiro [61] | ICC, FC |
| 13 | Int β1 subunit | Yamada et al. [62] | FB |
| 102DF5 | Int β1 subunit | Ylänne and Virtanen [63] | ICC, IP, FC |
| 90B10 | Int β2 subunit | Ylänne et al. [64] | ICC |
| AA3 | Int β4 subunit | Tamura et al. [65] | ICC |
| B5–1A9 | Int β5 subunit | Pasqualini et al. [66] | ICC |
| BRIC221 | Lutheran | Serotec, Oxford, England | ICC |
| Tra 1–60 | Tra 1–60 | Chemicon, Temecula, CA | ICC, FC |
| MC631 | SSEA‐3 | Solter and Knowles [67] Peter Andrews, Sheffield, UK | FC |
IP; immunoprecipitation, ICC; immunocytochemistry, FC; flow cytometry, FB; function blocking.
Labelled cells were lysed on ice in radioimmunoprecipitation assay (RIPA) buffer containing 10 mM Tris‐HCl, pH 7.2, 150 mM NaCl, 0.1% SDS, 1.0% Triton X‐100, 1% deoxycholate, 5 mM EDTA and protease inhibitor mixture. The cell sample was cleared by centrifugation. For ECM isolation, the cells were extracted with 0.5% deoxycholate in 10 mM Tris‐HCl, 150 mM NaCl, 1 mM protease inhibitor, pH 8.0. The remaining material was solubilized in RIPA buffer. The cell samples were supplemented with normal mouse or rat serum, and ECM specimens with normal mouse serum. The samples from cells were absorbed to GBPS beads pre‐coupled with MAb against Int α3, α5, α6 and β1 subunits (Table 1) and ECM specimens with MAbs to Lm chains as aforementioned.
After overnight immunoprecipitation, bound proteins from cell and matrix samples were first washed three times in RIPA buffer and bound proteins from medium were washed in phosphate buffered saline (PBS) with Triton X‐100. Then the proteins were eluted to Laemmli’s sample buffer and analysed by SDS‐PAGE. After immunoprecipitation the samples from hESC culture medium were exposed to Chondroitinase ABC treatment (0.02U chondroitinase ABC [Sigma] in 50 mM Tris‐HCl, pH 8.0, 50 mM sodium acetate) at +37°C for 8 hrs and thereafter treated as other samples. The following cell lines were used as positive controls: Jar [21] for Lm‐111 and Lm‐511, HPAE [24] or Lm‐411, and UT‐SCC‐43A [25] for Lm‐332. The antibody was omitted in negative controls.
Flow cytometry
hESCs were collected by trypsin‐EDTA (Invitrogen) treatment (4–5 min.), after which the cells were washed, counted and suspended in FACS staining buffer (5% foetal calf serum [Promocell, Heidelberg, Germany] in PBS). The cells were suspended in 50 μl of primary MAb solution (Table 1) and incubated on ice for 45 min. After three washes, the hESCs were incubated for 30 min. on ice with 2 μl of conjugated secondary antibodies, in the dark, washed and fixed with 0.1% paraformaldehyde in FACS staining buffer. Secondary conjugates were phycoerythrin‐coupled anti‐rat IgM for SSEA‐3 (BD Biosciences, San Jose, CA, USA), fluorescein‐isothiocyanate (FITC)‐coupled antimouse IgG or FITC‐coupled anti‐rat IgG (Invitrogen). The primary antibody was omitted in negative controls. Samples were run by FACSCalibur (BD Biosciences) using CellQuestPro software (BD Biosciences) and analysed by Flow Jo (Tree Star Inc., Ashland, OR, USA) software.
Immunocytochemistry
For indirect immunofluorescence the cells were cultured on glass cover slips and fixed in methanol at –20°C. After washing in PBS the cells were incubated with primary antibodies listed in Table 1. FITC‐coupled antimouse IgG (Fc‐fragment specific [Jackson Immunoresearch, West Grove, PA, USA]) was used as the secondary antibody, followed by MAb Tra1–60 and finally, after washing, the cells were incubated with goat anti‐mouse AlexaFluor 594 IgM (μ chain specific [Invitrogen]). The specimens were embedded in veronal glycerol buffer (1:1) (pH 8.4) and viewed with an AX70 Provis fluorescence microscope (Olympus, Tokyo, Japan). Images were acquired using a computer connected to a cooled digital camera mounted on the microscope.
RT‐PCR and quantitative PCR (qPCR) analysis
Total RNA was extracted using NucleoSpin RNA II kit (Macherey‐Nagel, Düren, Germany) without on‐column DNase treatment. DNase treatment was done separately as we have noticed that separate DNase treatment is more complete than on‐column treatment. After the DNase treatment, RNA was cleaned by using NucleoSpin RNA Clean‐up kit (Macherey‐Nagel).
Total RNA was reverse‐transcribed into cDNA by M‐MLV Reverse Transcriptase in RT reaction containing Oligo(dT)15 primers, the mix of all four dNTPs, and rRNasin (all from Promega, Madison, WI, USA). The cDNA amount was determined as the synthesized cDNA in a 20 μl RT reaction containing 1 μg total RNA. PCR was performed in a total volume of 25 μl containing 2.5 μl 10× PCR buffer with 15 mM MgCl2, 0.2 μl AmpliTaqGold 5U/μl (Applied Biosystems, Foster City, CA, USA), 2 μl 2.5 mM dNTPs mix, 1 μl of 10 μM mix of forward and reverse primers, 2 μl 50% dimethylsulfoxide (DMSO) and 2 μl RT reaction as a cDNA template. Polymerase was activated at 95°C for 7 min., followed by 36 cycles of 95°C, 20 sec.; 56.5°C, 30 sec.; 72°C, 30 sec., and the final extension at 72°C for 7 min. PCR products were analysed in 2.1% agarose gels. Primers for human Lu, B‐CAM, and cyclophilin G were described earlier [26]. The PCR product for human Lu (B‐CAM variant 1) is 115 bp, for human B‐CAM (variant 2) 109 bp and for human cyclophilin G (CG), 126bp.
For real‐time SYBR (a nucleic acid gel stain) Green qPCR, total RNA was reverse transcribed as described above. Each multiplication reaction, run in duplicate, contained 2 μl 10× PCR buffer (Applied Biosystems), 2 μl MgCl2 25 mM stock, 1.6 μl dNuTPs mix 2.5 mM each (Promega), 1.6 μl DMSO 50% stock, 5 μl mix of F/R primers (both 1.4 μM in mix), 1 μl RT reaction as a cDNA template, 0.16 μl AmpliTaq Gold DNA polymerase 5 U/μL (Applied Biosystems), 0.8 μl SYBR Green (1/500 dilution from stock SYBR Green I nucleic acid gel stain, 10,000× concentrate in DMSO [Invitrogen]), and DEPC H2O ad 20 μl. The reactions for the qPCR were prepared with a Corbett CAS‐1200 liquid handling system and the qPCR was performed using Corbett Rotor‐Gene 6000 (Corbett Life Science, Sydney, Australia) as follows: enzyme activation step at 95°C for 7 min. following 40 cycles of 95°C, 20 sec.; 56°C, 20 sec.; 72°C, 20 sec., followed by a melting step. Data were analysed according to the comparative Ct method (ΔΔCt) (Applied Biosystems, User Bulletin #2). Control samples were hESCs cultured on Matrigel in StemPro medium. The value ‘1’ represents the control in each qPCR result curve.
Primers used for qPCR:
-
1
Oct4 (F/R): 5′‐TTGGGCTCGAGAAGGATGTG‐3′/5′‐TCCTCTCGTTGTGCATAGTCG‐3′
-
2
(NM_00271; pos. 856–946; 91bp)
-
3
Sox2 (F/R): 5′‐GCCCTGCAGTACAACTCCAT‐3′/5′‐TGCCCTGCTGCGAGTAGGA‐3′
-
4
(NM_003106; pos. 1037–1121; 85 bp)
-
5
Goosecoid (GSC) (F/R): 5′‐GAGAACCTCTTCCAGGAGAC‐3′/
-
6
5′‐TTCTTAAACCAGACCTCCAC‐3′ (NM_173849; pos. 673–776; 104 bp)
-
7
Brachyury (Bra) (F/R): 5′‐CGCATGATCACCAGCCACTG‐3′/
-
8
5′‐TTTAAGAGCTGTGATCTCCTCG‐3′ (NM_003181; pos. 1031–1114; 84 bp)
-
9
Cyclophilin G (CG) (F/R): 5′‐CAATGGCCAACAGAGGGAAG‐3′/
-
10
5′‐CCAAAAACAACATGATGCCCA‐3′ (NM_004792; pos. 552–645; 94 bp)
Quantitative cell adhesion experiments
Fibronectin (Fn) was purified from outdated human plasma (Finnish Red Cross Blood Service, Helsinki, Finland) by Gelatin Sepharose 4B affinity chromatography (GE Healthcare‐Biosciences), according to Engvall and Ruoslahti [27]. Recombinant human Lm‐411 was produced in a mammalian expression system [28]. Lms‐111 [21] and ‐511 [22] were purified by immunoaffinity chromatography from the culture supernatant of Jar cells. Soluble recombinant Lutheran (Sol‐Lu) corresponding to the extracellular domain of Lu/B‐CAM was produced as described [12]. Briefly, adhesion experiments were performed using a method based on intracellular acid phosphatase assay, as described [29, 30]. Wells of 96‐well plates were first coated with distinct ECM proteins (4 μg/ml) at RT for 1 hr and then with 3% bovine serum albumin at RT for 1 hr. Alternatively, a monolayer of mEFs was plated 1 day before performing the experiments. When indicated, Sol‐Lu (10 μg/ml) was incubated at RT for 1 hr. Trypsin‐EDTA (Invitrogen) treated hESCs were washed by trypsin neutralizing solution (Promocell). 2 × 104 cells per pre‐coated well were plated with or without function blocking (FB) MAbs (Table 1) in hESC medium without serum replacement. After 2 hrs incubation in standard culture conditions at +37°C the wells were washed three times and the attached hESCs were exposed to phosphatase substrate (104 phosphatase substrate [Sigma], 6 mg/ml in 50 mM sodium acetate buffer with 0.1% Triton X‐100, pH 5.0). The reaction was stopped with 1M NaOH and absorbances were measured at 405 nm. Unspecific binding and background were measured by using either 3% bovine serum albumin treated wells or wells containing only mEFs. The experiments were performed in triplicate. Results were statistically analysed with two‐tailed Student’s t‐test.
Live‐cell imaging for the determination of cell growth rate
hESCs were passaged mechanically from Matrigel to Lm‐111, Lm‐511, Vitronectin, Matrigel, or Gelatin‐ (Sigma) coated 48‐wells in StemPro‐medium and let to adhere in a cell culture incubator for 24 hrs. Then, the culture medium was changed and the culture plate was transferred to a Cell‐IQ Cell Culturing Platform (ChipManTechnologies, Tampere, Finland). Selected positions of the wells were imaged every hour for 24 hrs. Then, the images were analysed for colony size and presence of dead cells, using protocols developed by ChipManTechnologies. Results are presented as relative changes in cell surface area in three replicate culture wells.
Results
hESCs synthesize and deposit laminins‐111 and ‐511
First we studied which Lm chains are synthesized by undifferentiated hESCs. By using metabolic labelling and MAbs specific for human Lm chains, we found that hESCs produce a limited set of Lm subunits, regardless of the culture conditions. Of the analysed Lm α chains (α1–5), only Lm α1 and α5 could be precipitated from the culture medium (Fig. 1a and b; Table 2). Also, Lm β1 and γ1 chains were precipitated from the medium. To ascertain the functionality of the Lm chains, we also isolated the ECM produced by hESCs. These experiments showed that the hESCs deposited Lm α1, α5, β1 and γ1 chains into the matrix (Fig. 1a and b; Table 2). By contrast, neither Lm α3 nor Lm α4 chains were detected in the hESC culture medium supernatant (Fig. 1c and d; Table 2) or in the hESC‐ECM material (not shown).
Figure 1.
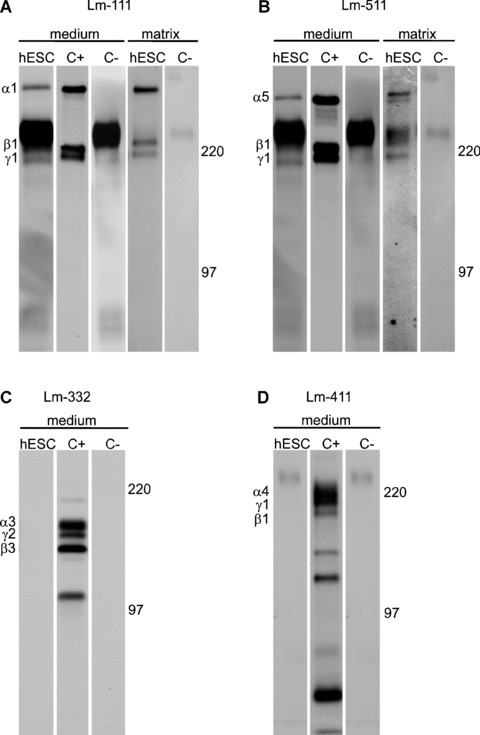
Production of laminin (Lm) chains by hESCs. hESCs were metabolically labelled with radioactive methionine followed by the immunoprecipitation of Lm chains. (a) and (b) hESCs synthesized and secreted chains of Lm‐111 (α1β1γ1) and Lm‐511 (α5β1γ1) into culture medium and matrix. (c) and (d) hESCs did not produce Lm‐332 or Lm‐411. C+; positive control, C–; negative control without antibody.
Table 2.
Examined laminin chains and their synthesis in hESCs
| Laminin chain | Method | Medium | Matrix |
|---|---|---|---|
| Lm α1 | IP, ICC | + | + |
| Lm α2 | ICC | − | − |
| Lm α3 | IP | − | − |
| Lm α4 | IP, ICC | − | − |
| Lm α5 | IP, ICC | + | + |
| Lm β1 | IP, ICC | + | + |
| Lm β2 | ICC | − | − |
| Lm γ1 | IP, ICC | + | + |
IP; immunoprecipitation, ICC; immunocytochemistry.
Undifferentiated hESCs contain integrin receptors for laminins
Flow cytometry, immunocytochemistry and immunoprecipitation analysis were carried out to find out which integrin subunits were present on hESCs (Fig. 2a and b; Table 3). Immunocytochemistry analysis was performed together with the pluripotency marker Tra1–60 to confirm the quality of the cells. Int β1 subunit was highly expressed, as 99% of the hESCs contained the antigen with high intensity (Fig. 2a) together with the pluripotency marker Tra1–60 (Fig. 2b). Lm binding integrin subunits Int α3 (95%) and α6 (98%), were also highly present on hESCs (Fig. 2a and b), while α1 and α2 subunits, specifically binding collagen, could not be found (Fig. 2a). Int αV (Fig. 2a and b) and β5 (Fig. 2b) subunits were strongly present in prominent point adhesion sites of hESCs (as suggested by lack of talin and vinculin, not shown), but not elsewhere in the cells. Also, Int α5 (90%) was present in most of the hESCs (Fig. 2a and b) while immunoreactivity for Int α2, α4, β3 or β4 subunits could not be found (not shown). Immunoprecipitation by MAbs against human integrin subunits confirmed that the hESCs contained Int β1, α3, α5 and α6 subunits produced by themselves (Fig. 2b). The results were highly similar in two different hESC lines in all tested culture conditions. Pluripotency markers Tra1–60 and SSEA3 were expressed (from 80% to 95%) by both hESC lines throughout the study (not shown).
Figure 2.
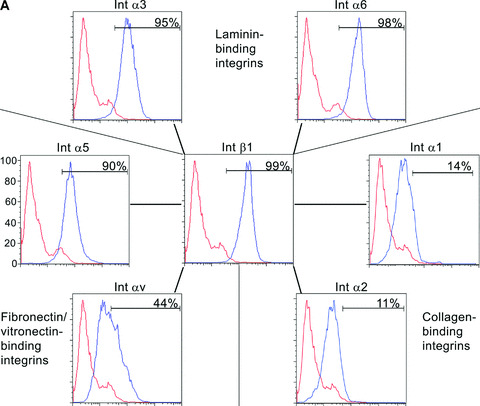
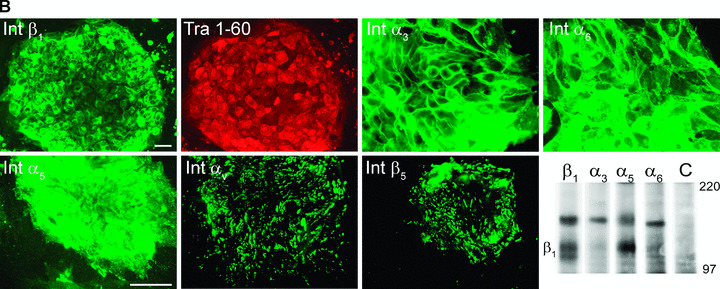
hESCs contain laminin (Lm‐), fibronectin‐ and vitronectin‐binding integrins. (a) Flow cytometry analysis showed that hESCs contained Int β1, α3, α6, α5 and αV subunits. Int β1 subunit can bind different Lms by pairing with Int α3 and α6 subunits, fibronectin with Int α5 subunit, and vitronectin with Int αV subunit. Functions of fibronectin and vitronectin receptors may overlap. hESCs did not contain collagen binding Integrins α1 and α2. (b) hESCs were double‐stained by monoclonal antibodies against integrin subunits and Tra1–60 antigen. Tra1–60‐positive hESCs showed immunoreactivity for Int β1, α3, α6 and α5 subunits in a cell surface‐confined manner (Tra1–60 shown only for Int β1). Int αV and β5 subunits were seen as a pattern resembling point adhesions. Scale bar, 50 μm. Immunoprecipitation confirmed production of Int β1, α3, α5 and α6 subunits.
Table 3.
Examined integrin subunits and their presence in hESCs
| Integrin subunit | Method | Localization |
|---|---|---|
| Int α1 | ICC, FC | ‐ |
| Int α2 | ICC, FC | ‐ |
| Int α3 | ICC, FC, IP | Cell surface |
| Int α4 | ICC, FC | ‐ |
| Int α5 | ICC, FC, IP | Cell surface |
| Int α6 | ICC, FC, IP | Cell surface |
| Int αv | ICC, FC | Point adhesions |
| Int β1 | ICC, FC, IP | Cell surface |
| Int β3 | ICC | ‐ |
| Int β4 | ICC | ‐ |
| Int β5 | ICC | Point adhesions |
IP; immunoprecipitation, ICC; immunocytochemistry, FC; flow cytometry.
Undifferentiated hESCs contain Lutheran glycoprotein
MAb against human Lu/B‐CAM uniformly stained undifferentiated hESC colonies with high intensity, together with Tra1–60 (Fig. 3a). Lu and B‐CAM arise from a single mRNA via alternative splicing and differ in the length of their cytoplasmic tail. Since the antibody against Lu/B‐CAM recognizes both variants of the proteins, we analysed the expression of these splice variants in hESCs by RT‐PCR. Both forms of the gene, B‐CAM variant 1(Lu) and B‐CAM variant 2(B‐CAM), were expressed in hESCs (Fig. 3b).
Figure 3.
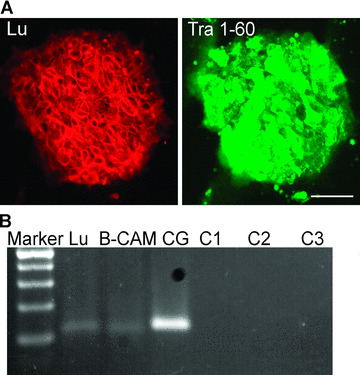
Undifferentiated hESCs contain a known Lm α5 receptor, Lutheran/B‐CAM. (a) Antibody against Lutheran/B‐CAM intensively stained Tra1–60‐positive hESCs. Scale bar, 50 μm. (b) Both mRNA variants of the same gene, 115 bp for Lutheran and 109 bp for B‐CAM, were expressed by hESCs. Negative controls; C1 for Lutheran, C2 for B‐CAM, C3 for Cyclophilin G.
hESCs adhere to laminin‐511 through lutheran/B‐CAM and Int α3β1
Next, we performed functional adhesion experiments to quantify hESC adhesion to distinct ECM and BM proteins in conditions devoid of serum and serum replacement. The experiments were performed with hESCs cultured in feeder‐free conditions. While these simplified conditions do not represent true culture conditions, our aim was to decipher interactions between the hESCs and the BM proteins at the receptor level. Based on findings performed by flow cytometry, immunoprecipitation and immunocytochemistry, we selected function blocking MAbs for certain highly expressed integrin receptor subunits to find out their functional properties in the adhesion of hESCs. The cells adhered well to Matrigel and fibronectin, both of which are known to function as potent cell adhesion substrata (Fig. 4a). Lu/B‐CAM receptor mediates adhesion to Lm α5 chain in many epithelial and endothelial cells [31]. Hence, we quantified adhesion of hESCs to Lm‐511 in presence of either function‐blocking MAb against Int β1 subunit alone, or function‐blocking MAb against Int α3 subunit alone. In addition, the adhesion was measured in presence of recombinant, soluble Lutheran (Sol‐Lu) alone, or Sol‐Lu together with function‐blocking MAb against Int α3 subunit. hESCs adhered strongly to purified human Lm‐511 (Fig. 4b). MAbs against Int α3β1 and Sol‐Lu inhibited hESC adhesion, thus suggesting that Lutheran mediates their adhesion to Lm‐511 in cooperation with Int α3β1 (P < 0.001) (Fig. 4b). Then, we quantified the adhesion of hESCs to mEFs either in presence of MAb against Int β1 or Sol‐Lu. Surprisingly, the adhesion to mEFs was not blocked by MAb against Int β1, but with sol‐Lu (Fig. 4c).
Figure 4.
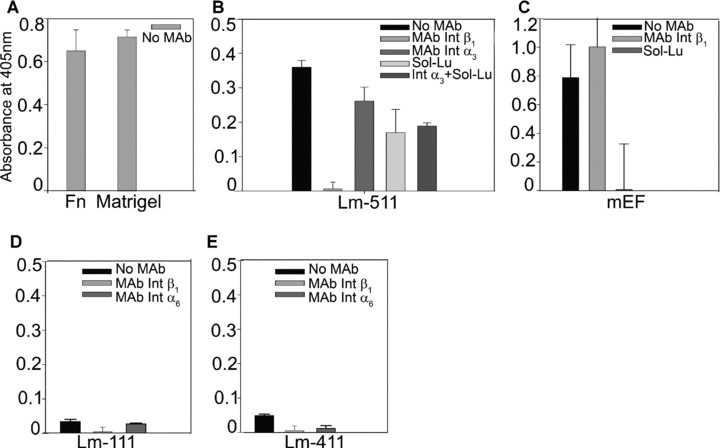
hESC adhesion to BM proteins was measured by quantitative adhesion experiments. Monoclonal antibodies (MAb) directed against various receptors, or recombinant Lutheran (Sol‐Lu) were used to determine functional receptors in hESCs. (a) hESCs adhered potently to positive control substrata, Matrigel and fibronectin. (b) Prominent hESC adhesion to purified human Lm‐511 was mediated via Int β1, α3 and Sol‐Lu (P < 0.001). (c) Sol‐Lu, but not MAb against Int β1, totally blocked adhesion to mEFs. (d) and (e) Adhesion to Lm‐111 or to Lm‐411 was poor. MAb against Int β1 prevented (P < 0.005) adhesion to Lm‐111. MAbs against Int β1 and α6 blocked adhesion to Lm‐411 (P < 0.001; P < 0.005).
Adhesion to purified human Lm‐111 was relatively weak and it was completely blocked by function‐blocking MAb against Int β1 subunit (P < 0.005) (Fig. 4d). Function‐blocking MAb against Int α6 tended to reduce the adhesion to Lm‐111, suggesting that Int α6β1 may be involved in the adhesion (Fig. 4d). However, this effect was not statistically significant. Figure 4e shows minor hESC adhesion to Lm‐411, which was completely blocked by function‐blocking MAb against either Int β1 (P < 0.001) or Int α6 (P < 0.005), suggesting that adhesion is mediated via Int α6β1. The adhesion of hESCs to purified human fibronectin and Matrigel was also significantly reduced by the Int β1 antibody (results not shown). The results were similar when the hESCs were cultured on mEFs.
BM proteins differ in their ability to maintain hESC self‐renewal
We measured hESC proliferation on Lm‐111, Lm‐511 or vitronectin in defined culture conditions. Cell growth was measured by changes in the hESC colony surface area in function of time with simultaneous monitoring of cellular morphology. The proliferation rates detected on hESCs growing on Matrigel or purified human Lm‐511 were highly comparable (Fig. 5a). Also vitronectin could support the growth, whereas proliferation was weak on purified human Lm‐111 and no proliferation was detected on gelatin (Fig. 5a). We then cultured hESCs for 5 passages on either Lm‐111, Lm‐511, or on heparin‐binding form of vitronectin (all purified from human sources) in StemPro culture medium. Typical undifferentiated morphology persisted on Lm‐511 (Fig. 5b, II and V) and on vitronectin (Fig. 5b, III and VI). However, distinct differentiation events occurred on Lm‐111 (arrows in Fig. 5b, I and IV). In order to further verify the self‐renewal capacity of the hESCs under the defined culture conditions we quantified gene expression levels of pluripotency marker genes Sox2 and Oct4, and early differentiation marker genes, Brachyury (Bra) and Goosecoid (GSC). Matrigel with StemPro was used as the reference culture condition, representing the value ‘1’ in each qPCR panel (Fig. 5c). The expression levels of Sox2 and Oct4 remained relatively stable in all conditions (Fig. 5c). However, the levels of Bra and GSC decreased during culture on Lm‐511 or vitronectin. In contrast, hESCs tended to differentiate when cultured on Lm‐111, as shown by increased expression of both Bra and GSC (Fig. 5c).
Figure 5.
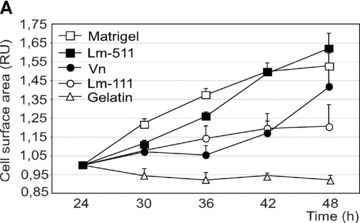
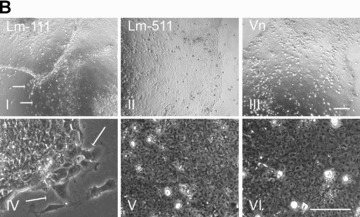
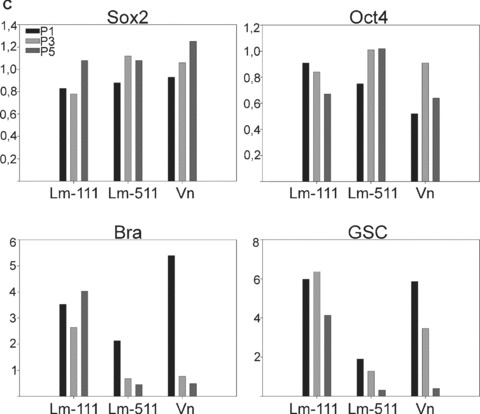
Individual laminin isoforms have distinct effects on the hESC self‐renewal. (a) Proliferation was measured in a continuous live‐cell imaging system. The area of hESC colonies in relative units is shown in function of time (hours). Results are presented as the mean ± S.E.M. for three culture wells. The growth rate was highest on Matrigel and on purified human Lm‐511. In contrast, proliferation on Lm‐111 was low. (b) hESCs cultured on purified human Lm‐111 (I and IV), Lm‐511 (II and V) or Vn (III and VI) in defined medium, StemPro, at passage 5. hESCs tended to differentiate on human Lm‐111 (I, IV; arrows) whereas typical characteristics of undifferentiated cells were present on Lm‐511 and on Vn. Scale bars, 100 μm. (c) qPCR analysis of hESCs cultured on BM proteins in StemPro, at passage 1, 3 and 5. The relative expression levels of the pluripotency marker genes, Sox2 and Oct4 sustained, while the expression of the differentiation markers GSC and Bra decreased during culture on Lm‐511 or Vn. In contrast, differentiation occurred when the hESCs were cultured on Lm‐111, as shown by the presence of GSC and Bra and a decreasing level of Oct4. Data are presented as the mean expression level relative to cells grown on Matrigel, determined in duplicate.
Discussion
Stem cell niches composed of neighbouring cells, ECM proteins, and soluble factors have been recognized crucial for regulating tissue specific stem cell self‐renewal and differentiation [32, 33, 34] and it has been proposed that ECM has an important regulatory role also for the hESC niche [35, 36]. In this study, we have analysed the production of Lm chains and the ECM and BM receptors by hESCs and further investigated the functional role of these receptors in adhesion to purified human BM proteins.
We show that Lm isoforms differ in their ability to support hESC proliferation and/or maintain their undifferentiated state, even in short‐term culture.
Our results show that hESCs produce Lm α1, α5, β1 and, γ1 chains and that those chains are also deposited as Lms‐111 and ‐511 into hESC‐produced ECM. According to our flow cytometry, immunocytochemistry and immunoprecipitation analyses the undifferentiated hESCs selectively contain Lm binding integrin β1, α3 and α6 subunits. In contrast, α1, α2 and α4 integrin subunits involved in collagen and/or Lm binding could not be detected in hESCs (not shown). Functional adhesion experiments confirmed that hESCs do adhere both to Lm‐111 and Lm‐511 utilizing their specific integrin receptors. In short‐term culture experiments especially Lm‐511 seemed to support both self‐renewal and proliferation of hESCs. The early differentiation markers, Brachyury and Goosecoid, were declined during cultures on Lm‐511 further strengthening its role as a biologically relevant BM component for undifferentiated hESCs. Supporting this, it has recently been shown that Lm‐511 supports the self‐renewal of mouse ES cells [37]. In contrast, Lm‐111 as such did not support growth or self‐renewal of the undifferentiated hESCs, as the same differentiation markers were constantly present when the hESCs were cultured on Lm‐111. Whether Lm‐111 has a role alone or in combination with other BM components in the maintenance or early differentiation of hESCs remains to be elucidated.
In previous studies, hESCs have been shown to have characteristics typical for epithelial cells [38, 39, 40]. Specifically, cells covering hESC colonies have a polarized structure and contain epithelial proteins, e.g. E‐cadherin. Of the studied BM proteins, Lm‐111 and Lm‐511 are, besides embryonic, also typical epithelial Lms. The same Lm chains have been shown to be essential for the formation of the embryonic BM and survival of the mouse embryo [6, 7].
It has also been suggested for mice that if the embryonic BM is not correctly formed, ICM cells are not able to polarize towards embryonic epiblastic cells, normally occurring prior to gastrulation [41, 42]. Thereby, it is reasonable that hESCs, as epithelial epiblast‐like cells, produce and deposit embryonic Lm chains. Taken together, we suggest that Lm‐111, and especially Lm‐511, are functionally important Lms for hESCs.
Majority of the hESCs showed intensive staining for Int α5, αV and β5 subunits that in conjunction with Int β1 are known to form a set of functional fibronectin and vitronectin receptors. The functionality of these integrins was also confirmed by adhesion and short term culture assays. Our results are in good agreement with previous studies, showing that both fibronectin and vitronectin can support hESCs in long‐term cultures [15, 43]. There are only few studies about the expression of integrins or Lms in early human embryos, the closest in vivo equivalents to hESC. In blastocyst/implantation stage of human embryos, a mainly trophectodermal expression of Int β1, β5 and α6 subunit proteins has been detected [44]. The human blastocyst also produces the Int αV subunit [9]. There is no evidence of the role of integrins in the commitment or maintenance of the ICM or trophectodermal cells and no data are available about the epiblastic/hypoblastic differentiation in the human embryo. An analysis of integrin expression of hESCs has recently appeared [43]. The only major difference between the results of Braam et al. and our study is that we did not detect immunoreactivity for Int α2.
Low Int α2 level has also been reported based on gene expression data [45]. The integrin β1 subunit is known to have a central role in various mammalian cell types and it was also found to be involved in interactions between hESCs and all the explored BM/ECM components in this study. The use of MAb blocking Int β1 function prevented the adhesion to BM proteins nearly totally. Blocking of Int α6 subunit function with MAb totally prevented hESC adhesion to Lm‐411. The result is in line with the results that Int α6β1 functions as a receptor for Lm‐111 in mouse embryonic and human epithelial cells [4, 21] as well as for Lm‐411 in human endothelial cells [24].
Interestingly, hESCs showed a high content of Lutheran/ B‐CAM (Lu/B‐CAM) glycoprotein on their surface. Lu/B‐CAM is a transmembrane receptor of the immunoglobulin superfamily and it is known to mediate cell adhesion to Lms‐511 and ‐521 independently or in concert with integrins [31, 46]. Our results show that independently of culture conditions hESC adhesion to Lm‐511 is mediated via cooperation of Lu/B‐CAM and Int α3β1. Importantly, Sol‐Lu also blocked hESC adhesion to mEFs in adhesion experiments. This is consistent with our unpublished observation that mEFs produce large amounts of Lm‐511, the target of Lu/B‐CAM. Little is known about the role of Lu/B‐CAM in early development. However, mice lacking Lutheran are viable, without severe defects [47]. The exact role of Lu/B‐CAM in hESC biology and culture adaptation remains to be examined.
Acknowledgements
We acknowledge Pipsa Kaipainen, Marja‐Leena Piironen, Cia Olsson, Eija Lönn and Emmi Hurskainen for skilled technical assistance and Dr. Samer Hussein for helpful discussions. Professors Peter Andrews, Manuel Patarroyo, Lloy J. Old, David Cheresh, Martin E. Hemler, Eva Engvall, Ulla Wever, Zena Werb and Kenneth M. Yamada are acknowledged for MAbs. This work was supported by funding for the ESTOOLS consortium under the Sixth Research Framework Program of the European Union, the Finnish Diabetes Research Foundation, the Sigrid Jusélius Foundation and the
Research Funds of the Helsinki University Hospital.
References
- 1. Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer . 2003; 3: 422–33. [DOI] [PubMed] [Google Scholar]
- 2. Scheele S, Nystrom A, Durbeej M, et al . Laminin isoforms in development and disease. J Mol Med . 2007; 85: 825–36. [DOI] [PubMed] [Google Scholar]
- 3. Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Ann Rev Cell Dev Biol . 2004; 20: 255–84. [DOI] [PubMed] [Google Scholar]
- 4. Ekblom P. Receptors for laminins during epithelial morphogenesis. Curr Opin Cell Biol . 1996; 8: 700–6. [DOI] [PubMed] [Google Scholar]
- 5. Gustafsson E, Fassler R. Insights into extracellular matrix functions from mutant mouse models. Exp Cell Res . 2000; 261: 52–68. [DOI] [PubMed] [Google Scholar]
- 6. Smyth N, Vatansever HS, Murray P, et al . Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J Cell Biol . 1999; 144: 151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miner JH, Li C, Mudd JL, et al . Compositional and structural requirements for laminin and basement membranes during mouse embryo implantation and gastrulation. Development . 2004; 131: 2247–56. [DOI] [PubMed] [Google Scholar]
- 8. Damsky C, Sutherland A, Fisher S. Extracellular matrix 5: adhesive interactions in early mammalian embryogenesis, implantation, and placentation. FASEB J . 1993; 7: 1320–9. [DOI] [PubMed] [Google Scholar]
- 9. Dubey AK, Cruz JR, Hartog B, et al . Expression of the alpha v integrin adhesion molecule during development of preimplantation human embryos. Fertil Steril . 2001; 76: 153–6. [DOI] [PubMed] [Google Scholar]
- 10. Cooper HM, Tamura RN, Quaranta V. The major laminin receptor of mouse embryonic stem cells is a novel isoform of the alpha 6 beta 1 integrin. J Cell Biol . 1991; 115: 843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Durbeej M, Larsson E, Ibraghimov‐Beskrovnaya O, et al . Non‐muscle alpha‐dystroglycan is involved in epithelial development. J Cell Biol . 1995; 130: 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kikkawa Y, Moulson CL, Virtanen I, et al . Identification of the binding site for the Lutheran blood group glycoprotein on laminin alpha 5 through expression of chimeric laminin chains in vivo. J Biol Chem . 2002; 277: 44864–9. [DOI] [PubMed] [Google Scholar]
- 13. Vainionpaa N, Kikkawa Y, Lounatmaa K, et al . Laminin‐10 and Lutheran blood group glycoproteins in adhesion of human endothelial cells. Am J Physiol . 2006; 290: C764–75. [DOI] [PubMed] [Google Scholar]
- 14. Xu C, Inokuma MS, Denham J, et al . Feeder‐free growth of undifferentiated human embryonic stem cells. Nat Biotechnol . 2001; 19: 971–4. [DOI] [PubMed] [Google Scholar]
- 15. Ludwig TE, Levenstein ME, Jones JM, et al . Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol . 2006; 24: 185–7. [DOI] [PubMed] [Google Scholar]
- 16. Bendall SC, Stewart MH, Menendez P, et al . IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature . 2007; 448: 1015–21. [DOI] [PubMed] [Google Scholar]
- 17. Li X, Chen Y, Scheele S, et al . Fibroblast growth factor signaling and basement membrane assembly are connected during epithelial morphogenesis of the embryoid body. J Cell Biol . 2001; 153: 811–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tanentzapf G, Devenport D, Godt D, et al . Integrin‐dependent anchoring of a stem‐cell niche. Nat Cell Biol . 2007; 9: 1413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. da Silva Meirelles L, Caplan AI, Nardi NB. In Search of the in vivo Identity of Mesenchymal Stem Cells. Stem Cells . 2008; 26: 2287–99. [DOI] [PubMed] [Google Scholar]
- 20. Mikkola M, Olsson C, Palgi J, et al . Distinct differentiation characteristics of individual human embryonic stem cell lines. BMC Dev Biol . 2006; 6: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Virtanen I, Gullberg D, Rissanen J, et al . Laminin alpha1‐chain shows a restricted distribution in epithelial basement membranes of fetal and adult human tissues. Exp Cell Res . 2000; 257: 298–309. [DOI] [PubMed] [Google Scholar]
- 22. Fujiwara H, Kikkawa Y, Sanzen N, et al . Purification and characterization of human laminin‐8. Laminin‐8 stimulates cell adhesion and migration through alpha3beta1 and alpha6beta1 integrins. J Biol Chem . 2001; 276: 17550–8. [DOI] [PubMed] [Google Scholar]
- 23. Ylanne J, Cheresh DA, Virtanen I. Localization of beta 1, beta 3, alpha 5, alpha v, and alpha IIb subunits of the integrin family in spreading human erythroleukemia cells. Blood . 1990; 76: 570–7. [PubMed] [Google Scholar]
- 24. Petajaniemi N, Korhonen M, Kortesmaa J, et al . Localization of laminin alpha4‐chain in developing and adult human tissues. J Histochem Cytochem . 2002; 50: 1113–30. [DOI] [PubMed] [Google Scholar]
- 25. Takkunen M, Grenman R, Hukkanen M, et al . Snail‐dependent and ‐independent epithelial‐mesenchymal transition in oral squamous carcinoma cells. J Histochem Cytochem . 2006; 54: 1263–75. [DOI] [PubMed] [Google Scholar]
- 26. Virtanen I, Banerjee M, Palgi J, et al . Blood vessels of human islets of Langerhans are surrounded by a double basement membrane. Diabetologia . 2008; 51: 1181–91. [DOI] [PubMed] [Google Scholar]
- 27. Engvall E, Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer . 1977; 20: 1–5. [DOI] [PubMed] [Google Scholar]
- 28. Kortesmaa J, Doi M, Patarroyo M, et al . Chondroitin sulphate modification in the alpha4 chain of human recombinant laminin‐8 (alpha4beta1gamma1). Matrix Biol . 2002; 21: 483–6. [DOI] [PubMed] [Google Scholar]
- 29. Prater CA, Plotkin J, Jaye D, et al . The properdin‐like type I repeats of human thrombospondin contain a cell attachment site. J Cell Biol . 1991; 112: 1031–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takkunen M, Ainola M, Vainionpaa N, et al . Epithelial‐mesenchymal transition downregulates laminin alpha5 chain and upregulates laminin alpha4 chain in oral squamous carcinoma cells. Histochem Cell Biol . 2008; 130: 509–25. [DOI] [PubMed] [Google Scholar]
- 31. Kikkawa Y, Miner JH. Review: Lutheran/B‐CAM: a laminin receptor on red blood cells and in various tissues. Connect Tissue Res . 2005; 46: 193–9. [DOI] [PubMed] [Google Scholar]
- 32. Scadden DT. The stem‐cell niche as an entity of action. Nature . 2006; 441: 1075–9. [DOI] [PubMed] [Google Scholar]
- 33. Xie T, Spradling AC. A niche maintaining germ line stem cells in the Drosophila ovary. Science . 2000; 290: 328–30. [DOI] [PubMed] [Google Scholar]
- 34. Conover JC, Notti RQ. The neural stem cell niche. Cell Tissue Res . 2008; 331: 211–24. [DOI] [PubMed] [Google Scholar]
- 35. Lensch MW, Daheron L, Schlaeger TM. Pluripotent stem cells and their niches. Stem Cell Rev . 2006; 2: 185–201. [DOI] [PubMed] [Google Scholar]
- 36. Bendall SC, Stewart MH, Bhatia M. Human embryonic stem cells: lessons from stem cell niches in vivo. Regen Med . 2008; 3: 365–76. [DOI] [PubMed] [Google Scholar]
- 37. Domogatskaya A, Rodin S, Boutaud A, et al . Laminin‐511, but not ‐332, ‐111 or ‐411 enables mouse embryonic stem cell self‐renewal in vitro. Stem Cells . 2008; 26: 2800–9. [DOI] [PubMed] [Google Scholar]
- 38. Ginis I, Luo Y, Miura T, et al . Differences between human and mouse embryonic stem cells. Dev Biol . 2004; 269: 360–80. [DOI] [PubMed] [Google Scholar]
- 39. Ullmann U, In’t Veld P, Gilles C, et al . Epithelial‐mesenchymal transition process in human embryonic stem cells cultured in feeder‐free conditions. Mol Hum Reprod . 2007; 13: 21–32. [DOI] [PubMed] [Google Scholar]
- 40. Van Hoof D, Braam SR, Dormeyers W, et al . Feeder‐free monolayer cultures of human embryonic stem cells express an epithelial plasma membrane protein profile. Stem Cells . 2008; 26: 2777–81. [DOI] [PubMed] [Google Scholar]
- 41. Li S, Edgar D, Fassler R, et al . The role of laminin in embryonic cell polarization and tissue organization. Dev Dell . 2003; 4: 613–24. [DOI] [PubMed] [Google Scholar]
- 42. Li S, Harrison D, Carbonetto S, et al . Matrix assembly, regulation, and survival functions of laminin and its receptors in embryonic stem cell differentiation. J Cell Biol . 2002; 157: 1279–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Braam SR, Zeinstra L, Litjens S, et al . Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self renewal via {alpha}V{beta}5 integrin. Stem Cells . 2008; 26: 2257–65. [DOI] [PubMed] [Google Scholar]
- 44. Bloor DJ, Metcalfe AD, Rutherford A, et al . Expression of cell adhesion molecules during human preimplantation embryo development. Mol Hum Reprod . 2002; 8: 237–45. [DOI] [PubMed] [Google Scholar]
- 45. Miyazaki T, Futaki S, Hasegawa K, et al . Recombinant human laminin isoforms can support the undifferentiated growth of human embryonic stem cells. Biochem Biophys Res Commun . 2008; 375: 27–32. [DOI] [PubMed] [Google Scholar]
- 46. Kikkawa Y, Sasaki T, Nguyen MT, et al . The LG1–3 tandem of laminin alpha5 harbors the binding sites of Lutheran/ basal cell adhesion molecule and alpha3beta1/alpha6beta1 integrins. J Biol Chem . 2007; 282: 14853–60. [DOI] [PubMed] [Google Scholar]
- 47. Rahuel C, Filipe A, Ritie L, et al . Genetic inactivation of the laminin alpha5 chain receptor Lu/BCAM leads to kidney and intestinal abnormalities in the mouse. Am J Physiol Renal Physiol . 2008; 294: F393–406. [DOI] [PubMed] [Google Scholar]
- 48. Leivo I, Engvall E. Merosin, a protein specific for basement membrane of Schwann cells, striated muscle, and trophoblast, is expressed late in nerve and muscle development. Proc Natl Acad Sci USA . 1988; 85 1544–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rousselle P, Lunstrum GP, Keene DR, et al . Kalinin: an epithelium‐specific basement membrane adhesion molecule that is a component of anchoring filaments. J Cell Biol . 1991; 114: 567–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wondimu Z, Geberhiwot T, Ingerpuu S, et al . An endothelial laminin isoform, laminin 8 (alpha4beta1gamma1), is secreted by blood neutrophils, promotes neutrophil migration and extravasation, and protects neutrophils from apoptosis. Blood . 2004; 104: 1859–66. [DOI] [PubMed] [Google Scholar]
- 51. Engvall E, Earwicker D, Haaparanta T, et al . Distribution and isolation of four laminin variants; tissue restricted distribution of heterotrimers assembled from five different subunits. Cell Regul . 1990; 1: 731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Virtanen I, Lohi J, Tani T, et al . Distinct changes in the laminin composition of basement membranes in human seminiferous tubules during development and degeneration. Am J Pathol . 1997; 150: 1421–31. [PMC free article] [PubMed] [Google Scholar]
- 53. Wewer UM, Thornell LE, Loechel F, et al . Extrasynaptic location of laminin beta 2 chain in developing and adult human skeletal muscle. Am J Pathol . 1997; 151: 621–31. [PMC free article] [PubMed] [Google Scholar]
- 54. Maatta M, Virtanen I, Burgeson R, et al . Comparative analysis of the distribution of laminin chains in the basement membranes in some malignant epithelial tumors: the alpha1 chain of laminin shows a selected expression pattern in human carcinomas. J Histochem Cytochem . 2001; 49: 711–26. [DOI] [PubMed] [Google Scholar]
- 55. Hemler ME, Brenner MB, McLean JM, et al . Antigenic stimulation regulates the level of expression of interleukin 2 receptor on human T cells. Proc Natl Acad Sci USA . 1984; 81: 2172–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Giltay JC, Brinkman HJ, Modderman PW, et al . Human vascular endothelial cells express a membrane protein complex immunochemically indistinguishable from the platelet VLA‐2 (glycoprotein Ia‐IIa) complex. Blood . 1989; 73: 1235–41. [PubMed] [Google Scholar]
- 57. Fradet Y, Cordon‐Cardo C, Thomson T, et al . Cell surface antigens of human bladder cancer defined by mouse monoclonal antibodies. Proc Natl Acad Sci USA . 1984; 81: 224–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wayner EA, Carter WG. Identification of multiple cell adhesion receptors for collagen and fibronectin in human fibrosarcoma cells possessing unique alpha and common beta subunits. J Cell Biol . 1987; 105: 1873–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hemler ME, Sanchez‐Madrid F, Flotte TJ, et al . Glycoproteins of 210,000 and 130,000 m.w. on activated T cells: cell distribution and antigenic relation to components on resting cells and T cell lines. J Immunol . 1984; 132: 3011–8. [PubMed] [Google Scholar]
- 60. Werb Z, Tremble PM, Behrendtsen O, et al . Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol . 1989; 109: 877–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cheresh DA, Spiro RC. Biosynthetic and functional properties of an Arg‐Gly‐Asp‐directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen, and von Willebrand factor. J Biol Chem . 1987; 262: 17703–11. [PubMed] [Google Scholar]
- 62. Yamada KM, Kennedy DW, Yamada SS, et al . Monoclonal antibody and synthetic peptide inhibitors of human tumor cell migration. Cancer Res . 1990; 50: 4485–96. [PubMed] [Google Scholar]
- 63. Ylanne J, Virtanen I. The Mr 140,000 fibronectin receptor complex in normal and virus‐transformed human fibroblasts and in fibrosarcoma cells: identical localization and function. Int J Cancer . 1989; 43: 1126–36. [DOI] [PubMed] [Google Scholar]
- 64. Ylanne J, Hormia M, Jarvinen M, et al . Platelet glycoprotein IIb/IIIa complex in cultured cells. Localization in focal adhesion sites in spreading HEL cells. Blood . 1988; 72: 1478–86. [PubMed] [Google Scholar]
- 65. Tamura RN, Rozzo C, Starr L, et al . Epithelial integrin alpha 6 beta 4: complete primary structure of alpha 6 and variant forms of beta 4. J Cell Biol . 1990; 111: 1593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pasqualini R, Bodorova J, Ye S, et al . A study of the structure, function and distribution of beta 5 integrins using novel anti‐beta 5 monoclonal antibodies. J Cell Sci . 1993; 105: 101–11. [DOI] [PubMed] [Google Scholar]
- 67. Solter D, Knowles BB. Developmental stage‐specific antigens during mouse embryogenesis. Curr Top Dev Biol . 1979; 13: 139–65. [DOI] [PubMed] [Google Scholar]


