Abstract
Background
Bone defects may occur because of severe trauma, nonunion, infection, or tumor resection. However, treatments for bone defects are often difficult and have not been fully established yet. We previously designed an efficient system of topical cutaneous application of carbon dioxide (CO2) using a novel hydrogel, which facilitates CO2 absorption through the skin into the deep area within a limb. In this study, the effect of topical cutaneous application of CO2 on bone healing was investigated using a rat femoral defect model.
Methods
In this basic research study, an in vivo bone defect model, fixed with an external fixator, was created using a rat femur. The affected limb was shaved, and CO2 was applied for 20 min/day, 5 days/week. In the control animals, CO2 gas was replaced with air. Radiographic, histological, biomechanical, and genetic assessments were performed to evaluate bone healing.
Results
Radiographically, bone healing rate was significantly higher in the CO2 group than in the control group at 4 weeks (18.2% vs. 72.7%). The degree of bone healing scored using the histopathological Allen grading system was significantly higher in the CO2 group than in the control group at 2 weeks (1.389 ± 0.334 vs. 1.944 ± 0.375). The ultimate stress, extrinsic stiffness, and failure energy were significantly greater in the CO2 group than in the control group at 4 weeks (3.2 ± 0.8% vs. 38.1 ± 4.8%, 0.6 ± 0.3% vs. 41.5 ± 12.2%, 2.6 ± 0.8% vs. 24.7 ± 5.9%, respectively.). The volumetric bone mineral density of the callus in micro-computed tomography analysis was significantly higher in the CO2 group than in the control group at 4 weeks (180.9 ± 43.0 mg/cm3 vs. 247.9 ± 49.9 mg/cm3). Gene expression of vascular endothelial growth factor in the CO2 group was significantly greater than that in the control group at 3 weeks (0.617 ± 0.240 vs. 2.213 ± 0.387).
Conclusions
Topical cutaneous application of CO2 accelerated bone healing in a rat femoral defect model. CO2 application can be a novel and useful therapy for accelerating bone healing in bone defects; further research on its efficacy in humans is warranted.
Keywords: CO2, Bone healing, Bone defect
Background
Bone defects can occur during and after treatments for bone tumor, osteomyelitis, and severe trauma, and they often lead to significant problems in the clinical setting. Treatments for these bone defects usually require external fixation procedures or microsurgical techniques, including ascension of a corticotomized bone fragment with an Ilizarov fixator [1], a free vascularized fibular flap [2, 3], an osteomyocutaneous flap [4], and Masquelet’s two-stage technique [5]. However, these clinically applicable therapies for bone defects are cumbersome and time-consuming, and treatment options are limited. Hence, basic and clinical research on bone healing that could lead to an improvement in current treatments for bone defects are urgently required.
These days, topical cutaneous application of CO2 is drawing attention in various fields, such as health, medical care, beauty, and sports [6–13]. These therapeutic effects are caused by an increase in the blood flow, microcirculation, and nitric-oxide-dependent neocapillary formation, as well as by an increase in the oxygen partial pressure in the local area known as the Bohr effect [10–14]. However, the detailed mechanism of these effects is still unknown.
We previously designed a novel system of topical cutaneous CO2 application by means of 100% CO2 gas and a hydrogel, which can facilitate CO2 absorption through the skin into the deep area within a limb [15, 16]. This easy and noninvasive system increased blood flow and oxygen dissociation from hemoglobin in the soft tissues surrounding a bone by way of an artificial Bohr effect [15]. Moreover, it was demonstrated that CO2 application significantly increased the gene expression of vascular endothelial growth factor (VEGF) in the rat muscle [16]. The effect of CO2 application in accelerating fracture repair via promoting angiogenesis, blood flow, and endochondral ossification was also demonstrated in a rat femoral fracture model [17]. A similar effect to that seen in a rat fracture model can be expected to be effective for bone defects as well. However, to date, the effect of topical cutaneous application of CO2 on bone defects has not been studied yet.
Therefore, in this study, we hypothesized that topical cutaneous application of CO2 accelerates bone healing and investigated the effect of CO2 application on bone healing using a rat femoral defect model.
Methods
Animal model
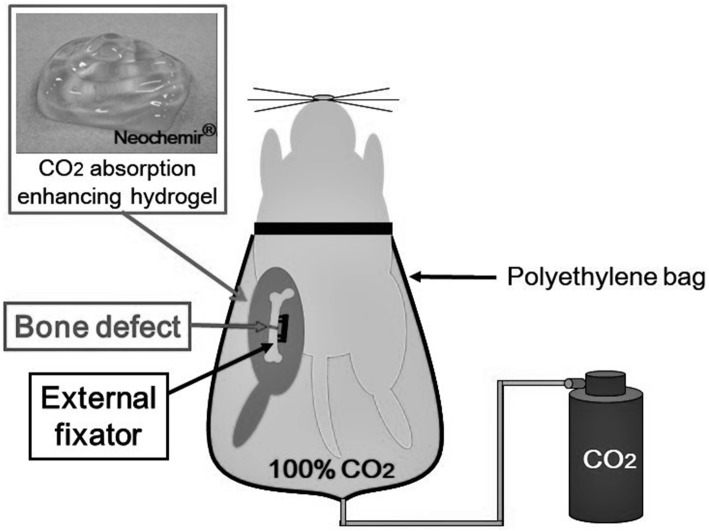
A total of 92 male Fischer 344 rats (Japan SLC, Hamamatsu, Japan) were used, with approval from the institutional ethical committee of our institute. Surgeries were performed on all animals at age 12 weeks under general anesthesia with isoflurane and pentobarbital. After exposing the femoral shaft, two pairs of tip-threaded 1.4-mm-diameter K-wires were placed in the proximal and distal femur. A bone defect of 1 mm was created between the proximal and distal pins by an oscillating saw, and a custom-made external fixator was attached to connect the pairs of pins (Fig. 1). Euthanasia was performed by intraperitoneal administration of a pentobarbital overdose before assessment.
Fig. 1.
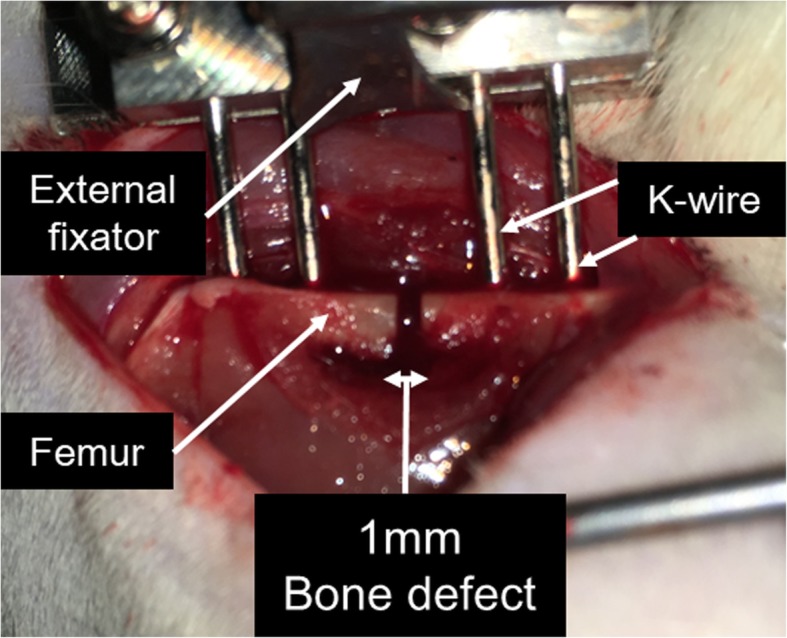
Representative intraoperative image. After exposing the femoral shaft, two pairs of tip-threaded 1.4-mm-diameter K-wires were placed in the proximal and distal femur. A bone defect of 1 mm was created between the proximal and distal pins by an oscillating saw, and a custom-made external fixator was attached to connect the pairs of pins
Topical cutaneous application of CO2
Rats were assigned into two groups: CO2 group (n = 46) and control group (n = 46). After sedation using a minimum dose of isoflurane, the hair of the affected limb was shaved and CO2 absorption enhancing hydrogel (NeoChemir Inc., Kobe, Japan) was applied. The hydrogel had a pH of 5.5 and consisted of carbomer, glycerin, sodium hydroxide, sodium alginate, sodium dihydrogen phosphate, methylparaben, and deionized water. In the CO2 group, the affected limb was sealed in a polyethylene bag filled with 100% CO2 for 20 min a day (Fig. 2). This treatment was performed 5 days a week. In the control group, the affected limb, coated in the same way with CO2 absorption enhancing hydrogel, was sealed in a polyethylene bag filled with air.
Fig. 2.
An illustration of the method of CO2 application in a rat femoral defect model. The affected limb was sealed in a polyethylene bag filled with 100% CO2 for 20 min a day
Radiographic assessment of bone healing
At 1, 2, 3, and 4 weeks after surgery, 58 animals (29 in the CO2 group and 29 in the control group) were anesthetized and fixed in the supine position with the hip joints fully abducted, and radiographs of the defect site were acquired. Radiographically, each callus on the two cortices on the lateral images were evaluated by three orthopedic surgeons blinded to the groups. Bone healing was defined as the absence of a bony gap or the presence of a bridging callus at both the anterior and posterior cortices.
Histological assessment for bone healing
At 2 and 4 weeks after surgery, the femur was harvested from 6 animals in each group, and the tissues were fixed in 4% paraformaldehyde, decalcified with ethylenediaminetetraacetic acid (EDTA), and embedded in paraffin wax. Sagittal sections were cut to a thickness of 5 μm and stained with Safranin-O/Fast Green for histological assessment of the bone defect area. The degree of bone healing was assessed with Allen’s grading system (grades 0 through 4), which was originally designed for histological evaluation of fracture healing [18]. Specimens were examined by three blinded examiners.
Biomechanical assessment of bone healing
Five femurs at 4 weeks after surgery were used for biomechanical evaluation. After euthanasia, the femur was dissected, and the muscle surrounding the defect site was removed. A standardized three-point bending test was performed with a load torsion and bending tester (MZ-500D, MZ-500S, Maruto Instrument Co., Ltd., Tokyo, Japan) for rats in both groups. The ultimate stress (N), extrinsic stiffness (N/mm), and failure energy (N.mm) were assessed by outsourcing to Kureha Special Laboratory, Co., Ltd. (Tokyo, Japan).
Micro-computed tomography (μ-CT) measurement of bone healing
At 4 weeks after surgery, the femur with bone defect was harvested, and the external fixator and pins were removed from the bone. For quantification of bone regeneration, μ-CT imaging analysis was performed. The femurs were scanned and evaluated using a μ-CT scanner (R_mCT2, RIGAKU, Tokyo, Japan). The region of interest (ROI) was set as 3 mm proximal and distal from the midline of the defect site on sagittal view. Tissue mineral density (TMD), total callus volume (TV), bone mineral content (BMC), and volumetric bone mineral density (vBMD; BMC/TV) of the callus were evaluated by TRI/3-D-BON (Ratoc System Engineering, Tokyo, Japan).
Assessment of gene expression
At 1, 2, and 3 weeks after surgery, gene expression was measured in 6 animals in each group by real-time polymerase chain reaction (PCR). Newly generated callus tissue was harvested. Total RNA was extracted from the tissue with a RNeasy Mini Kit (Qiagen, Valencia, California) and reverse-transcribed into single-stranded DNA with a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, California). Real-time PCR was performed in duplicate on the cDNA with an ABI PRISM 7700 Sequence Detection System and SYBR Green reagent (Applied Biosystems). We examined the expression of genes for VEGF to evaluate angiogenesis. The expression level of each gene was first normalized with respect to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), which served as an internal control, and the results are presented as the fold change relative to the control group (ΔΔCt method).
Statistical analysis
The Fisher’s exact test was used for radiographic assessment and the Mann-Whitney U-test was used for histological assessment, μ-CT assessment, biomechanical assessment, and genetic assessment with real-time PCR. A p-value less than 0.05 was considered statistically significant.
Results
Radiographic assessment of bone healing
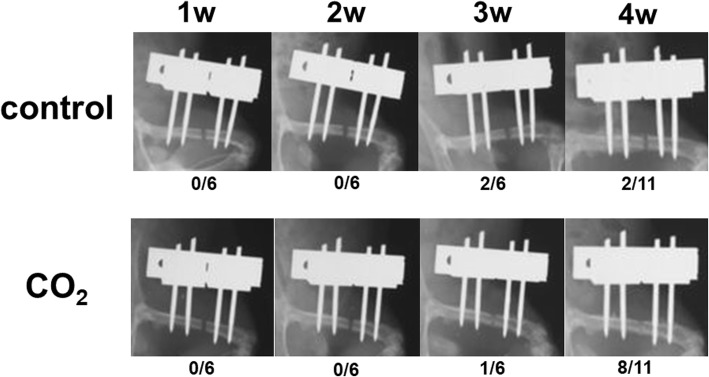
Representative radiographic findings of the control and CO2 groups at 1, 2, 3, and 4 weeks are shown in Fig. 3. At 4 weeks, 8 rats (72.7%) in the CO2 group achieved bone healing, whereas the femurs of 2 rats (18.2%) showed bone healing in the control group (Table 1). There was a significant difference in the bone healing rate between the two groups. (Fig. 4) However, there was no significant difference in the bone healing rate between both groups at 1, 2, and 3 weeks.
Fig. 3.
Radiographic evidence of bone healing in each group at 1, 2, 3, and 4 weeks after surgery. The number of samples with bone healing is represented under the radiograph
Table 1.
Healing and non-healing rates determined by radiographic assessment at 4 weeks
| Control | CO2 | |
|---|---|---|
| Healing | 2/11 (18.2%) | 8/11 (72.7%)* |
| Non-healing | 9/11 (81.8%) | 3/11 (27.3%) |
Bone healing was achieved in 8 rats (72.7%) in the CO2 group, whereas the femurs of 2 rats (18.2%) showed bone healing in the control group. There was a significant difference in the bone healing rate between the two groups.* p < 0.05 compared to the control group
Fig. 4.
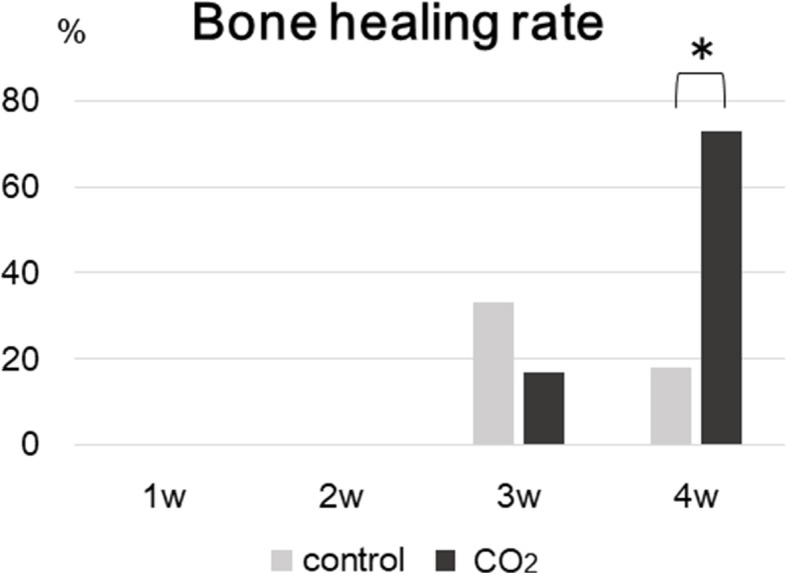
Bone healing rates in each group determined by radiographic assessment at 1, 2, 3, and 4 weeks. There was a significant difference in the bone healing rate between the two groups at 4 weeks. (*p < 0.05 in the indicated group)
Histological assessment for bone healing
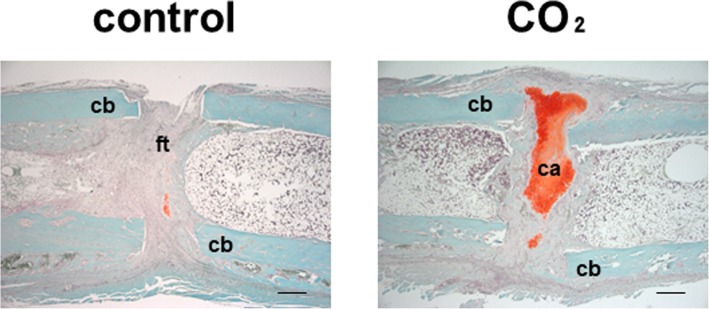
The representative histological findings of control and CO2 groups at 2 weeks are shown in Fig. 5. The area of cartilage stained red with Safranin-O was larger in the CO2 group than in the control group, which suggested that the degree of bone healing via endochondral ossification was more accelerated in the CO2 group than in the control group. The evaluation of histological bone healing with Allen’s grading score is shown in Fig. 6. A significant difference between the CO2 and control groups was detected at 2 weeks, but no significant difference was observed in the degree of bone healing between both groups at 4 weeks.
Fig. 5.
Representative histological sections stained with Safranin-O/Fast Green. cb = cortical bone, ca = cartilage, and ft. = fibrous tissue. Bar = 500 μm
Fig. 6.
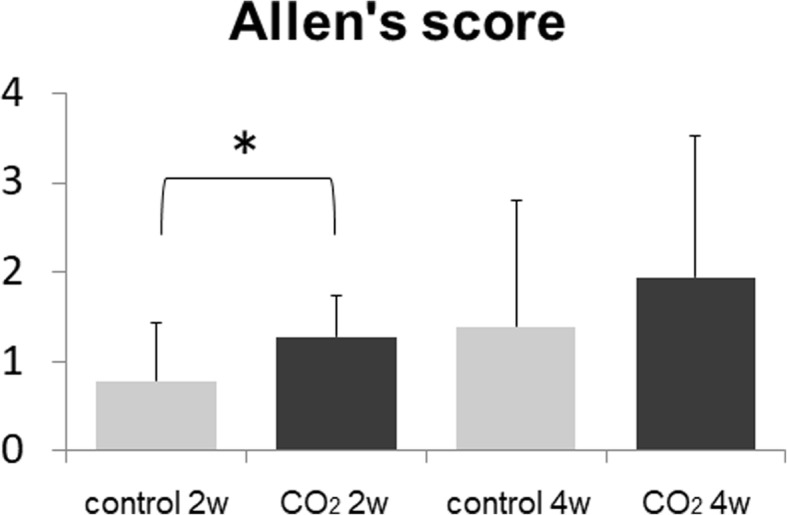
The degree of bone healing as indicated by the mean Allen’s score at 2 and 4 weeks. (n = 6 in each group) (*p < 0.05 in the indicated group)
Biomechanical assessment of bone healing
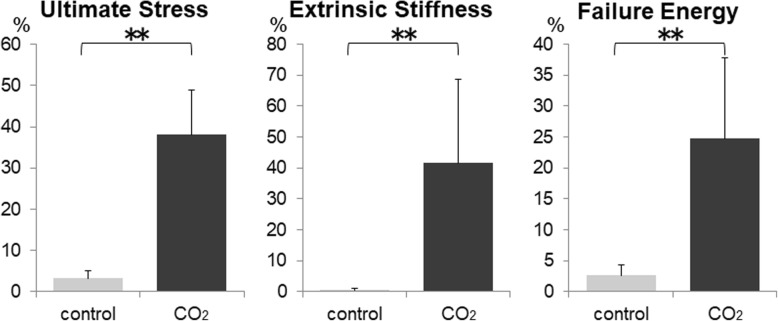
The ultimate stress, extrinsic stiffness, and failure energy of the defected femurs, that were expressed as a percentage of the value in the intact femur, were significantly higher in the CO2 group than those in the control group (3.2% vs. 38.1, 0.6% vs. 41.5, 2.6% vs. 24.7%, respectively.) (Fig. 7).
Fig. 7.
Biomechanical assessment of a femoral defect as assessed by the three-point bending test at 4 weeks. (n = 5 in each group) Values were normalized relative to the contralateral, intact femur. (**p < 0.01 in the indicated groups)
Micro-computed tomography (μ-CT) measurement of bone regeneration
A morphometrical assessment was performed with μ-CT at 4 weeks after surgery. The vBMD of the callus was significantly greater in the CO2 group than in the control group. No significant differences in TMD, TV, and BMC were observed between the control and CO2 groups. (Table 2).
Table 2.
Micro-computed tomography (μ-CT) measurement at 4 weeks
| Parameter | Control | CO2 |
|---|---|---|
| TMD (mg/cm3) | 605.6 ± 55.5 | 635.8 ± 55.2 |
| TV (cm3) | 0.062 ± 0.0097 | 0.063 ± 0.014 |
| BMC (mg) | 11.2 ± 2.83 | 15.7 ± 4.68 |
| vBMD (mg/cm3) | 180.9 ± 43.0 | 247.9 ± 49.9* |
The vBMD of the callus was significantly greater in the CO2 group than in the control group. * p < 0.05 compared to the control group. TMD tissue mineral density, TV total callus volume; BMC bone mineral content; vBMD volumetric bone mineral density (BMC/TV)
Assessment of gene expression
Real-time PCR was performed to evaluate the expression level of VEGF during bone healing. The gene expression of VEGF in the CO2 group was significantly higher than that in the control group at 3 weeks. (control group, 0.617 ± 0.240 vs. CO2 group, 2.213 ± 0.387) (Fig. 8) There were no significant differences between the groups at 1 and 2 weeks (Week 1: control group, 1.166 ± 0.130 vs. CO2 group, 3.335 ± 1.084; Week 2: control group, 2.313 ± 0.660 vs. CO2 group, 2.371 ± 0.558).
Fig. 8.
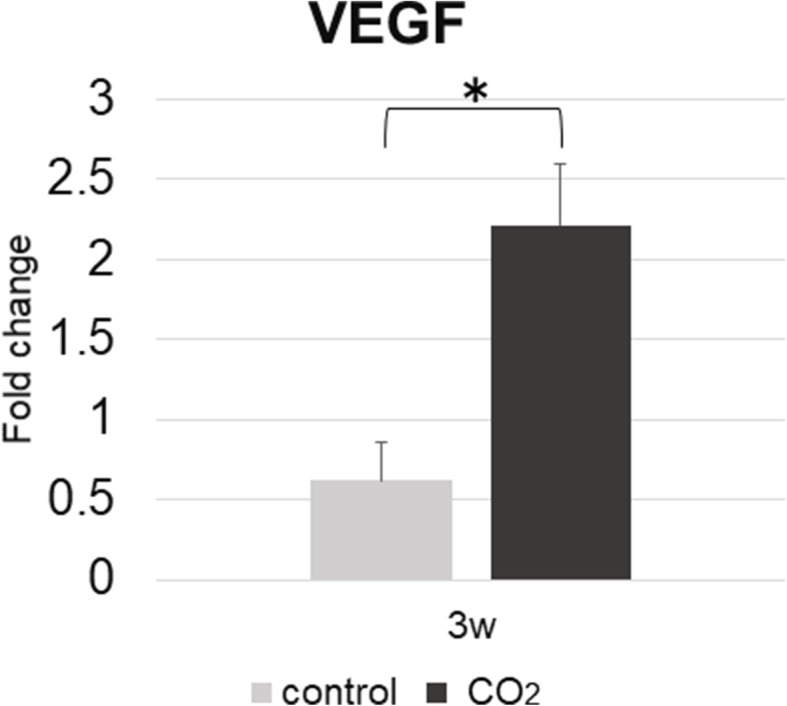
Expression level with standard error of VEGF of interest in each group at 3 weeks, which was measured by quantitative real-time PCR. (n = 6 in each group) (*p < 0.05 in the indicated group). VEGF, vascular endothelial growth factor
Discussion
The current study demonstrated that topical cutaneous application of CO2 increased the bone healing rate in a rat femoral defect model at 4 weeks after surgery. Sato et al. [19] reported that bone healing in rat models with 1-, 2-, and 3-mm femoral defects was observed at 8 weeks postoperatively. As a preliminary experiment, we created 2 mm bone defect models in rat femurs and no specimens achieved bone healing. However, bone healing was achieved in all specimens when creating 1 mm bone defect models. In the present study, we initially examined bone healing at 4 weeks as too early a time point to attain bone healing using 1 mm bone defect models in rat femurs that are expected to accomplish bone union eventually. Although there was a significant difference in bone healing rate between CO2 group and control group at 4 weeks, all specimens of the two groups did not attain bone healing. We confirmed that all of the 1-mm bone defect models in the two groups reached bone healing at 6 and 8 weeks postoperatively. Therefore, it was considered that 4 weeks after surgery was an appropriate time point to investigate the effect of topical cutaneous application of CO2 on bone healing.
The histological assessment in this study demonstrated a significant acceleration of bone healing in the CO2 group at as early as 2 weeks postoperatively. This may suggest that topical cutaneous application of CO2 in bone defect promoted endochondral ossification which occurred in early stage of osteogenesis. Considering fracture healing, Koga et al. studied the effect of transcutaneous CO2 application using a rat fracture model and reported that the gene expression of VEGF, Osterix, and Runx2 increased in the callus of the CO2/hydrogel group, indicating that angiogenesis and bone formation were promoted [17]. Angiogenesis is an important factor of bone repair, and it has been shown to serve as a means for inflammatory cells, cartilage precursor cells, and bone precursor cells, which metabolically transport oxygen and nutrients, to reach the injured site [20–22]. It is supposed that angiogenesis plays an important role in bone formation in a bone defect, as well as in fracture healing, and that CO2 application may contribute to acceleration of bone formation via promotion of angiogenesis. This can be supported by the result of real-time PCR in the current study. The expression level of VEGF was significantly higher in the CO2 group compared to the control group at 3 weeks postoperatively, suggesting enhanced angiogenesis in the bone defect site.
On the other hand, considering the result of histological assessment, it seems that endochondral ossification occurred before angiogenesis, which might be interpreted as an incompatibility with the process of ordinary bone formation. However, the gene expression of VEGF increased in the CO2 group at 2 weeks. Although this was not statistically significant, it might reflect accelerated angiogenesis in the earlier phase than endochondral ossification by CO2 treatment. As for the significant upregulation of VEGF in the CO2 group at 3 weeks, the reasons could be speculated as follows. Three weeks is considered the later phase of endochondral ossification and the time at which angiogenesis into newly formed chondral tissue actively occurs. The CO2 application might affect this angiogenesis following endochondral ossification, leading to a significant increase of VEGF expression. The detailed relationship between CO2 application and VEGF is still unknown, and further investigation is necessary to elucidate that.
Our findings suggest that topical cutaneous application of CO2 could be a beneficial treatment for bone defect accelerating bone regeneration. In a clinical setting, our CO2 application system is minimally invasive and relatively less costly [17]. However, additional experiments are needed to investigate whether this system could achieve bone healing in larger animals for clinical application.
This study has one limitation. The change in CO2 concentration in the bone defect area during topical cutaneous application of CO2 was not measured directly. Previously, we demonstrated that CO2 application significantly lowered the intracellular pH of human muscle [15]. Koga et al. suggest that the change in pH at the fracture site caused by CO2/hydrogel treatment may be one of the mechanisms leading to the promotion of angiogenesis and blood flow [17]. Further investigation is required to clarify whether CO2 is actually absorbed into the tissue at the bone defect area and whether promotion of endochondral ossification is directly induced by the CO2 itself or by a secondary reaction to the topical cutaneous application of CO2.
Conclusions
Topical cutaneous application of CO2 accelerated bone healing in a rat femoral defect model. Thus, CO2 application can be considered a novel and useful therapy for accelerating bone healing in bone defects, and further research on its efficacy in humans is warranted.
Acknowledgments
The authors would like to specially thank to Ms. M. Yasuda, Ms. K. Tanaka, and Ms. M. Nagata (Department of Orthopaedic Surgery, Kobe University Graduate School of Medicine) for their excellent technical assistance.
Funding
This work was supported by JSPS KAKENHI Grant Numbers JP26462266, JP17K10968. The funding sources had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BMC
Bone mineral content
- CO2
Carbon dioxide
- EDTA
Ethylenediaminetetraacetic acid
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- PCR
Polymerase chain reaction
- ROI
Region of interest
- TMD
Tissue mineral density
- TV
Total callus volume
- vBMD
BMC/TV, volumetric bone mineral density
- VEGF
Vascular endothelial growth factor
- μ-CT
micro-computed tomography
Authors’ contributions
YK1 and ST performed surgery and radiological, histological, biomechanical, and genetic assessments. YK1, TF, and TN analyzed all data, performed the statistical analysis and were major contributors in writing the manuscript. SL, KO, MA, YK2, TO, TM1, TM2, TA, YS, and RK conceived the study, and participated in its design and coordination. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All animal procedures were performed under the approval and guidance of the Animal Care and Use Committee of Kobe University Graduate School of Medicine.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yu Kuroiwa, Email: u_br1221@yahoo.co.jp.
Tomoaki Fukui, Email: tfukui@med.kobe-u.ac.jp.
Shunsuke Takahara, Email: shunsuketakahara@gmail.com.
Sang Yang Lee, Email: sangyang@beige.plala.or.jp.
Keisuke Oe, Email: keisuke5091@gmail.com.
Michio Arakura, Email: michioarakura@gmail.com.
Yohei Kumabe, Email: kumaclub0725@yahoo.co.jp.
Takahiro Oda, Email: taka.oda.0116@gmail.com.
Tomoyuki Matsumoto, Email: matsunt@med.kobe-u.ac.jp.
Takehiko Matsushita, Email: matsushi@med.kobe-u.ac.jp.
Toshihiro Akisue, Email: akisue@med.kobe-u.ac.jp.
Yoshitada Sakai, Email: yossie@med.kobe-u.ac.jp.
Ryosuke Kuroda, Email: kurodar@med.kobe-u.ac.jp.
Takahiro Niikura, Phone: +81-78-382-5985, Email: tniikura@med.kobe-u.ac.jp.
References
- 1.Trigui M, Ayadi K, Ellouze Z, Gdoura F, Zribi M, Keskes H. Treatment of bone loss on limbs by bone transport. Rev Chir Orthop. 2008;94:628–634. doi: 10.1016/j.rco.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Belt PJ, Dickinson IC, Theile DR. Vascularised free fibular flap in bone resection and reconstruction. Br J Plast Surg. 2005;58:425–430. doi: 10.1016/j.bjps.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 3.El-Gammal TA, Shiha AE, El-Deen MA, El-Sayed A, Kotb MM, Addosooki AI, Ragheb YF, Saleh WR. Management of traumatic tibial defects using free vascularized fibula or Ilizarov bone transport: a comparative study. Microsurgery. 2008;28:339–346. doi: 10.1002/micr.20501. [DOI] [PubMed] [Google Scholar]
- 4.Yazar S, Lin CH, Wei FC. One-stage reconstruction of composite bone and soft-tissue defects in traumatic lower extremities. Plast Reconstr Surg. 2004;114:1457–1466. doi: 10.1097/01.PRS.0000138811.88807.65. [DOI] [PubMed] [Google Scholar]
- 5.Masquelet AC, Fitoussi F, Begue T, Muller GP. Reconstruction of the long bones by the induced membrane and spongy autograft. Ann Chir Plast Esthet. 2000;45:346–353. [PubMed] [Google Scholar]
- 6.Berenbrink M. Evolution of vertebrate haemoglobins: histidine side chains, specific buffer value and Bohr effect. Respir Physiol Neurobiol. 2006;154:165–184. doi: 10.1016/j.resp.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Weber RE, Fago A. Functional adaptation and its molecular basis in vertebrate hemoglobins, neuroglobins and cytoglobins. Respir Physiol Neurobiol. 2004;144:141–159. doi: 10.1016/j.resp.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 8.Bohr C, Hasselbach K, Krogh A. On a biologically important influence exerted by the carbonic acid tension of the blood on its oxygen binding 1. Scand Arch Physiol. 1904;16:402–412. doi: 10.1111/j.1748-1716.1904.tb01382.x. [DOI] [Google Scholar]
- 9.Riggs A. The nature and significance of the Bohr effect in mammalian Hemoglobins. J Gen Physiol. 1960;43:737–752. doi: 10.1085/jgp.43.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irie H, Tatsumi T, Takamiya M, Zen K, Takahashi T, Azuma A, Tateishi K, Nomura T, Hayashi H, Nakajima N, Okigaki M, Matsubara H. Carbon dioxide–rich water bathing enhances collateral blood flow in ischemic Hindlimb via mobilization of endothelial progenitor cells and activation of NO-cGMP system. Circulation. 2005;111:1523–1529. doi: 10.1161/01.CIR.0000159329.40098.66. [DOI] [PubMed] [Google Scholar]
- 11.Pagourelias ED, Zorou PG, Tsaligopoulos M, Athyros VG, Karagiannis A, Efthimiadis GK. Carbon dioxide balneotherapy and cardiovascular disease. Int J Biometeorol. 2011;55:657–663. doi: 10.1007/s00484-010-0380-7. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto N, Hashimoto M. Immersion in CO2-rich water containing NaCl diminishes blood pressure fluctuation in anesthetized rats. Int J Biometeorol. 2007;52:109–116. doi: 10.1007/s00484-007-0102-y. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt J, Monnet P, Normand B, Fabry R. Microcirculatory and clinical effects of serial percutaneous application of carbon dioxide in primary and secondary Raynaud’s phenomenon. Vasa. 2005;34:93–100. doi: 10.1024/0301-1526.34.2.93. [DOI] [PubMed] [Google Scholar]
- 14.Jensen FB. Red blood cell pH, the Bohr effect, and other oxygenation-linked phenomena in blood O2 and CO2 transport. Acta Physiol Scand. 2004;182:215–227. doi: 10.1111/j.1365-201X.2004.01361.x. [DOI] [PubMed] [Google Scholar]
- 15.Sakai Y, Miwa M, Oe K, Ueha T, Koh A, Niikura T, Iwakura T, Lee SY, Tanaka M, Kurosaka M. A novel system for transcutaneous application of carbon dioxide causing an “artificial Bohr effect” in the human body. PLoS One. 2011;6:e24137. doi: 10.1371/journal.pone.0024137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oe K, Ueha T, Sakai Y, Niikura T, Lee SY, Koh A, Hasegawa T, Tanaka M, Miwa M, Kurosaka M. The effect of transcutaneous application of carbon dioxide (CO2) on skeletal muscle. Biochem Biophys Res Commun. 2011;407:148–152. doi: 10.1016/j.bbrc.2011.02.128. [DOI] [PubMed] [Google Scholar]
- 17.Koga T, Niikura T, Lee SY, Okumachi E, Ueha T, Iwakura T, Sakai Y, Miwa M, Kuroda R, Kurosaka M. Topical cutaneous CO2 application by means of a novel hydrogel accelerates fracture repair in rats. J Bone Joint Surg Am. 2014;96:2077–2084. doi: 10.2106/JBJS.M.01498. [DOI] [PubMed] [Google Scholar]
- 18.Allen HL, Wase A, Bear WT. Indomethacin and aspirin: effect of nonsteroidal anti-inflammatory agents on the rate of fracture repair in the rat. Acta Orthop Scand. 1980;51:595–600. doi: 10.3109/17453678008990848. [DOI] [PubMed] [Google Scholar]
- 19.Sato K, Watanabe Y, Harada N, Abe S, Matsushita T, Yamanaka K, Kaneko T, Sakai Y. Establishment of reproducible, critical-sized, femoral segmental bone defects in rats. Tissue Eng Part C Methods. 2014;20:1037–1041. doi: 10.1089/ten.tec.2013.0612. [DOI] [PubMed] [Google Scholar]
- 20.Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011;42:551–555. doi: 10.1016/j.injury.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ai-Aql ZS, Alagl AS, Graves DT, Gerstenfeld LC, Einhorn TA. Molecular mechanisms controlling bone formation during fracture healing and distraction osteogenesis. J Dent Res. 2008;87:107–118. doi: 10.1177/154405910808700215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurt DH, Michael D, Chancellor G, Mara S. Angiogenesis in Bone Regeneration. Injury. 2011;42:556–561. doi: 10.1016/j.injury.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.