Abstract
Background
Inhibition of the Na+/glucose co-transporter 2 is a new therapeutic strategy for diabetes. It is unclear how proximal loss of Na+ (and glucose) affects the subsequent Na+ transporters in the proximal tubule (PT), thick ascending limb of loop of Henle (TAL), distal convoluted tubule (DCT) and collecting duct (CD).
Methods
Mice on a high fat diet were administered 3 doses streptozotocin 6 days prior to oral dapagliflozin administration or vehicle for 18 days. A control group of lean mice were also included. Body weight and glucose were recorded at regular intervals during treatment. Renal Na+ transporters expression in nephron segments were analyzed by RT-qPCR and Western blot.
Results
Dapagliflozin treatment resulted in a significant reduction in body weight and blood glucose compared to vehicle-treated controls. mRNA results showed that Na+-hydrogen antiporter 3 (NHE3), Na+/phosphate cotransporter (NaPi-2a) and epithelial Na+ channel expression was increased, Ncx1, ENaCβ and ENaCγ expression declined (p all < 0.05), respectively, in dapagliflozin-treated mice when compared with saline vehicle mice. Na-K-2Cl cotransporters and Na-Cl cotransporter mRNA expression was not affected by dapagliflozin treatment. Na+/K+-ATPase (Atp1b1) expression was also increased significantly by dapagliflozin treatment, but it did not affect Atp1a1 and glucose transporter 2 expression. Western blot analysis showed that NaPi-2a, NHE3 and ATP1b1 expression was upregulated in dapagliflozin-treated diabetic mice when compared with saline vehicle mice (p < 0.05).
Conclusion
Our findings suggest that dapagliflozin treatment augments compensatory changes in the renal PT in diabetic mice.
Keywords: Dapagliflozin, Type 2 diabetes, Renal sodium transporters
Type 2 diabetes mellitus is a major health problem affecting 415 million people worldwide [1, 2]. This represents 8.3% of the adult population, with equal rates in women and men [3]. From 2012 to 2015, approximately 1.5–5.0 million deaths each year resulted from diabetes [4]. Diabetes and hypertension frequently occur together [5]. Hypertension in these patients further increases their already elevated cardiovascular risks, yet is difficult to manage. Indeed, in about half of the diabetic population, blood pressure targets are not met despite the use of multiple blood pressure lowering drugs, including diuretics [6, 7, 8].
Recently, dapagliflozin, a sodium-glucose co-transporter 2 (SGLT2) inhibitor, was introduced as a novel class of glucose-lowering agents for the treatment of type 2 diabetes. As SGLT2 is responsible for approximately 90% of the filtered glucose reabsorption in the proximal tubule (PT) segment 1 and 2 [9, 10, 11], its inhibition reduces renal glucose and sodium (Na+) reabsorption, leading to urinary glucose excretion and a reduction in blood glucose levels [12]. Therefore, dapagliflozin is an efficient novel drug to treat patients with type 2 diabetes mellitus [13, 14, 15, 16].
Some studies have also demonstrated that SGLT2 inhibitors exhibit an impressive diuretic effect and consequent blood pressure reduction, whereas others described modest effects on volume status [17, 18, 19, 20]. SGLT2 inhibition reduces PT Na+ reabsorption and thereby increases the distal tubular Na+ load, which inhibits the renin-angiotensin-aldosterone system activation [21]. The kidneys efficiently reabsorb 99% of filtered Na+ by the combined action of (i) the PT where 60–70% is reabsorbed via Na+-hydrogen antiporter 3 (NHE3), SGLT1 and SGLT2 [22]; (ii) the thick ascending limb (TAL) of Henle's loop that is responsible for 15–25% reabsorption via paracellular routes and Na-K-2Cl cotransporter (NKCC2); (iii) the distal convoluted tubule (DCT) that reabsorbs 15–25% via the thiazide-sensitive Na-Cl cotransporter (NCC) [23]; (iv) the collecting duct (CD) where the ENaC facilitates the reabsorption of the remaining 1–2% [10, 24].
A recent study has found that treatment with an SGLT2 inhibitor increases the expression of urea transporter-A1, aquaporin-2 and NKCC2 proteins [1]. However, a systematic analysis of the compensatory mechanisms that regulate renal Na+ reabsorption after SGLT2 treatment is lacking. Knowledge of which nephron segment compensates for proximal Na+ loss is of great fundamental and clinical interest, as this would provide the major pharmacological target for antihypertensive treatment.
The purpose of this study was to identify the compensatory impact of proximal Na+ wasting by the SGLT2 inhibitor, dapagliflozin, on renal Na+ transporters in high-fat diabetic mice.
Materials and Methods
The Following Primary Antibodies Were Used
NCC (Millipore, Billerica, MA, USA; #AB3553; immunoblotting [IB] 1: 2,000), and sheep anti NKCC2, IB 1: 2,000 [25], Na+/phosphate cotransporter (NaPi-2a; kind gift of Dr. Custer et al. [26]; IB 1: 2,000), NHE3 (Millipore, Billerica, MA, USA; # AB3085; IB 1: 500) [27], ATP1b1 (Merck KGaA, Darmstadt, Germany; # 05-382; IB 1: 500) [28]. Secondary antibodies were as follows: peroxidase conjugated goat anti-rabbit (Sigma-Aldrich; # A4914; IB 1: 10,000); peroxidase conjugated sheep anti-mouse (Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA; #515-035-003; IB 1: 10,000).
Buffers
Lysis buffer: 150 mM NaCl, 50 mM Tris-HCl (pH 7.5), 1 mM EDTA, 1 mM EGTA, 1 mM sodium-orthovanadate, 1% (v/v) Triton X-100, 10 mM sodium-glycerophosphate, 50 mM sodium fluoride, 0.27 M sucrose, 10 mM sodium pyrophosphate, containing freshly added tablet of complete protease inhibitor cocktail (Roche, Basel, Switzerland) and 0.1% (v/v) β-mercaptoethanol. SDS-PAGE sample buffer: 5× 10% (w/v) SDS, 10 mM β-mercaptoethanol, 50% (v/v) glycerol, 0.3 M Tris-HCl (pH 7.5), 0.05% (w/v) bromophenol blue. TBS-T (Tris-buffered saline, 0.1% [v/v] Tween 20): Tris-HCl (200 mM, pH 7.5), 0.15 M NaCl, and 0.2% (v/v) Tween-20.
Animal Model
Adult Swiss male mice (Harlan, Oxon, UK) at 16 weeks of age were housed in an air-conditioned room at 22 ± 2°C with 12: 12 h light/dark cycle. Mice had free access to high-fat diet (45% AFE Fat; Special Diet Services, Witham, UK; total energy 26.15 kJ/g). An additional lean group had free access to standard rodent chow (Teklad Global 18% Protein Rodent Diet; Harlan, UK; total energy 13.0 kJ/g). All animals were free to access drinking water and respective diet, and no adverse effects were observed during the entire experimental study. All experiments were performed according to the Principles of Laboratory Animal Care (NIH publication no. 86-23, revised 1985) and UK Home Office Regulations (UK Animals Scientific Procedures Act 1986).
Experimental Treatments
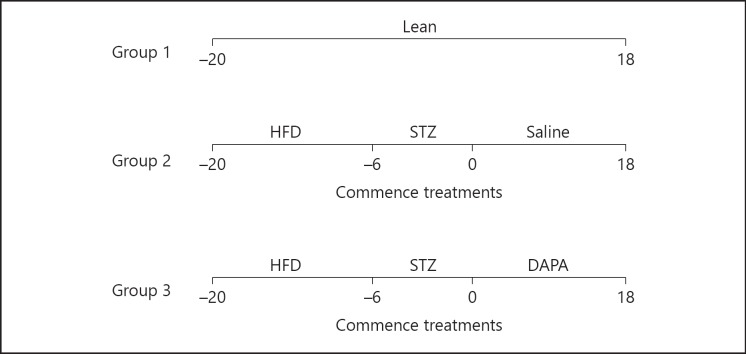
Mice commenced high-fat diet on day −20 and remained on this diet for the duration of the study. On day −6, streptozotocin (50 mg/kg; i.p.; Sigma-Aldrich, Dorset, UK) freshly prepared in ice-cold 0.1 M Na+ citrate buffer (HCl/pH 4.5) was administered 3 doses in total over a period of 6 days to induce diabetes. On day 0, one group of high-fat mice (n = 8) commenced daily treatment with dapagliflozin (1 mg/kg; p.o.; Stratech Scientific Ltd., Suffolk, UK) for 18 days, whereas high-fat control group (n = 8) received saline vehicle (0.9% w/v NaCl; p.o.) once-daily for the same time period. The volume for the oral gavage was 100 μL. A diagrammatic representation of the experimental design is shown in Figure 1.
Fig. 1.
Timeline for the experimental study. Group 1 (lean control): lean mice on normal diet for 38 days. Group 2 (high-fat controls): mice commenced a high fat diet on day −20 and subsequently received STZ treatment on day −6. At day 0, saline vehicle was administered for 18 days. Group 3 (high-fat dapagliflozin): mice commenced high fat diet on day −20 and subsequently received STZ on day −6. At day 0, dapagliflozin was administered for 18 days. Lean, lean control mice; HFD, high fat diet treatment; DAPA, dapagliflozin-treated mice; STZ, streptozotocin-treated mice.
Quantitative Analyses of Gene Expression
At study termination, total RNA was extracted from mouse kidney tissues with Trizol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. Subsequently, mouse RNA samples were subjected to DNase treatment to prevent genomic DNA contamination and the reverse transcriptase reaction was subsequently performed to synthesize cDNA [29]. mRNA levels of the target genes were determined by relative RT-qPCR following the MIQE guidelines 20 with a CFX96TM Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA) using iQTM SYBR Green Supermix (Bio Rad) detection of single PCR product accumulation. Each group had 8 kidneys and RT-qPCR experiments were commenced in triplicate. Primers for SGLT2, SGLT1, NHE3, NaPi-2a, NKCC2, NCC, Ncx1, ENaC (α, β, γ) were purchased from Biolegio BV (Nijmegen, Netherlands). In this study, gene expression levels were normalized to the expression levels of the standard species-specific reference genes glyceraldehyde 3-phosphate dehydrogenase. Here, the relative mRNA expression was analyzed using the Livak method (2–ΔΔCt). Primer sequences are shown in Table 1.
Table 1.
Primer sequences used for real-time quantitative RT-PCR
| Gene | Forward primer 5'–3' | Reverse primer 5'–3' |
|---|---|---|
| SGLT2 | ATGGAGCAACACGTAGAGGC | ACATAGACCACAAGCCAACACC |
| SGLT1 | TCTGTAGTGGCAAGGGGAAG | ACAGGGCTTCTGTGTCTTGG |
| NaPi-2a | AGGTGAGCTCCGCCATTCCGA | CCCTGCAAAAGCCCGCCTGA |
| NKCC2 | GGCTTGATCTTTGCTTTTGC | CCATCATTGAATCGCTCTCC |
| NCC | CTTCGGCCACTGGCATTCTG | GATGGCAAGGTAGGAGATGG |
| Ncx1 | TCCCTACAAAACTATTGAAGGCACA | TTTCTCATACTCCTCGTCATCGATT |
| ENaCα | GCTCAACCTTGACCTAGACCT | GCGGTGGAACTCGATCAGT |
| ENaCβ | GTCATCGGAACTTCACGCCTAT | TCCTCCTGACCGATGTCCAG |
| ENaCγ | TGACCTGCTTCTTCGATGGG | TTGCAGACCATACTCACTGCC |
| Atp1a1 | GGGGTTGGACGAGACAAGTAT | CGGCTCAAATCTGTTCCGTAT |
| Atp1b1 | ATCTCCTTCCGTCCTAATGACC | CTCGAAAATCATGTCGTCCTTCT |
| Glut2 | AGAAGACAAGATCACCGGAACC | TCACACCGATGTCATAGCCG |
| GAPDH | TAACATCAAATGGGGTGAGG | GGTTCACACCCATCACAAAC |
SGLT2, sodium-glucose co-transporter 2; NaPi-2a, Na+/phosphate cotransporter; NKCC2, Na-K-2Cl cotransporter; NCC, Na-Cl cotransporter; ENaCα, epithelial Na+ channel; Glut2, glucose transporter 2; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Protein Isolation
Kidney tissues were isolated from mice and homogenized in ice cold lysis buffer. The kidney lysates were clarified by centrifugation at 4°C for 15 min at 16, 110 g and supernatants stored at −80°C. Bradford method was used to determine protein concentrations according to the manufacturer's protocol (Bio-Rad).
Immunoblotting
Lysates (20 μg) in SDS sample buffer were added to electrophoresis on Criterion TGX precast gels (Bio-Rad) and then the gels were transferred to PVDF membranes. The membranes were blocked in TBS-T containing 5% (w/v) non-fat dry milk (NFDM) for 1 h at room temperature. Subsequently, they were immunoblotted at 4°C with primary antibody overnight. Next day, the blots were washed with TBS-T to remove unbound primary antibody and incubated with horseradish peroxidase conjugated secondary antibodies for 1 h at room temperature. After subsequent washes, the protein was visualized with chemiluminescent reagent (SuperSignal West femto/pico; Thermo Scientific, Waltham, MA, USA) and processed with the Bio-Rad ChemiDoc XRS. The NaPi-2a, NaPi-2c, NHE3, NCC, NKCC2 and ATP1b1 bands on the immunoblots were quantified with gel analyzer.
Statistical Analyses
Data is shown as means ± SEM. One-way ANOVA followed by Scheffe's test was used to examine the differences between groups. A p value < 0.05 was considered statistically significant.
Results
Effects of High-Fat Feeding and Dapagliflozin on Body Weight and Blood Glucose
Mice receiving the high-fat diet increased in body weight from day −20 to day 0 more than those receiving normal chow (p < 0.05). From day 0 to 18, high-fat control mice displayed a further modest increase in body weight (p < 0.05), whereas mice treated with dapagliflozin exhibited a significant reduction in body weight (p < 0.05). Body weights of lean mice did not differ during the study. High-fat control mice displayed increased blood glucose concentrations from day 0 to 18 (p < 0.05). In contrast, high-fat mice treated with dapagliflozin exhibited a marked reduction in blood glucose (p < 0.05). Glucose concentrations were unchanged in lean mice. Results are shown in Table 2.
Table 2.
Effects of high fat feeding and dapagliflozin on body weight and blood glucose
| Parameter | Lean (n = 8) |
HF + saline (n = 8) |
HF + DAPA (n = 8) |
|||
|---|---|---|---|---|---|---|
| 0 day | 18 days | 0 day | 18 days | 0 day | 18 days | |
| Body weight, g | 37.6±5.2 | 36±2.3 | 45.7±4.2 | 50.2±4.3a | 43.5±5.7 | 37.6±5.2b |
| Blood glucose, mM | 5.2±0.9 | 5.8±0.4 | 18.5±3.7 | 22.3±4.1a | 17.6±1.7 | 7.6±2.1b |
Data is mean ± SEM.
p < 0.05 vs. lean.
p < 0.05 vs. HF + saline.
Lean, lean control mice; HF + saline, saline vehicle diabetic mice; DAPA, dapagliflozin (1 mg/kg) treated diabetic mice. 0 day, day 0, saline vehicle and DAPA were administered; 18 days, day 18, saline vehicle and DAPA were administered for 18 days.
The Effect of Dapagliflozin on Na+ Transporter Expression in the PT
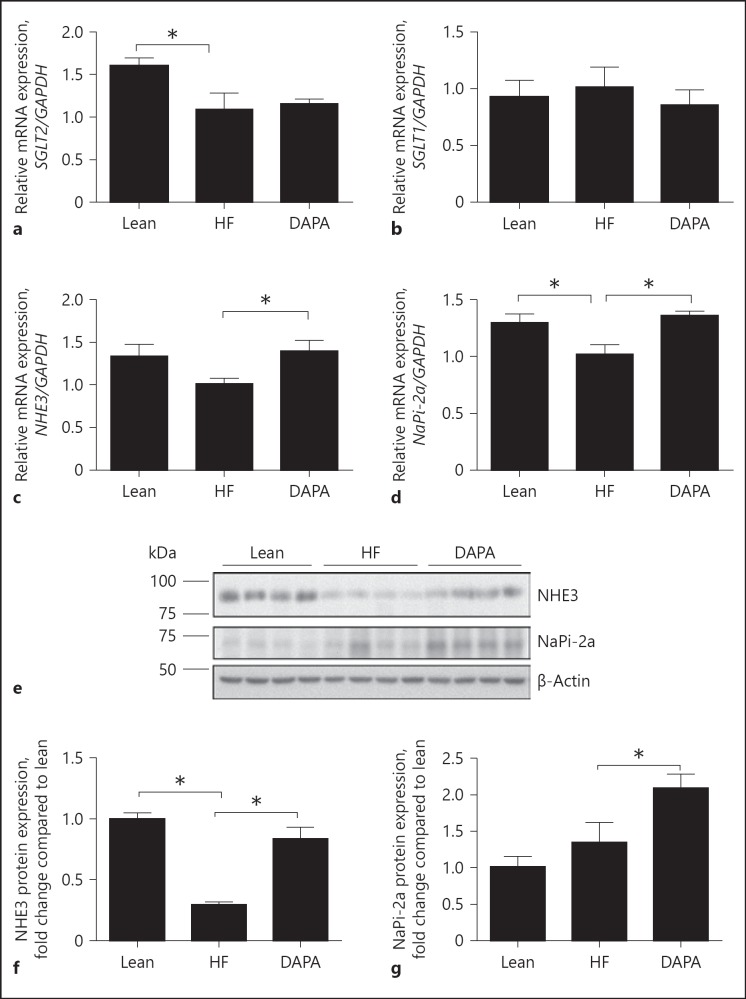
RT-qPCR was performed to analyze the renal expression of SGLT1, SGLT2, NHE3, NaPi-2a in the dapagliflozin-treated and control group mice. NHE3 and NaPi-2a gene expression was increased significantly by 33 and 34% (p < 0.05 each), respectively, in the dapagliflozin-treated mice compared to high-fat control group (Fig. 2c, d). In contrast, there were no significant differences in the expression of SGLT2 and SGLT1 (p > 0.2) between the dapagliflozin-treated group and the vehicle-control group (Fig. 2a, b). Protein expression level of NaPi-2a and NHE3 was further investigated by Western blot analysis (Fig. 2e). The expression of NaPi-2a and NHE3 was increased by 55 and 139% in the dapagliflozin-treatment group when compared with saline vehicle diabetic mice (p < 0.05; Fig. 2f, g).
Fig. 2.
Effect of dapagliflozin on the expression of the sodium transporters in the PT. a–d Quantitative RT-PCR analyses for the expression of SGLT2, SGLT1, NHE3 and NaPi-2a in the PT. e, f Immunoblot analysis of NaPi-2a (∼70 kDa), NHE3 (∼93 kDa) in mice fed the indicated diet. g, h Histograms for NaPi-2a, NHE3 Western blot results and normalized for β-actin expression. * p < 0.05. Lean, lean control mice; HF, saline vehicle mice; DAPA, dapagliflozin-treated mice. NHE3, Na+-hydrogen antiporter 3; NaPi-2a, Na+/phosphate cotransporter.
The Effect of Dapagliflozin on Na+ Transporter Expression in TAL and DCT
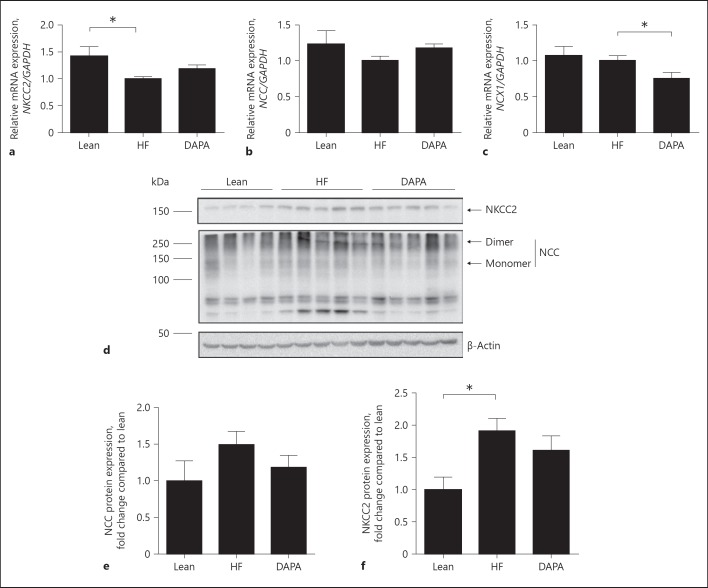
In order to study the effect of dapagliflozin on the expression of the Na+ transporters in the TAL and DCT, the expression of NKCC2, NCC and Ncx1 was analyzed by RT-qPCR. The expression of NKCC2 and NCC tended to increase by 23 and 17%, respectively, in the dapagliflozin treatment group (Fig. 3a, b). However, these changes did not reach statistical significance. The expression of Ncx1 decreased by 27% in the dapagliflozin treatment group when compared with that of the vehicle-control group (p < 0.05; Fig. 3c). Dapagliflozin treatment did not alter NCC and NKCC2 protein abundance when compared with saline vehicle diabetic mice (Fig. 3e, f).
Fig. 3.
Effect of dapagliflozin on the expression of the sodium transporters in the loop of Henle. a–c Quantitative RT-PCR analyses for the expression of NKCC2, NCC, and NCX1 in the loop of Henle. d Immunoblot analysis of NKCC2 (∼150 kDa), NCC (∼120 kDa) in mice fed the indicated diet. e, f Histograms for NKCC2, NCC Western blot results and normalized for β-actin expression. * p < 0.05. Lean, lean control mice; HF, saline vehicle mice; DAPA, dapagliflozin-treated mice. NKCC2, Na-K-2Cl cotransporter; NCC, Na-Cl cotransporter.
The Effect of Dapagliflozin on Na+ Transporter/Channel Expression in the CD
To further investigate the effect of proximal Na+ loss on the CD, we tested the expression of the ENaC (α, β and γ) by RT-qPCR. Dapagliflozin treatment increased the expression of epithelial Na+ channel (ENaCα) by 29% when compared with vehicle-control mice (p < 0.05) (Fig. 4a). In addition, the expression of ENaCβ and ENaCγ declined significantly by 13 and 26%, respectively, in the dapagliflozin-treated group compared to vehicle-controls (p < 0.05 each; Fig. 4b, c).
Fig. 4.
Effect of dapagliflozin on the expression of the sodium transporters in the DCT. a–c Quantitative RT-PCR analyses for the expression of ENaC α, ENaC β, and ENaC γ, in the DCT. * p < 0.05. Lean, lean control mice; HF, saline vehicle mice; DAPA, dapagliflozin-treated mice.
The Effect of Dapagliflozin on Expression of Na+-K+-ATPase and Glucose Transporters
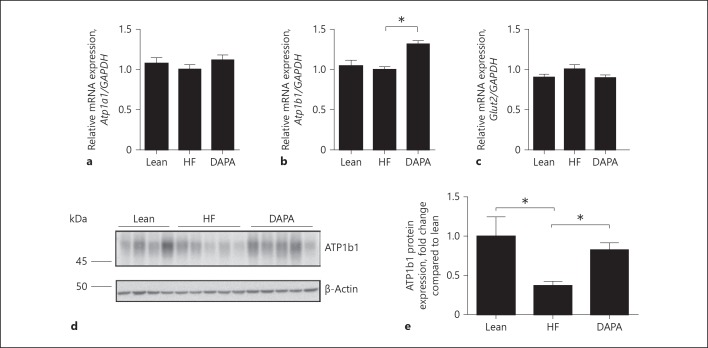
We also evaluated the effect of dapagliflozin on glucose transporter 2 (Glut2) and Na+-K+-ATPase transporters by RT-qPCR. The expression of Atp1b1 was significantly increased by 32% in the dapagliflozin-treated mice compared to that of the vehicle- control group (p < 0.05; Fig. 5b). The expression of Atp1a1 was not changed in the dapagliflozin-treated group (p > 0.2). Dapagliflozin treatment caused a slight reduction in the expression of Glut2 compared to the vehicle-control group, but this effect failed to reach statistical significance (p = 0.08; Fig. 5a, c). Western blot analysis was further used to investigate the protein expression level of ATP1b1 (Fig. 5d). ATP1b1 expression was increased by 123% in the dapagliflozin-treatment group when compared with saline vehicle diabetic mice (p < 0.05; Fig. 5e).
Fig. 5.
Effect of dapagliflozin on ATPase and Glut2. a–c Quantitative RT-PCR analyses for the expression of Atp1a1, Atp1b1, and Glut2. d Immunoblot analysis of ATP1b1 (∼50 kDa) in mice fed the indicated diet. e Histogram for ATP1b1 Western blot results and normalized for β-actin expression. * p < 0.05. Lean, lean control mice; HF, saline vehicle mice; DAPA, dapagliflozin-treated mice. GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Discussion
In this study, we demonstrated that the inhibition of SGLT2 increased the mRNA expression level of NHE3, NaPi-2a and ENaCα. Western blotting results show that the protein expression level of NHE3 and NaPi-2a was increased in dapagliflozin-treated mice. Moreover, ATP1b1 protein expression level was also increased. Our findings demonstrate that proximal inhibition of Na+ reabsorption via SGLT2 is compensated by an increased expression of local Na+ transporters in the PT but not in the TAL, DCT and CD.
The kidneys reabsorb large amounts of filtered glucose to clear urinary glucose, primarily through the Na+-dependent glucose co-transporter 2 (SGLT2) in the S1 segment of the PT. Inhibitors of SGLT2 are newly developed anti-diabetic agents and interfere with the pathway of physiological glucose reabsorption in the kidney. In this study, high fat and STZ induction significantly increased blood glucose when compared with lean group mice. In the dapagliflozin treatment group, we found that blood glucose level decreased significantly when compared with that of the vehicle-control group. In our study, STZ-induced diabetes decreased the SGLT2 expression. Albertoni Borghese et al. [30] have also demonstrated that STZ decreased the SGLT2 expression and activity. In contrast, the SGLT2 expression was increased in Akita/+ mice [31], humans [32] with type 2 diabetes, and alloxan-induced diabetic rats [33]. Indeed, the use of different diabetic models may, therefore, result in different SGLT2 expression. However, despite lower SGLT2 expression in STZ-induced mice, dapagliflozin treatment reduced blood glucose levels. We therefore expect that in other studies with higher SGLT2 expression, the observed effects may even be larger. Moreover, dapagliflozin treatment did not change SGLT2 expression in our experiment. The expression of SGLT2 does not necessarily alter upon dapagliflozin treatment. Other studies also found that pharmacological SGLT2 inhibition does not affect the expression of SGLT2 [31, 34]. Given the reduction of blood glucose concentrations in the dapagliflozin-treated group at day 18 when compared with saline vehicle mice, the lowered SGLT2 expression did not impair the effects of dapagliflozin treatment. Therefore, we do not expect that this has a major impact on our results.
SGLT2 inhibitors improve glucose control by inducing glycosuria, but they also reduce the reabsorption of Na+. Inhibition of Na+ reabsorption in the PT will switch on compensatory systems in more distally located segments to counteract the proximal Na+ loss. A recent study has shown that dapagliflozin treatment upregulated the expression of urea transporter-A1, aquaporin-2, and NKCC2 proteins [1]. However, little is known about the effects of SGLT2 inhibitors on the local and downstream Na+ transporters. We investigated how proximal Na+ wasting affects local and downstream Na+ transporters/channels' expression in high-fat diabetic mice.
The management of hypertension in diabetes is not without controversy [17]. Hence, the precise level at which anti-hypertensive therapy should be initiated and what the target blood pressure should be remain difficult issues. Many patients with type 2 diabetes receive multiple drugs to treat both hyperglycaemia and hypertension. The new class of SGLT2 inhibitors also induces renal Na+ wasting and, therefore, will have blood pressure-reducing properties. However, some studies report an impressive diuretic effect and consequent reduction in blood pressure, while others described modest effects on volume status [17, 35]. It is known that the compensatory capacity of the kidney is immense [36]. The inhibition of Na+ reabsorption in the PT will turn on compensatory systems in local or more distally located segments to counteract the proximal Na+ loss.
In this study, dapagliflozin treatment significantly increased the mRNA expression level of NHE3, NaPi-2a and ENaCα. In line with the mRNA expression level, Western blot results showed that NHE3, NaPi-2a also increased in the dapagliflozin treatment group when compared to that in saline vehicle mice. Therefore, our results suggest that the inhibition of Na+-glucose transporter 2 in the PT can turn on the local and downstream Na+ compensatory systems. Increased reabsorption of Na+ may elevate blood pressure in the type 2 diabetes [37, 38, 39]. Wang et al. [38] showed that a changed expression of Na+ transport in renal PT can be compensated by changes in more distal tubule in nephron, tubuloglomerular feedback and also by glomerular filtration rate adjustments. Nevertheless, extracellular fluid volume and consequently blood pressure can also be affected by the changed expression of sodium transport in PT. Sodium reabsorption initially happens at the PT apical membrane, therefore resulting in making apical sodium transport critical in adjusting the extracellular fluid volume and ultimately blood pressure control. Indeed, in polygenic human essential hypertension, the increase in sodium transport occurs at the PT and TAL of Henle [40, 41, 42] rather than in more distal nephron segments that is characteristic of monogenic hypertension [43]. Unfortunately, there were no plasma and urine electrolytes results in this study. Therefore, further studies are needed to better understand of the compensatory mechanisms of Na+ transporters distal from the PT to adjust blood pressure control.
SGLT2 is located in the S1 segment and accounts for 90% of the glucose reabsorption from the kidneys [44, 45, 46, 47]. Na+ absorption across the cell membrane creates an energy gradient that in turn allows the absorption of glucose. On the other side of the cell, Na+ is extruded through Na+-K+-ATPase into the bloodstream [48, 49, 50]. The concentration gradient within the cell, resulting from this exchange drives glucose reabsorption into the bloodstream via GLUT2 [51, 52, 53]. In order to evaluate if the increased expression of Na+ transporters in the PT and CD influenced the expression of Na+-K+-ATPase and GLUT2, we furthermore tested the expression of Atp1a1 and Atp1b1 and Glut2 transporters by RT-qPCR. The results revealed that the expression of Atp1b1 increased significantly in the dapagliflozin-treated group; however, the expression of Atp1a1 and Glut2 did not change. Furthermore, ATP1b1 protein expression level was also increased in the dapagliflozin-treated group. The increased expression of ATP1b1 may facilitate Na+ to be transported into the bloodstream, which could lead to salt retention and hypertension. These effects may blunt the potential BP-lowering effects of the SGLT2 inhibitor. This is also a risk for the type 2 diabetes patients to have hypertension [54].
In conclusion, we demonstrated that the SGLT2 inhibitor, dapagliflozin, increased the expression of NHE3, NaPi-2a in the PT. Furthermore, ATP1b1 was also upregulated, which may facilitate the uptake of the Na+ into the blood.
Ethics Statement
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors declare that they have no conflicts of interest to disclose.
Author Contributions
C.M., J.H.F.B., R.J.M.B., B.E.G., and J.G.J.H. designed the study; C.M. and P.J.M. performed experiments; C.M., J.H.F.B., P.J.M., V.A.G., R.J.M.B., B.E.G., and J.G.J.H. analyzed and interpreted data; C.M., J.H.F.B., P.J.M., V.A.G., R.J.M.B., and J.G.J.H. wrote the manuscript. J.H.F.B., B.E.G., R.J.M.B., and J.G.J.H. supervised the study; and all authors approved of the final manuscript.
Acknowledgement
This work was supported by grants from the Radboud Institute for Molecular Life Sciences (to Joost Hoenderop and Bastiaan de Galan).
References
- 1.Chen L, LaRocque LM, Efe O, Wang J, Sands JM, Klein JD. Effect of Dapagliflozin Treatment on Fluid and Electrolyte Balance in Diabetic Rats. Am J Med Sci. 2016 Nov;352((5)):517–523. doi: 10.1016/j.amjms.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi Y, Hu FB. The global implications of diabetes and cancer. Lancet. 2014 Jun;383((9933)):1947–8. doi: 10.1016/S0140-6736(14)60886-2. [DOI] [PubMed] [Google Scholar]
- 3.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010 a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012 Dec;380((9859)):2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Death rates. fall for 8 of 10 top causes; Alzheimer's up to no. 6. Hosp Health Netw. 2008 Jul;82((7)):127. [PubMed] [Google Scholar]
- 5.Campbell NR, Gilbert RE, Leiter LA, Larochelle P, Tobe S, Chockalingam A, Ward R, Morris D, Tsuyuki RT, Harris SB., Hypertension in people with type 2 diabetes Update on pharmacologic management. Can Fam Physician. 2011 Sep;57((9)):997–1002. [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung BM, Li C. Diabetes and hypertension is there a common metabolic pathway? Curr Atheroscler Rep. 2012 Apr;14((2)):160–166. doi: 10.1007/s11883-012-0227-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canale MP, Manca di Villahermosa S, Martino G, Rovella V, Noce A, De Lorenzo A, et al. Obesity-related metabolic syndrome mechanisms of sympathetic overactivity. Int J Endocrinol. 2013;2013:865965. doi: 10.1155/2013/865965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oliva RV, Bakris GL. Blood pressure effects of sodium-glucose co-transport 2 (SGLT2) inhibitors. J Am Soc Hypertens. 2014 May;8((5)):330–9. doi: 10.1016/j.jash.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Gorboulev V, Schürmann A, Vallon V, Kipp H, Jaschke A, Klessen D, et al. Na(+)-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes. 2012 Jan;61((1)):187–96. doi: 10.2337/db11-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vrhovac I, Balen Eror D, Klessen D, Burger C, Breljak D, Kraus O, et al. Localizations of Na(+)-D-glucose cotransporters SGLT1 and SGLT2 in human kidney and of SGLT1 in human small intestine and heart. Pflugers Arch. 2015 Sep;467((9)):1881–98. doi: 10.1007/s00424-014-1619-7. [DOI] [PubMed] [Google Scholar]
- 11.Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. 2011 Apr;91((2)):733–94. doi: 10.1152/physrev.00055.2009. [DOI] [PubMed] [Google Scholar]
- 12.Plosker GL. Dapagliflozin a review of its use in type 2 diabetes mellitus. Drugs. 2012 Dec;72((17)):2289–312. doi: 10.2165/11209910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Ptaszynska A, Johnsson KM, Parikh SJ, de Bruin TW, Apanovitch AM, List JF. Safety profile of dapagliflozin for type 2 diabetes pooled analysis of clinical studies for overall safety and rare events. Drug Saf. 2014 Oct;37((10)):815–29. doi: 10.1007/s40264-014-0213-4. [DOI] [PubMed] [Google Scholar]
- 14.Wilding JP, Woo V, Rohwedder K, Sugg J, Parikh S; Dapagliflozin 006 Study Group. Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin efficacy and safety over 2 years. Diabetes Obes Metab. 2014 Feb;16((2)):124–36. doi: 10.1111/dom.12187. [DOI] [PubMed] [Google Scholar]
- 15.Kaku K, Inoue S, Matsuoka O, Kiyosue A, Azuma H, Hayashi N, et al. Efficacy and safety of dapagliflozin as a monotherapy for type 2 diabetes mellitus in Japanese patients with inadequate glycaemic control a phase II multicentre, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2013 May;15((5)):432–40. doi: 10.1111/dom.12047. [DOI] [PubMed] [Google Scholar]
- 16.Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010 Oct;33((10)):2217–24. doi: 10.2337/dc10-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lovshin JA, Gilbert RE. Are SGLT2 inhibitors reasonable antihypertensive drugs and renoprotective? Curr Hypertens Rep. 2015 Jun;17((6)):551. doi: 10.1007/s11906-015-0551-3. [DOI] [PubMed] [Google Scholar]
- 18.Wright JT, Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, et al. SPRINT Research Group A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015 Nov;373((22)):2103–16. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014 Apr;85((4)):962–71. doi: 10.1038/ki.2013.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osorio H, Bautista R, Rios A, Franco M, Santamaría J, Escalante B. Effect of treatment with losartan on salt sensitivity and SGLT2 expression in hypertensive diabetic rats. Diabetes Res Clin Pract. 2009 Dec;86((3)):e46–e49. doi: 10.1016/j.diabres.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Vallon V, Thomson SC. Renal function in diabetic disease models the tubular system in the pathophysiology of the diabetic kidney. Annu Rev Physiol. 2012;74((1)):351–375. doi: 10.1146/annurev-physiol-020911-153333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujimoto M, Naito K, Kubota T. Electrochemical profile for ion transport across the membrane of proximal tubular cells. Membr Biochem. 1980;3((1-2)):67–97. doi: 10.3109/09687688009063879. [DOI] [PubMed] [Google Scholar]
- 23.Cadnapaphornchai MA, Kim YW, Gurevich AK, Summer SN, Falk S, Thurman JM, et al. Urinary concentrating defect in hypothyroid rats role of sodium, potassium, 2-chloride co-transporter, and aquaporins. J Am Soc Nephrol. 2003 Mar;14((3)):566–74. doi: 10.1097/01.asn.0000053417.33945.63. [DOI] [PubMed] [Google Scholar]
- 24.Palmer LG, Frindt G. Amiloride-sensitive Na channels from the apical membrane of the rat cortical collecting tubule. Proc Natl Acad Sci USA. 1986 Apr;83((8)):2767–70. doi: 10.1073/pnas.83.8.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Wijst J, Tutakhel OA, Bos C, Danser AH, Hoorn EJ, Hoenderop JG, et al. Effects of a high-sodium/low-potassium diet on renal calcium and phosphate handling. Am J Physiol Renal Physiol. 2018 Jul;315((1)):F110–F22. doi: 10.1152/ajprenal.00379.2017. [DOI] [PubMed] [Google Scholar]
- 26.Custer M, Lötscher M, Biber J, Murer H, Kaissling B. Expression of Na-P(i) cotransport in rat kidney localization by RT-PCR and immunohistochemistry. Am J Physiol. 1994 May;266((5 Pt 2)):F767–F774. doi: 10.1152/ajprenal.1994.266.5.F767. [DOI] [PubMed] [Google Scholar]
- 27.de Groot T, Sinke AP, Kortenoeven ML, Alsady M, Baumgarten R, Devuyst O, et al. Acetazolamide Attenuates Lithium-Induced Nephrogenic Diabetes Insipidus. J Am Soc Nephrol. 2016 Jul;27((7)):2082–91. doi: 10.1681/ASN.2015070796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gottardi CJ, Caplan MJ. Delivery of Na+ K(+)-ATPase in polarized epithelial cells. Science. 1993 Apr;260((5107)):552–4. doi: 10.1126/science.8386395. [DOI] [PubMed] [Google Scholar]
- 29.de Baaij JH, Kompatscher A, Viering DH, Bos C, Bindels RJ, Hoenderop JG. P2X6 Knockout Mice Exhibit Normal Electrolyte Homeostasis. PLoS One. 2016 Jun;11((6)):e0156803. doi: 10.1371/journal.pone.0156803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albertoni Borghese MF, Majowicz MP, Ortiz MC, Passalacqua MR, Sterin Speziale NB, Vidal NA. Expression and activity of SGLT2 in diabetes induced by streptozotocin relationship with the lipid environment. Nephron, Physiol. 2009;112((3)):45–52. doi: 10.1159/000214214. [DOI] [PubMed] [Google Scholar]
- 31.Vallon V, Gerasimova M, Rose MA, Masuda T, Satriano J, Mayoux E, et al. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am J Physiol Renal Physiol. 2014 Jan;306((2)):F194–F204. doi: 10.1152/ajprenal.00520.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quinn PG, Yeagley D. Insulin regulation of PEPCK gene expression a model for rapid and reversible modulation. Curr Drug Targets Immune Endocr Metabol Disord. 2005 Dec;5((4)):423–37. doi: 10.2174/156800805774912962. [DOI] [PubMed] [Google Scholar]
- 33.Freitas HS, Anhê GF, Melo KF, Okamoto MM, Oliveira-Souza M, Bordin S, et al. Na(+) -glucose transporter-2 messenger ribonucleic acid expression in kidney of diabetic rats correlates with glycemic levels involvement of hepatocyte nuclear factor-1alpha expression and activity. Endocrinology. 2008 Feb;149((2)):717–24. doi: 10.1210/en.2007-1088. [DOI] [PubMed] [Google Scholar]
- 34.Gembardt F, Bartaun C, Jarzebska N, Mayoux E, Todorov VT, Hohenstein B, et al. The SGLT2 inhibitor empagliflozin ameliorates early features of diabetic nephropathy in BTBR ob/ob type 2 diabetic mice with and without hypertension. Am J Physiol Renal Physiol. 2014 Aug;307((3)):F317–F325. doi: 10.1152/ajprenal.00145.2014. [DOI] [PubMed] [Google Scholar]
- 35.List JF, Woo V, Morales E, Tang W, Fiedorek FT. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care. 2009 Apr;32((4)):650–7. doi: 10.2337/dc08-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roth KS., Diagnosis of renal tubular transport disorders A guide for the clinician. Clin Pediatr (Phila) 1988 Oct;27((10)):463–70. doi: 10.1177/000992288802701001. [DOI] [PubMed] [Google Scholar]
- 37.Pratt JH. Central role for ENaC in development of hypertension. J Am Soc Nephrol. 2005 Nov;16((11)):3154–9. doi: 10.1681/ASN.2005050460. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Armando I, Upadhyay K, Pascua A, Jose PA. The regulation of proximal tubular salt transport in hypertension an update. Curr Opin Nephrol Hypertens. 2009 Sep;18((5)):412–20. doi: 10.1097/MNH.0b013e32832f5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu L, Dixit MP, Chen R, Dixit NM, Collins JF, Ghishan FK. Effects of angiotensin II on NaPi-IIa co-transporter expression and activity in rat renal cortex. Biochim Biophys Acta. 2004 Dec;1667((2)):114–21. doi: 10.1016/j.bbamem.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 40.Doris PA. Renal proximal tubule sodium transport and genetic mechanisms of essential hypertension. J Hypertens. 2000 May;18((5)):509–19. doi: 10.1097/00004872-200018050-00002. [DOI] [PubMed] [Google Scholar]
- 41.Ortiz PA, Garvin JL. Intrarenal transport and vasoactive substances in hypertension. Hypertension. 2001 Sep;38((3 Pt 2)):621–4. doi: 10.1161/hy09t1.093361. [DOI] [PubMed] [Google Scholar]
- 42.Staessen JA, Kuznetsova T, Zhang H, Maillard M, Bochud M, Hasenkamp S, et al. Blood pressure and renal sodium handling in relation to genetic variation in the DRD1 promoter and GRK4. Hypertension. 2008 Jun;51((6)):1643–50. doi: 10.1161/HYPERTENSIONAHA.107.109611. [DOI] [PubMed] [Google Scholar]
- 43.Ji W, Foo JN, O'Roak BJ, Zhao H, Larson MG, Simon DB, et al. Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nat Genet. 2008 May;40((5)):592–9. doi: 10.1038/ng.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujita Y, Inagaki N. Renal sodium glucose cotransporter 2 inhibitors as a novel therapeutic approach to treatment of type 2 diabetes clinical data and mechanism of action. J Diabetes Investig. 2014 May;5((3)):265–75. doi: 10.1111/jdi.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santer R, Calado J. Familial renal glucosuria and SGLT2 from a mendelian trait to a therapeutic target. Clin J Am Soc Nephrol. 2010 Jan;5((1)):133–41. doi: 10.2215/CJN.04010609. [DOI] [PubMed] [Google Scholar]
- 46.Kanai Y, Lee WS, You G, Brown D, Hediger MA. The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reabsorptive mechanism for D-glucose. J Clin Invest. 1994 Jan;93((1)):397–404. doi: 10.1172/JCI116972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nomura S. Renal sodium-dependent glucose cotransporter 2 (SGLT2) inhibitors for new anti-diabetic agent. Curr Top Med Chem. 2010;10((4)):411–8. doi: 10.2174/156802610790980567. [DOI] [PubMed] [Google Scholar]
- 48.Avner ED, Sweeney WE, Jr, Finegold DN, Piesco NP, Ellis D. Sodium-potassium ATPase activity mediates cyst formation in metanephric organ culture. Kidney Int. 1985 Sep;28((3)):447–55. doi: 10.1038/ki.1985.151. [DOI] [PubMed] [Google Scholar]
- 49.Ernst SA. Transport ATPase cytochemistry ultrastructural localization of potassium-dependent and potassium-independent phosphatase activities in rat kidney cortex. J Cell Biol. 1975 Sep;66((3)):586–608. doi: 10.1083/jcb.66.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Landon EJ, Jazab N, Forte L. Aldosterone and sodium-potassium-dependent ATPase activity of rat kidney membranes. Am J Physiol. 1966 Oct;211((4)):1050–6. doi: 10.1152/ajplegacy.1966.211.4.1050. [DOI] [PubMed] [Google Scholar]
- 51.Poudel RR. Renal glucose handling in diabetes and sodium glucose cotransporter 2 inhibition. Indian J Endocrinol Metab. 2013 Jul;17((4)):588–93. doi: 10.4103/2230-8210.113725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leloup C, Arluison M, Lepetit N, Cartier N, Marfaing-Jallat P, Ferré P, et al. Glucose transporter 2 (GLUT 2) expression in specific brain nuclei. Brain Res. 1994 Feb;638((1-2)):221–226. doi: 10.1016/0006-8993(94)90653-x. [DOI] [PubMed] [Google Scholar]
- 53.Thorens B. Molecular and cellular physiology of GLUT-2 a high-Km facilitated diffusion glucose transporter. Int Rev Cytol. 1992;137:209–38. doi: 10.1016/s0074-7696(08)62677-7. [DOI] [PubMed] [Google Scholar]
- 54.Ferrannini E, Cushman WC. Diabetes and hypertension the bad companions. Lancet. 2012 Aug;380((9841)):601–10. doi: 10.1016/S0140-6736(12)60987-8. [DOI] [PubMed] [Google Scholar]