Abstract
Background
The intensive use of chemical insecticides against mosquitoes has led to the development of widespread insecticide resistance. Control of Anopheles mosquitoes in malaria endemic areas of sub-Saharan Africa has become increasingly difficult. There is an urgent need for malaria control programmes to adopt more integrated mosquito management approaches that include sustainable, nonchemical solutions. The mermithid nematode Romanomermis iyengari is one of several natural control alternatives to synthetic pesticides for mosquito suppression. This study evaluated the effectiveness of the nematode R. iyengari for control of Anopheles gambiae.
Methods
The nematode R. iyengari was mass-produced, and pre-parasitic stage (J2) were used for laboratory and field experiments. In laboratory experiments, two concentrations of pre-parasitics (5 and 10 J2 per larva) were tested against first- (L1), second- (L2) and third-instar (L3) larvae of An. gambiae. Infected larvae were observed daily to determine their mortality rate and the number of post-parasitic nematodes emerging from dead larvae. In field experiments, 3500, 4000 and 5000 J2/m2 were sprayed in separate natural Anopheles breeding sites. After treatment, the larval mosquito density in the breeding sites was assessed every 5–7 days.
Results
Laboratory results showed that larval An. gambiae is susceptible to nematode infection: 100% L1 larvae died within 24 hours post-treatment, and 100% of both L2 and L3 larvae died within 7 days, regardless of nematode concentrations. The average number of post-parasitic nematodes emerging per larva increased with increasing nematode concentration. In field experiments, the monthly applications of 3500 to 5000 pre-parasitic nematodes per m2 eliminated larval mosquito development in Anopheles- and mixed breeding sites. Larval mosquito density dramatically decreased five days after the first treatment in all treated sites and was maintained at a very low level during the whole experimental period. Basically, only early instar larva were detected in treated sites throughout the test period. The average number of post-parasitic nematodes emerging per larva collected in treated sites was 1.45, 2, and 5.7 respectively for sites treated with 3500, 4000, and 5000 J2/m2.
Conclusions
Malaria mosquito larvae is susceptible to R. iyengari infection in West Africa. Parasitism intensity depends on tested nematode concentrations. Monthly application of 3500 J2/ m2 was enough to control effectively larval An. gambiae in wetlands and floodable locations in West Africa.
Keywords: Anopheles gambiae, Malaria, Entomopathogenic nematodes, Romanomermis iyengari, Vector control
Background
Mosquitoes are disease-carrying insects for chikungunya, dengue, yellow fever, and malaria which remains the deadliest disease worldwide [1, 2]. In the fight against malaria vectors, synthetic insecticides have been extensively used either for spraying breeding sites and homes, or for impregnating mosquito nets and curtains. The intensive use of chemical insecticides has led to the emergence of a resistance to control agents [3]. This resistance occurred for the organochlorine DDT, well-known for its shock effect and extreme persistence [4]. This has made the fight against mosquitoes increasingly difficult. While the immediate toxicity of most of the chemicals used is generally low, their continuous use over long periods can cause serious harm to public health, especially with regard to fertility and cancer [5, 6].
Given these problems, it is urgent to consider the use of alternative means for vector control. In this way, natural enemies of mosquitoes can be considered. Romanomermis iyengari (Mermithidae) is one of several species of entomopathogenic nematodes which parasitize and kill mosquito larvae [7].
Initial information on R. iyengari probably came from observations made by Ross [8] who reported the presence of mermithid nematodes in a sample of Culex larvae in India. Iyengar [9] found juvenile mermithid nematode parasites in the larvae of seven different species of anopheles. These nematodes were later described by Welch [10] as R. iyengari.
Various studies have demonstrated the effectiveness of these nematodes for mosquito control in some parts of the world. In Tajikistan, Vladimirova et al. [11] demonstrated that R. culicivorax and R. iyengari were much more effective against Anopheles than Culex mosquitoes. In Uzbekistan, Pridantseva et al. [12] demonstrated Anopheles infection with R. iyengari. Studies on mosquitoes conducted in Azerbaijan showed that R. iyengari infects both An. sacharovi and Cx. theileri [13]. The results of field work carried out in Cuba were particularly encouraging. Santamarina et al. [14] demonstrated that for a concentration of 1000 pre-parasitic R. iyengari per square metre of surface area, the percentage of larval reduction was 80–100% for Anopheles. In Mexico, a dose of 2000–3000 pre-parasitic juvenile R. iyengari per square metre produced an infection rate of approximately 85–100% in An. pseudopunctipennis larvae [15]. Similar results were also obtained in Brazil, where 12 natural sites of Anopheles were treated with 2000 infectious nematodes per square metre of surface. After a week, An. albitarsis and An. rondoni populations were reduced by 85–97% [16]. Despite the effectiveness of R. iyengari in regions where the climate is comparable to that of malaria endemic countries, this nematode has never been used in experiments in Africa, which accounts for 91% of deaths caused by malaria [17].
The present study has evaluated the potential of the entomopathogenic nematode R. iyengari for the biological control of Anopheles gambiae, the major vector of malaria in Africa.
Methods
Nematodes production
Production of R. iyengari in Benin [18] was initiated with eggs obtained from the Department of Nematology (University of California, Riverside, USA). Culex quinquefasciatus was used as host for the nematodes. Chickens (Gallus domesticus) were used to supply these mosquitoes with blood meals. A small plastic tray (12 × 8 × 6 cm) containing about 500 ml of water was provided in each mosquito cage for oviposition.
Four days after a blood meal, the egg rafts which contained about 120 eggs each, were collected and 6 of these were deposited in a plastic tray (25 × 15 × 12 cm) containing 2 l water. Five hundred plastic trays were installed on metal shelves in the nematode production room where temperature and relative humidity (RH) were 28 ± 2 °C and 70–90%, respectively. The containers were covered with a mesh screen to prevent oviposition by wild mosquitoes. When the larvae reached the second-instar stage (2 days), they were infected with preparasitic nematodes (second-stage juveniles, J2) of R. iyengari. The infection ratio was 3 J2 per mosquito larva. The nematodes were taken from cultures that had been stored for 8 weeks. These cultures were flooded with sterilised (chlorine-free) water to induce the eclosion of eggs and emergence of infective preparasites from the substrate. Fourteen hours after flooding the cultures (overnight), the water was decanted, and the concentration of nematodes in the water was calculated by volumetric dilution. After 8 days, the mosquito larva died and floated on the surface, indicating the end of the parasitic phase of the nematode. The water containing post-parasitic juveniles (J4) together with the dead mosquito larva and other debris was poured onto a sieve. The sieve containing the larvae and J4 was then placed in a container with clean water. After a few minutes, the J4 passed through the sieve into the clean water so that when the sieve was removed, the dead mosquito larva and debris were separated from the J4 that settled on the bottom of the container. The J4 were collected using a syringe and transferred to a glass beaker with clean water. The J4 were then washed thoroughly several times by sedimentation. Two grams (wet weight) of washed J4 (composed of 457.6 ± 1.38 females and 583 ± 0.59 males, as determined in 25 samples) were deposited in round plastic containers (13 cm diameter) with previously sterilised coconut coir fibres (35 g) and 500 ml sterilized (chlorine-free) water. About 3 h later (when all nematodes had moved into the substrate), the water was decanted, and the containers covered and stored for eight weeks so that the nematodes could reach sexual maturity, mate and deposit eggs. They were kept in a climate-controlled room at 27 ± 2 °C and 85% RH. Every week, condensation water droplets were removed from the containers with cotton tissue to prevent premature hatching of nematodes eggs.
Laboratory test for mosquito larvae susceptibility to R. iyengari nematode
To determine whether R. iyengari can infect and kill An. gambiae, tests were performed on the first three larval stages (L1, L2 and L3). The larvae used for these tests were collected in natural breeding sites of An. gambiae in Cotonou, Benin. Wild larvae were used to ensure the results could be extrapolated to field conditions.
The larvae were harvested on the day the tests were conducted. Once in the laboratory, the larvae were sorted and grouped by larval stage. For each stage, two batches of 100 larvae were established; one batch was infected with pre-parasitic nematodes and the other served as the untreated control group (zero nematode). Fish food was used to feed the experimental larvae throughout the trials. The larvae were incubated in 500 ml of distilled water.
The volumetric dilution method [19] was used to determine the concentration of suspended pre-parasitic nematodes. Concentrations of 5 and 10 pre-parasitic juvenile nematodes per larva were used for the tests.
Four days after infection, 20 larvae were transferred individually into Petri dishes containing 30 ml of distilled water for daily observation. The experimental larvae were kept in a room where the temperature is maintained at 27 °C and observed daily under a stereomicroscope to determine the mortality rate and number of post-parasitic nematodes emerging from dead larvae.
Efficacy tests of R. iyengari against mosquito larvae in a natural setting
Description of treated sites
Surveys were conducted in various districts to identify habitats of Anopheles larvae to be used for the experiments. Three different breeding sites were selected for the tests.
First site: yard of a house located in the 13th district of Cotonou, Benin (West Africa). In this yard, rainwater had stagnated for several months; the water was clear and covered an area of 150–320 m2 (depending on the rainfall). The water at this site was 46.66 cm deep on average and contained only An. gambiae larvae.
Second site: yard of another house located in the 13th district of Cotonou, where rainwater had also stagnated for several months. The water at this site covered an area of 20–50 m2 (depending on the rainfall) and was 32.33 cm deep on average; the water was clean but contained organic waste in some places. It was a mixed site containing An. gambiae and Cx. quinquefasciatus larvae.
Third site: flooded and uninhabited two-room hut, located in the 6th district of Cotonou. The water at the site was clear and covered an area of 20 m2 and was 30 cm deep on average. This site contained only An. gambiae larvae.
The physical and chemical parameters of these sites, i.e. temperature, pH, conductivity, salinity, redox percentage (POR) and total dissolved solids (TDS) are presented in Table 1.
Table 1.
Physical and chemical parameters of Anopheles and mixed breeding sites treated with R. iyengari
| Site 1 | Site 2 | Site 3 | |
|---|---|---|---|
| Temperature (°C) | 31.3 | 31.6 | 26.8 |
| pH | 9.49 | 9.85 | 7.99 |
| Salinity (mg/l) | 0 | 0 | 0.3 |
| Conductivity (µS/cm) | 333 | 317 | 996 |
| POR (mV) | − 135 | − 152 | − 57 |
| TDS (mg/l) | 164 | 156 | 496 |
Experimental procedure
Doses of 3500, 4000 and 5000 pre-parasitic nematodes (juvenile 2, J2) per square metre were applied at sites 1, 2 and 3, respectively. The nematodes were applied once a month at the three sites throughout the rainy season (approximately 5 months).
At each field site, the required nematode suspension was applied over the entire site with a knapsack sprayer. Immediately prior to each application, the larval mosquito density was measured at each site. A total of 3 liters of water was collected at different areas of the site with a 100 ml dipper. Using a Pasteur pipette, the number of larvae was counted and averaged to obtain the larval density expressed as the number of larvae per litre of water. After nematode spraying, the larval density in each site was measured at 5- or 7-day intervals to determine the larval mosquito population dynamics during the experimental period.
Additionally, five days after the first nematode application, at least 20 mosquito larvae were collected at each treated site and subsequently examined in the laboratory to determine if sprayed nematode had effectively infected the larvae. The sampled larvae were transferred individually into Petri dishes and observed daily under a stereomicroscope to determine their mortality rate and the infection intensity (number of post-parasitic nematodes emerging per larva).
Statistical analyses
For the laboratory tests, probit analysis was performed to determine if there was a significant difference between tested nematodes concentrations. Pearsonʼs correlation test was also performed to determine the correlation between nematode concentration and number of post-parasitic nematodes emerging per larva. The SPSS statistics package was used to perform the probit analysis and the correlation test.
For field experiments, the average density of mosquito larvae was calculated for each day of observation and the population dynamics of larval mosquitoes in treated sites was determined. The percentage reduction of mosquito larvae compared to the situation before the first treatment was also calculated for each day of observation; for this, the following formula was used [20]:
where LDpre is the larval density before treatment and LDpost is the larval density after treatment.
In addition, a mixed analysis of variance (ANOVA) on repeated measures was performed on the numbers of larvae counted after nematode spraying. This analysis was preferred over the traditional ANOVA because of a possible dependence between the number of larvae counted during an observation and that of a following one, thus violating one of the ANOVA conditions (independence between observations). In this model, the sites (which received different doses of pre-parasitic nematodes) were taken as the random factor, while the stages of larval development (L1, L2, L3, L4 and pupae) were considered the fixed factor. SASv9.2 software was used to perform the ANOVA test on repeated measures.
Results
Susceptibility of An. gambiae to nematode infection
The laboratory test showed that all L1 larvae died within 24 h after infection at an infection ratio of 10 J2 nematodes per larva, i.e. a mortality rate of 100%. For L2 larvae, the mortality rates were 0%, 30%, 75% and 100%, respectively, at the 4th, 5th, 6th and 7th day after treatment. Regarding L3 larvae, the rates were 0%, 25%, 90% and 100%, respectively, at the 4th, 5th, 6th and 7th day after treatment.
At an infection ratio of 5 J2 per larva, 100% of the L1 larvae were also killed within 24 h post-infection. For L2 larvae, the mortality rates were 0%, 15%, 90% and 100%, respectively, at the 4th, 5th, 6th and 7th day after treatment. Mortality rates for the L3 larvae were 0%, 15%, 65% and 100%, respectively, at the 4th, 5th, 6th and 7th day after treatment. No mortality was recorded in the control larvae during the laboratory tests.
Statistical analysis revealed no significant difference between the two tested nematode concentrations. For L2 larvae, the lethal times (LT50) were 5.45 and 5.46 days, respectively, for 5 and 10 nematodes per larva (χ2 = 19.56, df = 15, P = 0.189). Regarding L3 larvae, the LT50 were 5.70 and 5.35 days, respectively, for 5 and 10 nematodes per larva (χ2 = 10.45, df = 15; P = 0.791).
Table 2 shows the average number of post-parasitic nematodes emerging per larva for each larval stage of An. gambiae. No post-parasitic nematode emerged from L1 larvae regardless of the concentrations tested, and the L2 larvae generated more post-parasitic nematodes than the L3 larvae. Since the mortality rate of L1 larvae was 100% within 24 h after treatment, nematodes did not have sufficient time to complete their development and died therefore with the larvae. For L2 and L3 stages, nematodes developed normally within the larvae. These larvae died when post-parasitic nematodes started emerging (Fig. 1).
Table 2.
Average number of post-parasitic nematodes emerging per larval An. gambiae treated in laboratory
| Infectious nematodes concentration (J2 per larva) | Mean number ± SD of post-parasitic nematodes emerging per larva at each larval stage | ||
|---|---|---|---|
| L1 | L2 | L3 | |
| 10 | 0 | 7.6 ± 3.2 | 6.8 ± 4.4 |
| 5 | 0 | 4.7 ± 3.3 | 4.15 ± 2.3 |
Abbreviation: SD, standard deviation
Fig. 1.

Dead larvae with post-parasitic nematode emergence
Effectiveness of the R. iyengari against An. gambiae in the field
Effects of the dose of 3500 nematodes per m2
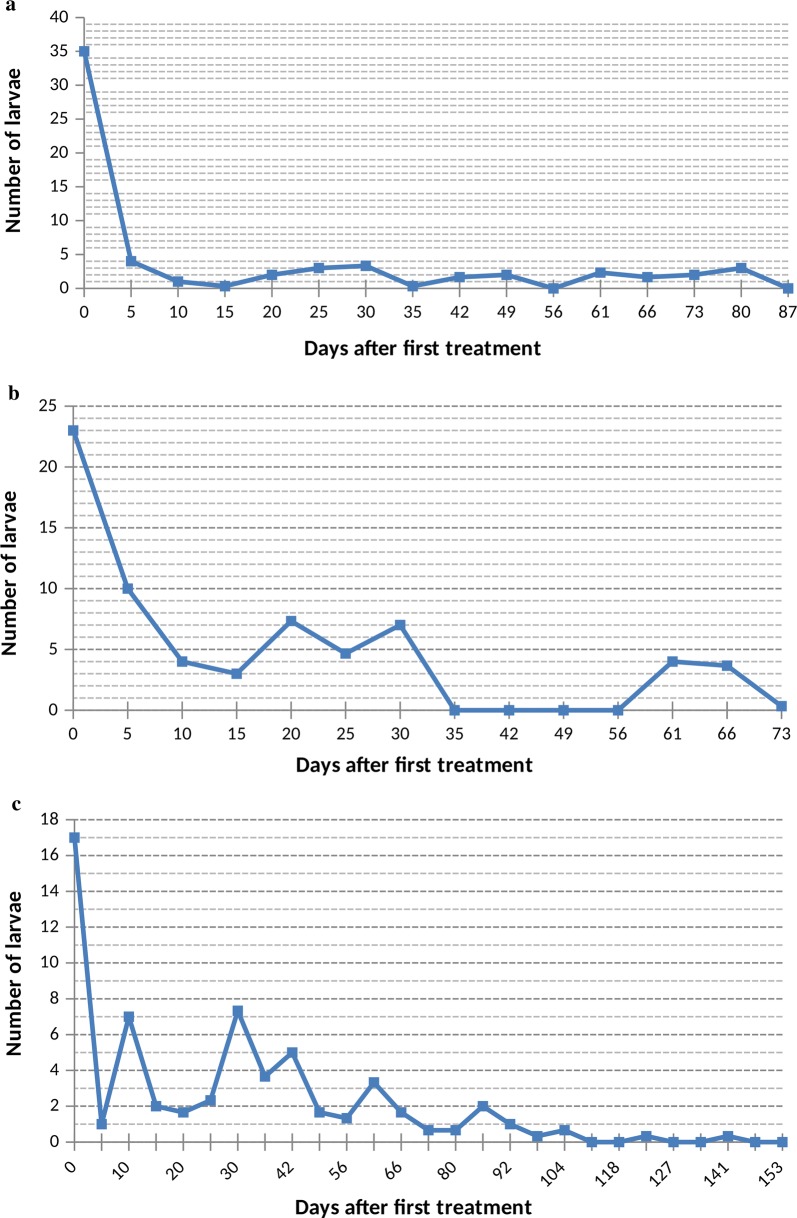
At the Anopheles site treated with 3500 pre-parasitic nematodes per square metre, the density of mosquito larvae dropped from 35 to 4 larvae per liter of water five days after the first treatment. Over the entire experiment period, larval density of Anopheles varied between 0 and 4 larvae per liter of water (Fig. 2a). Throughout the test period, the majority of larvae collected at this site were L1 and L2 larvae. Later stage larvae and pupae became very rare 5 days after the first treatment.
Fig. 2.
Larval mosquito population dynamics in breeding sites treated with nematodes. a 3500 juveniles 2 per m2. b 4000 juveniles 2. c 5000 juveniles 2
Daily observations in the laboratory of larvae sampled at this site 5 days after the first treatment revealed larval mortality rates of 0%, 0%, 20%, 40%, 50%, respectively, at the 5th, 6th, 7th, 8th and 9th day after treatment. The average number of post-parasitic nematodes emerging per larva was 1.45. These results confirm that the reduction in larval density recorded at this site can be attributed to the effect of R. iyengari.
Effects of the dose of 4000 nematodes per m2
The mixed site (containing both Anopheles and Culex larvae) was treated with 4000 J2 nematodes per square metre. Five days after the first treatment, the density of mosquito larvae dropped from 23 to 10 larvae per liter of water. Over the entire experiment period, larval density at this site varied between 0–7.33 larvae per liter of water (Fig. 2b). Throughout the test period, the majority of larvae sampled at this site were of L1 and L2 stages. Larvae of later stages and pupae also became very rare 5 days after the first treatment.
Daily observations in the laboratory of larvae sampled at this mixed site 5 days after the first treatment revealed larval mortality rates of 0%, 10%, 30%, 50% and 60%, respectively, at the 5th, 6th, 7th, 8th and 9th day after treatment. On average, 2 post-parasitic nematodes emerged per larva. Again, this confirms that the reduction in larval density recorded at this site can be attributed to the activity of R. iyengari.
Effects of the dose of 5000 nematodes per m2
At the Anopheles site treated with 5000 J2 per square metre, the density of mosquito larvae dropped from 17 to 1 larvae per litre of water five days after the first treatment. Throughout the test period, larval density at this site varied between 0–7.33 larvae per liter of water (Fig. 2c). Throughout the test duration, the majority of larvae sampled at this site were also L1- and L2-stage larvae. Here too, larvae at later stages and pupae became very rare 5 days after the first treatment.
Daily observations in the laboratory of larvae sampled at this site 5 days after the first treatment revealed larval mortality rates of 0%, 10%, 85% and 100%, respectively, at the 5th, 6th, 7th and 8th day after treatment, while the average number of post-parasitic nematodes emerging per larva was 5.7. These results also confirm that the reduction in larval density recorded at this site is also due to the action of R. iyengari.
Statistical analysis of larval densities observed at all treated sites showed that there is a highly significant difference between the number of larvae of different stages (L1, L2, L3 and L4), regardless of the length of time considered after first treatment of the sites (Table 3). This confirms that L1 and L2 larvae were dominant in the treated sites, indicating that these larvae which come from newly oviposited mosquito eggs did not have time to grow before being killed by R. iyengari.
Table 3.
Results of analysis of variance used in testing for site and stage effects
| Source | df | Type III SS | Mean square | F-value | P-value |
|---|---|---|---|---|---|
| Site | 2 | 5.5740741 | 2.7870370 | 0.71 | 0.5298 |
| Stage | 2 | 512.2345679 | 170.7448560 | 43.34 | 0.0002 |
| Error | 6 | 23.6358025 | 3.9393004 |
Discussion
This study has assessed the susceptibility of An. gambiae larvae to R. iyengari. Our results show that this nematode parasitizes and effectively kills larvae of An. gambiae, and the parasitic intensity (the number of nematodes parasitizing a larva) depends on the concentration of nematodes. The mortality rates observed for each larval stage indicate that L1 and L2 larvae are more susceptible to infection by the nematode, compared to later stage larvae. Authors who obtained similar results have attributed this to the fact that the thin cuticle of young larvae facilitates the invasion of pre-parasitic nematodes, which have more difficulty penetrating the thicker cuticles of older larvae [21–24].
Regardless of the nematode concentrations tested (10 or 5 J2 per larva), all L1 An. gambiae larvae died 24 hours after the infection, and 7 days were sufficient for later stage larvae to be killed by R. iyengari. These results indicate that R. iyengari can be effective in controlling this mosquito species in natural settings in Benin, West Africa.
In the field, larval density in the Anopheles and mixed breeding sites fell sharply 5 days after the nematode application. The larvae collected at treated sites and incubated in the laboratory also showed significant mortality with post-parasitic nematodes emerging from dead larvae. These data clearly indicate that the drastic drop in larval density observed in these sites 5 days after treatment is due to the parasitic nematode.
Various authors have reported similar levels of larval density reduction following the release of R. iyengari in countries such as Cuba and Mexico [14–16, 20, 21, 25–28]. Distinctive feature of the present study is that various nematode concentrations were tested, and results showed that there was no significant difference between these concentrations in the Anopheles and the mixed breeding sites. Doses of 3500, 4000 and 5000 pre-parasitic nematodes had the same effect, namely, the elimination of larval populations in the treated sites. This also confirms the results obtained in laboratory where concentrations of 5 and 10 pre-parasitic nematodes per larva produced the same mortality levels. These results demonstrate that a low dose of R. iyengari per square metre should be sufficient to control An. gambiae in West Africa.
A monthly release of pre-parasitic nematodes in the Anopheles and mixed breeding sites has led to the elimination of larval populations of An. gambiae, a major malaria vector in West Africa. The physicochemical data of water in treated sites were in the range considered to be optimal for allowing R. iyengari to reproduce and to continue parasitizing larval mosquitoes over a long period [12, 26, 29–31]. Perez-Pacheco et al. [20] indicated that a number of topographical and hydrological factors can play role in the effectiveness of R. iyengari against malaria vectors. In experiments conducted in Mexico, these authors obtained higher larval population reduction in the breeding site with stagnant water and lower salinity, compared to breeding sites with flowing water and high salinity. In the present study, high larval population reduction was achieved in all treated sites where water was also stagnant and salinity was about 0 mg/l. We can therefore conclude that characteristics of mosquito breeding sites in South Benin are favourable for the use of R. iyengari in malaria vector control.
Romanomermis iyengari is a parasite specific to mosquito larvae and its mass production is well established [32–37]. A large-scale production system of this worm has been developed for Africa. It uses endogenous and cost-effective materials which are locally available in sub-Saharan Africa. The production system employs three technicians and can produce monthly a sufficient amount of nematodes to treat at least 75 000 square metres of breeding sites [18]. Therefore, the insect parasitic nematode R. iyengari could be easily used as a component of integrated mosquito control programmes in malaria endemic countries.
Furthermore, R. iyengari is harmless for vertebrates. In experiments conducted by Gajanana et al. [38], live infective juveniles of R. iyengari were injected intravenously into animals such as mice, guinea pig, rabbit and chicken; results showed that the overall health of these vertebrates remained unchanged. In treated animals, the histology of organs such as liver, spleen, kidneys, lungs and the gastrointestinal system revealed no lesions linked to the nematode infection. In addition, Gambusia affinis fish exposed over several days to a large number of infective juvenile worms were not affected in any way. Thus, the use of this biological control agent raises no concern for the health of vertebrates.
In Senegal, West Africa, a nematode of the family Mermithidae was found in adults of An. gambiae, An. funestus and An. rufipes [39]. Although the species involved has not been identified, this discovery confirms that West African ecosystems are favourable to mermithid nematodes. Consequently, we believe that enhanced biological control of malaria vectors using R. iyengari would be a great success in West Africa.
Conclusions
Malaria mosquito larvae are susceptible to R. iyengari infection in West Africa. Parasitism intensity depends on tested nematode concentrations. Monthly application of 3500 J2/m2 was sufficient to control effectively larval An. gambiae in wetlands and floodable locations in West Africa.
Acknowledgements
Assistance of Applied Entomology students (Université d’Abomey-Calavi) is greatly appreciated. The “Edward A. Dickson Award for 2011” at University of California-Riverside supported the production and shipment of mermithid nematodes to the Université d’Abomey-Calavi.
Authors’ contributions
AZA, TBCA, RPP, and EGP conceived the project. AZA and TBCA conducted all Laboratory and Field experiments. All co-authors contributed to writing the manuscript. All authors read and approved the final manuscript.
Funding
We would like to thank Grand Challenges Canada (Grant no. 0122-01) for funding this study.
Availability of data and materials
The datasets used and/or analysed during the present study will be made available by the corresponding author upon reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ayaba Z. Abagli, Email: abaglizita@yahoo.fr
Thiery B. C. Alavo, Email: thieryalavo@hotmail.com
Rafael Perez-Pacheco, Email: rafaelperezpacheco@yahoo.com.
Edward G. Platzer, Email: edward.platzer@ucr.edu
References
- 1.Danis M, Gentilini M. Le paludisme fléau mondial. Rev Prat. 1998;48:254–257. [PubMed] [Google Scholar]
- 2.Gachot B, Bruneel F, Behr C. Paludisme pernicieux. Rev Prat. 2001;51:638–643. [PubMed] [Google Scholar]
- 3.Djogbénou L, Pasteur N, Akogbéto M, Weill M, Chandre F. Insecticide resistance in the Anopheles gambiae complex in Benin: a nationwide survey. Med Vet Entomol. 2011;25:256–267. doi: 10.1111/j.1365-2915.2010.00925.x. [DOI] [PubMed] [Google Scholar]
- 4.Balkew M, Elhassen I, Ibrahim M, Gebre-Michael T, Engers H. Very high DDT-resistant population of Anopheles pharoensis Theobald (Diptera: Culicidae) from Gorgora, northern Ethiopia. Parasite. 2006;13:327–329. doi: 10.1051/parasite/2006134327. [DOI] [PubMed] [Google Scholar]
- 5.de Jager C, Aneck-Hahn NH, Bornman MS, Farias P, Spanò M. DDT exposure levels and semen quality of young men from a malaria area in South Africa. Malar J. 2012;11(Suppl. 1):P21. doi: 10.1186/1475-2875-11-S1-P21. [DOI] [Google Scholar]
- 6.Carson R. Silent Spring. Boston: Houghton Mifflin; 1962. [Google Scholar]
- 7.Platzer EG. Mermithid nematodes. J Am Mosq Control Assoc. 2007;23:58–64. doi: 10.2987/8756-971X(2007)23[58:MN]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Ross R. Notes on the parasites of mosquitoes found in India between 1895 and 1899. J Hyg. 1906;6:101–108. doi: 10.1017/S0022172400002722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iyengar MOT. Parasitic nematodes of Anopheles in Bengal. In: Trans Far Eastern Assoc Trop Med 7th Congress. vol. 3. 1927. p. 128–35.
- 10.Welch HE. Romanomermis iyengari sp. nov. Nematoda: Mermithidae Braun, 1883. Pilot Register of Zoology, Card No. 4; 1964.
- 11.Vladimirova VV, Pridantseva EA, Gafurov AK, Muratova ME. Testing the mermithids Romanomermis iyengari and R. culicivorax for the control of blood-sucking mosquitoes in Tadznik SSR. Med Parazitol Parazit Bolezni. 1990;3:42–45. [PubMed] [Google Scholar]
- 12.Pridantseva EA, Lebedeva NI, Shcherban ZP, Kadyrova MK. Assessment of the possibility of using the mermithid Romanomermis iyengari for the control of mosquitoes in Uzbekistan. Med Parazitol Parazit Bolezni. 1990;1:15–17. [PubMed] [Google Scholar]
- 13.Alirzaev GU, Pridantseva EP, Vladimirova VV, Alekseev AN. Prospects for using Romanomermis culicivorax and Romanomermis iyengari (Nematoda: Mermithidae) for mosquito control in Azerbaijan USSR. Med Parazitol Parazit Bolezni. 1990;1:11–15. [PubMed] [Google Scholar]
- 14.Santamarina MA, Garcia AI, Gonzalez BR, Mijares AS, Avila IG, Broche RG. The ability of the nematode Romanomermis iyengari (Welch, 1964) (Nematoda: Mermithidae) to successfully parasitize mosquito larvae under natural conditions. Rev Cubana Med Trop. 1992;44:92–97. [PubMed] [Google Scholar]
- 15.Santamarina MA, Perez PR, Tomas-Martinez SH, Enrique CL, Flores AG. The Romanomermis iyengari parasite for Anopheles pseudopunctipennis suppression in natural habitats in Oaxaca State, Mexico. Pan Am J Public Health. 1999;5:23–28. doi: 10.1590/S1020-49891999000100004. [DOI] [PubMed] [Google Scholar]
- 16.Santamarina MA, Bellini AC. Mass produced Romanomermis iyengari (Nematoda: Mermithidae) applied to anopheline breeding sites in Boa Vista (Roraima), Brazil. Pan Am J Public Health. 2000;7:155–161. doi: 10.1590/s1020-49892000000300003. [DOI] [PubMed] [Google Scholar]
- 17.WHO. Rapport 2011 sur le paludisme dans le monde. 2011. http://www.who.int/malaria/world_malaria_report_2011/fr/index.html. Accessed 25 Apr 2012.
- 18.Alavo TBC, Abagli AZ, Pérez-Pacheco R, Platzer EG. Large-scale production of the malaria vector biocontrol agent Romanomermis iyengari (Nematoda: Mermithidae) in Benin, West Africa. Malar World J. 2015;6:1. doi: 10.5281/zenodo.10869973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen JJ, Willis OR. Procedures for the mass rearing of a mermithid parasite of mosquitoes. Mosq News. 1972;32:226–230. [Google Scholar]
- 20.Pérez-Pacheco R, Rodriguez-Hernandez C, Lara-Reyna J, Montes-Belmont R, Ruiz-Vega J. Control of the mosquito Anopheles pseudopunctipennis (Diptera: Culicidae) with Romanomermis iyengari (Nematoda: Mermithidae) in Oaxaca, Mexico. Biol Control. 2005;32:137–142. doi: 10.1016/j.biocontrol.2004.09.005. [DOI] [Google Scholar]
- 21.Pérez-Pacheco R, Rodriguez-Hernandez C, Lara-Reyna J, Montes-Belmont R, Ruiz-Vega J, Ramírez-Valverde G, et al. Parasitism of Romanomermis iyengari in larvae of three species of mosquito in laboratory, and of Anopheles pseudopunctipennis in the field. Agrociencia. 2004;38:413–421. [Google Scholar]
- 22.Achinelly MF, Micieli MV, Marti GA, Garcia JJ. Susceptibility of neotropical mosquito larvae (Diptera: Culicidae) and non-target aquatic organisms to the entomoparasitic nematode Strelkovimermis spiculatus; Poinar & Camino, 1986 (Nematoda: Mermithidae) Nematology. 2004;6:299–302. doi: 10.1163/1568541041217951. [DOI] [Google Scholar]
- 23.Santamarina MA. Actividad parasitaria de Romanomermis iyengari (Nematoda: Mermithidae) en criaderos naturales de larvas de mosquitos. Misc Zool. 1994;17:59–65. [Google Scholar]
- 24.Petersen JJ. Penetration and development of the mermithid nematode Reesimermis nielseni in eighteen species of mosquitoes. J Nematol. 1975;17:207–210. [PMC free article] [PubMed] [Google Scholar]
- 25.Pérez-Pacheco R, Santamarina MA, Martínez SH, Flores AG. Susceptibilidad de las larvas de Anopheles pseudopunctipennis al parasitismo del nematodo Romanomermis iyengari, estado de Oaxaca, México. Revis Cubana Med Trop. 1998;50:199–202. [PubMed] [Google Scholar]
- 26.Santamarina MA, Garcia AI, Rivera RJ, Solis MA. Release of Romanomermis iyengari (Nematoda: Mermithidae) to control Aedes taeniorhynchus (Diptera: Culicidae) in Punta del Este, Isla de la Juventud, Cuba. J Med Entomol. 1996;33:680–682. doi: 10.1093/jmedent/33.4.680. [DOI] [PubMed] [Google Scholar]
- 27.Santamarina MA, Garcia AI, Gonzalez BR, Mijares AS, Avila IG, Broche RG. Evaluation of the infective capacity of the parasitic nematode Romanomermis iyengari (Welch, 1964) (Nematoda: Mermithidae) in natural habitats of larval mosquitoes. Rev Cubana Med Trop. 1993;45:128–131. [PubMed] [Google Scholar]
- 28.Santamarina MA, Mijares AS. Parasitic activity of Romanomermis iyengari in natural breeding sites of mosquito larvae. Misc Zool. 1995;17:59–65. [Google Scholar]
- 29.Pailey KP, Jayachandran S. Factors inhibiting parasitism of mosquito larvae by the mermithid nematode Romanomermis iyengari in a polluted habitat. Indian J Med Res Sect B. 1987;86:469–474. [PubMed] [Google Scholar]
- 30.Pailey KP, Arunachalam N, Somachary N, Balaraman K. Infectivity of a mermithid nematode Romanomermis iyengari (Welch) in different conductivity levels under laboratory and field conditions. Indian J Exp Biol. 1991;29:570–581. [PubMed] [Google Scholar]
- 31.Pailey KP, Balaraman K. The effect of temperature on different stages of Romanomermis iyengari, a mermithid nematode parasite of mosquitoes. Mem Inst Oswaldo Cruz. 1994;89:635–642. doi: 10.1590/S0074-02761994000400022. [DOI] [PubMed] [Google Scholar]
- 32.Petersen JJ. Nematodes as biological control agents: part I. Mermithidae. Adv Parasitol. 1985;24:307–346. doi: 10.1016/S0065-308X(08)60565-5. [DOI] [PubMed] [Google Scholar]
- 33.Platzer EG, Mullens BA, Shamseldean MM. Mermithid nematodes. In: Grewal P, Ehlers R, Shapiro-Ilan D, editors. Nematodes as biocontrol agents. Wallinford: CABI Publishing; 2005. pp. 411–418. [Google Scholar]
- 34.Popiel I, Hominick WM. Nematodes as biological control agents: part II. Adv Parasitol. 1992;31:381–433. doi: 10.1016/S0065-308X(08)60025-1. [DOI] [Google Scholar]
- 35.Federici BA. The future of microbial insecticides as vector control agents. J Am Mosq Control Assoc. 1995;11:260–268. [PubMed] [Google Scholar]
- 36.Santamarina MA, Perez PR. Reduction of mosquito larval densities in natural sites after introduction of Romanomermis culicivorax (Nematoda: Mermithidae) in Cuba. J Med Entomol. 1997;34:1–4. doi: 10.1093/jmedent/34.1.1. [DOI] [PubMed] [Google Scholar]
- 37.Kerry BR, Hominick WM. Biological control. In: Lee DL, editor. The biology of nematodes. London: Taylor and Francis; 2002. pp. 483–509. [Google Scholar]
- 38.Gajanana A, Kazmi SJ, Bheema RUS, Suguna SG, Chandrahas RK. Studies on a nematode parasite (Romanomermis sp.: Mermithidae) of mosquito larvae in Pondicherry. Indian J Med Res. 1978;68:242–247. [PubMed] [Google Scholar]
- 39.Kobylinski KC, Sylla M, Black IVW, Foy BD. Mermithid nematodes found in adult Anopheles from southeastern Senegal. Parasit Vectors. 2012;5:131. doi: 10.1186/1756-3305-5-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the present study will be made available by the corresponding author upon reasonable request.