Abstract
Stem cell-based therapies exhibit profound therapeutic potential for treating various human diseases, including cancer. Among the cell types that can be used for this purpose, mesenchymal stem cells (MSCs) are considered as promising source of stem cells in personalized cell-based therapies. The inherent tumor-tropic property of MSCs can be used to target cancer cells. Although the impacts of MSCs on tumor progression remain elusive, they have been genetically modified or engineered as targeted anticancer agents which could inhibit tumor growth by blocking different processes of tumor. In addition, there are close interactions between MSCs and cancer stem cells (CSCs). MSCs can regulate the growth of CSCs through paracrine mechanisms. This review aims to focus on the current knowledge about MSCs-based tumor therapies, the opportunities and challenges, as well as the prospective of its further clinical implications.
1. Introduction
MSCs are nonhematopoietic cells that were first discovered from bone marrow and reported approximately 40 years ago by Friedenstein and his coworkers [1, 2]. Studies have shown that MSCs exist in a variety of tissues. To date, MSCs have been successfully isolated from various organs including brain, liver, lung, kidney, muscle, thymus, pancreas, skin, bone marrow adipose tissue, fetal tissues, and umbilical cord [3]. Also, MSCs are known as multipotent cells which can differentiate into adipocytes, myocytes, osteocytes, and chondrocytes [4–6]. In 2006, the International Society for Cellular Therapy proposed three minimal criteria to define human MSCs. They must express CD105, CD90, and CD73 and lack expression of CD45, CD34, CD14 or CD11b, CD79α or CD19, and HLA-DR surface molecules. Additionally, they must adhere to plastic in culture and differentiate into osteocytes, chondrocytes, and adipocytes [7]. In addition, MSCs possess unique immunophenotypic capacity, tissue-repair capacity, and immunoregulatory capacity [8]. Therefore, owing to their relative immune evasiveness and general immune dampening activities, MSCs can be utilized in an allogenic setting and are promising seed cells for cell therapy and tissue engineering [9]. Moreover, various preclinical trials suggest that MSCs show great potential for cancer treatment, although obstacles and risks were described [10].
Studies have shown that MSCs are capable of migrating directionally to specific tissues, which is termed as homing. The tropism property of MSCs into sites of injury and tumor makes them ideal vehicles for targeted tumor therapy, although the exact mechanism of MSCs homing is not completely understood. Ongoing preclinical trials suggest that MSCs are suitable targets for cell therapy in a variety of cancers. However, the antitumor effects of MSCs are still controversial. In various types of cancer, some studies have shown proliferative effects, while others demonstrate inhibitory effects of MSCs on tumors [11]. For example, MSCs have tumoricidal effects on liver, lung cancer cell lines, and pancreatic tumors in vitro and in vivo [12–14]. In contrary, it has been shown that MSCs are capable of enhancing progression and metastasis of types of tumor, such as breast cancer and colon cancer [15–18]. In addition, MSCs may exert therapeutic function through an immune evasive mechanism, which will protect MSCs from immune detection and prolong their persistence in vivo [9]. Moreover, the survival of MSCs in the tumor and biodistribution of MSCs should take more attention when designing a trial, which may influence the results of study. For example, although human MSCs were found by staining in the tumors 1 day after IV injection in a mice model, the cells almost were cleared after 1 week [19]. However, even after 11 weeks MSCs were still observed in the tumor, although at very low numbers [19]. In an in vivo study of colon cancer, exogenous MSCs were still able to regulate immune response of the tumor microenvironment even 1 year after the last MSCs injection [20]. In this review, we summarize recent advances of MSCs in the treatment of cancer and insights into potential strategies for cancer therapy.
2. MSCs and Cancer
2.1. Discrepancy in Impacts of MSCs on Tumor Progression
Extensive studies have been performed to investigate effects of MSCs on tumor in recent decades. However, this issue is still under debate. Controversial results have been reported. Several studies have shown that MSCs promote tumor progression and metastasis through influencing signaling pathway [18, 39], while other studies suggest that MSCs affect the pathways that can suppress both proliferation and apoptosis [13, 40].
Researches have demonstrated that MSCs would be recruited into tumor sites, promoting tumor growth, and angiogenesis through differentiating into cancer-associated myofibroblasts and secretion of proangiogenic cytokines (e.g., interleukin (IL)-6, vascular endothelial growth factor (VEGF), and transforming growth factor-β (TGF-β) [21–23]. In the meanwhile, the recruited MSCs also enhanced tumor metastasis via increasing lysyl oxidase [24]. Another tumor-promoting effect of MSCs is attributed to their protection role for breast cancer cells from immune clearance through modulating regulatory T cells and inhibiting natural killer (NK) cells and cytotoxic T lymphocyte (CTL) functions [25]. Furthermore, MSCs have been found to form a cancer stem cell niche in which tumor cells can preserve the potential to proliferate and sustain the malignant process [41]. Also, increasing evidences suggest that MSCs promote tumor angiogenesis through their potential to differentiate into pericytes or endothelial-like cells as well as by their secretion of trophic factors and cytokines, proangiogenic factors, growth factors, and plasminogen activator [42, 43]. Thus, MSCs promote tumor growth and metastasis through stimulation of angiogenesis, cancer stem cell niche maintenance, and immune protection. Moreover, it has also been showed that MSCs can affect tumor development and progression through miRNAs. In a xenograft tumor model, researchers demonstrated that human umbilical cord MSCs (hUCMSCs) powerfully promote the growth of lung adenocarcinoma (LUAD) cancer cells by transferring miR-410. The findings suggest that modification of hUCMSC-derived extracellular vesicles (hUCMSC-EVs) may be a promising therapeutic option for treatment of tumor [44]. In a mice model, study found that gastric cancer tissue-derived MSCs can significantly promote HGC-27 growth and migration via increasing the expression of miR-221, which may be as a novel biomarker in gastric cancer [45]. The studies supporting MSCs favor tumor growth are summarized in Table 1.
Table 1.
Favoring effect of MSCs on tumors.
| Author | MSC origin | Tumor model | MSC: tumor cell ratio | Outcomes | Mechanisms |
|---|---|---|---|---|---|
| Chaturvedi P et al. [17] | Human bone marrow-derived MSCs | Breast( MDA-MB-231, MDA-MB-43) | 1:1 coinjection | Increased metastasis | activation of the hypoxia-inducible factors (HIFs) |
|
| |||||
| Walter, M. et al. [21] | Human adipose stromal cells (ASCs) | Human breast cancer cell line MCF-7 | 1:1 coinjection | Increased migration and invasion | Secretion of IL-6 |
|
| |||||
| Tsai, Kuo–Shu et al. [22] | Human bone marrow-derived MSCs | Human colorectal cancer cell line HT-29 | 1:100 coinjection | Promoted tumor sphere formation and tumor initiation | IL-6 secreted by MSCs signaled through STAT3 |
|
| |||||
| Zhang, Ting et al. [23] | Human fetal bone marrow stem cells (hBM-MSCs) | 4T1 mouse mammary tumor cell line | 1:1 coinjection | Increased tumor growth | Neovascularization (secretion of macrophage inflammatory protein-2, vascular endothelial growth factor, transforming growth factor-beta and IL-6) |
|
| |||||
| El-Haibi, Christelle P. et al. [24] | Human bone marrow-derived MSCs | MDA-MB-231 and MCF7/Ras breast cancer cells | 1:1 coinjection | Enhanced metastasis | Increased de novo production of lysyl oxidase (LOX) |
|
| |||||
| Patel, Shyam A. et al. [25] | Human bone marrow-derived MSCs | Highly aggressive MDA-MB-231 breast adenocarcinoma, low-invasive MCF-7 breast adenocarcinoma, T47D breast adenocarcinoma, P815 murine mastocytoma | 1:1 (T47D and MSCs 2 × 105 /ml each) were added in 500 μl volumes to attain a 50:1 ratio of mononuclear fractions (PBMC)/MSC and PBMC/T47D | Protected breast cancer cells from immune clearance | Through Tregs, inhibited NK cell and CTL functions |
|
| |||||
| Chandler, Emily M. et al. [26] | Human adipose-derived stem cells (ADSCs) | MCF-7 and MDA-MB-231 | 1:1 co-injection | Promoted tumorigenesis and angiogenesis | Bidirectional signaling; ADSCs differentiated into cancer-associated myofibroblasts |
|
| |||||
| Gonzalez, Maria E. et al. [27] | Human breast cancer metastatic sites-derived MSCs | Breast cancer cell lines MDA-MB-231, MCF7, and MDA-MB-436 | MSCs were orthotopically injected into the mammary fat pads (1 × 106 cells/mouse) | Loss of DDR2 in MSCs impaired their ability to promote DDR2 phosphorylation in BC cells, as well as BC cell alignment, migration, and metastasis |
Reduced migration and metastasis |
In contrast, it has been shown that the unmodified MSCs have antitumor effects both in vitro and in different animal models of cancer, which is attributed to the factors secreted by MSCs that can suppress the proliferation of glioma, melanoma, hepatoma, and breast cancer cells [46–48]. Studies have indicated that MSCs exhibit antiglioma effect through inhibiting vascular growth in glioma cells, which is mediated by the downregulation of platelet-derived growth factor (PDGF)/PDGFR axis [28]. Also, human umbilical cord-derived MSCs (hUC-MSCs) have been shown to inhibit progression of breast cancer by inducing tumor cells death and suppressing angiogenesis [29]. Another study reported that human bone marrow-derived MSCs exhibit the potential to suppress the growth of breast cancer and inhibit lung metastasis by reducing their proliferative ability [30]. Furthermore, MSCs have been shown to have antiangiogenic effect both in vitro and in vivo [49]. MSCs also can inhibit tumor growth in a highly inflammatory and angiogenic Kaposi's sarcoma model [50]. Both in vitro and in vivo studies have shown that MSCs derived from fetal skin can inhibit the growth of human hepatocellular carcinoma (HCC) cells and can reduce cell proliferation, colony formation, and expression of oncogenes [48]. To conclude, MSCs play critical roles in processes of tumor angiogenesis, tumor immune response, and metastasis. The studies reporting MSCs inhibit tumor growth are summarized in Table 2.
Table 2.
Inhibitory effect of MSCs on tumors.
| Author | MSC origin | Tumor model | MSC: tumor cell ratio | Outcomes | Mechanisms |
|---|---|---|---|---|---|
| Ho, Ivy AW et al. [28] | Human bone marrow-derived MSCs | Primary human glioma cells | 1:1 (coinjection) | Reduction in tumor volume and vascular density | Secretion of soluble factors inhibiting endothelial progenitor cells recruitment and impaired tumor angiogenesis |
|
| |||||
| Leng, Liang et al. [29] | Human umbilical cord-derived MSCs | Human breast cancer cell line MDA-MB-231 | 1:1 (injection of MDA-MB-231 first, injection of MSCs 13 days later) | Antitumor effect | Inhibited tumor angiogenesis and induced cell apoptosis |
|
| |||||
| Meleshina, Aleksandra V. et al. [30] | Human bone marrow-derived MSCs | MDA-MB-231 human breast adenocarcinoma cell line | 1:1 (MDAMB-231-Turbo FP650 cells injection fist, injection of MSCs 10 days later) | Suppressed tumor growth and lung metastasis | Reduced proliferative activity of cancer cells |
|
| |||||
| Dasari, Venkata Ramesh et al. [31] | Human umbilical cord blood-derived MSCs | Two high-grade human glioma cell lines (SNB19 and U251) and two xenograft cell lines (4910 and 5310) | 1:4 (MSCs injection 7 days after tumor implantation) | Inhibited tumor growth | Upregulation of phosphatase and tensin homolog deleted on chromosome 10 (PTEN) in tumors induced cellular death through decreasing XIAP expression |
|
| |||||
| Xie, Chan et al. [32] | Human bone marrow-derived MSCs (interferon beta (IFN-β) modified) | HCC cell lines HepG2 and Huh7 | 300:1 (MSC injection 3 days after HCC inoculation) | Inhibition of HCC proliferation | Inhibition of AKT/FOCO3a pathway |
|
| |||||
| Wu, Ning et al. [33] | Human umbilical cords-derived MSCs (transfection of hepatocyte nuclear factor 4α (HNF4α) | Liver cancer cell lines HepG2 and SK-Hep-1 | 1:5 (MSC injection 24 h after tumor implantation) | Inhibited HCC proliferation and invasion | Downregulation of Wnt/β-catenin signaling in HCC cells |
|
| |||||
| François, Sabine et al. [20] | MSCs from human or rat bone marrow | Colorectal cancer cell lines (HT29, HCT-116, LS513, and CC531) | N/A | Attenuation of tumor progression | Modulation of immune component |
2.2. MSCs, Cancer Stem Cells and Cancer Microenvironment
Cancer remains as one of the most challenging diseases despite extensive studies have been performed and novel systemic treatment advances during recent decades. In particular, when cancer is diagnosed to have metastasized, treatments are much less successful; while it can often be treated successfully by surgery or local irradiation before it has spread [51]. Therefore, it is necessary and imperative to better understand the biological processes behind the progression of tumor cells towards metastasis.
Cancer cells in primary tumors reside in a complex microenvironment comprising numerous cell types, including endothelial cells of blood vessels, lymphatic circulation, fibroblasts, and various bone marrow-derived cells, such as macrophages and MSCs. It has been well documented that tumor cells secret chemokines, cytokines, and growth factors recruiting MSCs into the tumor sites. In turn, MSCs, as a component of tumor microenvironment, affect tumor growth and metastasis through secretion of cytokines and chemokines [52, 53]. Thus, the process of tumor progression has been regarded as a result of an evolving crosstalk between different cell types within the tumor and its surrounding host tissue and organ or tumor stroma [54].
Cancer stem cells (CSCs), which possess chemotherapy resistance, have been considered as the root of cancers and can resist chemotherapy, explaining cancer recurrence even many years after therapy is ended. The evidence that CSCs selectively resist therapy is provided by a multitude of observations in cell culture, animal models, and cancer patients. For example, direct analysis of apoptosis during cell culture showed that differentiated colon cancer cells are induced to die after chemotherapy, while CSCs from the same culture survive after toxic damage. Moreover, these surviving CSCs are able to reestablish the culture, indicating that they are responsible for therapy failure [55]. Chemotherapy-resistant CD133+ CSCs were also observed in lung and liver cancer [56, 57]. Similarly, the phenomenon of CSCs escape from therapy was also observed in xenograft studies. Chemotherapy treatment of xenotransplanted CSCs leads to an increase in CD133+ CSCs in the tumor [58]. This showed that CD133+ CSCs are more resistant to chemotherapy drugs in vivo compared to differentiated CD133+ cells. And breast CSCs and GBM CSCs isolated from patient specimens have showed selective resistance to a variety of chemotherapies [59, 60].
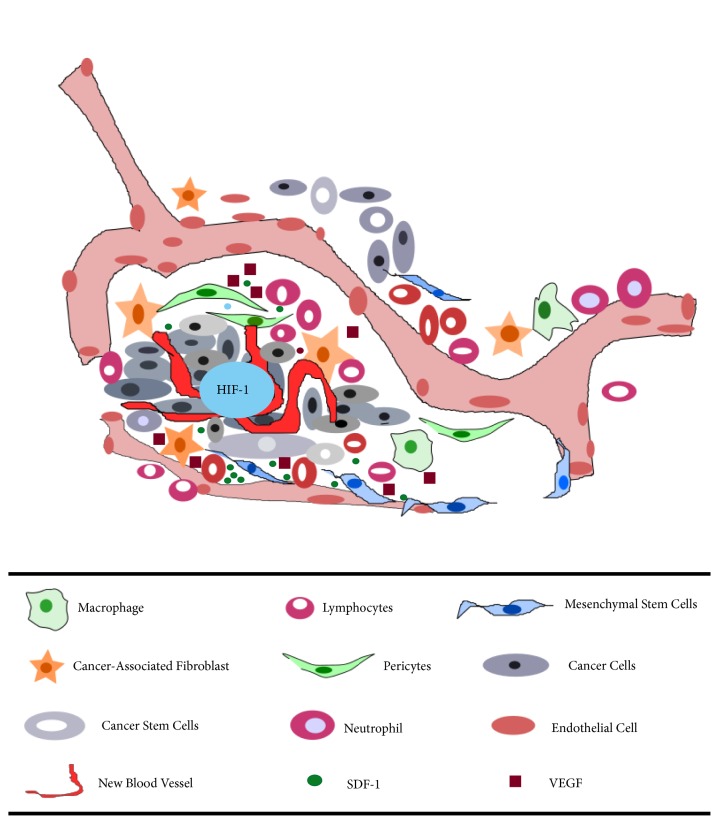
Furthermore, various studies have shown that the tumor stroma plays important roles in the survival, growth, and metastatic progression of cancer. In the hypoxic environment, the tumor stroma can increase its secretion of signaling proteins such as tumor necrosis factor-α (TNF-α), TGF-β, PDGF, and hepatocyte growth factor (HGF) [61]. In the meanwhile, tumor oxygenation status is tightly associated with its aggressive behavior. Experimental solid tumors contain a significant fraction of microregions that are chronically or transiently hypoxic. Hypoxia plays critical roles in tumor progression including tumor angiogenesis, mutation rate, metastasis and resistance to radiation and chemotherapy [62]. Many molecular pathways have been demonstrated to mediate these hypoxia-induced responses in tumors. Among them, hypoxia-inducible factor-1 (HIF-1) is a key signaling pathway regulating tumor responses to hypoxia [63]. Transiently hypoxic microenvironment in solid tumor may represent the stem cell niche to some extent, in which HIF-1α stabilization and activation of stromal-cell derived factor-1 (SDF1), VEGF, and Chemokine (C-X-C motif) Receptor 4 (CXCR4) occur, attracting MSCs homing and recruitment consequently [64, 65]. Furthermore, the state of tumor-induced hypoxia, which often perpetuates the inflammatory state, induces the secretion of numerous growth factors (e.g., endothelial growth factor-A, and fibroblast growth factor), thereby inducing MSCs recruitment and tumor growth through stimulation of tumor angiogenesis [23, 66]. The cancer microenvironment, MSCs, and CSCs are illustrated in Figure 1.
Figure 1.
The primary tumor microenvironment. Cancer cells in primary tumors are surrounded by a complex microenvironment that consists of numerous cells, including endothelial cells of the blood vessel, cancer-associated fibroblast, lymphocytes, neutrophil, MSCs, macrophages, cancer stem cells, and pericytes. Solid tumors contain a significant fraction of microregions that are chronically or transiently hypoxic, in which HIF-1 associated signaling pathway is activated, thus inducing the expression of various downstream genes, including VEGF and SDF-1.
3. MSCs and Antitumor Therapy
3.1. MSCs-Derived Exosomes as Vehicles for Antitumor Therapy
Exosomes are nano-sized (<100 nm) and lipid-bilayer-enclosed extracellular vesicles that are released by many types of cells. They are found to play critical roles in intercellular communication via the transfer of genetic molecules such as mRNA and microRNAs, as well as proteins [67]. A common characteristic of human cancers is the aberrant expressions of either oncogenes, oncomiRs, or tumor suppressors. The MSCs-derived exosomes which contain a variety of miRNAs can be taken up by cancer cells and function in them. For example, miR-100 has been found to be downregulated in all subtypes of breast cancer, including the luminal A, luminal B, basal-like, and human epidermal growth factor receptor 2 (HER2) subtypes [34]. It is enriched in MSCs-derived exosomes and could suppress in vitro angiogenesis through modulating the mTOR/HIF-1α/VEGF signaling axis in breast cancer cells [35].
However, to date, studies with controversial outcomes on MSC-derived exosomes in tumor progression have been reported, including promoted effects [36–38] and suppressive effects [68, 69], as summarized in Table 3. The controversy effects of MSCs-derived exosomes may result from different tissue-derived MSCs used and different component of exosomes applied, different protocols applied for exosome collection, as well as different cancer model and stages of cancer studied. In addition, there is also another issue that the exosomes secreted by MSCs are not created equal. Thus, comprehensive studies are required to advance our knowledge and concerns over cancer research and treatment using MSCs-derived exosomes. One possible approach for clinical application of MSCs-derived exosomes for cancer treatment is that MSCs should be genetically engineered for stable expression of some cancer killer genes before the isolation of exosomes from MSCs, just as Sueon Kim et al. reported for generating antigen-specific CD8+ T cells for adoptive cell therapies against viral infection and tumors [70].
Table 3.
Effects of MSCs-derived exosomes on tumors.
| Author | Exosome origin | Tumor model | Outcomes | Mechanisms |
|---|---|---|---|---|
| Li, Hongdan et al. [34] | Human bone marrow MSCs from patients undergoing hip-replacement surgery | Colon cancer cells (HCT-116, HT-29, and SW-480) | Increased the population of colon cancer stem cells | miR-142-3p in exosomes promoted the Notch signaling pathways by downregulating Numb |
|
| ||||
| Zhang, Yanling et al. [35] | Human omental adipose-derived MSCs from cancer-free female donors | Human EOC cell lines (SKOV3, A2780, and HO-8910) | Promoted cancer progression | Affect proteomic profile of tumor cells via paracrine mechanism |
|
| ||||
| Roccaro AM et al. [36] | Human bone marrow MSCs from normal or cancer patients | Multiple myeloma (MM) cells | MM BM-MSCs–derived exosomes promoted MM tumor growth, normal BM-MSC exosomes inhibited the growth of MM cells | Impact MM cell adhesion |
|
| ||||
| Makiko Ono et al. [37] | Human bone marrow MSCs | BM2 cells | Slowed tumor growth | Exosomal transfer of miR-23b and its suppression of MARCKS |
|
| ||||
| Reza AM et al. [38] | Human adipose MSCs | A2780 and SKOV-3 ovarian cancer cells | Inhibited proliferation of ovarian cancer cells | Upregulates proapoptotic molecules |
3.2. MSCs as Vehicles for Antitumor Therapy
MSCs have the characteristics of tumor tendency and avoidance of immune clearance; thus, it is promising that MSCs are utilized as vehicles to deliver anticancer treatments [71]. It has been demonstrated in a number of preclinical in vitro migration experiments and in various tumor models, such as hepatoma [72], leukemia cells [73], breast cancer [52], and osteosarcoma [74]. It may be an appropriate strategy that MSCs carrying anticancer drugs targeted treatment of tumors. For example, Bonomi et al. observed that MSCs-Paclitaxel (PTX) inhibit the proliferation of human myeloma cells in vitro 3D dynamic culture system [75]. The anticancer effect of MSCs-PTX has also been shown on pancreatic carcinoma cells in vitro [76]. MSCs are also promising tool for cisplatin (CDDP) delivery towards the tumor [71]. In addition, researches have shown that MSCs with suicide genes or apoptotic genes targeting for tumor is a promising approach. In vivo and in vitro studies have shown that the expression of interferon-γ in MSCs transfected by adenovirus can effectively kill glioma cells [77]. It is worth noting that in a model of lung metastasis of prostate cancer, MSCs expressing IFN-β could prolong the survival period, and its possible mechanism is that IFN-β could promote tumor cell apoptosis, inhibit angiogenesis, and increase the activity of natural killer cells [78]. Similarly, adenovirus-transfected MSCs expressing interferon-γ inhibit proliferation of leukemia cells and induce apoptosis of leukemia cells in vitro [79]. In models of lung metastatic carcinoma, a study has found that MSCs carrying TNF-related apoptosis-inducing ligand (TRAIL) reduce tumor growth and recurrence and inhibit the growth of lung metastatic foci in most mice [80]. Study has reported that in glioma mice, tumor tropism of umbilical cord MSCs carrying TRAIL was enhanced after irradiation and its proapoptotic effect on tumor cells was enhanced by MSCs-TRAIL [81]. In addition, the previous and our studies also have demonstrated that MSCs could be genetically modified with herpes simplex virus thymidine kinase (HSV-TK), and the cancer cells could be killed by HSV-TK/GCV suicide gene therapy [82–84]. A recent study showed that histone deacetylase inhibitors (HDACis) induced apoptosis of chemoresistant cells effectively, like CD123/CD47-positive cells, which were found as maybe serving as a key role for chemoresistance in tumor microenvironment. Furthermore, HDACis efficiently targeted and removed chemoresistant leukemia blasts in a xenograft AML mouse model [85].
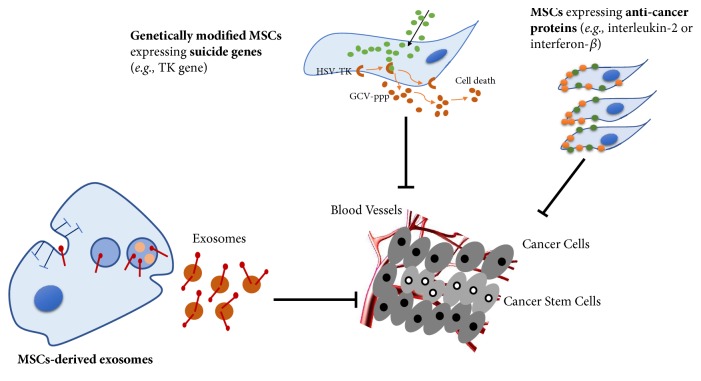
The immune system plays an important role in monitoring the growth of malignant cells. Therefore, stimulating the body's own immune system for antitumor treatment is a highly promising strategy. Interleukins (ILs) are cytokines that regulate inflammation and immune response and have been shown to exhibit antitumor effects through direct tumor-killing or active regulation of the endogenous immune system [86]. MSCs have been utilized to deliver interleukins that can improve the anticancer immune surveillance by activating NK cells and cytotoxic lymphocytes [86]. For example, the IL-18 secreted MSCs were correlative with enhanced T cell infiltration and antitumor immunity in mice bearing invasive and noninvasive gliomas [87]. Similarly, MSCs engineered to express IL-12 prevented metastasis into the lymph nodes and other internal organs as well as increased tumor cell apoptosis in mice bearing preestablished metastases of melanoma, breast, and hepatoma tumors [88]. Also, MSCs engineered to express IL-12 was tested in different mouse tumor models of melanoma and glioma [46]. Other immune-stimulatory molecules, like CX3C chemokine fractalkine (CX3CL), have also been engineered in MSCs. CX3CL1 is known as a strong T cell chemoattractant. Recent studies reveal that CX3CL1 is a driver of T cell migration to the omentum in esophagogastric adenocarcinoma (EAC). Previous research has shown that injection of an adenoviral vector expressing CX3CL1 can induce strong antitumor immune responses by activating both NK cells and T cells [89]. Similarly, intravenous or intratracheal delivery of MSCs-CX3CL1, activating T cells and NK cells, was observed to strongly inhibit process of lung metastasis and increase survival of mice carrying lung metastases cells [90, 91]. Taken together, the tumor-trophic homing capacity makes MSCs ideal cellular delivery vehicles for personalized cell-based targeted-cancer gene therapy. And the strategies of targeted-cancer therapy were summarized in Figure 2.
Figure 2.
Strategies of targeted-cancer therapy. MSCs have been utilized as vehicles to deliver anticancer treatments due to their tumor-tropic property. Genetically modified MSCs expressing suicide genes (e.g., TK gene) have been used to treat cancer effectively in vitro and in vivo through inducing cell death. Additionally, MSCs can be induced to express anticancer proteins (e.g., IL-2 or IFN-β), to generate prodrug activating enzymes or to deliver oncolytic viruses and the active drug for tumor-targeting. Simultaneously, MSCs-derived exosomes also suppressed tumor growth.
3.3. Inhibition of Migration for Antitumor Therapy
Tumor has been seen as a “wound that never heals” which enrolls MSCs in its microenvironment through production of paracrine and endocrine signals. So theoretically, inhibition of MSCs homing will prevent the growth of tumor. For instance, PDGF receptor β (PDGFRβ) has been reported to play an important role in recruitment of MSCs towards tumor sites [92]. And Simona Camorani et al. have demonstrated that interfere with the PDGFRβ-mediated crosstalk between BM-MSCs and tumor cells using a nuclease-resistant RNA aptamer could inhibit the migration of MSCs towards tumor cells and hampering tumor aggressiveness [93]. The classic signaling governing MSCs homing is SDF1-CXCR4 axis. SDF1 is highly expressed in active multiple myeloma, as well as in bone marrow sites of tumor metastasis, neutralizing SDF1 with a high-affinity L-RNA Spiegelmer to SDF-1 has been demonstrated to diminish the disease progression [94]. In tumor biology, a number of studies observed the requirement of Akt and Wnt signaling for the migration, invasion, and survival of tumor cells [95–97]. Recent studies have shown that MSCs are involved in mediating these signaling pathways to influence migration of tumors. In glioma cells and in the nude mice tumors, upregulation of PTEN by hUCBSC downregulated Akt and (phosphoinositide 3-kinase) PI3K signaling pathway results in the inhibition of migration [98]. Similarly, results of a study demonstrated that overexpression of HNF4α suppresses HCC progression by reducing hepatoma cell growth and metastasis through downregulating the Wnt/β-catenin signaling pathway [99].
4. Clinical Trials of MSCs in Cancer Therapy
A small number of registered clinical trials for the treatment of solid tumors with MSCs are underway. These trials have been inspired by successful preclinical trials, although some results have not been published yet. The first clinical trial of gastrointestinal tumors worldwide utilizing genetically engineered MSCs in humans has been reported (TREATME1) [100]; this trial uses MSCs-delivery of HSV-TK under the control of the CCL5 promoter. This is a successful phase I/II clinical trial. Another two registered clinical trials with MSCs have primarily focused on ovarian cancer. One of them is a phase I clinical trial sponsored by M.D. Anderson Cancer Center, in which human MSCs transfected with interferon beta (MSCs-IFNβ). The purposes of this clinical trial are to test the safety of MSCs-IFNβ and to find the highest tolerable dose of human MSCs-IFNβ that can be given to patients with ovarian cancer. Similarly, Mayo Clinic initiated a phase I/II trial to find the side effects and best dose of MSCs infected with oncolytic measles virus encoding NIS (MV-NIS) and to observe its effect on patients with ovarian cancer. In a clinical approach for the treatment of lung cancer, allogeneic MSCs expressing a full length version of TRAIL have been used. MSCs as gene-therapeutic vehicle aim to deliver the TRAIL. In addition, one clinical trial for treating liver cancer with MSCs is on the registry and is recruiting subjects. The purpose of this trial was to study whether MSCs may influence the outcome of graft versus host response in liver transplantation of liver cancer patients. In a phase I clinical test, allogeneic bone marrow-derived MSCs were infused in men with localized prostate cancer [101]. The primary objective was to assess safety and cancer-homing ability of MSCs. However, in this study, MSCs did not home primary tumors in sufficient levels to kill cancer cells or inhibit tumor growth. Although the treatment results have not been published or have not achieved the expected objectives, more attention and patience are needed to promote the clinical transformation of MSCs in tumor treatment. In summary, MSCs and their secreted exosomes have great potential for tumor therapy. Meanwhile, to accelerate the transformation from preclinical research to clinical application, more efficacy and safety of these therapeutic approaches need to be provided by preclinical studies.
5. Conclusions and Prospective
This article primarily discusses the recent progress of the complex roles of multipotent MSCs in tumor microenvironment, progression, and potential clinical applications. The function of CSCs in tumor microenvironment should be paid more attention to, which is critical for development of cancer cells. The roles of miRNAs and signaling pathways in tumor microenvironment need to be intensively studied, which may provide us with new means to accurately treat cancer. We should also pay more attention to the molecular mechanism of antitumorogenic activity of MSCs, which may improve the precision of targeted therapy. Importantly, reducing the growth stimulation and malignant transformation of MSCs in tumor targeted therapy will accelerate clinical transformation.
Acknowledgments
We apologize for the works not cited in the text owing to space restriction. This work was supported by National Natural Science Foundation of China (grant number 81473696, 81673999) and National Natural Science Foundation of Guangdong Province (grant number 2015A030313361; 2017A050506046). This work was also partially supported by the Guangdong Natural Science Funds for Distinguished Young Scholars (2015A030306037).
Abbreviations
- ADSCs:
Adipose-derived stem cells
- ASCs:
Adipose stromal cells
- BC:
Breast cancer
- CSCs:
Cancer stem cells
- CXCR4:
Chemokine (C-X-C motif) Receptor type 4
- CTL:
Cytotoxic T lymphocyte
- CX3CL:
CX3C chemokine fractalkine
- EAC:
Esophagogastric adenocarcinoma
- hBM-MSCs:
Human fetal bone marrow stem cells
- HCC:
Hepatocellular carcinoma
- HER2:
Growth factor receptor 2
- HGF:
Hepatocyte growth factor
- HIFs:
Hypoxia-inducible factors
- hUCMSCs:
Human umbilical cord MSCs
- HSV-TK:
Herpes simplex virus thymidine kinase
- hUCMSC-EVs:
hUCMSC-derived extracellular vesicles
- IL-6:
Interleukin-6
- IFN-β:
Interferon beta
- LUAD:
Lung adenocarcinoma
- LOX:
Lysyl oxidase
- MSCs:
Mesenchymal stem cells
- NK cells:
Natural killer cells
- PDGF:
Platelet-derived growth factor
- PI3K:
Phosphoinositide 3-kinase
- PDGFRβ:
PDGF receptor β
- PTEN:
Phosphatase and tensin homolog deleted on chromosome 10
- PTX:
Paclitaxel
- SDF1:
Stromal-cell derived factor-1
- TGF-β:
Transforming growth factor-β
- TNF-α:
Tumor necrosis factor-α
- TRAIL:
TNF-related apoptosis-inducing ligand
- VEGF:
Vascular endothelial growth factor.
Contributor Information
Gang Li, Email: gangli@cuhk.edu.hk.
Leilei Chen, Email: yutian_1010@sina.com.
Liangliang Xu, Email: xull-2016@gzucm.edu.cn.
Conflicts of Interest
The authors declare no financial conflicts of interest.
Authors' Contributions
Weiping Lin and Linfeng Huang contributed equally as the first authors.
References
- 1.Friedenstein A. J. Precursor cells of mechanocytes. International Review of Cytology-A Survey Of Cell Biology. 1976;47:327–359. doi: 10.1016/S0074-7696(08)60092-3. [DOI] [PubMed] [Google Scholar]
- 2.Friedenstein A. J., Chailakhjan R. K., Lalykina K. S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3(4):393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 3.Murphy M. B., Moncivais K., Caplan A. I. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Experimental & Molecular Medicine. 2013;45(11, article e54) doi: 10.1038/emm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zuk P. A., Zhu M., Mizuno H., et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Engineering Part A. 2001;7(2):211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 5.Minguell J. J., Erices A., Conget P. Mesenchymal stem cells. Experimental Biology and Medicine. 2001;226(6):507–520. doi: 10.1177/153537020122600603. [DOI] [PubMed] [Google Scholar]
- 6.Nombela-Arrieta C., Ritz J., Silberstein L. E. The elusive nature and function of mesenchymal stem cells. Nature Reviews Molecular Cell Biology. 2011;12(2):126–131. doi: 10.1038/nrm3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dominici M., Le Blanc K., Mueller I., et al. Minimal criteria for defining multipotent mesenchymal stromal cells. the international society for cellular therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y., Chen X., Cao W., Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nature Immunology. 2014;15(11):1009–1016. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- 9.Ankrum J. A., Ong J. F., Karp J. M. Mesenchymal stem cells: immune evasive, not immune privileged. Nature Biotechnology. 2014;32(3):252–260. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomes J. P. A., Assoni A. F., Pelatti M., Coatti G., Okamoto S. K., Zatz M. Deepening a simple question: Can MSCs be used to treat cancer? Anticancer Reseach. 2017;37(9):4747–4758. doi: 10.21873/anticanres.11881. [DOI] [PubMed] [Google Scholar]
- 11.Wong R. S. Y. Mesenchymal stem cells: angels or demons? Journal of Biomedicine and Biotechnology. 2011;2011:8. doi: 10.1155/2011/459510.459510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu T., Zhu K., Ke C., et al. Mesenchymal stem cells inhibited development of lung cancer induced by chemical carcinogens in a rat model. American Journal of Translational Research. 2017;9(6):2891–2900. [PMC free article] [PubMed] [Google Scholar]
- 13.Yulyana Y., Ho I. A. W., Sia K. C., et al. Paracrine factors of human fetal MSCs inhibit liver cancer growth through reduced activation of IGF-1R/PI3K/Akt signaling. Molecular Therapy. 2015;23(4):746–756. doi: 10.1038/mt.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kidd S., Caldwell L., Dietrich M., et al. Mesenchymal stromal cells alone or expressing interferon-beta suppress pancreatic tumors in vivo, an effect countered by anti-inflammatory treatment. Cytotherapy. 2010;12(5):615–625. doi: 10.3109/14653241003631815. [DOI] [PubMed] [Google Scholar]
- 15.Albarenque S. M., Zwacka R. M., Mohr A. Both human and mouse mesenchymal stem cells promote breast cancer metastasis. Stem Cell Research. 2011;7(2):163–171. doi: 10.1016/j.scr.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Shinagawa K., Kitadai Y., Tanaka M., et al. Mesenchymal stem cells enhance growth and metastasis of colon cancer. International Journal of Cancer. 2010;127(10):2323–2333. doi: 10.1002/ijc.25440. [DOI] [PubMed] [Google Scholar]
- 17.Chaturvedi P., Gilkes D. M., Wong C. C., et al. Hypoxia-inducible factor–dependent breast cancer–mesenchymal stem cell bidirectional signaling promotes metastasis. The Journal of Clinical Investigation. 2013;123(1):189–205. doi: 10.1172/JCI69244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong W., Tong Y., Li Y., et al. Mesenchymal stem cells in inflammatory microenvironment potently promote metastatic growth of cholangiocarcinoma via activating Akt/NF-kappaB signaling by paracrine CCL5. Oncotarget. 2017;8(43):73693–73704. doi: 10.18632/oncotarget.17793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee R., Yoon N., Reneau J., Prockop D. Preactivation of human MSCs with TNF-alpha enhances tumor-suppressive activity. Cell Stem Cell. 2012;11(6):825–835. doi: 10.1016/j.stem.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 20.François S., Usunier B., Forgue-Lafitte M., et al. Mesenchymal stem cell administration attenuates colon cancer progression by modulating the immune component within the colorectal tumor microenvironment. Stem Cells Translational Medicine. 2019;8(3):285–300. doi: 10.1002/sctm.18-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walter M., Liang S., Ghosh S., Hornsby P. J., Li R. Interleukin 6 secreted from adipose stromal cells promotes migration and invasion of breast cancer cells. Oncogene. 2009;28(30):2745–2755. doi: 10.1038/onc.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai K. S., Yang S. H., Lei Y. P., et al. Mesenchymal stem cells promote formation of colorectal tumors in mice. Gastroenterology. 2011;141(3):1046–1056. doi: 10.1053/j.gastro.2011.05.045. [DOI] [PubMed] [Google Scholar]
- 23.Zhang T., Lee Y. W., Rui Y. F., Cheng T. Y., Jiang X. H., Li G. Bone marrow-derived mesenchymal stem cells promote growth and angiogenesis of breast and prostate tumors. Stem Cell Research & Therapy. 2013;4(3, article 70) doi: 10.1186/scrt221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Haibi C. P., Bell G. W., Zhang J., et al. Critical role for lysyl oxidase in mesenchymal stem cell-driven breast cancer malignancy. Proceedings of the National Acadamy of Sciences of the United States of America. 2012;109(43):17460–17465. doi: 10.1073/pnas.1206653109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel S. A., Meyer J. R., Greco S. J., Corcoran K. E., Bryan M., Rameshwar P. Mesenchymal Stem Cells Protect Breast Cancer Cells through Regulatory T Cells: Role of Mesenchymal Stem Cell-Derived TGF-beta. The Journal of Immunology. 2010;184(10):5885–5894. doi: 10.4049/jimmunol.0903143. [DOI] [PubMed] [Google Scholar]
- 26.Chandler E. M., Seo B. R., Califano J. P., et al. Implanted adipose progenitor cells as physicochemical regulators of breast cancer. Proceedings of the National Acadamy of Sciences of the United States of America. 2012;109(25):9786–9791. doi: 10.1073/pnas.1121160109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalez M. E., Martin E. E., Anwar T., et al. Mesenchymal stem cell-induced DDR2 mediates stromal-breast cancer interactions and metastasis growth. Cell Reports. 2017;18(5):1215–1228. doi: 10.1016/j.celrep.2016.12.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ho I. A. W., Toh H. C., Ng W. H., et al. Human bone marrow-derived mesenchymal stem cells suppress human glioma growth through inhibition of angiogenesis. Stem Cells. 2013;31(1):146–155. doi: 10.1002/stem.1247. [DOI] [PubMed] [Google Scholar]
- 29.Leng L., Wang Y., He N., et al. Molecular imaging for assessment of mesenchymal stem cells mediated breast cancer therapy. Biomaterials. 2014;35(19):5162–5170. doi: 10.1016/j.biomaterials.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meleshina A. V., Cherkasova E. I., Shirmanova M. V., et al. Influence of mesenchymal stem cells on metastasis development in mice in vivo. Stem Cell Research & Therapy. 2015;6(1) doi: 10.1186/s13287-015-0003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dasari V. R., Kaur K., Velpula K. K., et al. Upregulation of PTEN in Glioma Cells by Cord Blood Mesenchymal Stem Cells Inhibits Migration via Downregulation of the PI3K/Akt Pathway. PLoS ONE. 2010;5(4) doi: 10.1371/journal.pone.0010350.e10350 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Xie C., Xie D.-Y., Lin B.-L., et al. Interferon-beta gene-modified human bone marrow mesenchymal stem cells attenuate hepatocellular carcinoma through inhibiting AKT/FOXO3a pathway. British Journal of Cancer. 2013;109(5):1198–1205. doi: 10.1038/bjc.2013.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu N., Zhang Y. L., Wang H. T., et al. Overexpression of hepatocyte nuclear factor 4 alpha in human mesenchymal stem cells suppresses hepatocellular carcinoma development through Wnt/beta-catenin signaling pathway downregulation. Cancer Biology & Therapy. 2016;17(5):558–565. doi: 10.1080/15384047.2016.1177675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen D., Sun Y., Yuan Y., et al. miR-100 induces epithelial-mesenchymal transition but suppresses tumorigenesis, migration and invasion. PLoS Genetics. 2014;10(2) doi: 10.1371/journal.pgen.1004177.e1004177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pakravan K., Babashah S., Sadeghizadeh M., et al. MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1alpha/VEGF signaling axis in breast cancer cells. Cellular Oncology. 2017;40(5):457–470. doi: 10.1007/s13402-017-0335-7. [DOI] [PubMed] [Google Scholar]
- 36.Li H., Li F. Exosomes from BM-MSCs increase the population of CSCs via transfer of miR-142-3p. British Journal of Cancer. 2018;119(6):744–755. doi: 10.1038/s41416-018-0254-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roccaro A. M., Sacco A., Maiso P., et al. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. The Journal of Clinical Investigation. 2013;123(4):1542–1555. doi: 10.1172/jci66517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y., Dong W., Wang J., Cai J., Wang Z. Human omental adipose-derived mesenchymal stem cell-conditioned medium alters the proteomic profile of epithelial ovarian cancer cell lines in vitro. OncoTargets and Therapy. 2017;10:1655–1663. doi: 10.2147/OTT.S129502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang W., Zhong W., Yuan J., et al. Involvement of Wnt/beta-catenin signaling in the mesenchymal stem cells promote metastatic growth and chemoresistance of cholangiocarcinoma. Oncotarget. 2015;6(39):42276–42289. doi: 10.18632/oncotarget.5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin H. D., Fong C.-Y., Biswas A., Choolani M., Bongso A. Human umbilical cord wharton's jelly stem cell conditioned medium induces tumoricidal effects on lymphoma cells through hydrogen peroxide mediation. Journal of Cellular Biochemistry. 2016;117(9):2045–2055. doi: 10.1002/jcb.25501. [DOI] [PubMed] [Google Scholar]
- 41.Ramasamy R., Lam E. W., Soeiro I., Tisato V., Bonnet D., Dazzi F. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia. 2007;21(2):304–310. doi: 10.1038/sj.leu.2404489. [DOI] [PubMed] [Google Scholar]
- 42.Spaeth E. L., Dembinski J. L., Sasser A. K., et al. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS ONE. 2009;4(4) doi: 10.1371/journal.pone.0004992.e4992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bexell D., Gunnarsson S., Tormin A., et al. Bone marrow multipotent mesenchymal stroma cells act as pericyte-like migratory vehicles in experimental gliomas. Molecular Therapy: The Journal of the American Society of Gene Therapy. 2009;17(1):183–190. doi: 10.1038/mt.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dong L., Pu Y., Zhang L., et al. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles promote lung adenocarcinoma growth by transferring miR-410. Cell Death & Disease. 2018;9(2):p. 218. doi: 10.1038/s41419-018-0323-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang M., Zhao C., Shi H., et al. Deregulated microRNAs in gastric cancer tissue-derived mesenchymal stem cells: novel biomarkers and a mechanism for gastric cancer. British Journal of Cancer. 2014;110(5):1199–1210. doi: 10.1038/bjc.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakamura K., Ito Y., Kawano Y., et al. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Therapy. 2004;11(14):1155–1164. doi: 10.1038/sj.gt.3302276. [DOI] [PubMed] [Google Scholar]
- 47.Qiao C., Xu W., Zhu W., et al. Human mesenchymal stem cells isolated from the umbilical cord. Cell Biology International. 2008;32(1):8–15. doi: 10.1016/j.cellbi.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 48.Qiao L., Xu Z., Zhao T., et al. Suppression of tumorigenesis by human mesenchymal stem cells in a hepatoma model. Cell Research. 2008;18(4):500–507. doi: 10.1038/cr.2008.40. [DOI] [PubMed] [Google Scholar]
- 49.Otsu K., Das S., Houser S. D., Quadri S. K., Bhattacharya S., Bhattacharya J. Concentration-dependent inhibition of angiogenesis by mesenchymal stem cells. Blood. 2009;113(18):4197–4205. doi: 10.1182/blood-2008-09-176198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khakoo A. Y., Pati S., Anderson S. A., et al. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi's sarcoma. The Journal of Experimental Medicine. 2006;203(5):1235–1247. doi: 10.1084/jem.20051921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chambers A. F., Groom A. C., MacDonald I. C. Dissemination and growth of cancer cells in metastatic sites. Nature Reviews Cancer. 2002;2(8):563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 52.Karnoub A. E., Dash A. B., Vo A. P., et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449(7162):557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 53.Zhu W., Xu W., Jiang R., et al. Mesenchymal stem cells derived from bone marrow favor tumor cell growth in vivo. Experimental and Molecular Pathology. 2006;80(3):267–274. doi: 10.1016/j.yexmp.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 54.Lorusso G., Rüegg C. The tumor microenvironment and its contribution to tumor evolution toward metastasis. Histochemistry and Cell Biology. 2008;130(6):1091–1103. doi: 10.1007/s00418-008-0530-8. [DOI] [PubMed] [Google Scholar]
- 55.Colak S., Zimberlin C. D., Fessler E. Decreased mitochondrial priming determines chemoresistance of colon cancer stem cells. Cell Death & Differentiation. 2014;21(7):1170–1177. doi: 10.1038/cdd.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bertolini G., Roz L., Perego P., et al. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proceedings of the National Acadamy of Sciences of the United States of America. 2009;106(38):16281–16286. doi: 10.1073/pnas.0905653106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma S., Lee T. K., Zheng B.-J., Chan K. W., Guan X.-Y. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene. 2008;27(12):1749–1758. doi: 10.1038/sj.onc.1210811. [DOI] [PubMed] [Google Scholar]
- 58.Todaro M., Alea M. P., di Stefano A. B., et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1(4):389–402. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 59.Eramo A., Ricci-Vitiani L., Zeuner A., et al. Chemotherapy resistance of glioblastoma stem cells. Cell Death & Differentiation. 2006;13(7):1238–1241. doi: 10.1038/sj.cdd.4401872. [DOI] [PubMed] [Google Scholar]
- 60.Ginestier C., Liu S., Diebel M. E., et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. The Journal of Clinical Investigation. 2010;120(2):485–497. doi: 10.1172/JCI39397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scheel C., Onder T., Karnoub A., Weinberg R. A., Talmadge J. E. Adaptation versus selection: the origins of metastatic behavior. Cancer Research. 2007;67(24):11476–11480. doi: 10.1158/0008-5472.CAN-07-1653. [DOI] [PubMed] [Google Scholar]
- 62.Almendros I., Gozal D. Intermittent hypoxia and cancer: undesirable bed partners? Respiratory Physiology & Neurobiology. 2018;256:79–86. doi: 10.1016/j.resp.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 63.Vaupel P., Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer and Metastasis Reviews. 2007;26(2):225–239. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- 64.Song C., Li G. CXCR4 and matrix metalloproteinase-2 are involved in mesenchymal stromal cell homing and engraftment to tumors. Cytotherapy. 2011;13(5):549–561. doi: 10.3109/14653249.2010.542457. [DOI] [PubMed] [Google Scholar]
- 65.Lin W., Xu L., Zwingenberger S., Gibon E., Goodman S. B., Li G. Mesenchymal stem cells homing to improve bone healing. Journal of Orthopaedic Translation. 2017;9:19–27. doi: 10.1016/j.jot.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spaeth E., Klopp A., Dembinski J., Andreeff M., Marini F. Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene Therapy. 2008;15(10):730–738. doi: 10.1038/gt.2008.39. [DOI] [PubMed] [Google Scholar]
- 67.Lee Y., El Andaloussi S., Wood M. J. A. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Human Molecular Genetics. 2012;21(1):R125–R134. doi: 10.1093/hmg/dds317.dds317 [DOI] [PubMed] [Google Scholar]
- 68.Reza A. M. M. T., Choi Y.-J., Yasuda H., Kim J.-H. Human adipose mesenchymal stem cell-derived exosomal-miRNAs are critical factors for inducing anti-proliferation signalling to A2780 and SKOV-3 ovarian cancer cells. Scientific Reports. 2016;6 doi: 10.1038/srep38498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ono M., Kosaka N., Tominaga N., et al. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Science Signaling. 2014;7(332) doi: 10.1126/scisignal.2005231. [DOI] [PubMed] [Google Scholar]
- 70.Kim S., Sohn H.-J., Lee H.-J., et al. Use of engineered exosomes expressing HLA and costimulatory molecules to generate antigen-specific CD8(+) T cells for adoptive cell therapy. Journal of Immunotherapy. 2017;40(3):83–93. doi: 10.1097/CJI.0000000000000151. [DOI] [PubMed] [Google Scholar]
- 71.Chulpanova D. S., Kitaeva K. V., Tazetdinova L. G., James V., Rizvanov A. A., Solovyeva V. V. Application of mesenchymal stem cells for therapeutic agent delivery in anti-tumor treatment. Frontiers in Pharmacology. 2018;9:p. 259. doi: 10.3389/fphar.2018.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang J., Hou L., Wu X., et al. Inhibitory effect of genetically engineered mesenchymal stem cells with Apoptin on hepatoma cells in vitro and in vivo. Molecular and Cellular Biochemistry. 2016;416(1-2):193–203. doi: 10.1007/s11010-016-2707-0. [DOI] [PubMed] [Google Scholar]
- 73.Pessina A., Coccè V., Pascucci L., et al. Mesenchymal stromal cells primed with Paclitaxel attract and kill leukaemia cells, inhibit angiogenesis and improve survival of leukaemia-bearing mice. British Journal of Haematology. 2013;160(6):766–778. doi: 10.1111/bjh.12196. [DOI] [PubMed] [Google Scholar]
- 74.Duchi S., Sotgiu G., Lucarelli E., et al. Mesenchymal stem cells as delivery vehicle of porphyrin loaded nanoparticles: effective photoinduced in vitro killing of osteosarcoma. Journal of Controlled Release. 2013;168(2):225–237. doi: 10.1016/j.jconrel.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 75.Bonomi A., Steimberg N., Benetti A., et al. Paclitaxel-releasing mesenchymal stromal cells inhibit the growth of multiple myeloma cells in a dynamic 3D culture system. Hematological Oncology. 2017;35(4):693–702. doi: 10.1002/hon.2306. [DOI] [PubMed] [Google Scholar]
- 76.Brini A. T., Coccè V., Ferreira L. M. J., et al. Cell-mediated drug delivery by gingival interdental papilla mesenchymal stromal cells (GinPa-MSCs) loaded with paclitaxel. Expert Opinion on Drug Delivery. 2016;13(6):789–798. doi: 10.1517/17425247.2016.1167037. [DOI] [PubMed] [Google Scholar]
- 77.Nakamizo A., Marini F., Amano T., et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Research. 2005;65(8):3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 78.Ren C., Kumar S., Chanda D., et al. Cancer gene therapy using mesenchymal stem cells expressing interferon-beta in a mouse prostate cancer lung metastasis model. Gene Therapy. 2008;15(21):1446–1453. doi: 10.1038/gt.2008.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li X., Lu Y., Huang W., et al. In vitro effect of adenovirus-mediated human Gamma Interferon gene transfer into human mesenchymal stem cells for chronic myelogenous leukemia. Hematological Oncology. 2006;24(3):151–158. doi: 10.1002/hon.779. [DOI] [PubMed] [Google Scholar]
- 80.Loebinger M. R., Eddaoudi A., Davies D., Janes S. M. Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer Research. 2009;69(10):4134–4142. doi: 10.1158/0008-5472.CAN-08-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim S. M., Oh J. H., Park S. A., et al. Irradiation enhances the tumor tropism and therapeutic potential of tumor necrosis factor-related apoptosis-inducing ligand-secreting human umbilical cord blood-derived mesenchymal stem cells in glioma therapy. Stem Cells. 2010;28(12):2217–2228. doi: 10.1002/stem.543. [DOI] [PubMed] [Google Scholar]
- 82.Uchibori R., Okada T., Ito T., et al. Retroviral vector-producing mesenchymal stem cells for targeted suicide cancer gene therapy. The Journal of Gene Medicine. 2009;11(5):373–381. doi: 10.1002/jgm.1313. [DOI] [PubMed] [Google Scholar]
- 83.Lee W. Y., Zhang T., Lau C. P., Wang C. C., Chan K. M., Li G. Immortalized human fetal bone marrow-derived mesenchymal stromal cell expressing suicide gene for anti-tumor therapy in vitro and in vivo. Cytotherapy. 2013;15(12):1484–1497. doi: 10.1016/j.jcyt.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 84.Song C., Xiang J., Tang J., et al. Thymidine kinase gene modified bone marrow mesenchymal stem cells as vehicles for antitumor therapy. Human Gene Therapy. 2011;22(4):439–449. doi: 10.1089/hum.2010.116. [DOI] [PubMed] [Google Scholar]
- 85.Yan B., Chen Q., Shimada K., et al. Histone deacetylase inhibitor targets CD123/CD47-positive cells and reverse chemoresistance phenotype in acute myeloid leukemia. Leukemia. 2018 doi: 10.1038/s41375-018-0279-6. [DOI] [PubMed] [Google Scholar]
- 86.Okada H., Pollack I. F. Cytokine gene therapy for malignant glioma. Expert Opinion on Biological Therapy. 2004;4(10):1609–1620. doi: 10.1517/14712598.4.10.1609. [DOI] [PubMed] [Google Scholar]
- 87.Xu X., Yang G., Zhang H., Prestwich G. D. Evaluating dual activity LPA receptor pan-antagonist/autotaxin inhibitors as anti-cancer agents in vivo using engineered human tumors. Prostaglandins & Other Lipid Mediators. 2009;89(3-4):140–146. doi: 10.1016/j.prostaglandins.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen X., Lin X., Zhao J., et al. A tumor-selective biotherapy with prolonged impact on established metastases based on cytokine gene-engineered MSCs. Molecular Therapy. 2008;16(4):749–756. doi: 10.1038/mt.2008.3. [DOI] [PubMed] [Google Scholar]
- 89.Xin H., Kikuchi T., Andarini S., et al. Antitumor immune response by CX3CL1 fractalkine gene transfer depends on both NK and T cells. European Journal of Immunology. 2005;35(5):1371–1380. doi: 10.1002/eji.200526042. [DOI] [PubMed] [Google Scholar]
- 90.Xin H., Kanehira M., Mizuguchi H., et al. Targeted delivery of CX3CL1 to multiple lung tumors by mesenchymal stem cells. Stem Cells. 2007;25(7):1618–1626. doi: 10.1634/stemcells.2006-0461. [DOI] [PubMed] [Google Scholar]
- 91.Xin H., Sun R., Kanehira M., et al. Intratracheal delivery of CX3CL1-expressing mesenchymal stem cells to multiple lung tumors. Molecular Medicine. 2009;15(9-10):321–327. doi: 10.2119/molmed.2009.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Veevers-Lowe J., Ball S. G., Shuttleworth A., Kielty C. M. Mesenchymal stem cell migration is regulated by fibronectin through alpha 5 beta 1-integrin-mediated activation of PDGFR-beta and potentiation of growth factor signals. Journal of Cell Science. 2011;124(8):1288–1300. doi: 10.1242/jcs.076935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Camorani S., Hill B. S., Fontanella R., et al. Inhibition of bone marrow-derived mesenchymal stem cells homing towards triple-negative breast cancer microenvironment using an Anti-PDGFR beta aptamer. Theranostics. 2017;7(14):3595–3607. doi: 10.7150/thno.18974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Roccaro A. M., Sacco A., Purschke W. G., et al. SDF-1 inhibition targets the bone marrow niche for cancer therapy. Cell Reports. 2014;9(1):118–128. doi: 10.1016/j.celrep.2014.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Monga S. P. Beta-Catenin Signaling and Roles in Liver Homeostasis, Injury, and Tumorigenesis. Gastroenterology. 2015;148(7):1294–1310. doi: 10.1053/j.gastro.2015.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chiurillo M. A. Role of the Wnt/beta-catenin pathway in gastric cancer: an in-depth literature review. World Journal of Experimental Medicine. 2015;5(2):84–102. doi: 10.5493/wjem.v5.i2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.King T. D., Suto M. J., Li Y. The Wnt/beta-catenin signaling pathway: a potential therapeutic target in the treatment of triple negative breast cancer. Journal of Cellular Biochemistry. 2012;113(1):13–18. doi: 10.1002/jcb.23350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dasari V. R., Kaur K., Velpula K. K., et al. Upregulation of PTEN in glioma cells by cord blood mesenchymal stem cells inhibits migration via downregulation of the PI3K/Akt pathway. PLoS ONE. 2010;5(4) doi: 10.1371/journal.pone.0010350.e10350 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 99.Wu N., Zhang Y., Wang H., et al. Overexpression of hepatocyte nuclear factor 4alpha in human mesenchymal stem cells suppresses hepatocellular carcinoma development through Wnt/beta-catenin signaling pathway downregulation. Cancer Biology & Therapy. 2016;17(5):558–565. doi: 10.1080/15384047.2016.1177675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Niess H., von Einem J. C., Thomas M. N., et al. Treatment of advanced gastrointestinal tumors with genetically modified autologous mesenchymal stromal cells (TREAT-ME1): study protocol of a phase I/II clinical trial. BMC Cancer. 2015;15(1):p. 237. doi: 10.1186/s12885-015-1241-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schweizer M. T., Wang H., Bivalacqua T. J., et al. A phase I study to assess the safety and cancer-homing ability of allogeneic bone marrow-derived mesenchymal stem cells in men with localized prostate cancer. Stem Cells Translational Medicine. 2019;8(5):441–449. doi: 10.1002/sctm.18-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]