Abstract
Objectives
Acute respiratory infections (ARIs) are a global cause of childhood morbidity. We compared temporal trends and socioeconomic disparities for ARI hospitalisations in young children across Western Australia, England and Scotland.
Design
Retrospective population-based cohort studies using linked birth, death and hospitalisation data.
Setting and participants
Population birth cohorts spanning 2000–2012 (Western Australia and Scotland) and 2003–2012 (England).
Outcome measures
ARI hospitalisations in infants (<12 months) and children (1–4 years) were identified through International Classification of Diseases, 10th edition diagnosis codes. We calculated admission rates per 1000 child-years by diagnosis and jurisdiction-specific socioeconomic deprivation and used negative binomial regression to assess temporal trends.
Results
The overall infant ARI admission rate was 44.3/1000 child-years in Western Australia, 40.7/1000 in Scotland and 40.1/1000 in England. Equivalent rates in children aged 1–4 years were 9.0, 7.6 and 7.6. Bronchiolitis was the most common diagnosis. Compared with the least socioeconomically deprived, those most deprived had higher ARI hospitalisation risk (incidence rate ratio 3.9 (95% CI 3.5 to 4.2) for Western Australia; 1.9 (1.7 to 2.1) for England; 1.3 (1.1 to 1.4) for Scotland. ARI admissions in infants were stable in Western Australia but increased annually in England (5%) and Scotland (3%) after adjusting for non-ARI admissions, sex and deprivation.
Conclusions
Admissions for ARI were higher in Western Australia and displayed greater socioeconomic disparities than England and Scotland, where ARI rates are increasing. Prevention programmes focusing on disadvantaged populations in all three countries are likely to translate into real improvements in the burden of ARI in children.
Keywords: acute respiratory infections, hospitalisation, socioeconomic disparity, international comparison, infant, population, record linkage
Strengths and limitations of this study.
We used population-level data from three countries to assess hospitalisation rates and changes over time for acute respiratory infections (ARIs) in children.
Analysis protocols and diagnosis coding was standardised across each country.
Hospitalisation rates for ARIs were described according to level of socioeconomic deprivation.
To control for changing admission thresholds within each country, we adjusted our models for all non-ARI admissions.
A limitation of this study is the different measures of socioeconomic deprivation available across the three countries.
Background
Acute respiratory infections (ARIs) including bronchiolitis, pneumonia and influenza are a major cause of hospitalisation in children worldwide, responsible for ~12 million annual episodes in children under 5 years of age.1 2 In England, the hospital admission rate for ARI increased by 40% from 1999 to 2010 among children aged <15 years3 and bronchiolitis was the most common reason for unplanned admissions in infants from 2010 to 2013.4 While hospitalisations for ARI doubled from 1992 to 2000 in Western Australia,5 they since stabilised 2000–2005.6 Vaccination programmes including influenza, pertussis and pneumococcal disease have been implemented in North America, Europe and Australia, but the majority of ARI hospitalisations in high-income countries are now caused by non-vaccine preventable viruses including respiratory syncytial virus (RSV), parainfluenza virus and human metapneumovirus.7
ARI hospitalisations are more common among children from poorer socioeconomic backgrounds.8 9 In addition to access to inadequate healthcare, risk factors for developing severe symptoms of ARIs, including prematurity, low birth weight, congenital anomalies, exposure to environmental tobacco smoke, damp and mould, and household overcrowding are all more common among children growing up in more deprived families in both high-income and low-income settings.10 11 Understanding the impact of socioeconomic disparities on ARI hospitalisations among children (both over time and between countries) can provide an estimate of the preventable proportion of ARI. Linkage of administrative health datasets provides a platform to investigate these trends in populations over many years. Additionally, the availability of comparable hospital admission datasets with similar coding systems using International Classification of Diseases, 10th edition (ICD-10) diagnosis codes allows comparison of hospitalisation rates among children for ARI according to deprivation level.
Using record linkage resources within Western Australia, England and Scotland, we conducted a comparative analysis of the three jurisdictions to investigate the hospitalisation rates for ARI in children aged <5 years. All three jurisdictions have publicly funded healthcare with free access to primary and public hospital care. Each jurisdiction has established childhood vaccination programmes targeting ARIs. This includes diphtheria, tetanus, pertussis, Haemophilus influenzae type B (three dose infant schedule), pneumococcal disease (2+1 schedule) and recently, seasonal influenza. Excluding influenza, vaccination coverage at age 12 months is >90% for all three jurisdictions.12 13 Our aim was to compare population-based hospitalisation rates by ARI diagnosis, age and level of socioeconomic deprivation and assess how ARI hospitalisation rates have changed over time.
Methods
Data sources and study populations
We conducted separate population-based birth cohort studies using administrative data from Western Australia, England and Scotland. Western Australia covers the western third of Australia, an area of 2.5 million square kilometres with a population of ~2.6 million,14 3.6% of whom identify as being Aboriginal and/or Torres Strait Islander (herein referred to as Aboriginal).15 Births were identified from the Midwives’ Notification System and Birth Register, deaths were identified from the death register and hospitalisations were recorded in the Hospital Morbidity Database Collection that provides full coverage of all hospital separations (hereafter referred to as hospitalisations). In the absence of a unique person identifier in Australia, extracted data were probabilistically linked by the Western Australian Data Linkage Branch using a series of demographic identifiers using an established best practice protocol.16 17 Aboriginal status was derived using a validated algorithm using Aboriginal identification information across all available records.18 England has a population of 53.9 million.19 The birth cohort was established by linking hospital birth and delivery records from the Hospital Episode Statistics database.20 Hospitalisations and deaths were identified via linkage to mortality registration data from the Office for National Statistics.21 Data linkage in England was carried out by National Health Service (NHS) Digital, using a deterministic algorithm based on the NHS number (a unique patient identifier in the English NHS), postcode, date of birth, sex and local hospital numbers. Scotland has a population of 5.3 million.19 The Scottish birth cohort was developed through linking data from birth registration and maternity databases.22 23 Hospitalisations and deaths were identified via linkage to the Scottish Morbidity Record 01 and mortality records using deterministic linkage carried out by the electronic Data Research and Innovation Service based on the Community Health Index number, a unique identifier recorded on all births and subsequent encounters within the Scottish NHS.
The datasets represented 99.9% of all births in Western Australia, 97.5% in England24 and 100% in Scotland with full coverage of inpatient and day admissions. Our study population comprised of singleton births in Western Australia and Scotland 2000–2012 and England 2003–2012. Multiple births were excluded due to a higher likelihood of linkage error. Children were followed from birth until their fifth birthday, date of death or 30 June 2013 (the end of follow-up) or (Scotland only) date of emigration, whichever occurred first.
Outcome measures
Our outcome measure was an ARI emergency hospitalisation for children in their first 5 years of life. All interhospital transfers were collapsed into a single admission. We identified hospitalisations for ARI using a selection of ICD-10 diagnosis codes (ICD-10-AM for Western Australia).25 Hospitalisation data for each jurisdiction provided a principal diagnosis code and up to 20 secondary diagnosis codes in Western Australia, 19 in England and 5 in Scotland. We identified ARI hospitalisations using the principal diagnosis code and all the available additional diagnosis codes as six diagnostic groups: whooping cough (A37), influenza (J09-J11), pneumonia (J12-J18, B59, B05.2, B37.1, B01.2), bronchitis (J20, J40), bronchiolitis (J21) and unspecified acute lower respiratory infection (ALRI) (J22). Consistent with our previous Western Australian work,6 ARI hospitalisations within 14 days of a previous ARI hospitalisation were classified as a single infection episode. In such cases, we applied a hierarchical diagnosis algorithm6 within the readmission set in order to code an overall principal diagnosis. This algorithm ranked diagnoses in order of disease severity: whooping cough, pneumonia, bronchiolitis, influenza, unspecified ALRI and bronchitis. Children with missing data on sex or deprivation were excluded from the analyses. Deaths due to ARI in these populations are rare and our data would be not sufficiently powered to assess mortality rates in this cohort, especially for Western Australia and Scotland. As such we do not report ARI-related mortality rates here and focus our outcome measure on ARI-related hospitalisations.
Exposure measures
It is known that hospitalisation rates for ARI are higher in infants aged <12 months than those aged older than 12 months. Thus, we assessed hospitalisations for ARI in infants aged <12 months and young children aged 1–4 years at time of admission. Other exposure measures of interest were sex, level of socioeconomic deprivation and admission year. In Western Australia, socioeconomic deprivation was measured through the index of relative disadvantage (IRSD), one of the four socioeconomic indexes for areas derived by the Australian Bureau of Statistics.26 The IRSD score is derived from 17 different variables including low income, internet connection, unemployment and education.26 Scores were grouped into collectors district, the smallest unit for population-based analyses which, on average, consist of ~200 dwellings. For England, socioeconomic deprivation was measured through the index of multiple deprivation (IMD), based on seven domains of deprivation including income, employment, education, crime, barriers to housing and living environment.27 IMD scores are measured at lower super output area level, covering an average of 1200–1500 households. For Scotland, deprivations scores were based on the Carstairs index, based on four variables including car ownership, male unemployment, overcrowding and low occupational social class. The Carstairs index is measured at postcode sector level, which contains an average of 5000 people.28 In all jurisdictions, socioeconomic deprivation scores were based on mother’s residential address at time of her child’s delivery and were grouped into four levels based on a country-level ranking with the lowest scores representing the most socioeconomically deprived.
Statistical analysis
Consistent methodology was applied to the assembled datasets in the three jurisdictions. We calculated hospitalisation rates per 1000 child-years at risk for each diagnostic grouping of ARI (as principal diagnosis). To assess the impact of including additional diagnosis codes, we compared hospitalisation rates derived using the principal diagnosis code only with rates derived from using the principal plus all additional diagnosis codes (any diagnosis). We used any diagnosis to assess ARI rates by socioeconomic deprivation and year of admission. We present age-specific hospitalisation rates with 95% CIs and where appropriate, rates were compared using incidence rate ratios (IRRs) with 95% CIs. To assess temporal trends, we plotted annual hospitalisation rates in the two age groups for each jurisdiction by admission year for all ARIs and bronchiolitis, pneumonia and unspecified ALRIs. We also used negative binomial regression models to assess linear temporal trends in infant hospitalisations from 2001 to 2012 (Western Australia and Scotland) and 2004 to 2012 (England). Year of admission was included as a linear term in the models, and the natural logarithm of child-years at risk was included as an offset in the models. Trends over time in ARI admission rates were assumed to be statistically significant if the Wald test p value for the coefficient for the linear year term was <0.05. Models were adjusted for sex and the four-level socioeconomic indicator and we present IRRs with 95% CIs. In order to control for overall trends in hospitalisation, we also adjusted the models for the number of all non-ARI emergency admissions.29 All data analyses were conducted within each jurisdiction in Stata V.14.0.30
Patient and public involvement
A community reference group located in Western Australia was consulted during the conduct of this study. No individual patients were involved.
Results
A total of 337 909 (Western Australia), 5 939 009 (England) and 699 590 (Scotland) births were included in the study (see online supplementary table 1). There were 14 480 infant hospitalisations for ARI as a principal diagnosis in Western Australia, 217 985 for England and 26 103 for Scotland giving overall infant hospitalisation rates of 44.3/1000 child-years for Western Australia, 40.7/1000 for Scotland and 40.1/1000 for England. In all jurisdictions, bronchiolitis had the highest hospitalisation rates accounting for 79% of ARI admissions in infants in Western Australia, 79% in England and 84% in Scotland (table 1). ARI hospitalisation rates in infants were higher in Western Australia compared with England and Scotland across all ARI diagnoses, most notably for pneumonia, where rates were 1.4–2.2 times higher compared with England and Scotland. The only exception was for unspecified ALRI where the hospitalisation rate in infants was 70% higher in England than in Scotland and Western Australia. ARI hospitalisation rates in children aged 1–4 years were 19% higher in Western Australia compared with England and Scotland (table 1). The most common ARI principal diagnosis among children aged 1–4 years was pneumonia in Western Australia (42%) and unspecified ALRI in England (54.6%) and Scotland (43.9%). Consequently, hospitalisation rates for pneumonia in Western Australian children aged 1–4 years were 1.5–1.8 times higher than England and Scotland.
Table 1.
Number of admissions and hospitalisation rate for ARI by diagnostic category by principal diagnosis in infants aged <1 year and children aged 1–4 years in Western Australia, England and Scotland
| Diagnosis | Western Australia | England | Scotland | |||
| n (%) | Rate (95% CI)* | n (%) | Rate (95% CI)* | n (%) | Rate (95% CI)* | |
| <1 year† | ||||||
| Whooping cough | 220 (1.6) | 0.7 (0.6 to 0.8) | 2395 (1.1) | 0.5 (0.4 to 0.5) | 372 (1.4) | 0.6 (0.5 to 0.6) |
| Pneumonia | 1278 (9.4) | 4.1 (3.9 to 4.4) | 15 592 (7.2) | 2.9 (2.9 to 3.0) | 1245 (4.8) | 1.9 (1.8 to 2.1) |
| Bronchiolitis | 10 652 (78.7) | 34.4 (33.8 to 35.1) | 171 805 (78.8) | 32.2 (32.1 to 32.4) | 22 021 (84.4) | 34.3 (33.9 to 34.8) |
| Influenza | 407 (3.0) | 1.3 (1.2 to 1.4) | 1627 (0.7) | 0.3 (0.3 to 0.3) | 426 (1.6) | 0.7 (0.6 to 0.7) |
| Unspecified ALRI | 809 (6.0) | 2.6 (2.4 to 2.8) | 24 563 (11.3) | 4.6 (4.5 to 4.7) | 1797 (6.9) | 2.8 (2.7 to 2.9) |
| Bronchitis | 169 (1.2) | 0.5 (0.5 to 0.6) | 2003 (0.9) | 0.4 (0.4 to 0.4) | 242 (0.9) | 0.4 (0.3 to 0.4) |
| All ARI | 13 535 (100.0) | 43.7 (43.0 to 44.5) | 217 985 (100.0) | 40.1 (40.7 to 41.1) | 26 103 (100.0) | 40.7 (40.2 to 41.2) |
| 1–4 years‡ | ||||||
| Whooping cough | 33 (0.4) | 0.04 (0.03 to 0.06) | 95 (0.1) | 0.008 (0.007 to 0.01) | 23 (0.2) | 0.01 (0.01,0.02) |
| Pneumonia | 3031 (41.6) | 3.7 (3.6 to 3.9) | 29 741 (33.2) | 2.5 (2.5 to 2.6) | 3411 (26.9) | 2.1 (2.0 to 2.1) |
| Bronchiolitis | 1893 (26.0) | 2.3 (2.2 to 2.4) | 8283 (9.2) | 0.7 (0.7 to 0.7) | 3141 (24.7) | 1.9 (1.8 to 2.0) |
| Influenza | 366 (5.0) | 0.4 (0.4 to 0.5) | 1714 (1.9) | 0.2 (0.1 to 0.2) | 392 (3.1) | 0.2 (0.2 to 0.3) |
| Unspecified ALRI | 1767 (24.3) | 2.2 (2.1 to 2.3) | 48 910 (54.6) | 4.2 (4.1 to 4.2) | 5570 (43.9) | 3.3 (3.3 to 3.4) |
| Bronchitis | 195 (2.7) | 0.2 (0.2 to 0.3) | 859 (1.0) | 0.1 (0.1 to 0.1) | 161 (1.3) | 0.1 (0.1 to 0.1) |
| All ALRI | 7285 (100.0) | 9.0 (8.8 to 9.2) | 89 602 (100.0) | 7.6 (7.6 to 7.7) | 12 698 (100.0) | 7.6 (7.5 to 7.8) |
*Rate is per 1000/child-years.
†2001–2012 for Western Australia and Scotland; 2004–2012 for England.
‡2005–2012 for Western Australia and Scotland; 2008–2012 for England.
ALRI, acute lower respiratory infection; ARI, acute respiratory infection.
bmjopen-2018-028710supp001.pdf (103KB, pdf)
When ARI hospitalisations were identified based on any diagnosis compared with principal diagnosis only, the difference in hospitalisation rates varied across diagnoses with the most notable difference for unspecified ALRI in Western Australia where rates were 1.5 (95% CI 1.4 to 1.6) times higher in infants when using any diagnosis compared with principal diagnosis only (see online supplementary table 2).
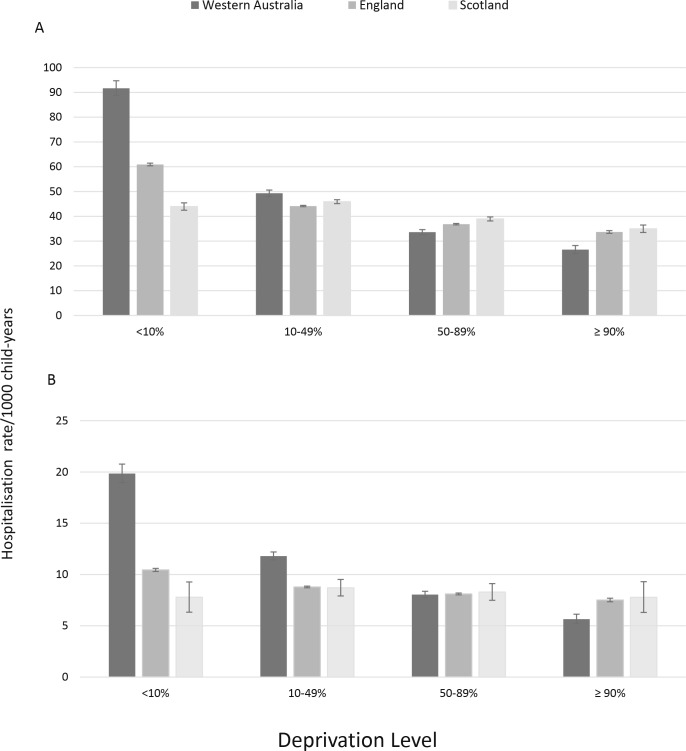
ARI hospitalisation rates were higher for children from the most socioeconomically deprived areas. The association with deprivation was greatest in Western Australia and more marked in infants compared with young children aged 1–4 years (figure 1). The relative difference in ARI hospitalisation rates between the most and least deprived infants was 3.5 (95% CI 3.2 to 3.7) in Western Australia, 1.8 for England and 1.3 for Scotland with similar patterns in children aged 1–4 years (figure 1). In multivariable models, level of socioeconomic deprivation was significantly associated with all ARI categories in all infants but most notably in Western Australia, and in particular, pneumonia (IRR 6.9, 95% CI 5.6 to 8.6) and unspecified ALRI (IRR 8.9, 95% CI 6.7 to 11.8; table 2).
Figure 1.
Hospitalisation rates for ARI in Western Australia, England and Scotland by level of socioeconomic deprivation for (A) infants (<1 year) and (B) young children (1–4 years). Those in the <10% level represent the most deprived and those ≥90% represent those least deprived. ARI, acute respiratory infection.
Table 2.
Risk of hospitalisation for bronchiolitis, pneumonia, unspecified ALRI and overall ARI from log-linear modelling in infants aged <1 year in Western Australia, England and Scotland
| Exposure | Western Australia | England | Scotland |
| IRR (95% CI) | IRR (95% CI) | IRR (95% CI) | |
| Bronchiolitis | |||
| Year* | 0.99 (0.98 to 1.00) | 1.05 (1.04 to 1.07) | 1.04 (1.03 to 1.05) |
| Male | Reference | Reference | Reference |
| Female | 0.68 (0.64 to 0.72) | 0.68 (0.63 to 0.74) | 0.70 (0.64 to 0.77) |
| Deprivation <10% | 3.34 (3.02 to 3.71) | 1.94 (1.73 to 2.19) | 1.28 (1.16 to 1.42) |
| Deprivation 10%–49% | 2.04 (1.75 to 2.37) | 1.48 (1.07 to 2.06) | 1.29 (0.89 to 1.87) |
| Deprivation 50%–89% | 1.36 (1.19 to 1.55) | 1.18 (0.95 to 1.45) | 1.09 (0.85 to 1.40) |
| Deprivation ≥90% | Reference | Reference | Reference |
| Non-ARI admissions | 1.00 (1.00 to 1.00) | 1.00 (1.00 to 1.00) | 1.00 (1.00 to 1.00) |
| Pneumonia | |||
| Year* | 0.94 (0.93 to 0.96) | 0.98 (0.97 to 0.99) | 0.97 (0.94 to 0.99) |
| Male | Reference | Reference | Reference |
| Female | 0.75 (0.67 to 0.84) | 0.76 (0.70 to 0.82) | 0.80 (0.67 to 0.97) |
| Deprivation 0%–10% | 6.91 (5.59 to 8.56) | 1.47 (1.30 to 1.66) | 1.09 (0.88 to 1.37) |
| Deprivation 10%–49% | 3.26 (2.49 to 4.28) | 0.90 (0.65 to 1.25) | 0.74 (0.39 to 1.43) |
| Deprivation 50%–89% | 1.66 (1.29 to 2.13) | 0.86 (0.70 to 1.07) | 0.80 (0.51 to 1.25) |
| Deprivation >90% | Reference | Reference | Reference |
| Non-ARI admissions | 1.00 (1.00 to 1.00) | 1.00 (1.00 to 1.00) | 1.00 (1.00 to 1.00) |
| Unspecified ALRI | |||
| Year* | 0.95 (0.93 to 0.97) | 1.06 (1.05 to 1.07) | 1.02 (1.00 to 1.04) |
| Male | Reference | Reference | Reference |
| Female | 0.62 (0.54 to 0.71) | 0.65 (0.61 to 068) | 0.73 (0.62 to 0.85) |
| Deprivation <10% | 8.90 (6.69 to 11.83) | 1.81 (1.66 to 1.98) | 0.93 (0.78 to 1.12) |
| Deprivation 10%–49% | 4.18 (2.93 to 5.96) | 1.34 (1.06 to 1.70) | 0.84 (0.48 to 1.48) |
| Deprivation 50%–89% | 1.96 (1.40 to 2.73) | 1.11 (0.95 to 1.30) | 0.85 (0.58 to 1.26) |
| Deprivation >90% | Reference | Reference | Reference |
| Non-ARI admissions | 1.00 (1.00 to 1.00) | 1.00 (1.00 to 1.00) | 1.00 (1.00 to 1.00) |
| Total ARI | |||
| Year* | 0.99 (0.98 to 1.00) | 1.05 (1.04 to 1.06) | 1.03 (1.02 to 1.04) |
| Male | Reference | Reference | Reference |
| Female | 0.68 (0.65 to 0.72) | 0.69 (0.64 to 0.73) | 0.71 (0.65 to 0.77) |
| Deprivation 0%–10% | 3.85 (3.50 to 4.21) | 1.87 (1.70 to 2.06) | 1.25 (1.14 to 1.37) |
| Deprivation 10%–49% | 2.22 (1.95 to 2.54) | 1.41 (1.07 to 1.85) | 1.25 (0.88 to 1.77) |
| Deprivation 50%–89% | 1.42 (1.26 to 1.60) | 1.14 (0.96 to 1.36) | 1.08 (0.85 to 1.36) |
| Deprivation >90% | Reference | Reference | Reference |
| Non-ARI admissions | 1.00 (1.00 to 1.00) | 1.00 (1.00 to 1.00) | 1.00 (1.00 to 1.00) |
*Year included as a linear term.
ALRI, acute lower respiratory infection; ARI, acute respiratory infection; IRR, incidence rate ratio.
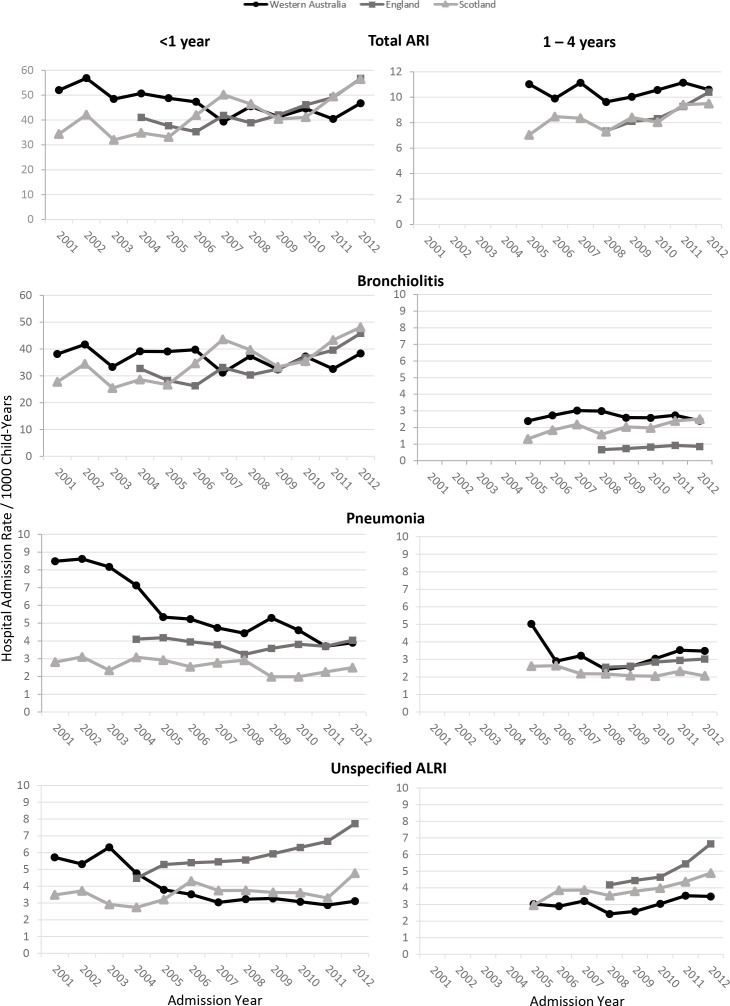
Overall, ARI hospitalisation rates have increased in England and Scotland, but declined (infants) or remained stable (children aged 1–4 years) in Western Australia (figure 2). After adjusting for sex, deprivation and non-ARI emergency hospitalisations, the ARI hospitalisation rate among infants increased by 5% per year in England (IRR 1.05, 95% CI 1.04 to 1.07) and by 3% per year (IRR 1.03, 95% CI 1.02 to 1.04) in Scotland with no statistically significant trend in Western Australia (IRR 0.99, 95% CI 0.98 to 1.00; table 2, figure 2). Similar results were seen for bronchiolitis admissions in infants.
Figure 2.
Hospitalisation rates by year of admission for infants (<1 year) and children (1–4 years) in Western Australia, England and Scotland for ARI, bronchiolitis, pneumonia and unspecified ALRI. ALRI, acute lower respiratory infection; ARI, acute respiratory infection.
Diverging trends were seen with pneumonia and unspecified ALRI across the three jurisdictions with pneumonia hospitalisation rates in infants declining in Western Australia from 9.0/1000 in 2002 to 3.9/1000 in 2012 while rates remained steady around 3–4/1000 in England and 2–3/1000 in Scotland (figure 2). After adjusting for sex, socioeconomic deprivation and non-ARI admissions, the annual decline in pneumonia hospitalisations was 6% in Western Australia (IRR 0.94, 95% CI 0.93 to 0.96), 2% in England and 3% in Scotland (table 2). Unspecified ALRI declined in Western Australia annually by 5% but increased by 6% and 2% annually in England and Scotland (table 2).
Discussion
ARI, particularly bronchiolitis, continues to be an important cause of infant and childhood hospitalisation. The availability of linked administrative data in three economically similar jurisdictions with publicly funded healthcare systems afforded us the opportunity to compare ARI hospitalisation rates in children. Overall, admission rates were highest in Western Australia and decreasing or remaining stable but increasing in England and Scotland. The relative differences in ARI admission rates between children from the most socioeconomically deprived areas to the least deprived areas were largest in Western Australia.
The interpretation of hospitalisation trends across countries is complex. We have found higher rates of ARI admissions in Western Australia compared with England and Scotland which could mean a higher incidence in ARI, a higher risk of developing more severe symptoms, or differences in diagnostic coding or hospital admission thresholds. A recent study comparing admission rates between England and Ontario finding substantially higher rates in England was partly explained by differing admission thresholds from differential waiting practices and policies in emergency departments.4 Comparisons of asthma admissions from national hospital data in Finland and Sweden noted diverging trends citing differences in national coding guidelines and subsequent altered admission thresholds.31 In an attempt to control for changing admissions thresholds over time within each jurisdiction, we adjusted our multivariable models for the overall trend in non-ARI emergency hospital use. However, we could not adjust for differing thresholds between countries. Emergency hospitalisations are increasing at a faster rate in England compared with other parts of the UK32 and our data here suggest that hospitalisations due to unspecified ALRI and bronchiolitis in England are contributing to that increase. It is also possible that diverging trends are a result of diagnostic shifts in that for the same clinical presentations, a diagnosis of unspecified ALRI is given in England while other non-specific codes (including codes we have not assessed) are given in Western Australia and Scotland. The use of additional diagnosis codes for ARI seemed more frequent in Western Australia compared with England and Scotland and should be taken into consideration for future comparative studies using ICD diagnosis codes.
Hospitalisation rates for ARI were significantly associated with level of socioeconomic deprivation, consistent with an earlier analysis in England.33 This association was strongest in Western Australia with IRRs for those in the most deprived level in the order of 3.9 for all ARIs, up to 8.9 for unspecified ALRI. There appeared a linear relationship with level of deprivation and rates of ARI in Western Australia while rates in all levels (bar the most deprived) not differing in England and Scotland. Western Australian data were inclusive of Aboriginal children, an Indigenous population with higher levels of socioeconomic disadvantage34 compared with their non-Aboriginal peers and a significantly higher burden of pneumonia worldwide,6 35 36 despite reductions in the 2000s and further reductions seen in our results here, most likely due to the positive impact of pneumococcal vaccination.6 37 This most likely explains the higher rates of pneumonia seen in Western Australia compared with England and Scotland. We have previously reported that hospitalisation rates for all ARIs are five to seven times higher in young Aboriginal children compared with non-Aboriginal children.9 Aboriginal children also suffer a disproportionate burden of RSV,38 the major cause of bronchiolitis which could explain the higher bronchiolitis rates in Western Australia than in England and Scotland. However, level of socioeconomic deprivation has been associated with hospitalisations for respiratory infections in both Aboriginal and non-Aboriginal children,9 so the contribution of Aboriginal children alone cannot explain the higher socioeconomic disparities seen here. Indeed, when Aboriginal children were removed from the analysis, the socioeconomic disparities remained, although slightly lessened, and were still higher than England and Scotland (eg, the IRR for most deprived children for all ARI reduced from 3.9 to 2.1 and for unspecified ALRI reduced from 8.9 to 2.9 (data not shown)). Respiratory infections continue to be a source of health inequalities among disadvantaged children worldwide. Geographical remoteness is more of an issue in Western Australia due to its sheer geographical size in comparison to England and Scotland. The lack of adequate primary care in rural and remote Australia39 which is often coupled with lower socioeconomic levels could be driving higher hospitalisation rates. Nevertheless, these important findings highlight the need for targeted prevention programmes such as smoking cessation, improved housing and timely vaccination for key respiratory pathogens for the most disadvantaged populations in all three jurisdictions.
Since 2013, the UK had been rolling out a universal seasonal influenza vaccination programme for children aged 2–16 years and from 2018, all Australian states and territories offer free seasonal influenza vaccine to children aged between 6 months and 5 years. Our study period was prior to this time. Relative to other ARI diagnoses, recorded influenza hospitalisation rates are low. Assessing the impact of the universal childhood vaccination programme for influenza is likely to be challenging without linking national-level birth cohorts to infection surveillance and vaccination data. This has already been implemented in Scotland.40 There is also renewed interest in preventing morbidity due to RSV with vaccination.41 Understanding the baseline hospitalisation rates for RSV–bronchiolitis and pneumonia prior to when vaccination is available is critical to aid in implementation and for its ongoing evaluation postimplementation.
We conducted our analysis on near total population birth cohorts in each jurisdiction and thus our outcome measures have narrow CIs and minimum selection bias. An additional strength of the population-based cohort design is standardisation of analysis protocols and the provision of large numbers allowing us to assess temporal trends and associations with less common infections. The hospital morbidity database systems used in all three jurisdictions have the same population coverage of all inpatient admissions and day surgeries further adding to the validity of our estimates. Although Western Australia is a state within Australia and we have made comparisons to country wide data for England and Scotland, the rate of cross-border hospitalisation from Western Australia to other Australian states is very low.42
However, our study does have some limitations. The socioeconomic deprivation scores used were jurisdiction specific and included different items to represent disadvantage. In addition, area-level socioeconomic deprivation was only measured at birth. Therefore, the observed association between area-level socioeconomic deprivation and the rate of ARI admissions may be subject to increasing measurement error as the child’s age increases. How socioeconomic deprivation is associated with morbidity due to ARI at the primary care level is unknown but perhaps likely to aid in explaining disparities in socioeconomic deprivation that we have seen here. While primary care data are more readily available in England and Scotland, limited data with adequate diagnostic information are available for population-based studies in Western Australia. As previously alluded to, there also may be differences in admission thresholds across the three jurisdictions that may explain some higher admission rates across countries. A comparison of emergency department presentations in conjunction with hospitalisations for ARI could be useful here, although diagnostic information from emergency department data is limited,43 and no individual level data on emergency department visits exist in Scotland. Additionally, through our experience of linking routine laboratory data to hospital data in Western Australia, we are aware of unspecific ICD codes that are associated with detections of respiratory viral pathogens.44 We did not include such ICD codes (eg, viral infection of unspecified site ‘B34’) in this analysis. However, we would not expect the potential exclusion of ARI hospitalisations to alter the direction of our results in terms of association with socioeconomic deprivation.
Conclusions
Population-based administrative data from economically similar developed countries provide a powerful tool to conduct international comparative studies that can compare and contrast the epidemiology of, and healthcare responses to, respiratory infections. Western Australia experiences higher admissions in children for ARI and a greater disparity in rates according to level of socioeconomic deprivation. Rates are overall slightly lower in England and Scotland but are increasing, particularly in England. These findings suggest that prevention programmes focusing on disadvantaged populations in all three countries are likely to translate into real improvements in the burden of ARI in children. We are planning to use these administrative data to assess effectiveness of interventions (such as vaccination) and how this may affect disparities in ARI admissions rates according to socioeconomic deprivation.
Supplementary Material
Acknowledgments
We would like to acknowledge the Western Australian Data Custodians, and particularly Alexandra Merchant and Mikhalina Dombrovskaya from the Western Australian Data Linkage Branch, for their assistance and support in collating the data for Western Australia. Source data from England can be accessed by researchers applying to the Health and Social Care Information Centre for England. Copyright © 2017, reused with the permission of NHS Digital. All rights reserved. This research benefits from and contributes to the NIHR Children and Families Policy Research Unit, but was not commissioned by the NIHR Policy Research Programme.
Footnotes
Contributors: HCM, CCB and PH conceived the study design. PF assisted with data cleaning and coding in Western Australia. AZ and MV assisted with data extraction for England and Scotland. Statistical analysis was conducted by HCM (Western Australia) and PH (England and Scotland) with expert advice from NdK with critical revisions for intellectual content from CCB and RG. HCM drafted the first manuscript with PH. All authors read and approved the final manuscript.
Funding: This work was supported by a National Health and Medical Research Council Project Grant (1045668), a University of Western Australia Research Collaboration Award (to HCM) and a Wesfarmers Centre of Vaccines and Infectious Diseases Seed Grant (to HCM, CCB, and PH). CCB and HCM are supported by National Health and Medical Research Council Fellowships (1034254 to HCM and 1111596 to CCB). PH was funded by a National Institute for Health Research postdoctoral fellowship, reference number PDF-2013-06-004. AZ’s PhD studentship is funded by awards to establish the Farr Institute of Health Informatics Research, London, from the Medical Research Council, Arthritis Research UK, British Heart Foundation, Cancer Research UK, Chief Scientist Office, Economic and Social Research Council, Engineering and Physical Sciences Research Council, National Institute for Health Research, National Institute for Social Care and Health Research, and Wellcome Trust (grant MR/K006584/1). AZ is also supported by the Administrative Data Research Centre for England (funded by the Economic and Social Research Council). MV’s PhD studentship is funded by the UBEL DTP of the Economic and Social Research Council. MV was also supported by the Policy Research Unit in the Health of Children, Young People and Families (reference 109/00017), which is funded by the Department of Health Policy Research Programme at UCL. This is an independent report commissioned and funded by the Department of Health.
Disclaimer: The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
Competing interests: None declared.
Ethics approval: Approval to use the Western Australian data was granted by the Western Australian Department of Health Human Research Ethics Committee, the Western Australian Aboriginal Health Ethics Committee and the Western Australian Data Linkage Branch. We have a data sharing agreement with National Health Service (NHS) Digital to use a deidentified extract of Hospital Episode Statistics for research into children’s use of secondary care services; therefore, we did not require ethical approval to use English datasets. For Scotland, approvals were obtained from the Public Benefit and Privacy Panel for Health and Social Care, reference number 1516-0405.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: We cannot share the individual-level data used for this study under our agreements with the data providers. The datasets analysed during the current study can be applied for from the Western Australian Data Linkage System (Western Australia; http://www.datalinkage-wa.org.au/), NHS Digital (England; http://content.digital.nhs.uk) and the electronic Data Research and Innovation Service (Scotland; http://www.isdscotland.org/Products-and-Services/eDRIS/). Derived data from these datasets for each jurisdiction are within the paper. No additional data are available.
Correction notice: This article has been corrected since it was published. The license information has been updated.
Patient consent for publication: Not required.
References
- 1. Nair H, Simões EA, Rudan I, et al. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet 2013;381:1380–90. 10.1016/S0140-6736(12)61901-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shi T, McAllister DA, O’Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017;390:946–58. 10.1016/S0140-6736(17)30938-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gill PJ, Goldacre MJ, Mant D, et al. Increase in emergency admissions to hospital for children aged under 15 in England, 1999–2010: national database analysis. Arch Dis Child 2013;98 10.1136/archdischild-2012-302383 [DOI] [PubMed] [Google Scholar]
- 4. Harron K, Gilbert R, Cromwell D, et al. International comparison of emergency hospital use for infants: data linkage cohort study in Canada and England. BMJ Qual Saf 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moore H, Burgner D, Carville K, et al. Diverging trends for lower respiratory infections in non-Aboriginal and Aboriginal children. J Paediatr Child Health 2007;43:451–7. [DOI] [PubMed] [Google Scholar]
- 6. Moore HC, Lehmann D, de Klerk N, et al. Reduction in disparity for pneumonia hospitalisations between Australian Indigenous and non-Indigenous children. J Epidemiol Community Health 2012;66:489–94. [DOI] [PubMed] [Google Scholar]
- 7. Anderson AJ, Snelling TL, Moore HC, et al. Advances in Vaccines to Prevent Viral Respiratory Illnesses in Children. Paediatr Drugs 2017;19:523–31. [DOI] [PubMed] [Google Scholar]
- 8. Cakmak S, Hebbern C, Cakmak JD, et al. The modifying effect of socioeconomic status on the relationship between traffic, air pollution and respiratory health in elementary schoolchildren. J Environ Manage 2016;177:1–8. [DOI] [PubMed] [Google Scholar]
- 9. Moore HC, de Klerk N, Richmond P, et al. A retrospective population-based cohort study identifying target areas for prevention of acute lower respiratory infections in children. BMC Public Health 2010;10:757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. MacIntyre EA, Gehring U, Mölter A, et al. Air pollution and respiratory infections during early childhood: an analysis of 10 European birth cohorts within the ESCAPE Project. Environ Health Perspect 2014;122:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smith KR, Samet JM, Romieu I, et al. Indoor air pollution in developing countries and acute lower respiratory infections in children. Thorax 2000;55:518–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Health AGDo. The Australian Immunisation Handbook. 10th edn: Australian Government, 2017. [Google Scholar]
- 13. Public Health England. Quarterly vaccination coverage statistics for children aged up to five years in the UK (COVER programme): July to September 2018, 2018.
- 14. ABS. 3101.0 - Australian Demographic Statistics, Jun 2014. Australian Bureau of Statistics, 2015. Available: http://www.abs.gov.au/AUSSTATS/abs@.nsf/Lookup/3101.0Main+Features1Jun%202014?OpenDocument
- 15. HealthInfoNet. What details do we know about the Indigenous population?: Australian Indigenous HealthInfoNet, 2015. Available: http://www.healthinfonet.ecu.edu.au/health-facts/health-faqs/aboriginal-population
- 16. Holman CD, Bass AJ, Rosman DL, et al. A decade of data linkage in Western Australia: strategic design, applications and benefits of the WA data linkage system. Aust Health Rev 2008;32:766–77. [DOI] [PubMed] [Google Scholar]
- 17. Kelman CW, Bass AJ, Holman C. Research use of linked health data—a best practice protocol. Aust N Z J Public Health 2002;26:251–5. [DOI] [PubMed] [Google Scholar]
- 18. Christenen D, Davis G, Draper G, et al. Evidence for the use of an algorithm in resolving inconsistent and missing Indigenous status in administrative data collections. Aust J Soc Issues 2014;49:423–43. [Google Scholar]
- 19. Office for National Statistics. 2011 Census: Population Estimates for the United Kingdom, March 2011, 2012. Available: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/bulletins/2011censuspopulationestimatesfortheunitedkingdom/2012-12-17 [Accessed 6 Feb 2018].
- 20. Herbert A, Wijlaars L, Zylbersztejn A, et al. Data Resource Profile: Hospital Episode Statistics Admitted Patient Care (HES APC). Int J Epidemiol 2017;46:1093–1093i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. NHS Digital. A Guide to Linked Mortality Data from Hospital Episode Statistics and the Office for National Statistics, 2015. Available: http://content.digital.nhs.uk/media/11668/HES-ONS-Mortality-Data-Guide/pdf/mortality_guide.pdf [Accessed 6 Feb 2018].
- 22. Administrative Data Liaison Service. Birth Registrations - National Records of Scotland, 2016. http://www.adls.ac.uk/national-records-of-scotland/birth-registrations/?detail
- 23. Administrative Data Liaison Service. SMR02 – Maternity Inpatient and Day Case dataset, 2016. http://www.adls.ac.uk/nhs-scotland/maternity-inpatient-and-day-case-smr02/?detail
- 24. Harron K, Gilbert R, Cromwell D, et al. Linking Data for Mothers and Babies in De-Identified Electronic Health Data. PLoS ONE 2016;11:e0164667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roberts RF, Innes KC, Walker SM. Introducing ICD-10-AM in Australian hospitals. Med J Aust 1998;169:S32–5. [DOI] [PubMed] [Google Scholar]
- 26. Pink B. An Introduction to Socio-Economic Indexes for Areas (SEIFA) - Information paper: Australian Bureau of Statistics, Commonwealth of Australia, 2008. [Google Scholar]
- 27. Smith T, Noble M, Noble S, et al. The English Indices of Deprivation 2015: Technical Report: Department for Communities and Local Government, 2015. [Google Scholar]
- 28. Brown D, Allik M, Dundas R, et al. Carstairs Scores for Scottish Postcode Sectors, Datazones & Output Areas from the 2011 Census: University of Glasgow, 2014. [Google Scholar]
- 29. Gonzalez-Izquierdo A, Cortina-Borja M, Woodman J, et al. Maltreatment or violence-related injury in children and adolescents admitted to the NHS: comparison of trends in England and Scotland between 2005 and 2011. BMJ Open 2014;4:e004474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. StataCorp. Stata Statistical Software. Release 14. College Station, TX: StataCorp LP, 2015. [Google Scholar]
- 31. Kivistö JE, Protudjer JLP, Karjalainen J, et al. Trends in paediatric asthma hospitalisations – differences between neighbouring countries. Thorax 2017. [DOI] [PubMed] [Google Scholar]
- 32. Department of Health. Emergecny admissions to hospital: managing the demand: National Audit Office, 2013. [Google Scholar]
- 33. Hawker JI, Olowokure B, Sufi F, et al. Social deprivation and hospital admission for respiratory infection:: an ecological study. Respir Med 2003;97:1219–24. [DOI] [PubMed] [Google Scholar]
- 34. Zubrick SR, Lawrence D, Silburn S, et al. The Western Australian Aboriginal Child Health Survey: The Health of Aboriginal Children and Young People. 1st edn Perth, Western Australia: Telethon Institute for Child Health Research, 2004. [Google Scholar]
- 35. Holman RC, Hennessy TW, Haberling DL, et al. Increasing trend in the rate of infectious disease hospitalisations among Alaska Native people. Int J Circumpolar Health 2013;72:20994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. O’Grady K-Ann F, Lee Katherine J, Carlin John B, et al. Increased risk of hospitalization for acute lower respiratory tract infection among Australian Indigenous infants 5-23 months of age following pneumococcal vaccination: A cohort study. Clin Infect Dis 2010;50:970–8. [DOI] [PubMed] [Google Scholar]
- 37. Jardine A, Menzies RI, McIntyre PB. Reduction in hospitalizations for pneumonia associated with the introduction of a pneumococcal conjugate vaccination schedule without a booster dose in Australia. Pediatr Infect Dis J 2010;29:607–12. 10.1097/INF.0b013e3181d7d09c [DOI] [PubMed] [Google Scholar]
- 38. Moore HC, Lim FJ, Fathima P, et al. Exploring RSV epidemiology in Australian chidlren: estimates of the population burden and effectiveness of a maternal vaccine. Malaga, Spain: RSV Vaccines for the World, 2017. [Google Scholar]
- 39. Thomas SL, Wakerman J, Humphreys JS. Ensuring equity of access to primary health care in rural and remote Australia - what core services should be locally available? Int J Equity Health 2015;14:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hardelid P, Verfuerden M, McMenamin J, et al. Risk factors for admission to hospital with laboratory-confirmed influenza in young children: birth cohort study. Eur Respir J 2017;50:1700489. [DOI] [PubMed] [Google Scholar]
- 41. World Health Organization. RSV Vaccine Research and Develoment Technology Roadmap. 2017.
- 42. Spilsbury K, Rosman D, Alan J, et al. Cross border hospital use: analysis using data linkage across four Australian states. Med J Aust 2015;202:582–6. [DOI] [PubMed] [Google Scholar]
- 43. Moore HC, de Klerk N, Jacoby P, et al. Can linked emergency department data help assess the out-of-hospital burden of acute lower respiratory infections? A population-based cohort study. BMC Public Health 2012;12:703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lim F, Blyth CC, Fathima P, et al. Record linkage study of the pathogen-specific burden of respiratory viruses in children. Influenza Other Respir Viruses 2017;11:502–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-028710supp001.pdf (103KB, pdf)