Abstract
Measurements of the intracellular state of mammalian cells often require probes or molecules to breach the tightly regulated cell membrane. Mammalian cells have been shown to grow well on vertical nanoscale structures in vitro, going out of their way to reach and tightly wrap the structures. A great deal of research has taken advantage of this interaction to bring probes close to the interface or deliver molecules with increased efficiency or ease. In turn, techniques have been developed to characterize this interface. Here, we endeavor to survey this research with an emphasis on the interface as driven by cellular mechanisms.
Keywords: vertical structures, biointerface, electrophysiology, molecular delivery, electron microscopy
In the pursuit of a complete understanding of the human body and its associated pathophysiologies, biological scientists and engineers have focused on developing tools that measure single cells. A portion of these tools seek to measure the state of the interior of cells which immediately introduces significant difficulties. Mammalian cells are tightly regulated systems delineated by a complex and active lipid bilayer membrane and measuring inside cells usually requires the insertion of a probe (solvated, solid state, photonic, etc.) through this membrane or placement of a probe very close to the membrane. However, due to the selectivity of the membrane, many approaches are untenable whether due to impermeability or toxicity or invasivity to the measurement of interest. Nanoscale vertical structures have emerged in recent years as a new approach to ameliorating some of these problems. In this review, we endeavor to show that the unique cell-structure interface has enabled significant progress in making heretofore impossible measurements of mammalian cellular systems.
Fabrication.
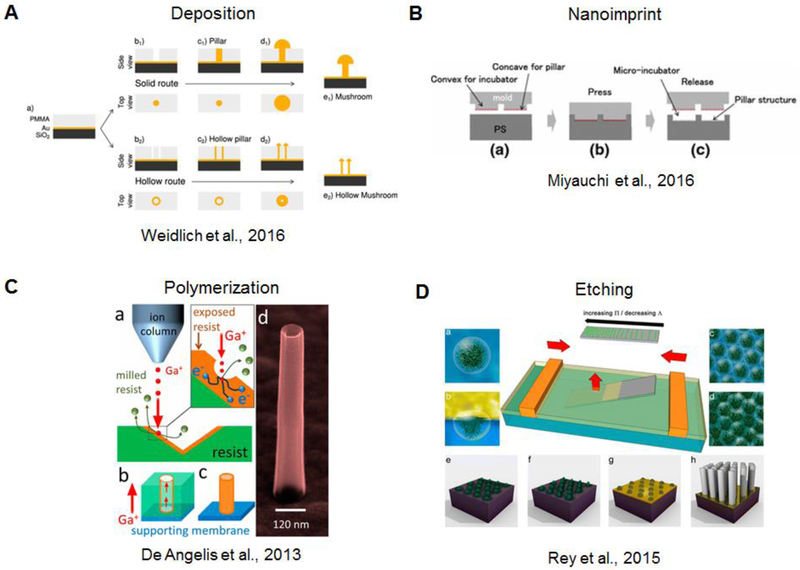
Micro- and nanopatterning tools allow the fabrication of vertical micro and nanostructures in a large variety of materials and geometries. When creating array of structures the main limitation lies in the space between adjacent structures to avoid fabrication defects and overlapping features. When vertical structures are fabricated with the final aim of coupling them with biological cells, biocompatibility is a fundamental requirement. Thus, the fabrication process should not introduce any surface modification to the material which is toxic to cells. Deposition of conductive materials (i.e. metals, conjugated polymers) is commonly used to induce vertical growth through an aperture which is typically created through a polymer, i.e. resist and is a result of an antecedent lithography process (Ozel et al. 2017). Alternatively, assisted deposition can be used to construct the vertical feature from the bottom-up (Martiradonna et al. 2012; C. Xie et al. 2012). Both techniquese allow for the creation of create vertical structures with two different conductive layers (Nick, Thielemann, and Schlaak 2014). In the case of electrodeposition, depending on the lithographic pattern, a variety of geometries (Jahed et al. 2014) can be fabricated as shown in the work of Weidleich et al., for instance, where solid and hollow-like structures were created by finely controlling gold electrodeposition (Figure 1A) (Weidlich et al. 2017). Fabrication of structures which protrude vertically from a support material can utilize imprinting and etching processes. In the imprinting process, there is a mold which is first pressed into a support material to mold it, and then released. (Figure 1B) (Miyauchi et al. 2016). For polymers, a recent innovation uses a focused ion beam to locally and precisely polymerize a certain resist (Figure 1C) (De Angelis et al. 2013). Over the years, many etching processes have been proposed which generally use a masking material. This can be a sacrificial metal previously patterned via lithography (Zhao et al. 2017) or a layer of nanoparticles (Figure 1D) (Cheung et al. 2006; Rey et al. 2016).
Figure 1.
Overview of fabrication methods for vertical structures. A) Deposition of material through a resist aperture via electrodeposition (Weidlich et al. 2017). B) Nanoimprinting utilizes a mold-based pressing process(Miyauchi et al. 2016). C) Polymerization via focused gallium ion beam (De Angelis et al. 2013). D) Etching process through particle templating (Rey et al. 2016).
Physical interaction with cells.
In general cells are placed on vertical structures in a suspension, landing on the surface by gravity. This leads to a spontaneous arrangement of the cell either directly on the top of the structures or on the flat area first from which cells migrate and adhere to the structures. Vertical structures can reach very high aspect ratios and some cases have suggested spontaneous penetration into the cytosol (Robinson et al. 2012; Shalek et al. 2012), although this is disputed (Prinz 2015; Hanson et al. 2012). Alternatively, cells can be directly printed on vertical structures arrays via an inkjet printing process (D. Lee et al. 2016). Cells also can be placed on vertical structures by applying an external force to induce the contact at the interface. One approach is to centrifugate cells with the vertical structure devices and cells can be forced into contact with the structures, even being penetrated intracellularly, as shown for biodegradable silicon and diamond nanoneedles (Ciro Chiappini, Martinez, et al. 2015; C. Chiappini et al. 2015; Wang et al. 2015). Other physical processes could potentially favor a close interaction with an enforcing molecular mechanism at the cell-structure interface. For instance, photoactive structures have been shown to interface with cells in the work of Tang et al., where the generation of H2S was pobed by a nitrogen-doped nanodot/nanowire (Tang et al. 2014). Magnetic force also has potential. A recent study shows that cells and iron-coated vertical nanoneedles of different shapes are biocompatible (Kavaldzhiev et al. 2017). In order to narrow the subject matter, we have chosen to focus on only those examples of a “passively”-constructed cell-structure interface where the cells forge the interface without large external forces. For a survey of force-driven cell-structure interfaces, we suggest the recent review by Chiappini (Ciro Chiappini 2017).
Overview.
The interface between cells and vertical structures has been the topic of recent reviews emphasizing cell capture, molecular delivery, and semiconducting nanowires (Chang et al. 2016; Kwak et al. 2015; Ciro Chiappini 2017; Zhou, Dai, and Lieber 2017; Prinz 2015). In this review, we focus on four aspects of the interaction between cells and vertical structures. In particular, we will discuss two main applications of those structures presented in literature as an alternative tool to traditional patch clamp for electrophysiology of cells. In fact, the effective electrical coupling to electrogenic cells and vertical micro and nanoelectrodes will be presented with a particular attention to the electrical modelling as key step for recording and stimulation capabilities. Then, we will explore a variety of examples of intracellular molecule delivery and its effect on cells in terms of morphological re-arrangement, gene expression, and potential to use vertical structures for cellular assays. In the second part of this review, we will discuss how cells interact with vertical structures at the nanoscale and how far the field has gone to precisely determine the interaction of cellular compartments with vertical structures by means of microscopy techniques.
1. Nanopillar electrophysiology
1.1. History and Motivation
Throughout the 20th century, genetic tools reached new heights of precision and robustness for a wide variety of ends, from understanding the basis of disease to identifying the vast diversity of cell types found in the human body. Of great intrigue in the latter pursuit was the structuring of the brain. With the ability to distinguish cells on a genomic level came the push to understand how brain structure gives rise to human behavior, development, and disease. While genetics provides information on one level, the picture would benefit from functional information.
Electrophysiology is one such functional aspect of the neurological picture. Scientists and doctors have been measuring and manipulating neuronal electrophysiology since at least the 1930s (Davis 1939), but the depth of knowledge has increased periodically. Each era of electrophysiology is roughly defined by the available technology for electronic measurement and recording. Recently, the expansion in computer memory capacity and the miniaturization of electronics has paralleled the desire to measure the network activity of neurons (Alivisatos et al. 2013; van de Burgt et al. 2017). Thus, the multielectrode array emerged as a method to monitor an area with dozens of recording sites reporting local field potentials. These local field potentials give information about clusters of cells close to a given electrode site such that experimental conditions could be correlated with changes in electrical behavior across the network. This field has developed in parallel to that of nanoelectrodes, driven by advances in CMOS technology which enable ever-higher electrode dobienensity (Müller et al. 2015; Obien et al. 2014; Berdondini et al. 2009; Eversmann et al. 2003; Frey et al. 2010).
Bioelectrical activity is a result of concerted action by various ion channels present in the cell membrane. Much of the channel-specific information encoded in the action potential shape is lost in field potential measurements.
The development of lithography techniques capable of constructing nanoscale electrodes has advanced the available technology to a new era in which intracellular potentials can be recorded using multielectrode array architecture. These devices were initially proven on cardiac cell systems due to the robustness of these cells, and there are independent applications of nanoelectrodes to cardiac disease and drug development (see section 1.4). In the last couple of years, reports of nanoelectrode recordings from mammalian neurons have become more common, fulfilling the promise of the technology (Robinson et al. 2012; R. Liu et al. 2017; Dipalo et al. 2017; Ojovan et al. 2015).
1.2. Nanopillar electrode technologies for intracellular recording
To simplify matters, the term “nanopillar” as used in this review encompass the wide variety of geometries and sizes, from sub-100 nm needle-like structures, to 1000 nm mushroom-like structures. Individual groups utilize various terms to emphasize certain aspects of their technology. We view all these geometries as related by the fact that the electrodes are a free-standing vertical structures with features that are of a length-scale commensurate with mammalian cellular dimensions. The nanopillar electrode technology and its various iterations share the common objective to record intracellular action potentials and to do so in a multiplexed, long term fashion--over the course of days. Toward this goal, the approaches under development are diverse, carrying their distinct advantages and drawbacks.
Methods of intracellular signal access.
One approach is to gain and maintain physical intracellular access for the duration of the experiment, thereby achieving a continuous stream of high signal-to-noise ratio voltage data. A single mammalian cell maintains homeostasis by employing a tight boundary, the cell membrane, which is decorated with selective protein complexes that permit and detect entities on a chemical basis. With knowledge of the chemical makeup of this boundary, scientists have had some success chemically engineering molecular functionalization which dupes the cell into allowing such entities as nanotubes and nanopillars to continuously penetrate the cell membrane for prolonged periods (Qing et al. 2014; Almquist and Melosh 2010).
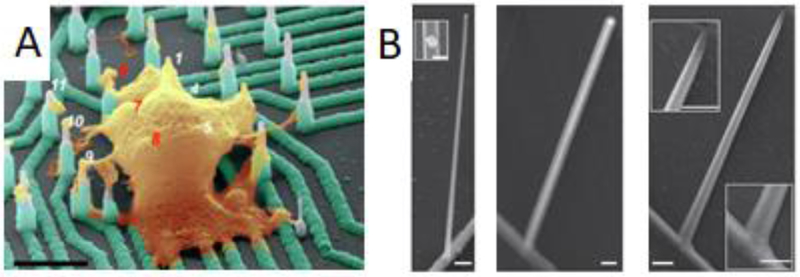
Researchers have also taken the tack of designing vertical electrodes with the idea that cells will deploy native machinery that engulf vertical structures tightly (Hai, Kamber, et al. 2009; Santoro, Dasgupta, et al. 2014). While these electrodes remain outside of the cell membrane as shown by electron microscopy, the electrodes record intracellular-shaped potentials (Figure 2A). Currently, this is explained by a reduced resistance of the membrane patch proximal to the electrodes by means of a recruitment of ion channels to engulfment sites (Rabieh et al. 2016; Shmoel et al.2016). While the achievement of an extracellular electrode recording intracellular potentials with high integrity would greatly benefit long-term measurements, there is some evidence that the persistence of the impedance at the cell membrane-electrode junction distorts the recorded signal shape (Shmoel et al. 2016).
Figure 2.
Vertical structures for electrophysiology. A) Doped silicon nanoelectrodes record intracellular action potentials from primary and stem cell-derived neurons. Scale bar: 4 um. (R. Liu et al. 2017) B) Nanotube channels increase field effect transistor performance. Left: germanium branch on silicon nanowire. Inset gold nanodot on nanowire. Middle: structure after coating with aluminum oxide. Right: Hollow nanotube forms the transistor channel after etching of germanium core. Scale bars: 200 nm for all but left inset (100 nm). (Duan et al. 2011)
A third approach is to utilize an iteration of the now-ubiquitous biological method of electroporation (Neumann et al. 1982) to repeatedly gain temporary low impedance electrical access to the cytoplasm. The combination of large local electric field gradients and intimate cell membrane-electrode contact particular to nanoelectrodes enables the use of small potentials (~1–5 V) to achieve poration and, thereby, minimize heat-induced cytotoxicity (Rabieh et al. 2016; C. Xie et al. 2012; Fendyur and Spira 2012; Abbott et al. 2017; Lin et al. 2014, 2017). This method takes practical advantage of the presence of stimulating electronics that often accompany the recording system, but has the cost of a recording blind period immediately after electroporation as the amplifiers recover from the influx of charge.
An alternative approach is plasmonic optoporation which has the same philosophy as electroporation but avoids its recording blind time by separating the poration mechanism (photonic) from the recording mechanism (electronic). Nanoelectrodes made of a plasmonic material, i.e. commonly gold, can transduce focused laser excitation into hot electrons which in turn excite local water molecules inducing a shock wave. This shock wave acts to porate the cell membrane, yielding electrical access (Messina et al. 2015; Dipalo et al. 2017). Besides the method of intracellular access, the nanoelectrode toolbox is diversified by the electrical properties of the probe.
Materials of nanoelectrodes.
A wide variety of materials have been used to construct metallic nanoelectrodes, among them gold (Dipalo et al. 2017; Ojovan et al. 2015; Rabieh et al. 2016; Shmoel et al. 2016; Hai, Shappir, and Spira 2010; Hai and Spira 2012), platinum (Abbott et al. 2017; C. Xie et al. 2012; Lin et al. 2017), doped silicon (Robinson et al. 2012; R. Liu et al. 2017), and iridium oxide (Lin et al. 2014). The criteria by which nanoelectrodes are primarily judged are their biocompatibility, low electrochemical impedance at electrophysiological frequencies, and stability in aqueous solutions of neutral pH. Various other features yield increased functionality such as the vast array of gold functionalization chemistry or the advanced fabrication methods developed for silicon. Finally, a material may be chosen based on its tunable geometry (Weidlich et al. 2017).
With the use of metallic electrodes come their limitations. First, the noise level of voltaic measurements increases with decreasing electrode surface area (Equations 1 and 2) (Gesteland et al. 1959) so, to this point, work has been done to augment the surface area of electrodes increasing the porosity of the surface (Seker et al. 2010; Brüggemann et al. 2011; Heim et al. 2012; Wesche et al. 2012; Chapman et al. 2015; Y. H. Kim et al. 2015). For example, multiple nanoelectrodes are often connected to a single electric pad to increase the surface area. Second, the fabrication of metallic electrodes mostly necessitates top-down lithographic methods, limiting the creative chemical tricks that can be exploited for novel, smaller, or heterogeneous geometries.
| (Eq. 1) |
| (Eq. 2) |
where Vrms, noise is the root mean square noise voltage, k is Boltzmann’s constant, T is theabsolute temperature, Z is the electrochemical impedance, Δf is the frequency band and A isthe surface area.
In contrast, semiconducting electrodes, typically used in a field-effect transistor (FET) configuration for electrophysiology, experience great freedom in fabrication methods and have starkly different limitations (Figure 2B) (Duan et al. 2011). These FET devices come in a variety of forms, including those with graphene sheet and silicon nanowire channels (Cohen-Karni et al. 2010; Patolsky et al. 2006) but in keeping with the theme of this review, we will focus on 3D nanowire-like structures. For an expert review of this topic more broadly defined, we recommend that recently written by Zhang and Lieber (Zhang and Lieber 2016).
We posit that, on some level, the increased functionality of nanopillar electrodes derives from their size. It is no coincidence, then, that some of the most powerful examples of intracellular probes have been semiconducting nanowires due to the increased control over device size and composition. Vapor-liquid-solid growth from nanoparticles provide much of this control and allow for fabrication of FETs with device elements on the order of ion channels in size. NanoFETs are active devices and therefore currently require custom electronics to maintain the potential of maximum transconductance for each electrode while recording microvolt fluctuations. While transistors can achieve a lower noise floor with increasing source-drain current up to a certain point, the drive current trades off with heating of the device which can have cytotoxic effects (Harrison 2008; Abbott et al. 2017). Finally, as they currently stand, the small dimensions of freestanding nanowires lead to increased fragility from an engineering manufacturability perspective. Thus, at the current stage of nanoelectrode development, one must weigh many factors before deciding precisely what electrode is best for the task at hand.
1.3. Mechanistic understanding
Circuit models.
Given the enhanced signal-to-noise ratio of nanoelectrode measurements over those of planar electrodes (C. Xie et al. 2012), the question remains: what drives this enhancement? Various groups can produce the variety of electrodes just described, but there is as yet no satisfactory mechanistic understanding of why the nanopillar-cell interface begets such improved device performance. The first instinct in the field has been to explicate the system in of an equivalent electronic circuit. Utilizing circuit analogies for electrophysiological systems is not new; traditional sharp electrode electrophysiologists have been using circuit element descriptions since at least the 1960s (Strickholm 1961). The system in question is highly complex as deterministic, high sensitivity electronics interface with a complex electrochemical cell which includes a stochastic biological system. Circuit models simplify the picture and have been used for chip-based electrophysiology devices since the first use of transistors to measure leech neurons by Fromherz, et al. (Fromherz et al. 1991).
In the last few years, circuit models for nanoelectrodes have converged to a consensus framework with three separate groups using the same circuit to explain their observed phenomena (Figure 3A) (Abbott et al. 2017; Rabieh et al. 2016; Dipalo et al. 2017). The circuit is composed of four modules: (1) the cell, (2) the electrode, (3) the amplifier electronics, and (4) the interface. The cell is represented as a voltage source surrounded by the complex impedance of the membrane. This complex impedance is modeled as a resistor (Rm) and capacitor (Cm) in parallel, the resistor deriving from the ion channels which mediate an ionic current, and the capacitor deriving from the lipid bilayer which maintains a charge separation. Importantly, the membrane impedance is separated into a “junctional” impedance (that portion of the membrane in close proximity to the electrode surface) and the non-junctional impedance corresponds to the remaining membrane area. This separation is supported by experimental evidence that the nanoelectrodes’ sensing is highly local to the interface (C. Xie et al. 2012). The electrode is represented by a complex electrochemical interface impedance with the charge transfer resistance in parallel with the double layer capacitance. The amplifier circuitry commonly includes a parasitic feedline capacitance (Cp), an input capacitance (Cin), and a high input impedance, non-inverting operational amplifier. Finally, the interface between the cell and electrode is characterized by a single “seal” resistance (Rseal) which is an analog of the physical cleft formed as the cell engulfs the electrode and prevents current from passing from the junctional space to the grounded bath solution.
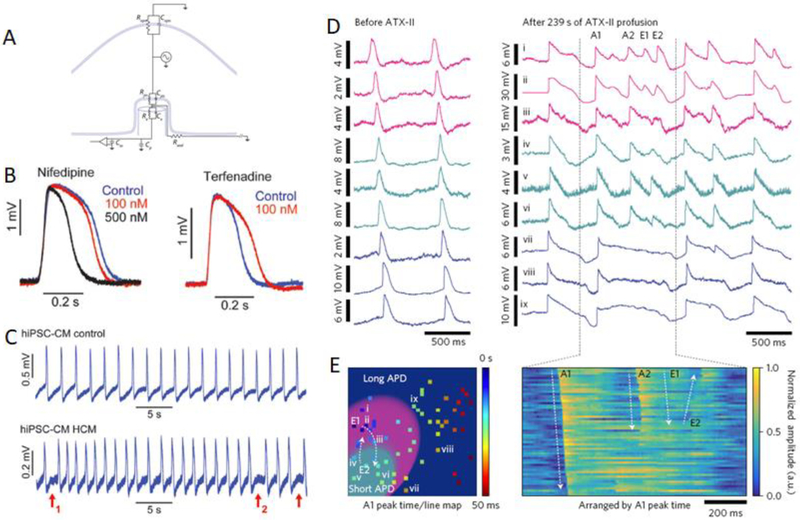
Figure 3.
A) General circuit model of the cell-electrode interface. B) Drug test using nanoelectrodes and human embryonic stem cell-derived cardiomyocytes. C) Nanoelectrodes enable measurement of arrhythmia in patient-derived cardiomyocyte culture. (Lin et al. 2017) D) Parallel recordings of rat ventricular cardiomyocytes before (left) and after (right) administration of a Na+ ion channel blocker. E) The ability to record with high spatial resolution across a tissue-scale area allows for conclusions about regional arrhythmia in response to drugs. 54 pixels recorded by the CMOS array labeled
In this milieu of resistors and capacitors, there are two focal points. The first, which has been clear since the observation of nanoelectrodes’ unique efficacy, is the seal resistance, Rseal. The cleft formed between cells and nanostructures is measurably smaller compared to the cell-flat substrate cleft. (see section 3) And since Rseal can be considered as the grounding resistor in a voltage divider in the model, the larger Rseal, the better the integrity of the recorded signal amplitude (i.e. “coupling coefficient”). The second point addresses the difference between recording intracellular-like signals versus extracellular signals. An action potential as recorded by an intracellular electrode (e.g. sharp microelectrode or whole-cell patch clamp) is monophasic and resolves the contributions of various ion channels. If the action potential is recorded extracellularly by a planar electrode, the membrane impedance acts as a differentiator, yielding a biphasic spike. Broadly, the relative magnitudes of Rjm, Cjm, Re, and Ce determine whether a nanoelectrode records an intracellular-like signal versus an extracellular signal. Recent work by groups using mushroom microelectrodes (Zhu et al. 2016; Ojovan et al. 2015; Rabieh et al. 2016; Shmoel et al. 2016) and nanopillars (Abbott et al. 2017; Lin et al. 2017; Dipalo et al. 2017; R. Liu et al. 2017) have attributed their intracellular-like signals to different ways of manipulating these variables.
Both groups of researchers agree that Rseal should be as large as possible to record with the best signal-to-noise ratio possible. Additionally, each group has suggested that Rjm is the critical parameter in the Rjm/Cjm/Re/Ce interplay. In the case of micro-mushrooms, electrical recordings from which have been pioneered by the group of Micha Spira, intracellular-like action potentials are recorded while the electrode remains fully outside of the cell. They posit that such a situation comes about because ion channels are recruited to the engulfment site, reducing Rjm by multiple orders of magnitude. They show in simulations that this can lead to phase-preserving capacitive matching which in turn leads to intracellular-like signal recording. In contrast, groups working with nanopillar-pillar induced poration posit that the membrane pores electrically short Rjm and Cjm, leading to a purely resistive path to the cytoplasm and intracellular action potential recording. The two proposed mechanisms of action lead to different paths for improvement. In the case of micro-mushrooms, schemes to further recruit ion channels to the junctional membrane area would lead to improved signal, although questions of physiological invasiveness accompany such initiatives. For nanopillars, methods to increase Rseal would proportionally increase recorded amplitudes. Development on these two fronts is ongoing.
1.4. Practical applications of nanoelectrodes
While understanding nanoelectrodes’ mechanism of action is important for establishing future design rules, non-optimized electrodes have already made practical impact. In a 2014 study, Burridge, et al. utilized platinum nanopillars to measure induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs) (Burridge et al. 2014). The nanopillars were used to confirm patch clamp measurements of cardiomyocyte subtype--whether the cells were nodal-, atrial-, or ventricular-like. The measurements found that over 60% were ventricular-like at day 25–30 after differentiation. A total of 20 cells contributed to these statistics. The electrodes thus showed promise to increase the functional monitoring throughput of stem cell-to-cardiomyocyte differentiation.
Another study by Lin, et al. utilized the same platinum nanopillars for further measurements of iPSC-CMs (Lin et al. 2017). First, nanopillar measurements were compared extensively with patch clamp measurements to ensure that there were minimal perturbations caused by the electrode. Next, a population of iPSC-CMs was monitored for nearly a month and the subtype determined at two time points--days 25–32 and days 62–69 with 92 and 54 cells, respectively. Significantly, the population changed from 61% ventricular and 13% nodal to 94% ventricular and 0% nodal over that time period. Last, the nanoelectrodes were used to carry out drug testing and disease monitoring on patient-derived iPSC-CMs. Dose tests for the cardioactive drugs nifedipine, cisapride, and terfenadine all displayed their characteristic effects on a control cell line (Figure 3B). Then cells from patients with hypertrophic cardiomyopathy and long-QT syndrome were measured on the nanoelectrodes and distinct features characterized the difference between diseased cells and control cells (Figure 3C). These results indicated that this platform could be utilized for therapeutic screening for efficacy and safety.
A third study by Abbott, et al. is the most recent study to offer practical insights using nanoelectrodes (Abbott et al. 2017). The study utilizes platinum-coated silicon oxide nanopillars which have been fabricated on a 1024 CMOS electrode array. This number of recording sites enables more powerful conclusions about the effect of therapies on cardiac networks. While novel therapeutics effect single cells, they can also have consequences across a network in ways that are indecipherable locally. With a dense culture of neonatal rat ventricular cardiomyocytes covering the nanoelectrode array, ATX-II, which is an Na+ channel toxin, was added to the culture. Other studies have shown that ATX-II can recapitulate the phenotype caused by congenital long-QT syndrome type 3 (Shryock et al. 2013). The study goes on to show that intracellular recordings across the network indicate a region-specific changes in action potential duration (Figure 3D,E). The overall arrhythmia in the culture caused by ATX-II can then be traced to regional polarization dynamics and the spatial arrangement of longer- and shorter-action potential duration regions. So much for the applications of nanopillars to cardiac electrophysiology, we mentioned at the outset that much of the motivation for nanopillars was to measure neuronal behavior. While neuronal studies are increasing in frequency, there is still a vast application space that is untapped in neuronal studies, from network development, to disease, to in vivo monitoring. We will now switch to a survey of non-electrical measurements of cells using nanopillars.
2. Molecular delivery and cellular assays
The impetus to interface nanopillars with mammalian cells did not originate in electrophysiology, however, but in molecular delivery. A major portion of biological research, from fundamental to clinical is mediated by modulating the preponderance of intracellular biomolecules. This type of perturbation can modify a signalling cascade to affect a specific cellular behavior or can initiate a cascade which affects protein production. The DNA, RNA, proteins, and various other molecules to be delivered can vary from small and robust to bulky and physicochemically delicate, adding complexity to the question of how they should be delivered. Traditional methods, like parallel plate electroporation, chemical transfection (e.g. lipofection, calcium phosphate), and viral transduction, can be const-effective and robust in certain situations, but preclude a wide variety of desired perturbations. Some of the complications that plague these methods include cell death, cargo-dependent efficiency, and cell-type-dependent variability. Thus when nanopillars were discovered to interface tightly with mammalian cells and potentially penetrate the membrane (W. Kim et al. 2007), the first application was to delivery--to test whether the pillars could overcome the aforementioned hurdles. Much of the impactful gains by nanopillars can be found in this field (Shalek et al. 2012; C. Chiappini et al. 2015; Shalek et al. 2010; Ciro Chiappini, Martinez, et al. 2015; Harding et al. 2016; Zhu et al. 2016; Matsumoto et al. 2016; Zu et al. 2016; Ciro Chiappini, Campagnolo, et al. 2015). And from the pillar motif, nanostraw-based delivery emerged wherein microfludic access to the cytosol could enhance experimental control (Caprettini et al. 2017; Messina et al. 2015; VanDersarl, Xu, and Melosh 2012; X. Xie, Xu, Leal-Ortiz, et al. 2013; Xu et al. 2017).
Subsequently, when it was found that mammalian cells would maintain this tight membrane-nanomaterial interface over a period of days, a variety of other cell-analytical technology soon developed. This included, naturally, albeit more painstakingly, sampling molecules from the intracellular space (Cao et al. 2017; S. Choi et al. 2016). Highly local fluorescence and electrochemical sampling have also been shown in the vein of enhanced sensors (Rawson et al. 2016; Shashaani et al. 2016; C. Xie et al. 2011). Finally, a new motif has arisen in interrogating cellular mechanotransduction and curvature sensing pathways using ordered arrays of nanopillars (Hanson et al. 2015; Zhao et al. 2017).
2.1. Nanopillar-mediated molecular delivery
Nanopillars for every application, but perhaps foremost for delivery exhibit different efficacy dependent upon their design. In fabricating nanopillars there are generally six parameters that are tuned: diameter, height, density, geometry, porosity and material. Optimizing these parameters can depend on cell type or cell size, and part of the difficulty with reaching a consensus on the mechanism of action of this technology may depend on this variability. Other parameter choices come from an application-specific standpoint; for example, an ordered array of pillars of defined density can yield control over the maximum number of delivery sites per cell (Shalek et al. 2012; Yosef et al. 2013). Similarly, porosity and surface area can determine loading capacity (Ciro Chiappini, Martinez, et al. 2015; C. Chiappini et al. 2015; Ciro Chiappini, Campagnolo, et al. 2015). Once the pillars are fabricated, cargo, from small molecule dyes to siRNAs, are coated onto the pillars prior to cell seeding. Then, after the cells adhere to the pillars, it takes a variable amount of time before the cargo is delivered intracellularly, between 0.5 and 48 hours (depending on the system), with high transfection efficiencies.
The nanopillar technology has been mainly promoted as having the ability to deliver a wide variety of cargo with high efficiency, but an additional benefit is the ability to surreptitiously bypass cellular machinery in the process. This reduces perturbations to the cell which have confounded some areas of research. In the following section, we will examine two examples of pillar-based delivery which exhibit both powerful applications of the technology as well as the complicated picture surrounding their mechanism of action.
Applications of nanopillar-mediated delivery.
In one of the earliest examples of nanopillar technology impacting the broader biomedical research community, Shalek, et al. investigate how intracellular signaling in immune cells contributes to chronic lymphocytic leukemia (CLL) (Shalek et al. 2012). Immune cells are a member of the aforementioned class of cells perturbed by transfection methods. The authors describe how all conventional methods have poor efficiency, cause inflammation (which can be initiated by endocytic pathway upregulation), or outright kill the cells, thus preventing some important genetic studies of immune cells. They first discuss their silicon nanopillar design decisions--using various immune cell types with different nanopillar device geometries, two trends emerged. For one, they posit that cells which typically grow in suspension are best treated with longer, sharper pillars at higher density, whereas the trend is the opposite for adherent cells. And second, they are able to establish the trend that the density and diameter of nanopillars must be scaled to match size and the height to facilitate adhesion and penetration. Later in the course of the study, the authors show via gene expression analysis that successful delivery of genetic agents using nanopillars does not activate those confounding endocytic or inflammatory pathways. Furthermore, immune sensing mechanisms themselves were not activated compared to cells on planar silicon. Thereafter, the authors are able to correlate wide variation in CLL patient outcomes, including response to treatment, with their B-cell responses to gene silencing.
The study was not an isolated case for the utility of nanopillar-mediated delivery. Some of the same authors used the technique again in a study which enabled the dissection of the pathway which governs T-cell differentiation (Yosef et al. 2013). With the success of the method and the evidence that the pillars gained direct access to the cytosol without activating endocytic processes, a breaking and tight wrapping of the membrane about the pillar sidewalls seemed most likely. Yet other studies have evidence that without forceful insertion of the pillars into cells, intracellular penetration is inconsistent (Zhu et al. 2016).
In one such study, Chiappini, et al. interface HeLa cervical cancer cells in vitro with porous Si nanopillars in two different situations and compare delivery efficacy (C. Chiappini et al. 2015). In one case, the cells are seeded on the nanopillars and monitored for molecular delivery in a “passive” delivery (Figure 4A). In the other case, cells are seeded on a planar substrate and subsequently sandwiched by an array of nanopillars from above and the sandwich is centrifuged to force the pillars into the cell--an “active” delivery. The authors find that delivery occurs after around four hours in the passive case and immediately in the active case. Investigating this further, they attempt passive delivery at 4 °C (albeit in a no-centrifugation, pillars-on-top configuration) compared to with-centrifugation delivery. This low temperature inhibits encotycosis. They find that after four hours, no delivery has occurred in the zero-force sample, indicating that temperature-dependent cellular processes are responsible for the uptake of molecular cargo from nanopillars. This is in apparent contrast to the immune cell study in which no immune or endocytic response was elicited during the delivery process, suggesting that the picture of delivery is different across pillar or cell types. Chiappini, et al. go on to demonstrate that, using force, they can deliver a plasmid into rat superficial tissues (VEGF into skin and back muscle) in order to promote neovascularization of those tissues. These results are seemingly consistent with those of Hanson, et al. who utilized transmission electron microscopy to characterize the nanopillar-cell interface utilizing quartz pillars and rat embryonic cortical neurons (Hanson et al. 2012). They found that the cell membrane wrapped entirely around pillars less than 300 nm in diameter and up to 2 μm in height. Again, however, different cell and pillar types were used, leaving questions open as to the generality of the conclusions.
Figure 4.
Strategies for enhanced molecular delivery. A) Nanostraw device schematic. B) Azidosugar delivery schematic. (Xu et al. 2017) C) Electron micrograph and cross section of HeLa cells on porous silicon nanoneedles. Scale bars: left, 5 um, right, 2 um. (C. Chiappini et al. 2015)
2.2. Nanostraw-mediated molecular delivery
Agnostic of the precise mechanism by which nanopillars deliver their cargo, evidence has accumulated that they have great utility for time-independent delivery. Yet a variety of biological questions focus upon intracellular signaling dynamics and, for this, solid nanopillar delivery does not suffice. The ability to fabricate hollow structures with nanopillar-like dimensions enabled these dynamics studies. The hollow nanostraws are hollow through their substrate which is mounted on a fluid reservoir (VanDersarl, Xu, and Melosh 2012). This reservoir enables precise timing of delivery as well as concentration control. Of interest to the broader issue under review--the cell-sensor interface--the first demonstration of nanostraw delivery found that 100 nm outer diameter straws achieved delivery while 750 nm diameter straws did not (after 24 h, using HeLa and Chinese hamster ovary (CHO) cells) (VanDersarl, Xu, and Melosh 2012). While nanostraws are able to deliver without additional active mechanisms, the efficiency is low. Recently, low voltage electroporation (X. Xie, Xu, Leal-Ortiz, et al. 2013; Caprettini et al. 2017) and optoporation (Messina et al. 2015) have been used to induce membrane permeability and increase delivery efficiency.
Application of nanostraw-mediated delivery.
One example of nanostraws facilitating broader research progress was published recently by Xu, et al. on delivering probes of protein glycosylation (Figure 4B,C) (Xu et al. 2017). In studying metabolic activity, the impermeability of some otherwise desirable probes, such as negatively charged, phosphate-modified sugars, narrows the available toolbox. Xu, et al. demonstrate that nanostraws with 100 nm outer diameter and 2 um height enable direct intracellular delivery of cell-impermeable cargo after 24 h using CHO cells. They show that nanostraw-mediated delivery efficiencies are comparable to those for cell permeable molecules. With this capability, researchers can then direct their chemical probes to a particular metabolic step, rather than playing complex, high attrition tricks like feeding a permeable probe precursor several metabolic steps before the step of interest. Again, nanopillar-like geometries are shown to surmount barriers to intracellular measurement and perturbation.
Quantification and modeling of nanostraw penetration.
Given the variation in results from various groups and systems there still is room for debate over whether penetration events depend on the time-scale, the pillar geometry, the cell type, or all of the above and more. One important contribution to this discussion has come from the temporal resolution afforded by nanostraws. By exploiting metal-ion quenching of enhanced green fluorescent protein (eGFP), Xu, et al. were able to measure the timeframe for penetration and the frequency with which penetration events occur (Xu et al. 2014). CHO cells constitutively expressing eGFP were seeded on nanostraws (100 nm in diameter and 1 to 2 um tall) at which point Co2+ ions were delivered via the straws, causing quenching of cytosolic eGFP. In this way, penetration events could be monitored with high temporal resolution which eliminates confounding possibilities such as endocytic uptake. The study found that the percent of cell-nanostraw interfaces which lead to a penetration event is 6–12% and that this penetration is sustained (>30 min.) Furthermore, the authors found that enhancing cell-substrate adhesion by coating the nanostraws with polyornithine or fibronectin increased the percentage of penetration events and shortened the timescale on which they occurred. They conclude that while spontaneous penetration may be infrequent, with enough delivery sites per cell, the technology is robust for its intended ends.
Subsequent mechanical modeling from the same group, using Co2+ quenching and electron microscopy data, indicates that the energetic barrier to penetrating the cell membrane is sufficiently high to explain the infrequent penetration measured (X. Xie et al. 2015). The modeling also enables the broader conclusion that the nanopillar/nanostraw penetration depends on both the adhesive force and the cell membrane stiffness such that soft or stiff cells with low adhesive forces are less likely to allow penetration. And in another study, they demonstrate that both the cell membrane and basal cytoskeleton work in concert to resist penetration, leading to the idea that there could be chemical means to facilitate penetration (Aalipour et al. 2014). Thus as nanopillar geometries and use cases evolve, nanostraws will continue to be a means of determining their mechanism of action.
2.3. Intracellular analysis
Nanotechnology has borne plentiful fruit partially because the technology is of the order of size of cells’ native machinery and salient environmental features. This point is emphatically made by the techniques enumerated in this section which are impressively diverse, and which each enable novel biological studies of unmatched precision or cellular access.
Sampling concentrations of molecules from the intracellular space is powerful in identifying cell type, phase, and activation state. Many routinely-used methods for such studies require cell lysis, precluding the opportunity to study a given cell or population longitudinally. More recent methods use fluorescent tags to avoid lysis, but these are subject to constraints on multiplexing due to spectral overlap and concerns over invasiveness. Nanopillar-based platforms represent a solid-state solution which negligibly perturbs many signaling pathways and can therefore be used for sampling intracellular contents.
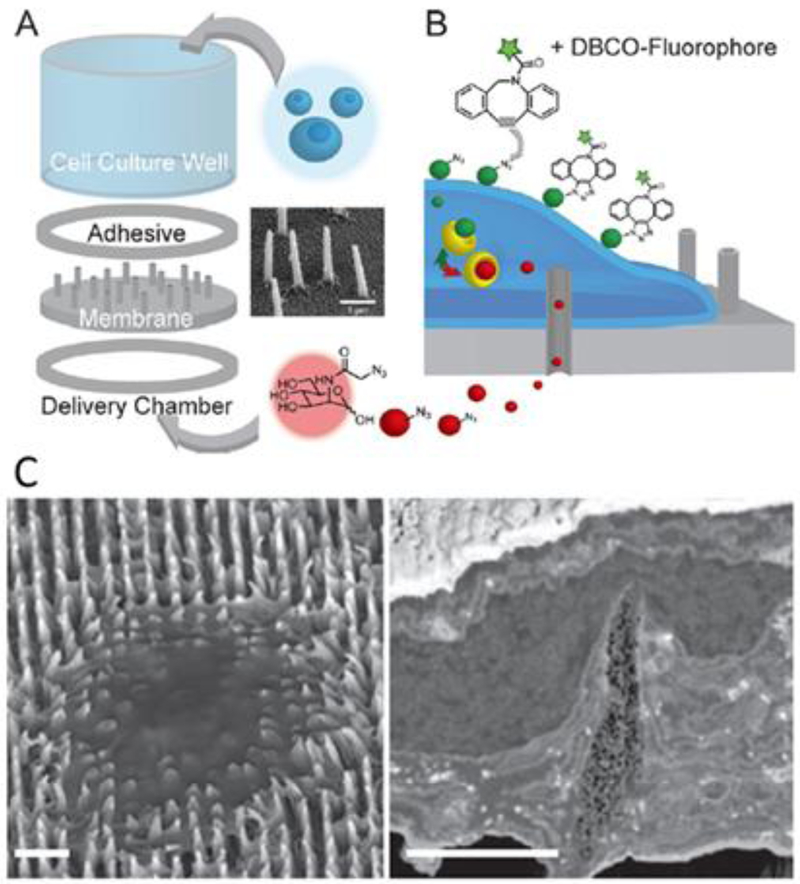
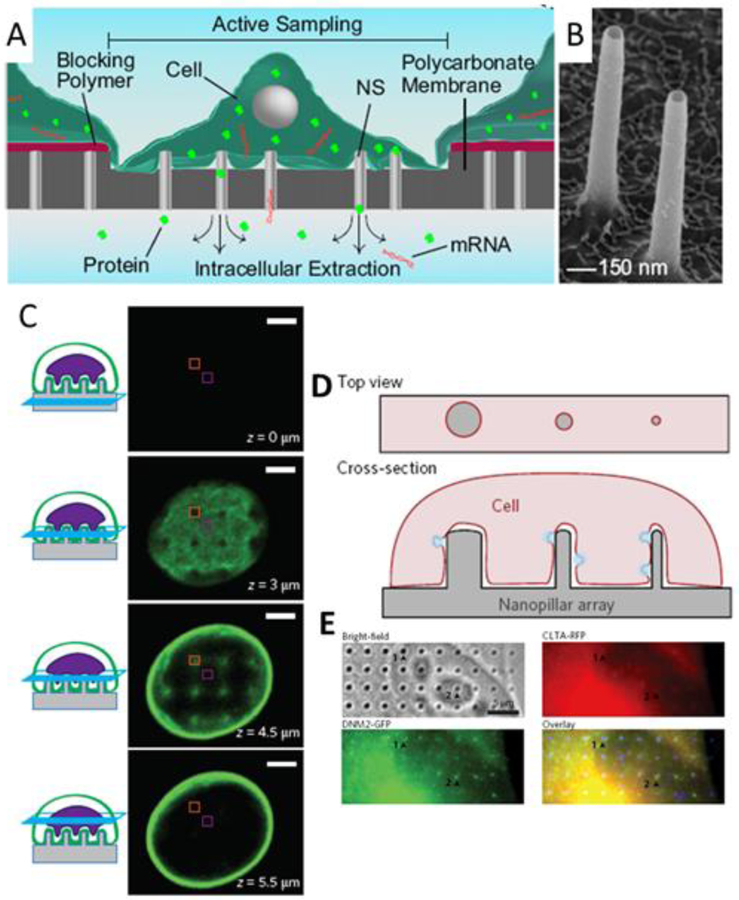
Nanostraws, which were previously discussed in regard to molecular delivery (Section 2.2), were shown by Cao, et al. to sample ~10% of mobile intracellular contents on each of a series of days (Figure 5A,B) (Cao et al. 2017). Before each sampling event, electroporation pulses were applied between an electrode in the liquid reservoir under the cells and a counter electrode in solution above the cells. This guarantees that each nanostraw has access to the cytosol of an engulfing cell. The study shows that sampling does not significantly affect cell viability (for CHO cells, astrocytes, and hiPSC-CMs) and can resolve concentration fluctuations of 41 of 48 mRNAs in hiPSC-CMs with high integrity (compared to a lysis-based method) over the course of three days. The technology thus has the potential to facilitate studies of stem cell differentiation or general intracellular dynamics.
Figure 5.
Cellular assays. A) Schematic of nanostraw sampling of intracellular molecular contents. (Cao et al. 2017) B) SEM of alumina nanostraws. (Cao et al. 2017) C) Confocal z-stack of images depicting nanopillar-induced nuclear deformation. The nuclear envelope is labeled with GFP-Sun2. Scale bars 3 um. (Hanson et al. 2015) D) Schematic of Nanopillar-induced curvature for studies of endocytosis. E) Collocalized immunostaining for clathrin and dynamin2 allows correlation between degree of curvature and protein accumulation. (Zhao et al. 2017)
In contrast to the indiscriminate, longitudinal sampling by nanostraws, nanopillars have been used to interrogate particular protein-protein interactions in pull-down assays using a heterogeneous material-based approach which does not necessitate a separation step (S. Choi et al. 2016). Nanopillars can be further used to enhance extant techniques because of they passively promote a uniquely tight interface between the probe and cell membrane. Electrochemical measurements have been demonstrated using nanoelectrodes (Rawson et al. 2016) which could lead to intracellular monitoring of the electrochemical driving force that affects cellular activity. Additionally, sub-wavelength diameter pillars were shown to enable confinement of interrogating light to evanescent wave excitation, thus allowing highly local fluorescent imaging (C. Xie et al. 2011).
Nanopillar-induced cell perturbation.
Finally, a new motif has arisen in biological study using nanopillars as a result of cells’ affinity to wrap nanopillars and engulf them at sufficiently low density. Membrane curvature has been suggested to initiate intracellular signaling (McMahon and Gallop 2005; J. Liu et al. 2009) while the mechanical properties of various cellular components are critical to biological properties such as migration. Nanopillars enable highly regular perturbations of these parameters--cell membrane curvature, cytoskeletal structure, and cellular mechanics--and thereby can provide increased measurement accuracy and reduced inter-measurement variation compared to prior methods. In one study, nanopillars in conjunction with electron microscopy were shown to induce nuclear deformation thereby allowing the modeling of cytoskeletal contributions to nanopillar-induced deformation and mechanotransduction (Figure 5C) (Hanson et al. 2015). A second study utilizes nanopillars and nanobars to induce precisely defined curvature, demonstrate curvature-dependence of clathrin-mediated endocytosis, and identify which proteins involved in the endocytic pathway sense curvature (Figure 5D,E) (Zhao et al. 2017).
Efforts that have been made to understand the interface which is critical to all these advances (Xu et al. 2014; Santoro, Dasgupta, et al. 2014; Buch-Månson et al. 2015; X. Xie et al. 2015; X. Xie, Xu, Angle, et al. 2013). The shared conclusion across studies using various biological systems, materials, and geometries, is that the interface is a function of many parameters: cell type, and pillar geometry, and density. Entire fields of research have spawned to take advantage of and characterize these complexities, so we will move on from nanopillar applications to cover those fields.
3. Interaction mechanisms of cells and vertical structures at different scales
Surface interaction of cells with nanopillars.
In parallel to the models deployed to explain device performance, experimental studies have highlighted how cells interact generally at the macroscale with vertical structures. When cells are placed on such structures, their interactions depend on the geometry, dimensions, and spacing between adjacent structures. When cells interact with vertical structures, they initiate a bending conformation surrounding the structure and stabilize contact points along the structures via membrane curvature, focal adhesion complex, and accumulation of actin and endocytic vesicles (Hanson et al. 2015; Zhao et al. 2017; X. Liu et al. 2017; Viela et al. 2016; Seo et al. 2017). In general, it is clear that cells wraps around the structures very tightly (Santoro, Dasgupta, et al. 2014; Persson et al. 2013; Ojovan et al. 2015; Buch-Månson et al. 2017), similar to the way in which cells phagocytose macromolecules in their surroundings (Hai, Dormann, et al. 2009). Cells effectively adapt to the vertical structures and the wrapping process results in membrane ruffling around the structures’ surfaces such that the plasma membrane is only separated 10–50 nm from the structures’ edges. This is very similar to an engulfment phenomenon and probably happens both at the moment of seeding as well as during cellular migration processes. Besides this biomechanical re-arrangement of the cell in response to diverse vertical structures, we will now explore a variety of effects and variations that vertical structures induce in cells in terms of migration, proliferation, differentiation, and more general morphological changes as they have been presented in the literature.
Migration and proliferation of cells on pillars.
Diverse types of cells adhere and proliferate (C.-H. Choi et al. 2007; Nomura et al. 2005; Hu et al. 2010) on pillar substrates and spread on high aspect ratio vertical nanostructures while maintaining their physiological activity (S. Lee et al.2015). However, when vertical structures are involved, the perturbation of cells has to be minimized (Persson et al. 2013). In the work of Persson et al. (Persson et al. 2015), the effect on metabolism of vertical nanowire length vs. nanowire density has been explored. Surprisingly, few nanowires are capable of immobilizing mouse fibroblasts while a ‘bed of nails’ configuration allows cells to freely move. In addition, the rate of cell proliferation decreases with the increase of nanowire density.
Morphology.
Cells sense protruding structures as topographical cues which induce different responses, i.e. polarity (Bucaro et al. 2012), stretching (Oh et al. 2009; Zou et al. 2017; Santoro et al. 2013), with the response depending upon the cells’ type and structures’ material and geometry. A macroscale cellular rearrangement happens and concerns primarily cytoskeleton and plasma membrane. This macroscale response affects the overall behavior of cells in networks and pillars can be used to tune certain properties of complex cell assemblies. In fact, it has been shown that for glioma cells, a collective behavior which leads to tumor-like aggregate formation is typically shown when cells are in contact with large pillars (>2 um) where hypothetically cells tend to grow on the upper surface of the pillars rather than gain an intimate contact at the interface between the pillar and the flat support from which they are protruding (Jahed et al. 2016).
Pillars can be advantageous when in contact with neuronal cells to pin individual cells in a sort of nanofabricated trap (C. Xie et al. 2010) and, moreover, those cues induce multiple synaptic connections at network level as has been shown for InP nanowires arrays (Gautam et al. 2017). In light of this clear guidance effect on neuronal cells, a well-defined pillars array with a certain number of nodes can be employed to create a network of neurons with a limited number of connections which simplifies the investigation of signal propagation within the network (Santoro, Panaitov, and Offenhäusser 2014).
Differentiation.
In some cases, the topographical cues can have a reprogramming effect and induce specific cell differentiation and reorganization (Dalby et al. 2007; Brammer et al. 2011; Wilkinson et al. 2002). Vertical silicon nanocolumn arrays have been used for creating spheroid-like architectures from mouse induced pluripotent cells (H. Kim et al. 2016). In the work of Wei et al. (Wei et al. 2017), mesenchymal cells (MSC) have been coupled to polypyrrole nanotubes. Polypyrrole is a conjugated polymer and is sensitive to potential changes. In fact, by applying a negative potential nanotubes become much sharper like nanotips. This geometrical change of the vertical structures interestingly determines the differentiation of MSC into osteoblasts. Thus, it is possible to dynamically tune cell fate and use vertical structures to reprogram cellular functionalities.
With all the evidence of significant cellular response to vertical structures, the question becomes what does the interface look like under different conditions. In the next section we will survey the relevant techniques and some of their findings.
4. Resolution of the cell-vertical structure interface
4.1. Optical methods
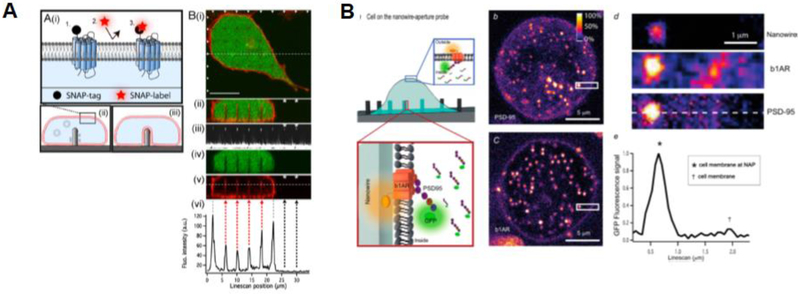
When cells are examined on planar surfaces, optical microscopy can be a suitable tool for understanding various parameters and can determined precisely how cells interact with the material surface. Understanding which process take place at the interface remains a main challenge in the biointerface community, particularly attempting to analyze mechanisms at the nanoscale via microscopy. A first example was given by Lambacher and Fromherz who used FLIC-microscopy to determine the adhesion properties of a labelled plasma membrane on structure silicon oxide. In fact, considering the reflective properties of this material, it is possible to investigate the fluorescent dye intensity as a function of the oxide thickness and distance of the plasma membrane from the material surface (Lambacher and Fromherz 1996). Similarly this could be calculated for a giant vesicle (Fromherz et al. 1999) and for supported lipid bilayers with immobilized proteins (Kiessling and Tamm 2003). Another approach is to use surface plasmon resonance to precisely determined the gap distance between a surface of a material and cells. As presented in the work for Toma et al., depending on the surface functionalization of the material, cells adhere differently and the gap distance depends also on the effective dimension of the functionalization molecule (K. Toma, Kano, and Offenhäusser 2014). In the case of vertical structures, the system geometry does not allow the use of optical microscopy most of the times for reflection limitations. However, successful attempts have been performed. Berthing et al. have demonstrated that confocal z-stack imaging can resolve the plasma membrane domain in contact with high aspect ratio (2–11 um) nanowires functionalized with aminosilane and polyethyleneimine. The plasma membrane was labelled with a SNAP-tag in the outer layer of the membrane. This optimal fluorescence labelling revealed that independent of structure dimensions and functionalization, the nanowire remains encapsulated in the extracellular domain of the cell (Figure 6A) (Berthing et al. 2012). Recently, nanowire aperture probes (NAP) have been used for photonic excitation. In this way, The nanowire acts as an aperture, which focuses the incoming excitation photon flux and can guide the fluorophore emission properties. In perspective, this approach allows the visualization of small molecules acting at the interface between cells and vertical structures (Figure 6B) (Frederiksen et al. 2016).
Figure 6:
Fluorescence microscopy of cells and vertical structures. A) SNAP labelled cell membrane tightly wraps around nanowires. (Berthing et al. 2012) B) NAP for molecular detection of cells and nanostructures. (Frederiksen et al. 2016)
4.2. Scanning electron microscopy - SEM
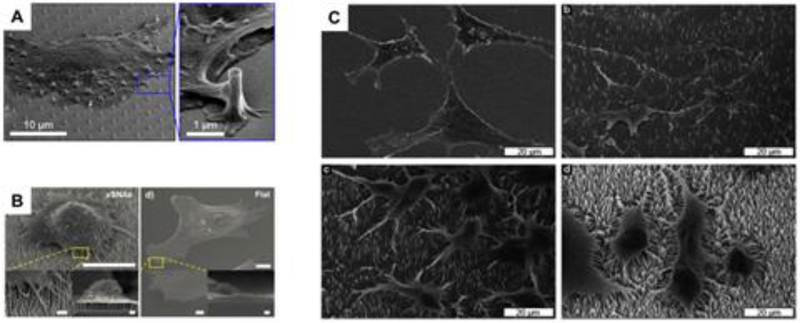
While optical microscopy relies on the labelling of specific molecules to understand how vertical nanostructures interact with cells, scanning electron microscopy allows an overview of the cell/structure interaction particularly focusing on adhesion properties. For SEM investigations, cells are chemically fixed and processed in order to preserve the integrity of the cells and vertical structures. These investigations are crucial in understanding how cells adhere and spread over time on vertical structures. Together with optical microscopy, SEM studies allow the visualization of cell bodies and their protrusions approaching vertical structures (Figure 7A) (Santoro et al. 2017; Berthing et al. 2012) and, in the case of flexible materials, the deformation force applied by the cells can be calculated (Viela et al. 2016). Furthermore, in the case of solid inflexible vertical structures coupled to cells with long protrusions, i.e. neuronal cells, a clear directionality effect has been shown as these cells grow on large pillar arrays made of gold (Panaitov et al. 2011; Santoro, Panaitov, and Offenhäusser 2014), platinum (Martiradonna et al. 2012; Sileo et al. 2013), and indium phosphide (Gautam et al. 2017). In particular, it has been shown that neuronal cells can grow on vertical structures and form tight junctions at the nanopillar surface. Depending on pillar surface roughness, an SEM investigation has shown that neuronal cells have preferential attachment points along superhydrophobic pillar bodies. Smooth pillars result in attachment at the base while nanostructured pillars induce attachment points at the top of the pillar body (Limongi et al. 2013). In general, SEM gives a very detailed macroscopic visualization of the cell morphology on diverse surfaces. The spreading of cells can be investigated as shown for fibroblasts grown on vertical nanostructures vs. planar material (Figure 7B) (S. Lee et al. 2015). Moreover, cells’ morphology can be monitored and by changing the vertical structures’ density (Figure 7C). In fact, Persson et al., show the cellular response to a transition from a flat surface to sparse structures to a very high density of nanostructures. Elongated cell processes develop dramatically in the presence of a bed-of-nails configuration which highlights how cells effectively form protrusions as final contact points to high aspect ratio nanostructured surfaces (Persson et al. 2015).
Figure 7.
SEM of cells vertical structures. A) Cardiomyocytes on quartz nanopillars with protrusions approach the pillar surface. (Santoro et al. 2017) B) Comparison of fibroblast on vertical structures vs. flat surface. (S. Lee et al. 2015)(Lee, et al. 2015) C) cells spreading on vertical structures at different densities. (Persson et al. 2015)
4.3. Transmission electron microscopy - TEM
To investigate the interface of cells and vertical structures with nanometer resolution, it is necessary to use electron microscopy-based techniques. In this way, it is possible to resolve the plasma membrane and its attachment points on the structures and the response of intracellular compartments, ie. nuclei and vesicles, to the presence of vertical structures (Hanson et al. 2015; Zhao et al. 2017). When TEM is involved, typically thin lamellae are prepared via mechanical sectioning, i.e. microtomes. This must be done after the removal of the vertical structures which can be done by chemical or physical etching. TEM allows the visualization of areas of interest with resolution of a few nanometers. First attempts to characterize the cell-substrate interface have been carried out on planar devices, as shown in the work of Wrobel et al. (Wrobel et al. 2008). More recent approaches have involved dissociated cells adhering to vertical structures. It has been shown that the average distance between cells and vertical nanostructures is reduced compared to flat surfaces (Hanson et al. 2012). This close interaction proven by the accurate measurement of the interstitial space between cells and nanopillars give an important insight into the nanopillars’ capabilities of trap cells and limit to some extent cell mobility as discussed in section 3.1. In particular for electrophysiology applications, the determination of the interstitial space becomes essential as it is directly connected to the electrical coupling and quality of the recording/stimulation capabilities of the nanostructured electrode. Gold mushroom-shaped vertical structures, vertically aligned carbon nanotubes and silicon-based nanowires exhibited high coupling to neuronal cells at both somas and branching extensions (Fendyur et al. 2011; Hai, Kamber, et al. 2009; Spira et al. 2007; Shmoel et al. 2016; R. Liu et al. 2017; Jeong et al. 2017). Similar observations were found for cardiomyocyte cells (Lin et al. 2014) and their more complex architectures in myotubes (Rabieh et al. 2016).
4.4. Scanning electron microscopy and focused ion beam - SEM/FIB
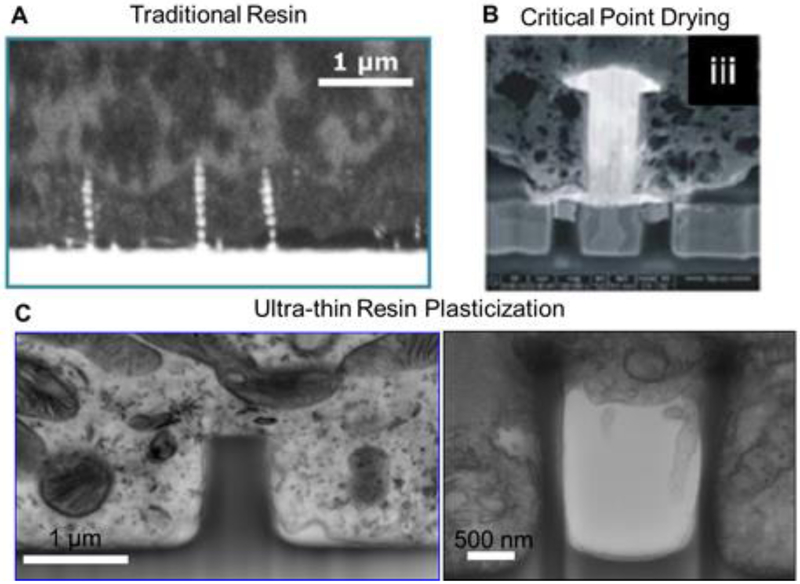
While TEM has the fundamental advantage of high resolution and contrast which allows the visualization of intracellular structures as well as the plasma membrane, it has major interface-preservation limitations. In order to prepare a section from the specimen, a mechanical knife is used to cut through the cell-resin matrix but it is not capable of cutting the substrate material. To overcome this, support materials are etched away and this process can introduce artifacts at the interface. For this reason, alternative sectioning procedures have been explored. Among them, a high energy ion beam represents an optimal tool to selectively etch an area of interest and, most importantly, through a large variety of materials (Munroe 2009). In this way, cross sections can be created which simultaneously preserve the position of the cell and the support material. Most ion beams are equipped in a dual beam configuration with an electron beam such that sectioning and imaging can be performed in situ in a short time window (Narayan and Subramaniam 2015). The preparation of biological samples can be mainly carried out in two ways. The first consists in resin embedding similar to that for TEM specimens. In this case, the embedding includes the support material which is not removed. The ion beam penetrates through the resin-cell matrix and the support to yield a cross section (Figure 8A). It has been shown that this approach is suitable for imaging supports with vertical structures of diverse aspect ratios and to map their interaction with cells (Wierzbicki et al. 2013; Persson et al. 2013). Alternatively, a thin lamella can be prepared and moved to a TEM system for further image acquisition (R. Liu et al. 2017). However, to locate an area of interest is very difficult because of the presence of a large resin matrix (mm thickness). The second approach is to perform a dehydration process by exchanging the water with other liquid components first and then use a hard drying procedure, i.e. via liquid CO2 in critical point drying. The drying procedure allows a direct visualization of whole cells on the support material under SEM such that an area of interest can be located and a cross section can be created by FIB (Ha et al. 2014; Santoro, Panaitov, and Offenhäusser 2014; M. Toma et al.2017). Even though many attempts have been made to limit typical curtaining effects at the interface of cells and vertical structures (Santoro, Neumann, et al. 2014; Friedmann et al. 2011), intracellular structures are unlikely to be preserved and multiple cavities are visible in correspondence to intracellular structures. Furthermore, the space between the plasma membrane and vertical structures is difficult to resolve (Figure 8B) (Panaitov et al. 2011; Braeken et al. 2009; Santoro, Dasgupta, et al. 2014; Sileo et al. 2013; Harding et al. 2016; Seyock et al. 2017; Van Meerbergen et al. 2008). To overcome this limitation, a new specimen preparation, named “ultra-thin plasticization”, has been developed to combine the structural support of resin and the possibility to identify an area of interest by SEM for further FIB sectioning (Belu et al.2016). While in the first approach mentioned earlier the resin polymerization is carried out to ensure that a large matrix surrounds both cells and support, in this new approach excess resin is removed before polymerization by a solvent wash. In this way, both cells and structures are clearly distinguishable and small cellular features, i.e. filopodia and lamellipodia, are preserved on the structures. While this new approach represents an appropriate alternative to hard drying procedure for visualization of whole-cell morphology in SEM, further FIB processing necessitates the use of heavy metal staining for intracellular structure resolution (C. Chiappini et al. 2015). In the work of Santoro et al., an advanced ultra-thin plasticization method has been developed to resolve intracellular compartments and, moreover, to establish a procedure to create cross sections of dissociated cells on diverse nanostructured materials. In fact, it has been shown that the interface can be investigated by SEM/FIB for quartz nanostructures (pillars, nanoletters, nanotubes), silicon-based nanostructures, and polymers (PEDOT:PSS) (Figure 8C). Furthermore, this method allows three-dimensional representation of a volume of interest via sequential cross sectioning and automated segmented reconstruction of the nanostructured support (Santoro et al. 2017).
Figure 8.
Various interface preparation protocols. A) Traditional resin embedding method. (Wierzbicki et al., 2013) B) Critical point drying (Van Meerbergen et al., 2008) C) Ultra-thin resin plasticization. (Santoro et al., 2017)
5. Conclusion & Outlook
Since the first experiments interfacing cells with high aspect ratio vertical structures roughly a decade ago, the field of nano-biosensors has only gained momentum, using new geometries and materials to probe a wide variety of cellular processes in vitro. The field has stimulated growth in other areas, too, as the cell-material interface begs characterization. We hope to have shown that this interface is unique in yielding such unparalleled performance in a variety of assays while being driven directly by cells.
We believe that the applications of the cell-material interface will continue to grow, but also look to recent results in vivo which suggest that nanotechnology will make a claim to increased performance there, too. From brain-implanted flexible electrode meshes (C. Xie et al. 2015; Xiang, Liu, and Lee 2016; Luan et al. 2017; Gonzales et al. 2017), to nanostraw devices packaged for ingestion (Fox et al. 2016), there is a vast application space for tissue-material interfaces. It is uncertain whether the same interfacial dynamics will hold at the tissue level and new tools will be required to answer this question as well.
6. References
- Aalipour Amin, Xu Alexander M., Sergio Leal-Ortiz Craig C. Garner, and Melosh Nicholas A.. 2014. “Plasma Membrane and Actin Cytoskeleton as Synergistic Barriers to Nanowire Cell Penetration.” Langmuir: The ACS Journal of Surfaces and Colloids 30 (41): 12362–67. [DOI] [PubMed] [Google Scholar]
- Abbott Jeffrey, Ye Tianyang, Qin Ling, Jorgolli Marsela, Gertner Rona S., Ham Donhee, and Park Hongkun. 2017. “CMOS Nanoelectrode Array for All-Electrical Intracellular Electrophysiological Imaging.” Nature Nanotechnology 12 (5): 460–66. [DOI] [PubMed] [Google Scholar]
- Alivisatos A. Paul, Alivisatos A. Paul, Andrews Anne M., Boyden Edward S., Chun Miyoung, Church George M., Deisseroth Karl, et al. 2013. “Nanotools for Neuroscience and Brain Activity Mapping.” ACS Nano 7 (3): 1850–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almquist Benjamin D., and Melosh Nicholas A.. 2010. “Fusion of Biomimetic Stealth Probes into Lipid Bi layer Cores.” Proceedings of the National Academy of Sciences of the United States of America 107 (13): 5815–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belu A, Schnitker J, Bertazzo S, Neumann E, Mayer D, Offenhäusser A, and Santoro F. 2016. “Ultra-Thin Resin Embedding Method for Scanning Electron Microscopy of Individual Cells on High and Low Aspect Ratio 3D Nanostructures.” Journal of Microscopy 263 (1): 78–86. [DOI] [PubMed] [Google Scholar]
- Berdondini Luca, Imfeld Kilian, Maccione Alessandro, Tedesco Mariateresa, Neukom Simon, Koudelka-Hep Milena, and Martinoia Sergio. 2009. “Active Pixel Sensor Array for High Spatio-Temporal Resolution Electrophysiological Recordings from Single Cell to Large Scale Neuronal Networks.” Lab on a Chip 9 (18): 2644–51. [DOI] [PubMed] [Google Scholar]
- Berthing Trine, Bonde Sara, Rostgaard Katrine R., Madsen Morten Hannibal, Sørensen Claus B., Nygård Jesper, and Martinez Karen L.. 2012. “Cell Membrane Conformation at Vertical Nanowire Array Interface Revealed by Fluorescence Imaging.” Nanotechnology 23 (41): 415102. [DOI] [PubMed] [Google Scholar]
- Braeken D, Huys R, Jans D, Loo J, Severi S, Vleugels F, Borghs G, Callewaert G, and Bartic C. 2009. “Local Electrical Stimulation of Single Adherent Cells Using Three-Dimensional Electrode Arrays with Small Interelectrode Distances.” In 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society 10.1109/iembs.2009.5333871. [DOI] [PubMed] [Google Scholar]
- Brammer Karla S., Choi Chulmin, Frandsen Christine J., Oh Seunghan, and Jin Sungho. 2011. “Hydrophobic Nanopillars Initiate Mesenchymal Stem Cell Aggregation and Osteo-Differentiation.” Acta Biomaterialia 7 (2): 683–90. [DOI] [PubMed] [Google Scholar]
- Brüggemann D, Wolfrum B, Maybeck V, Mourzina Y, Jansen M, and Offenhäusser A. 2011. “Nanostructured Gold Microelectrodes for Extracellular Recording from Electrogenic Cells.” Nanotechnology 22 (26): 265104. [DOI] [PubMed] [Google Scholar]
- Bucaro Michael A., Vasquez Yolanda, Hatton Benjamin D., and Aizenberg Joanna. 2012. “Fine-Tuning the Degree of Stem Cell Polarization and Alignment on Ordered Arrays of High-Aspect-Ratio Nanopillars.” ACS Nano 6 (7): 6222–30. [DOI] [PubMed] [Google Scholar]
- Buch-Månson Nina, Bonde Sara, Bolinsson Jessica, Berthing Trine, Nygård Jesper, and Martinez Karen L.. 2015. “Towards a Better Prediction of Cell Settling on Nanostructure Arrays-Simple Means to Complicated Ends.” Advanced Functional Materials 25 (21): 3246–55. [Google Scholar]
- Buch-Månson Nina, Kang Dong-Hee, Kim Dongyoon, Kyung Eun Lee Myung-Han Yoon, and Martinez Karen L.. 2017. “Mapping Cell Behavior across a Wide Range of Vertical Silicon Nanocolumn Densities.” Nanoscale 9 (17): 5517–27. [DOI] [PubMed] [Google Scholar]
- Yoeri van de Burgt, Lubberman Ewout, Fuller Elliot J., Keene Scott T., Faria Grégorio C., Agarwal Sapan, Marinella Matthew J., Talin A. Alec, and Salleo Alberto. 2017. “A Non-Volatile Organic Electrochemical Device as a Low-Voltage Artificial Synapse for Neuromorphic Computing.” Nature Materials 16 (4): 414–18. [DOI] [PubMed] [Google Scholar]
- Burridge Paul W., Matsa Elena, Shukla Praveen, Lin Ziliang C., Churko Jared M., Ebert Antje D., Lan Feng, et al. 2014. “Chemically Defined Generation of Human Cardiomyocytes.” Nature Methods 11 (8): 855–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Yuhong, Hjort Martin, Chen Haodong, Birey Fikri, Leal-Ortiz Sergio A., Han Crystal M., Santiago Juan G., Pa§ca Sergiu P., Wu Joseph C., and Melosh Nicholas A.. 2017. “Nondestructive Nanostraw Intracellular Sampling for Longitudinal Cell Monitoring.” Proceedings of the National Academy of Sciences of the United States of America 114 (10): E1866–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprettini Valeria, Cerea Andrea, Melle Giovanni, Lovato Laura, Capozza Rosario, Huang Jian-An, Tantussi Francesco, Dipalo Michele, and De Angelis Francesco. 2017. “Soft Electroporation for Delivering Molecules into Tightly Adherent Mammalian Cells through 3D Hollow Nanoelectrodes.” Scientific Reports 7 (1): 8524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Lingqian, Hu Jiaming, Chen Feng, Chen Zhou, Shi Junfeng, Yang Zhaogang, Li Yiwen, and Lee Ly James. 2016. “Nanoscale Bio-Platforms for Living Cell Interrogation: Current Status and Future Perspectives.” Nanoscale 8 (6): 3181–3206. [DOI] [PubMed] [Google Scholar]
- Chapman Christopher A. R., Chen Hao, Stamou Marianna, Biener Juergen, Biener Monika M., Lein Pamela J., and Seker Erkin. 2015. “Nanoporous Gold as a Neural Interface Coating: Effects of Topography, Surface Chemistry, and Feature Size.” ACS Applied Materials & Interfaces 7 (13): 7093–7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung CL, Nikolic RJ, Reinhardt CE, and Wang TF. 2006. “Fabrication of Nanopillars by Nanosphere Lithography.” Nanotechnology 17 (5): 1339. [Google Scholar]
- Chiappini C, De Rosa E, Martinez JO, Liu X, Steele J, Stevens MM, and Tasciotti E.2015. “Biodegradable Silicon Nanoneedles Delivering Nucleic Acids Intracellularly Induce Localized in Vivo Neovascularization.” Nature Materials 14 (5): 532–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappini Ciro. 2017. “Nanoneedle-Based Sensing in Biological Systems.” ACS Sensors. 10.1021/acssensors.7b00350. [DOI] [PubMed] [Google Scholar]
- Chiappini Ciro, Campagnolo Paola, Almeida Carina S., Nima Abbassi-Ghadi Lesley W. Chow, Hanna George B., and Stevens Molly M.. 2015. “Mapping Local Cytosolic Enzymatic Activity in Human Esophageal Mucosa with Porous Silicon Nanoneedles.” Advanced Materials 27 (35): 5147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappini Ciro, Martinez Jonathan O., Enrica De Rosa Carina S. Almeida, Tasciotti Ennio, and Stevens Molly M.. 2015. “Biodegradable Nanoneedles for Localized Delivery of Nanoparticles in Vivo: Exploring the Biointerface.” ACS Nano 9 (5): 5500–5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Chang-Hwan, Hagvall Sepideh H., Wu Benjamin M., Dunn James C. Y., Beygui Ramin E., and Kim Chang-Jin CJ. 2007. “Cell Interaction with Three-Dimensional Sharp-Tip Nanotopography.” Biomaterials 28 (9): 1672–79. [DOI] [PubMed] [Google Scholar]
- Choi Sojoong, Kim Hyunju, Kim So Yeon, and Yang Eun Gyeong. 2016. “Probing Protein Complexes inside Living Cells Using a Silicon Nanowire-Based Pull-down Assay.” Nanoscale 8 (22): 11380–84. [DOI] [PubMed] [Google Scholar]
- Cohen-Karni Tzahi, Qing Quan, Li Qiang, Fang Ying, and Lieber Charles M.. 2010. “Graphene and Nanowire Transistors for Cellular Interfaces and Electrical Recording.” Nano Letters 10 (3): 1098–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalby Matthew J., Gadegaard Nikolaj, Tare Rahul, Andar Abhay, Riehle Mathis O., Herzyk Pawel, Wilkinson Chris D. W., and Oreffo Richard O. C.. 2007. “The Control of Human Mesenchymal Cell Differentiation Using Nanoscale Symmetry and Disorder.” Nature Materials 6 (12): 997–1003. [DOI] [PubMed] [Google Scholar]
- Davis H 1939. “Electrical Phenomena of the Brain and Spinal Cord.” Annual Review of Physiology 1 (1): 345–62. [Google Scholar]
- Angelis De, Francesco Mario Malerba, Patrini Maddalena, Miele Ermanno, Das Gobind, Toma Andrea, Zaccaria Remo Proietti, and Di Fabrizio Enzo. 2013. “3D Hollow Nanostructures as Building Blocks for Multifunctional Plasmonics.” Nano Letters 13 (8): 3553–58. [DOI] [PubMed] [Google Scholar]
- Dipalo Michele, Amin Hayder, Lovato Laura, Moia Fabio, Caprettini Valeria, Messina Gabriele C., Tantussi Francesco, Berdondini Luca, and Francesco De Angelis. 2017. “Intracellular and Extracellular Recording of Spontaneous Action Potentials in Mammalian Neurons and Cardiac Cells with 3D Plasmonic Nanoelectrodes.” Nano Letters 17 (6): 3932–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Xiaojie, Gao Ruixuan, Xie Ping, Tzahi Cohen-Karni Quan Qing, Hwan Sung Choe Bozhi Tian, Jiang Xiaocheng, and Lieber Charles M.. 2011. “Intracellular Recordings of Action Potentials by an Extracellular Nanoscale Field-Effect Transistor.” Nature Nanotechnology 7 (3): 174–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eversmann B, Jenkner M, Hofmann F, Paulus C, Brederlow R, Holzapfl B, Fromherz P, et al. 2003. “A 128 X 128 Cmos Biosensor Array for Extracellular Recording of Neural Activity.” IEEE Journal of Solid-State Circuits 38 (12): 2306–17. [Google Scholar]
- Fendyur Anna, Mazurski Noa, Shappir Joseph, and Spira Micha E.. 2011. “Formation of Essential Ultrastructural Interface between Cultured Hippocampal Cells and Gold Mushroom-Shaped MEA-Toward ‘IN-CELL’ Recordings from Vertebrate Neurons.” Frontiers in Neuroengineering 4 (December): 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendyur Anna, and Spira Micha E.. 2012. “Toward on-Chip, in-Cell Recordings from Cultured Cardiomyocytes by Arrays of Gold Mushroom-Shaped Microelectrodes.” Frontiers in Neuroengineering 5 (August): 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox Cade B., Cao Yuhong, Nemeth Cameron L., Chirra Hariharasudhan D., Chevalier Rachel W., Xu Alexander M., Melosh Nicholas A., and Desai Tejal A.. 2016. “Fabrication of Sealed Nanostraw Microdevices for Oral Drug Delivery.” ACS Nano 10 (6): 5873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen Rune S., Esther Alarcon-Llado Peter Krogstrup, Bojarskaite Laura, Nina Buch-Månson Jessica Bolinsson, Nygård Jesper, Morral Anna Fontcuberta i., and Martinez Karen L.. 2016. “Nanowire-Aperture Probe: Local Enhanced Fluorescence Detection for the Investigation of Live Cells at the Nanoscale.” ACS Photonics 3 (7): 1208–16. [Google Scholar]
- Frey Urs, Sedivy Jan, Heer Flavio, Pedron Rene, Ballini Marco, Mueller Jan, Bakkum Douglas, et al. 2010. “Switch-Matrix-Based High-Density Microelectrode Array in CMOS Technology.” IEEE Journal of Solid-State Circuits 45 (2): 467–82. [Google Scholar]
- Friedmann Andrea, Hoess Andreas, Cismak Andreas, and Heilmann Andreas. 2011. “Investigation of Cell-Substrate Interactions by Focused Ion Beam Preparation and Scanning Electron Microscopy.” Acta Biomaterialia 7 (6): 2499–2507. [DOI] [PubMed] [Google Scholar]
- Fromherz P, Kiessling V, Kottig K, and Zeck G. 1999. “Membrane Transistor with Giant Lipid Vesicle Touching a Silicon Chip.” Applied Physics A: Materials Science & Processing 69 (5): 571–76. [Google Scholar]
- Fromherz P, Offenhäusser A, Vetter T, and Weis J. 1991. “A Neuron-Silicon Junction: A Retzius Cell of the Leech on an Insulated-Gate Field-Effect Transistor.” Science 252 (5010): 1290–93. [DOI] [PubMed] [Google Scholar]
- Gautam Vini, Naureen Shagufta, Shahid Naeem, Gao Qian, Wang Yi, Nisbet David, Jagadish Chennupati, and Daria Vincent R.. 2017. “Engineering Highly Interconnected Neuronal Networks on Nanowire Scaffolds.” Nano Letters 17 (6): 3369–75. [DOI] [PubMed] [Google Scholar]
- Gesteland R, Howland B, Lettvin J, and Pitts W. 1959. “Comments on Microelectrodes.” Proceedings of the IRE 47 (11): 1856–62. [Google Scholar]
- Gonzales Daniel L., Badhiwala Krishna N., Vercosa Daniel G., Avants Benjamin W., Liu Zheng, Zhong Weiwei, and Robinson Jacob T.. 2017. “Scalable Electrophysiology in Intact Small Animals with Nanoscale Suspended Electrode Arrays.” Nature Nanotechnology 12 (7): 684–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai Aviad, Dormann Ada, Shappir Joseph, Yitzchaik Shlomo, Bartic Carmen, Borghs Gustaaf,Langedijk JPM, and Spira Micha E.. 2009. “Spine-Shaped Gold Protrusions Improve the Adherence and Electrical Coupling of Neurons with the Surface of Micro-Electronic Devices.” Journal of the Royal Society, Interface / the Royal Society 6 (41): 1153–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai Aviad, Kamber Dotan, Malkinson Guy, Erez Hadas, Mazurski Noa, Shappir Joseph, and Spira Micha E.. 2009. “Changing Gears from Chemical Adhesion of Cells to Flat Substrata toward Engulfment of Micro-Protrusions by Active Mechanisms.” Journal of Neural Engineering 6 (6): 066009. [DOI] [PubMed] [Google Scholar]
- Hai Aviad, Shappir Joseph, and Spira Micha E.. 2010. “Long-Term, Multisite, Parallel, in-Cell Recording and Stimulation by an Array of Extracellular Microelectrodes.” Journal of Neurophysiology 104 (1): 559–68. [DOI] [PubMed] [Google Scholar]
- Hai Aviad, and Spira Micha E.. 2012. “On-Chip Electroporation, Membrane Repair Dynamics and Transient in-Cell Recordings by Arrays of Gold Mushroom-Shaped Microelectrodes.” Lab on a Chip 12 (16): 2865–73. [DOI] [PubMed] [Google Scholar]
- Hanson Lindsey, Ziliang Carter Lin Chong Xie, Cui Yi, and Cui Bianxiao. 2012. ”Characterization of the Cell-Nanopillar Interface by Transmission Electron Microscopy.” Nano Letters 12 (11): 5815–20. [DOI] [PubMed] [Google Scholar]
- Hanson Lindsey, Zhao Wenting, Lou Hsin-Ya, Ziliang Carter Lin Seok Woo Lee, Chowdary Praveen, Cui Yi, and Cui Bianxiao. 2015. “Vertical Nanopillars for in Situ Probing of Nuclear Mechanics in Adherent Cells.” Nature Nanotechnology 10 (6): 554–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha Qing, Yang Gao, Ao Zhuo, Han Dong, Niu Fenglan, and Wang Shutao. 2014. “RapidFibroblast Activation in Mammalian Cells Induced by Silicon Nanowire Arrays.” Nanoscale 6 (14): 8318–25. [DOI] [PubMed] [Google Scholar]
- Harding Frances J., Surdo Salvatore, Delalat Bahman, Cozzi Chiara, Elnathan Roey, Gronthos Stan, Voelcker Nicolas H., and Barillaro Giuseppe. 2016. “Ordered Silicon Pillar Arrays Prepared by Electrochemical Micromachining: Substrates for High-Efficiency Cell Transfection.” ACS Applied Materials & Interfaces 8 (43): 29197–202. [DOI] [PubMed] [Google Scholar]
- Harrison RR 2008. “The Design of Integrated Circuits to Observe Brain Activity.” Proceedings of the IEEE 96 (7): 1203–16. [Google Scholar]
- Heim Matthias, Rousseau Lionel, Reculusa Stéphane, Urbanova Veronika, Mazzocco Claire, Joucla Sébastien, Bouffier Laurent, et al. 2012. “Combined Macro-/mesoporous Microelectrode Arrays for Low-Noise Extracellular Recording of Neural Networks.” Journal of Neurophysiology 108 (6): 1793–1803. [DOI] [PubMed] [Google Scholar]
- Hu Walter, Crouch Adam S., Miller Danielle, Aryal Mukti, and Luebke Kevin J.. 2010. “Inhibited Cell Spreading on Polystyrene Nanopillars Fabricated by Nanoimprinting and in Situ Elongation.” Nanotechnology 21 (38): 385301. [DOI] [PubMed] [Google Scholar]
- Jahed Zeinab, Lin Peter, Seo Brandon B., Verma Mohit S., Gu Frank X., Tsui Ting Y., and Mofrad Mohammad R. K.. 2014. “Responses of Staphylococcus Aureus Bacterial Cells to Nanocrystalline Nickel Nanostructures.” Biomaterials 35 (14): 4249–54. [DOI] [PubMed] [Google Scholar]
- Jahed Zeinab, Zareian Ramin, Yeung Yeung Chau Brandon B. Seo, West Mary, Tsui Ting Y., Wen Weijia, and Mofrad Mohammad R. K.. 2016. “Differential Collective- and Single-Cell Behaviors on Silicon Micropillar Arrays.” ACS Applied Materials & Interfaces 8 (36): 23604–13. [DOI] [PubMed] [Google Scholar]
- Jeong Du Won, Gook Hwa Kim Na Yeon Kim, Lee Zonghoon, Jung Sang Don, and Lee Jeong-O. 2017. “A High-Performance Transparent Graphene/vertically Aligned Carbon Nanotube (VACNT) Hybrid Electrode for Neural Interfacing.” RsC Advances 7 (6): 3273–81. [Google Scholar]
- Kavaldzhiev Mincho, Jose Efrain Perez Yurii Ivanov, Bertoncini Andrea, Liberale Carlo, and Kosel Jürgen. 2017. “Biocompatible 3D Printed Magnetic Micro Needles.” Biomedical Physics & Engineering Express 3 (2): 025005. [Google Scholar]
- Kiessling Volker, and Tamm Lukas K.. 2003. “Measuring Distances in Supported Bilayers by Fluorescence Interference-Contrast Microscopy: Polymer Supports and SNARE Proteins.” Biophysical Journal 84 (1): 408–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Hyunju, Dong Hee Kang Kyung Hee Koo, Lee Seyeong, Kim Seong-Min, Kim Janghwan, Yoon Myung-Han, Kim So Yeon, and Yang Eun Gyeong. 2016. “Vertical Nanocolumn-Assisted Pluripotent Stem Cell Colony Formation with Minimal Cell-Penetration.” Nanoscale 8 (42): 18087–97. [DOI] [PubMed] [Google Scholar]
- Kim Woong, Ng Jennifer K., Kunitake Miki E., Conklin Bruce R., and Yang Peidong. 2007. “Interfacing Silicon Nanowires with Mammalian Cells.” Journal of the American Chemical Society 129 (23): 7228–29. [DOI] [PubMed] [Google Scholar]
- Kim Yong Hee, Gook Hwa Kim Ah Young Kim, Young Hwan Han Myung-Ae Chung, and Jung Sang-Don. 2015. “In Vitroextracellular Recording and Stimulation Performance of Nanoporous Gold-Modified Multi-Electrode Arrays.” Journal of Neural Engineering 12 (6): 066029. [DOI] [PubMed] [Google Scholar]
- Kwak Minsuk, Han Lin, Chen Jonathan J., and Fan Rong. 2015. “Interfacing Inorganic Nanowire Arrays and Living Cells for Cellular Function Analysis.” Small 11 (42): 5600–5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambacher Armin, and Fromherz Peter. 1996. “Fluorescence Interference-Contrast Microscopy on Oxidized Silicon Using a Monomolecular Dye Layer.” Applied Physics A: Materials Science & Processing 63 (3): 207–16. [Google Scholar]
- Lee Donggyu, Lee Daehee, Won Yulim, Hong Hyeonaug, Kim Yongjae, Song Hyunwoo, Jae-Chul Pyun Yong Soo Cho, Ryu Wonhyoung, and Moon Jooho. 2016. “Insertion of Vertically Aligned Nanowires into Living Cells by Inkjet Printing of Cells.” Small 12 (11): 1446–57. [DOI] [PubMed] [Google Scholar]
- Lee Seyeong, Kim Dongyoon, Kim Seong-Min, Kim Jeong-Ah, Kim Taesoo, Kim Dong-Yu, and Yoon Myung-Han. 2015. “Polyelectrolyte Multilayer-Assisted Fabrication of Non-Periodic Silicon Nanocolumn Substrates for Cellular Interface Applications.” Nanoscale 7 (35): 14627–35. [DOI] [PubMed] [Google Scholar]
- Limongi Tania, Cesca Fabrizia, Gentile Francesco, Marotta Roberto, Ruffilli Roberta, Barberis Andrea, Dal Maschio Marco, et al. 2013. “Nanostructured Superhydrophobic Substrates Trigger the Development of 3D Neuronal Networks.” Small 9 (3): 402–12. [DOI] [PubMed] [Google Scholar]
- Lin Ziliang Carter, McGuire Allister F., Burridge Paul W., Matsa Elena, Lou Hsin-Ya, Wu Joseph C., and Cui Bianxiao. 2017. “Accurate Nanoelectrode Recording of Human Pluripotent Stem Cell-Derived Cardiomyocytes for Assaying Drugs and Modeling Disease.” Microsystems & Nanoengineering 3: 16080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Ziliang Carter, Xie Chong, Osakada Yasuko, Cui Yi, and Cui Bianxiao. 2014. “Iridium Oxide Nanotube Electrodes for Sensitive and Prolonged Intracellular Measurement of Action Potentials.” Nature Communications 5: 3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Jian, Sun Yidi, Drubin David G., and Oster George F.. 2009. “The Mechanochemistry of Endocytosis.” PLoS Biology 7 (9): e1000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Ren, Chen Renjie, Elthakeb Ahmed T., Sang Heon Lee Sandy Hinckley, Khraiche Massoud L., Scott John, et al. 2017. “High Density Individually Addressable Nanowire Arrays Record Intracellular Activity from Primary Rodent and Human Stem Cell Derived Neurons.” Nano Letters 17 (5): 2757–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Xiangnan, Liu Ruili, Gu Yexin, and Ding Jiandong. 2017. “Nonmonotonic Self-Deformationof Cell Nuclei on Topological Surfaces with Micropillar Array.” ACS Applied Materials & Interfaces 9 (22): 18521–30. [DOI] [PubMed] [Google Scholar]
- Luan Lan, Wei Xiaoling, Zhao Zhengtuo, Siegel Jennifer J., Potnis Ojas, Tuppen Catherine A., Lin Shengqing, et al. 2017. “Ultraflexible Nanoelectronic Probes Form Reliable, Glial Scar-Free Neural Integration.” Science Advances 3 (2): e1601966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martiradonna Luigi, Quarta Luca, Sileo Leonardo, Schertel Andreas, Maccione Alessandro, Simi Alessandro, Dante Silvia, Scarpellini Alice, Berdondini Luca, and De Vittorio Massimo. 2012. “Beam Induced Deposition of 3D Electrodes to Improve Coupling to Cells.” Microelectronic Engineering 97 (September): 365–68. [Google Scholar]
- Matsumoto Daisuke, Yamagishi Ayana, Saito Megumi, Rao Sathuluri Ramachandra, Silberberg Yaron R., Iwata Futoshi, Kobayashi Takeshi, and Nakamura Chikashi. 2016. “Mechanoporation of Living Cells for Delivery of Macromolecules Using Nanoneedle Array.” Journal of Bioscience and Bioengineering 122 (6): 748–52. [DOI] [PubMed] [Google Scholar]
- McMahon Harvey T., and Gallop Jennifer L.. 2005. “Membrane Curvature and Mechanisms of Dynamic Cell Membrane Remodelling.” Nature 438 (7068): 590–96. [DOI] [PubMed] [Google Scholar]
- Messina Gabriele C., Dipalo Michele, Rosanna La Rocca Pierfrancesco Zilio, Caprettini Valeria, Remo Proietti Zaccaria Andrea Toma, Tantussi Francesco, Berdondini Luca, and De Angelis Francesco. 2015. “Spatially, Temporally, and Quantitatively Controlled Delivery of Broad Range of Molecules into Selected Cells through Plasmonic Nanotubes.” Advanced Materials 27 (44): 7145–49. [DOI] [PubMed] [Google Scholar]
- Miyauchi Akihiro, Kuwabara Kosuke, Hasegawa Mitsuru, and Ogino Masahiko. 2016. “Large-Area Nanoimprint and Application to Cell Cultivation.” Applied Physics A: Materials Science & Processing 122 (4). 10.1007/s00339-016-9744-0. [DOI] [Google Scholar]
- Müller Jan, Ballini Marco, Livi Paolo, Chen Yihui, Radivojevic Milos, Shadmani Amir, Viswam Vijay, et al. 2015. “High-Resolution CMOS MEA Platform to Study Neurons at Subcellular, Cellular, and Network Levels.” Lab on a Chip 15 (13): 2767–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munroe PR 2009. “The Application of Focused Ion Beam Microscopy in the Material Sciences.” Materials Characterization 60 (1): 2–13. [Google Scholar]
- Narayan Kedar, and Subramaniam Sriram. 2015. “Focused Ion Beams in Biology.” Nature Methods 12 (11): 1021–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann E, Schaefer-Ridder M, Wang Y, and Hofschneider PH. 1982. “Gene Transfer into Mouse Lyoma Cells by Electroporation in High Electric Fields.” The EMBO Journal 1 (7): 841–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nick Christoph, Thielemann Christiane, and Schlaak Helmut F.. 2014. “PEDOT:PSS Coated Gold Nanopillar Microelectrodes for Neural Interfaces.” In 2014 International Conference on Manipulation, Manufacturing and Measurement on the Nanoscale (3M-NANO), 160–65. IEEE. [Google Scholar]
- Nomura Shinobu, Kojima Hiroko, Ohyabu Yoshimi, Kuwabara Kosuke, Miyauchi Akihiro, and Uemura Toshimasa. 2005. “Cell Culture on Nanopillar Sheet: Study of HeLa Cells on Nanopillar Sheet.” Japanese Journal of Applied Physics 44 (37): L1184–86. [DOI] [PubMed] [Google Scholar]
- Obien Marie Engelene J., Deligkaris Kosmas, Bullmann Torsten, Bakkum Douglas J., and Frey Urs. 2014. “Revealing Neuronal Function through Microelectrode Array Recordings.” Frontiers in Neuroscience 8: 423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh Seunghan, Brammer Karla S., Li Y. S. Julie, Teng Dayu, Engler Adam J., Chien Shu, and Jin Sungho. 2009. “Stem Cell Fate Dictated Solely by Altered Nanotube Dimension.” Proceedings of the National Academy of Sciences of the United States of America 106 (7): 2130–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojovan Silviya M., Rabieh Noha, Shmoel Nava, Erez Hadas, Maydan Eilon, Cohen Ariel, and Spira Micha E.. 2015. “A Feasibility Study of Multi-Site, intracellular Recordings from Mammalian Neurons by Extracellular Gold Mushroom-Shaped Microelectrodes.” Scientific Reports 5 (September): 14100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozel Tuncay, Zhang Benjamin A., Gao Ruixuan, Day Robert W., Lieber Charles M., and Nocera Daniel G.. 2017. “Electrochemical Deposition of Conformal and Functional Layers on High Aspect Ratio Silicon Micro/Nanowires.” Nano Letters 17 (7): 4502–7. [DOI] [PubMed] [Google Scholar]
- Panaitov Gregory, Thiery Simon, Hofmann Boris, and Offenhäusser Andreas. 2011. “Fabrication of Gold Micro-Spine Structures for Improvement of Cell/device Adhesion.” Microelectronic Engineering 88 (8): 1840–44. [Google Scholar]
- Patolsky Fernando, Timko Brian P., Yu Guihua, Fang Ying, Greytak Andrew B., Zheng Gengfeng, and Lieber Charles M.. 2006. “Detection, Stimulation, and Inhibition of Neuronal Signals with High-Density Nanowire Transistor Arrays.” Science 313 (5790): 1100–1104. [DOI] [PubMed] [Google Scholar]
- Persson Henrik, Carsten Købler Kristian Mølhave, Samuelson Lars, Tegenfeldt Jonas O., Oredsson Stina, and Prinz Christelle N.. 2013. “Fibroblasts Cultured on Nanowires Exhibit Low Motility, Impaired Cell Division, and DNA Damage.” Small 9 (23): 4006–16, 3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson Henrik, Li Zhen, Tegenfeldt Jonas O., Oredsson Stina, and Prinz Christelle N.. 2015. “From Immobilized Cells to Motile Cells on a Bed-of-Nails: Effects of Vertical Nanowire Array Density on Cell Behaviour.” Scientific Reports 5 (December): 18535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz Christelle N. 2015. “Interactions between Semiconductor Nanowires and Living Cells.” Journal of Physics. Condensed Matter: An Institute of Physics Journal 27 (23): 233103. [DOI] [PubMed] [Google Scholar]
- Qing Quan, Jiang Zhe, Xu Lin, Gao Ruixuan, Mai Liqiang, and Lieber Charles M.. 2014. “Free-Standing Kinked Nanowire Transistor Probes for Targeted Intracellular Recording in Three Dimensions.” Nature Nanotechnology 9 (2): 142–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabieh Noha, Ojovan Silviya M., Shmoel Nava, Erez Hadas, Maydan Eilon, and Micha E. Spira. 2016. “On-Chip, Multisite Extracellular and Intracellular Recordings from Primary Cultured Skeletal Myotubes.” Scientific Reports 6 (November): 36498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson FJ, Cole MT, Hicks JM, Aylott JW, Milne WI, Collins CM, Jackson SK, Silman NJ, and Mendes PM. 2016. “Electrochemical Communication with the inside of Cells Using Micro-Patterned Vertical Carbon Nanofibre Electrodes.” Scientific Reports 6 (December): 37672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey By Marcel, Elnathan Roey, Ditcovski Ran, Geisel Karen, Zanini Michele, Miguel-Angel Fernandez-Rodriguez Vikrant V. Naik, et al. 2016. “Fully Tunable Silicon Nanowire Arrays Fabricated by Soft Nanoparticle Templating.” Nano Letters 16 (1): 157–63. [DOI] [PubMed] [Google Scholar]
- Robinson Jacob T., Jorgolli Marsela, Shalek Alex K., Yoon Myung-Han, Gertner Rona S., and Park Hongkun. 2012. “Vertical Nanowire Electrode Arrays as a Scalable Platform for Intracellular Interfacing to Neuronal Circuits.” Nature Nanotechnology 7 (3): 180–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro Francesca, Dasgupta Sabyasachi, Schnitker Jan, Auth Thorsten, Neumann Elmar, Panaitov Gregory, Gompper Gerhard, and Offenhäusser Andreas. 2014. “Interfacing Electrogenic Cells with 3D Nanoelectrodes: Position, Shape, and Size Matter.” ACS Nano 8(7): 6713–23. [DOI] [PubMed] [Google Scholar]
- Santoro Francesca, Neumann Elmar, Panaitov Gregory, and Offenhäusser Andreas. 2014. “FIB Section of Cell-electrode Interface: An Approach for Reducing Curtaining Effects.” Microelectronic Engineering 124: 17–21. [Google Scholar]
- Santoro Francesca, Panaitov Gregory, and Offenhäusser Andreas. 2014. “Defined Patterns of Neuronal Networks on 3D Thiol-Functionalized Microstructures.” Nano Letters 14 (12): 6906–9. [DOI] [PubMed] [Google Scholar]
- Santoro Francesca, Schnitker Jan, Panaitov Gregory, and Offenhäusser Andreas. 2013. “On Chip Guidance and Recording of Cardiomyocytes with 3D Mushroom-Shaped Electrodes.” Nano Letters 13 (11): 5379–84. [DOI] [PubMed] [Google Scholar]
- Santoro Francesca, Zhao Wenting, Joubert Lydia-Marie, Duan Liting, Schnitker Jan, van de Burgt Yoeri, Lou Hsin-Ya, et al. 2017. “Revealing the Cell-Material Interface with Nanometer Resolution by Focused Ion Beam/Scanning Electron Microscopy.” ACS Nano 11 (8): 8320–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seker Erkin, Berdichevsky Yevgeny, Begley Matthew R., Reed Michael L., Staley Kevin J., and Yarmush Martin L.. 2010. “The Fabrication of Low-Impedance Nanoporous Gold Multiple-Electrode Arrays for Neural Electrophysiology Studies.” Nanotechnology 21 (12): 125504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo Brandon B., Jahed Zeinab, Coggan Jennifer A., Yeung Yeung Chau Jacob L. Rogowski, Gu Frank X., Wen Weijia, Mofrad Mohammad R. K., and Tsui Ting Yiu. 2017. “Mechanical Contact Characteristics of PC3 Human Prostate Cancer Cells on Complex-Shaped Silicon Micropillars.” Materials 10 (8). 10.3390/ma10080892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyock Silke, Maybeck Vanessa, Scorsone Emmanuel, Rousseau Lionel, Clément Hébert Gaëlle Lissorgues, Bergonzo Philippe, and Offenhäusser Andreas. 2017. “Interfacing Neurons on Carbon Nanotubes Covered with Diamond.” RSC Advances 7 (1): 153–60. [Google Scholar]
- Shalek Alex K., Gaublomme Jellert T., Wang Lili, Yosef Nir, Chevrier Nicolas, Andersen Mette S., Robinson Jacob T., et al. 2012. “Nanowire-Mediated Delivery Enables Functional Interrogation of Primary Immune Cells: Application to the Analysis of Chronic Lymphocytic Leukemia.” Nano Letters 12 (12): 6498–6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalek Alex K., Robinson Jacob T., Karp Ethan S., Jin Seok Lee Dae-Ro Ahn, Yoon Myung-Han, Sutton Amy, et al. 2010. “Vertical Silicon Nanowires as a Universal Platform for Delivering Biomolecules into Living Cells.” Proceedings of the National Academy of Sciences of the United States of America 107 (5): 1870–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shashaani Hani, Faramarzpour Mahsa, Hassanpour Morteza, Namdar Nasser, Alikhani Alireza, and Abdolahad Mohammad. 2016. “Silicon Nanowire Based Biosensing Platform for Electrochemical Sensing of Mebendazole Drug Activity on Breast Cancer Cells.” Biosensors & Bioelectronics 85 (November): 363–70. [DOI] [PubMed] [Google Scholar]
- Shmoel Nava, Rabieh Noha, Ojovan Silviya M., Erez Hadas, Maydan Eilon, and Spira Micha E.. 2016. “Multisite Electrophysiological Recordings by Self-Assembled Loose-Patch-like Junctions between Cultured Hippocampal Neurons and Mushroom-Shaped Microelectrodes.” Scientific Reports 6 (June): 27110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shryock John C., Song Yejia, Rajamani Sridharan, Antzelevitch Charles, and Belardinelli Luiz. 2013. “The Arrhythmogenic Consequences of Increasing Late INa in the Cardiomyocyte.” Cardiovascular Research 99 (4): 600–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sileo Leonardo, Pisanello Ferruccio, Quarta Luca, Maccione Alessandro, Simi Alessandro, Berdondini Luca, De Vittorio Massimo, and Martiradonna Luigi. 2013. “Electrical Coupling of Mammalian Neurons to Microelectrodes with 3D Nanoprotrusions.” Microelectronic Engineering 111 (November): 384–90. [Google Scholar]
- Spira Micha E., Kamber Dotan, Dormann Ada, Cohen Ariel, Bartic Carmen, Borghs Gustaf, Langedijk JPM, Yitzchaik Shlomo, Shabthai Keren, and Shappir Josef. 2007. “Improved Neuronal Adhesion to the Surface of Electronic Device by Engulfment of Protruding Micro-Nails Fabricated on the Chip Surface.” In TRANSDUCERS 2007 – 2007 International Solid-State Sensors, Actuators and Microsystems Conference, 1247–50. IEEE. [Google Scholar]
- Strickholm A 1961. “Impedance of a Small Electrically Isolated Area of the Muscle Cell Surface.” The Journal of General Physiology 44 (6): 1073–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Jing, Zhang Yueyu, Kong Biao, Wang Yongcheng, Da Peimei, Li Jun, Elzatahry Ahmed A., Zhao Dongyuan, Gong Xingao, and Zheng Gengfeng. 2014. “Solar-Driven Photoelectrochemical Probing of Nanodot/nanowire/cell Interface.” Nano Letters 14 (5): 2702–8. [DOI] [PubMed] [Google Scholar]
- Toma Koji, Kano Hiroshi, and Offenhäusser Andreas. 2014. “Label-Free Measurement of Cell-Electrode Cleft Gap Distance with High Spatial Resolution Surface Plasmon Microscopy.” ACS Nano 8 (12): 12612–19. [DOI] [PubMed] [Google Scholar]
- Toma Mana, Belu Andreea, Mayer Dirk, and Offenhäusser Andreas. 2017. “Flexible Gold Nanocone Array Surfaces as a Tool for Regulating Neuronal Behavior.” Small 13 (24). 10.1002/smll.201700629. [DOI] [PubMed] [Google Scholar]
- VanDersarl Jules J., Xu Alexander M., and Melosh Nicholas A.. 2012. “Nanostraws for Direct Fluidic Intracellular Access.” Nano Letters 12 (8): 3881–86. [DOI] [PubMed] [Google Scholar]
- Van Meerbergen B, Jans K, Loo J, Reekmans G, Braeken D, Seon-Ah C, Bonroy K, et al. 2008. “Peptide-Functionalized Microfabricated Structures for Improved on-Chip Neuronal Adhesion.” Conference Proceedings:… Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Conference 2008: 1833–36. [DOI] [PubMed] [Google Scholar]
- Viela Felipe, Granados Daniel, Ayuso-Sacido Angel, and Rodríguez Isabel. 2016. “Biomechanical Cell Regulation by High Aspect Ratio Nanoimprinted Pillars.” Advanced Functional Materials 26 (31): 5599–5609. [Google Scholar]
- Wang Zixun, Yang Yang, Xu Zhen, Wang Ying, Zhang Wenjun, and Shi Peng. 2015. “Interrogation of Cellular Innate Immunity by Diamond-Nanoneedle-Assisted Intracellular Molecular Fishing.” Nano Letters 15 (10): 7058–63. [DOI] [PubMed] [Google Scholar]
- Weidlich Sabrina, Krause Kay J., Schnitker Jan, Wolfrum Bernhard, and Offenhäusser Andreas. 2017. “MEAs and 3D Nanoelectrodes: Electrodeposition as Tool for a Precisely Controlled Nanofabrication.” Nanotechnology 28 (9): 095302. [DOI] [PubMed] [Google Scholar]
- Wei Yan, Mo Xiaoju, Zhang Pengchao, Li Yingying, Liao Jingwen, Li Yongjun, Zhang Jinxing, et al. 2017. “Directing Stem Cell Differentiation via Electrochemical Reversible Switching between Nanotubes and Nanotips of Polypyrrole Array.” ACS Nano 11 (6): 5915–24. [DOI] [PubMed] [Google Scholar]
- Wesche Manuel, Hüske Martin, Yakushenko Alexey, Brüggemann Dorothea, Mayer Dirk, Offenhäusser Andreas, and Wolfrum Bernhard. 2012. “A Nanoporous Alumina Microelectrode Array for Functional Cell-Chip Coupling.” Nanotechnology 23 (49): 495303. [DOI] [PubMed] [Google Scholar]
- Wierzbicki Rafat, Carsten Købler, Mikkel R. B. Jensen, Joanna Lopacińska, Michael S. Schmidt, Skolimowski Maciej, Abeille Fabien, Qvortrup Klaus, and Kristian Mølhave. 2013. “Mapping the Complex Morphology of Cell Interactions with Nanowire Substrates Using FIB-SEM.” PloS One 8 (1): e53307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson CDW, Riehle M, Wood M, Gallagher J, and Curtis ASG. 2002. “The Use of Materials Patterned on a Nano- and Micro-Metric Scale in Cellular Engineering.” Materials Science and Engineering: C 19 (1–2): 263–69. [Google Scholar]
- Wrobel Günter, Matthias Höller, Sven Ingebrandt, Dieluweit Sabine, Sommerhage Frank, Hans Peter Bochem, and Andreas Offenhäusser. 2008. “Transmission Electron Microscopy Study of the Cell-Sensor Interface.” Journal of the Royal Society, Interface / the Royal Society 5 (19): 213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Zhuolin, Liu Jingquan, and Lee Chengkuo. 2016. “A Flexible Three-Dimensional Electrode Mesh: An Enabling Technology for Wireless Brain-computer Interface Prostheses.” Microsystems & Nanoengineering 2: 16012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Chong, Hanson Lindsey, Cui Yi, and Cui Bianxiao. 2011. “Vertical Nanopillars for Highly Localized Fluorescence Imaging.” Proceedings of the National Academy of Sciences of the United States of America 108 (10): 3894–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Chong, Hanson Lindsey, Xie Wenjun, Lin Ziliang, Cui Bianxiao, and Cui Yi. 2010. “Noninvasive Neuron Pinning with Nanopillar Arrays.” Nano Letters 10 (10): 4020–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Chong, Lin Ziliang, Hanson Lindsey, Cui Yi, and Cui Bianxiao. 2012. “Intracellular Recording of Action Potentials by Nanopillar Electroporation.” Nature Nanotechnology 7 (3): 185–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Chong, Liu Jia, Fu Tian-Ming, Dai Xiaochuan, Zhou Wei, and Lieber Charles M.. 2015. “Three-Dimensional Macroporous Nanoelectronic Networks as Minimally Invasive Brain Probes.” Nature Materials 14 (12): 1286–92. [DOI] [PubMed] [Google Scholar]
- Xie Xi, Aalipour Amin, Gupta Sneha V., and Melosh Nicholas A.. 2015. “Determining the Time Window for Dynamic Nanowire Cell Penetration Processes.” ACS Nano 9 (12): 11667–77. [DOI] [PubMed] [Google Scholar]
- Xie Xi, Xu Alexander M., Angle Matthew R., Tayebi Noureddine, Verma Piyush, and Melosh Nicholas A.. 2013. “Mechanical Model of Vertical Nanowire Cell Penetration.” Nano Letters 13 (12): 6002–8. [DOI] [PubMed] [Google Scholar]
- Xie Xi, Xu Alexander M., Leal-Ortiz Sergio, Cao Yuhong, Garner Craig C., and Melosh Nicholas A.. 2013. “Nanostraw-Electroporation System for Highly Efficient Intracellular Delivery and Transfection.” ACS Nano 7 (5): 4351–58. [DOI] [PubMed] [Google Scholar]
- Xu Alexander M., Aalipour Amin, Leal-Ortiz Sergio, Mekhdjian Armen H., Xie Xi, Dunn Alexander R., Garner Craig C., and Melosh Nicholas A.. 2014. “Quantification of Nanowire Penetration into Living Cells.” Nature Communications 5 (April): 3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Alexander M., Wang Derek S., Shieh Peyton, Cao Yuhong, and Melosh Nicholas A.. 2017. “Direct Intracellular Delivery of Cell-Impermeable Probes of Protein Glycosylation by Using Nanostraws.” Chembiochem: A European Journal of Chemical Biology 18 (7): 623–28. [DOI] [PubMed] [Google Scholar]
- Yosef Nir, Shalek Alex K., Gaublomme Jellert T., Jin Hulin, Lee Youjin, Awasthi Amit, Wu Chuan, et al. 2013. “Dynamic Regulatory Network Controlling TH17 Cell Differentiation.” Nature 496 (7446): 461–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Anqi, and Lieber Charles M.. 2016. “Nano-Bioelectronics.” Chemical Reviews 116 (1): 215–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Wenting, Hanson Lindsey, Lou Hsin-Ya, Akamatsu Matthew, Chowdary Praveen D., Santoro Francesca, Marks Jessica R., et al. 2017. “Nanoscale Manipulation of Membrane Curvature for Probing Endocytosis in Live Cells.” Nature Nanotechnology 12 (8): 750–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Wei, Dai Xiaochuan, and Lieber Charles M.. 2017. “Advances in Nanowire Bioelectronics.” Reports on Progress in Physics 80 (1): 016701. [DOI] [PubMed] [Google Scholar]
- Zhu Xiaoyue, Yuen Muk Fung, Li Yan, Zhang Zhenyu, Ai Fujin, Yang Yang, Yu Peter K. N., Zhu Guangyu, Zhang Wenjun, and Chen Xianfeng. 2016. “Intracellular Delivery: Diamond-Nanoneedle-Array-Facilitated Intracellular Delivery and the Potential Influence on Cell Physiology (Adv. Healthcare Mater. 10/2016).” Advanced Healthcare Materials 5 (10): 1116. [DOI] [PubMed] [Google Scholar]
- Zou Yang, Feng Hongqing, Ouyang Han, Jin Yiming, Yu Min, Liu Zhuo, and Li Zhou. 2017. “The Modulation Effect of the Convexity of Silicon Topological Nanostructures on the Growth of Mesenchymal Stem Cells.” RSC Advances 7 (28): 16977–83. [Google Scholar]
- Zu Yingbo, Huang Shuyan, Lu Yang, Liu Xuan, and Wang Shengnian. 2016. “Size Specific Transfection to Mammalian Cells by Micropillar Array Electroporation.” Scientific Reports 6 (December): 38661. [DOI] [PMC free article] [PubMed] [Google Scholar]